Abstract
Despite numerous clinical trials over the past 2 decades, the overall survival for children diagnosed with diffuse intrinsic pontine glioma (DIPG) remains 9–10 months. Radiation therapy is the only treatment with proven effect and novel therapies are needed. Epidermal growth factor receptor variant III (EGFRvIII) is the most common variant of the epidermal growth factor receptor and is expressed in many tumor types but is rarely found in normal tissue. A peptide vaccine targeting EGFRvIII is currently undergoing investigation in phase 3 clinical trials for the treatment of newly diagnosed glioblastoma (GBM), the tumor in which this variant receptor was first discovered. In this study, we evaluated EGFRvIII expression in pediatric DIPG samples using immunohistochemistry with a double affinity purified antibody raised against the EGFRvIII peptide. Staining of pediatric DIPG histological samples revealed expression in 4 of 9 cases and the pattern of staining was consistent with what has been seen in EGFRvIII transfected cells as well as GBMs from adult trials. In addition, analysis of tumor samples collected immediately post mortem and of DIPG cells in culture by RT-PCR, western blot analysis, and flow cytometry confirmed EGFRvIII expression. We were therefore able to detect EGFRvIII expression in 6 of 11 DIPG cases. These data suggest that EGFRvIII warrants investigation as a target for these deadly pediatric tumors.
Keywords: Diffuse intrinsic brain stem glioma (DIPG), Epidermal growth factor variant III (EGFRvIII), Immunotherapy, Cancer vaccine
Introduction
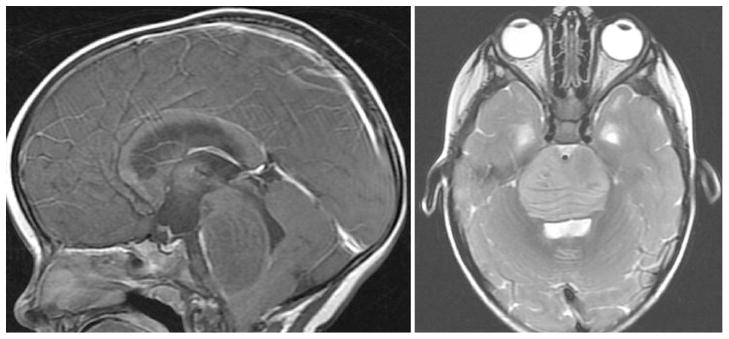
Brain tumors are the most common cause of solid cancer mortality in children [1]. Pediatric brain stem tumors account for 10–20% of pediatric brain tumors and the majority of these brain stem tumors are the diffuse intrinsic pontine glioma (DIPG) type [2, 3]. The prognosis for this group of children is dismal as median survival remains 9–10 months [4–7]. Radiation is the only modality effective in reducing tumor burden, and surgical resection has not demonstrated a role due to the diffuse spread of tumor throughout the brain stem. Over the last 20 years, there have been numerous clinical trials studying different combinations and timing of radiation and chemotherapies failing to show significant prolongation of survival [8]. One of the main difficulties in the treatment of this tumor is its highly invasive and diffuse spread through the brainstem parenchyma (Fig. 1). Under these circumstances, local therapies are ineffective and systemic therapies are limited by the blood brain barrier and lack of specificity. New systemic therapies are needed, and unique approaches from immunotherapy for other tumors have emerged over the past two decades with promising results. Immunotherapy has the advantage of being both tumor specific and systemic in delivery.
Fig. 1.
MRI Sagittal T1-post contrast (left) and Axial T2 (right) demonstrating a diffusely infiltrative brainstem lesion
The epidermal growth factor receptor (EGFR) has been shown to be important in cell growth and regulation [9]. It is amplified in a number of tumor types including glioblastoma (GBM) [10, 11]. Several compounds that specifically inhibit EGFR and other members of the family are being used as cancer therapeutics and are currently being investigated further [12]. However, the presence of EGFR on normal human tissue leads to side effects with these drugs. Epidermal growth factor variant III (EGFRvIII) is the most common variant of this receptor and is present in many different cancer types but not in normal tissue [13]. This mutation was first identified in human GBM [14, 15] where it is present in 24–67% of GBMs and has recently been detected in pediatric high grade gliomas [16]. It results from fusion of exon 1 to exon 8 of the EGFR gene which results in a novel glycine [14, 15]. EGFRvIII is constitutively active in these tumors and can lead directly to cancer phenotypes [17]. The novel peptide sequence including the glycine is the basis for a peptide vaccine targeting EGFRvIII in adult GBM, now called Rindopepimut (generic name) or CDX-110 (provisional Celldex name). EGFRvIII is an attractive cancer target as the antigen is specific to tumor cells and because cells producing EGFRvIII have an enhanced capacity for unregulated growth, survival, invasion, and recruitment of new tumor blood vessels. There have been preclinical models that have shown the efficacy of this approach for brain cancer [18, 19], and human clinical trials have demonstrated improved overall survival and an EGFRvIII specific immune response in newly diagnosed adult GBM patients treated with the vaccine[20–22].
Initial studies of DIPG demonstrated that wild type EGFR is amplified and overexpressed in DIPGs, but the study did not include analysis of EGFRvIII expression [23]. Since EGFRvIII has significant promise for the treatment of adult glial tumors, we evaluated if this variant was present in pediatric brain stem gliomas. As surgery is not indicated in this disease, however, DIPG tissue is extremely difficult to obtain. Fortunately, we were able to obtain tissue from 11 different cases. If expression of this variant receptor were present in DIPG, EGFRvIII targeted approaches against these tumors could be warranted. Based on the promising success of the EGFRvIII vaccine in GBM, this work has the potential to improve the survival of patients with this fatal pediatric brain tumor.
Materials and methods
Immunohistochemistry (IHC)
Slides from formalin-fixed, paraffin-embedded blocks of 9 diffuse intrinsic pontine glioma autopsy cases at Massachusetts General Hospital and Dana-Farber Cancer Institute were obtained under Stanford University internal review board (IRB) protocol# 15608. These slides were obtained from post mortem autopsy samples and were confirmed to be from classic DIPG patients. IHC was performed with an affinity purified EGFRvIII antibody. Slides were deparaffinized in xylene and then rehydrated with ethanol and then double distilled water (ddH20). Hydrogen peroxide was used to block unspecific sites, and the slides were placed in diva decloaker (BioCare Medical) and microwaved for antigen retrieval. After a cooling down period, the slides were blocked with background sniper universal serum (Biocare Medical), washed with PBS and then incubated with the double affinity purified EGFRvIII antibody overnight at 4°C. Slides were then washed with PBS and incubated in secondary antibody, HRP anti-Rabbit (Biocare, Medical). After another PBS wash, expression was detected by betazoid DAB (Biocare Medical). The slides were then counterstained with hematoxylin. For peptide competition reactions, the primary antibody was first incubated with 100-fold molar excess of LEEKKGNYVVTDHC peptide for 1 h at 37°C prior to the overnight incubation. Slides of GBM samples known to express EGFRvIII were used as positive controls and secondary antibody alone and peptide competition reactions were used for negative controls.
Tumor sphere culture and tumor lysates
Tumor spheres were generated from diffuse intrinsic pontine glioma tissue obtained from a 5-year-old child 14 h after death. Cells were grown under neural stem cell conditions as described in Monje et al. [24] after informed consent from the family and approval from the IRB. Briefly, tissue was dissociated by collagenase IV (1 mg/ml), treated with ACK/RBC lysis buffer (0.15 M NH4Cl, 1.0 mM KHCO3 and 0.1 mM Na2-EDTA) and resuspended in tumor stem media consisting of Neurobasal-A (Invitrogen), B27-A (Invitrogen), human-bFGF (20 ng/ml), human-EGF (20 ng/ml) (Shenandoah, Biotech), human PDGFab (20 ng/ml) (Peprotech) and heparin (10 ng/ml). Fresh growth factors were added every 3rd day and fresh media added every 5th day. Tumor tissue lysates were also obtained from this 5-year-old child as well as from a child who had succumbed to his DIPG. The pathologist performed the post mortem analysis within 8 h of his death and placed the tumor tissue into liquid nitrogen.
Reverse transcriptase polymerase chain reaction (RT-PCR)
Total RNA was extracted from DIPG tumor spheres, tumor tissue, and U87MG and U87MG-EGFRvIII cells (generous donation from Dr. Donald O’Rourke), following a standard protocol (TRIzol®, Invitrogen), and stored at −80°C. RT-PCR was performed as previously described [25]. Briefly, primers for RT-PCR were designed for EGFRvIII and glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The RT-PCR reaction mixture contained 1 μg of total RNA, deoxyribonucleotide triphosphates, QIAGEN buffer, DMSO, Roche Protector RNase inhibitor, QIAGEN onestep RT-PCR enzyme mix, and double-distilled water. The mixture was first incubated at 52°C for 45 min, 60°C for 1 min, 52°C for 30 min, and then 95°C for 15 min. The mixture was then subjected to 40 cycles. Each cycle consisted of 93°C for 30 s, 60°C for 1 min, 72°C for 45 s, with a final extension of 72°C for 10 min. PCR products were then run on a 2% agarose gel and stained with ethidium bromide.
Flow cytometry
Flow cytometry analysis was performed as previously described [25]. Briefly, tumor sphere cells were stained with a polyclonal antibody anti-EGFRvIII and anti-CD133/1-APC (Miltenyi) followed by anti-rabbit IgG-alexa-488. For intracellular staining, surface EGFRvIII was stained as before with anti-EGFRvIII and anti-rabbit IgG-PE, fixed and stained with BD cytofix and wash/perm buffer I (BD Biosciences) and subsequently stained with anti-Nestin-Alexa647 antibody (BD Biosciences). Analysis was carried out on an Aria-II FACS machine (BD Biosciences) at the Stanford University FACS facility or the Institute for Stem Cell Biology and Regenerative Medicine FACS Core. BD™ CompBeads were used for fluorescence compensation of the two dyes. Appropriate isotype controls were used to control for non-specific isotype background.
Western blot analysis
Western blot analysis was performed as previously described [25]. Tumor sphere cells and tumor tissue were lysed in a modified RIPA buffer and cleared by centrifugation. Extracted proteins were separated on 4–20% Tris–glycine SDS-PAGE gels and transferred to nitrocellulose membrane for immunoblot analysis. EGFRvIII was detected using the affinity purified anti-EGFRvIII antibody.
EGFRvIII antibody
The EGFRvIII antibody was an affinity purified antibody raised against the EGFRvIII peptide (LEEKKGNYVVTDHC) corresponding to the mutant EGFRvIII junction representing the fusion of exons 1–8 of the EGFR gene [26], which is identical to the antigen for the CDX-110 vaccine [27]. This antibody was double affinity purified, first with affinity purification to obtain antibodies against the EGFRvIII peptide. Then, this antibody preparation was passed over affinity columns containing peptides from the two normal portions of the receptor, LEEKKC and NYVVTDHC, where the terminal cysteine is added for purposes of conjugation. This double affinity purified antibody is monospecific for EGFRvIII and does not recognize wild type EGFR.
Fluorescence in situ hybridization
A passage 20 DIPG-1 neurosphere culture was harvested and dissociated using Tryple (Invitrogen) and slides prepared by standard cytogenetic methodology. A slide was pretreated for hybridization by standard protocol with the VP2000™ slide pretreatment instrument (Abbott Molecular). Briefly, the slide was incubated in 2xSSC solution at 73 °C, digested with a 10% pepsin solution at 37°C (Protease I, VP2000™ protease buffer; Abbott Molecular) and dehydrated in an ethanol series. The air-dried slide was co-denatured with the EGFR/D7Z1 dual-color probe combination (Abbot Molecular) using a Vysis® HYBrite instrument at 75°C for 1 min and hybridized for 16 h at 37°C. The hybridization was sequentially washed in 0.4×SSC pH 7.0 at 73°C for 2 min and 2×SSC/0.3% NP-40 pH 7.0 for 1 min, counterstained with DAPI and examined with an Olympus BX51 microscope equipped with a 100× oil immersion objective, appropriate fluorescent filters, and CytoVision® imaging software (Leica). The slide was then qualitatively scored for signal copy number for both target EGFR (red) and centromeric D7Z1 (green) control signals.
Results
EGFRvIII is detected in DIPG autopsy samples by IHC
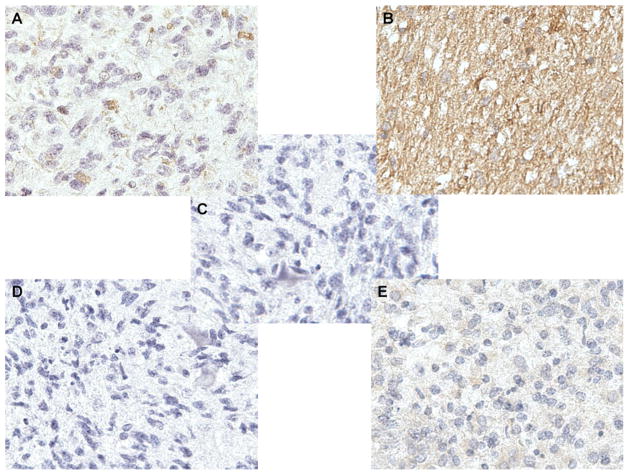
To determine the expression status of EGFRvIII in DIPG tumors we collected 9 different autopsy samples from Massachusetts General Hospital and Dana-Farber Cancer Institute and conducted immunohistochemistry (IHC) using an EGFRvIII specific antibody. The antibody was previously shown to be highly specific for EGFRvIII and does not cross react with wild type EGFR [13, 26]. IHC revealed that 4 cases had expression of EGFRvIII (Fig. 2a, b) while 5 cases had no significant staining (Fig. 2e). To verify that the EGFRvIII antibody was specific we conducted a peptide competition with a 100-fold molar excess of an EGFRvIII peptide which completely abolished staining (Fig. 2d). In addition, the 4 cases that were positive for EGFRvIII had a pattern of staining consistent with what has been seen in GBM cell lines transduced with EGFRvIII, as well as GBM samples from the adult human trials. These patterns include: localization to the perinuclear regions consistent with the golgi apparatus (Fig. 2a) and strong staining throughout the cytoplasm (Fig. 2b). Lastly, we performed a stain with the secondary alone to control for nonspecific binding (Fig. 2c). Together, our findings demonstrate that ~40% of the DIPG samples expressed EGFRvIII.
Fig. 2.
Immunohistochemistry of pediatric diffuse intrinsic pontine glioma samples reveal EGFRvIII expression. a Example of a case revealing staining in a perinuclear pattern by the EGFRvIII antibody. b Example of a case revealing a diffuse staining pattern by the EGFRvIII antibody. c A negative control example of a case being stained only by secondary antibody. d A negative control example of a case being stained by the EGFRvIII antibody after peptide competition. e Example of a tumor that did not express EGFRvIII by IHC
EGFRvIII is detected in fresh tumor samples and tumor spheres isolated from a pediatric diffuse pontine glioma
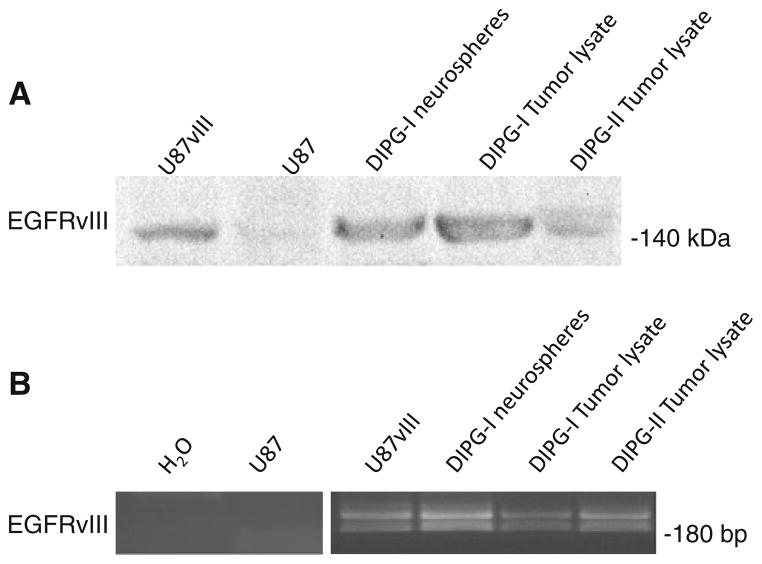
To expand on our initial findings we analyzed two pediatric DIPG. One sample came from an immediate post-mortem examination of a 5-year-old child that demonstrated an anaplastic astrocytoma, WHO grade III (DIPG-1). The second was an immediate post mortem sample placed in liquid nitrogen from a child that succumbed in an institution in Texas (DIPG-2). To determine the expression of EGFRvIII, we used the affinity purified EGFRvIII antibody. We verified the specificity of the antibody by conducting a western analysis studying a GBM cell line (U87MG) that was stably transduced with EGFRvIII (U87vIII). Consistent with previous results, only the U87vIII cells had the appropriately sized band of ~140 kDa for EGFRvIII, and no band was present that corresponded to wild type EGFR (~170 kDa) (Fig. 5a). Both DIPG-1 and DIPG-2 had EGFRvIII expression, but interestingly DIPG-1 had similar protein levels as U87vIII suggesting that both samples had high expression of EGFRvIII (Fig. 5a). To confirm that these two samples had EGFRvIII expression, we analyzed EGFRvIII mRNA levels using RT-PCR. We designed primers that yielded a 186 bp product that was unique to EGFRvIII mRNA. Our results indicate that both DIPG samples had EGFRvIII RNA, but surprisingly DIPG-1 had lower levels of mRNA compared to DIPG-2 even though DIPG-1 had high protein expression (Fig. 5b). To verify that the primers were specific, we used U87MG and U87vIII RNA samples, as well as a water control. In addition, we used primers to GAPDH to confirm that equal amounts of RNA were loaded (data not shown). Our findings reiterate that adult and pediatric DIPG tumors may have EGFRvIII expression.
Fig. 5.
Western blot and RT-PCR analysis reveals EGFRvIII expression in the diffuse pontine glioma neurospheres and tumor samples: a Western blot analysis showed the presence of EGFRvIII in DIPG neurospheres, tumor lysate and U87MG-vIII (positive control) but not in the U87MG cell lysate. b RT-PCR was performed on total RNA from DIPG neurospheres and tumor samples. Water and U87MG cells were used as a negative control and total RNA from U87MG-EGFRvIII cells were used as a positive control. EGFRvIII was detected in the tumor and neurosphere samples as well as the positive control but not the negative control
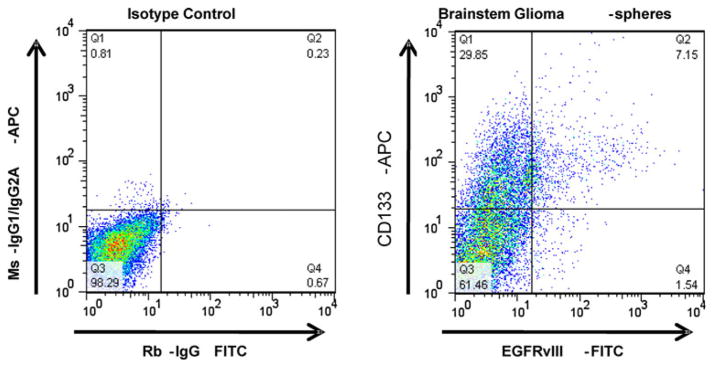
One major drawback of using established immortalized cell lines from primary tumor samples is that they often do not phenocopy the original tumor. In order to further analyze the relationship of EGFRvIII in DIPG, we needed to generate a cell line that could accurately represent this tumor. Consequently, we derived tumor spheres from the DIPG-1 sample. Tumor spheres were previously shown to recapitulate all the phenotypes seen in the original tumor making them a more ideal tumor model than other cell lines [28]. To generate these spheres, fresh tumor tissue was harvested using sterile technique and plated in a chemically defined media designed to propagate tumor spheres. These spheres contain many stem cell characteristics, including the ability to re-constitute all neural and astrocytic lineages. A recent report demonstrated that the DIPG tumor spheres derived from DIPG-1 were able to phenocopy the DIPG tumor when intracranially injected into mice [24] indicating that DIPG-1 accurately represents the DIPG tumor. Western analysis and RT-PCR analysis verified that the DIPG-1 tumor spheres had EGFRvIII expression (Fig. 5a, b). In addition, we performed fluorescence in situ hybridization (FISH) to evaluate EGFR gene amplification. Visual assessment of EGFR FISH demonstrated a majority of cells with unequivocal EGFR amplification, defined as innumerable EGFR signal (>10 signals per cell) (Fig. 3). In addition, since we were able to culture these cells, we were able to conduct flow cytometry. FACS was performed on live, unfixed passage 2 DIPG-1 tumor spheres stained with the highly specific EGFRvIII antibody (see Materials and methods for further details) followed by detection with anti-rabbit-FITC. Rabbit whole IgG was used as an IgG background control. We observed a distinct population (~9%) of cells which showed surface expression of EGFRvIII (Fig. 4). We also analyzed the tumor spheres for CD133 expression. CD133 is an established marker for cancer stem cells. This revealed that ~37% of cells were CD133+, but ~7% were positive for both markers. If there was no co-association between the two markers, the expected fraction of double positive cells would be only ~3% suggesting a potential link between EGFRvIII and cancer stem cells in DIPG. To verify the maintenance of EGFRvIII expression in this line we analyzed a late passage (>20) DIPG-1 cells for EGFRvIII expression along with a known neural stem cell marker Nestin. We found that in late passage cells EGFRvIII strongly correlated with Nestin expression suggesting a selection for EGFRvIII + tumor progenitor cells in the neurosphere culture system (Supplemental Fig. 1).
Fig. 3.
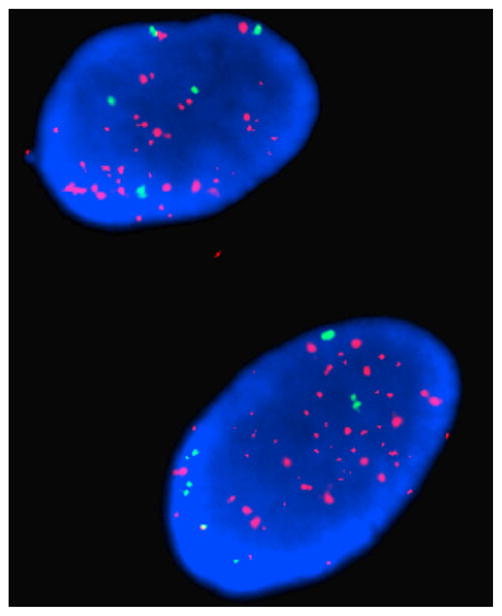
FISH reveals EGFR gene amplification. EGFR (red) and centromeric D7Z1 (green) control signals shows innumerable EGFR signal (>10 signals per cell)
Fig. 4.
Flow cytometric analysis of DIPG neurospheres reveal EGFRvIII surface expression. Live unfixed cells were analyzed for EGFRvIII surface expression using a 2-step staining protocol. Positive cells were scored as those which showed fluorescence above that of rabbit IgG alone. Flow cytometric analysis of neurospheres grown from DIPG-I showed expression of both EGFRvIII and CD133. EGFRvIII was surface expressed in about 9% of the cells
Discussion
DIPGs are highly aggressive tumors of childhood with an extremely poor prognosis. Therapeutic advances for this devastating cancer have been limited due to the poor understanding of its basic biology, secondary to a lack of available tissue or valid animal model for study. Using a variety of techniques, we have for the first time demonstrated EGFRvIII expression in 6 of 11 pediatric diffuse intrinsic pontine gliomas. Other groups have studied EGFRvIII expression in DIPG but have not been able to conclusively show its expression. One possible reason for this discrepancy is the lack of highly specific commercially available antibodies for EGFRvIII. Many of these antibodies cross react with the wild type EGFR or other non-specific proteins or comparatively have low affinity when used for IHC. The use of these commercially available antibodies might suggest that the DIPG case has amplification and enhanced expression of wild type EGFR, but not EGFRvIII expression. For example, through our findings we discovered that the DIPG-1 derived cells had EGFR amplification (Fig. 3). However, by using our highly specific EGFRvIII antibody we demonstrated expression of this variant (Fig. 5). The EGFRvIII antibody used in this study was thoroughly tested to verify that it does not cross react with other antigens [26]. Even though our antibody is specific it is still limited by the abundance of the protein and the accessibility of the antigen region. To this end we also analyzed the mRNA levels in our samples using RT-PCR to verify EGFRvIII expression.
As previously mentioned, one of the most difficult aspects of studying DIPG is the low availability of tumor samples. A larger sample size would be ideal, however, DIPG tissue is extremely difficult to obtain as it is an inoperable disease. We spent 2 years obtaining the 11 samples in our study. While our initial sample size (n = 9) was small and the analysis was limited to IHC, we were able to discover two additional tumor samples from primary pediatric DIPG patients which allowed us to confirm our results with RT-PCR, western blot and flow cytometry. These two samples reinforce the finding that EGFRvIII expression occurs in DIPG and eliminates potential artifacts that may have occurred due to preparation of the 9 samples from Massachusetts General Hospital and Dana-Farber Cancer Institute. In addition, while our results demonstrate that EGFRvIII is present in the 6 of 11 tumors, we do not know conclusively that the expression levels are adequate for targeting. Our results do show that DIPG-1 had similar protein expression as a cell line stably transduced with EGFRvIII (U87vIII). This cell line has been used as a positive control to screen for EGFRvIII antibodies and for genetically modified T-lymphocytes redirected to kill EGFRvIII expressing gliomas [29]. Consequently, our findings suggest that at least a population of DIPG tumors may have expression that is readily high enough for therapeutic targeting.
The data presented in this study suggests that EGFRvIII warrants investigation as a target for these deadly pediatric tumors. We are currently recruiting children with newly diagnosed diffuse intrinsic pontine gliomas into a phase 1 trial where they will receive the EGFRvIII peptide vaccine after conventional radiation therapy (http://med.stanford.edu/clinicaltrials/detail.do?studyId=4642).
Supplementary Material
Acknowledgments
We would like to acknowledge Dr. David Louis and Dr. Keith Ligon for providing the paraffin embedded slides from Massachusetts General Hospital and Dana-Farber Cancer Institute. We would like to thank Dr. Hannes Vogel for reviewing the immunohistochemistry slides.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s11060-012-0842-3) contains supplementary material, which is available to authorized users.
Conflict of interest The authors declare that they have no conflict of interest with the sponsor of this research.
Contributor Information
Gordon Li, Email: gordonli@stanford.edu, Department of Neurosurgery, Stanford University School of Medicine, 1201 Welch Rd, MSLS, P309, Stanford, CA 94305, USA.
Siddhartha S. Mitra, Department of Neurosurgery, Stanford University Medical Center, 300 Pasteur Drive, Stanford, CA 94305, USA. Institute of Stem Cell Biology and Regenerative Medicine, Ludwig Center for Cancer Stem Research and Medicine, Stanford University Medical Center, 300 Pasteur Drive, Stanford, CA 94305, USA
Michelle Monje, Department of Neurology, Stanford University Medical Center, 300 Pasteur Drive, Stanford, CA 94305, USA.
Kristy N. Henrich, Department of Neurosurgery, Stanford University Medical Center, 300 Pasteur Drive, Stanford, CA 94305, USA
C. Dana Bangs, Department of Pathology, Cytogenetics Laboratory, Stanford University Medical Center, 300 Pasteur Drive, Stanford, CA 94305, USA.
Ryan T. Nitta, Department of Neurosurgery, Stanford University Medical Center, 300 Pasteur Drive, Stanford, CA 94305, USA
Albert J. Wong, Email: ajwong@stanford.edu, Department of Neurosurgery, Stanford University Medical Center, 300 Pasteur Drive, Stanford, CA 94305, USA
References
- 1.Central brain tumor registry of the United States. 2009. [Google Scholar]
- 2.Freeman CR, Farmer JP. Pediatric brain stem gliomas: a review. Int J Radiat Oncol Biol Phys. 1998;40(2):265. doi: 10.1016/s0360-3016(97)00572-5. [DOI] [PubMed] [Google Scholar]
- 3.Recinos PF, Sciubba DM, Jallo GI. Brainstem tumors: where are we today? Pediatr Neurosurg. 2007;43(3):192. doi: 10.1159/000098831. [DOI] [PubMed] [Google Scholar]
- 4.Bernier-Chastagner V, et al. Topotecan as a radiosensitizer in the treatment of children with malignant diffuse brainstem gliomas: results of a French Society of Paediatric Oncology Phase II Study. Cancer. 2005;104(12):2792. doi: 10.1002/cncr.21534. [DOI] [PubMed] [Google Scholar]
- 5.Korones DN, et al. Treatment of children with diffuse intrinsic brain stem glioma with radiotherapy, vincristine and oral VP-16: a Children’s Oncology Group phase II study. Pediatr Blood Cancer. 2008;50(2):227. doi: 10.1002/pbc.21154. [DOI] [PubMed] [Google Scholar]
- 6.Mandell LR, et al. There is no role for hyperfractionated radiotherapy in the management of children with newly diagnosed diffuse intrinsic brainstem tumors: results of a Pediatric Oncology Group phase III trial comparing conventional vs. hyperfractionated radiotherapy. Int J Radiat Oncol Biol Phys. 1999;43(5):959. doi: 10.1016/s0360-3016(98)00501-x. [DOI] [PubMed] [Google Scholar]
- 7.Packer RJ, et al. Outcome of children with brain stem gliomas after treatment with 7800 cGy of hyperfractionated radiotherapy. A Childrens Cancer Group Phase I/II Trial. Cancer. 1994;74(6):1827. doi: 10.1002/1097-0142(19940915)74:6<1827::aid-cncr2820740628>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 8.Hargrave D, Bartels U, Bouffet E. Diffuse brainstem glioma in children: critical review of clinical trials. Lancet Oncol. 2006;7(3):241. doi: 10.1016/S1470-2045(06)70615-5. [DOI] [PubMed] [Google Scholar]
- 9.Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer. 2005;5(5):341. doi: 10.1038/nrc1609. [DOI] [PubMed] [Google Scholar]
- 10.Libermann TA, et al. Amplification, enhanced expression and possible rearrangement of EGF receptor gene in primary human brain tumours of glial origin. Nature. 1985;313(5998):144. doi: 10.1038/313144a0. [DOI] [PubMed] [Google Scholar]
- 11.Wong AJ, et al. Increased expression of the epidermal growth factor receptor gene in malignant gliomas is invariably associated with gene amplification. Proc Natl Acad Sci USA. 1987;84(19):6899. doi: 10.1073/pnas.84.19.6899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ciardiello F, Tortora G. EGFR antagonists in cancer treatment. N Engl J Med. 2008;358(11):1160. doi: 10.1056/NEJMra0707704. [DOI] [PubMed] [Google Scholar]
- 13.Moscatello DK, et al. Frequent expression of a mutant epidermal growth factor receptor in multiple human tumors. Cancer Res. 1995;55(23):5536. [PubMed] [Google Scholar]
- 14.Humphrey PA, et al. Anti-synthetic peptide antibody reacting at the fusion junction of deletion-mutant epidermal growth factor receptors in human glioblastoma. Proc Natl Acad Sci USA. 1990;87(11):4207. doi: 10.1073/pnas.87.11.4207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong AJ, et al. Structural alterations of the epidermal growth factor receptor gene in human gliomas. Proc Natl Acad Sci USA. 1992;89(7):2965. doi: 10.1073/pnas.89.7.2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bax DA, et al. EGFRvIII deletion mutations in pediatric high-grade glioma and response to targeted therapy in pediatric glioma cell lines. CLin Cancer Res. 2009;15(18):5753. doi: 10.1158/1078-0432.CCR-08-3210. [DOI] [PubMed] [Google Scholar]
- 17.Li G, Wong AJ. EGF receptor variant III as a target antigen for tumor immunotherapy Expert. Rev Vaccines. 2008;7(7):977. doi: 10.1586/14760584.7.7.977. [DOI] [PubMed] [Google Scholar]
- 18.Heimberger AB, et al. Epidermal growth factor receptor VIII peptide vaccination is efficacious against established intra-cerebral tumors. Clin Cancer Res. 2003;9(11):4247. [PubMed] [Google Scholar]
- 19.Moscatello DK, Ramirez G, Wong AJ. A naturally occurring mutant human epidermal growth factor receptor as a target for peptide vaccine immunotherapy of tumors. Cancer Res. 1997;57(8):1419. [PubMed] [Google Scholar]
- 20.Sampson JH, et al. American society of clinical oncology. Chicago: 2008. Effect of EGFRvIII-targeted vaccine (CDX-110) on immune response and TTP when given with simultaneous standard and continuous temozolomide in patients with GBM; p. 26. [Google Scholar]
- 21.Sampson JH, et al. Tumor-specific immunotherapy targeting the EGFRvIII mutation in patients with malignant glioma. Semin Immunol. 2008;20(5):267. doi: 10.1016/j.smim.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmittling RJ, et al. Detection of humoral response in patients with glioblastoma receiving EGFRvIII-KLH vaccines. J Immunol Methods. 2008;339(1):74. doi: 10.1016/j.jim.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 23.Gilbertson RJ, et al. ERBB1 is amplified and overexpressed in high-grade diffusely infiltrative pediatric brain stem glioma. CLin Cancer Res. 2003;9(10 Pt 1):3620. [PubMed] [Google Scholar]
- 24.Monje M, et al. Hedgehog-responsive candidate cell of origin for diffuse intrinsic pontine glioma. Proc Natl Acad Sci USA. 2011;108(11):4453. doi: 10.1073/pnas.1101657108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Gordon, et al. Pineal parenchymal tumor of intermediate differentiation: clinicopathologic report and evaluation of epidermal growth factor receptor variant III expression. Neurosurgery. 2010;66(5):963–968. doi: 10.1227/01.NEU.0000367726.49003.F1. [DOI] [PubMed] [Google Scholar]
- 26.Moscatello DK, et al. Frequent expression of a mutant epidermal growth factor receptor in multiple human tumors. Cancer Res. 1995;55(23):5536. [PubMed] [Google Scholar]
- 27.Okamoto I, et al. Expression of constitutively activated EGFRvIII in non-small cell lung cancer. Cancer Sci. 2003;94(1):50. doi: 10.1111/j.1349-7006.2003.tb01351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guerrero-Cazares H, Chaichana KL, Quinones-Hinojosa A. Neurosphere culture and human organotypic model to evaluate brain tumor stem cells. Methods Mol Biol. 2009;568:73–83. doi: 10.1007/978-1-59745-280-9_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bullain SS, et al. Genetically engineered T cells to target EGFRvIII expressing glioblastoma. J Neurooncol. 2009;94(3):373. doi: 10.1007/s11060-009-9889-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.