Abstract
The concise and enantioselective total syntheses of (+)-naseseazines A and B are described. Our regioselective and directed dimerization of diketopiperazines provides their critical C3–Csp2 linkages, an assembly with plausible biogenetic relevance. We revise the absolute stereochemistry of (+)-naseseazines A and B.
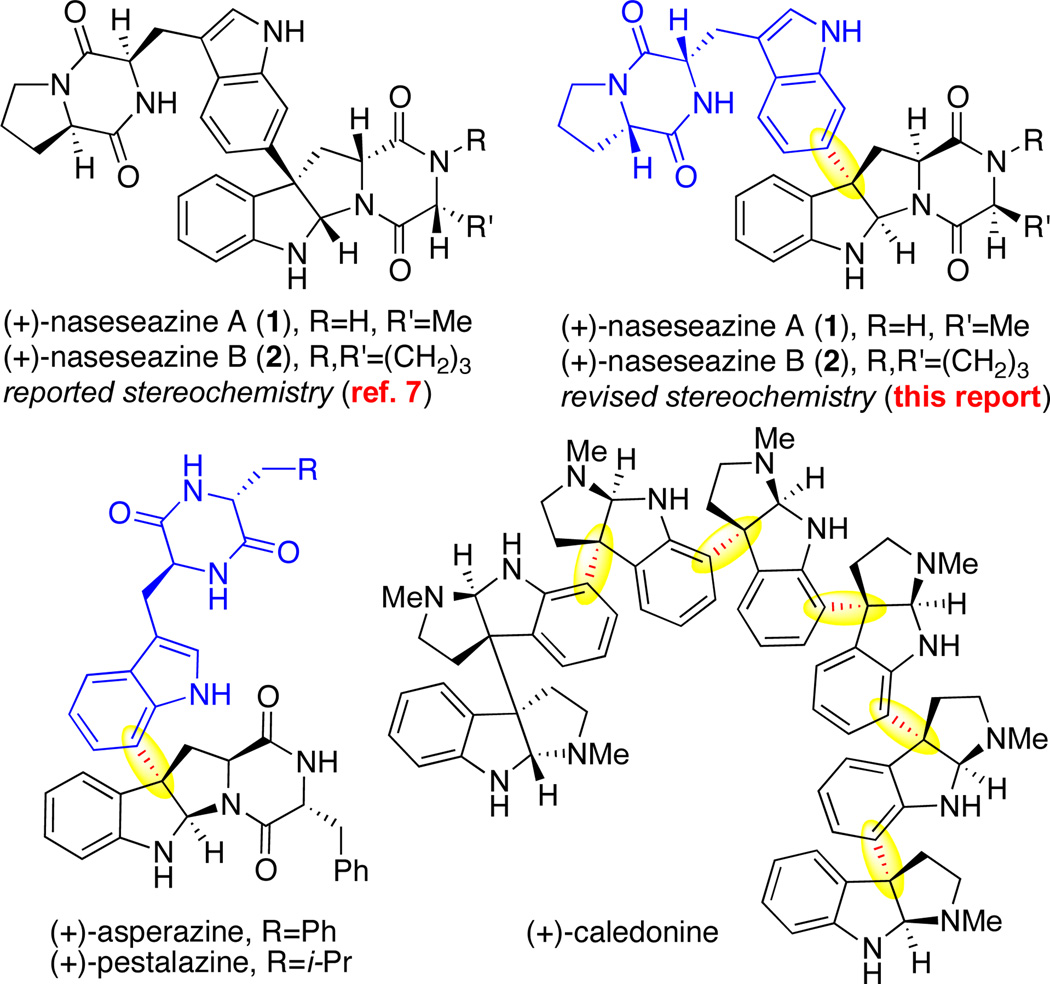
Dimeric diketopiperazine alkaloids, which comprise an important class of natural products, possess an expansive repertoire of biological activities intimately mirroring their structural diversity.1 An important source of diversity of great strategic relevance to their synthesis is the nature of the dimeric linkage. In recent years, much progress has been made toward the rapid construction of the C3sp3–C3'sp32,3,4 as well as the C3sp3–N1'5 bond connectivity. In this report, we aim to broaden the spectrum of dimerization modes that are predisposed for rapid disconnection. We describe herein a Friedel–Crafts based method for the regioselective and directed C3-functionalization of advanced diketopiperazine intermediates employing a range of π-nucleophiles. The concision and broad relevance of this approach to the synthesis of C3-arylated hexahydropyrroloindole alkaloids6 (Figure 1) are highlighted through the first total syntheses of Fijan actinomycete Streptomyces sp. derived alkaloids (+)-naseseazines A (1) and B (2),7 an endeavor culminating in their stereochemical revision.
Figure 1.
Representative C3-arylated hexahydropyrroloindole alkaloids. Revised structures of (+)-naseseazines A and B (1–2).
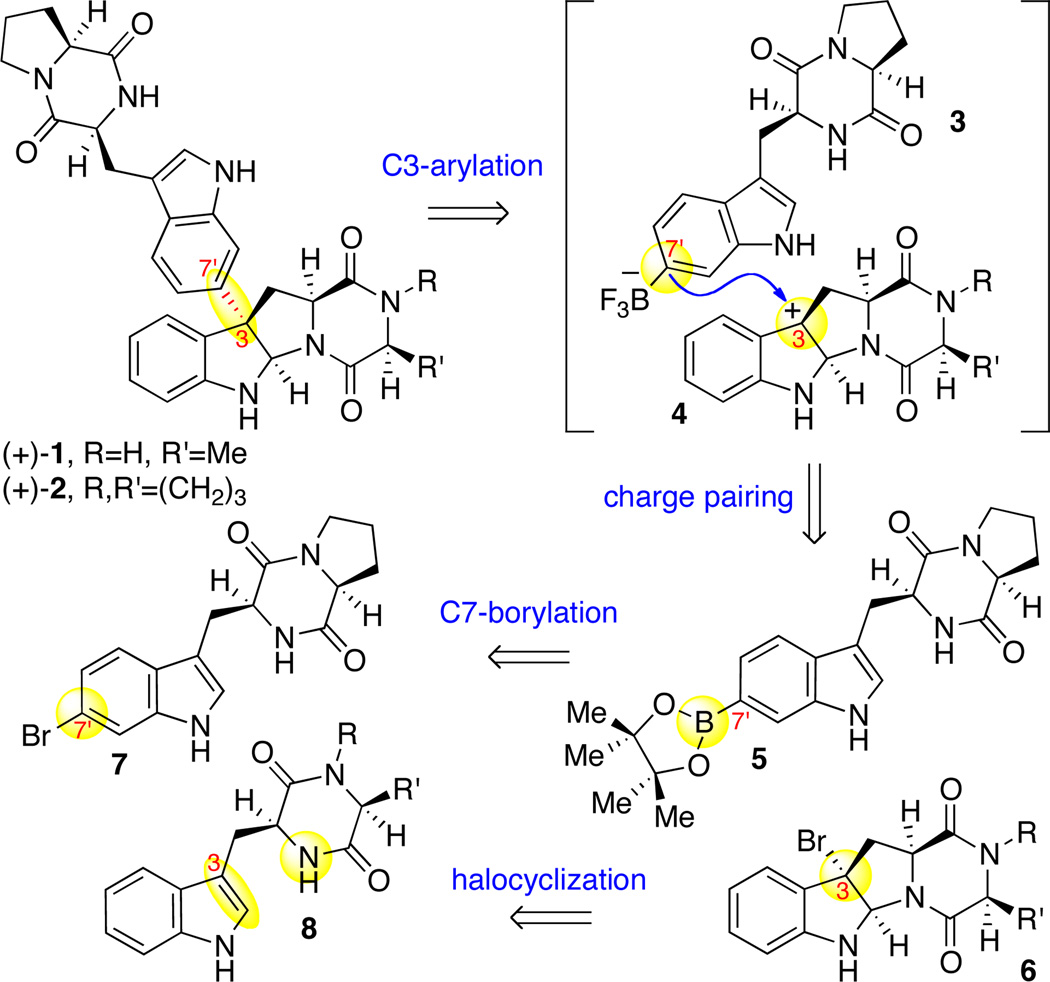
In deference to our desire for a maximally convergent synthesis of (+)-naseseazines A (1) and B (2), and consistent with a plausible hypothesis for their biogenesis,8,9 our retrosynthetic analysis commenced with the disconnection of the C3–C7'10 bonds of (+)-1 and (+)-2, affording two diketopiperazine fragments of comparable complexity (Scheme 1). Tetracyclic bromide 6 could be derived from the bromocyclization of diketopiperazine 8,3b whereas pinacolboronate 5 could be prepared from the corresponding bromodiketopiperazine 7. We envisioned that ionization of tetracyclic bromide 6 would provide the necessary C3-electrophile,11,12 while conversion of the pinacolboronate function of diketopiperazine 5 to the corresponding trifluoroborate13 would offer the necessary C7'-nucleophilic partner for a directed and regioselective sp3−sp2 bond formation (Scheme 1).
Scheme 1.
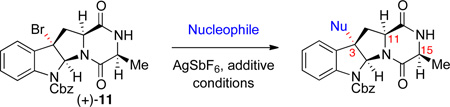
Retrosynthetic analysis of (+)-naseseazines A and B (1–2).
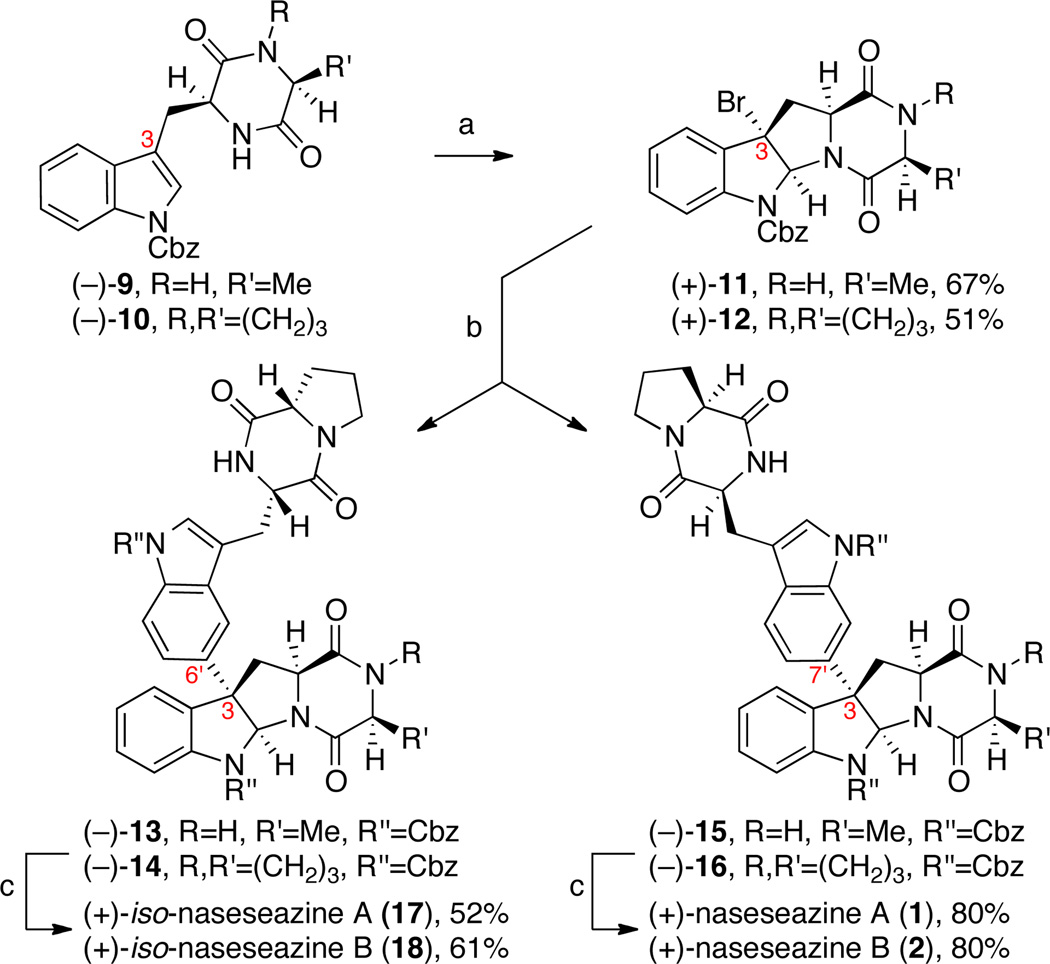
In our first generation approach, alanine and proline diketopiperazines (−)-9 and (−)-10, the requisite tetracyclic bromides en route to the corresponding naseseazine alkaloids, were synthesized in 89% and 71% yields, respectively, from readily available Nα-Boc-Nin-Cbz-l-tryptophan methyl ester.14 Exposure of diketopiperazines (−)-9 and (−)-10 to pyridinium tribromide in 2,2,2-trifluoroethanol afforded the respective tetracyclic bromides (+)-11 and (+)-12 in 67% and 51% yields as single diastereomers (Scheme 2). In possession of all the necessary components, we sought to validate the feasibility of our planned Friedel–Crafts-based strategy for C3-arylation at this juncture. Treating a solution of diketopiperazine (−)-10 (1.5 equiv) and tetracyclic bromide (+)-11 (1 equiv) with silver hexafluoroantimonate in nitroethane under optimized conditions (vide infra), afforded a mixture of regioisomeric dimers (−)-13 and (−)-15 in 53% combined yield ((−)-13:(−)-15 = 1:1.4). Similarly, the same proline derived diketopiperazine (−)-10 could be coupled to tetracyclic bromide (+)-12 to give regioisomeric dimers (−)-14 and (−)-16 in 47% combined yield ((−)-14:(−)-16 = 1:1.4). Hydrogenolytic removal of the carboxybenzyl groups on compounds 13–16, provided the first access to (+)-iso-naseseazines A (17) and B (18) as well as our target alkaloids (+)-1 and (+)-2, respectively (Scheme 2). Notably, selectivity was observed in favor of the regioisomer with the desired C3–C7' bond connectivity during the coupling reaction.8 While we were pleased with the utility of this new mode of heterodimerization, the low level of regioselectivity in this unguided union prompted further refinement of our new strategy toward a more selective and directed assembly of complex diketopiperazine fragments.
Scheme 2.
First generation synthesis of (+)-naseseazines A (1) and B (2).a
a Conditions: (a) PyHBr3, 2,2,2-trifluoroethanol, 23 °C. (b) (−)-10, AgSbF6, EtNO2, 23 °C; for (+)-11, 13:15=1:1.4, 53%; for (+)-12, 14:16=1:1.4, 47%. (c) H2, Pd/C, AcOH, 23 °C.
We anticipated that the regioselective arylation of tetracyclic bromide (+)-11 could be achieved by installation of a directing group at an appropriate position of the π-nucleophile.15,16 Importantly, we wished to increase the ratio of (−)-15 and (−)-16 to (−)-13 and (−)-14, respectively. In the early stages of our optimization studies, we selected thiophene, with its innate preference for reaction at the 2-position (Table 1, entry 1, 19:20 = 6.2:1), as our model system. While 3-cuprate, 3-trimethylstannyl, and 3-zinc chloride thiophene derivatives were ineffective as directed nucleophiles in the C3-arylation of bromide (+)-11, the use of the corresponding potassium trifluoroborate derivative proved promising.15,17 We expected that using 18-crown-6 as an additive would confer multiple benefits: increased solubility of the potassium trifluoroborate salt, enhanced nucleophilicity of the arene via ion pairing with the electrophile, and facilitated trifluoroborate elimination by further dissociation of the potassium-aryltrifluoroborate ion pair.18 Indeed, when silver hexafluoroantimonate was introduced to a solution of tetracyclic bromide (+)-11, potassium 3-thiophenetrifluoroborate, and 18-crown-6 in dichloromethane at 23 °C, we obtained the desired C3-arylated product as a mixture of regioisomers favoring the desired C3-adduct (19:20 = 1:1.3). Sub-ambient temperatures were then examined to enhance the regioselectivity for C3; however, selectivity did not improve noticeably at 0 °C in dichloromethane, and conversion to the product was not observed at lower temperatures. Thus, we turned to higher dielectric-constant media to facilitate the ionization event.
Table 1.
Lewis acid-promoted C3-nucleophile substitution.
 | ||||
|---|---|---|---|---|
| Entry | Nucleophile | Solvent, Temp (°C) | Yield (%)a | |
| 1 | CH2Cl2, 23 | 77 (6.3:1)b | ||
| 2 | 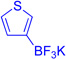 |
EtNO2, −45c | 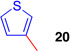 |
50 (17:1)b |
| 3 | CH2Cl2, 23 | 60 | ||
| 4 | CH2Cl2, 23 | 52 | ||
| 5 | CH2Cl2, 23 | 91 | ||
| 6 | 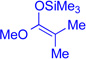 |
CH2Cl2, 23 | 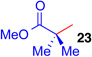 |
89 |
| 7 | CH2Cl2, 23 | 50 | ||
| 8 | 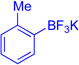 |
EtNO2, −45c | 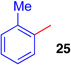 |
26 (3.9:1)d |
| 9 | 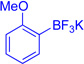 |
EtNO2, −45c | 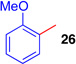 |
54 (2.4:1)d |
| 10 | EtNO2, 23c | 59 | ||
Isolated yield; average of two experiments; AgSbF6 (2.0 equiv) and nucleophile (2.0 equiv).
Regioisomeric ratios determined by 1H NMR. Major regioisomer shown.
18-crown-6 (2.0 equiv).
The minor regioisomer is the para-adduct.
Nitrile-based solvents such as acetonitrile and benzonitrile enabled ionization; however, tight solvent-carbocation interactions significantly impeded the nucleophilic reaction, and hydroxylation19 and Ritter reaction adducts20 formed in significant amounts as principal byproducts. Similarly, use of N,N'-dimethylformamide resulted exclusively in hydroxyl and formate addition products.21 Ultimately, we discovered that the use of nitroalkane solvents was most effective for the desired transformation. The level of regioselection improved for the C3-thiophenyl adduct at −25 °C in nitromethane (19:20 = 1:2.8). Under optimal conditions, treatment of (+)-11 with 3-thiophenetrifluoroborate at −45 °C in nitroethane provided the desired product with an excellent level of regioselection (Table 1, entry 2, 50%, 19:20 = 1:17).9
Encouraged by the efficiency and high regioselectivity observed in the trifluoroborate-directed thiophenylation of bromide (+)-11, we sought to examine the generality of this chemistry with respect to other π-nucleophiles. Table 1 demonstrates the substrate scope for the nucleophilic addition into the C3-position of the tetracyclic bromide (+)-11: allyltri-n-butylstannane (Table 1, entry 3, 60%), allyltrimethylsilane (Table 1, entry 4, 52%), as well as (isopropenyloxy) trimethylsilane (Table 1, entry 5, 91%) act as suitable nucleophiles. Notably, vicinal quaternary stereocenters can also be installed efficiently, as demonstrated by the addition of methyl trimethysilyl dimethylketene acetal (Table 1, entry 6, 89%). Electron rich arenes, such as thiophene, (Table 1, entry 1, 76%) and electron neutral arenes, best represented by toluene (Table 1, entry 7, 50%), are also able to incorporate efficiently. Toluene itself adds with exclusive selectivity for the para-position, a preference that can be reversed using the trifluoroborate group.
The regioselectivity of aryltrifluoroborate addition, although moderate, is eroded in the presence of ortho-substituents such as in ortho-tolyltrifluoroborate (Table 1, entry 8, 25:24 = 3.5:1) and 2-methoxyphenyl trifluoroborate (Table 1, entry 9, 26:26' = 2.7:1), likely reflecting a sterically demanding transition state in the formation of quaternary stereocenters. Unfortunately, electron deficient arenes, such as potassium 4-acetylphenyl-trifluoroborate, lead to exclusive C3-fluorination via fluoride transfer from the aryltrifluoroborate. Nucleophiles without a competent electrofuge, such as styrene, result in polymerization. This problem can be circumvented through use of a trifluoroborate group, which likely eliminates faster than a proton (Table 1, entry 10, 59%). In general, the efficiency of the reactions appears to correlate well with the relative nucleophilicity of the compounds.22 The mildness of the reaction conditions is also of particular note. The utility of the method in late-stage applications is highlighted by the preservation of stereochemical integrity at C11 and C15, epimerizable stereocenters3b,5e consequential for the further derivatization of advanced intermediates.3c
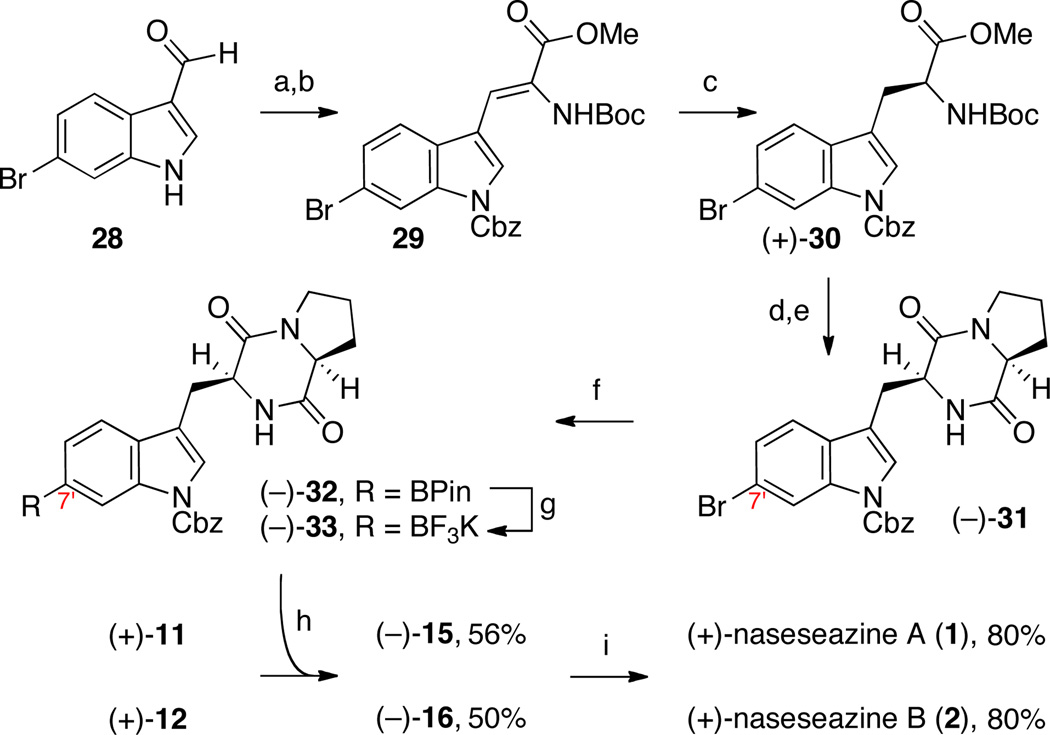
Our planned strategy for the directed union of advanced stage diketopiperazine fragments for the synthesis of (+)-naseseazines A (1) and B (2) required the synthesis of the trifluoroborate (−)-33 (Scheme 3). Starting from commercially available 6-bromo-3-carboxaldehyde (28), benzyloxycarbonylation followed by a Horner–Wadsworth–Emmons reaction with Boc-α-phosphonoglycine trimethylester afforded enamide 29 in 97% yield (2-steps).23 Catalytic asymmetric hydrogenation of the olefin using (S,S)-Et-DUPHOS-Rh (1.8 mol%) efficiently provided the bromotryptophan (+)-30 in 97% yield and >99% ee.24 An expeditious two-step sequence afforded the bromocyclodipeptide (−)-31 in 75% yield from bromotryptophan derivative (+)-30 without a need for chromatography. Our initial efforts to introduce the pinacol boronic ester function via palladium-catalyzed cross-coupling with pinacolborane25 were mired by undesired carboxybenzyl deprotection as well as the formation of significant amounts of a C7'-reduction byproduct. After significant experimentation, we recognized that employing Buchwald’s aminobiphenyl-(XPhos)PdCl precatalyst (34, 5 mol%),26 XPhos (15 mol%), K3PO4 (3 equiv), and bis(pinacolato)diboron (3 equiv) in DMSO at 60 °C effectively afford the desired pinacol boronate (−)-32 in 65% yield (Scheme 3). Treatment of the pinacol boronate (−)-32 with aqueous potassium hydrogen difluoride gave the key potassium trifluoroborate (−)-33 in 88% yield.13
Scheme 3.
Concise and directed synthesis of (+)-naseseazines A (1) and B (2).a
a Conditions: (a) CbzCl, Et3N, DMAP, CH2Cl2, 100%. (b) Boc-α-phosphonoglycine trimethylester, DBU, CH2Cl2, 97%. (c) H2 (80 psi), (S,S)-Et-DUPHOS-Rh (1.8 mol%), CH2Cl2, MeOH, 97%, >99% ee. (d) TFA, CH2Cl2; EDC•HCl, HOBt, Et3N, Boc-l-Pro. (e) TFA, CH2Cl2; NH4OH, MeOH, 75% (2-steps). (f) 2-aminobiphenyl(XPhos)PdCl (5 mol%), XPhos (15 mol%), (BPin)2, K3PO4, DMSO, 60 °C, 65%. (g) KHF2 (aq.), MeOH, 88%. (h) AgSbF6, 18-crown-6, EtNO2, 23 °C. (i) H2, Pd/C, AcOH, 23 °C.
Treatment of alanine-derived tetracyclic bromide (+)-11 (1 equiv) with tetracyclic potassium trifluoroborate (−)-33 (1.5 equiv) in the presence of silver hexafluoroantimonate and 18-crown-6 in nitroethane at 23 °C afforded the desired (−)-dicarboxybenzyl naseseazine A (15) in 56% yield with complete regioselection (Scheme 3). Under identical conditions, exposure of proline derived tetracyclic bromide (+)-12 (1 equiv) with tetracyclic potassium trifluoroborate (−)-33 (1.5 equiv) gave (−)-dicarboxybenzyl naseseazine B (16) in 50% yield as a single regioisomer (Scheme 3). Excitingly, the ability to override the innate nucleophilic tendencies of the indole substructure through an appropriately positioned directing group has broad implications toward the synthesis of related congeners of different regioisomeric constitutions about the C3sp3–Csp2 dimeric linkage, such as in (+)-asperazine,27 (+)-pestalazine,28 as well as the entire superfamily of oligomeric cyclotryptamines,1 represented by (+)-caledonine (Figure 1).29
Hydrogenolytic removal of the benzyloxycarbonyl groups from (−)-15 and (−)-16 with palladium on carbon in acetic acid provided (+)-naseseazine A (1) {[α]22D = +123 (c 0.12, CH3OH); lit. [α]23D = +139 (c 0.10, CH3OH)}7 and (+)-naseseazine B (2) {[α]22D = +101 (c 0.23, CH3OH); lit. [α]23D = +95 (c 0.08, CH3OH)},7 respectively, each in 80% yield (Scheme 3). All of the spectroscopic data for (+)-1 and (+)-2 matched those reported in the literature.7 The agreement between the optical rotation sign of our synthetic samples of naseseazine A (+)-1 and B (+)-2 and those of the natural alkaloids stands in direct contrast to the predicted absolute stereochemistry of (+)-1 and (+)-2 based on C3 Marfey’s analysis30 of degradation products. Given the use of l-amino acid derivatives in our synthesis of (+)-naseseazine A (1) and B (2), we revise the absolute stereochemistry of these dimeric natural alkaloids (Figure 1).
We have developed a general approach to dimeric diketopiperazine alkaloids containing a C3sp3–Csp2 linkage, with the solution yielding a concise 9-step total synthesis of (+)-naseseazines A (1) and B (2). Our mild and highly regioselective Friedel–Crafts based coupling strategy, featuring the formation of quaternary stereogenic centers, facilitated the late-stage union of advanced diketopiperazine structures in a convergent manner. Our total syntheses of (+)-1 and (+)-2 have also enabled the revision of the absolute stereochemistry of these natural products. This new fragment assembly strategy, ideated from a close adherence to biogenetically relevant intermediates and transformations, highlights the power of a strategic framework guided by retrobiosynthetic31 analysis.
Supplementary Material
Acknowledgement
We acknowledge financial support by NIH-NIGMS (GM089732), Amgen, and DuPont. M.M. is a Camille Dreyfus Teacher-Scholar. J.K. acknowledges a National Defense Science and Engineering Graduate Fellowship. We thank Dr. Omar K. Ahmad and Dr. Nicolas Boyer for helpful discussions. We are grateful to Prof. R. J. Capon for a communication regarding the discrepancy between our respective stereochemical assignments of (+)-naseseazines A and B.
Footnotes
Supporting Information. Experimental procedures, spectroscopic data, and copies of UV-vis, 1H, 13C, and 19F NMR spectra. This material is free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Hino T, Nakagawa M. In: The Alkaloids: Chemistry and Pharmacology. Brossi A, editor. Vol. 34. New York: Academic Press; 1989. pp. 1–75. [Google Scholar]; (b) Anthoni U, Christophersen C, Nielsen PH. In: Alkaloids Chemical and Biological Perspectives. Pelletier SW, editor. Vol. 13. London: Pergamon; 1999. pp. 163–236. [Google Scholar]
- 2.(a) Hendrickson JB, Rees R, Goschke R. Proc. Chem. Soc. 1962:383. [Google Scholar]; (b) Hino T, Yamada S. Tetrahedron Lett. 1963;4:1757. [Google Scholar]; (c) Scott AI, McCapra F, Hall ES. J. Am. Chem. Soc. 1964;86:302. doi: 10.1021/ja01017a041. [DOI] [PubMed] [Google Scholar]; (d) Nakagawa M, Sugumi H, Kodato S, Hino T. Tetrahedron Lett. 1981;22:5323. [Google Scholar]; (e) Fang CL, Horne S, Taylor N, Rodrigo R. J. Am. Chem. Soc. 1994;116:9480. [Google Scholar]; (f) Link JT, Overman LE. J. Am. Chem. Soc. 1996;118:8166. [Google Scholar]; (g) Overman LE, Paone DV, Stearns BA. J. Am. Chem. Soc. 1999;121:7702. [Google Scholar]; (h) Somei M, Osikiri N, Hasegawa M, Yamada F. Heterocycles. 1999;51:1237. [Google Scholar]; (i) Overman LE, Larow JF, Stearns BA, Vance JM. Angew. Chem. Int. Ed. 2000;39:213. doi: 10.1002/(sici)1521-3773(20000103)39:1<213::aid-anie213>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]; (j) Ishikawa H, Takayama H, Aimi N. Tetrahedron Lett. 2002;43:5637. [Google Scholar]; (k) Matsuda Y, Kitajima M, Takayama H. Heterocycles. 2005;65:1031. [Google Scholar]
- 3.(a) Movassaghi M, Schmidt MA. Angew. Chem. Int. Ed. 2007;46:3725. doi: 10.1002/anie.200700705. [DOI] [PubMed] [Google Scholar]; (b) Movassaghi M, Schmidt MA, Ashenhurst JA. Angew. Chem. Int. Ed. 2008;47:1485. doi: 10.1002/anie.200704960. [DOI] [PubMed] [Google Scholar]; (c) Kim J, Ashenhurst J, Movassaghi M. Science. 2009;324:238. doi: 10.1126/science.1170777. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Kim J, Movassaghi M. J. Am. Chem. Soc. 2010;132:14376. doi: 10.1021/ja106869s. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Movassaghi M, Ahmad OK, Lathrop SP. J. Am. Chem. Soc. doi: 10.1021/ja2057852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.For recent applications of our cobalt-mediated dimerization of indole derivatives, see: Pérez-Balado C, de Lera ÁR. Org. Lett. 2008;10:3701. doi: 10.1021/ol8013073. Pérez-Balado C, Rodríguez-Graña P, de Lera ÁR. Chem. Eur. J. 2009;15:9928. doi: 10.1002/chem.200901056. Iwasa E, Hamashima Y, Fujishiro S, Higuchi E, Ito A, Yoshida M, Sodeoka M. J. Am. Chem. Soc. 2010;132:4078. doi: 10.1021/ja101280p. Foo K, Newhouse T, Mori I, Takayama H, Baran PS. Angew. Chem. Int. Ed. 2011;50:2716. doi: 10.1002/anie.201008048.
- 5.(a) Matsuda Y, Kitajima M, Takayama H. Org. Lett. 2008;10:125. doi: 10.1021/ol702637r. [DOI] [PubMed] [Google Scholar]; (b) Newhouse T, Baran PS. J. Am. Chem. Soc. 2008;130:10886. doi: 10.1021/ja8042307. [DOI] [PubMed] [Google Scholar]; (c) Espejo VR, Rainier JD. J. Am. Chem. Soc. 2008;130:12894. doi: 10.1021/ja8061908. [DOI] [PubMed] [Google Scholar]; (d) Newhouse T, Lewis CA, Baran PS. J. Am. Chem. Soc. 2009;131:6360. doi: 10.1021/ja901573x. [DOI] [PubMed] [Google Scholar]; (e) Espejo VR, Li XB, Rainier JD. J. Am. Chem. Soc. 2010;132:8282. doi: 10.1021/ja103428y. [DOI] [PubMed] [Google Scholar]; (f) Espejo VR, Rainier JD. Org. Lett. 2010;12:2154. doi: 10.1021/ol100672z. [DOI] [PubMed] [Google Scholar]; (g) Pérez-Balado C, de Lera ÁR. Org. Biomol. Chem. 2010;8:5179. doi: 10.1039/c0ob00531b. [DOI] [PubMed] [Google Scholar]
- 6.For syntheses of C3-arylated cyclotryptamines, see: Govek SP, Overman LE. J. Am. Chem. Soc. 2001;123:9468. doi: 10.1016/j.tet.2007.05.127. Kodanko JJ, Overman LE. Angew. Chem, Int. Ed. 2003;42:2528. doi: 10.1002/anie.200351261. Govek SP, Overman LE. Tetrahedron. 2007;63:8499. Kodanko JJ, Hiebert S, Peterson EA, Sung L, Overman LE, De Moura Linck V, Goerck GC, Amador TA, Leal MB, Elisabetsky E. J. Org. Chem. 2007;72:7909. doi: 10.1021/jo7013643. Movassaghi M, Schmidt MA, Ashenhurst JA. Org. Lett. 2008;10:4009. doi: 10.1021/ol8015176.
- 7.Raju R, Piggott AM, Conte M, Aalbersberg WGL, Feussner K, Capon RJ. Org. Lett. 2009;11:3862. doi: 10.1021/ol901466r. [DOI] [PubMed] [Google Scholar]
- 8.We consider a biosynthetic hypothesis in which dimerization occurs at an advanced stage using well-elaborated tryptophan systems, such as cyclodipeptides, to be plausible; see Scheme S1.
- 9.See Supporting Information for details.
- 10.For the systematic positional numbering system used throughout this report, see page S3 in the Supporting Information.
- 11.For electrophilic activation of a C3-selenocyclotryptamine, see Depew KM, Marsden SP, Zatorska D, Zatorski A, Bornmann WG, Danishefsky SJ. J. Am. Chem. Soc. 1999;121:11953. Iwaki T, Yamada F, Funaki S, Somei M. Heterocycles. 2005;65:1811.
- 12.For addition to C3 of oxindoles, see Nicolaou KC, Chen DY-K, Huang X, Ling T, Bella M, Snyder SA. J. Am. Chem. Soc. 2004;126:12888. doi: 10.1021/ja040092i. Fuchs JR, Funk RL. Org. Lett. 2005;7:677. doi: 10.1021/ol047532v. Magnus P, Turnbull R. Org. Lett. 2006;8:3497. doi: 10.1021/ol061191z. Cheung CM, Goldberg FW, Magnus P, Russell CJ, Turnbull R, Lynch V. J. Am. Chem. Soc. 2007;129:12320. doi: 10.1021/ja0744448. England DB, Merey G, Padwa A. Org. Lett. 2007;9:3805. doi: 10.1021/ol7016438. Grant CD, Krische MJ. Org. Lett. 2009;11:4485. doi: 10.1021/ol9018562.
- 13.(a) Molander GA, Ellis N. Acc. Chem. Res. 2007;40:275. doi: 10.1021/ar050199q. [DOI] [PubMed] [Google Scholar]; (b) Vedejs E, Chapman RW, Fields SC, Lin S, Schrimpf MR. J. Org. Chem. 1995;60:3020. [Google Scholar]
- 14.For a single-step synthesis from commercially available Nα-Boc-l-Trp-OMe see: Kiso Y, Inai M, Kitagawa K, Akita T. Chem. Lett. 1983;5:739. doi: 10.1248/cpb.31.1818.
- 15.Petasis NA, Akritopoulou I. Tetrahedron Lett. 1993;34:583. [Google Scholar]
- 16.For the use of potassium heteroaryl- and styrenyl-trifluoroborates in Friedel–Crafts alkylation reactions, see: Lee S, MacMillan DWC. J. Am. Chem. Soc. 2007;129:15438. doi: 10.1021/ja0767480.
- 17.Transmetalation of the aryltrifluoroborate to silver appears to be slower than the rate of C3 ionization. Addition of PhAg (2 equiv) to a solution of (+)-11 in CH2Cl2 at 23 °C did not provide the desired product. For silver transmetalation of aryl boronic acids, see: Furuya T, Ritter T. Org. Lett. 2009;11:2860. doi: 10.1021/ol901113t.
- 18.For the addition of phenyltrifluoroborate (2 equiv) to (+)-11 (1 equiv) in nitromethane using AgSbF6 (2 equiv) as the ionization promoter, a C3-hydroxylated product was observed (38% yield) along with the desired adduct (38% yield). In contrast, inclusion of 18-crown-6 (2 equiv) resulted in formation of the desired product (78% yield).
- 19.C3-hydroxylated products likely arise from hydrolysis of an activated intermediate upon aqueous quench.
- 20.Reaction of PhBF3K (2 equiv) with (+)-11 (1 equiv) and AgSbF6 (2 equiv) in MeCN at 23 °C resulted in the C3-phenyl adduct (59% yield) and a C3-acetamide adduct (41% yield).
- 21.Reaction of PhBF3K (2 equiv) with (+)-11 (1 equiv) and AgSbF6 (2 equiv) in DMF at 23 °C resulted in the C3-hydroxylated product (22% yield) and a C3-formate adduct (72% yield).
- 22.Mayr H, Bug T, Gotta MF, Hering N, Irrgang B, Janker B, Kempf B, Loos R, Ofial AR, Remennikov G, Schimmel H. J. Am. Chem. Soc. 2001;123:9500. doi: 10.1021/ja010890y. [DOI] [PubMed] [Google Scholar]
- 23.Schmidt U, Griesser H, Leitenberger V, Lieberknecht A, Mangold R, Meyer R, Reidl B. Synthesis. 1992:487. [Google Scholar]
- 24.(a) Burk MJ, Allen JG, Kiesman WF. J. Am. Chem. Soc. 1998;120:657. [Google Scholar]; (b) Wang W, Xiong C, Yang J, Hruby VJ. Tetrahedron Lett. 2001;42:7717. [Google Scholar]
- 25.(a) Billingsley KL, Buchwald SL. J. Org. Chem. 2008;73:5589. doi: 10.1021/jo800727s. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Billingsley KL, Barder TE, Buchwald SL. Angew. Chem. Int. Ed. 2007;46:5359. doi: 10.1002/anie.200701551. [DOI] [PubMed] [Google Scholar]; (c) Ishiyama T, Murata M, Miyaura N. J. Org. Chem. 1995;60:7508. [Google Scholar]
- 26.Kinzel T, Zhang Y, Buchwald SL. J. Am. Chem. Soc. 2010;132:14073. doi: 10.1021/ja1073799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Varoglu M, Corbett T, Valeriote F, Crews P. J. Org. Chem. 1997;62:7078. doi: 10.1021/jo970568z. [DOI] [PubMed] [Google Scholar]
- 28.Ding G, Jiang L, Guo L, Chen X, Zhang H, Che Y. J. Nat. Prod. 2008;71:1861. doi: 10.1021/np800357g. [DOI] [PubMed] [Google Scholar]
- 29.Jannic V, Gueritte F, Laprevote O, Serani L, Martin MT, Sevenet T, Potier P. J. Nat. Prod. 1999;62:838. doi: 10.1021/np9805387. [DOI] [PubMed] [Google Scholar]
- 30.Ratnayake R, Fremlin LJ, Lacey E, Gill JH, Capon RJ. J. Nat. Prod. 2008;71:403. doi: 10.1021/np070589g. [DOI] [PubMed] [Google Scholar]
- 31.Movassaghi M, Siegel DS, Han S. Chem. Sci. 2010;1:561. doi: 10.1039/c0sc00351d. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.