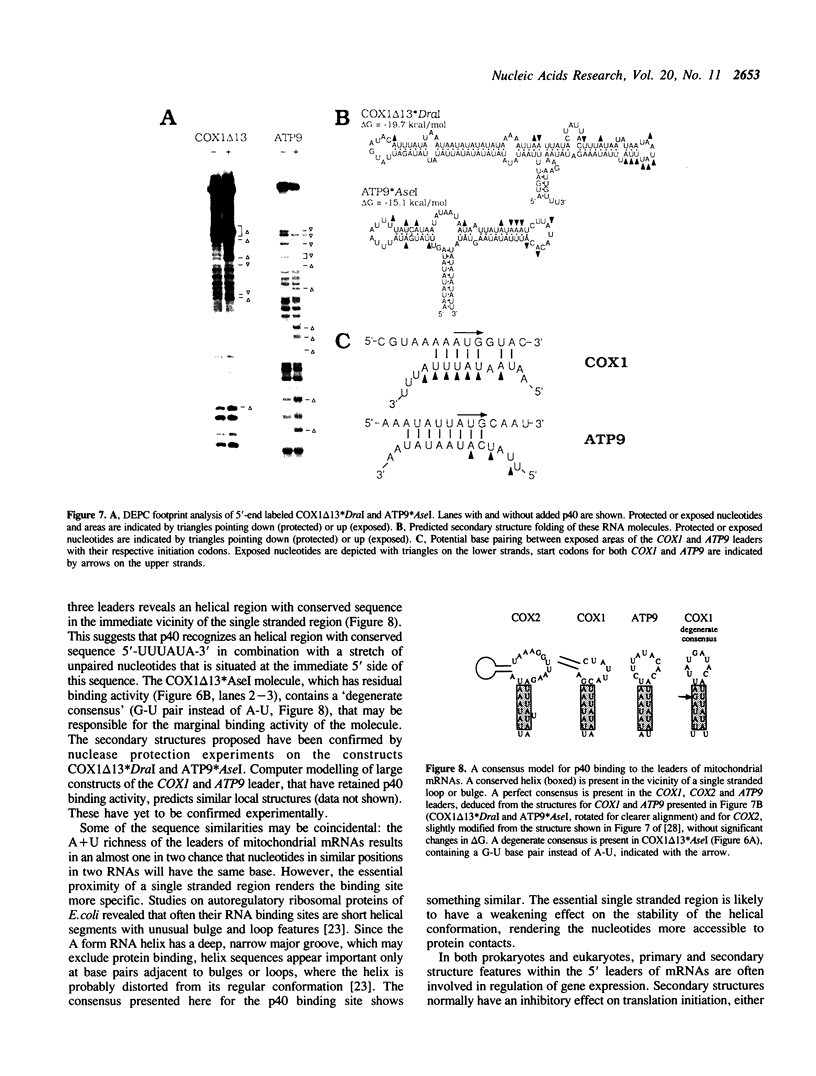
Abstract
An abundant yeast mitochondrial 40 kDa protein (p40) binds with high specificity to the 5'-untranslated region of cytochrome c oxidase subunit II (COX2) mRNA. Using mobility shift and competition assays, we show here that purified p40 complexes with the leaders of all eight mitochondrial mRNAs of Saccharomyces cerevisiae. The location of the protein binding site on the different leaders is not conserved with respect to the AUG start codon. In vitro RNA footprint and deletion experiments have been used to define the p40-binding site on the leaders of COX1 and ATP9 mRNAs. Nucleotides at, and near, a single stranded region are protected or exposed for DEPC modification by binding of p40 to these leaders. Removal of this region from the COX1 messenger shows that it is essential for the protein-RNA interaction. While no obvious sequence similarity can be detected between the single stranded regions in different leaders, a nearby helical segment is conserved. A consensus model for p40-RNA interactions is presented and the possible biological function of p40 is discussed.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackerman S. H., Gatti D. L., Gellefors P., Douglas M. G., Tzagoloff A. ATP13, a nuclear gene of Saccharomyces cerevisiae essential for the expression of subunit 9 of the mitochondrial ATPase. FEBS Lett. 1991 Jan 28;278(2):234–238. doi: 10.1016/0014-5793(91)80124-l. [DOI] [PubMed] [Google Scholar]
- Altuvia S., Locker-Giladi H., Koby S., Ben-Nun O., Oppenheim A. B. RNase III stimulates the translation of the cIII gene of bacteriophage lambda. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6511–6515. doi: 10.1073/pnas.84.18.6511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen T., Johnsen M., Fiil N. P., Friesen J. D. RNA secondary structure and translation inhibition: analysis of mutants in the rplJ leader. EMBO J. 1984 Jul;3(7):1609–1612. doi: 10.1002/j.1460-2075.1984.tb02018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway L., Wickens M. Modification interference analysis of reactions using RNA substrates. Methods Enzymol. 1989;180:369–379. doi: 10.1016/0076-6879(89)80112-0. [DOI] [PubMed] [Google Scholar]
- Costanzo M. C., Fox T. D. Control of mitochondrial gene expression in Saccharomyces cerevisiae. Annu Rev Genet. 1990;24:91–113. doi: 10.1146/annurev.ge.24.120190.000515. [DOI] [PubMed] [Google Scholar]
- Costanzo M. C., Fox T. D. Product of Saccharomyces cerevisiae nuclear gene PET494 activates translation of a specific mitochondrial mRNA. Mol Cell Biol. 1986 Nov;6(11):3694–3703. doi: 10.1128/mcb.6.11.3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costanzo M. C., Fox T. D. Specific translational activation by nuclear gene products occurs in the 5' untranslated leader of a yeast mitochondrial mRNA. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2677–2681. doi: 10.1073/pnas.85.8.2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costanzo M. C., Seaver E. C., Fox T. D. At least two nuclear gene products are specifically required for translation of a single yeast mitochondrial mRNA. EMBO J. 1986 Dec 20;5(13):3637–3641. doi: 10.1002/j.1460-2075.1986.tb04693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decoster E., Simon M., Hatat D., Faye G. The MSS51 gene product is required for the translation of the COX1 mRNA in yeast mitochondria. Mol Gen Genet. 1990 Oct;224(1):111–118. doi: 10.1007/BF00259457. [DOI] [PubMed] [Google Scholar]
- Dekker P. J., Papadopoulou B., Grivell L. A. Properties of an abundant RNA-binding protein in yeast mitochondria. Biochimie. 1991 Dec;73(12):1487–1492. doi: 10.1016/0300-9084(91)90182-z. [DOI] [PubMed] [Google Scholar]
- Dieckmann C. L., Mittelmeier T. M. Nuclearly-encoded CBP1 interacts with the 5' end of mitochondrial cytochrome b pre-mRNA. Curr Genet. 1987;12(6):391–397. doi: 10.1007/BF00434815. [DOI] [PubMed] [Google Scholar]
- Dieckmann C. L., Tzagoloff A. Assembly of the mitochondrial membrane system. CBP6, a yeast nuclear gene necessary for synthesis of cytochrome b. J Biol Chem. 1985 Feb 10;260(3):1513–1520. [PubMed] [Google Scholar]
- Draper D. E. How do proteins recognize specific RNA sites? New clues from autogenously regulated ribosomal proteins. Trends Biochem Sci. 1989 Aug;14(8):335–338. doi: 10.1016/0968-0004(89)90167-9. [DOI] [PubMed] [Google Scholar]
- Gold L. Posttranscriptional regulatory mechanisms in Escherichia coli. Annu Rev Biochem. 1988;57:199–233. doi: 10.1146/annurev.bi.57.070188.001215. [DOI] [PubMed] [Google Scholar]
- Grivell L. A. Nucleo-mitochondrial interactions in yeast mitochondrial biogenesis. Eur J Biochem. 1989 Jul 1;182(3):477–493. doi: 10.1111/j.1432-1033.1989.tb14854.x. [DOI] [PubMed] [Google Scholar]
- Haffter P., McMullin T. W., Fox T. D. Functional interactions among two yeast mitochondrial ribosomal proteins and an mRNA-specific translational activator. Genetics. 1991 Feb;127(2):319–326. doi: 10.1093/genetics/127.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson R. J., Howell M. T., Kaminski A. The novel mechanism of initiation of picornavirus RNA translation. Trends Biochem Sci. 1990 Dec;15(12):477–483. doi: 10.1016/0968-0004(90)90302-r. [DOI] [PubMed] [Google Scholar]
- Kloeckener-Gruissem B., McEwen J. E., Poyton R. O. Identification of a third nuclear protein-coding gene required specifically for posttranscriptional expression of the mitochondrial COX3 gene is Saccharomyces cerevisiae. J Bacteriol. 1988 Mar;170(3):1399–1402. doi: 10.1128/jb.170.3.1399-1402.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Comparison of initiation of protein synthesis in procaryotes, eucaryotes, and organelles. Microbiol Rev. 1983 Mar;47(1):1–45. doi: 10.1128/mr.47.1.1-45.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Structural features in eukaryotic mRNAs that modulate the initiation of translation. J Biol Chem. 1991 Oct 25;266(30):19867–19870. [PubMed] [Google Scholar]
- Kozak M. The scanning model for translation: an update. J Cell Biol. 1989 Feb;108(2):229–241. doi: 10.1083/jcb.108.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelis U., Körte A., Rödel G. Association of cytochrome b translational activator proteins with the mitochondrial membrane: implications for cytochrome b expression in yeast. Mol Gen Genet. 1991 Nov;230(1-2):177–185. doi: 10.1007/BF00290666. [DOI] [PubMed] [Google Scholar]
- Mittelmeier T. M., Dieckmann C. L. CBP1 function is required for stability of a hybrid cob-oli1 transcript in yeast mitochondria. Curr Genet. 1990 Dec;18(5):421–428. doi: 10.1007/BF00309911. [DOI] [PubMed] [Google Scholar]
- Mueller D. M., Getz G. S. Steady state analysis of mitochondrial RNA after growth of yeast Saccharomyces cerevisiae under catabolite repression and derepression. J Biol Chem. 1986 Sep 5;261(25):11816–11822. [PubMed] [Google Scholar]
- Nomura M., Gourse R., Baughman G. Regulation of the synthesis of ribosomes and ribosomal components. Annu Rev Biochem. 1984;53:75–117. doi: 10.1146/annurev.bi.53.070184.000451. [DOI] [PubMed] [Google Scholar]
- Papadopoulou B., Dekker P., Blom J., Grivell L. A. A 40 kd protein binds specifically to the 5'-untranslated regions of yeast mitochondrial mRNAs. EMBO J. 1990 Dec;9(12):4135–4143. doi: 10.1002/j.1460-2075.1990.tb07636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne M. J., Schweizer E., Lukins H. B. Properties of two nuclear pet mutants affecting expression of the mitochondrial oli1 gene of Saccharomyces cerevisiae. Curr Genet. 1991 May;19(5):343–351. doi: 10.1007/BF00309594. [DOI] [PubMed] [Google Scholar]
- Poutre C. G., Fox T. D. PET111, a Saccharomyces cerevisiae nuclear gene required for translation of the mitochondrial mRNA encoding cytochrome c oxidase subunit II. Genetics. 1987 Apr;115(4):637–647. doi: 10.1093/genetics/115.4.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rödel G., Fox T. D. The yeast nuclear gene CBS1 is required for translation of mitochondrial mRNAs bearing the cob 5' untranslated leader. Mol Gen Genet. 1987 Jan;206(1):45–50. doi: 10.1007/BF00326534. [DOI] [PubMed] [Google Scholar]
- Rödel G. Two yeast nuclear genes, CBS1 and CBS2, are required for translation of mitochondrial transcripts bearing the 5'-untranslated COB leader. Curr Genet. 1986;11(1):41–45. doi: 10.1007/BF00389424. [DOI] [PubMed] [Google Scholar]
- Schimmel P. RNA pseudoknots that interact with components of the translation apparatus. Cell. 1989 Jul 14;58(1):9–12. doi: 10.1016/0092-8674(89)90395-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theil E. C. Regulation of ferritin and transferrin receptor mRNAs. J Biol Chem. 1990 Mar 25;265(9):4771–4774. [PubMed] [Google Scholar]
- Wulczyn F. G., Kahmann R. Translational stimulation: RNA sequence and structure requirements for binding of Com protein. Cell. 1991 Apr 19;65(2):259–269. doi: 10.1016/0092-8674(91)90160-z. [DOI] [PubMed] [Google Scholar]
- Zuker M., Stiegler P. Optimal computer folding of large RNA sequences using thermodynamics and auxiliary information. Nucleic Acids Res. 1981 Jan 10;9(1):133–148. doi: 10.1093/nar/9.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]