Abstract
Cystic fibrosis (CF) is a disease characterized by airway infection, inflammation, remodeling, and obstruction that gradually destroy the lungs. Direct delivery of the cystic fibrosis transmembrane conductance regulator (CFTR) gene to airway epithelia may offer advantages, as the tissue is accessible for topical delivery of vectors. Yet, physical and host immune barriers in the lung present challenges for successful gene transfer to the respiratory tract. Advances in gene transfer approaches, tissue engineering, and novel animal models are generating excitement within the CF research field. This review discusses current challenges and advancements in viral and nonviral vectors, cell-based therapies, and CF animal models.
Introduction
Cystic fibrosis (CF) is the most common lethal monogenic disease among Caucasians.1,2 It affects multiple organs including the pancreas, sweat glands, intestines, liver, and reproductive tract. However, the respiratory disease, characterized by progressive airway infection and inflammation, is the most common cause of the morbidity and mortality in CF patients.1,2 CF is a recessive disease caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene, which encodes an anion channel regulated by ATP hydrolysis and phosphorylation.3,4 CF is an attractive candidate for gene therapy because heterozygotes are phenotypically normal and the target cells lining the intrapulmonary airways are potentially accessible for vector delivery via aerosol or other topical strategies.
Since the CFTR gene was first cloned in 1989,3,4,5 several gene therapy strategies for correction of CF lung disease have been investigated. However, the development of safe and efficient vector systems remains a major challenge. This is due, in part, to the multiple, sophisticated pulmonary barriers that have evolved to clear or prevent the uptake of foreign particles.6 Thick secretions and the secondary effects of chronic infection and inflammation in the CF lung present additional barriers to gene transfer. An understanding of the obstacles gene transfer vectors face is required to devise successful strategies for gene transfer to the airway epithelium. Early intervention is likely an important component of optimal gene transfer for CF.
The respiratory epithelia lining the conducting airway are comprised of many cell types (Figure 1) including ciliated, nonciliated, basal, and goblet cells. CFTR is expressed in ciliated cells lining the surface epithelium and submucosal gland ducts7 as well as in serous cells of submucosal glands.8 Johnson and colleagues reported that expression of CFTR in as few as 6–10% of airway epithelia is sufficient to restore the function of chloride ion transport.9 However, another study suggested higher levels of correction (25%) may be required to normalize sodium ion transport and mucociliary clearance.10 Further studies are required to determine the exact gene transfer targets and the level of CFTR correction required to prevent or slow disease progression.
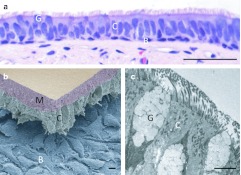
Figure 1.
Cell types comprising the human tracheal and bronchial airway epithelium. (a) Light microscopy of hematoxylin and eosin stained bronchus airway epithelium reveals the abundance of goblet cells (G), ciliated cells (C), basal cells (B). (Bar = 50 µm). (b) Freeze fractured scanning electron microscopy separates the ciliated cell layer from basal cells and the basement membrane. The mucus or gel layer (M) overlaying the ciliated cells remains intact (Bar = 10 µm). (c) Transmission electron microscopy of human bronchial airway epithelium highlights the abundance of cilia and the morphology of goblet cells (Bar = 10 µm).
The first clinical trial for CF took place in 1993. Since then, 25 gene therapy clinical trials have been conducted.11 Among these, 10 used adenovirus (Ad) vectors, 6 involved adeno-associated virus (AAV2) vectors, and 9 used nonviral vectors. The vectors were generally well-tolerated in the subjects. In some studies, the Cl− transport defect was partially and transiently corrected.12,13,14,15,16,17,18,19,20 Here, we will review important barriers to vector delivery, and recent developments in viral and nonviral vectors, cell-based therapies, as well as CF animal models.
Current Gene and Cell Therapy Strategies
Gene addition
The majority of gene transfer strategies pursue gene addition, in which the wild-type CFTR complementary DNA (cDNA) is delivered to cells with a viral or nonviral vector. Viral vector systems under investigation for CF pulmonary applications include lentiviral (LV) vectors, helper-dependent Ad (HD-Ad), and AAV. LV vectors are widely used integrating systems. These vectors integrate across transcriptional units21 and have less risk of insertional mutagenesis than early generation γ-retrovirus vectors.22 Importantly, LV vectors can transduce dividing and nondividing cells and support pseudotyping with glycoproteins from many enveloped viruses. Pseudotyping allows targeting to specific tissues and may enhance vector stability.23
Gene addition can also be achieved using non-integrating viral vectors such as Ad and AAV. Both vectors are encapsidated. Although the risk of insertional mutagenesis is less,24 achieving lifelong expression may require repeated administration. Ad vectors showed promise in early studies; however, the immunologic responses to capsid proteins and vector-encoded proteins reduced enthusiasm for use of Ad vectors in airway epithelium transduction. Interestingly, combining the epithelial cell-specific keratin 18 expression cassette with HD-Ad vectors, devoid of all viral-coding sequences, resulted in significantly longer transgene expression and less inflammation upon airway epithelium transduction.25 AAV vectors are nonpathogenic, less immunogenic, and also transduce both dividing and nondividing cells.26 Twelve different AAV capsid serotypes with more than 100 variants transduce respiratory epithelia to varying degrees.27,28,29
Nonviral integrating vectors such as bacteriophage ϕC3130 and DNA transposons (Sleeping Beauty31,32 and piggybac33) or non-integrating nonviral vectors such as nanoparticles34 and plasmids35 may also be used for gene addition. Most nonviral vectors exhibit a lower transfection efficiency in airway epithelia compared to viral vectors.36 Plasmid-based vectors are susceptible to endosomal and cytoplasmic degradation. On the other hand, plasmid-based vectors may be less immunogenic compared to viral vectors. The transgene expression and persistence from nonviral vectors can be improved by the selection of promoters. For example, Hyde et al.35 demonstrated that a hybrid promoter, human cytomegalovirus enhancer coupled to elongation factor 1α, conferred prolonged, high level of transgene expression in murine lungs. They also reported that transgene codon optimization increased protein translation. A recent study in an ovine large animal model identified the cationic lipid 67 (GL67A) as the most efficacious vehicle for delivery of a CFTR-expressing plasmid.37 In vivo delivery of nonviral vectors remains a challenge, but steady progress has been made.
Gene correction
Gene correction is an alternative approach by which repair of the defective gene sequences occurs through homologous recombination (HR). Zinc-finger nucleases (ZFNs) are increasingly used to enhance the frequency of HR at designated loci. ZFNs possess a DNA-binding domain, encoded by site-specific zinc-finger motifs, and a DNA-cleaving domain, derived from the nonspecific bacterial FokI endonuclease.38 A pair of ZFNs binds to opposite DNA strands of a specific target locus near a mutation, dimerize their cleavage domains, and introduce a double-stranded break into DNA. The double-stranded break is corrected via HR by co-delivery of a wild-type repair template provided transiently. CFTR-specific ZFNs constructed using oligomerized pool engineering (OPEN) bind and cleave near the ΔF508 mutation in CFTR.39 The frequency of cleavage by CFTR-specific ZFNs delivered by plasmids was ~1.2%.39 To further advance this approach, additional progress is needed to improve the delivery of the reagents and enhance the efficiencies of double-stranded breaks and HR. It is expected that delivery using viral vectors would increase the efficiency of delivery to epithelial cells.
ZFNs may also be used for gene addition. In this case, instead of targeting repair of the genomic locus, “safe harbor” loci such as AAVS1,40 CCR5,41 or Rosa26,42 may be chosen for gene insertion. The recent discovery of the ability of engineered transcription activator-like effector (TALE) nucleases to introduce site-specific double-stranded breaks provides an alternative reagent to ZFNs.43,44,45 The application of ZFNs as well as TALE nucleases for in vivo CF treatment is challenging. The use of these reagents in ex vivo cell-based approaches may provide more efficient gene correction.
Cell and tissue engineering
In addition to vector-based gene transfer strategies, cell-based approaches are under investigation. The stem cell properties of self-renewal and differentiation into specific cell types raise the possibility of their use for the treatment of many diseases, including CF.
Several sources of stem cells demonstrated their ability to develop epithelial characteristics. Bone marrow (BM)-derived stem cells and mesenchymal stem cells are capable of differentiating into respiratory epithelia.46,47,48 Wang et al.47 isolated mesenchymal stem cells from homozygous ΔF508 CF patients and conferred CFTR expression by transducing cells with a Moloney murine leukemia virus vector carrying the CFTR cDNA. Cyclic adenosine monophosphate (AMP) stimulation resulted in apical chloride secretion in CFTR-transduced mesenchymal stem cells from CF patients. Wong et al.49 recently discovered cell populations in mouse and human bone marrow that express Clara cell markers and differentiate into several epithelial lineages. Kajstura and colleagues50 identified human lung stem cells expressing c-kit, a known marker for hematopoietic stem cells and human cardiac stem cells, within normal human lung tissues. Interestingly, injections of these human lung stem cells into damaged murine lungs resulted in the formation of chimeric conducting airways and pulmonary vessels. Since identifying appropriate cell populations that can differentiate into and function within the lung has been a major obstacle, these findings could lead to advances in cell-based therapy for lung diseases including CF.
Complementation of CFTR using transplanted stem cells was also determined. Lori and colleagues51 transplanted BM-derived cells collected from wild-type mice into CFTR-null mice. A small percentage of cells differentiated into airway epithelia (0.025%), with some expressing CFTR protein (0.01%). Bruscia et al.52 transplanted wild-type CFTR carrying BM cells into irradiated CFTR-null mice. Very low levels of BM-derived epithelia and CFTR-expressing cells were detected. The same results were observed when transplantation of BM-derived cells was carried out in newborn mice. Cell-based therapies could be an important application for the gene correction strategies discussed earlier. One can envision collecting or deriving stem cells from a patient, correcting the genetic defect ex vivo, and reimplanting corrected cells, or implanting stem cells on a tissue-based or synthetic matrix.53,54
Currently, stem cell-based approaches to treat CF lung disease have not achieved the efficiencies of delivery and engraftment needed for therapy. In order to restore CFTR function, the number of stem cells that can differentiate into airway epithelia must be increased. In addition, new strategies to induce cell differentiation and homing to the epithelium need to be identified since stem cells only differentiate after lung injury. Bioluminescent and fluorescent imaging techniques may be used to assess short- and long-term efficacy of preclinical cell therapy studies in vivo; however, safety concerns, such as the immunogenic potential of exogenous stem cells in the lung, will also need to be addressed.
Stem cells were also used to create cell lines carrying mutations specific to disease. Pickering and colleagues55 created human embryonic stem cell lines carrying a homozygous mutation of ΔF508 CFTR. Somers et al.56 generated induced pluripotent stem cells from dermal fibroblasts of CF patients. These cells may be useful for drug screening, development of techniques to generate and transplant epithelia, as well as examining the toxicity and efficacy of gene transfer in vitro.
Perhaps the most difficult hurdle for cell-based therapy is the identification of efficient means to achieve sufficient engraftment into the airway epithelium. Macchiarini and colleagues57 demonstrated successful transplantation of ex vivo engineered donor trachea. In this single patient report, cells along with major histocompatibility class antigens were removed from the tissue scaffold, followed by colonizing with patient-derived epithelia and lung-derived chondrocytes. The repopulated graft was successfully transplanted into the left mainstem bronchus, providing clinical improvement.
Progress has also been made in the field of whole lung engineering. Recently, Petersen et al.58 and Ott et al.59 generated rat lung tissue ex vivo. A scaffold of decellularized rat lung was seeded with epithelia and vascular endothelial cells. The engineered lung tissues had a similar mechanical phenotype as native lung tissue in vitro. Interestingly, the engineered lungs could support gas exchange for a short time when implanted into rats. Although exciting, these studies are still in early preclinical stages. A major challenge for a corrected stem cell-based approach for CF treatment is devising a cell delivery or tissue engineering strategy to replace epithelial cells in multiple generations of the conducting airways. Improvements in techniques and further evaluation in appropriate animal models could lead to new treatment strategies.
Physical barriers
The lungs have evolved multiple barriers to prevent foreign particles and pathogens from accessing airway cells (shown schematically, Figure 2). The conducting airway surface is lined by a ciliated epithelium. Cilia are bathed in the periciliary fluid layer (sol). The mucus (gel) layer (Figure 1b), another important physical barrier, covers the periciliary fluid layer. Mucins, which are secreted by surface airway goblet cells and submucosal glands, are primary components of mucus.60 The mucus layer traps inhaled particles and removes them by mucociliary clearance.61 An apical surface glycocalyx, composed of carbohydrate, glycoproteins, and polysaccharides, is another barrier. It binds inhaled particles and prevents them from reaching cell surface receptors.62
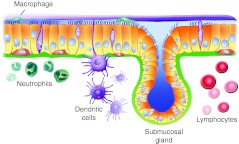
Figure 2.
Schematic representation of airway epithelia and potential barriers to viral and nonviral vectors. Ciliated and nonciliated epithelia (yellow) line the conducting airway surface with their basolateral surfaces interacting with the basal lamina (green). Basal cells (orange) are an important progenitor cell type. Submucosal glands are a major source of secreted liquid, host defense factors, and mucins. The mucus (gel) layer (purple) covers the periciliary fluid (sol) layer (blue) in which cilia are submerged. Macrophages circulate in the periciliary environment and engulf inhaled particles, including vectors. Neutrophils, dendritic cells, as well as lymphocytes represent additional barriers and sentinels for the adaptive immune system in airways.
To inhibit mucociliary clearance, Sinn et al.63 demonstrated that the formulation of Ad5, AAV5, or GP64-pseudotyped FIV vectors with viscoelastic gels (carboxymethylcellulose or methylcellulose) greatly enhanced their transduction efficiency. Presumably, such viscoelastic gels allow the virus to interact with cellular receptors for a longer period of time. Disruption of the mucus layer is another strategy for enhancing gene transfer. Ferrari and colleagues64 showed increased nonviral gene transfer in vitro and in vivo using the mucolytic agent N-acetylcysteine lysinate (nacystelyn) or the anticholinergic drug glycopyrrolate. Pretreatment with nacystelyn followed by administration of Ad vectors in conjunction with the polycation diethylaminoethyl (DEAE)-Dextran increased gene transfer to the airways of mice.65 In addition, the anti-inflammatory property of nacystelyn reduces airway inflammation.66 Suk et al.67 demonstrated that pretreatment with N-acetylcysteine alone or in combination with recombinant human DNase improved diffusion of the nonviral gene carrier, poly-L-lysine conjugated with a 10 kDa polyethylene glycol segment (CK30PEG10k) (PEGylated Poly-L-lysine DNA nanoparticles),34 across sputum. Intranasal pretreatment with N-acetylcysteine before CK30PEG10k/DNA nanoparticle delivery increased gene expression in murine lungs with mucus hypersecretion due to Pseudomonas aeruginosa lipopolysaccharide induction. In addition, McLachlan and colleagues68 demonstrated in sheep airways that pretreatment with the antimuscarinic agent glycopyrrolate improved transgene expression when the cationic polymer polyethyleneimine formulated with empty plasmid DNA was delivered by aerosol.
Access to receptors expressed on the basolateral surface of epithelium can be achieved by transiently disrupting the tight junctions using calcium chelating agents, such as ethyleneglycol-bis-(2-aminoethylether)-N,N,N′,N′-tetraacetic acid (EGTA),69 or the nonionic detergent, polidocanol.70 Sodium caprate increased the transduction efficiency of Ad vectors by disrupting claudin-1, a major component of the tight junctions.71 Stocker and colleagues72 demonstrated that pretreatment with lysophosphatidylcholine (LPC) followed by a single dose of HIV-1 based LV vector resulted in the prolonged expression of transgenes for the lifetime limit of 24-months in mice. Cmielewski et al.73 also reported improvement of LV vector-mediated gene transduction by pretreatment with LPC or LPC variants in vivo. They demonstrated that LPC disrupts junctional complexes in airway epithelia, allowing viral vectors to access basolateral receptors.73
Although the disruption of tight junctions may create concerns for clinical use, pseudotyping LV vectors with apical targeting envelopes such as the Zaire strain of the Ebola virus (EboZ),74,75 influenza hemagglutinin from fowl plague virus (FPV),76 or the glycoprotein from baculovirus (GP64)77 holds promise. These pseudotyped LV vectors were shown to efficiently transduce airway epithelium from the apical surface. Mitomo and colleagues78 generated simian immunodeficiency virus (SIV) pseudotyped with Sendai virus envelope proteins, hemgglutinin-neuraminidase, and fusion protein. Administration of F/N-pseudotyped SIV vector to nares of mice resulted in transgene expression for over 1 year. Furthermore, vector readministration was feasible. In addition, the F/N-pseudotyped SIV vector expressed functional CFTR chloride channels in vitro.
Other vectors with natural airway tropism are useful in CFTR gene transfer. Kwilas et al.79 demonstrated that respiratory syncytial virus carrying the CFTR gene could transduce and correct the anion transport defect in primary human airway epithelial cell cultures derived from CF patients. Zhang et al.80,81 reported that human recombinant parainfluenza virus type 3 efficiently transduced the apical surface of human airway epithelia and supported transient gene expression in respiratory epithelia of rhesus macaques in vivo. The limitations of transient expression and immune responses need to be addressed to move these vector systems forward.
Another method to improve transduction efficiency is by creating hybrid AAV vectors. Excoffon and colleagues82 generated AAV2.5T, a chimera of AAV2 and AAV5 with one point mutation, by DNA shuffling of cap genes and selection on the apical surfaces of human airway epithelia. AAV2.5T increased the apical airway epithelia transduction efficiency by 100-fold, in part through its better binding capacity. AAV2.5T carrying a CFTR cDNA restored Cl− transport function in CF epithelia to wild-type level. Li et al.83 also identified two AAV variants with improved transduction efficiency and the ability to partially correct the Cl− transport defect in human airway epithelia.
AAV9 poorly transduces the conducting airways.84 Bell et al.85 and Shen et al.86 identified terminal galactose as a cellular receptor for AAV9. Administration of an AAV9 vector coincident with neuraminidase treatment, which cleaves sialic acid linkages, increased the transduction efficiency in murine lungs. This improvement was due to the exposure of terminal galactose residues on the apical surface of conducting airway epithelia. Pretreatment of mice with neuraminidase increased the transduction efficiency about onefold in murine nasal airways. Approximately 140 times more lacZ-positive cells/field were observed in conducting airway of neuraminidase-treated mice compared to non-treated mice. Since AAV9 has been successfully readministered in the presence of neutralizing antibodies and supports stable and prolonged expression of transgene in vivo,84 it may be an interesting candidate for further development.
Immune barriers
Innate and adaptive immune responses are major obstacles for successful gene transfer. The lung has multilayered, sophisticated defense mechanisms which protect the host from pathogens. Important players in this response include macrophages, dendritic cells, neutrophils, and lymphocytes (Figure 2). Pathogen recognition receptors trigger acute and transient innate immune responses through detection of pathogen-associated molecular patterns. Toll-like receptors, the antiviral cytoplasmic helicases (RIG-I and MDA5), and nucleotide oligomerization domain-like receptors are among the pathogen recognition receptors expressed in the airway epithelium. The recognition of pathogen molecules, as well as some gene transfer vectors, results in the secretion of inflammatory cytokines and maturation of antigen presenting cells.
Ad vectors are rapidly taken up by alveolar macrophages, inducing secretion of proinflammatory cytokines and chemokines, and orchestrating a strong innate immune response.87 In animal studies, 70% of Ad vectors are eliminated within 24 hours by this process.88 Alveolar macrophages also inhibited retrovirus-mediated gene transfer to airway epithelia in vitro.89
The secretion of interferons (IFN) is important for host defense against viruses. Type I IFNs and type III IFNs induce the expression of many genes whose products help establish an antiviral state, inhibiting viral replication and cell proliferation.90,91 Type I IFNs also activate antigen presenting cells and natural killer cells.92 Zhu and colleagues93 demonstrated that Ad vectors trigger secretion of type I IFNs from both plasmacytoid dendritic cells (pDCs) and non-pDCs. Blocking type I IFN by neutralizing antibodies allowed increased transgene expression and decreased inflammation. The same group showed that AAV vectors also induce type I IFN production by pDCs through toll-like receptor 9 (TLR9) recognition of viral DNA.94 Brown et al.95 reported that the intravenous administration of an HIV vector induced IFNαβ responses in mouse liver and spleen. When LV vectors were administered to IFNαβR−/− mice, transduction improved and persistent transgene expression for over 5 weeks was observed in mouse hepatocytes. Type I IFNs are likely an obstacle for viral vector-mediated gene transfer, though the responses may be very cell-type specific.
Nonviral vectors are generally less immunogenic than viral vectors. However, administration of CFTR cDNA-GL67 complexes to the lower airways of CF patients elicited inflammatory responses in clinical trials.19,96 The presence of CG dinucleotides (CpG) in DNA plasmids triggers the inflammatory response via TLR9. Administration of CpG-free plasmid reduced inflammation and allowed sustained expression of a CFTR transgene in murine lungs.35 Interestingly, even a single CpG in a DNA plasmid may be sufficient to trigger an innate immune response.35 These results indicate that careful design and production of plasmid DNA is important for successful nonviral gene transfer.
Adaptive immune responses triggered by vector antigens or vector-encoded proteins can limit transgene persistence. Ad vectors induce strong CD8+ T-cell responses to both the transgene product and vector antigens.97 Although they are much weaker when compared to Ad vectors, AAV vectors can also elicit CD8+ T-cells. AAV vectors induce a CD8+ T-cell response to a transgene in mice preimmunized with Ad vectors expressing the same transgene.98 AAV vector capsids can also trigger memory CD8+ T-cell proliferation in humans.99 Limberis and colleagues100 demonstrated that delivery of HIV-based LV vectors to the lungs induced a cytotoxic T-cell response to the transgene. While decreased transgene expression was seen in wild-type mice after intratracheal delivery of vesicular stomatitis virus-G (VSV-G) pseudotyped LV, sustained transgene expression was observed throughout the experiment in recombination-activating gene-deficient mice lacking B and T cells.
Preexisting antibodies against Ad and AAV are found in many individuals since Ad and/or AAV infections are common.101,102 This is a potential obstacle for gene transfer, especially when repeated administration might be required. Studies of repeat administration will be important as the field advances and new candidate vectors for clinical trials are selected. To prevent inhibition of gene transfer by adaptive immune responses, transient immunosuppression can be applied. Cao et al.103 demonstrated that cyclophosphamide treatment during the primary administration of HD-Ad vectors significantly improved transduction efficiency by ~3.5-fold compared to non-treated when HD-Ad vectors were readministered to murine lungs. This increased gene transfer was due to inhibition of neutralizing antibody production against Ad and reduced infiltration of CD4+ T and CD8+ T-cells by cyclophosphamide. Importantly, transduction efficiency after the readministration of HD-Ad vectors in immunosuppressed mice was similar to the control mice receiving a single dose of HD-Ad vector with or without cyclophosphamide treatment. As to whether these procedures are applicable to CF patients, additional studies in larger animal models will be required. The risks of immunosuppression in persons with CF would need to be balanced by the therapeutic benefits. Targeting interventions early in the disease course and before the onset of chronic infection and inflammation may have a more favorable risk-benefit ratio.
Coating the vector capsid with polyethylene glycol (PEG) can mask neutralizing antibody epitopes and reduce CD8+ T cell responses. Croyle and colleagues104 demonstrated that PEGylation of Ad vectors reduced CD8+ T cell responses and the production of neutralizing antibodies against Ad capsids after intratracheal delivery in murine lungs. Prolonged expression of the transgene (from 4 to 42 days) was also observed. In addition, they demonstrated that alternating the formulations of PEG between doses is necessary to allow efficient gene transfer during repeated administration. Zhong and colleagues105 demonstrated that formulating an Ad vector with anionic liposomes improved the duration of gene expression in murine airways and reduced neutralizing antibody responses against Ad following a single intratracheal vector administration. Price et al.106 reported that an Ad vector formulated with liposomes, composed of the anti-inflammatory cationic lipid dexamethasone-spermine (DS) and the neutral lipid dioleoylphosphatidhylethanolamine (DOPE), allowed homologous Ad vector readministration in murine lungs. Formulation with DS/DOPE reduced neutralizing antibody production as well as infiltration of CD4+ and CD8+ T-cells to the site of vector delivery. Evasion of antibody neutralization can also be achieved through mutating the neutralizing epitopes on viral vector capsids by site-directed mutagenesis93,94 or by directed evolution.107
It is possible that a therapeutic transgene may elicit immune responses, as autoimmunity against the CFTR gene product has been reported. Limberis et al.108 demonstrated that human CFTR expression elicited CD8+ T cell responses when Ad vectors carrying human CFTR cDNA were delivered to CFTR knockout, heterozygote, and wild-type mice. Intratracheal delivery induced more effective CFTR-specific T-cell responses compared to intranasal delivery. A minor T-cell response to an epitope conserved between human and mouse CFTR was observed in CFTR knockout mice but not wild-type mice. These results suggest that CFTR mutations associated with loss of protein translation, specifically class I mutations, may more likely elicit CFTR-specific T-cell responses.109 Further studies are required to understand the implications of these animal and in vitro studies.
Implications From New Animal Models
A lack of animal models presenting phenotypes similar to those of humans with CF has impeded studies of disease pathogenesis and new therapies. Mice have served as models in the majority of in vivo studies, and several different CF mouse models are available.110 Most of the CF mouse models exhibit severe gastrointestinal complications at weaning, which leads to death in a large fraction of animals without special diets or complementation with a human CFTR gene expressed in a gut.111 While the lungs are severely affected in CF patients, CF mice have minimal spontaneous lung pathology. There are several possible reasons for these species differences including the presence of alternative Ca2+-mediated Cl− secretory pathways.111 Large animals, including sheep37,68 have proven very useful in vector scale up studies, safety analyses, evaluation of immune responses, efficiency studies, and assessment of vector delivery/distribution. However, there is currently no available ovine CFTR loss of function model. Advances in gene-targeting technologies, including zinc finger and TALE nucleases, may facilitate CFTR gene disruption in additional species.
Recently, new CF animal models have been developed. Rogers and colleagues112,113 generated CFTR-null and CFTR-ΔF508 heterozygote pigs and subsequently CFTR-ΔF508 homozygous animals.114 Advantages of the pig as a CF model include lung anatomy, physiology, histology, and biochemistry that are more similar to humans.115 In addition, pigs are more homologous to humans genetically, have a larger body size, and longer life spans. CF pigs manifest several phenotypes present in humans with CF. Loss of CFTR function in pigs results in exocrine pancreatic destruction, pancreatic insufficiency, focal biliary cirrhosis, and micro gallbladder.112,116 The penetrance of meconium ileus is 100% in CF pigs. This form of intestinal obstruction is observed in about 15% of newborn humans with CF. CF pig lungs exhibit no inflammation at birth, but interestingly their lung tissue was less frequently sterile compared to wild-type littermates.114,117 When challenged with Staphylococcus aureus intratracheally, CF pigs exhibited reduced bacterial eradication compared to wild-type. The animals spontaneously developed lung disease within the first month after birth characterized by bacterial infection, inflammation, airway injury, and remodeling.117 The lung disease manifestations were heterogeneous and severity varied from mild to severe.117 These findings suggest that defects in bacterial eradication lead to inflammation and the development of lung disease.
Another new CF animal model is the ferret. Sun et al.118 demonstrated that CFTR−/− ferrets develop meconium ileus with 75% penetrance, pancreatic disease, liver disease, and their lungs are often spontaneously colonized with bacteria including Streptococcus and Staphylococcus species within the first 4 weeks after birth. Progressive development of lung disease, as well as defects in bacterial clearance have also been observed in newborn CF ferrets challenged with bacteria (J.F. Engelhardt, The University of Iowa, unpublished observation).
Importantly, both models spontaneously develop lung disease and recapitulate several features of CF disease progression observed in humans. The availability of these new animal models should provide new insights into disease pathogenesis and provide opportunities for testing treatment strategies, including gene- and cell-based therapies.
Importance of preclinical model choice
Preclinical models must be carefully chosen based on the goal of the studies. Each model has advantages and disadvantages that must be considered for each vector system.
For example, tripartite motif protein 5α (TRIM5α) restricts retroviral infection in a species-specific manner by blocking the early postentry phase of retroviral infection. Thus, while TRIM5α of Old World monkeys blocks HIV-1 infection, it blocks SIV only moderately.119 Human TRIM5α blocks N-tropic murine leukemia virus (N-MLV)120,121 and equine infectious anemia virus,122 but not HIV-1 infection.119 Restriction by TRIM5α is initiated by the recognition of incoming retroviral capsids by the PRYSPRY domain, which is followed by rapid disassembly of the viral capsids.123 TRIM5α bound to the capsids of restriction-sensitive virus is degraded via the proteasome,124 causing reduction of reverse-transcribed products.125 Due to the species-specific restriction by TRIM5α, outcomes of studies with lentiviral vectors may vary depending on the vectors and animal models selected. Therefore, recognition of the species-specific restriction patterns of TRIM5α should help guide the selection of animal models for preclinical studies with LV vectors. It is important to be mindful of the evolving literature of host- and virus-specific restriction factors to consider their implications for the design of informative preclinical studies.
Liu and colleagues29 conducted a comparative study of the transduction profiles of AAV vector serotypes 1, 2, and 5 in pig, ferret, mouse, and human polarized airway epithelia. They demonstrated that AAV serotype preferences for transduction in pig, ferret, and human airway epithelia were similar, but differed in mouse airway epithelia. Furthermore, the receptors required by each AAV vector serotype for transduction were different among species. While AAV1 and AAV5 required N-linked sialic acid receptors for transduction of human and mouse airway epithelia, these vectors did not require the same receptor for transduction of pig and ferret airway epithelia. Their results indicate that while pigs and ferrets are suitable models to study AAV-mediated gene transfer, they may not be appropriate models to examine the mechanism of AAV transduction to airways. In order to select relevant systems to evaluate new therapies, additional comparative studies of vectors of interest in available animal models are required.
Concluding Remarks and Perspective
The efficacy and safety of vectors applicable for CF pulmonary gene therapy have been improved through intensive studies. Better strategies to overcome host immune responses and physical barriers are still needed to increase the gene transfer efficiency to airway epithelia. Further development of new or modified vectors may also enhance airway epithelial cell transduction. Cell-based therapies for CF are still at a very early stage. However, the ability to generate rat lung tissue ex vivo is a novel prospect. As the appropriate cell populations capable of efficiently differentiating into airway epithelium are identified and methods for engraftment are further optimized, cell-based therapies may be an attractive treatment for CF. New animal models are providing significant new insights into CF pathophysiology. They will also help in developing new therapies, as well as in evaluating the effectiveness and adverse effects of such treatments. Significant progress has been made in the 22 years since the CFTR gene was first discovered. Continued advancements will bring us closer to a gene therapy strategy to treat CF lung disease.
Acknowledgments
This work was supported by NIH grants: P01 HL-51670, P01 HL- 091842, and the Roy J. Carver Charitable Trust. We also acknowledge the support of the In Vitro Models and Cell Culture Core, Gene Transfer Vector Core, and Cell Morphology Core, partially supported by the Center for Gene Therapy for Cystic Fibrosis (NIH P30 DK-54759) and the Cystic Fibrosis Foundation. We thank Thomas Moninger and David Meyerholz for images presented in Figure 1. We thank Erin Burnight, Dibyakanti Mandal, Ashley Peterson, Shyam Ramachandran, for critically reviewing the manuscript. We thank Mary Moye-Rowley for assistance with illustrations. The authors declared no conflict of interest.
REFERENCES
- Welsh MJ, Tsui LC, Boat TF., and, Beaudet AL.1995Cystic fibrosis Scriver CR, Beaudet AL, Sly WS., and, Valle D.eds). The Metabolic and Molecular Basis of Inherited Disease7th edn., vol. 3. McGraw-Hill: New York; pp. 3799–3876. [Google Scholar]
- Davis PB, Drumm M., and, Konstan MW. Cystic fibrosis. Am J Respir Crit Care Med. 1996;154:1229–1256. doi: 10.1164/ajrccm.154.5.8912731. [DOI] [PubMed] [Google Scholar]
- Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z.et al. (1989Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA Science 2451066–1073. [DOI] [PubMed] [Google Scholar]
- Rommens JM, Iannuzzi MC, Kerem B, Drumm ML, Melmer G, Dean M.et al. (1989Identification of the cystic fibrosis gene: chromosome walking and jumping Science 2451059–1065. [DOI] [PubMed] [Google Scholar]
- Kerem B, Rommens JM, Buchanan JA, Markiewicz D, Cox TK, Chakravarti A.et al. (1989Identification of the cystic fibrosis gene: genetic analysis Science 2451073–1080. [DOI] [PubMed] [Google Scholar]
- Griesenbach U, Geddes DM., and, Alton EW. Gene therapy for cystic fibrosis: an example for lung gene therapy. Gene Ther. 2004;11 suppl. 1:S43–S50. doi: 10.1038/sj.gt.3302368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreda SM, Mall M, Mengos A, Rochelle L, Yankaskas J, Riordan JR.et al. (2005Characterization of wild-type and deltaF508 cystic fibrosis transmembrane regulator in human respiratory epithelia Mol Biol Cell 162154–2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt JF, Zepeda M, Cohn JA, Yankaskas JR., and, Wilson JM. Expression of the cystic fibrosis gene in adult human lung. J Clin Invest. 1994;93:737–749. doi: 10.1172/JCI117028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson LG, Olsen JC, Sarkadi B, Moore KL, Swanstrom R., and, Boucher RC. Efficiency of gene transfer for restoration of normal airway epithelial function in cystic fibrosis. Nat Genet. 1992;2:21–25. doi: 10.1038/ng0992-21. [DOI] [PubMed] [Google Scholar]
- Zhang L, Button B, Gabriel SE, Burkett S, Yan Y, Skiadopoulos MH.et al. (2009CFTR delivery to 25% of surface epithelial cells restores normal rates of mucus transport to human cystic fibrosis airway epithelium PLoS Biol 7e1000155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griesenbach U., and, Alton EW. Gene transfer to the lung: lessons learned from more than 2 decades of CF gene therapy. Adv Drug Deliv Rev. 2009;61:128–139. doi: 10.1016/j.addr.2008.09.010. [DOI] [PubMed] [Google Scholar]
- Zabner J, Couture LA, Gregory RJ, Graham SM, Smith AE., and, Welsh MJ. Adenovirus-mediated gene transfer transiently corrects the chloride transport defect in nasal epithelia of patients with cystic fibrosis. Cell. 1993;75:207–216. doi: 10.1016/0092-8674(93)80063-k. [DOI] [PubMed] [Google Scholar]
- Hay JG, McElvaney NG, Herena J., and, Crystal RG. Modification of nasal epithelial potential differences of individuals with cystic fibrosis consequent to local administration of a normal CFTR cDNA adenovirus gene transfer vector. Hum Gene Ther. 1995;6:1487–1496. doi: 10.1089/hum.1995.6.11-1487. [DOI] [PubMed] [Google Scholar]
- Zabner J, Ramsey BW, Meeker DP, Aitken ML, Balfour RP, Gibson RL.et al. (1996Repeat administration of an adenovirus vector encoding cystic fibrosis transmembrane conductance regulator to the nasal epithelium of patients with cystic fibrosis J Clin Invest 971504–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplen NJ, Alton EW, Middleton PG, Dorin JR, Stevenson BJ, Gao X.et al. (1995Liposome-mediated CFTR gene transfer to the nasal epithelium of patients with cystic fibrosis Nat Med 139–46. [DOI] [PubMed] [Google Scholar]
- Gill DR, Southern KW, Mofford KA, Seddon T, Huang L, Sorgi F.et al. (1997A placebo-controlled study of liposome-mediated gene transfer to the nasal epithelium of patients with cystic fibrosis Gene Ther 4199–209. [DOI] [PubMed] [Google Scholar]
- Porteous DJ, Dorin JR, McLachlan G, Davidson-Smith H, Davidson H, Stevenson BJ.et al. (1997Evidence for safety and efficacy of DOTAP cationic liposome mediated CFTR gene transfer to the nasal epithelium of patients with cystic fibrosis Gene Ther 4210–218. [DOI] [PubMed] [Google Scholar]
- Zabner J, Cheng SH, Meeker D, Launspach J, Balfour R, Perricone MA.et al. (1997Comparison of DNA-lipid complexes and DNA alone for gene transfer to cystic fibrosis airway epithelia in vivo J Clin Invest 1001529–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alton EW, Stern M, Farley R, Jaffe A, Chadwick SL, Phillips J.et al. (1999Cationic lipid-mediated CFTR gene transfer to the lungs and nose of patients with cystic fibrosis: a double-blind placebo-controlled trial Lancet 353947–954. [DOI] [PubMed] [Google Scholar]
- Konstan MW, Davis PB, Wagener JS, Hilliard KA, Stern RC, Milgram LJ.et al. (2004Compacted DNA nanoparticles administered to the nasal mucosa of cystic fibrosis subjects are safe and demonstrate partial to complete cystic fibrosis transmembrane regulator reconstitution Hum Gene Ther 151255–1269. [DOI] [PubMed] [Google Scholar]
- Wu X, Li Y, Crise B., and, Burgess SM. Transcription start regions in the human genome are favored targets for MLV integration. Science. 2003;300:1749–1751. doi: 10.1126/science.1083413. [DOI] [PubMed] [Google Scholar]
- Biffi A, Bartolomae CC, Cesana D, Cartier N, Aubourg P, Ranzani M.et al. (2011Lentiviral vector common integration sites in preclinical models and a clinical trial reflect a benign integration bias and not oncogenic selection Blood 1175332–5339. [DOI] [PubMed] [Google Scholar]
- Cronin J, Zhang XY., and, Reiser J. Altering the tropism of lentiviral vectors through pseudotyping. Curr Gene Ther. 2005;5:387–398. doi: 10.2174/1566523054546224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacein-Bey-Abina S, Von Kalle C, Schmidt M, McCormack MP, Wulffraat N, Leboulch P.et al. (2003LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1 Science 302415–419. [DOI] [PubMed] [Google Scholar]
- Toietta G, Koehler DR, Finegold MJ, Lee B, Hu J., and, Beaudet AL. Reduced inflammation and improved airway expression using helper-dependent adenoviral vectors with a K18 promoter. Mol Ther. 2003;7 5 Pt 1:649–658. doi: 10.1016/s1525-0016(03)00059-5. [DOI] [PubMed] [Google Scholar]
- Michelfelder S., and, Trepel M. Adeno-associated viral vectors and their redirection to cell-type specific receptors. Adv Genet. 2009;67:29–60. doi: 10.1016/S0065-2660(09)67002-4. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Voutetakis A, Afione S, Zheng C, Mandikian D., and, Chiorini JA. Adeno-associated virus type 12 (AAV12): a novel AAV serotype with sialic acid- and heparan sulfate proteoglycan-independent transduction activity. J Virol. 2008;82:1399–1406. doi: 10.1128/JVI.02012-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbert CL, Allen JM., and, Miller AD. Adeno-associated virus type 6 (AAV6) vectors mediate efficient transduction of airway epithelial cells in mouse lungs compared to that of AAV2 vectors. J Virol. 2001;75:6615–6624. doi: 10.1128/JVI.75.14.6615-6624.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Luo M, Guo C, Yan Z, Wang Y., and, Engelhardt JF. Comparative biology of rAAV transduction in ferret, pig and human airway epithelia. Gene Ther. 2007;14:1543–1548. doi: 10.1038/sj.gt.3303014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aneja MK, Imker R., and, Rudolph C. Phage phiC31 integrase-mediated genomic integration and long-term gene expression in the lung after nonviral gene delivery. J Gene Med. 2007;9:967–975. doi: 10.1002/jgm.1090. [DOI] [PubMed] [Google Scholar]
- Ivics Z, Hackett PB, Plasterk RH., and, Izsvák Z. Molecular reconstruction of Sleeping Beauty, a Tc1-like transposon from fish, and its transposition in human cells. Cell. 1997;91:501–510. doi: 10.1016/s0092-8674(00)80436-5. [DOI] [PubMed] [Google Scholar]
- Belur LR, Podetz-Pedersen K, Frandsen J., and, McIvor RS. Lung-directed gene therapy in mice using the nonviral Sleeping Beauty transposon system. Nat Protoc. 2007;2:3146–3152. doi: 10.1038/nprot.2007.460. [DOI] [PubMed] [Google Scholar]
- Ding S, Wu X, Li G, Han M, Zhuang Y., and, Xu T. Efficient transposition of the piggyBac (PB) transposon in mammalian cells and mice. Cell. 2005;122:473–483. doi: 10.1016/j.cell.2005.07.013. [DOI] [PubMed] [Google Scholar]
- Ziady AG, Gedeon CR, Miller T, Quan W, Payne JM, Hyatt SL.et al. (2003Transfection of airway epithelium by stable PEGylated poly-L-lysine DNA nanoparticles in vivo Mol Ther 8936–947. [DOI] [PubMed] [Google Scholar]
- Hyde SC, Pringle IA, Abdullah S, Lawton AE, Davies LA, Varathalingam A.et al. (2008CpG-free plasmids confer reduced inflammation and sustained pulmonary gene expression Nat Biotechnol 26549–551. [DOI] [PubMed] [Google Scholar]
- Griesenbach U., and, Alton EW. Current status and future directions of gene and cell therapy for cystic fibrosis. BioDrugs. 2011;25:77–88. doi: 10.2165/11586960-000000000-00000. [DOI] [PubMed] [Google Scholar]
- McLachlan G, Davidson H, Holder E, Davies LA, Pringle IA, Sumner-Jones SG.et al. (2011Pre-clinical evaluation of three non-viral gene transfer agents for cystic fibrosis after aerosol delivery to the ovine lung Gene Ther 18996–1005. [DOI] [PubMed] [Google Scholar]
- Kim YG, Cha J., and, Chandrasegaran S. Hybrid restriction enzymes: zinc finger fusions to Fok I cleavage domain. Proc Natl Acad Sci USA. 1996;93:1156–1160. doi: 10.1073/pnas.93.3.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeder ML, Thibodeau-Beganny S, Osiak A, Wright DA, Anthony RM, Eichtinger M.et al. (2008Rapid “open-source” engineering of customized zinc-finger nucleases for highly efficient gene modification Mol Cell 31294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockemeyer D, Soldner F, Beard C, Gao Q, Mitalipova M, DeKelver RC.et al. (2009Efficient targeting of expressed and silent genes in human ESCs and iPSCs using zinc-finger nucleases Nat Biotechnol 27851–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez EE, Wang J, Miller JC, Jouvenot Y, Kim KA, Liu O.et al. (2008Establishment of HIV-1 resistance in CD4+ T cells by genome editing using zinc-finger nucleases Nat Biotechnol 26808–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer M, de Angelis MH, Wurst W., and, Kühn R. Gene targeting by homologous recombination in mouse zygotes mediated by zinc-finger nucleases. Proc Natl Acad Sci USA. 2010;107:15022–15026. doi: 10.1073/pnas.1009424107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian M, Cermak T, Doyle EL, Schmidt C, Zhang F, Hummel A.et al. (2010Targeting DNA double-strand breaks with TAL effector nucleases Genetics 186757–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Huang S, Jiang WZ, Wright D, Spalding MH, Weeks DP.et al. (2011TAL nucleases (TALNs): hybrid proteins composed of TAL effectors and FokI DNA-cleavage domain Nucleic Acids Res 39359–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JC, Tan S, Qiao G, Barlow KA, Wang J, Xia DF.et al. (2011A TALE nuclease architecture for efficient genome editing Nat Biotechnol 29143–148. [DOI] [PubMed] [Google Scholar]
- Kotton DN, Ma BY, Cardoso WV, Sanderson EA, Summer RS, Williams MC.et al. (2001Bone marrow-derived cells as progenitors of lung alveolar epithelium Development 1285181–5188. [DOI] [PubMed] [Google Scholar]
- Wang G, Bunnell BA, Painter RG, Quiniones BC, Tom S, Lanson NA., Jret al. (2005Adult stem cells from bone marrow stroma differentiate into airway epithelial cells: potential therapy for cystic fibrosis Proc Natl Acad Sci USA 102186–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macpherson H, Keir P, Webb S, Samuel K, Boyle S, Bickmore W.et al. (2005Bone marrow-derived SP cells can contribute to the respiratory tract of mice in vivo J Cell Sci 118Pt 112441–2450. [DOI] [PubMed] [Google Scholar]
- Wong AP, Keating A, Lu WY, Duchesneau P, Wang X, Sacher A.et al. (2009Identification of a bone marrow-derived epithelial-like population capable of repopulating injured mouse airway epithelium J Clin Invest 119336–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajstura J, Rota M, Hall SR, Hosoda T, D'Amario D, Sanada F.et al. (2011Evidence for human lung stem cells N Engl J Med 3641795–1806. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Loi R, Beckett T, Goncz KK, Suratt BT., and, Weiss DJ. Limited restoration of cystic fibrosis lung epithelium in vivo with adult bone marrow-derived cells. Am J Respir Crit Care Med. 2006;173:171–179. doi: 10.1164/rccm.200502-309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruscia EM, Price JE, Cheng EC, Weiner S, Caputo C, Ferreira EC.et al. (2006Assessment of cystic fibrosis transmembrane conductance regulator (CFTR) activity in CFTR-null mice after bone marrow transplantation Proc Natl Acad Sci USA 1032965–2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungebluth P, Alici E, Baiguera S, Le Blanc K, Blomberg P, Bozóky B.et al. (2011Tracheobronchial transplantation with a stem-cell-seeded bioartificial nanocomposite: a proof-of-concept study Lancet 3781997–2004. [DOI] [PubMed] [Google Scholar]
- Ingenito EP, Sen E, Tsai LW, Murthy S., and, Hoffman A. Design and testing of biological scaffolds for delivering reparative cells to target sites in the lung. J Tissue Eng Regen Med. 2010;4:259–272. doi: 10.1002/term.237. [DOI] [PubMed] [Google Scholar]
- Pickering SJ, Minger SL, Patel M, Taylor H, Black C, Burns CJ.et al. (2005Generation of a human embryonic stem cell line encoding the cystic fibrosis mutation ΔF508, using preimplantation genetic diagnosis Reprod Biomed Online 10390–397. [DOI] [PubMed] [Google Scholar]
- Somers A, Jean JC, Sommer CA, Omari A, Ford CC, Mills JA.et al. (2010Generation of transgene-free lung disease-specific human induced pluripotent stem cells using a single excisable lentiviral stem cell cassette Stem Cells 281728–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macchiarini P, Jungebluth P, Go T, Asnaghi MA, Rees LE, Cogan TA.et al. (2008Clinical transplantation of a tissue-engineered airway Lancet 3722023–2030. [DOI] [PubMed] [Google Scholar]
- Petersen TH, Calle EA, Zhao L, Lee EJ, Gui L, Raredon MB.et al. (2010Tissue-engineered lungs for in vivo implantation Science 329538–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott HC, Clippinger B, Conrad C, Schuetz C, Pomerantseva I, Ikonomou L.et al. (2010Regeneration and orthotopic transplantation of a bioartificial lung Nat Med 16927–933. [DOI] [PubMed] [Google Scholar]
- Thornton DJ., and, Sheehan JK. From mucins to mucus: toward a more coherent understanding of this essential barrier. Proc Am Thorac Soc. 2004;1:54–61. doi: 10.1513/pats.2306016. [DOI] [PubMed] [Google Scholar]
- Knowles MR., and, Boucher RC. Mucus clearance as a primary innate defense mechanism for mammalian airways. J Clin Invest. 2002;109:571–577. doi: 10.1172/JCI15217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stonebraker JR, Wagner D, Lefensty RW, Burns K, Gendler SJ, Bergelson JM.et al. (2004Glycocalyx restricts adenoviral vector access to apical receptors expressed on respiratory epithelium in vitro and in vivo: role for tethered mucins as barriers to lumenal infection J Virol 7813755–13768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinn PL, Shah AJ, Donovan MD., and, McCray PB., Jr Viscoelastic gel formulations enhance airway epithelial gene transfer with viral vectors. Am J Respir Cell Mol Biol. 2005;32:404–410. doi: 10.1165/rcmb.2004-0410OC. [DOI] [PubMed] [Google Scholar]
- Ferrari S, Kitson C, Farley R, Steel R, Marriott C, Parkins DA.et al. (2001Mucus altering agents as adjuncts for nonviral gene transfer to airway epithelium Gene Ther 81380–1386. [DOI] [PubMed] [Google Scholar]
- Kushwah R, Oliver JR, Cao H., and, Hu J. Nacystelyn enhances adenoviral vector-mediated gene delivery to mouse airways. Gene Ther. 2007;14:1243–1248. doi: 10.1038/sj.gt.3302968. [DOI] [PubMed] [Google Scholar]
- Gregory LG, Harbottle RP, Lawrence L, Knapton HJ, Themis M., and, Coutelle C. Enhancement of adenovirus-mediated gene transfer to the airways by DEAE dextran and sodium caprate in vivo. Mol Ther. 2003;7:19–26. doi: 10.1016/s1525-0016(02)00021-7. [DOI] [PubMed] [Google Scholar]
- Suk JS, Boylan NJ, Trehan K, Tang BC, Schneider CS, Lin JM.et al. (2011N-acetylcysteine enhances cystic fibrosis sputum penetration and airway gene transfer by highly compacted DNA nanoparticles Mol Ther 191981–1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan G, Baker A, Tennant P, Gordon C, Vrettou C, Renwick L.et al. (2007Optimizing aerosol gene delivery and expression in the ovine lung Mol Ther 15348–354. [DOI] [PubMed] [Google Scholar]
- Wang G, Zabner J, Deering C, Launspach J, Shao J, Bodner M.et al. (2000Increasing epithelial junction permeability enhances gene transfer to airway epithelia In vivo Am J Respir Cell Mol Biol 22129–138. [DOI] [PubMed] [Google Scholar]
- Parsons DW, Grubb BR, Johnson LG., and, Boucher RC. Enhanced in vivo airway gene transfer via transient modification of host barrier properties with a surface-active agent. Hum Gene Ther. 1998;9:2661–2672. doi: 10.1089/hum.1998.9.18-2661. [DOI] [PubMed] [Google Scholar]
- Coyne CB, Kelly MM, Boucher RC., and, Johnson LG. Enhanced epithelial gene transfer by modulation of tight junctions with sodium caprate. Am J Respir Cell Mol Biol. 2000;23:602–609. doi: 10.1165/ajrcmb.23.5.4164. [DOI] [PubMed] [Google Scholar]
- Stocker AG, Kremer KL, Koldej R, Miller DS, Anson DS., and, Parsons DW. Single-dose lentiviral gene transfer for lifetime airway gene expression. J Gene Med. 2009;11:861–867. doi: 10.1002/jgm.1368. [DOI] [PubMed] [Google Scholar]
- Cmielewski P, Anson DS., and, Parsons DW. Lysophosphatidylcholine as an adjuvant for lentiviral vector mediated gene transfer to airway epithelium: effect of acyl chain length. Respir Res. 2010;11:84. doi: 10.1186/1465-9921-11-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobinger GP, Weiner DJ, Yu QC., and, Wilson JM. Filovirus-pseudotyped lentiviral vector can efficiently and stably transduce airway epithelia in vivo. Nat Biotechnol. 2001;19:225–230. doi: 10.1038/85664. [DOI] [PubMed] [Google Scholar]
- Medina MF, Kobinger GP, Rux J, Gasmi M, Looney DJ, Bates P.et al. (2003Lentiviral vectors pseudotyped with minimal filovirus envelopes increased gene transfer in murine lung Mol Ther 8777–789. [DOI] [PubMed] [Google Scholar]
- McKay T, Patel M, Pickles RJ, Johnson LG., and, Olsen JC. Influenza M2 envelope protein augments avian influenza hemagglutinin pseudotyping of lentiviral vectors. Gene Ther. 2006;13:715–724. doi: 10.1038/sj.gt.3302715. [DOI] [PubMed] [Google Scholar]
- Sinn PL, Burnight ER, Hickey MA, Blissard GW., and, McCray PB., Jr Persistent gene expression in mouse nasal epithelia following feline immunodeficiency virus-based vector gene transfer. J Virol. 2005;79:12818–12827. doi: 10.1128/JVI.79.20.12818-12827.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitomo K, Griesenbach U, Inoue M, Somerton L, Meng C, Akiba E.et al. (2010Toward gene therapy for cystic fibrosis using a lentivirus pseudotyped with Sendai virus envelopes Mol Ther 181173–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwilas AR, Yednak MA, Zhang L, Liesman R, Collins PL, Pickles RJ.et al. (2010Respiratory syncytial virus engineered to express the cystic fibrosis transmembrane conductance regulator corrects the bioelectric phenotype of human cystic fibrosis airway epithelium in vitro J Virol 847770–7781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Bukreyev A, Thompson CI, Watson B, Peeples ME, Collins PL.et al. (2005Infection of ciliated cells by human parainfluenza virus type 3 in an in vitro model of human airway epithelium J Virol 791113–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Limberis MP, Thompson C, Antunes MB, Luongo C, Wilson JM.et al. (2010a-Fetoprotein gene delivery to the nasal epithelium of nonhuman primates by human parainfluenza viral vectors Hum Gene Ther 211657–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Excoffon KJ, Koerber JT, Dickey DD, Murtha M, Keshavjee S, Kaspar BK.et al. (2009Directed evolution of adeno-associated virus to an infectious respiratory virus Proc Natl Acad Sci USA 1063865–3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Zhang L, Johnson JS, Zhijian W, Grieger JC, Ping-Jie X.et al. (2009Generation of novel AAV variants by directed evolution for improved CFTR delivery to human ciliated airway epithelium Mol Ther 172067–2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limberis MP., and, Wilson JM. Adeno-associated virus serotype 9 vectors transduce murine alveolar and nasal epithelia and can be readministered. Proc Natl Acad Sci USA. 2006;103:12993–12998. doi: 10.1073/pnas.0601433103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell CL, Vandenberghe LH, Bell P, Limberis MP, Gao GP, Van Vliet K.et al. (2011The AAV9 receptor and its modification to improve in vivo lung gene transfer in mice J Clin Invest 1212427–2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen S, Bryant KD, Brown SM, Randell SH., and, Asokan A. Terminal N-linked galactose is the primary receptor for adeno-associated virus 9. J Biol Chem. 2011;286:13532–13540. doi: 10.1074/jbc.M110.210922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zsengellér Z, Otake K, Hossain SA, Berclaz PY., and, Trapnell BC. Internalization of adenovirus by alveolar macrophages initiates early proinflammatory signaling during acute respiratory tract infection. J Virol. 2000;74:9655–9667. doi: 10.1128/jvi.74.20.9655-9667.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worgall S, Leopold PL, Wolff G, Ferris B, Van Roijen N., and, Crystal RG. Role of alveolar macrophages in rapid elimination of adenovirus vectors administered to the epithelial surface of the respiratory tract. Hum Gene Ther. 1997;8:1675–1684. doi: 10.1089/hum.1997.8.14-1675. [DOI] [PubMed] [Google Scholar]
- McCray PB, Jr, Wang G, Kline JN, Zabner J, Chada S, Jolly DJ.et al. (1997Alveolar macrophages inhibit retrovirus-mediated gene transfer to airway epithelia Hum Gene Ther 81087–1093. [DOI] [PubMed] [Google Scholar]
- van Boxel-Dezaire AH, Rani MR., and, Stark GR. Complex modulation of cell type-specific signaling in response to type I interferons. Immunity. 2006;25:361–372. doi: 10.1016/j.immuni.2006.08.014. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Hamming OJ, Ank N, Paludan SR, Nielsen AL., and, Hartmann R. Type III interferon (IFN) induces a type I IFN-like response in a restricted subset of cells through signaling pathways involving both the Jak-STAT pathway and the mitogen-activated protein kinases. J Virol. 2007;81:7749–7758. doi: 10.1128/JVI.02438-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theofilopoulos AN, Baccala R, Beutler B., and, Kono DH. Type I interferons (alpha/beta) in immunity and autoimmunity. Annu Rev Immunol. 2005;23:307–336. doi: 10.1146/annurev.immunol.23.021704.115843. [DOI] [PubMed] [Google Scholar]
- Zhu J, Huang X., and, Yang Y. Innate immune response to adenoviral vectors is mediated by both Toll-like receptor-dependent and -independent pathways. J Virol. 2007;81:3170–3180. doi: 10.1128/JVI.02192-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Huang X., and, Yang Y. The TLR9-MyD88 pathway is critical for adaptive immune responses to adeno-associated virus gene therapy vectors in mice. J Clin Invest. 2009;119:2388–2398. doi: 10.1172/JCI37607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown BD, Sitia G, Annoni A, Hauben E, Sergi LS, Zingale A.et al. (2007In vivo administration of lentiviral vectors triggers a type I interferon response that restricts hepatocyte gene transfer and promotes vector clearance Blood 1092797–2805. [DOI] [PubMed] [Google Scholar]
- Ruiz FE, Clancy JP, Perricone MA, Bebok Z, Hong JS, Cheng SH.et al. (2001A clinical inflammatory syndrome attributable to aerosolized lipid-DNA administration in cystic fibrosis Hum Gene Ther 12751–761. [DOI] [PubMed] [Google Scholar]
- Yang Y, Li Q, Ertl HC., and, Wilson JM. Cellular and humoral immune responses to viral antigens create barriers to lung-directed gene therapy with recombinant adenoviruses. J Virol. 1995;69:2004–2015. doi: 10.1128/jvi.69.4.2004-2015.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siders WM, Shields J, Kaplan J, Lukason M, Woodworth L, Wadsworth S.et al. (2009Cytotoxic T lymphocyte responses to transgene product, not adeno-associated viral capsid protein, limit transgene expression in mice Hum Gene Ther 2011–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mingozzi F, Maus MV, Hui DJ, Sabatino DE, Murphy SL, Rasko JE.et al. (2007CD8(+) T-cell responses to adeno-associated virus capsid in humans Nat Med 13419–422. [DOI] [PubMed] [Google Scholar]
- Limberis MP, Bell CL, Heath J., and, Wilson JM. Activation of transgene-specific T cells following lentivirus-mediated gene delivery to mouse lung. Mol Ther. 2010;18:143–150. doi: 10.1038/mt.2009.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirmule N, Propert K, Magosin S, Qian Y, Qian R., and, Wilson J. Immune responses to adenovirus and adeno-associated virus in humans. Gene Ther. 1999;6:1574–1583. doi: 10.1038/sj.gt.3300994. [DOI] [PubMed] [Google Scholar]
- Halbert CL, Miller AD, McNamara S, Emerson J, Gibson RL, Ramsey B.et al. (2006Prevalence of neutralizing antibodies against adeno-associated virus (AAV) types 2, 5, and 6 in cystic fibrosis and normal populations: Implications for gene therapy using AAV vectors Hum Gene Ther 17440–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H, Yang T, Li XF, Wu J, Duan C, Coates AL.et al. (2011Readministration of helper-dependent adenoviral vectors to mouse airway mediated via transient immunosuppression Gene Ther 18173–181. [DOI] [PubMed] [Google Scholar]
- Croyle MA, Chirmule N, Zhang Y., and, Wilson JM. “Stealth” adenoviruses blunt cell-mediated and humoral immune responses against the virus and allow for significant gene expression upon readministration in the lung. J Virol. 2001;75:4792–4801. doi: 10.1128/JVI.75.10.4792-4801.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Z, Han J, Wan Y, Zhang Z., and, Sun X. Anionic liposomes enhance and prolong adenovirus-mediated gene expression in airway epithelia in vitro and in vivo. Mol Pharm. 2011;8:673–682. doi: 10.1021/mp100404q. [DOI] [PubMed] [Google Scholar]
- Price AR, Limberis MP, Wilson JM., and, Diamond SL. Pulmonary delivery of adenovirus vector formulated with dexamethasone-spermine facilitates homologous vector re-administration. Gene Ther. 2007;14:1594–1604. doi: 10.1038/sj.gt.3303031. [DOI] [PubMed] [Google Scholar]
- Maheshri N, Koerber JT, Kaspar BK., and, Schaffer DV. Directed evolution of adeno-associated virus yields enhanced gene delivery vectors. Nat Biotechnol. 2006;24:198–204. doi: 10.1038/nbt1182. [DOI] [PubMed] [Google Scholar]
- Limberis MP, Figueredo J, Calcedo R., and, Wilson JM. Activation of CFTR-specific T Cells in cystic fibrosis mice following gene transfer. Mol Ther. 2007;15:1694–1700. doi: 10.1038/sj.mt.6300210. [DOI] [PubMed] [Google Scholar]
- Rowntree RK., and, Harris A. The phenotypic consequences of CFTR mutations. Ann Hum Genet. 2003;67 Pt 5:471–485. doi: 10.1046/j.1469-1809.2003.00028.x. [DOI] [PubMed] [Google Scholar]
- Fisher JT, Zhang Y., and, Engelhardt JF. Comparative biology of cystic fibrosis animal models. Methods Mol Biol. 2011;742:311–334. doi: 10.1007/978-1-61779-120-8_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubb BR., and, Boucher RC. Pathophysiology of gene-targeted mouse models for cystic fibrosis. Physiol Rev. 1999;79 suppl. 1:S193–S214. doi: 10.1152/physrev.1999.79.1.S193. [DOI] [PubMed] [Google Scholar]
- Rogers CS, Stoltz DA, Meyerholz DK, Ostedgaard LS, Rokhlina T, Taft PJ.et al. (2008Disruption of the CFTR gene produces a model of cystic fibrosis in newborn pigs Science 3211837–1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers CS, Hao Y, Rokhlina T, Samuel M, Stoltz DA, Li Y.et al. (2008Production of CFTR-null and CFTR-DeltaF508 heterozygous pigs by adeno-associated virus-mediated gene targeting and somatic cell nuclear transfer J Clin Invest 1181571–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostedgaard LS, Meyerholz DK, Chen JH, Pezzulo AA, Karp PH, Rokhlina T.et al. (2011The ?F508 mutation causes CFTR misprocessing and cystic fibrosis-like disease in pigs Sci Transl Med 374ra24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers CS, Abraham WM, Brogden KA, Engelhardt JF, Fisher JT, McCray PB., Jret al. (2008The porcine lung as a potential model for cystic fibrosis Am J Physiol Lung Cell Mol Physiol 295L240–L263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerholz DK, Stoltz DA, Pezzulo AA., and, Welsh MJ. Pathology of gastrointestinal organs in a porcine model of cystic fibrosis. Am J Pathol. 2010;176:1377–1389. doi: 10.2353/ajpath.2010.090849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoltz DA, Meyerholz DK, Pezzulo AA, Ramachandran S, Rogan MP, Davis GJ.et al. (2010Cystic fibrosis pigs develop lung disease and exhibit defective bacterial eradication at birth Sci Transl Med 229ra31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Sui H, Fisher JT, Yan Z, Liu X, Cho HJ.et al. (2010Disease phenotype of a ferret CFTR-knockout model of cystic fibrosis J Clin Invest 1203149–3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stremlau M, Owens CM, Perron MJ, Kiessling M, Autissier P., and, Sodroski J. The cytoplasmic body component TRIM5alpha restricts HIV-1 infection in Old World monkeys. Nature. 2004;427:848–853. doi: 10.1038/nature02343. [DOI] [PubMed] [Google Scholar]
- Hatziioannou T, Perez-Caballero D, Yang A, Cowan S., and, Bieniasz PD. Retrovirus resistance factors Ref1 and Lv1 are species-specific variants of TRIM5alpha. Proc Natl Acad Sci USA. 2004;101:10774–10779. doi: 10.1073/pnas.0402361101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perron MJ, Stremlau M, Song B, Ulm W, Mulligan RC., and, Sodroski J. TRIM5alpha mediates the postentry block to N-tropic murine leukemia viruses in human cells. Proc Natl Acad Sci USA. 2004;101:11827–11832. doi: 10.1073/pnas.0403364101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keckesova Z, Ylinen LM., and, Towers GJ. The human and African green monkey TRIM5alpha genes encode Ref1 and Lv1 retroviral restriction factor activities. Proc Natl Acad Sci USA. 2004;101:10780–10785. doi: 10.1073/pnas.0402474101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perron MJ, Stremlau M, Lee M, Javanbakht H, Song B., and, Sodroski J. The human TRIM5alpha restriction factor mediates accelerated uncoating of the N-tropic murine leukemia virus capsid. J Virol. 2007;81:2138–2148. doi: 10.1128/JVI.02318-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rold CJ., and, Aiken C. Proteasomal degradation of TRIM5alpha during retrovirus restriction. PLoS Pathog. 2008;4:e1000074. doi: 10.1371/journal.ppat.1000074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JL, Campbell EM, Wu X, Vandegraaff N, Engelman A., and, Hope TJ. Proteasome inhibition reveals that a functional preintegration complex intermediate can be generated during restriction by diverse TRIM5 proteins. J Virol. 2006;80:9754–9760. doi: 10.1128/JVI.01052-06. [DOI] [PMC free article] [PubMed] [Google Scholar]