Abstract
Two tRNA molecules at the ribosomal A- and P-sites, with a relatively small angle between the planes of the L-shaped molecules, can be arranged in two mutually exclusive orientations. In one (the 'R'-configuration), the T-loop of the A-site tRNA faces the D-loop of the P-site tRNA, whereas in the other (the 'S'-configuration) the D-loop of the A-site tRNA faces the T-loop of the P-site tRNA. A number of stereochemical arguments, based on the crystal structure of 'free' tRNA, favour the R-configuration. In the ribosome, the CCA-ends of the tRNA molecules are 'fixed' at the base of the central protuberance (the peptidyl transferase centre) of the 50S subunit, and the anticodon loops lie in the neck region (the decoding site) of the 30S subunit. The translocation step is essentially a rotational movement of the tRNA from the A- to the P-site, and there is convincing evidence that the A-site must be located nearest to the L7/L12 protuberance of the 50S subunit. The mRNA in the two codon-anticodon duplexes lies on the 'inside' of the 'elbows' of the tRNA molecules (in both the S-type and R-type configurations), and runs up between the two molecules from the A- to the P-site in the 3' to 5'-direction. These considerations have the consequence that in the S-configuration the mRNA in the codon-anticodon duplexes is directed towards the 50S subunit, whereas in the R-configuration it is directed towards the 30S subunit. The results of site-directed cross-linking experiments, in particular cross-links to mRNA at positions within or very close to the codons interacting with A- or P-site tRNA, favour the latter situation. This conclusion is in direct contradiction to other current models for the arrangement of mRNA and tRNA on the ribosome.
Full text
PDF
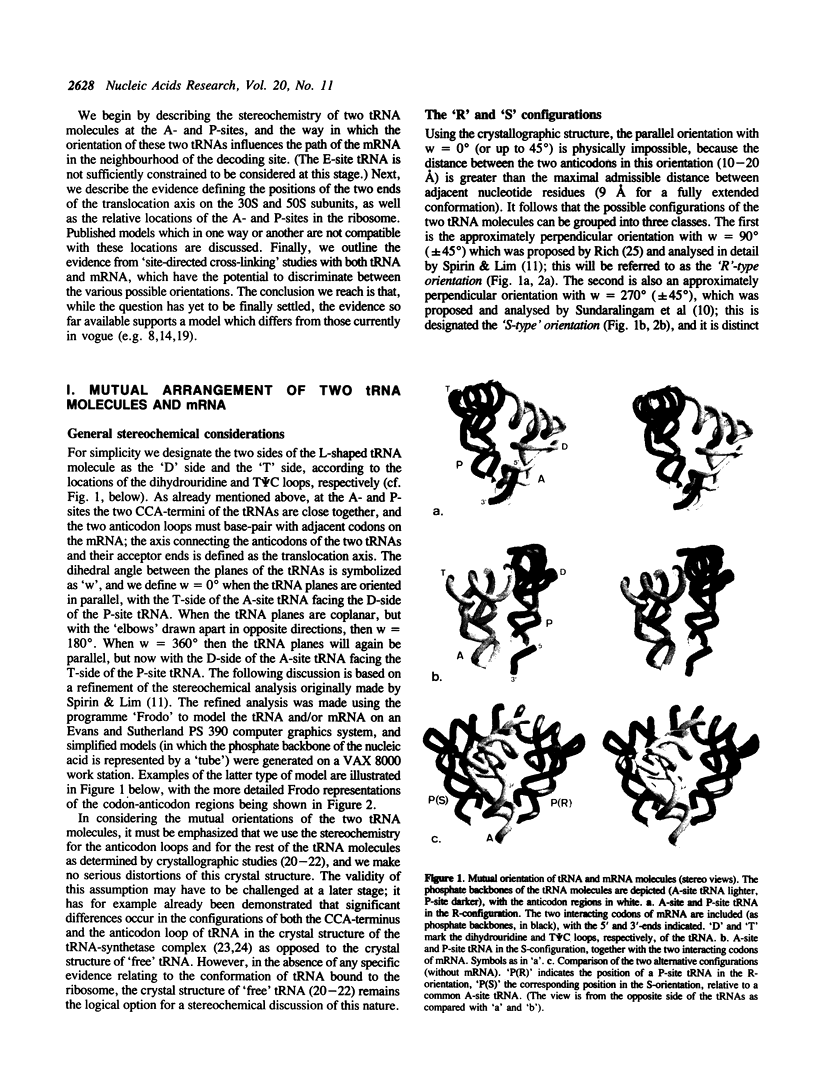






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdurashidova G. G., Tsvetkova E. A., Budowsky E. I. Determination of tRNA nucleotide residues directly interacting with proteins in the post- and pretranslocated ribosomal complexes. FEBS Lett. 1990 Sep 3;269(2):398–401. doi: 10.1016/0014-5793(90)81202-y. [DOI] [PubMed] [Google Scholar]
- Abdurashidova G. G., Tsvetkova E. A., Budowsky E. I. Direct tRNA-protein interactions in ribosomal complexes. Nucleic Acids Res. 1991 Apr 25;19(8):1909–1915. doi: 10.1093/nar/19.8.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdurashidova G. G., Tsvetkova E. A., Budowsky E. I. Nucleotide residues of tRNA, directly interacting with proteins within the complex of the 30 S subunit of E. coli ribosome with poly(U) and NAcPhe-tRNA(Phe). FEBS Lett. 1989 Jan 30;243(2):299–302. doi: 10.1016/0014-5793(89)80149-8. [DOI] [PubMed] [Google Scholar]
- Barta A., Steiner G., Brosius J., Noller H. F., Kuechler E. Identification of a site on 23S ribosomal RNA located at the peptidyl transferase center. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3607–3611. doi: 10.1073/pnas.81.12.3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernabeu C., Lake J. A. Nascent polypeptide chains emerge from the exit domain of the large ribosomal subunit: immune mapping of the nascent chain. Proc Natl Acad Sci U S A. 1982 May;79(10):3111–3115. doi: 10.1073/pnas.79.10.3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boublik M., Hellmann W., Roth H. E. Localization of ribosomal proteins L7L12 in the 50 S subunit of Escherichia coli Ribosomes by electron microscopy. J Mol Biol. 1976 Nov 15;107(4):479–490. doi: 10.1016/s0022-2836(76)80079-4. [DOI] [PubMed] [Google Scholar]
- Boutorin A. S., Clark B. F., Ebel J. P., Kruse T. A., Petersen H. U., Remy P., Vassilenko S. A study of the interaction of Escherichia coli elongation factor-Tu with aminoacyl-tRNAs by partial digestion with cobra venom ribonuclease. J Mol Biol. 1981 Nov 5;152(3):593–608. doi: 10.1016/0022-2836(81)90271-0. [DOI] [PubMed] [Google Scholar]
- Branlant C., Korobko V., Ebel J. P. The binding site of protein L1 on 23-S ribosomal RNA from Escherichia coli. 3. Nucleotide sequence. Eur J Biochem. 1976 Nov 15;70(2):471–482. doi: 10.1111/j.1432-1033.1976.tb11038.x. [DOI] [PubMed] [Google Scholar]
- Brimacombe R., Atmadja J., Stiege W., Schüler D. A detailed model of the three-dimensional structure of Escherichia coli 16 S ribosomal RNA in situ in the 30 S subunit. J Mol Biol. 1988 Jan 5;199(1):115–136. doi: 10.1016/0022-2836(88)90383-x. [DOI] [PubMed] [Google Scholar]
- Capel M. S., Kjeldgaard M., Engelman D. M., Moore P. B. Positions of S2, S13, S16, S17, S19 and S21 in the 30 S ribosomal subunit of Escherichia coli. J Mol Biol. 1988 Mar 5;200(1):65–87. doi: 10.1016/0022-2836(88)90334-8. [DOI] [PubMed] [Google Scholar]
- De Groot N., Panet A., Lapidot Y. The binding of purified Phe-tRNA and peptidyl-tRNA Phe to Escherichia coli ribosomes. Eur J Biochem. 1971 Dec 10;23(3):523–527. doi: 10.1111/j.1432-1033.1971.tb01649.x. [DOI] [PubMed] [Google Scholar]
- Dontsova O., Kopylov A., Brimacombe R. The location of mRNA in the ribosomal 30S initiation complex; site-directed cross-linking of mRNA analogues carrying several photo-reactive labels simultaneously on either side of the AUG start codon. EMBO J. 1991 Sep;10(9):2613–2620. doi: 10.1002/j.1460-2075.1991.tb07803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douthwaite S., Garrett R. A., Wagner R. Comparison of Escherichia coli tRNAPhe in the free state, in the ternary complex and in the ribosomal A and P sites by chemical probing. Eur J Biochem. 1983 Mar 15;131(2):261–269. doi: 10.1111/j.1432-1033.1983.tb07258.x. [DOI] [PubMed] [Google Scholar]
- Döring T., Greuer B., Brimacombe R. The three-dimensional folding of ribosomal RNA; localization of a series of intra-RNA cross-links in 23S RNA induced by treatment of Escherichia coli 50S ribosomal subunits with bis-(2-chloroethyl)-methylamine. Nucleic Acids Res. 1991 Jul 11;19(13):3517–3524. doi: 10.1093/nar/19.13.3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evstafieva A. G., Shatsky I. N., Bogdanov A. A., Semenkov Y. P., Vasiliev V. D. Localization of 5' and 3' ends of the ribosome-bound segment of template polynucleotides by immune electron microscopy. EMBO J. 1983;2(5):799–804. doi: 10.1002/j.1460-2075.1983.tb01503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank J., Penczek P., Grassucci R., Srivastava S. Three-dimensional reconstruction of the 70S Escherichia coli ribosome in ice: the distribution of ribosomal RNA. J Cell Biol. 1991 Nov;115(3):597–605. doi: 10.1083/jcb.115.3.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank J., Radermacher M., Wagenknecht T., Verschoor A. Studying ribosome structure by electron microscopy and computer-image processing. Methods Enzymol. 1988;164:3–35. doi: 10.1016/s0076-6879(88)64032-8. [DOI] [PubMed] [Google Scholar]
- Gebhardt-Singh E., Sprinzl M. Ser-tRNAs from bovine mitochondrion form ternary complexes with bacterial elongation factor Tu and GTP. Nucleic Acids Res. 1986 Sep 25;14(18):7175–7188. doi: 10.1093/nar/14.18.7175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girshovich A. S., Bochkareva E. S., Vasiliev V. D. Localization of elongation factor Tu on the ribosome. FEBS Lett. 1986 Mar 3;197(1-2):192–198. doi: 10.1016/0014-5793(86)80325-8. [DOI] [PubMed] [Google Scholar]
- Girshovich A. S., Kurtskhalia T. V., Ovchinnikov YuA, Vasiliev V. D. Localization of the elongation factor G on Escherichia coli ribosome. FEBS Lett. 1981 Jul 20;130(1):54–59. doi: 10.1016/0014-5793(81)80664-3. [DOI] [PubMed] [Google Scholar]
- Gold L. Posttranscriptional regulatory mechanisms in Escherichia coli. Annu Rev Biochem. 1988;57:199–233. doi: 10.1146/annurev.bi.57.070188.001215. [DOI] [PubMed] [Google Scholar]
- Gornicki P., Nurse K., Hellmann W., Boublik M., Ofengand J. High resolution localization of the tRNA anticodon interaction site on the Escherichia coli 30 S ribosomal subunit. J Biol Chem. 1984 Aug 25;259(16):10493–10498. [PubMed] [Google Scholar]
- Hausner T. P., Atmadja J., Nierhaus K. H. Evidence that the G2661 region of 23S rRNA is located at the ribosomal binding sites of both elongation factors. Biochimie. 1987 Sep;69(9):911–923. doi: 10.1016/0300-9084(87)90225-2. [DOI] [PubMed] [Google Scholar]
- Johnson A. E., Adkins H. J., Matthews E. A., Cantor C. R. Distance moved by transfer RNA during translocation from the A site to the P site on the ribosome. J Mol Biol. 1982 Mar 25;156(1):113–140. doi: 10.1016/0022-2836(82)90462-4. [DOI] [PubMed] [Google Scholar]
- Joshi R. L., Faulhammer H., Chapeville F., Sprinzl M., Haenni A. L. Aminoacyl RNA domain of turnip yellow mosaic virus Val-RNA interacting with elongation factor Tu. Nucleic Acids Res. 1984 Oct 11;12(19):7467–7478. doi: 10.1093/nar/12.19.7467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao T., Miller D. L., Abo M., Ofengand J. Formation and properties of a covalent complex between elongation factor Tu and Phe-tRNA bearing a photoaffinity probe on its 3-(3-amino-3-carboxypropyl)uridine residue. J Mol Biol. 1983 May 25;166(3):383–405. doi: 10.1016/s0022-2836(83)80091-6. [DOI] [PubMed] [Google Scholar]
- Kiesewetter S., Ott G., Sprinzl M. The role of modified purine 64 in initiator/elongator discrimination of tRNA(iMet) from yeast and wheat germ. Nucleic Acids Res. 1990 Aug 25;18(16):4677–4682. doi: 10.1093/nar/18.16.4677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. H., Suddath F. L., Quigley G. J., McPherson A., Sussman J. L., Wang A. H., Seeman N. C., Rich A. Three-dimensional tertiary structure of yeast phenylalanine transfer RNA. Science. 1974 Aug 2;185(4149):435–440. doi: 10.1126/science.185.4149.435. [DOI] [PubMed] [Google Scholar]
- Kirillov S. V., Makarov E. M., Semenkov YuP Quantitative study of interaction of deacylated tRNA with Escherichia coli ribosomes. Role of 50 S subunits in formation of the E site. FEBS Lett. 1983 Jun 27;157(1):91–94. doi: 10.1016/0014-5793(83)81122-3. [DOI] [PubMed] [Google Scholar]
- Lake J. A. Aminoacyl-tRNA binding at the recognition site is the first step of the elongation cycle of protein synthesis. Proc Natl Acad Sci U S A. 1977 May;74(5):1903–1907. doi: 10.1073/pnas.74.5.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake J. A. Ribosome structure determined by electron microscopy of Escherichia coli small subunits, large subunits and monomeric ribosomes. J Mol Biol. 1976 Jul 25;105(1):131–139. doi: 10.1016/0022-2836(76)90200-x. [DOI] [PubMed] [Google Scholar]
- Lührmann R., Eckhardt H., Stöffler G. Codon-anticodon interaction at the ribosomal peptidyl-site. Nature. 1979 Aug 2;280(5721):423–425. doi: 10.1038/280423a0. [DOI] [PubMed] [Google Scholar]
- Maassen J. A., Möller W. Elongation factor G-dependent binding of a photoreactive GTP analogue to Escherichia coli ribosomes results in labeling of protein L11. J Biol Chem. 1978 Apr 25;253(8):2777–2783. [PubMed] [Google Scholar]
- Maassen J. A., Möller W. Photochemical cross-linking of elongation factor G to 70-S ribosomes from Escherichia coli by 4-(6-formyl-3-azidophenoxy)butyrimidate. Eur J Biochem. 1981 Apr;115(2):279–285. doi: 10.1111/j.1432-1033.1981.tb05235.x. [DOI] [PubMed] [Google Scholar]
- Malkin L. I., Rich A. Partial resistance of nascent polypeptide chains to proteolytic digestion due to ribosomal shielding. J Mol Biol. 1967 Jun 14;26(2):329–346. doi: 10.1016/0022-2836(67)90301-4. [DOI] [PubMed] [Google Scholar]
- McDonald J. J., Rein R. A stereochemical model of the transpeptidation complex. J Biomol Struct Dyn. 1987 Apr;4(5):729–744. doi: 10.1080/07391102.1987.10507675. [DOI] [PubMed] [Google Scholar]
- Mitchell P., Osswald M., Brimacombe R. Identification of intermolecular RNA cross-links at the subunit interface of the Escherichia coli ribosome. Biochemistry. 1992 Mar 24;31(11):3004–3011. doi: 10.1021/bi00126a023. [DOI] [PubMed] [Google Scholar]
- Mitchell P., Osswald M., Schueler D., Brimacombe R. Selective isolation and detailed analysis of intra-RNA cross-links induced in the large ribosomal subunit of E. coli: a model for the tertiary structure of the tRNA binding domain in 23S RNA. Nucleic Acids Res. 1990 Aug 11;18(15):4325–4333. doi: 10.1093/nar/18.15.4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moazed D., Noller H. F. Binding of tRNA to the ribosomal A and P sites protects two distinct sets of nucleotides in 16 S rRNA. J Mol Biol. 1990 Jan 5;211(1):135–145. doi: 10.1016/0022-2836(90)90016-F. [DOI] [PubMed] [Google Scholar]
- Moazed D., Noller H. F. Interaction of tRNA with 23S rRNA in the ribosomal A, P, and E sites. Cell. 1989 May 19;57(4):585–597. doi: 10.1016/0092-8674(89)90128-1. [DOI] [PubMed] [Google Scholar]
- Moazed D., Noller H. F. Sites of interaction of the CCA end of peptidyl-tRNA with 23S rRNA. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3725–3728. doi: 10.1073/pnas.88.9.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moazed D., Robertson J. M., Noller H. F. Interaction of elongation factors EF-G and EF-Tu with a conserved loop in 23S RNA. Nature. 1988 Jul 28;334(6180):362–364. doi: 10.1038/334362a0. [DOI] [PubMed] [Google Scholar]
- Moras D., Comarmond M. B., Fischer J., Weiss R., Thierry J. C., Ebel J. P., Giegé R. Crystal structure of yeast tRNAAsp. Nature. 1980 Dec 25;288(5792):669–674. doi: 10.1038/288669a0. [DOI] [PubMed] [Google Scholar]
- Nagano K., Takagi H., Harel M. The side-by-side model of two tRNA molecules allowing the alpha-helical conformation of the nascent polypeptide during the ribosomal transpeptidation. Biochimie. 1991 Jul-Aug;73(7-8):947–960. doi: 10.1016/0300-9084(91)90136-o. [DOI] [PubMed] [Google Scholar]
- Olson H. M., Grant P. G., Cooperman B. S., Glitz D. G. Immunoelectron microscopic localization of puromycin binding on the large subunit of the Escherichia coli ribosome. J Biol Chem. 1982 Mar 10;257(5):2649–2656. [PubMed] [Google Scholar]
- Olson H. M., Lasater L. S., Cann P. A., Glitz D. G. Messenger RNA orientation on the ribosome. Placement by electron microscopy of antibody-complementary oligodeoxynucleotide complexes. J Biol Chem. 1988 Oct 15;263(29):15196–15204. [PubMed] [Google Scholar]
- Olson H. M., Nicholson A. W., Cooperman B. S., Glitz D. G. Localization of sites of photoaffinity labeling of the large subunit of Escherichia coli ribosomes by arylazide derivative of puromycin. J Biol Chem. 1985 Aug 25;260(18):10326–10331. [PubMed] [Google Scholar]
- Olson H. M., Olah T. V., Cooperman B. S., Glitz D. G. Immune electron microscopic localization of dinitrophenyl-modified ribosomal protein S19 in reconstituted Escherichia coli 30 S subunits using antibodies to dinitrophenol. J Biol Chem. 1988 Apr 5;263(10):4801–4806. [PubMed] [Google Scholar]
- Paulsen H., Robertson J. M., Wintermeyer W. Topological arrangement of two transfer RNAs on the ribosome. Fluorescence energy transfer measurements between A and P site-bound tRNAphe. J Mol Biol. 1983 Jun 25;167(2):411–426. doi: 10.1016/s0022-2836(83)80342-8. [DOI] [PubMed] [Google Scholar]
- Podkowinski J., Gornicki P. Neighbourhood of the central fold of the tRNA molecule bound to the E. coli ribosome--affinity labeling studies with modified tRNAs carrying photoreactive probes attached to the dihydrouridine loop. Nucleic Acids Res. 1991 Feb 25;19(4):801–808. doi: 10.1093/nar/19.4.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podkowiński J., Górnicki P. Ribosomal proteins S7 and L1 are located close to the decoding site of E. coli ribosome--affinity labeling studies with modified tRNAs carrying photoreactive probes attached adjacent to the 3'-end of the anticodon. Nucleic Acids Res. 1989 Nov 11;17(21):8767–8782. doi: 10.1093/nar/17.21.8767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabahakaran M., Harvey S. C. Models for two tRNAs bound to successive codons on mRNA on the ribosome. J Biomol Struct Dyn. 1989 Aug;7(1):167–179. doi: 10.1080/07391102.1989.10507758. [DOI] [PubMed] [Google Scholar]
- Prince J. B., Taylor B. H., Thurlow D. L., Ofengand J., Zimmermann R. A. Covalent crosslinking of tRNA1Val to 16S RNA at the ribosomal P site: identification of crosslinked residues. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5450–5454. doi: 10.1073/pnas.79.18.5450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rheinberger H. J., Sternbach H., Nierhaus K. H. Three tRNA binding sites on Escherichia coli ribosomes. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5310–5314. doi: 10.1073/pnas.78.9.5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinke-Appel J., Jünke N., Stade K., Brimacombe R. The path of mRNA through the Escherichia coli ribosome; site-directed cross-linking of mRNA analogues carrying a photo-reactive label at various points 3' to the decoding site. EMBO J. 1991 Aug;10(8):2195–2202. doi: 10.1002/j.1460-2075.1991.tb07755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson J. M., Wintermeyer W. Effect of translocation on topology and conformation of anticodon and D loops of tRNAPhe. J Mol Biol. 1981 Sep 5;151(1):57–79. doi: 10.1016/0022-2836(81)90221-7. [DOI] [PubMed] [Google Scholar]
- Robertus J. D., Ladner J. E., Finch J. T., Rhodes D., Brown R. S., Clark B. F., Klug A. Structure of yeast phenylalanine tRNA at 3 A resolution. Nature. 1974 Aug 16;250(467):546–551. doi: 10.1038/250546a0. [DOI] [PubMed] [Google Scholar]
- Rould M. A., Perona J. J., Söll D., Steitz T. A. Structure of E. coli glutaminyl-tRNA synthetase complexed with tRNA(Gln) and ATP at 2.8 A resolution. Science. 1989 Dec 1;246(4934):1135–1142. doi: 10.1126/science.2479982. [DOI] [PubMed] [Google Scholar]
- Ruff M., Krishnaswamy S., Boeglin M., Poterszman A., Mitschler A., Podjarny A., Rees B., Thierry J. C., Moras D. Class II aminoacyl transfer RNA synthetases: crystal structure of yeast aspartyl-tRNA synthetase complexed with tRNA(Asp). Science. 1991 Jun 21;252(5013):1682–1689. doi: 10.1126/science.2047877. [DOI] [PubMed] [Google Scholar]
- Ryabova L. A., Selivanova O. M., Baranov V. I., Vasiliev V. D., Spirin A. S. Does the channel for nascent peptide exist inside the ribosome? Immune electron microscopy study. FEBS Lett. 1988 Jan 4;226(2):255–260. doi: 10.1016/0014-5793(88)81434-0. [DOI] [PubMed] [Google Scholar]
- Shatsky I. N., Bakin A. V., Bogdanov A. A., Vasiliev V. D. How does the mRNA pass through the ribosome? Biochimie. 1991 Jul-Aug;73(7-8):937–945. doi: 10.1016/0300-9084(91)90135-n. [DOI] [PubMed] [Google Scholar]
- Sköld S. E. Chemical cross-linking of elongation factor G to both subunits of the 70-S ribosomes from Escherichia coli. Eur J Biochem. 1982 Oct;127(2):225–229. doi: 10.1111/j.1432-1033.1982.tb06859.x. [DOI] [PubMed] [Google Scholar]
- Smith D., Yarus M. tRNA-tRNA interactions within cellular ribosomes. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4397–4401. doi: 10.1073/pnas.86.12.4397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spirin A. S. Location of tRNA on the ribosome. FEBS Lett. 1983 Jun 13;156(2):217–221. doi: 10.1016/0014-5793(83)80499-2. [DOI] [PubMed] [Google Scholar]
- Spirin A. S. Ribosomal translocation: facts and models. Prog Nucleic Acid Res Mol Biol. 1985;32:75–114. doi: 10.1016/s0079-6603(08)60346-3. [DOI] [PubMed] [Google Scholar]
- Sprinzl M., Wagner T., Lorenz S., Erdmann V. A. Regions of tRNA important for binding to the ribosomal A and P sites. Biochemistry. 1976 Jul 13;15(14):3031–3039. doi: 10.1021/bi00659a015. [DOI] [PubMed] [Google Scholar]
- Stade K., Rinke-Appel J., Brimacombe R. Site-directed cross-linking of mRNA analogues to the Escherichia coli ribosome; identification of 30S ribosomal components that can be cross-linked to the mRNA at various points 5' with respect to the decoding site. Nucleic Acids Res. 1989 Dec 11;17(23):9889–9908. doi: 10.1093/nar/17.23.9889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner G., Kuechler E., Barta A. Photo-affinity labelling at the peptidyl transferase centre reveals two different positions for the A- and P-sites in domain V of 23S rRNA. EMBO J. 1988 Dec 1;7(12):3949–3955. doi: 10.1002/j.1460-2075.1988.tb03281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern S., Weiser B., Noller H. F. Model for the three-dimensional folding of 16 S ribosomal RNA. J Mol Biol. 1988 Nov 20;204(2):447–481. doi: 10.1016/0022-2836(88)90588-8. [DOI] [PubMed] [Google Scholar]
- Stöffler G., Stöffler-Meilicke M. Immunoelectron microscopy of ribosomes. Annu Rev Biophys Bioeng. 1984;13:303–330. doi: 10.1146/annurev.bb.13.060184.001511. [DOI] [PubMed] [Google Scholar]
- Tischendorf G. W., Zeichhardt H., Stöffler G. Architecture of the Escherichia coli ribosome as determined by immune electron microscopy. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4820–4824. doi: 10.1073/pnas.72.12.4820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tischendorf G. W., Zeichhardt H., Stöffler G. Determination of the location of proteins L14, L17, L18, L19, L22, L23 on the surface of the 5oS ribosomal subunit of Escherichia coli by immune electron microscopy. Mol Gen Genet. 1974;134(3):187–208. doi: 10.1007/BF00267715. [DOI] [PubMed] [Google Scholar]
- Vasiliev V. D. Morphology of the ribosomal 30S subparticle according to electron microscopic data. Acta Biol Med Ger. 1974;33(5-6):779–793. [PubMed] [Google Scholar]
- Vasiliev V. D., Selivanova O. M., Baranov V. I., Spirin A. S. Structural study of translating 70 S ribosomes from Escherichia coli. I. Electron microscopy. FEBS Lett. 1983 May 2;155(1):167–172. doi: 10.1016/0014-5793(83)80232-4. [DOI] [PubMed] [Google Scholar]
- Vester B., Garrett R. A. The importance of highly conserved nucleotides in the binding region of chloramphenicol at the peptidyl transfer centre of Escherichia coli 23S ribosomal RNA. EMBO J. 1988 Nov;7(11):3577–3587. doi: 10.1002/j.1460-2075.1988.tb03235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATSON J. D. THE SYNTHESIS OF PROTEINS UPON RIBOSOMES. Bull Soc Chim Biol (Paris) 1964;46:1399–1425. [PubMed] [Google Scholar]
- WETTSTEIN F. O., NOLL H. BINDING OF TRANSFER RIBONUCLEIC ACID TO RIBOSOMES ENGAGED IN PROTEIN SYNTHESIS: NUMBER AND PROPERTIES OF RIBOSOMAL BINDING SITES. J Mol Biol. 1965 Jan;11:35–53. doi: 10.1016/s0022-2836(65)80169-3. [DOI] [PubMed] [Google Scholar]
- Wagenknecht T., Carazo J. M., Radermacher M., Frank J. Three-dimensional reconstruction of the ribosome from Escherichia coli. Biophys J. 1989 Mar;55(3):455–464. doi: 10.1016/S0006-3495(89)82839-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walleczek J., Schüler D., Stöffler-Meilicke M., Brimacombe R., Stöffler G. A model for the spatial arrangement of the proteins in the large subunit of the Escherichia coli ribosome. EMBO J. 1988 Nov;7(11):3571–3576. doi: 10.1002/j.1460-2075.1988.tb03234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikman F. P., Siboska G. E., Petersen H. U., Clark B. F. The site of interaction of aminoacyl-tRNA with elongation factor Tu. EMBO J. 1982;1(9):1095–1100. doi: 10.1002/j.1460-2075.1982.tb01302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wintermeyer W., Robertson J. M. Transient kinetics of transfer ribonucleic acid binding to the ribosomal A and P sites: observation of a common intermediate complex. Biochemistry. 1982 Apr 27;21(9):2246–2252. doi: 10.1021/bi00538a038. [DOI] [PubMed] [Google Scholar]
- Wower J., Hixson S. S., Zimmermann R. A. Labeling the peptidyltransferase center of the Escherichia coli ribosome with photoreactive tRNA(Phe) derivatives containing azidoadenosine at the 3' end of the acceptor arm: a model of the tRNA-ribosome complex. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5232–5236. doi: 10.1073/pnas.86.14.5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wower J., Zimmermann R. A. A consonant model of the tRNA-ribosome complex during the elongation cycle of translation. Biochimie. 1991 Jul-Aug;73(7-8):961–969. doi: 10.1016/0300-9084(91)90137-p. [DOI] [PubMed] [Google Scholar]
- Wurmbach P., Nierhaus K. H. Codon-anticodon interaction at the ribosomal P (peptidyl-tRNA)site. Proc Natl Acad Sci U S A. 1979 May;76(5):2143–2147. doi: 10.1073/pnas.76.5.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Heel M., Stöffler-Meilicke M. Characteristic views of E. coli and B. stearothermophilus 30S ribosomal subunits in the electron microscope. EMBO J. 1985 Sep;4(9):2389–2395. doi: 10.1002/j.1460-2075.1985.tb03944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]