Abstract
Study Objective:
It is well established that brain metabolism is higher during wake and rapid eye movement (REM) sleep than in nonrapid eye movement (NREM) sleep. Most of the brain's energy is used to maintain neuronal firing and glutamatergic transmission. Recent evidence shows that cortical firing rates, extracellular glutamate levels, and markers of excitatory synaptic strength increase with time spent awake and decline throughout NREM sleep. These data imply that the metabolic cost of each behavioral state is not fixed but may reflect sleep-wake history, a possibility that is investigated in the current report.
Design:
Chronic (4d) electroencephalographic (EEG) recordings in the rat cerebral cortex were coupled with fixed-potential amperometry to monitor the extracellular concentration of oxygen ([oxy]) and lactate ([lac]) on a second-by-second basis across the spontaneous sleep-wake cycle and in response to sleep deprivation.
Setting:
Basic sleep research laboratory.
Patients or Participants:
Wistar Kyoto (WKY) adult male rats.
Interventions:
N/A.
Measurements and Results:
Within 30-60 sec [lac] and [oxy] progressively increased during wake and REM sleep and declined during NREM sleep (n = 10 rats/metabolite), but with several differences. [Oxy], but not [lac], increased more during wake with high motor activity and/or elevated EEG high-frequency power. Meanwhile, only the NREM decline of [lac] reflected sleep pressure as measured by slow-wave activity, mirroring previous results for cortical glutamate.
Conclusions:
The observed state-dependent changes in cortical [lac] and [oxy] are consistent with higher brain metabolism during waking and REM sleep in comparison with NREM sleep. Moreover, these data suggest that glycolytic activity, most likely through its link with glutamatergic transmission, reflects sleep homeostasis.
Citation:
Dash MB; Tononi G; Cirelli C. Extracellular levels of lactate, but not oxygen, reflect sleep homeostasis in the rat cerebral cortex. SLEEP 2012;35(7):909–919.
Keywords: Lactate, oxygen, in vivo amperometry, sleep, rat, cerebral cortex, EEG, slow-wave activity
INTRODUCTION
Slow-wave activity (SWA), the electroencephalographic (EEG) power in the 0.5-4 Hz range during nonrapid eye movement (NREM) sleep, is a well-established electrophysiologic correlate of sleep pressure. The longer and/or more “intense” wake is, the higher SWA is at sleep onset.1 The cellular mechanisms underlying sleep homeostasis remain elusive, but recent evidence suggests a link between sleep need and neuronal activity and plasticity.2 In the brain most of the energy is used to support glutamatergic activity,3 and glucose consumption is stoichiometrically coupled to glutamate-glutamine cycling.4 It is not surprising, therefore, that brain metabolism is higher in wake and rapid eye movement (REM) sleep than in NREM sleep5–8 (e.g., because most cortical neurons are glutamatergic and mean cortical firing rates are higher in wake and REM sleep than in NREM sleep).9,10 Moreover, wake increases messenger RNA levels of α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) receptor subunits and of proteins implicated in glutamate synthesis and glutamate receptor clustering.11–13 Lack of sleep also increases cortical synaptic expression of GluR1-containing AMPA receptors14 and strengthens cortical glutamatergic synapses,15 whereas NREM sleep results in removal of AMPA receptors and long-term synaptic depression.14,16
However, recent evidence also shows that within each behavioral state cortical firing rates are higher after prolonged wake and lower after sleep.17 Moreover, cortical extracellular glutamate levels progressively increase with time spent spontaneously awake and decline throughout NREM sleep, and the rate of decline correlates with the SWA decline.18 Extracellular levels of adenosine, a by-product of energy metabolism, also increase with time spent awake in basal forebrain and to some extent in cortex, and this buildup has been causally linked to sleep need.19 Overall, these results suggest that the metabolic cost of each behavioral state is not fixed but may reflect sleep-wake history, and brain metabolism, glutamatergic tone, and sleep homeostasis may be linked. However, these hypotheses have not been tested directly. Furthermore, although oxidative phosphorylation of glucose is the main source of adenosine triphosphate (ATP) in the brain,20 glycolysis is also important, providing lactate as a fuel for neurons21 and rapidly generating energy within astrocytic filopodia22 and postsynaptic densities23 that are too small to contain mitochondria. Nevertheless, whether there is a specific link between sleep homeostasis and oxidative or glycolytic aspects of brain metabolism remains unclear.
To address these issues we performed EEG recordings in the rat cerebral cortex coupled with fixed-potential amperometry, to monitor the extracellular concentration of oxygen ([oxy]) and lactate ([lac]) during sleep, wake, and sleep deprivation, on a second-by-second basis for up to 4 days.
MATERIALS AND METHODS
Surgical Procedures
Male Wistar Kyoto rats (n = 20; 300-350 g at time of surgery; Harlan) were individually housed in recording chambers (clear Plexiglas enclosure, 36.5 × 25 × 46 cm in a sound-attenuating box) in a controlled environment (24 ± 1 °C; 12h light/dark cycle, lights on at 10:00; food and water ad libitum). Under isoflurane anesthesia 4 silver screws serving as EEG electrodes were affixed to the scalp: 2 anterior (mm from bregma: anteroposterior (AP) +2 or +3, mediolateral (ML) +3 or +0), 1 parietal (AP −2.3, ML −3.8), and 1 cerebellar (AP −11, ML +1). Silver wire electrodes were implanted in the nuchal muscles to monitor the electromyogram (EMG). Microelectrode arrays (MEAs; see the following paragraphs) were implanted in either frontal cortex (n = 10; AP +2, ML −3 dorsoventral (DV) −1.5) or prefrontal cortex (n = 10; AP +3.2, ML −0.8, DV −4.5). One Ag/AgCl reference electrode for the MEAs was placed occipitally (AP-6.5, ML +3). Dental cement was used to affix the electrodes to the animal's skull. Recordings began immediately following surgery. All animal procedures followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals and facilities were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Wisconsin-Madison, and were inspected and accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC).
Sleep-Wake Recordings
To record the EEG and EMG, electrodes were connected to a commutator with a flexible cable and signals were sent to a polygraph (Grass Technologies, Model 7P5B, West Warwick, RI) to be amplified and filtered (EEG and LFP signals: high-pass at 0.1 Hz; EMG signals: high-pass at 5 Hz, low-pass at 70 Hz). All signals were notch-filtered at 60 Hz. Analog signals were digitized with the A/D board (National Instruments, Austin, TX) and sampled at 200 Hz (VitalRecorder, Kissei Comtec, Nagano, Japan). Vigilance states were determined offline by visual inspection of EEG and EMG signals and scored in 4-sec epochs as either wake (low-voltage, high-frequency EEG, high EMG), NREM sleep (high-voltage, low-frequency EEG, low EMG), or REM sleep (low-voltage, high-frequency EEG, low EMG activity). Vigilance state could be determined for all epochs. Wake epochs were further classified offline as quiet or active according to the amount of EMG activity (see Results).
Microelectrode Preparation
Ceramic-based MEAs (Center for Microelectrode Technology, Lexington, KY) containing 4 platinum/iridium electrodes (15 μM × 333 μM) were modified for chronic implantation in freely moving rats as previously described.24 Assembled MEAs were briefly (approximately 5 sec) submerged in Nafion® (Sigma Aldrich, St. Louis, MO) and then oven-dried (185°C for 5 min). Nafion® acts as a charge-dependent exclusion layer to limit interference in vivo from electroactive analytes without disrupting detection of the analyte of interest. For electrodes used to record [lac], an additional coating solution of l-lactate oxidase (LacOx; MP Biomedicals, Solon, OH), glutaraldehyde (Sigma Aldrich) and bovine serum albumin (BSA, Sigma Aldrich) was applied to 2 recording electrodes. LacOx catalyzes the conversion of lactate to pyruvate and hydrogen peroxide. The ensuing oxidation of hydrogen peroxide at the surface of the recording electrodes produces a current that is proportional to the concentration of lactate. Glutaraldehyde and BSA were included to affix the LacOx to the recording electrodes. The remaining 2 recording electrodes were coated with only glutaraldehyde and BSA to measure background current from possible interferents only (sentinel electrodes). Signal and sentinel channels are in close proximity in the MEA, with a distance of 100μ separating the edge of the signal channels from the edge of the sentinel channels, and therefore sample the same extracellular milieu. Subtracting the current sensed by sentinel electrodes from the current sensed by signal electrodes provides a signal reflecting [lac], removes background current produced by other analytes, and reduces noise.24,25 No additional coatings were needed to record [oxy] because oxygen can be reduced directly at the surface of the electrode without need for an electroactive intermediate.26–28
Electrode Calibration and In Vivo Amperometry
Prior to implantation in freely moving rats, each microelectrode was calibrated in vitro to ensure proper selectivity and sensitivity for the desired analyte. Microelectrodes were submerged in 40 ml of 0.05 M phosphate-buffered saline (PBS, pH 7.4) maintained by a water bath at 37°C (Gaymar Co., Orchard Park, NY). Fixed potential amperometry using the FAST-16 potentiostat (Fast Analytical Sensing Technology, Quanteon, Lexington, KY) was implemented to record either oxygen (-0.7 V vs. Ag/AgCl reference) or lactate (+0.7 V) concentrations. To calibrate oxygen electrodes the response of the electrodes to nitrogen-bubbled PBS, oxygen-saturated PBS, and supersaturated PBS (by directly bubbling oxygen in solution) was recorded. Electrodes were only used if the amount of current between nitrogen-bubbled and oxygen- saturated PBS differed by > 30 nA, to ensure sensitivity to changes in [oxy] across a range exceeding those expected to be observed in vivo. To calibrate lactate electrodes, the response of signal and sentinel electrodes to the major electroactive interferent in the brain, ascorbic acid (250 μM; Sigma Aldrich), as well as to l-lactate (250 μM increments) and hydrogen peroxide was recorded. Lactate electrodes for in vivo recordings were only used if the response of the signal channels to lactate was more than 20 times their response to ascorbic acid, and the response of signal and sentinel channels to hydrogen peroxide did not differ by more than 10%.
Once calibrated, MEAs were implanted in freely behaving rats and fixed-potential amperometry was performed using a 4-channel potentiostat (FAST-16). Implantable MEAs were connected to a headstage amplifier (20 pA / mV for oxygen; 2 pA / mV for lactate; Rat Hat; Quanteon) to amplify current produced from reduction (oxygen) or oxidation (lactate) at the surface of the electrodes. Analog signals from the MEA were sent to the potentiostat and furthered amplified (lactate: 10×; oxygen: 2×). Analog outputs from the potentiostat were then digitized with an A/D board (National Instruments) and recorded to hard drive with a sampling rate of 200 Hz (VitalRecorder, Kissei Comtec) simultaneously with EEG and EMG signals. Chronic recordings began immediately after surgery and lasted 4 days postsurgery. Amperometric, EEG, and EMG signals were recorded continuously across each day, with the exception of a few minutes at light's onset each day when recordings were restarted. In a subset of animals (n = 5 each) [lac] or [oxy] was recorded during a 3-hr sleep deprivation period (beginning at light's onset) and during subsequent recovery sleep. During the sleep deprivation, we continuously observed the behavior and polysomnographic recordings of the rat. To maintain wakefulness, rats were given novel objects to promote exploratory behaviors (paper towels and toys of various shapes and sizes) or activated by acoustic stimuli (e.g., tapping on the cage) whenever behavioral or electrographic (slow waves or low tone EMG) signs of drowsiness were observed. Importantly, rats were not touched or handled directly nor were they disturbed when they were spontaneously awake, feeding, or drinking. This method minimized stress while maintaining arousal across the 3-hr of the deprivation procedure.
Throughout this report, [lac] and [oxy] are reported as percentages of the daily mean instead of as absolute concentrations. Although theoretically the precise concentration of an analyte can be determined from a calibration curve of known standards, in practice this can fail to be true for a number of reasons. The microenvironment of the neuropil surrounding the implanted electrode, when compared with the typical calibration, may produce differences in the diffusion coefficients of the analyte,29 partially occlude the implanted electrode and thereby alter the recording surface area,30 and/or alter the shape and size of the diffusion layer at the electrodes surface.31 Consequently, the current produced in vivo may not directly equate to that produced during an in vitro calibration.32 Reporting absolute concentrations, therefore, can be confounded by technical shortcomings, introduces unwarranted variability, and may be misleading. As such, we have chosen to report our findings as relative values rather than absolute concentrations. However, it should be noted that despite a high degree of variability across subjects (ranging from 0.11 mM to 1.45 mM), on average, the [lac] recorded in the current study was 0.49 ± 0.14 mM, consistent with previous reports of a typical extracellular [lac] of 0.4-1.25 mM in cortex.8,33 Because our calibration procedure did not establish a quantitative relationship between [oxy] and the amount of current from our electrodes, we are unable to report the absolute value of oxygen concentrations recorded in vivo.
Postprocessing and Data Analysis
Recorded signals were imported into Matlab (Mathworks, Natick, MA) and processed with custom scripts. Amperometric signals were low-pass filtered (chebyshev type II, passband = 18 Hz, stopband = 19 Hz) to remove any high-frequency noise and subsequently binned and averaged over 4-sec epochs to facilitate comparisons with scored behavioral state. For [lac], current from sentinel channels was subtracted from signal channels to minimize nonspecific effects due to interferents. Using a 30-min moving window across each day, artifacts (< 5% of total recording time) were identified (data points > or < 5 standard deviations from the mean) and removed. Delay periods for wake and NREM sleep episodes were calculated by finding the 4-sec epoch in which the minimum (wake) and maximum (NREM) concentration of either lactate or oxygen occurred in the first 2 min after episode onset. All power spectra included in the analyses of band-limited power (BLP) and SWA were calculated in Matlab for each 4-sec epoch across the day by means of a Fast Fourier Transorm (FFT; Hamming window, 0.25 Hz resolution). All statistical tests were performed using Matlab or Statistica (Statsoft, Tulsa, OK) and all data are presented as mean ± standard error of the mean.
RESULTS
State-Dependent Changes in Extracellular [lac] and [oxy]
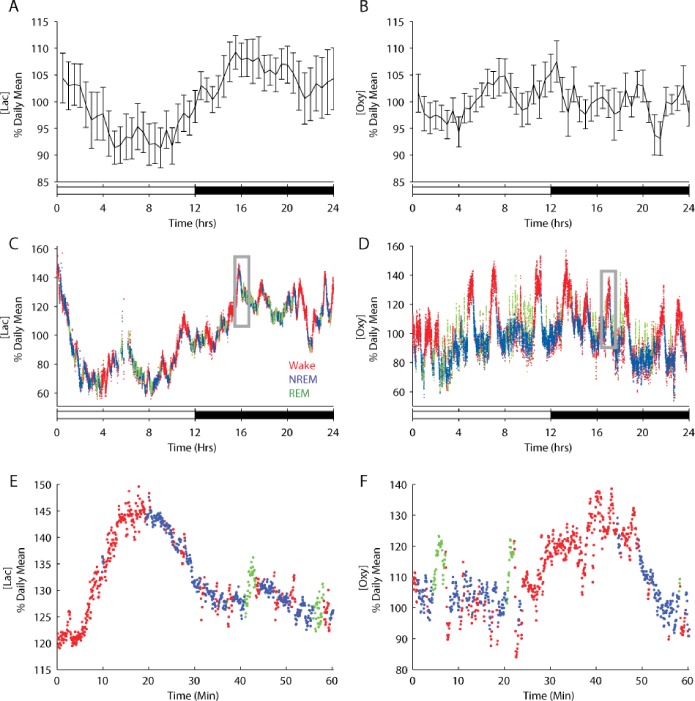
We used in vivo amperometry, which has a temporal resolution of < 1 sec,24,25 and recorded extracellular [lac] or [oxy] continuously for 4 days in the frontal or prefrontal cortex of freely behaving rats (n = 10 rats each). EEG and EMG activity were also simultaneously recorded and were used to characterize behavioral state (in 4-sec epochs) offline. Rats were recorded during the week immediately after surgery, when amperometric signal strength is maximal. Despite the proximity of the recordings to surgery, sleep-wake patterns appeared normal: all animals showed a clear circadian rest-activity rhythm, sleeping 57.53 ± 0.99 % of the light period, when rats typically have the most consolidated sleep, and 34.37 ± 1.01 % of the dark period. Across the entire 24-hr period, rats spent on average 52.15 ± 0.80 % of total time awake, 40.91 ± 0.78 % in NREM sleep, and 6.94 ± 0.25 % in REM sleep, values similar to those of fully recovered rats.34,35 [Lac] declined during the first 5 hr of the light period and then leveled off for the remaining 7 hr (Figure 1A). In the first part of the night, when rats typically have long, consolidated bouts of wake, [lac] increased, reaching a peak 4 hr after dark's onset. Overall, [lac] was significantly lower during the light period than during the dark period (% daily mean, 95.68 ± 1.34 versus 105.36 ± 1.45; t (9) = −3.50, P < 0.01; dependent samples t test, 2-tailed). [Oxy], in contrast, did not differ significantly between the light and the dark period (% daily mean, 100.32 ± 0.62 versus 99.81 ± 0.75; t (9) = 0.32, P = 0.76) (Figure 1B).
Figure 1.
Increases in [lac] and [oxy] are typically observed during waking and REM sleep, behavioral states characterized by elevated neuronal activity, whereas these energy metabolites typically decrease during NREM sleep. (A and B) The average [lac] and [oxy] across the 24-hr period (n = 10 rats each). [Lac] was significantly lower in the light period than in the dark period (P < 0.05) (C and D) [Lac] and [oxy] in the prefrontal cortex of individual rats are depicted for each 4-sec epoch of wake (red), NREM sleep (blue), and REM sleep (green) during undisturbed baseline days. The boxed regions in C and D can be seen at higher resolution in E and F, respectively.
To investigate whether these 24-hr profiles could arise from sleep-wake dependent changes we directly compared behavioral state as determined by the EEG and EMG to concurrent [lac] and [oxy]. Visual inspection of individual recordings showed that both [lac] and [oxy] depended on behavioral state, and did so in a similar way, usually increasing during wake and REM sleep and declining during NREM sleep (Figure 1C-F). To quantify the magnitude of these changes we then focused on consolidated episodes of wake, NREM and REM sleep (those whose duration was at least equal to the mean episode duration for each behavioral state), and obtained a profile of the average changes in [lac] and [oxy] as a function of behavioral state (Figure 2). This analysis confirmed that [lac] and [oxy] increase during wake and REM sleep and decrease during NREM sleep (% daily mean/min; [lac] wake +0.346 ± 0.04, REM +0.522 ± 0.18, NREM −0.345 ± 0.09; [oxy] wake +1.17 ± 0.05, REM +1.85 ± 0.24, NREM −0.630 ± 0.05).
Figure 2.
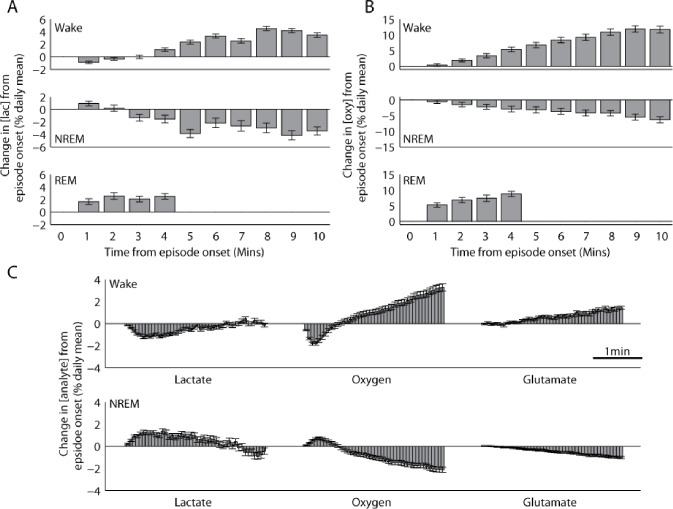
After a short delay (< 1 min), [lac] and [oxy] increase during waking and REM sleep and decrease during NREM sleep. Changes in [lac] (A) or [oxy] (B) during wake, NREM sleep, and REM sleep relative to the behavioral state onset (n = 10 rats each). Because of intrinsic variability in episode duration, data (mean ± standard error of the mean) presented here are shown to include all time points at which at least 15% of all episodes for that behavioral state can contribute. (C) Average cortical concentration of lactate, oxygen, and glutamate in 4-sec epochs for the first 3-min after wake or NREM sleep onset. Glutamate results were derived from data previously collected by Dash et al.18
Interestingly, changes in [lac] and [oxy] were delayed relative to the onset of sleep and wake as defined by the EEG and EMG (Figure 2C). After the transition to wake, [lac] initially declined for 40.89 ± 2.28 sec before typically increasing throughout the rest of the episode. On the other hand, after NREM sleep onset, [lac] initially increased for 36.89 ± 3.94 sec before exhibiting the typical decline during this behavioral state. [Oxy] exhibited a similar trend, with an initial decrease for 27.20 ± 6.53 sec at the onset of wake and an initial increase for 36.44 ± 7.23 sec at the onset of NREM sleep. The duration of these delay periods was variable for oxygen, perhaps reflecting the distance from each electrode to the nearest capillary and/or arteriole, because local oxygen pressure can vary as a function of the distance to these oxygen sources.36 Of note, no delays were observed in previous recordings of glutamate concentrations,18Figure 2C), which began increasing during wake and decreasing during NREM sleep at the episode's onset. Thus, changes in [lac] and [oxy] are not only delayed relative to behavioral state transitions as defined by the EEG, but also as compared with changes in glutamatergic activity. These results suggest that [lac] and [oxy] changes do not reflect behavioral state per se, but rather arise as a consequence of changes in neuronal and/or glutamatergic activity that are characteristic of each behavioral state.
Motor Activity, EEG BLP, and Changes in [lac] and [oxy]
Changes in both [lac] and [oxy] showed substantial variability across and within individual episodes of sleep or wake (Figure 1C-F). This variability may be of functional significance, because neuronal activity is not homogenous within each behavioral state, but rather displays a wide array of task-dependent and spontaneous alterations.17,37 To test this possibility we assessed the relationship between [lac] and [oxy] changes and two indices that may reflect ongoing activity in frontal/prefrontal cortices: locomotion and EEG BLP. Notably, the cortical areas in which we recorded [lac] and [oxy] are both contained within the rat's motor cortex as defined by microstimulation mapping.38
First, the raw EMG signal for the entire day was rectified and then averaged across 4-sec to produce an index of motor activity for every 4-sec behavioral state epoch. The magnitude of this index corresponds to the amount of locomotor activity that occurred during that 4-sec epoch. Using this index of motor activity, we classified all wake episodes as either mostly active or mostly quiet, depending on whether these episodes contained greater than or less than the mean locomotor activity across all wake episodes, respectively. We found that [lac] increased to a similar extent in active and quiet wake (% daily mean/min, 0.295 ± 0.06 versus 0.422 ± 0.05; t (9) = 1.12, P = 0.29; Figure 3A) and the same was true for [glu] (according to data from Dash and colleagues18; 0.622 ± 0.07 versus 0.540 ± 0.06; t (7) = 0.78, P = 0.45; Figure 3C). In contrast, [oxy] increased almost twice as much during active wake than during quiet wake (1.37 ± 0.06 versus 0.72 ± 0.03; t (9) = 3.45, P < 0.01; Figure 3B). Thus, [oxy] in cortical areas involved in motor activity varies as a consequence of different levels of locomotor activity, and these changes do not appear to be driven by changes in [glu]. Because the episodes of mostly active wake as defined here included brief periods of inactivity (and vice versa), we also measured changes in [lac] or [oxy] during each 4-sec waking epoch, taking advantage of the high temporal resolution of fixed potential amperometry. A threshold, below and above which all wake epochs were classified as quiet wake and active wake, respectively, was determined by calculating the 95th percentile of EMG activity during all NREM sleep episodes. This threshold ensured that the identified “quiet wake” epochs were only those that contained minimal amounts of EMG activity. Behavioral analysis of video recordings in a subset of animals (n = 5) confirmed that this method accurately identified periods of inactivity during wake as quiet wake, whereas activities such as exploring, grooming, and feeding were all scored as active wake. The average increase in [oxy] during active wake was 261.53 ± 48.88 % more than during quiet wake (t (9) = 3.21, P < 0.05), whereas [lac] did not differ between quiet and active waking periods (t (9) = −0.61, P = 0.56).
Figure 3.
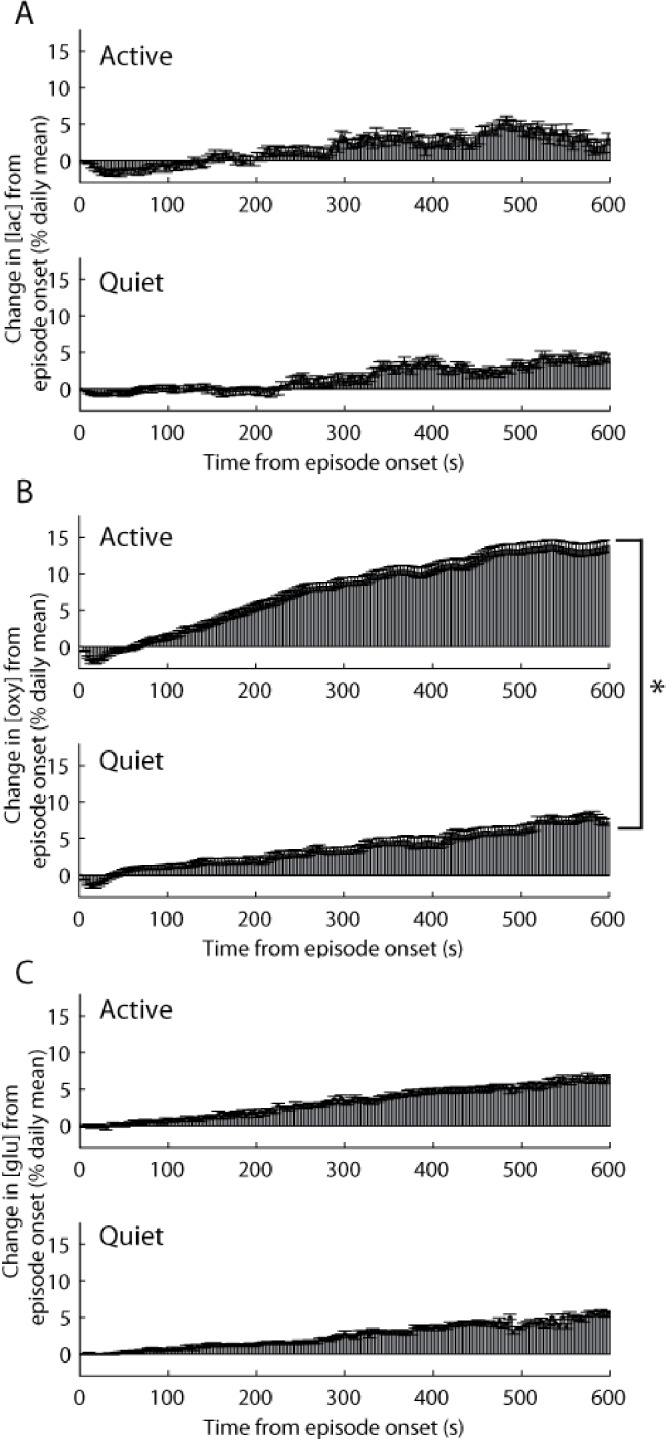
During waking, [oxy] in cortical areas activated by movement increases more when locomotor activity is high, whereas [lac] or [glu] do not change in association with locomotor activity. Each panel depicts the concentration (mean ± standard error of the mean) of lactate (A), oxygen (B), or glutamate (C) in 4-sec epochs across all episodes of mostly active or mostly quiet wake. All episodes were classified as either active or quiet based on total electromyographic activity across the duration of the wake episode. *P < 0.01. Glutamate results were derived from data previously collected by Dash et al.18
We then examined the relationship between EEG BLP and cortical [lac] and [oxy]. BLP reflects the amount of activity across a particular frequency range in the EEG or local field potential signal. To obtain a rough index of ongoing neuronal activity we calculated BLP for 8 distinct frequency bands for each 4-sec epoch of wake (see Materials and Methods). Wake epochs were subdivided into quartiles based on total power for each band and the average change in [lac], [oxy], or [glu] was calculated within each quartile (Figure 4A-C). We found no significant relationship between changes in [lac] during wake and either higher or lower frequency power (higher, F(3,27) = 1.89, P = 0.16; lower, F(3,27) = 0.84, P = 0.48). Similarly, no significant relationship between changes in [glu] during wake and either higher or lower frequency power was observed (higher, F(3,21) = 0.54, P = 0.66; lower, F(3,21) = 1.92, P = 0.16). By contrast, wake changes in [oxy] were modulated in association with changes in BLP, such that [oxy] increased more during epochs with the greatest power in the high-frequency range (22–100 Hz) than during epochs with the least amount of high-frequency power (repeated measures analysis of variance, F(3,27) = 6.95 P < 0.01; post hoc Fisher least significant difference). Moreover, [oxy] displayed an inverse relationship with low frequency power (0.5-18 Hz), increasing significantly more during epochs with the least power in the low frequencies (F(3,27) = 4.62 P < 0.01; post hoc Fisher least significant difference). Thus, wake changes in [oxy] are associated with acute changes in neuronal activity as indexed by high-frequency BLP, as well as by acute changes in motor activity, whereas changes in [lac] do not show a significant relationship with either motor activity or BLP. These cortical metabolites, therefore, appear to respond preferentially to different types of activity during wake.
Figure 4.
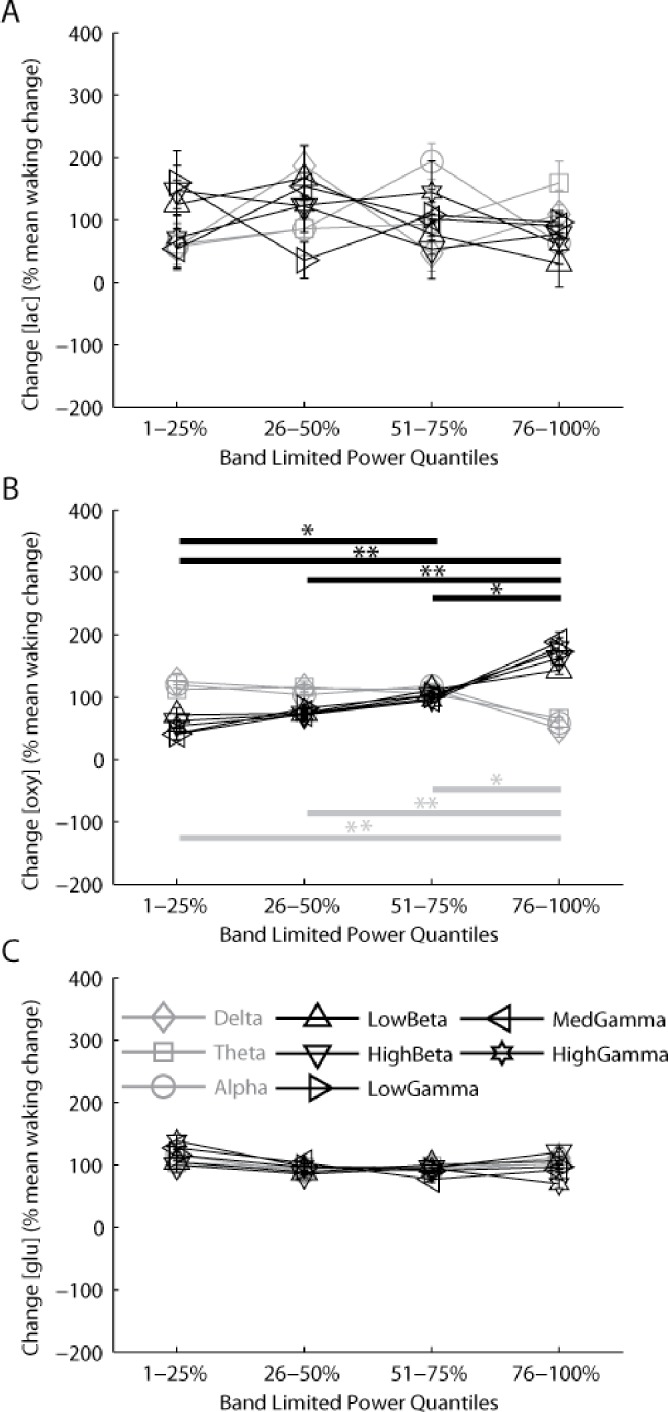
[Oxy] changes in association with the amount of high- and low-frequency electroencephalographic (EEG) band-limited power (BLP; an index of cortical activity), whereas [lac] and [glu] show no relationship with BLP at any frequency. BLP was calculated for every 4-sec epoch of wake in 3 lower frequency windows (gray lines); Delta (0.5-4Hz), Theta (5-9Hz), Alpha (10-18Hz) and 5 higher frequency windows (black lines): LowBeta (22-30), HighBeta (30.25-40Hz), LowGamma (40.25-59Hz), MedGamma (61-80Hz), HighGamma (80.25-100Hz). Changes in [lac] (A), [oxy] (B), and [glu] (C) during each 4-sec epoch of wake were binned across 4 quartiles of increasing power within each frequency band and are presented as mean ± standard error of the mean. Post hoc testing for lower frequencies (0.5-18 Hz) and higher frequencies (22-100 Hz), Fisher least significant difference: *P < 0.05; **P < 0.01. Glutamate results were derived from data previously collected by Dash et al.18
Sleep Pressure and Changes in [lac] and [oxy]
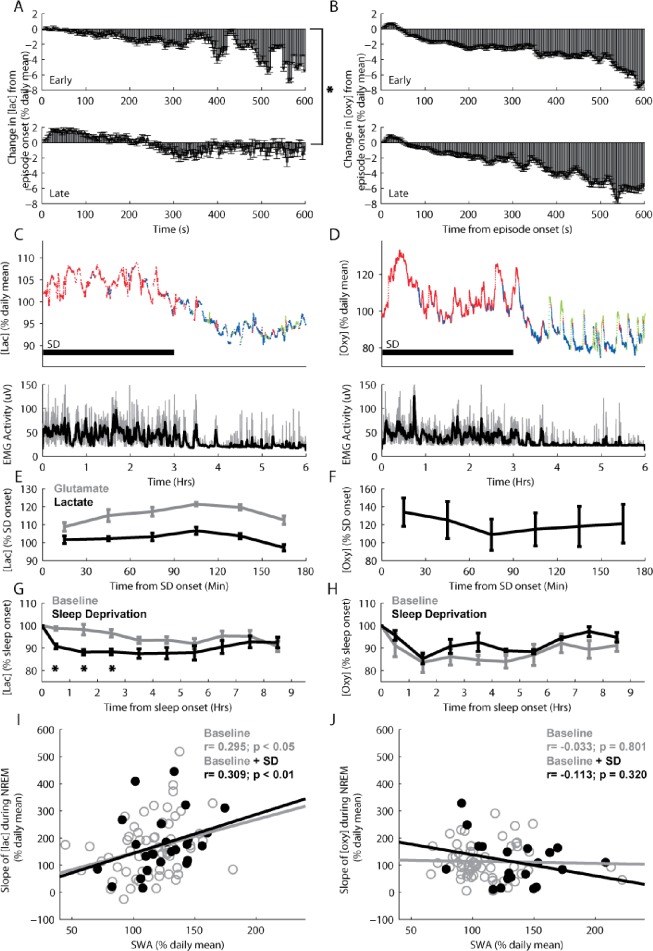
Sleep pressure as measured by SWA is highest at sleep onset and dissipates in the course of sleep.1 We previously reported that cortical [glu] also declines during NREM sleep, and that the rate of this decline correlates with the SWA decline.18 To investigate whether the decline in [lac] and/or [oxy] during NREM sleep are also affected by sleep pressure we compared changes in these metabolites during episodes of NREM sleep that occur early in the light period (first 4 hr after light's onset, when SWA is high) to late NREM episodes (last 4 hr of the light period, when SWA is low). [Lac] declined significantly more during early than during late sleep (% daily mean/min, −0.549 ± 0.08 versus −0.101 ± 0.09; t (9) = 4.62, P < 0.01; Figure 5A). In contrast, [oxy] declined to a similar extent during early and late sleep (-0.72 ± 0.05 versus −0.59 ± 0.07; t (9) = 0.41, P = 0.69; Figure 5B), suggesting that spontaneous changes in sleep pressure were associated with changes in [lac], but not with changes in [oxy]. To confirm this result a subset of animals (n = 5/metabolite) were sleep deprived for 3 hr starting at the onset of the light period. Throughout the time of enforced wake, periods of large increases in [oxy], which appeared concurrent with large bouts of motor activity, were interspersed with phases in which [oxy] declined steeply, remained stable, or increased slightly (Figure 5D and F). In contrast, changes in [lac] showed little relationship to EMG activity, but rather increased across the 3 hr, only declining toward the end of the deprivation when sleep pressure was at its highest (Figure 5C and E). These changes in [lac] during sleep deprivation were similar to those previously observed for [glu] (Figure 5E; data from Dash and colleagues18). Overall, the results during enforced wake are consistent with those observed during baseline, with [lac] following changes in sleep pressure and [oxy] reflecting more acute changes in motor activity.
Figure 5.
During both spontaneous and recovery nonrapid eye movement (NREM) sleep after sleep deprivation, increased sleep pressure is associated with larger declines in [lac], whereas [oxy] does not change in association with sleep pressure. Average changes in [lac] (A) and [oxy] (B) during episodes of early (hr 1-4 after lights onset) and late (hr 8-12 after lights onset) spontaneous NREM sleep. (C and D) Top panels: average [lac] and [oxy] for each 4-sec epoch of wake (red), NREM sleep (blue), and REM sleep (green) during 3 hr of sleep deprivation and ensuing 3 hr of recovery sleep in individual rats. Bottom panels: electromyographic activity is depicted for each 4-sec epoch (gray) along with a 1-min moving average overlay (black). (E and F) Average [lac], [glu], or [oxy] (n = 5 each) across the 3-hr sleep deprivation. Concentrations were averaged across 30-min bins and plotted at the middle time point for each bin. (G and H) Average [lac] and [oxy] (relative to sleep onset) during the first 9 hr from light onset (baseline days) and during the first 9 hr after the end of sleep deprivation (n = 5 rats each). Concentrations were binned and averaged across 1-hr intervals and plotted at the middle time point for each bin. (I and J) The relationship between the rates of decline in [lac] and [oxy] and slow-wave activity (SWA) during NREM sleep following either undisturbed baseline (empty gray circles; n = 10 rats each) or sleep deprivation (filled black circles; n = 5 rats each). SWA and analyte concentrations were binned and averaged for 2-hr intervals across the light period. *P < 0.05. Glutamate results were derived from data previously collected by Dash et al.18
We then compared changes in [lac] and [oxy] during baseline sleep to changes during the recovery period after sleep deprivation. Sleep deprivation yielded the expected increase in SWA during ensuing recovery sleep in comparison with baseline sleep for all animals. Overall, SWA during the first 3 hr of recovery sleep increased by 128.29 ± 3.35% over baseline in those animals in which [lac] was recorded, and by 126.02 ± 2.38% in those rats in which [oxy] was measured. Despite this increased sleep intensity during recovery sleep, [oxy] declined to a similar extent during recovery and baseline sleep (Figure 5H; main effect of condition: F (1,8) = 0.32, P = 0.59; Condition × Time: F (4,32) = 0.21, P = 0.93), whereas [lac] declined more during recovery sleep compared with baseline (Figure 5G; main effect of condition: F (1,8) = 15.25, P < 0.01; Condition × Time: F (4,32) = 2.82, P < 0.05). To more directly assess the relationship between sleep pressure and [lac] and [oxy], we calculated SWA during all episodes of NREM sleep and compared these values to the observed changes in these metabolites. We found that the rate of decline in [lac] during NREM sleep was significantly correlated with SWA, both during baseline (r = 0.295; P < 0.05) and when sleep deprivation data were included (r = 0.309; P < 0.01, Figure 5I), whereas no correlation was found for [oxy] (Figure 5H, baseline, r = −0.033; P = 0.801; baseline + standard deviation, r = −0.113; P = 0.320; Figure 5J). Thus, [lac], but not [oxy], changes along with sleep pressure.
DISCUSSION
We found overall similarities and specific differences in the way [oxy] and [lac] are affected by sleep and wake. Both metabolites increased progressively during wake and REM sleep and decreased during NREM sleep. The [lac] findings are in line with previous studies showing elevated [lac] during wake and REM sleep compared with NREM sleep,8,39,40 although in those experiments the rapid and progressive nature of these changes was not examined, nor were the changes across the 24-hr cycle and after sleep deprivation. Our results are also consistent with many studies that showed that wake and REM sleep have higher energy demand than NREM sleep,5–8 and [lac] and [oxy] predominantly reflect supply, rather than consumption, with the supply typically exceeding consumption. Acute neuronal activation, for instance, which increases metabolic demands, also increases [lac], with the exception of a 10- 12-sec initial decrease thought to reflect rapid uptake and consumption before lactate production is activated.41 Similarly, acute neuronal activation increases [oxy], again with the possible exception of an initial 1- 6-sec decrease.42–44 Thus, both acute (previous studies) and more chronic (this report) conditions of increased cortical neuronal activity result in higher levels of [lac] and [oxy].
Importantly, we also found that cortical levels of [oxy] and [lac] progressively increased during wake for up to approximately 1 hr, the maximal duration of spontaneous wake episodes in our animals (68.34 ± 8.99 min, n = 20 rats). These findings are in line with previous studies suggesting that the cost of wake is not fixed but may increase with wake duration, due to a progressive increase in synaptic strength.14–16,45,46–47 Note, however, that although [oxy] and [lac] showed a progressive buildup during episodes of spontaneous wake, there was no obvious increasing trend after the first 1-2 hr of sleep deprivation. This is consistent with converging evidence showing that molecular,2 biochemical (cortical [glu]),18 and metabolic (activity of mitochondrial enzymes)48 markers that are higher during spontaneous wake relative to sleep only increase slightly during the beginning of sleep deprivation (for approximately 1-3 hr) and moreover do not increase further as sleep deprivation is prolonged. In the current study, we observed that [lac] exhibited a similar pattern, increasing only at the beginning of the deprivation before declining over the last hour of prolonged wakefulness. Together, these data may indicate that the quality of waking activity may change as sleep pressure increases across the duration of the deprivation. This change in waking quality may account for the decline in [lac] despite continued waking at the end of the deprivation, consistent with recent findings that clearly demonstrate that, toward the end of a short sleep deprivation (3-4 hr), local groups of neurons progressively go ′off-line’ as they normally do during NREM sleep,49 despite the fact that the rats look entirely awake, with movement and a scalp EEG typical of wake.
We also found that changes in [lac] and [oxy] were delayed by several seconds relative to behavioral state onset and to changes in extracellular [glu]. It is unlikely that these delays were due to a technical artifact, because the response time of the electrodes (< 1 sec) is much faster than the delays and delays were not observed in our previous [glu] recordings using a similar technique. Most likely, therefore, the delays reflect the time it takes for metabolic processes to respond to changes in energy demand at behavioral state transitions. Of note, the duration of the delays was variable, especially for oxygen. [Lac] most likely reflects local lactate production and subsequent efflux through monocarboxylate transporters, because physiologic lactate levels in the blood largely do not affect cortical [lac] levels.50,51 By contrast, intracortical [oxy] correlates with cerebral blood flow both across baseline and activated conditions.26,52 Thus, delays for oxygen may be more affected by the distance from each electrode to the nearest capillary and/or arteriole, because local oxygen pressure can vary as a function of the distance to these oxygen sources.36
Although [lac] is the local product of glycolysis, [oxy] reflects blood-derived supply used to sustain both oxidative phosphorylation and aerobic glycolysis. Thus, despite the similarities, it is perhaps not surprising that we found differences in the way wake and sleep affected the 2 metabolites. First, [oxy] increased mainly when the rats were actively moving, consistent with numerous reports of motor tasks increasing blood flow to cortical areas involved in motor processing (reviewed by Toma and Nakai37). [Oxy] also increased more during wake epochs that contained elevated high-frequency BLP and reduced low-frequency BLP. Elevated EEG power in high frequencies (40–200 Hz) has repeatedly been shown to correlate with neuronal firing rate, dendritic inputs, and/or synchrony.53–55 Cerebral blood flow is coupled to ongoing neuronal activity56 and both [oxy]57 and the hemodynamic response58–60 correlate more with high-frequency power than with neuronal firing rates. Thus, [oxy] may be particularly sensitive to changes in dendritic input and/or neuronal synchrony.
[Lac], in contrast, was not associated with changes in locomotion or BLP, characteristics shared with [glu]. The dynamics of the [lac] decline during sleep were also very similar to those of [glu] (see the next paragraphs), suggesting that changes in [lac] may primarily reflect changes in the uptake of extracellular glutamate. One major source of [lac] is astrocytic glycolysis. Astrocytic glycolysis is activated in response to elevated extracellular concentrations of K+ and/or glutamate21,61,62 producing lactate and providing ATP to fuel the Na, K-ATPase membrane pump activity necessary to maintain glutamate uptake.63,64 Neurons may then use lactate to support synaptic activity65 and plasticity, for example, to provide intermediates for synaptic biosynthesis.66 Because increasing evidence shows that waking activity results in a net increase in synaptic strength whereas sleep favors synaptic renormalization,14–16,45–47 it is tempting to speculate that the observed increase in [lac] plays an important role in maintaining these newly strengthened synapses. Indeed, experimental block of lactate production and/or delivery to neurons abolishes hippocampal long-term potentiation and long-term memory formation.67
[Lac] and [oxy] also showed important differences during sleep. Specifically, during NREM sleep the rate of decline of [oxy] did not correlate with SWA, a measure of sleep intensity and sleep need.1 In both rodents and humans increases in sleep pressure are typically associated with increases in the low-frequency EEG power in both wake68,69 and NREM sleep.1 However, as described previously, changes in [oxy] during wake were negatively correlated with changes in low-frequency EEG power, and positively correlated with changes in high-frequency EEG power. In stark contrast, the rate of decline of [lac] did reflect sleep pressure, because it was higher during early rather than late sleep and it correlated with SWA. Why should only [lac] be linked to the need for sleep? Although we can only speculate, one clue may be that the dynamics of [lac] during NREM sleep and its link with SWA mirrored those previously found for [glu], strengthening the connection between production of lactate and sleep-wake changes in [glu]. Because lactate production may disproportionately occur within astrocytes,21 these observations also hint at a role for astrocytes in contributing to sleep homeostasis, consistent with previous studies showing a link between sleep regulation and gliotransmission.70,71 Regardless of the cell type responsible for lactate's production, our results point to a role for lactate and the processes that lead to its extracellular buildup in sleep homeostasis. One previous study provided evidence for such a role in subcortical areas: it was found that sleep deprivation in young rats resulted in increased [lac] in the basal forebrain as well as increase in SWA during recovery sleep, whereas in older rats neither [lac] nor SWA were affected by sleep loss.72 Thus, at least in the basal forebrain, it appears that glutamatergic activity and lactate accumulation are essential regulators of sleep homeostasis. Interestingly, the same study also found that in young but not in older rats [lac] in the basal forebrain correlated with the wake EEG power in the high theta frequency, considered to be a measure of cortical arousal.72 In the current study, we did not observe a similar relationship between [lac] in the cerebral cortex and waking EEG theta power, nor were we able to characterize a specific aspect of waking activity that leads to the accumulation of cortical lactate. In the future, it will be important to use our method to test how waking activity leads to lactate accumulation in different brain regions, whether the accumulation of lactate causally affects subsequent sleep need, and if so, whether this occurs in some but not all brain areas.
These results point to a role for glycolytic processes in sleep homeostasis, but do not rule out a similar role for oxidative phosphorylation. A recent study linked the ATP levels during early NREM sleep to sleep homeostasis,73 but future experiments should investigate whether the sleep-wake history is reflected in progressive changes in mitochondrial enzymes’ activity and/or in the number or rate of movement of mitochondria.
When interpreting the results of this study, it is important to consider a number of limitations inherent to the experimental design and methodology. Amperometric recordings can typically measure only 1 analyte at a time and thus we were unable to simultaneously assess how behavioral state affects [lac] and [oxy] in the same animal. Moreover, although it is likely that [lac] and [oxy] predominantly reflect supply rather than demand (see previous paragraphs), without independent measures of consumption and/or supply of these metabolites we cannot definitively conclude whether their observed changes arise from changes in supply, demand, or a combination thereof. Additionally, our recordings were confined to prefrontal/motor cortical regions. We have conducted pilot recordings of [lac] in barrel cortex and [oxy] in visual cortex and observed qualitatively similar results to those presented here. However, more experiments are needed to determine whether the observed relationships between these energy metabolites, EEG power, and sleep homeostasis is similar throughout cortex. Finally, the association between [lac] and sleep homeostasis is strictly correlative. Thus, future experiments investigating whether lactate accumulation during waking has a causal role in mediating the need for sleep will be necessary to further understand the relationships between waking activity, glycolysis, and sleep homeostasis.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Tononi has consulted for Philips Respironics and has been involved in a research study in humans supported by Philips Respironics. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This work was funded by the National Institute of Mental Health (P20 MH077967 to Dr. Cirelli), by the NIH Director's Pioneer award (to Dr. Tononi), and by a grant from the James S. McDonnell Foundation.
REFERENCES
- 1.Achermann P, Borbely AA. Mathematical models of sleep regulation. Front Biosci. 2003;8:s683–93. doi: 10.2741/1064. [DOI] [PubMed] [Google Scholar]
- 2.Tononi G, Cirelli C. Sleep function and synaptic homeostasis. Sleep Med Rev. 2006;10:49–62. doi: 10.1016/j.smrv.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 3.Attwell D, Laughlin SB. An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab. 2001;21:1133–45. doi: 10.1097/00004647-200110000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Sibson NR, Dhankhar A, Mason GF, et al. Stoichiometric coupling of brain glucose metabolism and glutamatergic neuronal activity. Proc Natl Acad Sci U S A. 1998;95:316–21. doi: 10.1073/pnas.95.1.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kennedy C, Gillin JC, Mendelson W, et al. Local cerebral glucose utilization in non-rapid eye movement sleep. Nature. 1982;297:325–7. doi: 10.1038/297325a0. [DOI] [PubMed] [Google Scholar]
- 6.Madsen PL, Vorstrup S. Cerebral blood flow and metabolism during sleep. Cerebrovascular and Brain Metabolism Reviews. 1991;3:281–96. [PubMed] [Google Scholar]
- 7.Netchiporouk L, Shram N, Salvert D, Cespuglio R. Brain extracellular glucose assessed by voltammetry throughout the rat sleep-wake cycle. Eur J Neurosci. 2001;13:1429–34. doi: 10.1046/j.0953-816x.2001.01503.x. [DOI] [PubMed] [Google Scholar]
- 8.Shram N, Netchiporouk L, Cespuglio R. Lactate in the brain of the freely moving rat: voltammetric monitoring of the changes related to the sleep-wake states. Eur J Neurosci. 2002;16:461–6. doi: 10.1046/j.1460-9568.2002.02081.x. [DOI] [PubMed] [Google Scholar]
- 9.Desiraju T. Discharge properties of neurons of the parietal association cortex during states of sleep and wakefulness in the monkey. Brain Res. 1972;47:69–75. doi: 10.1016/0006-8993(72)90252-1. [DOI] [PubMed] [Google Scholar]
- 10.Noda H, Adey WR. Neuronal activity in the association cortex of the cat during sleep, wakefulness and anesthesia. Brain Res. 1973;54:243–59. doi: 10.1016/0006-8993(73)90047-4. [DOI] [PubMed] [Google Scholar]
- 11.Cirelli C, Tononi G. Gene expression in the brain across the sleep-waking cycle. Brain Res. 2000;885:303–21. doi: 10.1016/s0006-8993(00)03008-0. [DOI] [PubMed] [Google Scholar]
- 12.Cirelli C, Gutierrez CM, Tononi G. Extensive and divergent effects of sleep and wakefulness on brain gene expression. Neuron. 2004;41:35–43. doi: 10.1016/s0896-6273(03)00814-6. [DOI] [PubMed] [Google Scholar]
- 13.Maret S, Dorsaz S, Gurcel L, et al. Homer1a is a core brain molecular correlate of sleep loss. Proc Natl Acad Sci U S A. 2007;104:20090–5. doi: 10.1073/pnas.0710131104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vyazovskiy VV, Cirelli C, Pfister-Genskow M, et al. Molecular and electrophysiological evidence for net synaptic potentiation in wake and depression in sleep. Nat Neurosci. 2008;11:200–8. doi: 10.1038/nn2035. [DOI] [PubMed] [Google Scholar]
- 15.Liu ZW, Faraguna U, Cirelli C, et al. Direct evidence for wake-related increases and sleep-related decreases in synaptic strength in rodent cortex. J Neurosci. 2010;30:8671–5. doi: 10.1523/JNEUROSCI.1409-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lante F, Toledo-Salas JC, Ondrejcak T, et al. Removal of synaptic Ca(2)+-permeable AMPA receptors during sleep. J Neurosci. 2011;31:3953–61. doi: 10.1523/JNEUROSCI.3210-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vyazovskiy VV, Olcese U, Lazimy YM, et al. Cortical firing and sleep homeostasis. Neuron. 2009;63:865–78. doi: 10.1016/j.neuron.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dash MB, Douglas CL, Vyazovskiy VV, et al. Long-term homeostasis of extracellular glutamate in the rat cerebral cortex across sleep and waking states. J Neurosci. 2009;29:620–9. doi: 10.1523/JNEUROSCI.5486-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Porkka-Heiskanen T, Kalinchuk AV. Adenosine, energy metabolism and sleep homeostasis. Sleep Med Rev. 2011;15:123–35. doi: 10.1016/j.smrv.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 20.Gjedde A, Marrett S, Vafaee M. Oxidative and nonoxidative metabolism of excited neurons and astrocytes. J Cereb Blood Flow Metab. 2002;22:1–14. doi: 10.1097/00004647-200201000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Magistretti PJ. Role of glutamate in neuron-glia metabolic coupling. Am J Clin Nutr. 2009;90:875S–80S. doi: 10.3945/ajcn.2009.27462CC. [DOI] [PubMed] [Google Scholar]
- 22.Hertz L, Peng L, Dienel GA. Energy metabolism in astrocytes: high rate of oxidative metabolism and spatiotemporal dependence on glycolysis/glycogenolysis. J Cereb Blood Flow Metab. 2007;27:219–49. doi: 10.1038/sj.jcbfm.9600343. [DOI] [PubMed] [Google Scholar]
- 23.Wu K, Aoki C, Elste A, et al. The synthesis of ATP by glycolytic enzymes in the postsynaptic density and the effect of endogenously generated nitric oxide. Proc Natl Acad Sci U S A. 1997;94:13273–8. doi: 10.1073/pnas.94.24.13273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rutherford EC, Pomerleau F, Huettl P, et al. Chronic second-by-second measures of L-glutamate in the central nervous system of freely moving rats. J Neurochem. 2007;102:712–22. doi: 10.1111/j.1471-4159.2007.04596.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burmeister JJ, Gerhardt GA. Self-referencing ceramic-based multisite microelectrodes for the detection and elimination of interferences from the measurement of L-glutamate and other analytes. Anal Chem. 2001;73:1037–42. doi: 10.1021/ac0010429. [DOI] [PubMed] [Google Scholar]
- 26.Lowry JP, Boutelle MG, Fillenz M. Measurement of brain tissue oxygen at a carbon past electrode can serve as an index of increases in regional cerebral blood flow. J Neurosci Methods. 1997;71:177–82. doi: 10.1016/s0165-0270(96)00140-9. [DOI] [PubMed] [Google Scholar]
- 27.Bazzu G, Puggioni GG, Dedola S, et al. Real-time monitoring of brain tissue oxygen using a miniaturized biotelemetric device implanted in freely moving rats. Anal Chem. 2009;81:2235–41. doi: 10.1021/ac802390f. [DOI] [PubMed] [Google Scholar]
- 28.McHugh SB, Fillenz M, Lowry JP, et al. Brain tissue oxygen amperometry in behaving rats demonstrates functional dissociation of dorsal and ventral hippocampus during spatial processing and anxiety. Eur J Neurosci. 2011;33:322–37. doi: 10.1111/j.1460-9568.2010.07497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Benoit-Marand M, Suaud-Chagny MF, Gonon F. Presynaptic regulation of extracellular dopamine as studied by continuous amperometry in anesthetized animals. In: Michael A, Borland L, editors. Electrochemical methods for neuroscience. Boca Raton, FL: CRC Press; 2007. [PubMed] [Google Scholar]
- 30.Wang J. Electrochemical glucose biosensors. Chem Rev. 2008;108:814–25. doi: 10.1021/cr068123a. [DOI] [PubMed] [Google Scholar]
- 31.Robinson DL, Hermans A, Seipel AT, Wightman RM. Monitoring rapid chemical communication in the brain. Chem Rev. 2008;108:2554–84. doi: 10.1021/cr068081q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilson GS, Gifford R. Biosensors for real-time in vivo measurements. Biosens Bioelectron. 2005;20:2388–2403. doi: 10.1016/j.bios.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 33.Siesjo BK. Brain energy metabolism. New York: Wiley; 1978. [Google Scholar]
- 34.Alfoldi P, Franken P, Tobler I, Borbely AA. Short light-dark cycles influence sleep stages and EEG power spectra in the rat. Behav Brain Res. 1991;43:125–31. doi: 10.1016/s0166-4328(05)80062-2. [DOI] [PubMed] [Google Scholar]
- 35.Fisher SP, Sugden D. Endogenous melatonin is not obligatory for the regulation of the rat sleep-wake cycle. Sleep. 2010;33:833–40. doi: 10.1093/sleep/33.6.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vovenko E. Distribution of oxygen tension on the surface of arterioles, capillaries and venules of brain cortex and in tissue in normoxia: an experimental study on rats. Pflugers Arch. 1999;437:617–23. doi: 10.1007/s004240050825. [DOI] [PubMed] [Google Scholar]
- 37.Toma K, Nakai T. Functional MRI in human motor control studies and clinical applications. Magn Reson Med Sci. 2002;1:109–20. doi: 10.2463/mrms.1.109. [DOI] [PubMed] [Google Scholar]
- 38.Neafsey EJ, Bold EL, Haas G, et al. The organization of the rat motor cortex: a microstimulation mapping study. Brain Res. 1986;396:77–96. doi: 10.1016/s0006-8993(86)80191-3. [DOI] [PubMed] [Google Scholar]
- 39.Richter D, Dawson RM. Brain metabolism in emotional excitement and in sleep. Am J Physiol. 1948;154:73–9. doi: 10.1152/ajplegacy.1948.154.1.73. [DOI] [PubMed] [Google Scholar]
- 40.Cocks JA. Change in the concentration of lactic acid in the rat and hamster brain during natural sleep. Nature. 1967;215:1399–400. doi: 10.1038/2151399a0. [DOI] [PubMed] [Google Scholar]
- 41.Hu Y, Wilson GS. A temporary local energy pool coupled to neuronal activity: fluctuations of extracellular lactate levels in rat brain monitored with rapid-response enzyme-based sensor. J Neurochem. 1997;69:1484–90. doi: 10.1046/j.1471-4159.1997.69041484.x. [DOI] [PubMed] [Google Scholar]
- 42.Thompson JK, Peterson MR, Freeman RD. Single-neuron activity and tissue oxygenation in cerebral cortex. Science. 2003;299:1070–2. doi: 10.1126/science.1079220. [DOI] [PubMed] [Google Scholar]
- 43.Offenhauser N, Thomsen K, Caesar K, Lauritzen M. Activity-induced tissue oxygenation changes in rat cerebellar cortex: interplay of postsynaptic activation and blood flow. J Physiol. 2005;565:279–94. doi: 10.1113/jphysiol.2005.082776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li B, Freeman RD. High-resolution neurometabolic coupling in the lateral geniculate nucleus. J Neurosci. 2007;27:10223–9. doi: 10.1523/JNEUROSCI.1505-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gilestro GF, Tononi G, Cirelli C. Widespread changes in synaptic markers as a function of sleep and wakefulness in Drosophila. Science. 2009;324:109–12. doi: 10.1126/science.1166673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bushey D, Tononi G, Cirelli C. Sleep and synaptic homeostasis: structural evidence in Drosophila. Science. 2011;332:1576–81. doi: 10.1126/science.1202839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hulse BK, Landsness EC, Sarasso S, et al. A postsleep decline in auditory evoked potential amplitude reflects sleep homeostasis. Clin Neurophysiol. 2011;122:1549–55. doi: 10.1016/j.clinph.2011.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nikonova EV, Vijayasarathy C, Zhang L, et al. Differences in activity of cytochrome C oxidase in brain between sleep and wakefulness. Sleep. 2005;28:21–7. doi: 10.1093/sleep/28.1.21. [DOI] [PubMed] [Google Scholar]
- 49.Vyazovskiy VV, Olcese U, Hanlon EC, et al. Local sleep in awake rats. Nature. 2011;472:443–7. doi: 10.1038/nature10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kuhr WG, van den Berg CJ, Korf J. In vivo identification and quantitative evaluation of carrier-mediated transport of lactate at the cellular level in the striatum of conscious, freely moving rats. J Cereb Blood Flow Metab. 1988;8:848–56. doi: 10.1038/jcbfm.1988.142. [DOI] [PubMed] [Google Scholar]
- 51.Boumezbeur F, Petersen KF, Cline GW, et al. The contribution of blood lactate to brain energy metabolism in humans measured by dynamic 13C nuclear magnetic resonance spectroscopy. J Neurosci. 2010;30:13983–91. doi: 10.1523/JNEUROSCI.2040-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Masamoto K, Kershaw J, Ureshi M, et al. Apparent diffusion time of oxygen from blood to tissue in rat cerebral cortex: implication for tissue oxygen dynamics during brain functions. J Appl Physiol. 2007;103:1352–8. doi: 10.1152/japplphysiol.01433.2006. [DOI] [PubMed] [Google Scholar]
- 53.Nir Y, Mukamel R, Dinstein I, et al. Interhemispheric correlations of slow spontaneous neuronal fluctuations revealed in human sensory cortex. Nat Neurosci. 2008;11:1100–8. doi: 10.1038/nn.2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mukamel R, Nir Y, Harel M, et al. Invariance of firing rate and field potential dynamics to stimulus modulation rate in human auditory cortex. Hum Brain Mapp. 2010;32:1181–93. doi: 10.1002/hbm.21100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ray S, Crone NE, Niebur E, et al. Neural correlates of high-gamma oscillations (60-200 Hz) in macaque local field potentials and their potential implications in electrocorticography. J Neurosci. 2008;28:11526–36. doi: 10.1523/JNEUROSCI.2848-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Keri S, Gulyas B. Four facets of a single brain: behaviour, cerebral blood flow/metabolism, neuronal activity and neurotransmitter dynamics. Neuroreport. 2003;14:1097–1106. doi: 10.1097/00001756-200306110-00001. [DOI] [PubMed] [Google Scholar]
- 57.Viswanathan A, Freeman RD. Neurometabolic coupling in cerebral cortex reflects synaptic more than spiking activity. Nat Neurosci. 2007;10:1308–12. doi: 10.1038/nn1977. [DOI] [PubMed] [Google Scholar]
- 58.Logothetis NK, Pauls J, Augath M, et al. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412:150–7. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- 59.Niessing J, Ebisch B, Schmidt KE, et al. Hemodynamic signals correlate tightly with synchronized gamma oscillations. Science. 2005;309:948–51. doi: 10.1126/science.1110948. [DOI] [PubMed] [Google Scholar]
- 60.Zaehle T, Frund I, Schadow J, et al. Inter- and intra-individual covariations of hemodynamic and oscillatory gamma responses in the human cortex. Front Hum Neurosci. 2009;3:8. doi: 10.3389/neuro.09.008.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Demestre M, Boutelle M, Fillenz M. Stimulated release of lactate in freely moving rats is dependent on the uptake of glutamate. J Physiol. 1997;499(Pt 3):825–32. doi: 10.1113/jphysiol.1997.sp021971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bittner CX, Valdebenito R, Ruminot I, et al. Fast and reversible stimulation of astrocytic glycolysis by K+ and a delayed and persistent effect of glutamate. J Neurosci. 2011;31:4709–13. doi: 10.1523/JNEUROSCI.5311-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pellerin L, Magistretti PJ. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci U S A. 1994;91:10625–9. doi: 10.1073/pnas.91.22.10625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Uehara T, Sumiyoshi T, Itoh H, Kurachi M. Role of glutamate transporters in the modulation of stress-induced lactate metabolism in the rat brain. Psychopharmacology (Berl) 2007;195:297–302. doi: 10.1007/s00213-007-0881-1. [DOI] [PubMed] [Google Scholar]
- 65.Barros LF, Deitmer JW. Glucose and lactate supply to the synapse. Brain Res Rev. 2010;63:149–59. doi: 10.1016/j.brainresrev.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 66.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–33. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Suzuki A, Stern SA, Bozdagi O, et al. Astrocyte-neuron lactate transport is required for long-term memory formation. Cell. 2011;144:810–23. doi: 10.1016/j.cell.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Finelli LA, Baumann H, Borbely AA, Achermann P. Dual electroencephalogram markers of human sleep homeostasis: correlation between theta activity in waking and slow-wave activity in sleep. Neuroscience. 2000;101:523–9. doi: 10.1016/s0306-4522(00)00409-7. [DOI] [PubMed] [Google Scholar]
- 69.Vyazovskiy VV, Tobler I. Theta activity in the waking EEG is a marker of sleep propensity in the rat. Brain Res. 2005;1050:64–71. doi: 10.1016/j.brainres.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 70.Halassa MM, Florian C, Fellin T, et al. Astrocytic modulation of sleep homeostasis and cognitive consequences of sleep loss. Neuron. 2009;61:213–9. doi: 10.1016/j.neuron.2008.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fellin T, Halassa MM, Terunuma M, et al. Endogenous nonneuronal modulators of synaptic transmission control cortical slow oscillations in vivo. Proc Natl Acad Sci U S A. 2009;106:15037–42. doi: 10.1073/pnas.0906419106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wigren HK, Rytkonen KM, Porkka-Heiskanen T. Basal forebrain lactate release and promotion of cortical arousal during prolonged waking is attenuated in aging. J Neurosci. 2009;29:11698–707. doi: 10.1523/JNEUROSCI.5773-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dworak M, McCarley RW, Kim T, et al. Sleep and brain energy levels: ATP changes during sleep. J Neurosci. 2010;30:9007–16. doi: 10.1523/JNEUROSCI.1423-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]