Abstract
Study Objective:
To test the hypothesis that recurrent sleep curtailment will result in decreased physical activity in adults at risk for type 2 diabetes.
Design:
Two-condition 2-period randomized crossover study.
Setting:
University General Clinical Research Center.
Participants:
Eighteen healthy patients with parental history of type 2 diabetes (9 females and 9 males, age 27 yr [standard deviation 3], body mass index 23.7 [2.3] kg/m2).
Interventions:
Two week-long inpatient sessions with 8.5 or 5.5-hr nighttime sleep opportunity. Participants who exercised regularly (39%) could follow their usual exercise routines during both sessions.
Measurements and Results:
Sleep and total body movement were measured by wrist actigraphy and waist accelerometry. Subjective mood and vigor was assessed using visual analog scales. The main outcome was the comparison of total activity counts between sleep conditions. Ancillary endpoints included changes in sedentary, light, and moderate plus vigorous activity, and their association with changes in mood and vigor. Daily sleep was reduced by 2.3 hr (P < 0.001) and total activity counts were 31% lower (P = 0.020) during the 5.5-hr time-in-bed condition. This was accompanied by a 24% reduction in moderate-plus-vigorous activity time (P = 0.005) and more sedentary behavior (+21 min/day; P = 0.020). The decrease in daily activity during the 5.5-hr time-in-bed condition was seen mostly in participants who exercised regularly (-39 versus −4% in exercisers versus nonexercisers; P = 0.027). Sleep loss-related declines in physical activity correlated strongly with declines in subjective vigor (R = 0.90; P < 0.001).
Conclusions:
Experimental sleep restriction results in decreased amount and intensity of physical activity in adults at risk for type 2 diabetes.
Citation:
Bromley LE; Booth JN; Kilkus JM; Imperial JG; Penev PD. Sleep restriction decreases the physical activity of adults at risk for type 2 diabetes. SLEEP 2012;35(7):977-984.
Keywords: Physical activity, sleep restriction, regular exercise, sedentary behavior, familial risk of diabetes
INTRODUCTION
Adults with a parental history of type 2 diabetes have a high risk of developing the disease, particularly in the setting of physical inactivity and excessive weight gain.1 Lifestyle modification combining diet-induced weight loss and increased physical activity can ameliorate the metabolic risk and reduce the incidence of type 2 diabetes in such high-risk populations.2,3 In the Diabetes Prevention Program, regular physical activity helped to maintain lost weight and reduced the incidence of diabetes in participants who did not meet weight-loss targets but achieved activity goals.3 Twin studies, which controlled for differences in genetic susceptibility to obesity, also indicate that individual physical activity has a considerable effect on total and visceral adiposity.4 Thus, the identification of modifiable factors that promote or interfere with the maintenance of adequate physical activity in at-risk individuals could lead to improved strategies for metabolic disease prevention.
Many people currently sleep fewer than 6 hr per night5 and self-reports of such short sleep have been associated with increased incidence of obesity and diabetes.6–8 Because individuals with inadequate sleep commonly complain of excessive daytime sleepiness and decreased physical functioning,9 it has been hypothesized that reduced physical activity is one of the factors that mediate the association of chronic sleep loss with metabolic morbidity. Indeed, recent data from our laboratory show that free-living adults with familial risk for type 2 diabetes who habitually curtail their sleep have reduced amounts of total daily movement and more sedentary behavior.10 However, these observational data could not determine whether recurrent sleep loss may have a causal role for the lower physical activity of short sleepers. The current study tested the hypothesis that experimental sleep restriction under carefully controlled conditions will decrease the amount of total daily movement and time spent in moderate and vigorous physical activity in adults with parental history of type 2 diabetes.
PATIENTS AND METHODS
Study Participants
Healthy adults ages 22-31 yr with body mass index (BMI) between 20.0 and 27.0 kg/m2 who had at least one parent with type 2 diabetes were recruited through local advertisements (a BMI cutoff of 28.0 kg/m2 was used for two male volunteers with athletic stature). Fasting weight and height were measured in the morning using a calibrated scale (Scale-Tronix Inc., Wheaton, IL) and stationary Harpenden stadiometer (Holtain, Crymych, Wales). We excluded subjects who had any acute or chronic medical condition; self-reported sleep problems (Pittsburgh Sleep Quality Index global sleep disturbance score > 7), night work, or habitual daytime naps; recent (< 4 wk) travel across time zones; history of irregular menstrual periods or pregnancy during the past year; depressed mood (Center for Epidemiologic Studies of Depression score > 15 confirmed by clinical interview); excessive alcohol intake (> 14 drinks/wk for men; > 7 drinks/wk for women); use of tobacco, prescription, over-the-counter, or illegal drugs and supplements that can affect sleep or metabolism; and abnormal findings on physical examination or laboratory testing including complete blood counts, comprehensive metabolic and thyroid function panels, 12-lead electrocardiogram and a 75-g oral glucose challenge. Study candidates kept sleep and activity logs while following their usual lifestyle at home, and their free-living activity-rest patterns were assessed by wrist and waist accelerometry (Actiwatch-64 and Actical; Mini-Mitter Respironics Inc., Bend, OR) as described previously.10 Using this information, we enrolled participants who either engaged in voluntary exercise more than twice and accumulated at least 90 min of moderate or 40 min of vigorous exercise in an average week (regular exercise category) or did not have such regular exercise habits (no regular exercise category). All participants completed one night of screening polysomnography (Neurofax-1100 EEG Acquisition System, Nihon-Kohden, Foothill Ranch, CA) to exclude sleep pathology.10
Experimental Protocol
Participants who did not exercise regularly at home were enrolled in a randomized crossover experiment including two 7-day inpatient periods with 8.5- versus 5.5-hr nightly sleep opportunity. Regular exercisers completed a similar randomized crossover protocol that included two longer 14-day inpatient periods with 8.5 versus 5.5-hr nightly sleep opportunity. Because the experimental sleep intervention during the first week of both studies was identical, this report presents the pooled data of participants with and without regular exercise habits during this corresponding 7-day period. The experimental protocols were registered (ClinicalTrials.gov Identifiers: NCT00721019 and NCT00724087) and approved by the Institutional Review Board of the University of Chicago. Research volunteers gave written informed consent and were paid for their participation. During each study, participants were admitted as inpatients in the Clinical Research Center and exposed to 7 nights with fixed time in bed (8.5 or 5.5 hr/night) on two separate occasions in random order at least 3 wk apart (median time between treatments: 2.4 mo; interquartile range: 2.0, 3.2 mo). Order of treatment was determined using random number tables. Female study participants completed both sleep conditions during the same phase of their menstrual cycle. To modify overnight sleep duration without changes in circadian phase, the usual going-to-bed and getting-out-of-bed times of the participants at home were moved proportionally closer together or further apart.
To expose participants to comparable “occupational” activity during each study, they performed office-like tasks such as working on a computer, entering data, making phone calls, designing, reading, writing, researching, and retrieving information for 6 hr/day, sitting at a desk in their room. The effect of sleep restriction on subjective vigor and affect was assessed using a set of visual analog scales (VAS) twice daily at approximately 09:00 in the morning and 15:00 in the afternoon.11 Participants spent the remaining waking hours engaged in indoor leisure activities and had free access to the Internet, telephone, TV, videos, computer games, newspapers, magazines, and books in their room. Those who exercised regularly were given free access to all on-campus athletic facilities, whereas those who did not were allowed to spend up to 60 min/day outside of the Clinical Research Center on the university campus. Participants were not aware that their physical activity is one of the outcomes of the study. They were informed that the purpose of the experiment was to examine the effects of sleep restriction on substrate (glucose) metabolism, which will be reported elsewhere. No naps were allowed and a member of the research staff monitored the safety and compliance of the participants during all waking hours, and accompanied them when they were outside of the laboratory. Continuous wrist actigraphy and waist accelerometry were used to monitor daily sleep and measure the physical activity of each participant during the study.10
Participants received an individualized 3-day rotating menu during both sleep conditions. A registered dietitian interviewed all participants to determine their food preferences and developed individualized, nutritionally balanced weight-maintenance meal plans. Caffeinated beverages were allowed only with breakfast and lunch, if needed to match usual caffeine consumption at home. The study diet had an initial caloric content equal to 1.5 (no-exercise group) and 1.7 (exercise group) times the resting metabolic rate of the participants at the time of screening with 50% (standard deviation 2%) of the energy from carbohydrate, 34% (2%) from fat, and 16% (1%) from protein. Daily calories were divided among breakfast (approximately 25%, 08:00-09:00), lunch (approximately 30%, 12:30-13:30), dinner (approximately 35%, 18:30-19:30) and an evening snack (approximately 10%, 21:00). Participants were weighed every morning before breakfast and, if needed, the daily energy content of their meals was adjusted to avoid > 1% changes in body weight. The caloric content and macronutrient composition of meals and snacks consumed during each study was calculated using Food Processor software (ESHA Research, Salem, OR). Calorie counts for one female participant who did not exercise were incomplete and were not used for further analysis.
Data Analysis and Statistics
Nighttime sleep was scored automatically with Actiware Sleep version 3.4 (Mini-Mitter Respironics Inc., Bend, OR) using a medium sensitivity setting of 40, as described previously.10 Daily sleep time was calculated as the sum of all minutes scored as sleep during the scheduled time in bed. Sleep efficiency was calculated as the percent of time in bed that was scored as sleep. Sleep onset latency was defined as the time between lights off and sleep onset. Daily sleep data were averaged across each experimental condition. Morning and afternoon VAS scores of vigor and affect were averaged for each day and then across sleep conditions. Activity records were analyzed using version 2.12 of the Actical software (Mini-Mitter Respironics Inc., Bend, OR), which translates accelerations recorded in 1-min epochs into categoric measures of time spent in light, moderate, and vigorous physical activity, based on an algorithm with estimated intensity cutoffs of < 3.0 metabolic equivalents (METS), ≥ 3 to < 6 METS, and ≥ 6.0 METS.10 Actical output included total number of activity counts, and the minutes of sedentary time, light and moderate plus vigorous physical activity during each 24-hr period. Results were averaged to obtain measures of total daily movement (activity counts/day) and intensity distribution of physical activity (min/day) during each sleep condition. To account for the contribution of sleep duration to total sedentary time, we subtracted the average sleep time (measured by Actiwatch) from the 24-hr sedentary time (measured by Actical) of each participant to calculate the amount of sedentary time when he or she was awake.
Weight maintenance was assessed using paired t test comparisons of average body weight, its coefficient of variability, and the difference between initial and final body weight during each sleep condition. The effect of experimental sleep restriction (5.5 versus 8.5-hr time in bed as a within-subject factor) on the total activity count (primary outcome measure) and time spent in sedentary, light, and moderate plus vigorous physical activity (ancillary endpoints) was examined using repeated-measures analysis of variance (RM-ANOVA) with exercise category (regular exercise versus no regular exercise) as a between-subject factor. The same approach was used to assess the effects of reduced sleep on subjective ratings of mood and vigor. All RM-ANOVA models were re-run with order of treatment (1st versus 2nd inpatient admission) and sex as additional between-subject factors. There were no significant main effects or interactions with sleep for order of treatment and sex. Because these factors did not influence the final results, they were not included in the reported analyses. Partial correlation analyses, controlling for age, BMI, sex, race/ethnicity, and exercise category were used to investigate whether sleep loss-related declines in subjective mood and vigor will be associated with changes in physical activity between the two sleep conditions. All analyses were performed using SPSS 19.0 (SPSS Inc. IBM, Chicago, IL). Testing of ancillary endpoints was done without adjustment for multiple comparisons. Results are reported as mean (SD) in the text.
RESULTS
Twenty participants (9 in the regular exercise category) completed the study. One female long-distance runner, who sustained a stress fracture during the home washout period after the 8.5-hr time-in-bed condition and stopped running prior to her second admission, and one male competitive underwater swimmer, whose physical activity in the pool could not be measured by accelerometry, were excluded from analysis. The characteristics of the remaining 18 participants at the time of screening are summarized in Table 1. Nine participants were studied in the 8.5-hr time-in-bed condition first; the other 9 started with the 5.5-h time-in-bed condition first.
Table 1.
Participant characteristics and screening polysomnography data

The average body weight of the participants and its variability were comparable between the two sleep conditions (Table 2). There was no difference between initial and final body weight during each study period (8.5-hr time in bed: 70.9 (11.5) versus 70.8 (11.5) kg, P = 0.68; 5.5-hr time in bed: 69.8 (11.6) versus 69.9 (11.7) kg, P = 0.58). Energy intake during the 8.5 and 5.5-hr time-in-bed condition averaged 38 (7) and 36 (6) kcal/kg/day, respectively, in participants with regular exercise habits, and 32 (2) and 32 (3) kcal/kg/day in those who did not exercise.
Table 2.
Weight maintenance, sleep, vigor, affect, and physical activity during each sleep condition

Daily sleep measured by actigraphy was decreased by 2 hr, 17 min (22 min) during the 5.5-hr compared with the 8.5-hr time-in-bed condition (P < 0.001; Table 2). As expected, sleep curtailment was accompanied by faster sleep onset, increased sleep efficiency, and considerably lower daytime vigor with approximately 50% higher ratings of subjective sleepiness, weariness, and effort (all P < 0.01; Table 2). Self-ratings of sadness, calm, tension, and global affect did not differ between the two sleep conditions, but participants felt less happy when their sleep was curtailed (P < 0.05; Table 2).
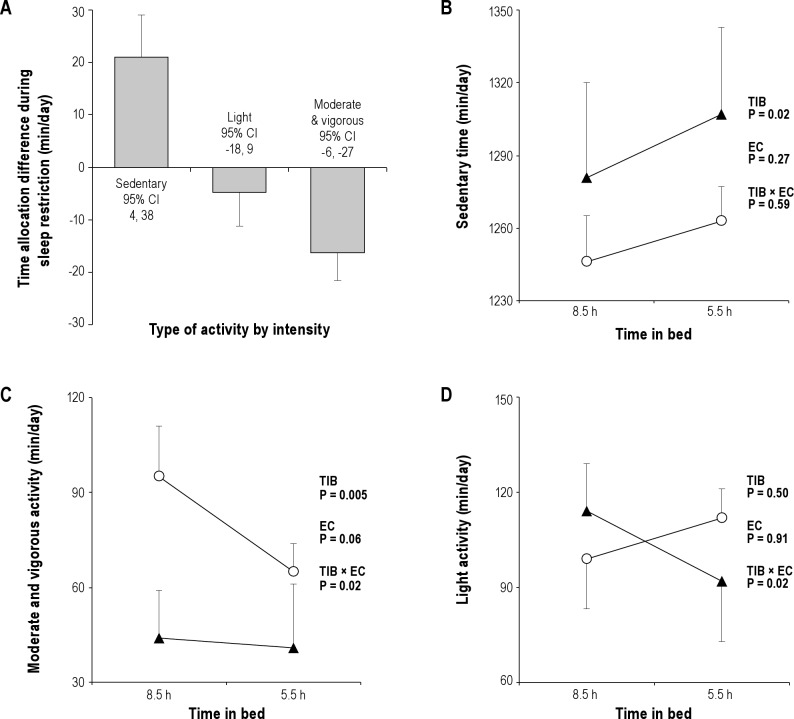
Compared with the 8.5-hr time-in-bed condition, participants had 31% fewer total activity counts (-52982 counts/24hr; 95% confidence interval [CI]: −9623 to −96341; P = 0.020; Figure 1A), spent 24% less time engaged in moderate plus vigorous physical activity (-16 min/24 hr; 95% CI: −6 to −27; P = 0.005; Figure 2A), and were more sedentary (+21 min/24 hr; 95% CI: 4 to 38; P = 0.020; Figure 2A) when their sleep was curtailed. Participants who had regular exercise habits and were allowed to exercise during the study had more total daily movement compared with those in the no-exercise category (+135634 counts/24 hr; 95% CI: 34373 to 236895; P = 0.012; Figure 1B), and were responsible for most of the sleep-related decrease in physical activity during the 5.5-hr time-in-bed condition (-39 versus −4% in exercisers versus nonexercisers; P = 0.027 for exercise category × time-in-bed interaction; Figure 1B). The shift toward lower-intensity physical activity and more sedentary time during the 5.5-hr time-in-bed condition (Figure 2A) also differed in participants with and without regular exercise. Regular exercisers reallocated almost a third of their moderate plus vigorous activity time during the 8.5-hr time-in-bed condition to less intense light and sedentary activities when their sleep was curtailed (-30 [12] min/24 hr; −31%; Figure 2B-D). Participants in the no-exercise category showed little change in daily time spent in moderate plus vigorous physical activity during the 5.5-h time-in-bed condition (-3 [3] min/24 hr; −7%; P = 0.020 for exercise category × time-in-bed interaction; Figure 2C). Instead, they reallocated time spent in light-intensity physical activity during the 8.5-hr time-in-bed condition to more sedentary behaviors during the 5.5-hr time-in-bed condition (P = 0.020 for exercise category × time-in-bed interaction; Figure 2D).
Figure 1.
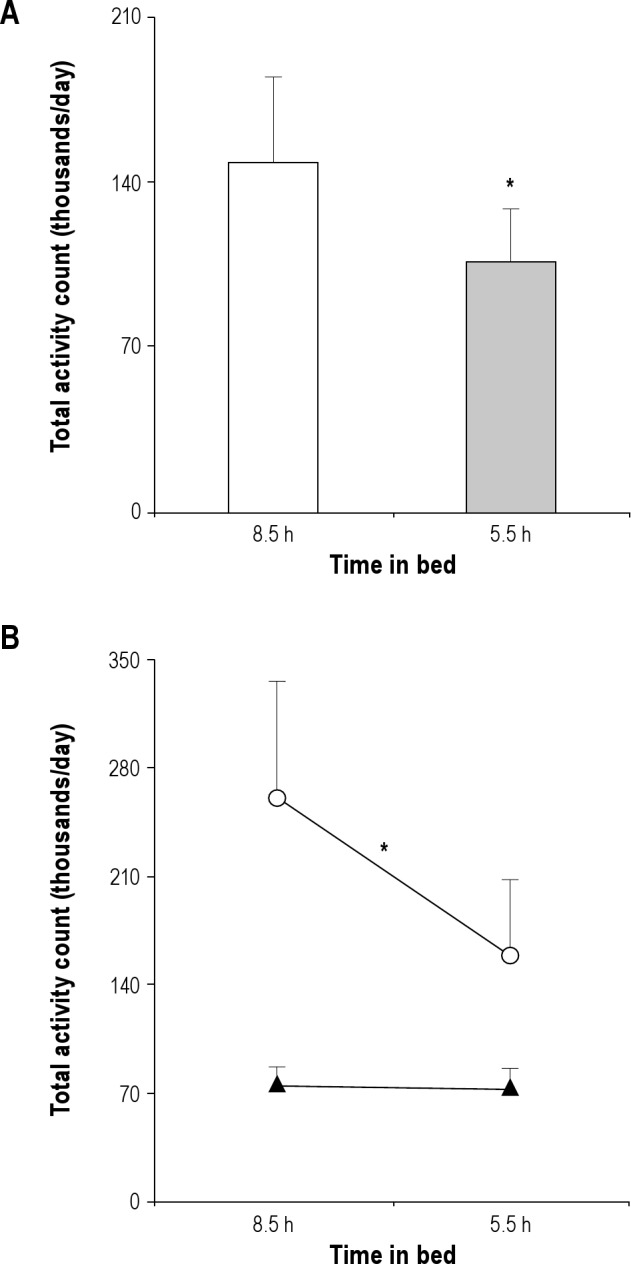
(A) Mean (+standard error, SE) total activity counts during the 8.5-h (open bar) and 5.5-h time-in-bed (shaded bar) experimental condition. *P = 0.020 for the effect of time in bed using repeated-measures analysis of variance with exercise category as a between-subject factor. (B) Mean (+SE) total activity counts during each sleep condition in the regular exercise (open circles) and no exercise (solid triangles) group of participants. *In addition to the main effect of time-in-bed condition (P = 0.020), there was a main effect of exercise versus no exercise category (P = 0.012) and a significant interaction between exercise category and time-in-bed condition (P = 0.027).
Figure 2.
(A) Estimated mean (± standard error, SE) change in daily time allocation to sedentary, light, and moderate plus vigorous physical activity during the 5.5-hr compared with the 8.5-hr time-in-bed condition, based on repeated-measures analysis of variance with exercise category as a between-subject factor. (B) Mean (+ SE) daily time spent sedentary during each sleep condition in the regular exercise (open circles) and no exercise (solid triangles) group of participants. (C) Mean (+ SE) daily time allocated to moderate plus vigorous physical activity during each sleep condition in the regular exercise (open circles) and no exercise (solid triangles) group of participants. (D) Mean (± SE) daily time allocated to light-intensity physical activity during each sleep condition in the regular exercise (open circles) and no exercise (solid triangles) group of participants. Repeated-measures analysis of variance was used to derive reported P values for the effect of time-in-bed (TIB) condition as a within-subject factor, exercise category (EC) as a between-subject factor, and their interaction (TIB × EC).
Partial correlation analysis controlling for individual age, BMI, sex, race/ethnicity, and habitual exercise category showed that sleep loss-related decrease in global vigor was strongly associated with the observed decline in total activity counts (R = 0.90; P < 0.001) and time spent in moderate plus vigorous physical activity (R = 0.73; P = 0.011). The strongest contributors to this relationship were the change in subjective alertness (R = 0.89 with change in daily activity count, P < 0.001; R = 0.79 with change in moderate plus vigorous activity time, P = 0.004) and weariness (R = −0.76 with change in daily activity counts, P = 0.006; R = −0.58 with change in moderate plus vigorous activity time, P = 0.060). Self-rated declines in alertness were also associated with increasing amounts of sedentary time during the 5.5-hr time-in-bed condition (R = −0.63; P = 0.037). There was no significant association between changes in physical activity and subjective affect.
DISCUSSION
Physical activity is a complex behavior that plays an important role in diabetes prevention and can be influenced by a number of biologic, psychosocial, and environmental factors. We tested the hypothesis that experimental sleep restriction in adults with familial risk for type 2 diabetes will result in decreased physical activity. Indeed, when the sleep of participants was reduced by 2.3 hr/day, they had 31% fewer daily activity counts, spent 24% less time engaged in moderate plus vigorous physical activity, and became more sedentary (Figures 1 and 2). This sleep loss-related decrease in activity was seen mostly in participants with regular exercise habits, who were allowed to exercise during the study – on average, they reallocated 30 min of their daily moderate plus vigorous activity time to less intense light and sedentary activities during the 5.5-hr time-in-bed condition. The decline in total movement and time spent in moderate plus vigorous activity during the 5.5-hr time-in-bed condition correlated strongly with the corresponding reduction in subjective vigor. These findings are consistent with the hypothesis that chronic sleep insufficiency can result in reduced amounts and intensity of everyday physical activity, and thus contribute to the risk of obesity and type 2 diabetes in susceptible individuals.
Limited by the reliability of questionnaires based on subjective recall,5,12 cross-sectional analyses of self-reported sleep and physical activity in adults have given inconsistent results showing either positive,13–15 negative,7 or no significant association.6,16,17 Rare observational reports including objective monitoring of sleep or physical activity in samples of rural versus urban-dwelling and very active versus less physically active individuals have also reached conflicting conclusions.10,17–19 Only a handful of experimental studies have examined the effects of sleep deprivation on daily activity. Schmid et al. found that overnight sleep restriction results in less total movement and a shift toward lower-intensity activities on the following day.20 Similar experiments in participants confined to the laboratory showed a higher percentage of inactivity time after overnight sleep deprivation.21 In contrast, Brondel et al. observed that overnight sleep restriction was followed by more movement during a subsequent day with increased food intake, structured morning tasks, and free-living activity after lunch,22 whereas Bosy-Westphal et al. did not find effects of sleep restriction and increased food intake (2 days with time in bed of 6 hr/night and 1 day with 4 hr/night) on daily activity.23 Finally, St-Onge et al. performed a crossover inpatient study of 9 women and 9 men exposed to 5 nights with time in bed of 4.0 versus 9.0 hr/night and caloric restriction (approximately 400 fewer kcal/day) until night 4 followed by uncontrolled ad libitum food intake after that: the average of the total activity counts recorded after the 1st, 2nd, 4th and 5th night (night 3 was followed by a day of repeated blood sampling when participants had in-dwelling catheters and their movements were restricted) showed no difference between sleep conditions.24 The comparison of these studies to our results is limited, because all of them involved a shorter period of sleep restriction and/or uncontrolled food intake. Both positive and negative energy balance can alter and confound the effects of sleep loss on physical activity.18,25–28 In addition, the consequences of brief sleep deprivation are likely to change as participants adapt to recurrent exposure18,25–28 and may not capture the changes in daily activity of people who exercise only a few times per week. Nevertheless, the decrease in activity during the short-sleep condition of the present study was very similar to the effects of experimental sleep restriction described by Schmid et al.20 and Roehrs et al.21
In addition to having fewer total activity counts, participants spent less time in moderate plus vigorous physical activity and remained more sedentary when their sleep was curtailed (Figures 1 and 2). Schmid et al.20 and St-Onge et al.24 found a similar shift away from time spent in high-intensity physical activity and in favor of lower-intensity physical activity in response to experimental sleep restriction. Consistent with these data, sleep deprivation was shown to decrease individual endurance and physical exercise performance on tasks with moderate and high intensity.29–32 In addition to changes in muscle metabolism,32 these effects have been attributed to increased perception of effort, negative affect, decreased vigor, and reduced motivation related to sleep loss.30–32 In a similar fashion, individuals with inadequate sleep often complain of excessive daytime sleepiness and report decreased physical functioning.9 In the current study, the sleep loss-related decline in total daily movement was strongly correlated with changes in subjective alertness, weariness, and global vigor. Importantly, our experimental sleep restriction was not accompanied by significant deterioration in global affect (Table 2) and the decline in physical activity during the short-sleep condition was not associated with changes in dysphoric or depressed mood.
Total sleep deprivation results in 20-30% higher overnight energy expenditure33 followed by compensatory declines in the waking metabolic rate during morning bed rest34 and energy expenditure during a subsequent night of recovery sleep.35 Compensation for increased energy expenditure in activity related to experimental sleep fragmentation during the night was also present in respiratory chamber experiments, where 24-hr energy expenditure did not change despite multiple periodic nighttime awakenings.36 The existence of such adaptations to acute sleep loss raises the possibility that the observed decrease in physical activity in the current study may be another compensatory mechanism to offset the metabolic cost of extended wakefulness during the 5.5-hr time-in-bed condition.25–28 Participants with regular exercise habits were responsible for most of the change (Figure 1B), which suggests that this group may be particularly vulnerable to the inhibitory effect of insufficient sleep on physical activity. However, participants were exposed to a highly sedentary inpatient environment that minimized total daily movement in the nonexercise group irrespective of the assigned sleep condition. In agreement with prior reports,37 exercisers and nonexercisers had similar 24-hr activity counts when monitored at home (Table 1). However, many of the habitual activities of nonexercisers (shopping, cooking, house cleaning, walking for transportation, etc.) were eliminated upon their admission to the sleep laboratory and their 24-hr activity counts decreased (free-living conditions versus 8.5-hr timeinbed: P < 0.001; Figure 1B). In contrast, ensuring that regular exercisers had uninterrupted access to all athletic facilities while in the laboratory allowed them to maintain a level of physical activity comparable to that at home (free-living conditions versus 8.5-hr time in bed: P = 0.879; Figure 1B). The absence of changes in daytime activity in respiratory-chamber studies of total sleep deprivation could be related to a similar `floor effect'.35,36 As a result, it remains possible that free-living individuals without regular exercise habits will also exhibit significant declines in everyday physical activity when their sleep is curtailed.
From a clinical point of view, experimental sleep curtailment reduced total activity counts in adults at risk for type 2 diabetes by approximately 30%, and regular exercisers reallocated 30 min of their daily moderate plus vigorous physical activity to less intense light and sedentary activities. If persistent under free-living conditions, these changes can have important health implications. Higher amounts of total body movement have been associated with improved insulin sensitivity and better glucose tolerance,38–40 and moderate plus vigorous physical activity has a well-established role in the prevention of type 2 diabetes.2,3 For instance, walking for exercise at least 2.5 hr versus less than 1 hr per wk in the Finnish Diabetes Prevention Study was associated with a more than 60% lower risk of incident diabetes.2 In addition, when sleep was curtailed, participants had longer waking hours at their disposal but devoted all additional out-of-bed time and more (+21 min/day; Figure 2A) to various sedentary behaviors. Increased sedentary time has been associated with insulin resistance, abnormal glucose metabolism, and greater metabolic risk.40,41 Importantly, free-living adults with parental history of type 2 diabetes who habitually curtail their sleep show remarkably similar changes in the intensity of their everyday activities.10
This study had several strengths and limitations. We studied carefully screened healthy individuals with parental history of type 2 diabetes to avoid the confounding effects of obesity, sleep disorders, and poor general and emotional health on physical activity. Targeting individuals with increased risk for type 2 diabetes was important to inform further research on sleep and metabolic disease prevention. Using a well-controlled weight-maintenance diet under identical laboratory conditions, we were able to keep the weight of the study participants stable while experimentally manipulating their average sleep duration from > 7 hr/day (an epidemiologic sleep category with low metabolic risk) to < 6 hr/day (a category with increased metabolic risk).8 Despite its strengths, the study included a small number of participants with regular exercise habits, and physical activity was monitored during a relatively short period of time. Although the inpatient metabolic ward environment facilitates the maintenance of stable energy balance and allows precise manipulations of sleep, it limits the diversity of occupational, volitional, and incidental activities and social interactions, which may modify the effect of insufficient sleep on everyday physical activity outside of the laboratory.
In conclusion, experimental sleep restriction under well-controlled laboratory conditions decreased the amount and intensity of physical activity in adults with increased risk for type 2 diabetes. These results support the hypothesis that a reduction in physical activity in response to sleep loss is one of the causal pathways for the association of chronic sleep insufficiency with metabolic morbidity. Additional intervention studies are needed to define the magnitude and reversibility of this effect in everyday life settings.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank all volunteers for their participation, and the staff of the University of Chicago General Clinical Research Center and Luis Alcantar in the Department of Medicine for their excellent technical assistance. Preliminary data from this study were presented in abstract form at the 2010 Annual Meeting of the Endocrine Society. This work was supported by NIH grants R01-HL089637, CTSA-RR024999, and P60-DK020595.
REFERENCES
- 1.Sargeant LA, Wareham NJ, Khaw KT. Family history of diabetes identifies a group at increased risk for the metabolic consequences of obesity and physical inactivity in EPIC-Norfolk: a population-based study. The European Prospective Investigation into Cancer. Int J Obes. 2000;24:1333–9. doi: 10.1038/sj.ijo.0801383. [DOI] [PubMed] [Google Scholar]
- 2.Laaksonen DE, Lindstrom J, Lakka TA, et al. Physical activity in the prevention of type 2 diabetes: the Finnish diabetes prevention study. Diabetes. 2005;54:158–65. doi: 10.2337/diabetes.54.1.158. [DOI] [PubMed] [Google Scholar]
- 3.Hamman RF, Wing RR, Edelstein SL, et al. Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes Care. 2006;29:2102–7. doi: 10.2337/dc06-0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Samaras K, Kelly PJ, Chiano MN, Spector TD, Campbell LV. Genetic and environmental influences on total-body and central abdominal fat: the effect of physical activity in female twins. Ann Intern Med. 1999;130:873–82. doi: 10.7326/0003-4819-130-11-199906010-00002. [DOI] [PubMed] [Google Scholar]
- 5.Lauderdale DS, Knutson KL, Yan LL, Liu K, Rathouz PJ. Self-reported and measured sleep duration: how similar are they? Epidemiology. 2008;19:838–45. doi: 10.1097/EDE.0b013e318187a7b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patel SR, Malhotra A, White DP, Gottlieb DJ, Hu FB. Association between reduced sleep and weight gain in women. Am J Epidemiol. 2006;164:947–54. doi: 10.1093/aje/kwj280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaput JP, Despres JP, Bouchard C, Tremblay A. The association between sleep duration and weight gain in adults: a 6-year prospective study from the Quebec Family Study. Sleep. 2008;31:517–23. doi: 10.1093/sleep/31.4.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cappuccio FP, D'Elia L, Strazzullo P, Miller MA. Quantity and quality of sleep and incidence of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2010;33:414–20. doi: 10.2337/dc09-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weaver TE, Laizner AM, Evans LK, et al. An instrument to measure functional status outcomes for disorders of excessive sleepiness. Sleep. 1997;20:835–43. [PubMed] [Google Scholar]
- 10.Booth JN, Bromley L, Darukhanavala A, Whitmore HR, Imperial J, Penev P. Reduced physical activity in adults at risk for type 2 diabetes who curtail their sleep. Obesity (Silver Spring) 2012;20:278–84. doi: 10.1038/oby.2011.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Monk TH. A visual analogue scale technique to measure global vigor and affect. Psychiatry Research. 1989;27:89–99. doi: 10.1016/0165-1781(89)90013-9. [DOI] [PubMed] [Google Scholar]
- 12.Shephard RJ. Limits to the measurement of habitual physical activity by questionnaires. Br J Sports Med. 2003;37:197–206. doi: 10.1136/bjsm.37.3.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu X, Uchiyama M, Kim K, et al. Sleep loss and daytime sleepiness in the general adult population of Japan. Psychiatry Res. 2000;93:1–11. doi: 10.1016/s0165-1781(99)00119-5. [DOI] [PubMed] [Google Scholar]
- 14.Ohida T, Kamal AM, Uchiyama M, et al. The influence of lifestyle and health status factors on sleep loss among the Japanese general population. Sleep. 2001;24:333–8. doi: 10.1093/sleep/24.3.333. [DOI] [PubMed] [Google Scholar]
- 15.Patel SR, Blackwell T, Redline S, et al. The association between sleep duration and obesity in older adults. Int J Obes. 2008;32:1825–34. doi: 10.1038/ijo.2008.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Imaki M, Hatanaka Y, Ogawa Y, Yoshida Y, Tanada S. An epidemiological study on relationship between the hours of sleep and life style factors in Japanese factory workers. J Physiol Anthropol Appl Human Sci. 2002;21:115–20. doi: 10.2114/jpa.21.115. [DOI] [PubMed] [Google Scholar]
- 17.Youngstedt SD, Perlis ML, O'Brien PM, et al. No association of sleep with total daily physical activity in normal sleepers. Physiol Behav. 2003;78:395–401. doi: 10.1016/s0031-9384(03)00004-0. [DOI] [PubMed] [Google Scholar]
- 18.Montgomery I, Trinder J, Paxton SJ. Energy expenditure and total sleep time: effect of physical exercise. Sleep. 1982;5:159–68. doi: 10.1093/sleep/5.2.159. [DOI] [PubMed] [Google Scholar]
- 19.Evans DS, Snitker S, Wu SH, et al. Habitual sleep/wake patterns in the Old Order Amish: heritability and association with non-genetic factors. Sleep. 2011;34:661–9. doi: 10.1093/sleep/34.5.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmid SM, Hallschmid M, Jauch-Chara K, et al. Short-term sleep loss decreases physical activity under free-living conditions but does not increase food intake under time-deprived laboratory conditions in healthy men. Am J Clin Nutr. 2009;90:1476–82. doi: 10.3945/ajcn.2009.27984. [DOI] [PubMed] [Google Scholar]
- 21.Roehrs T, Turner L, Roth T. Effects of sleep loss on waking actigraphy. Sleep. 2000;23:793–7. [PubMed] [Google Scholar]
- 22.Brondel L, Romer MA, Nougues PM, Touyarou P, Davenne D. Acute partial sleep deprivation increases food intake in healthy men. Am J Clin Nutr. 2010;91:1550–9. doi: 10.3945/ajcn.2009.28523. [DOI] [PubMed] [Google Scholar]
- 23.Bosy-Westphal A, Hinrichs S, Jauch-Chara K, et al. Influence of partial sleep deprivation on energy balance and insulin sensitivity in healthy women. Obes Facts. 2008;1:266–73. doi: 10.1159/000158874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.St-Onge MP, Roberts AL, Chen J, et al. Short sleep duration increases energy intakes but does not change energy expenditure in normal-weight individuals. Am J Clin Nutr. 2011;94:410–6. doi: 10.3945/ajcn.111.013904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. N Engl J Med. 1995;332:621–8. doi: 10.1056/NEJM199503093321001. [DOI] [PubMed] [Google Scholar]
- 26.Nedeltcheva AV, Kilkus JM, Imperial J, Kasza K, Schoeller DA, Penev PD. Sleep curtailment is accompanied by increased intake of calories from snacks. Am J Clin Nutr. 2009;89:126–33. doi: 10.3945/ajcn.2008.26574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nedeltcheva AV, Kilkus JM, Imperial J, Schoeller DA, Penev PD. Insufficient sleep undermines dietary efforts to reduce adiposity. Ann Intern Med. 2010;153:435–41. doi: 10.1059/0003-4819-153-7-201010050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosenbaum M, Leibel RL. Adaptive thermogenesis in humans. Int J Obes (Lond) 2010;34(Suppl 1):S47–55. doi: 10.1038/ijo.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen HI. Effects of 30-h sleep loss on cardiorespiratory functions at rest and in exercise. Med Sci Sports Exerc. 1991;23:193–8. [PubMed] [Google Scholar]
- 30.Rodgers CD, Paterson DH, Cunningham DA, et al. Sleep deprivation: effects on work capacity, self-paced walking, contractile properties and perceived exertion. Sleep. 1995;18:30–8. doi: 10.1093/sleep/18.1.30. [DOI] [PubMed] [Google Scholar]
- 31.Oliver SJ, Costa RJ, Laing SJ, Bilzon JL, Walsh NP. One night of sleep deprivation decreases treadmill endurance performance. Eur J Appl Physiol. 2009;107:155–61. doi: 10.1007/s00421-009-1103-9. [DOI] [PubMed] [Google Scholar]
- 32.Skein M, Duffield R, Edge J, Short MJ, Mundel T. Intermittent-sprint performance and muscle glycogen after 30 h of sleep deprivation. Med Sci Sports Exerc. 2011;43:1301–11. doi: 10.1249/MSS.0b013e31820abc5a. [DOI] [PubMed] [Google Scholar]
- 33.Boyle PJ, Scott JC, Krentz AJ, Nagy RJ, Comstock E, Hoffman C. Diminished brain glucose metabolism is a significant determinant for falling rates of systemic glucose utilization during sleep in normal humans. J Clin Invest. 1994;93:529–35. doi: 10.1172/JCI117003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Benedict C, Hallschmid M, Lassen A, et al. Acute sleep deprivation reduces energy expenditure in healthy men. Am J Clin Nutr. 2011;93:1229–36. doi: 10.3945/ajcn.110.006460. [DOI] [PubMed] [Google Scholar]
- 35.Jung CM, Melanson EL, Frydendall EJ, Perreault L, Eckel RH, Wright KP. Energy expenditure during sleep, sleep deprivation and sleep following sleep deprivation in adult humans. J Physiol. 2011;589:235–44. doi: 10.1113/jphysiol.2010.197517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hursel R, Rutters F, Gonnissen HK, Martens EA, Westerterp-Plantenga MS. Effects of sleep fragmentation in healthy men on energy expenditure, substrate oxidation, physical activity, and exhaustion measured over 48 h in a respiratory chamber. Am J Clin Nutr. 2011;94:804–8. doi: 10.3945/ajcn.111.017632. [DOI] [PubMed] [Google Scholar]
- 37.Westerterp KR. Pattern and intensity of physical activity. Nature. 2001;410:539. doi: 10.1038/35069142. [DOI] [PubMed] [Google Scholar]
- 38.Ekelund U, Griffin SJ, Wareham NJ. Physical activity and metabolic risk in individuals with a family history of type 2 diabetes. Diabetes Care. 2007;30:337–42. doi: 10.2337/dc06-1883. [DOI] [PubMed] [Google Scholar]
- 39.Balkau B, Mhamdi L, Oppert JM, et al. Physical activity and insulin sensitivity: the RISC study. Diabetes. 2008;57:2613–8. doi: 10.2337/db07-1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Healy GN, Wijndaele K, Dunstan DW, et al. Objectively measured sedentary time, physical activity, and metabolic risk: the Australian Diabetes, Obesity and Lifestyle Study (AusDiab) Diabetes Care. 2008;31:369–71. doi: 10.2337/dc07-1795. [DOI] [PubMed] [Google Scholar]
- 41.Helmerhorst HJ, Wijndaele K, Brage S, Wareham NJ, Ekelund U. Objectively measured sedentary time may predict insulin resistance independent of moderate- and vigorous-intensity physical activity. Diabetes. 2009;58:1776–9. doi: 10.2337/db08-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]