SUMMARY
Epithelial cell-cell junctions, organized by adhesion proteins and the underlying actin cytoskeleton, are considered to be stable structures maintaining the structural integrity of tissues. Contrary to the idea that α-catenin links the adhesion protein E-cadherin through β-catenin to the actin cytoskeleton, in the accompanying paper we report that α-catenin does not bind simultaneously to both E-cadherin-β-catenin and actin filaments. Here we demonstrate that α-catenin exists as a monomer or a homodimer with different binding properties. Monomeric α-catenin binds more strongly to E-cadherin-β-catenin, whereas the dimer preferentially binds actin filaments. Different molecular conformations are associated with these different binding states, indicating that α-catenin is an allosteric protein. Significantly, α-catenin directly regulates actin-filament organization by suppressing Arp2/3-mediated actin polymerization, likely by competing with the Arp2/3 complex for binding to actin filaments. These results indicate a new role for α-catenin in local regulation of actin assembly and organization at sites of cadherin-mediated cell-cell adhesion.
INTRODUCTION
Epithelial cell-cell junctions are organized by adhesion proteins and the underlying actin cytoskeleton. They provide adaptable interfaces that can respond to signals for cell movement during convergent extension in gastrulation (Keller, 2002) or changes in cell shape during tube formation (Lubarsky and Krasnow, 2002) but also provide a constant permeability barrier between different biological compartments in the body. Analysis of how migrating cells initiate cell-cell adhesion has revealed dramatic changes in membrane dynamics and organization of the actin cytoskeleton. Migrating cells have characteristic forward-moving lamellipodia produced by rapid Arp2/3-nucleated assembly of a branched actin network perpendicular to the leading edge (Pollard and Borisy, 2003). Upon cell-cell adhesion, lamellipodial activity is reduced over the contacting area, and there is a concomitant reorganization of actin filaments (Adams et al., 1998; Ehrlich et al., 2002; Omelchenko et al., 2001; Vaezi et al., 2002; Vasioukhin et al., 2001); electron microscopy of simple epithelial cells indicates the formation of bundles of actin filaments parallel to the contacting membranes (Hirokawa et al., 1983), an organization very different from that of branched actin in lamellipodia. It is unknown how engagement of cadherins between migrating cells causes these dramatic changes in actin-filament assembly and organization.
The intracellular domain of cadherins binds cytoplasmic proteins that are thought to recruit and organize actin filaments (Gumbiner, 2000; Jamora and Fuchs, 2002). These molecular linkages (Nagafuchi and Takeichi, 1988; Ozawa et al., 1989) include high-affinity binding of β-catenin to the cadherin cytoplasmic domain (Huber et al., 2001) and a lower-affinity interaction between β-catenin and α-catenin (Pokutta and Weis, 2000). Since α-catenin can also bind actin filaments in vitro (Pokutta et al., 2002; Rimm et al., 1995), it is widely accepted that α-catenin bound to the cadherin-β-catenin complex bridges these components to actin. In addition, many actin binding proteins have been reported to bind α-catenin, including vinculin and α-actinin (Kobielak and Fuchs, 2004), suggesting that they could also link the cadherin-catenin complex to the actin cytoskeleton. Furthermore, the Rho family of small GTPases (Braga, 2002; Fukata and Kaibuchi, 2001) and the actin nucleators formin (Kobielak et al., 2004) and the Arp2/3 complex (Kovacs et al., 2002) are involved in cell-cell adhesion and may regulate actin dynamics around the cadherin-catenin complex.
In the accompanying paper (Yamada et al., 2005 [this issue of Cell]), experiments with purified proteins demonstrated that the ternary complex of cadherin-β-catenin-α-catenin does not bind directly to actin filaments or indirectly through vinculin or α-actinin. Moreover, live-cell imaging showed that the cadherin-catenin complex has dynamics that are very different from those of actin, consistent with the lack of a stable linkage between the cadherin-catenin complex and actin in cells.
The inability of α-catenin to bind simultaneously to the cadherin-β-catenin complex and actin indicates that it may function as a molecular switch, whereby binding to one partner changes the ability to interact with the other. Here we provide evidence that these changes are associated with distinct conformational states of α-catenin and that dimerization of α-catenin influences its ability to selectively bind to β-catenin or actin. Given the highly dynamic properties of the actin network at cell-cell contacts, we further examined the role of α-catenin in regulating actin assembly. We show that α-catenin suppresses actin polymerization by the Arp2/3 complex, suggesting that assembly and clustering of the cadherin-catenin complex at cell-cell contacts may provide a pool of α-catenin that can locally regulate actin dynamics and organization.
RESULTS
Native and Recombinant α-Catenin Exist as Monomer and Homodimer
Purified α-catenin has been reported to be a homodimer in solution (Koslov et al., 1997). The crystal structure of the N-terminal domain of α-catenin revealed that the dimerization domain overlaps the β-catenin binding domain (Pokutta and Weis, 2000). Since the stoichiometry of the α-catenin-β-catenin heterodimer is 1:1, the α-catenin homodimer would have to dissociate before α-catenin could bind β-catenin (Pokutta and Weis, 2000). Thus, the molecular state of α-catenin (monomer, homodimer, heterodimer with β-catenin) appears to be critical for interactions with potential binding partners.
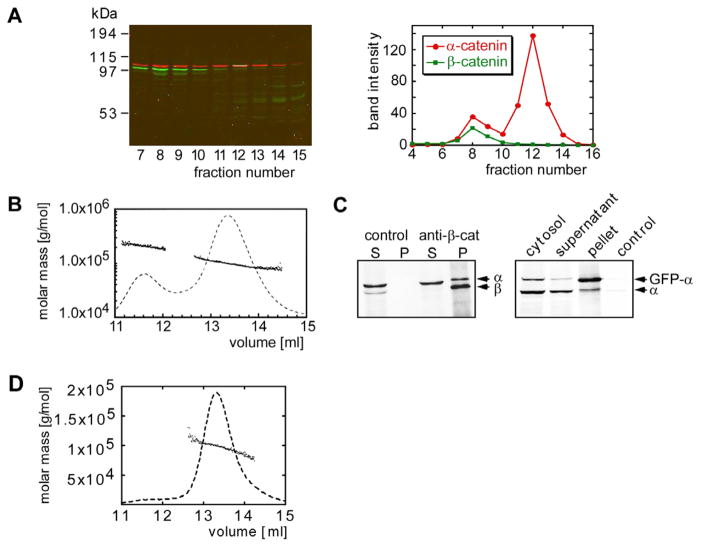
A pool of α-catenin is found in MDCK cell cytoplasm, and another is associated with the E-cadherin-β-catenin complex at the plasma membrane (Hinck et al., 1994). Fractionation of a 100,000 ×g cytosol supernatant by gel filtration revealed two peaks of α-catenin (Figure 1A) with elution volumes similar to those of purified recombinant His-tagged α-catenin (Figure 1B). The molecular mass of the two peaks of His-tagged α-catenin, determined by multiangle light scattering (MALS), corresponded to an α-catenin monomer and homodimer, respectively (Figure 1B). Data from several independent experiments indicated that 60%–75% of cytosolic α-catenin in MDCK cells was monomeric (Figure 1A), and the proportion of monomer was independent of the cell plating density (data not shown).
Figure 1. Oligomeric State of α-Catenin.
(A) Superdex 200 gel filtration chromatography of MDCK cell cytosol. Fractions of the gel filtration run were analyzed by SDS-PAGE and subsequent Western blotting. α-catenin (red) and β-catenin (green) were identified in the column fractions that correspond to the peak fractions of the recombinant α-catenin monomer and dimer. Band intensities of the α-catenin and β-catenin bands were plotted versus the fraction number.
(B) Molecular mass versus elution volume distribution plot obtained from a MALS experiment. The trace of the light scattering signal of the 90° angle detector is shown as a dashed line.
(C) The left-hand gel shows immunoprecipitation using anti-β-catenin antibodies of fraction 8 of the Superdex 200 gel filtration run of a MDCK cell lysate shown in (A). The supernatant and pellet were analyzed by Western blotting using anti-α-catenin and anti-β-catenin antibodies. Protein A beads with no antibody coupled were used as a control. The gel on the right shows formation of mixed dimers between GFP-α-catenin and endogenous α-catenin, as analyzed by immunoprecipitation of the cytosol fraction of MDCK cells expressing GFP-α-catenin with anti-GFP-antibody. The gel was blotted with anti-α-catenin antibody. The pellet of an immunoprecipitate using an anti-GST-antibody is shown as a control.
(D) MALS analysis of the βα-catenin molecular mass.
Although the first peak of α-catenin observed in the cytosol fractionation can be superimposed on the elution profile of the recombinant α-catenin homodimer, we found that endogenous β-catenin also coeluted with this peak. Since recombinant β-catenin elutes at a smaller apparent molecular weight (e.g., Figures 2A and 2B), the observed coelution of β-catenin and α-catenin may indicate that there is a β-/α-catenin heterodimer in MDCK cell cytosol. Indeed, α-catenin coimmunoprecipitated with β-catenin from the higher-molecular-weight peak (Figure 1C). However, a β-/α-catenin heterodimer does not account for all α-catenin in this peak, as endogenous α-catenin can be coimmunoprecipitated with a green fluorescent protein-α-catenin fusion protein (GFP-α-catenin), indicating the presence of α-catenin homodimer in cytosol (Figure 1C). Comparison of the amount of α-catenin in the pellet and supernatant after coimmunoprecipitation with β-catenin indicates that approximately 20% of α-catenin in the higher-molecular-weight fraction was bound to β-catenin, with approximately 80% an α-catenin homodimer. Thus, in contrast to previous reports, purified α-catenin can exist as a monomer and homodimer in solution, and most of endogenous α-catenin in MDCK cell cytosol is monomeric.
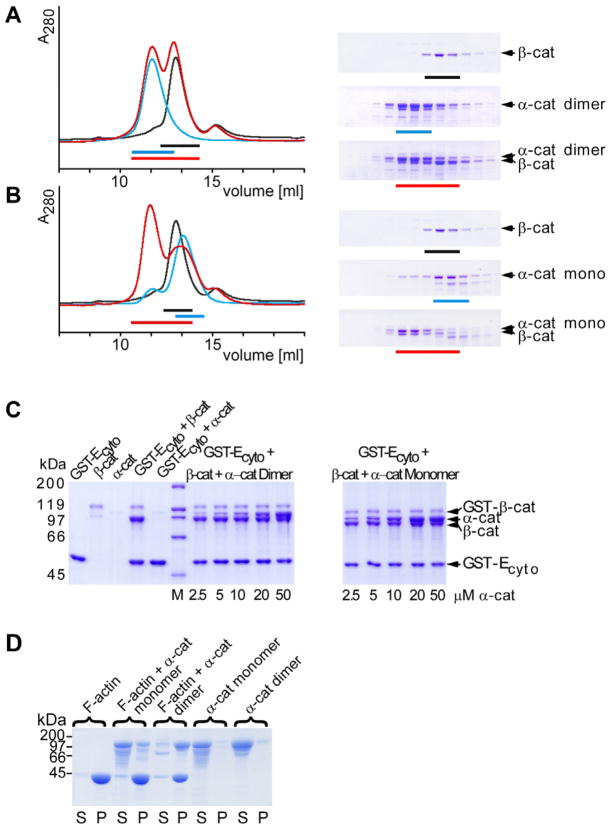
Figure 2. β-Catenin and Actin Binding Activity of α-Catenin Monomer and Dimer.
(A) Superdex 200 gel filtration chromatography of the α-catenin dimer and β-catenin incubated overnight (red line) and of the individual proteins, α-catenin dimer (blue) and β-catenin (black). Fractions analyzed by SDS-PAGE are shown for the individual runs on the right. Peak fractions are indicated by colored bars.
(B) Gel filtration chromatrography as described in (A) with the α-catenin monomer.
(C) GST-E-cadherin cytoplasmic domain (10 μM) and β-catenin (10 μM) were incubated with α-catenin monomer or dimer at the indicated concentrations. Protein complexes were isolated on GST-agarose beads and analyzed by SDS-PAGE. Background binding of contaminating uncleaved GST-β-catenin is seen in the β-catenin-containing samples.
(D) Sedimentation of monomeric and dimeric α-catenin in the presence and absence of F-actin. Supernatant S containing the unbound protein and pellet P containing actin bound protein were analyzed by SDS-PAGE.
The concentration of α-catenin in the MDCK cell cytosol was estimated from α-catenin Western blots by comparing the intensities of bands from cytosol to those from known amounts of input recombinant α-catenin. The most intense monomer fractions had a concentration of 7 nM. Accounting for cell volume and dilution during cell lysis and column chromatography, we obtain an estimate of 0.6 μM α-catenin in cytosol. Recombinant α-catenin appears as a mixture of monomer and dimer when run on gel filtration columns at concentrations of 2–20 μM (e.g., Figure 1B). This places the homodimerization constant in the micromolar range, which is consistent with the observation that the majority of cytosolic α-catenin is monomeric.
α-Catenin Monomer and Homodimer Show Preferential Binding to β-Catenin and Actin, Respectively
Since α-catenin forms a 1:1 complex with β-catenin and the homodimerization and β-catenin binding domains of α-catenin overlap (Pokutta and Weis, 2000), we hypothesized that α-catenin monomer should bind more readily to β-catenin. Formation of the β-catenin-α-catenin complex was monitored in solution by gel filtration chromatography. Mixtures of α-catenin and β-catenin were applied to an analytical gel filtration column at 26 μM, which then became diluted approximately 10-fold on the column. Under these conditions, α-catenin homodimer did not bind β-catenin, and both proteins eluted separately (Figure 2A), whereas the majority of α-catenin monomer eluted in a 1:1 complex with β-catenin (Figure 2B). We assessed whether the presence of E-cadherin affects the relative affinities of α-catenin monomer or homodimer for β-catenin. Pull-down assays were established using glutathione-agarose beads to capture bacterially expressed GST-E-cadherin cytoplasmic domain (GST-Ecyto), full-length recombinant β-catenin, and increasing amounts of full-length His-tagged α-catenin monomer or homodimer; note that recombinant GST-Ecyto and β-catenin bind with 36 nM affinity in a 1:1 stoichiometry in solution (Huber et al., 2001; Choi et al., 2006). The resulting bead bound complexes showed that the α-catenin monomer has a higher apparent affinity for β-catenin than the homodimer (saturation reached at approximately 20 μM monomer versus 50 μM for the homodimer) (Figure 2C). Gel filtration and pull-down assays using plakoglobin, a homolog of β-catenin that also binds E-cadherin and α-catenin, gave similar results (see Figure S1 in the Supplemental Data available with this article online).
We tested whether α-catenin monomer and homodimer have different apparent affinities for actin filaments in an in vitro actin pelleting assay. Greater than 90% of the α-catenin homodimer pelleted with actin filaments, whereas less than 40% of the monomer bound (Figure 2D). As a fraction of purified α-catenin monomer rapidly converts to the homodimer (see above), it is impossible to obtain samples that are 100% pure monomeric α-catenin. Therefore, it is possible that the α-catenin bound to actin filaments in this experiment is due to contaminating homodimer and that the monomer does not bind actin filaments under these conditions.
In the accompanying paper (Yamada et al., 2005), we showed that binding of α-catenin to β-catenin and actin is mutually exclusive. This was confirmed by using the chimeric β-catenin-α-catenin protein, which mimics the interaction of the two proteins by covalently linking the α-catenin binding site of β-catenin to the β-catenin binding domain of α-catenin (Pokutta and Weis, 2000). Gel filtration chromatography (data not shown) and MALS (Figure 1D) demonstrated that the β-catenin-α-catenin chimera is monomeric. Therefore, the inability of the chimera to bind to actin could be due to its oligomeric state. Alternatively, binding of β-catenin induces a conformation of α-catenin that is unable to bind actin.
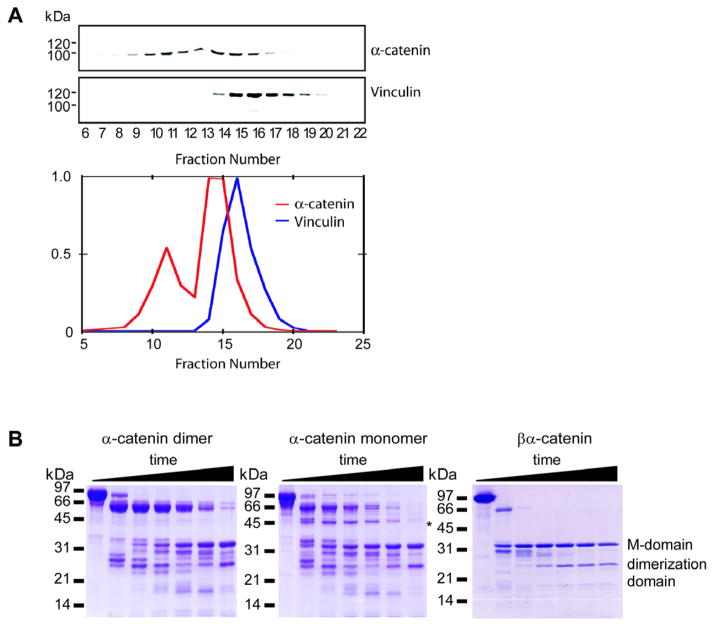
The inability of α-catenin to bind both β-catenin and actin simultaneously suggests that the N- and C-terminal domains of α-catenin are allosterically coupled, whereby binding to one partner alters its ability to bind to the other. Interestingly, the homologous protein vinculin shows the opposite behavior: the head and tail regions of vinculin bind to talin and actin, respectively, only if an autoinhibitory head-tail interaction is relieved (Bass et al., 2002; Johnson and Craig, 1995). Purified vinculin behaves as a globular protein on a sizing column, whereas monomeric α-catenin displays a considerably larger apparent molecular mass (Figure 3A), indicating a more extended, open conformation. Moreover, we could not detect an interaction between the N- and C-terminal domains of α-catenin (S.P., unpublished data). The βα-catenin chimera has a gel filtration elution profile similar to that of monomeric α-catenin, implying that its inability to bind to actin is not due to an interaction between α-catenin N- and C-terminal domains. Furthermore, proteolytic-sensitivity experiments indicate that the α-catenin monomer has a conformation different from that of the βα-catenin chimera (Figure 3B). Thus, the inability of α-catenin to bind actin in the presence of β-catenin is probably due to conformational changes produced when α-catenin monomer binds to the β-catenin-E-cadherin complex. Further structural studies will be needed to analyze these conformational changes in α-catenin.
Figure 3. Conformational Properties of α-Catenin and Comparison to Vinculin.
(A) Vinculin elutes at a lower apparent molecular weight on a gel filtration column than α-catenin. MDCK cell cytosol was analyzed by Superdex 200 gel filtration chromatography. Fractions were analyzed by SDS-PAGE and subsequent Western blotting for α-catenin and vinculin. Intensities of the α-catenin and vinculin bands were plotted versus the fraction number.
(B) Proteolytic sensitivity of α-catenin monomer, homodimer, and β-catenin-α-catenin chimera. SDS-PAGE of α-catenin monomer, dimer, and βα-catenin chimera incubated for 0 min, 5 min, 15 min, 30 min, 1 hr, 2 hr, and 4 hr with trypsin. The asterisk indicates a degradation product unique to the α-catenin monomer. Molecular-weight markers are indicated on the left. The indicated dimerization and M domains were identified previously as comprising residues 82–264 and 385–651, respectively (Pokutta et al., 2002; Pokutta and Weis, 2000).
Exchange between Cytoplasmic and Membrane Bound Pools of α-Catenin
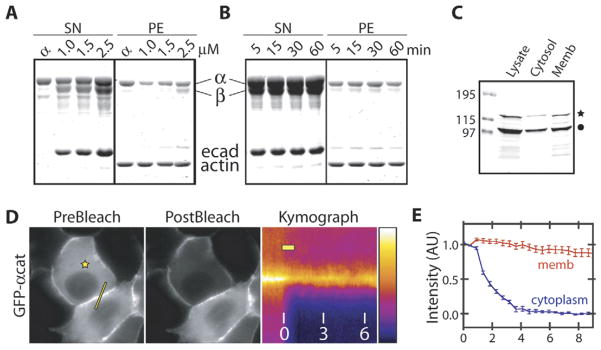
The mutually exclusive binding of α-catenin to β-catenin or actin implies that α-catenin must dissociate from the E-cadherin-β-catenin complex before it can bind to actin. This was tested by incubating actin filaments with preassembled, gel filtration-purified cadherin-catenin complex containing a 1:1:1 molar ratio of E-cadherin cytoplasmic domain, β-catenin, and α-catenin at a series of concentrations (Figure 4A) or for different times (Figure 4B). The actin filaments were then pelleted, and bound proteins were identified. E-cadherin or β-catenin did not pellet above background levels (see also Yamada et al., 2005). However, a significant fraction of α-catenin dissociated from the complex and pelleted with actin filaments independently of incubation time (Figure 4B).
Figure 4. Exchange of α-Catenin from Cadherin-β-Catenin Complexes.
(A and B) Sedimentation of actin filaments in the presence of preassembled E-cadherin-β-catenin-α-catenin complex. Preassembled cadherin-catenin complex was isolated by gel filtration and mixed with actin filaments while varying the concentration of the complex (A) and incubation time (B) and centrifuged to sediment actin filaments and associating proteins. E-cadherin and β-catenin did not pellet above background levels. Lane α did not contain E-cadherin or β-catenin.
(C) Cellular distribution of endogenous and GFP-α-catenin following detergent extraction of MDCK cells stably expressing GFP-α-catenin. Lysates were run on SDS-PAGE and Western blotted with anti-α-catenin antibody. Endogenous and GFP-tagged α-catenin are indicated with the filled circle and star, respectively.
(D) Pre- and postbleach images and the corresponding kymograph of GFP-α-catenin at cell-cell contacts. The stars designate the location of the photo-bleaching laser spot, and lines indicate the intensity profile plotted in the kymograph. The bar in the kymograph shows the duration of photobleaching by the laser, and numbers are time in minutes. The fluorescence intensity scale is pseudocolored as shown.
(E) Time-dependent intensity profile of cytoplasmic (blue) and membrane bound (red) pools of GFP-α-catenin after FLIP as shown in (D). Data points are averages of 15 independent experiments, and the error bars represent the SEM. Time (min) is depicted on the x axis.
The data obtained from the purified, recombinant proteins show that α-catenin can exchange between an E-cadherin-β-catenin complex and actin filaments in solution. We next tested whether this exchange occurs in cells. It was shown in the accompanying paper that GFP-labeled catenins display significant fluorescence recovery after photobleaching at the membrane (Yamada et al., 2005). Fluorescence recovery of peripheral membrane proteins can occur in two ways: lateral diffusion along the membrane surface in association with an integral membrane protein or exchange with the cytoplasmic pool. There is a significant cytoplasmic pool of α-catenin that could exchange with membrane bound α-catenin (Figure 4C). To test the extent of α-catenin exchange between these pools, we measured the fluorescence loss of GFP-α-catenin at cell-cell contacts while continuously photobleaching the cytoplasmic pool of GFP-α-catenin (fluorescence loss in photobleaching, FLIP; Figure 4D and Movie S1). The membrane bound pool of α-catenin, measured by subtracting the cytoplasmic fluorescence intensity from that at cell-cell contacts, decreased by an average of ~10% over a period of 9 min (n = 15; Figures 4D and 4E), suggesting that a fraction of membrane bound α-catenin dissociated from β-catenin and entered the cytoplasm. Since the fluorescence intensity of cytoplasmic GFP-β-catenin was near background levels (see Yamada et al., 2005), as expected (Näthke et al., 1994), we could not reliably measure the dynamics of membrane bound GFP-β-catenin.
It is possible that the observed exchange of α-catenin, as well as the recovery of GFP-tagged cadherins and catenins reported in the accompanying paper, is due to endocytosis of cadherin-catenin complexes from the membrane and fusion of cadherin-containing cytoplasmic vesicles at the membrane. However, no vesicle fusion events that coincided with the fluorescence recovery of E-cadherin or catenins were detected, although the temporal and spatial resolution of our microscope may not be sufficient to reliably detect these events. Moreover, the half-life of E-cadherin in MDCK cells is ~5 hr (Shore and Nelson 1991), which converts to a turnover of ~1% of E-cadherin over the period that the recovery was measured. In addition, the putative recycling pool of cadherin vesicles is <2% of total E-cadherin (Le et al., 1999). Thus, these mechanisms may contribute to a small portion of α-catenin turnover, but direct exchange with the cytosolic pool is most likely to be the dominant mechanism.
α-Catenin Suppresses Arp2/3-Mediated Actin Polymerization
The mobility of the cadherin-catenin complex is quantitatively different from those of the membrane-associated and cytoplasmic pools of actin and several other actin binding proteins, observations consistent with in vitro binding experiments showing that the cadherin-catenin complex does not bind actin (Yamada et al., 2005). Nevertheless, actin is associated with cell-cell contacts, and actin dynamics are important for induction of cell-cell adhesion (Adams et al., 1998; Ehrlich et al., 2002; Vaezi et al., 2002), indicating that cadherin-mediated cell-cell adhesion somehow induces changes in actin organization and dynamics. Therefore, we examined whether α-catenin can influence Arp2/3 complex-mediated actin assembly, which plays a central role in actin assembly and branching in lamellipodia (Pollard and Borisy, 2003) and is localized to sites of initiating cell-cell contact (Kovacs et al., 2002).
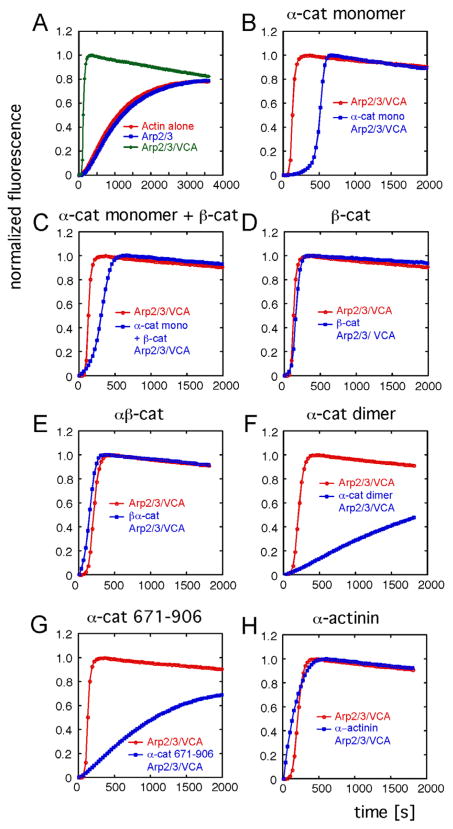
Actin polymerization in the presence of α-catenin, purified Arp2/3 complex, and the activation domain (VCA) of the Wiskott-Aldrich syndrome protein (WASp-VCA) was measured in a standard pyrene-actin fluorescence assay. As expected, Arp2/3 complex alone had little effect on actin polymerization, but it strongly enhanced actin polymerization in the presence of WASp-VCA (Figure 5A; Prehoda et al., 2000). The presence of α-catenin monomer or homodimer had little or no effect on actin polymerization (data not shown). However, in the presence of the Arp2/3 complex and WASp-VCA, α-catenin monomer increased the initial lag phase of actin polymerization (Figure 5B). This effect was significantly reduced by the addition of an equimolar amount of β-catenin (Figure 5C); β-catenin alone did not have an effect on actin polymerization in the presence of Arp2/3-WASp (Figure 5D). The βα-catenin chimera (βα-cat), which mimics the β-catenin-α-catenin interaction and does not bind actin filaments (Yamada et al., 2005), also had no effect on actin polymerization induced by the Arp2/3 complex and WASp-VCA (Figure 5E).
Figure 5. α-Catenin Suppresses Arp2/3-Mediated Actin Dynamics.
Effect of α-catenin, β-catenin, and α-actinin on Arp2/3-mediated actin polymerization measured by pyrene-actin assay. Assembly reactions contained 5 μM actin containing 10% pyrene actin, 50 nM Arp2/3 complex, 50 nM WASp-VCA, and 5 μM of the indicated protein.
(A) Actin alone or in the presence of Arp2/3 complex with and without VCA.
(B–H) Arp2/3-mediated actin polymerization in the presence or absence of α-catenin monomer (B), α-catenin monomer and β-catenin (C), β-catenin (D), βα-catenin chimera (E), α-catenin dimer (F), α-catenin tail domain aa 671–906 (G), or α-actinin (H).
In contrast to monomeric α-catenin, α-catenin homodimer completely suppressed actin polymerization in the presence of the Arp2/3 complex and WASp-VCA at 5 μM concentration (Figure 5F). The actin binding domain of α-catenin (α-cat 671–906), which is also a dimer (S.P., unpublished data), suppressed actin polymerization induced by Arp2/3 complex and WASp-VCA to an extent similar to that of the α-catenin homodimer (Figure 5G). It is possible that bundling of actin filaments by α-catenin homodimers (Rimm et al., 1995) sterically inhibited the Arp2/3 complex from binding to actin filaments, thereby suppressing actin polymerization. However, addition of α-actinin, another actin-bundling protein, did not suppress actin polymerization induced by the Arp2/3 complex and WASp-VCA at 5 μM concentration (Figure 5H).
α-Catenin Competes with Arp2/3 for Binding to Actin Filaments at High Concentrations
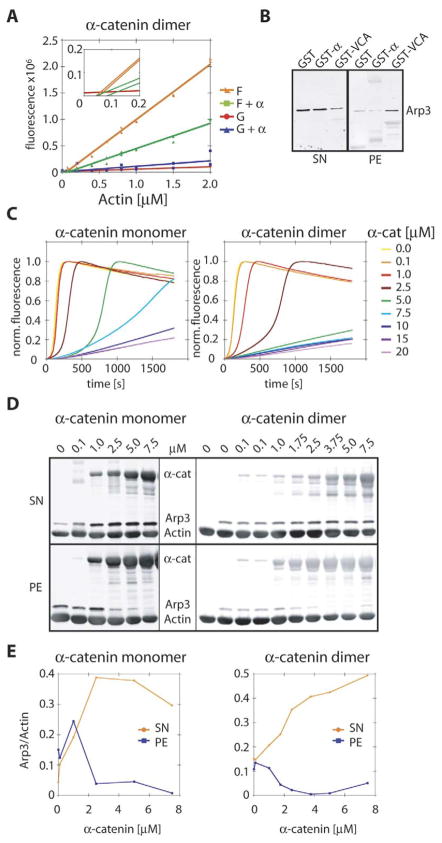
We sought to elucidate the molecular mechanism by which α-catenin suppresses Arp2/3-WASp-VCA-stimulated actin polymerization. We first tested whether α-catenin capped the growing barbed end of actin filaments or sequestered actin monomers by measuring the critical concentration for actin polymerization, which increases when the barbed end of actin filaments are capped. The critical concentration of actin in the presence and absence of 5 μM α-catenin was not significantly different (0.1 μM and 0.07 μM, respectively; Figure 6A). In the presence of α-catenin, the slope of the F-actin curve decreased, which is probably due to changes in pyrene fluorescence when α-catenin binds to actin filaments. The G-actin fluorescence curve was not significantly altered by the presence of α-catenin (Figure 6A), and G-actin does not bind to GST-α-catenin in pull-down experiments (data not shown). Finally, we asked whether suppression of actin polymerization in the presence of the Arp2/3 complex and WASp-VCA might be due to direct binding of α-catenin to, and sequestration of, the Arp2/3 complex. However, purified Arp2/3 complex did not bind to either α-catenin monomer or homodimer (Figure 6B). Thus, α-catenin does not appear to cap the growing barbed end of actin filaments or sequester either G-actin or the Arp2/3 complex.
Figure 6. α-Catenin Competes with Arp2/3 for Binding to Actin Filaments.
(A) Critical concentration of actin in the presence or absence of 5 μM α-catenin homodimer. Insert shows intersection of curves corresponding to the critical concentration. F = actin in polymerization buffer; G = actin in G buffer.
(B) Binding of Arp2/3 to GST-α-catenin or GST-WASp-VCA. Anti-Arp3 Western blot of bead binding assay of GST, GST-α-catenin (GST-α), and GST-WASp-VCA (GST-VCA) with purified Arp2/3 complex. SN = supernatant; PE = pellet.
(C) Concentration dependence of α-catenin monomer and homodimer on Arp2/3 and VCA-stimulated actin polymerization measured in a pyrene-actin assay. α-catenin at concentrations between 0.1 and 20 μM was added to 5 μM actin containing 10% pyrene actin, 50 nM Arp2/3 complex, and 50 nM WASp-VCA, and actin-filament assembly was monitored by sedimentation (see [D]).
(D) Western blot of F-actin pellet (PE) and supernatant (SN) of samples from polymerization assays in (C) after reaching equilibrium (t > 2 hr). The Western blot was probed with anti-α-catenin (anti-mouse λ680) and anti-Arp3 (anti-rabbit λ800), quantified, and reprobed with anti-actin (anti-mouse λ800) without stripping, causing all three antibody signals to be visible in the λ800 channel.
(E) Quantification of Western blot band intensities from (D). Arp3 intensities were normalized against actin intensity in each lane and plotted against the α-catenin concentration.
The suppression of Arp2/3-mediated actin polymerization by α-catenin was concentration dependent (Figure 6C) and correlated with α-catenin binding to actin filaments (Figures 6D and 6E). In the pyrene-actin polymerization assay, suppression began at 1 μM and 2.5 μM and reached full inhibition at 7.5 μM and 15 μM for α-catenin homodimer and monomer, respectively. At intermediate concentrations (1–2.5 μM for homodimer; 2.5–5 μM for monomer), α-catenin increased the lag phase of actin polymerization, but actin polymerization eventually proceeded rapidly. Although the concentration of α-catenin monomer and homodimer required for complete suppression appears to differ only by a factor of two, we have to take into account that we are unable to isolate α-catenin monomer without some contaminating homodimer. Therefore, the actual difference between the suppressive activities of α-catenin monomer and homodimer is likely to be more pronounced than that observed in these experiments. Addition of β-catenin reduced the inhibitory effect of α-catenin (Figure 5C); since the α-catenin-β-catenin complex does not bind to actin filaments (Yamada et al., 2005), the presence of β-catenin reduces the effective concentration of α-catenin that can bind actin. In an actin cose-dimentation assay, the amount of Arp2/3 complex bound to actin filaments decreased as the concentration of α-catenin increased (Figures 6D and 6E). Taken together, these results indicate that the suppressive effect of α-catenin on Arp2/3-mediated actin polymerization is likely due to direct competition with the Arp2/3 complex for binding to actin filaments.
DISCUSSION
Cell-cell contacts are considered to be stable structures that maintain the structural integrity of tissues and are thought to be formed by clustering of cell-adhesion proteins through binding between opposed extracellular domains and linkage through the cytoplasmic domain to the underlying actin cytoskeleton (Gumbiner, 2000; Jamora and Fuchs, 2002). The cadherin cytoplasmic domain binds with high affinity to β-catenin (Huber et al., 2001), which in turn binds with weaker affinity to α-catenin (Pokutta and Weis, 2000). Given that α-catenin binds to actin filaments (Pokutta et al., 2002; Rimm et al., 1995) and to other actin binding proteins such as vinculin and α-actinin (Hazan et al., 1997; Knudsen et al., 1995; Watabe-Uchida et al., 1998; Weiss et al., 1998), it was reasonable to assume that α-catenin bound to the cadherin-β-catenin complex also binds directly or indirectly to actin filaments. However, direct tests of this model failed. In the accompanying paper, we showed that α-catenin does not bind simultaneously to the cadherin-catenin complex and actin filaments (Yamada et al., 2005). These results predict that interactions between the cadherin-catenin complex and underlying actin cytoskeleton in cells might be very dynamic rather than being static as has been assumed.
Although direct interactions between the cadherin complex and actin filaments were not verified experimentally, there is a considerable body of work concluding that some sort of interaction of actin filaments and the cadherin-catenin complex is important in cell-cell adhesion. Cytochalasin D and latrunculin A, which change the dynamic organization of the actin cytoskeleton, reduce adhesion and weaken cell-cell contacts (Angres et al., 1996; Chu et al., 2004; Imamura et al., 1999; Matsuzaki et al., 1990). However, these drugs have global effects on actin organization that are not restricted to effects on only cell-cell contacts. Genetic deletion of α-catenin potentially provides a more direct approach to disrupt the putative cadherin-actin linkage. Cell-cell adhesion in α-catenin null cells is reduced and could be rescued by re-expression of α-catenin (Bullions et al., 1997; Hirano et al., 1992; van Hengel et al., 1997; Watabe et al., 1994; Watabe-Uchida et al., 1998). However, it is noted that cell-cell adhesion occurred in some α-catenin null cells (Maeno et al., 1999), presumably because there was sufficient cadherin on the cell surface to initiate cell-cell adhesion. α-catenin null cells have also been used to express chimeras between α-catenin-E-cadherin (Imamura et al., 1999; Nagafuchi et al., 1994; Ozawa and Kemler, 1998), α-catenin-vinculin (Ozawa and Kemler, 1998; Watabe-Uchida et al., 1998), and α-catenin-formin-1 (Kobielak et al., 2004), all of which partially rescue cell-cell adhesion. However, no direct evidence was presented in those studies that these chimeric proteins bound actin filaments. Moreover, our findings that the molecular and functional properties of α-catenin are altered upon binding to β-catenin—in particular, that the α-catenin/β-catenin complex cannot bind actin—demonstrate that the use of E-cadherin-α-catenin chimeras cannot recapitulate the behavior of the cadherin-catenin complex at the membrane. It is possible that expression of some of these chimeric proteins could locally change actin dynamics or simply increase the amount of cadherin at the cell surface to a level that can partially rescue cell-cell adhesion.
Although it is surprising that a stable linkage does not exist between cadherins and the underlying actin cytoskeleton, cell-cell adhesion is a dynamic process during embryonic development, wound healing, and cancer cell metastasis (Takeichi, 1995; Thiery, 2002). This may require a more dynamic interaction between cadherin and the actin cytoskeleton rather than the static, stable linkage proposed in previous models. In addition, it is noteworthy that, in most cell types, cadherins are not the only means of cell-cell adhesion. Many other adhesion proteins are expressed, including members of the nectin occludin/claudin, JAM, and desmosomal cadherin families (Gestsios et al., 2004; Takai and Nakanishi, 2003), all of which are thought to interact directly or indirectly with the actin or intermediate filament cytoskeletons and thereby contribute to cell-cell adhesion.
There are dramatic changes in membrane and actin dynamics associated with the formation of cell-cell adhesions. Initial cell-cell contact formation is driven by overlapping membrane lamellipodia from contacting cells. These lamellipodia are regulated by actin polymerization and branching induced by the Arp2/3 complex (Kovacs et al., 2002) and local activation of the Rho family of small GTPases (Braga, 2002; Fukata and Kaibuchi, 2001). However, lamellipodial activity decays as the cadherin-catenin complex accumulates and the contacts mature into stable cell-cell junctions (Ehrlich et al., 2002; Vaezi et al., 2002). It is not known what regulates this contact-dependent decrease of membrane activity, which presumably depends upon a decrease in Arp2/3-mediated actin polymerization. It is interesting to note, in this context, that decreased levels of α-catenin result in increased membrane activity in hippocampal neurons, while overexpression of α-catenin suppress membrane activity (Abe et al., 2004), indicating that α-catenin directly regulates membrane protrusive activity. Furthermore, keratinocytes from α-catenin knockout mice are characterized by loss of contact inhibition and increased migratory activity (Vasioukhin et al., 2001).
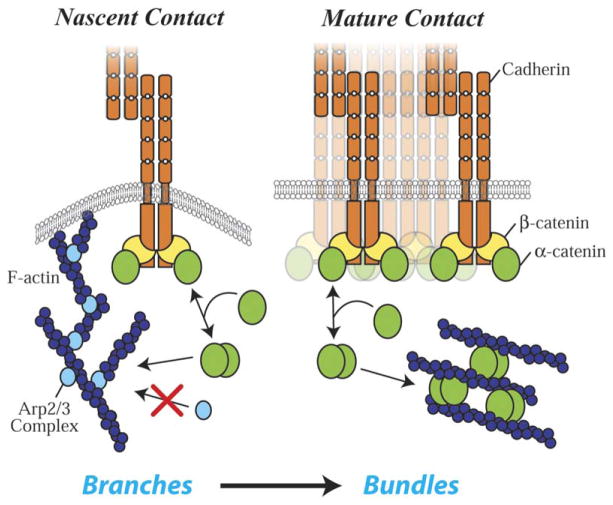
How might changes in both actin assembly and organization (from networks to bundles; see Hirokawa et al., 1983) that drive the transition from active lamellipodia to quiescent contacts (see Ehrlich et al., 2002) be coordinated during the formation of cell-cell adhesions? Our finding that α-catenin suppresses Arp2/3-mediated actin polymerization in a concentration-dependent manner, combined with the actin bundling activity of α-catenin (Rimm et al., 1995), may provide an explanation (Figure 7). The cytoplasmic α-catenin monomer concentration (0.6 μM) is too low to bind actin significantly and would need to be concentrated to bind actin and to dimerize. A significant increase in the local concentration of α-catenin at the membrane occurs upon accumulation of the cadherin-catenin complex at nascent contacts. This cadherin bound pool of α-catenin can exchange with the cytoplasmic pool (Figure 4); note that we probably underestimate the amount of exchange because we cannot directly measure it locally at cell-cell contacts. Although the local concentrations of α-catenin and Arp2/3 immediately adjacent to contacting membranes are unknown, a 10-fold increase in local concentration of α-catenin would be sufficient for α-catenin to compete with the Arp2/3 complex for actin filaments (Figure 7). This would suppress formation of branched actin networks and inhibit lamellipodial activity and would also favor formation of α-catenin homodimers that bundle actin filaments (Rimm et al., 1995), resulting in a reorganization of actin filaments and a change in membrane dynamics underneath the junction (Figure 7). It has also been proposed that formins, which promote formation of linear actin cables, are recruited to the adherens junction by α-catenin (Kobielak et al., 2004). If so, α-catenin would serve as a switch that turns off Arp2/3-mediated branched-actin-network formation required for lamellipodial activity during the initiation of adhesion and turn on α-catenin-mediated bundling of actin filaments and formation of linear cables by formins during maturation of the adherens junction. While further work is needed to test specific tenets of this hypothesis, our results provide new mechanistic insights into many aspects of the local dynamics of actin and membranes associated with cell-cell contacts not accounted for in previous models.
Figure 7. Model of α-Catenin Function in Regulating Actin Dynamics and Organization.
Initial cell-cell contact is mediated by interactions of cadherins present on the membranes of lamellipodia. Clustering of cadherins at the nascent contacts leads to accumulation of cadherin-catenin complexes. A high local concentration of α-catenin is produced when it dissociates from these complexes. α-catenin, which exists as monomer or homodimer, competes with Arp2/3 complex for actin filaments (the dimer more potently than the monomer), thereby suppressing Arp2/3-mediated actin assembly that drives lamellipodia. α-catenin also bundles actin filaments, which may contribute to the reorganization of actin in the mature contact.
EXPERIMENTAL PROCEDURES
Protein Expression and Purification
E-cadherin cytoplasmic domain, β-catenin, plakoglobin, α-catenin, and the β-catenin-α-catenin chimera were expressed and purified as described in the accompanying paper (Yamada et al., 2005).
Chromatography
Analytical size exclusion chromatography was done in 20 mM Tris (pH 8.0), 150 mM NaCl, and 1 mM DTT on a Superdex 200 column. Proteins were injected at a 25.6 μM concentration. For binding studies, proteins were mixed and incubated overnight at 4°C.
Multiangle Light Scattering
α-catenin monomer and βα-catenin were analyzed on a Superdex 200 column attached to a UV detector followed by a multiangle light scattering (MALS) DAWN EOS (Wyatt Technology, Santa Barbara) and a refractive index (RI) detector. The system was equilibrated with a buffer containing 20 mM Tris (pH 8.0), 150 mM NaCl, and 1 mM DTT, and the response of the light scattering detector was normalized by measuring the signal of monomeric bovine serum albumin (BSA). A value of 0.185 ml/g was assumed for the dn/dc of BSA and α-catenin. Scattering intensities measured at nine different angles (detectors 7–15) were used for data analysis. The molecular weight of the protein was calculated with the ASTRA IV program using the signal from the MALS and the RI detector.
Limited Proteolysis
Full-length α-catenin monomer and dimer and βα-catenin were incubated at 0.01 mg/ml trypsin (Sigma) at a protein concentration of 14.3 μM in 20 mM Tris (pH 8.0), 150 mM NaCl, and 1 mM DTT. After the indicated time, the reaction was stopped by addition of gel loading buffer and boiling for 4 min. Samples were analyzed by SDS-PAGE.
Microscopy and Image Analysis
Methods are described in the accompanying paper (Yamada et al., 2005).
Actin Pelleting, Pyrene Polymerization, and Critical-Concentration Assay
Actin was prepared from chicken pectoral muscle as described (Spudich and Watt, 1971) and was further purified by gel filtration. Pyrene-labeled actin was purchased from Cytoskeleton Inc. Arp2/3 complex and WASp-VCA were generous gifts from Dr. M.J. Footer (Stanford University). Actin polymerization rates were measured by the change of pyrene-actin fluorescence upon incorporation into actin filaments using a Fluorolog3 Spectrofluorometer (Jobin Yvon Horiba). The final concentration of actin, α-catenin monomer/dimer, α-catenin tail domain, β-catenin, α-actinin, and βα-catenin was 5 μM; the concentration of Arp 2/3 and VCA was 50 nM in the assays or as indicated. The critical concentration of actin polymerization was determined in F buffer (20 mM Tris [pH 8.0], 50 mM KCl, 2 mM MgCl2, 0.2 mM CaCl2, 0.5 mM DTT) or G buffer (5 mM Tris [pH 8.0], 0.2 mM CaCl2, 0.2 mM ATP, 0.5 mM DTT) as described (Mullins and Machesky, 2000). Samples from pyrene assays were collected, incubated for 2 hr at room temperature, and centrifuged at 435,000 × g (Beckman TL-100) for 7 min. Supernatant and pellet were analyzed by SDS-PAGE and Western blotting using mouse anti-α-catenin, rabbit anti-Arp3, and mouse anti-actin, and the resulting blots were scanned with the Odyssey infrared imaging system (LI-COR, Inc.).
Supplementary Material
Acknowledgments
We thank Drs. Susan Craig and Matt Footer for reagents, Sofiya Fridman and Jackie Benjamin for technical assistance, and Byron DeLaBarre for assistance with MALS analysis. This work was supported by National Institutes of Health grant R01 GM35527 (to W.J.N.), National Institutes of Health grant R01 GM56169 (to W.I.W.), and a predoctoral fellowship from Boehringer Ingelheim Fonds (to F.D.).
Footnotes
Supplemental Data include one figure and one movie and can be found with this article online at http://www.cell.com/cgi/content/full/123/5/903/DC1/.
References
- Abe K, Chisaka O, Van Roy F, Takeichi M. Stability of dendritic spines and synaptic contacts is controlled by α-N-catenin. Nat Neurosci. 2004;7:357–363. doi: 10.1038/nn1212. [DOI] [PubMed] [Google Scholar]
- Adams CL, Chen YT, Smith SJ, Nelson WJ. Mechanisms of epithelial cell-cell adhesion and cell compaction revealed by high-resolution tracking of E-cadherin-green fluorescent protein. J Cell Biol. 1998;142:1105–1119. doi: 10.1083/jcb.142.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angres B, Barth A, Nelson WJ. Mechanism for transition from initial to stable cell-cell adhesion: kinetic analysis of E-cadherin-mediated adhesion using a quantitative adhesion assay. J Cell Biol. 1996;134:549–557. doi: 10.1083/jcb.134.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass MD, Patel B, Barsukov IG, Fillingham IJ, Mason R, Smith BJ, Bagshaw CR, Critchley DR. Further characterization of the interaction between the cytoskeletal proteins talin and vinculin. Biochem J. 2002;362:761–768. doi: 10.1042/0264-6021:3620761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga VM. Cell-cell adhesion and signaling. Curr Opin Cell Biol. 2002;14:546–556. doi: 10.1016/s0955-0674(02)00373-3. [DOI] [PubMed] [Google Scholar]
- Bullions LC, Notterman DA, Chung LS, Levine AJ. Expression of wild-type α-catenin protein in cells with a mutant α-catenin gene restores both growth regulation and tumor suppressor activities. Mol Cell Biol. 1997;17:4501–4508. doi: 10.1128/mcb.17.8.4501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H-J, Huber AH, Weis WI. Thermodynamics of β-catenin-ligand interactions. The roles of the N- and C-terminal tails in modulating binding affinity. J Biol Chem. 2006 doi: 10.1074/jbc.M511338200. in press. [DOI] [PubMed] [Google Scholar]
- Chu YS, Thomas WA, Eder O, Pincet F, Perez E, Thiery JP, Dufour S. Force measurements in E-cadherin-mediated cell doublets reveal rapid adhesion strengthened by actin cytoskeleton remodeling through Rac and Cdc42. J Cell Biol. 2004;167:1183–1194. doi: 10.1083/jcb.200403043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich JS, Hansen MD, Nelson WJ. Spatio-temporal regulation of Rac1 localization and lamellipodia dynamics during epithelial cell-cell adhesion. Dev Cell. 2002;3:259–270. doi: 10.1016/s1534-5807(02)00216-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukata M, Kaibuchi K. Rho-family GTPases in cadherin-mediated cell-cell adhesion. Nat Rev Mol Cell Biol. 2001;2:887–897. doi: 10.1038/35103068. [DOI] [PubMed] [Google Scholar]
- Gestsios S, Huen AC, Green KJ. Working out the strength and flexibility of desmosomes. Nat Rev Mol Cell Biol. 2004;5:271–281. doi: 10.1038/nrm1356. [DOI] [PubMed] [Google Scholar]
- Gumbiner BM. Regulation of cadherin adhesive activity. J Cell Biol. 2000;148:399–403. doi: 10.1083/jcb.148.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazan RB, Kang L, Roe S, Borgen PI, Rimm DL. Vinculin is associated with the E-cadherin adhesion complex. J Biol Chem. 1997;272:32448–32453. doi: 10.1074/jbc.272.51.32448. [DOI] [PubMed] [Google Scholar]
- Hinck L, Nathke IS, Papkoff J, Nelson WJ. Dynamics of cadherin/catenin complex formation: novel protein interactions and pathways of complex assembly. J Cell Biol. 1994;125:1327–1340. doi: 10.1083/jcb.125.6.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano S, Kimoto N, Shimoyama Y, Hirohashi S, Takeichi M. Identification of a neural α-catenin as a key regulator of cadherin function and multicellular organization. Cell. 1992;70:293–301. doi: 10.1016/0092-8674(92)90103-j. [DOI] [PubMed] [Google Scholar]
- Hirokawa N, Keller TC, 3rd, Chasan R, Mooseker MS. Mechanism of brush border contractility studied by the quick-freeze, deep-etch method. J Cell Biol. 1983;96:1325–1336. doi: 10.1083/jcb.96.5.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber AH, Stewart DB, Laurents DV, Nelson WJ, Weis WI. The cadherin cytoplasmic domain is unstructured in the absence of β-catenin: a possible mechanism for regulating cadherin turnover. J Biol Chem. 2001;276:12301–12309. doi: 10.1074/jbc.M010377200. [DOI] [PubMed] [Google Scholar]
- Imamura Y, Itoh M, Maeno Y, Tsukita S, Nagafuchi A. Functional domains of α-catenin required for the strong state of cadherin-based cell adhesion. J Cell Biol. 1999;144:1311–1322. doi: 10.1083/jcb.144.6.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamora C, Fuchs E. Intercellular adhesion, signalling and the cytoskeleton. Nat Cell Biol. 2002;4:E101–E108. doi: 10.1038/ncb0402-e101. [DOI] [PubMed] [Google Scholar]
- Johnson RP, Craig SW. F-actin binding site masked by the intramolecular association of vinculin head and tail domains. Nature. 1995;373:261–264. doi: 10.1038/373261a0. [DOI] [PubMed] [Google Scholar]
- Keller R. Shaping the vertebrate body plan by polarized embryonic cell movements. Science. 2002;298:1950–1954. doi: 10.1126/science.1079478. [DOI] [PubMed] [Google Scholar]
- Knudsen KA, Soler AP, Johnson KR, Wheelock MJ. Interaction of α-actinin with the cadherin/catenin cell-cell adhesion complex via α-catenin. J Cell Biol. 1995;130:67–77. doi: 10.1083/jcb.130.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobielak A, Fuchs E. α-catenin: at the junction of intercellular adhesion and actin dynamics. Nat Rev Mol Cell Biol. 2004;5:614–625. doi: 10.1038/nrm1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobielak A, Pasolli HA, Fuchs E. Mammalian formin-1 participates in adherens junctions and polymerization of linear actin cables. Nat Cell Biol. 2004;6:21–30. doi: 10.1038/ncb1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koslov ER, Maupin P, Pradhan D, Morrow JS, Rimm DL. α-catenin can form asymmetric homodimeric complexes and/or heterodimeric complexes with β-catenin. J Biol Chem. 1997;272:27301–27306. doi: 10.1074/jbc.272.43.27301. [DOI] [PubMed] [Google Scholar]
- Kovacs EM, Goodwin M, Ali RG, Paterson AD, Yap AS. Cadherin-directed actin assembly: E-cadherin physically associates with the Arp2/3 complex to direct actin assembly in nascent adhesive contacts. Curr Biol. 2002;12:379–382. doi: 10.1016/s0960-9822(02)00661-9. [DOI] [PubMed] [Google Scholar]
- Le TL, Yap AS, Stow JL. Recycling of E-cadherin: a potential mechanism for regulating cadherin dynamics. J Cell Biol. 1999;146:219–232. [PMC free article] [PubMed] [Google Scholar]
- Lubarsky B, Krasnow MA. Making and shaping biological tubes. Cell. 2002;112:19–28. doi: 10.1016/s0092-8674(02)01283-7. [DOI] [PubMed] [Google Scholar]
- Maeno Y, Moroi S, Nagashima H, Noda T, Shiozaki H, Monden M, Tsukuta S, Nagafuchi A. α-catenin-deficient F9 cells differentiate into signet ring cells. Am J Pathol. 1999;154:1323–1328. doi: 10.1016/s0002-9440(10)65385-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki F, Mege RM, Jaffe SH, Friedlander DR, Gallin WJ, Goldberg JI, Cunningham BA, Edelman GM. cDNAs of cell adhesion molecules of different specificity induce changes in cell shape and border formation in cultured S180 cells. J Cell Biol. 1990;110:1239–1252. doi: 10.1083/jcb.110.4.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins RD, Machesky LM. Actin assembly mediated by Arp2/3 complex and WASP family proteins. Methods Enzymol. 2000;325:214–237. doi: 10.1016/s0076-6879(00)25445-1. [DOI] [PubMed] [Google Scholar]
- Nagafuchi A, Takeichi M. Cell binding function of E-cadherin is regulated by the cytoplasmic domain. EMBO J. 1988;7:3679–3684. doi: 10.1002/j.1460-2075.1988.tb03249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagafuchi A, Ishihara S, Tsukita S. The roles of catenins in the cadherin-mediated cell adhesion: functional analysis of E-cadherin-α-catenin fusion molecules. J Cell Biol. 1994;127:235–245. doi: 10.1083/jcb.127.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Näthke IS, Hinck L, Swedlow JR, Papkoff J, Nelson WJ. Defining interactions and distributions of cadherin and catenin complexes in polarized epithelial cells. J Cell Biol. 1994;125:1341–1352. doi: 10.1083/jcb.125.6.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omelchenko T, Fetisova E, Ivanonva O, Bonder EM, Feber H, Vasiliev JM, Gelfand IM. Contact interactions between epitheliocytes and fibroblasts: formation of heterotypic cadherin-containing adhesion sites is accompanied by local cytoskeletal reorganization. Proc Natl Acad Sci USA. 2001;98:8632–8637. doi: 10.1073/pnas.151247698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa M, Kemler R. Altered cell adhesion activity by pervanadate due to the dissociation of a-catenin from the E-cadherin catenin complex. J Biol Chem. 1998;273:6166–6170. doi: 10.1074/jbc.273.11.6166. [DOI] [PubMed] [Google Scholar]
- Ozawa M, Baribault H, Kemler R. The cytoplasmic domain of the cell adhesion molecule uvomorulin associates with three independent proteins structurally related in different species. EMBO J. 1989;8:1711–1717. doi: 10.1002/j.1460-2075.1989.tb03563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokutta S, Weis WI. Structure of the dimerization and β-catenin binding region of α-catenin. Mol Cell. 2000;5:533–543. doi: 10.1016/s1097-2765(00)80447-5. [DOI] [PubMed] [Google Scholar]
- Pokutta S, Drees F, Takai Y, Nelson WJ, Weis WI. Biochemical and structural definition of the l-afadin- and actin-binding sites of α-catenin. J Biol Chem. 2002;277:18868–18874. doi: 10.1074/jbc.M201463200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard TD, Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 2003;112:453–465. doi: 10.1016/s0092-8674(03)00120-x. [DOI] [PubMed] [Google Scholar]
- Prehoda KE, Scott JA, Mullins RD, Lim WA. Integration of multiple signals through cooperative regulation of the N-WASP-Arp2/3 complex. Science. 2000;290:801–806. doi: 10.1126/science.290.5492.801. [DOI] [PubMed] [Google Scholar]
- Rimm DL, Koslov ER, Kebriaei P, Cianci CD, Morrow JS. α1(E)-catenin is an actin-binding and -bundling protein mediating the attachment of F-actin to the membrane adhesion complex. Proc Natl Acad Sci USA. 1995;92:8813–8817. doi: 10.1073/pnas.92.19.8813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore EM, Nelson WJ. Biosynthesis of the cell adhesion molecule uvomorulin (E-cadherin) in Madin-Darby canine kidney epithelial cells. J Biol Chem. 1991;266:19672–19680. [PubMed] [Google Scholar]
- Spudich JA, Watt S. The regulation of rabbit skeletal muscle contraction. I Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J Biol Chem. 1971;246:4866–4871. [PubMed] [Google Scholar]
- Takai Y, Nakanishi H. Nectin and afadin: novel organizers of intercellular junctions. J Cell Sci. 2003;116:17–27. doi: 10.1242/jcs.00167. [DOI] [PubMed] [Google Scholar]
- Takeichi M. Morphogenetic roles of classic cadherins. Curr Opin Cell Biol. 1995;7:619–627. doi: 10.1016/0955-0674(95)80102-2. [DOI] [PubMed] [Google Scholar]
- Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- Vaezi A, Bauer C, Vasioukhin V, Fuchs E. Actin cable dynamics and Rho/Rock orchestrate a polarized cytoskeletal architecture in the early steps of assembling a stratified epithelium. Dev Cell. 2002;3:367–381. doi: 10.1016/s1534-5807(02)00259-9. [DOI] [PubMed] [Google Scholar]
- van Hengel J, Gohon L, Bruyneel E, Vermeulen S, Cornelissen M, Mareel M, von Roy F. Protein kinase C activation upregulates intercellular adhesion of alpha-catenin-negative human colon cancer cell variants via induction of desmosomes. J Cell Biol. 1997;137:1103–1116. doi: 10.1083/jcb.137.5.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasioukhin V, Bauer C, Begenstein L, Wise B, Fuchs E. Hyperproliferation and defects in epithelial polarity upon conditional ablation of α-catenin in skin. Cell. 2001;104:605–617. doi: 10.1016/s0092-8674(01)00246-x. [DOI] [PubMed] [Google Scholar]
- Watabe M, Nagafuchi A, Tsukita S, Takeichi M. Induction of polarized cell-cell association and retardation of growth by activation of the E-cadherin–catenin adhesion system in a dispersed carcinoma line. J Cell Biol. 1994;127:247–256. doi: 10.1083/jcb.127.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watabe-Uchida M, Uchida N, Imamura Y, Nagafuchi A, Fujimoto K, Uemura T, Vermeulen S, van Roy F, Adamson ED, Takeichi M. α-catenin-vinculin interaction functions to organize the apical junctional complex in epithelial cells. J Cell Biol. 1998;142:847–857. doi: 10.1083/jcb.142.3.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss EE, Kroemker M, Rüdiger AH, Jockusch BM, Rüdiger M. Vinculin is part of the cadherin-catenin junctional complex: complex formation between α-catenin and vinculin. J Cell Biol. 1998;141:755–764. doi: 10.1083/jcb.141.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada S, Pokutta S, Drees F, Weis WI, Nelson WJ. Deconstructing the cadherin-catenin-actin complex. Cell. 2005;123(this issue):889–901. doi: 10.1016/j.cell.2005.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.