Abstract
Circadian rhythms describe biological phenomena that oscillate with an ≈24-hour cycle. These rhythms include blood pressure, body temperature, hormone levels, the number of immune cells in blood, and the sleep-wake cycle. In this paper, we will focus on common genes between species that are responsible for determining the circadian behavior, especially some transcription factors (i.e., switch genes) that serve to regulate many circadian rhythm genes. The intent of this summary is to introduce the common molecular mechanism of biological clocks between flies and humans and then to describe the research from three laboratories that was presented in the session.
The alternating of day and night of the earth’s cycle is so reliable that it is not surprising that animals, plants, and bacteria adjust their behavior and physiology (for a review, see ref. 1). Circadian rhythms are a ubiquitous adaptation of all organisms to the most predictable of environmental challenges. A biological rhythm that persists under constant conditions and has a period of ≈1 day is called “circadian” (circa, “around”; dian, “day”).
Until very recently, the molecules underlying the oscillation have remained unknown. Perturbations of such oscillations by inhibitors of RNA or protein synthesis suggest that such molecules are involved (2).
An approach that has been successful in unraveling mechanisms is the use of genetic alterations. The first and second clock mutants discovered in the fruit fly, Drosophila melanogaster, are period and timeless genes (3–5).
In fruit flies, the abundance of mRNA and protein products of the period and timeless genes cycle for ≈24 hours in specific sites of the fly brain (6). Maki Kaneko et al. talked about these putative pacemaker cells in the fruit fly brain by using these molecular oscillation as a marker (7). In the adult head, protein studies showed that per is rhythmically expressed in specific sites, the lateral neurons located between the central brain and the optic lobes. Lateral neurons are considered as the putative pacemaker cells for the adult fly’s locomoter activity rhythm.
Kaneko et al. (7) demonstrated that the products of per and tim are detectable in a limited number of neurons in the larval brain. The expression patterns in several such cells is cyclical. Among these neurons, five laterally located cells express. PERIOD (PER) from the early larval stage, suggesting that they may be responsible for the larval time keeping of eclosion and locomoter activity. Another interesting finding is a cluster of neurons with the cyclical expression of per and tim in antiphase to the lateral neurons. The results imply the presence of multiple oscillators involved in rhythms of different physiological or behavioral processes in a single organism. Kaneko et al. (7) also described the anatomical characterization of the wiring patterns of the pacemaker neurons by using per promoter-dependent reporter gene expression. Such a molecular anatomical approach should bring a new insight into the functional mapping of this brain system. Furthermore, the comparison between mammalian and fly clock cells [i.e., suprachiasmatic nucleus (SCN) and lateral neurons] should clarify evolutionary relationship between these systems.
The circadian control of transcription provides an entry point to analyze the cis-acting regulatory elements and trans-acting factors through which the clock may regulate many clock-controlled gene expressions (6). These putative cis-acting regulatory elements, named the “time-box” (8), are assumed to be located in the promoter and enhancer region of clock-controlled genes. Furthermore, the clock-controlled responsive element (6) or time-box may regulate the endogenous circadian physiological phenomena under constant conditions. Most recently, a possible candidate for the time-box has been identified in the promoter region of the Drosophila period gene (9). Although per has been proposed to mediate mRNA cycling through transcriptional repression, direct interaction between per and DNA is very unlikely because of the lack of a DNA-binding domain in PER. Hardin’s group extensively analyzed the promoter region of per gene in studies using per-lacZ fusion gene transgenic flies (9). They identified a circadian transcriptional enhancer within a 69-bp DNA fragment containing an E-box upstream of per gene, which is responsible for the night-time activation of per gene expression. The E-box is a known binding site for the basic helix-loop-helix class of transcription factors.
Recently, the strongest candidate yet for a trans-acting factor in the oscillator is Clock, cloned by using a forward-genetic strategy (10). Takahashi’s group (10) isolated and analyzed locomoter activity of circadian mutant mouse strains. The Clock mutant exhibited long period becoming arrhythmic after several days in constant darkness. Takahashi and colleagues (10) successfully cloned the responsible gene and identified the mutation in the protein coding region of the Clock gene. Interestingly enough, the Clock protein contains a protein–protein binding domain (PAS), which is located in the Drosophila per gene and a basic helix-loop-helix motif for DNA-binding. Moreover, Takahashi and colleagues (10) were able to completely rescue the long period and arrhythmic phenotype of clock mutant mice by transfer of the normal clock gene.
Ravi Allada et al. described the common molecular components focusing on Clock, which is responsible for the circadian rhythm generation in both flies and humans (11). Allada and his colleagues (11) screened chemically mutagenized flies looking for mutants that alter or abolish circadian rhythmicity of locomoter activity and found a new arrhythmic mutant, initially called Jrk. Jrk flies express low levels of period and timeless proteins because of reduced levels of transcription. The gene was identified and exhibits striking sequence conservation with the mammalian circadian rhythm gene, Clock; hence, Allada et al. (11) renamed this fly gene dClock. Like mouse clock, Drosophila clock contains basic helix-loop-helix and PAS domains as well as a transcriptional activation domain.
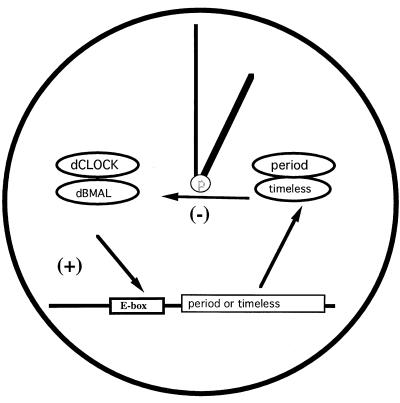
Recent works from both mammals and flies suggest that the protein partners of CLOCK are also evolutionarily conserved (named BMAL) (12, 13). CLOCK-BMAL dimmers were shown to bind to the promoter region of period and timeless genes and to transactivate both genes in flies. Furthermore, PERIOD-TIMELESS (PER-TIM) expression represses CLOCK-BMAL-mediated reporter induction. Thus, a negative feedback model has been proposed (Fig. 1).
Figure 1.
The negative feedback model of molecular biological clock. Recent studies from both mammals and flies suggest that the protein partners of clock are also evolutionary conserved (named BMAL). CLOCK-BMAL dimers were shown to transactivate the expression of period and timeless genes. Furthermore, PER-TIM plays a role as the repressor of CLOCK-BMAL-mediated reporter induction.
In mammals, the SCN in the hypothalamus is considered to be a major pacemaker for circadian rhythm phenomena, as demonstrated by many anatomical and physiological studies (14). Recently, three homologues of Drosophila period gene were reported in mouse and human (15). Despite the existence of three mammalian period homologues that show mRNA circadian oscillation in the suprachiasmatic nucleus in the mouse brain, no functional implication of circadian locomoter behavior has been reported.
To clarify whether the mammalian per homologue might be involved in the circadian rhythm of locomoter behavior of mammals, Ishida’s group has cloned a rat per homologue and has made arrhythmic SCN-lesioned rats to monitor circadian rhythms in peripheral tissues (16).
To test whether rhythmic expression of rat PERIOD 2 (RPER2) mRNA is observed in tissues other than the SCN, Northern blot analysis was carried out on tissues from the eye, brain, heart, lung spleen, liver, and kidneys. Interestingly enough, all of the tissues tested showed rhythmic expression of RPER2 mRNA, although the night/day ratio was different in each tissue. RPER2 behaves as a mammalian homologue of the Drosophila period gene (16) because its circadian expression was high at night throughout a wide variety of tissues as period is in Drosophila.
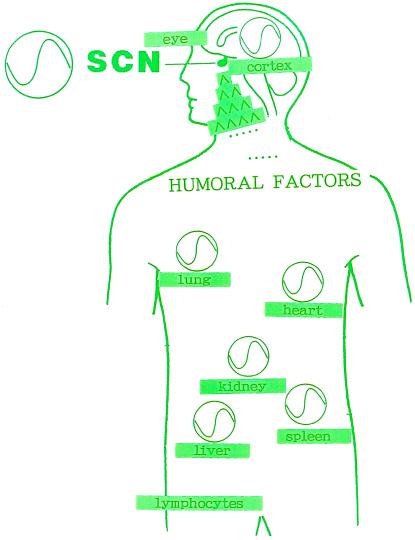
Because the SCN is considered as a circadian clock pacemaker in mammals, Ishida’s group (16) tested whether multiple tissue circadian expression of RPER2 mRNA is affected by an SCN lesion. Surprisingly enough, the rhythmic nature of the multiple tissue expression of RPER2 was completely abolished by the SCN lesion. The multiple tissue expression of RPER2 is therefore under the control of the SCN. This is the first report to indicate that multitissue circadian rhythm is governed by a mammalian brain clock, the SCN of hypothalamus. The data also suggest that a mammalian per homologue (RPER2) might be involved in the circadian rhythm of locomoter behavior in mammals, because loss of circadian expression of RPER2 mRNA in the whole body occurred when the circadian locomoter activity of rats was lost. To clarify such a problem, we have to make transgenic animals having a loss-of-function or a gain-of-function mutation in the RPER2 gene. The fact that the rhythmic expression of RPER2 mRNA in several tissues completely depends on the SCN suggests that some signals are needed to maintain coordinately the rhythm of the whole body (Fig. 2). An SCN transplantation study also suggests the importance of humoral factors from the SCN (17). Such humoral factors from the SCN might be important to generate the circadian rhythmic expression of RPER2 gene in peripheral tissues. Thus, it appears, as in the case of developmental biology, that key molecules of this biological clock are well conserved between flies and mammals. The common molecular clock mechanism from bacteria (18) to human might be envisioned in the near future.
Figure 2.
The master clock (SCN) governs the peripheral tissue rhythm in mammals. The fact that the rhythmic expression of RPER2 mRNA in several tissues completely depends on the SCN suggests that some signals (Humoral Factors) are needed to maintain coordinately the rhythm of the whole body.
Acknowledgments
We thank Drs. Masao Ito (Riken, Wako, Japan), Tasuka Honjo (Kyoto Univ., Kyoto), and Michio Ooishi (Kazusa DNA Research, Kazusa, Japan) for their encouragement of this field.
ABBREVIATIONS
- per, period
tim, timeless
- SCN
suprachiasmatic nucleus
- PAS
period arnt sim
- RPER2
Rat PERIOD 2
- TIM
TIMELESS
- BMAL
brain and muscle arnt-like
References
- 1.Bünning E. The Physiological Clock. New York: Springer; 1967. [Google Scholar]
- 2.Feldman J F. Proc Nat Acad Sci USA. 1967;57:1080. doi: 10.1073/pnas.57.4.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Konopka R J, Benzer S. Proc Nat Acad Sci USA. 1971;68:2112–2116. doi: 10.1073/pnas.68.9.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sehgel A, Price J L, Man B, Young M. Science. 1994;263:1603–1606. doi: 10.1126/science.8128246. [DOI] [PubMed] [Google Scholar]
- 5.Matsumoto A, Tomioka K, Chiba Y, Tammura T. Mol Cell Biol. 1999;19:4343–4354. doi: 10.1128/mcb.19.6.4343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takahashi J S. Annu Rev Neurosci. 1995;18:531–553. doi: 10.1146/annurev.ne.18.030195.002531. [DOI] [PubMed] [Google Scholar]
- 7.Kaneko M, Helfrich-Förster C, Hall J C. J Neurosci. 1997;17:6745–6760. doi: 10.1523/JNEUROSCI.17-17-06745.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kako K, Ishida N. Neurosci Res. 1998;31:257–264. doi: 10.1016/s0168-0102(98)00054-6. [DOI] [PubMed] [Google Scholar]
- 9.Hao H, Allen D L, Hardin P E. Mol Cell Biol. 1997;17:3687–3693. doi: 10.1128/mcb.17.7.3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.King D P, Zhao Y, Sangoram A M, Wilsbacher L D, Tanaka M, Antoch M P, Steeves T D L, Vitaterna M H, Kornhauser J M, Lowrey P L, et al. Cell. 1997;89:641–653. doi: 10.1016/s0092-8674(00)80245-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Allada R, White N E, Venus So W, Hall J C, Rosbash M. Cell. 1998;93:791–804. doi: 10.1016/s0092-8674(00)81440-3. [DOI] [PubMed] [Google Scholar]
- 12.Rutila J E, Suri V, Le M, Venus So W, Rosbash M, Hall J C. Cell. 1998;93:805–814. doi: 10.1016/s0092-8674(00)81441-5. [DOI] [PubMed] [Google Scholar]
- 13.Darlington T K, Wager-Smith K, Ceriani M F, Staknis D, Gekakis N, Steeves T D L, Weitz C J, Takahashi J S, Kay S A. Science. 1998;280:1599–1603. doi: 10.1126/science.280.5369.1599. [DOI] [PubMed] [Google Scholar]
- 14.Meijer J H, Rietveld W J. Physiol Rev. 1989;69:671–707. doi: 10.1152/physrev.1989.69.3.671. [DOI] [PubMed] [Google Scholar]
- 15.Dunlup J C. Cell. 1999;96:271–290. doi: 10.1016/s0092-8674(00)80566-8. [DOI] [PubMed] [Google Scholar]
- 16.Sakamoto K, Nagase T, Fukui H, Horikawa K, Okada T, Tanaka H, Sato K, Miyake Y, Ohara O, Kako K, Ishida N. J Biol Chem. 1998;273:27039–27042. doi: 10.1074/jbc.273.42.27039. [DOI] [PubMed] [Google Scholar]
- 17.Silver R, LeSauter J, Tresco P A, Lehman M N. Nature (London) 1996;382:810–813. doi: 10.1038/382810a0. [DOI] [PubMed] [Google Scholar]
- 18.Kondo T, Tsirenomas N F, Golden S S, Johnson C H, Kutsuna S, Ishiura M. Science. 1996;266:1233–1236. doi: 10.1126/science.7973706. [DOI] [PubMed] [Google Scholar]