Abstract
Genetic variants that modify brain gene expression may also influence risk for human diseases. We measured expression levels of 24,526 transcripts in brain samples from the cerebellum and temporal cortex of autopsied subjects with Alzheimer's disease (AD, cerebellar n = 197, temporal cortex n = 202) and with other brain pathologies (non–AD, cerebellar n = 177, temporal cortex n = 197). We conducted an expression genome-wide association study (eGWAS) using 213,528 cisSNPs within ±100 kb of the tested transcripts. We identified 2,980 cerebellar cisSNP/transcript level associations (2,596 unique cisSNPs) significant in both ADs and non–ADs (q<0.05, p = 7.70×10−5–1.67×10−82). Of these, 2,089 were also significant in the temporal cortex (p = 1.85×10−5–1.70×10−141). The top cerebellar cisSNPs had 2.4-fold enrichment for human disease-associated variants (p<10−6). We identified novel cisSNP/transcript associations for human disease-associated variants, including progressive supranuclear palsy SLCO1A2/rs11568563, Parkinson's disease (PD) MMRN1/rs6532197, Paget's disease OPTN/rs1561570; and we confirmed others, including PD MAPT/rs242557, systemic lupus erythematosus and ulcerative colitis IRF5/rs4728142, and type 1 diabetes mellitus RPS26/rs1701704. In our eGWAS, there was 2.9–3.3 fold enrichment (p<10−6) of significant cisSNPs with suggestive AD–risk association (p<10−3) in the Alzheimer's Disease Genetics Consortium GWAS. These results demonstrate the significant contributions of genetic factors to human brain gene expression, which are reliably detected across different brain regions and pathologies. The significant enrichment of brain cisSNPs among disease-associated variants advocates gene expression changes as a mechanism for many central nervous system (CNS) and non–CNS diseases. Combined assessment of expression and disease GWAS may provide complementary information in discovery of human disease variants with functional implications. Our findings have implications for the design and interpretation of eGWAS in general and the use of brain expression quantitative trait loci in the study of human disease genetics.
Author Summary
Genetic variants that regulate gene expression levels can also influence human disease risk. Discovery of genomic loci that alter brain gene expression levels (brain expression quantitative trait loci = eQTLs) can be instrumental in the identification of genetic risk underlying both central nervous system (CNS) and non–CNS diseases. To systematically assess the role of brain eQTLs in human disease and to evaluate the influence of brain region and pathology in eQTL mapping, we performed an expression genome-wide association study (eGWAS) in 773 brain samples from the cerebellum and temporal cortex of ∼200 autopsied subjects with Alzheimer's disease (AD) and ∼200 with other brain pathologies (non–AD). We identified ∼3,000 significant associations between cisSNPs near ∼700 genes and their cerebellar transcript levels, which replicate in ADs and non–ADs. More than 2,000 of these associations were reproducible in the temporal cortex. The top cisSNPs are enriched for both CNS and non–CNS disease-associated variants. We identified novel and confirmed previous cisSNP/transcript associations for many disease loci, suggesting gene expression regulation as their mechanism of action. These findings demonstrate the reproducibility of the eQTL approach across different brain regions and pathologies, and advocate the combined use of gene expression and disease GWAS for identification and functional characterization of human disease-associated variants.
Introduction
Expression quantitative trait loci (eQTL) are genomic loci that influence levels of gene transcripts and can be mapped by genetic linkage in families or eGWAS in unrelated populations [1]. eQTLs are distinct from other complex trait loci, because they directly identify the target gene, since the transcript trait is a reflection of the mRNA level from a single gene. Furthermore, eQTLs imply regulation of gene expression as the mechanism of action for the underlying variants. Recently, few studies identified an enrichment of eQTLs from lymphocytes [2] and lymphoblasts [3] amongst human complex disease and trait GWAS loci, suggesting that eQTLs may be useful in mapping human disease-associated variants.
Most human eQTL mapping studies to date assessed immortalized lymphoblastoid cell lines [4], [5], [6], [7], [8], [9], [10], [11], [12] and family-based samples from the CEPH [4], [5], [6], [7], [8], [13] (Centre d'Etude du polymorphisme humain) or HapMap [10], [11], [14], [15] repositories. Multiple other small and large scale eQTL studies investigated other tissues and populations including lymphocytes [16], monocytes [17], T-cells [18], fibroblasts [18], skin [19], subcutaneous and omental adipose tissue [20], [21], bone [22], liver [23] and brain [24], [25].
Despite the assumption that brain eQTLs would also influence human diseases and traits, there are no systematic gene mapping studies for human diseases that utilize brain gene expression phenotypes. Furthermore, the brain region most relevant for such studies and the influence of brain pathology on eQTL mapping studies are largely unknown. To address these issues, we performed an eQTL using cerebellar tissue from 197 subjects with Alzheimer's disease (AD) neuropathology and 177 with other pathologies (non–AD). We validated the results in a different brain region using temporal cortex samples from 202 ADs and 197 non–ADs (Supplementary Tables 1 and 2 in Dataset S1), 85% of whom overlapped with the cerebellar group. We evaluated significant cisSNPs from our study for association with human diseases/traits using a GWAS catalog [26]. We also assessed our significant eGWAS cisSNPs for association with two central nervous system (CNS) diseases, progressive supranuclear palsy (PSP) [27] and AD risk [28], using two recent GWAS for these diseases.
Our results demonstrate the power of the brain eQTL approach in the identification and characterization of many human CNS and non–CNS disease-associated variants. This study also highlights the remarkable reproducibility of human eQTLs across different brain regions and pathologies, which has implications for the design of eGWAS in general. Combined assessment of eQTLs and disease risk loci can be instrumental in mapping disease genes with regulatory variants.
Results
Brain eGWAS
Levels of 24,526 transcripts for 18,401 genes were measured in 773 brain samples from the cerebellum and temporal cortex of ∼200 ADs and ∼200 non–ADs, using WG-DASL assays. Nearly 70% of all probes could be detected in >75% of the samples tested. All autopsied subjects were genotyped for 313,330 single nucleotide polymorphisms (SNPs) from Illumina HumanHap300-Duo Genotyping BeadChips, as part of the Mayo AD GWAS [29]. An eGWAS testing association of transcript levels with cisSNPs was performed using multivariable linear regression correcting for APOE ε4 dosage, age at death, gender and multiple technical variables. False discovery rate (FDR)-based q values [30] (q) were used for corrections of multiple testing.
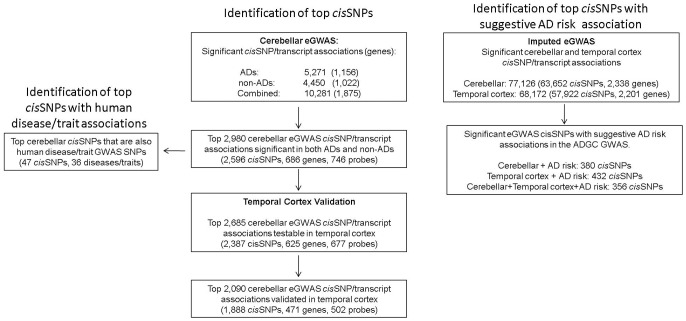
To achieve internal replication, we first analyzed the ADs and non–ADs separately. In our cerebellar eGWAS, at q<0.05, there were 5,271 significant cisSNP/transcript associations (1,156 unique genes) in the AD, 4,450 (1,022 unique genes) in the non–AD and 10,281 (1,875 unique genes) in the combined datasets. Q-Q plots suggested a clear excess of significant results (Figure 1, Figure S1a–S1d). 2,980 cisSNP/transcript associations (2,596 unique cisSNPs, 686 unique genes) were significant at q<0.05 in both ADs and non–ADs (Table 1, Supplementary Table 3 in Dataset S1, Figure S2). The direction and magnitude of associations in both groups demonstrate remarkable similarities (Pearson's correlation coefficient = 0.98, p<0.0001). The box plots depicted for some of these top associations (Figure S3a–S3c) demonstrate this replication in ADs and non–ADs. Most associations have an additive or dominant pattern with respect to the minor allele.
Figure 1. Summary of brain eGWAS and human disease associations.
Table 1. Examples of top cerebellar eGWAS cisSNP/transcript associations.
| NON–AD | AD | ALL | |||||||||||||
| CHR | SNP | PROBE | SYMBOL | P | PBonf | Q | Beta | P | PBonf | Q | Beta | P | PBonf | Q | Beta |
| 11 | rs11552421 | ILMN_1651745 | TMEM25 | 1.44.E-68 | 6.37.E-63 | 1.14.E-58 | 2.69 | 1.67.E-82 | 7.41.E-77 | 7.26.E-71 | 2.65 | 3.50.E-153 | 1.55.E-147 | 3.10.E-138 | 2.67 |
| 5 | rs3776455 | ILMN_1718932 | MTRR | 6.70.E-64 | 2.97.E-58 | 1.90.E-54 | 1.30 | 4.06.E-70 | 1.80.E-64 | 7.41.E-60 | 1.12 | 6.37.E-133 | 2.83.E-127 | 7.34.E-120 | 1.20 |
| 6 | rs1096699 | ILMN_1694711 | MAD2L1BP | 6.09.E-56 | 2.70.E-50 | 4.86.E-47 | 1.31 | 6.54.E-60 | 2.90.E-54 | 5.35.E-50 | 1.22 | 1.41.E-116 | 6.25.E-111 | 5.44.E-104 | 1.26 |
| 12 | rs10843881 | ILMN_2345908 | DDX11 | 7.87.E-54 | 3.49.E-48 | 3.15.E-45 | -1.06 | 5.59.E-58 | 2.48.E-52 | 9.92.E-49 | −1.06 | 1.48.E-112 | 6.57.E-107 | 2.39.E-100 | −1.06 |
| 12 | rs2708389 | ILMN_1730477 | TAS2R43 | 9.30.E-59 | 4.12.E-53 | 1.29.E-49 | 1.13 | 5.26.E-53 | 2.34.E-47 | 2.23.E-44 | 1.01 | 2.80.E-112 | 1.24.E-106 | 2.39.E-100 | 1.07 |
| 12 | rs1971762 | ILMN_1807798 | ATP5G2 | 1.52.E-44 | 6.73.E-39 | 4.49.E-37 | 0.96 | 4.76.E-59 | 2.11.E-53 | 2.11.E-49 | 1.04 | 1.10.E-104 | 4.87.E-99 | 1.64.E-93 | 1.01 |
| 21 | rs2838859 | ILMN_2376667 | POFUT2 | 1.19.E-48 | 5.28.E-43 | 8.06.E-41 | −0.81 | 1.17.E-53 | 5.20.E-48 | 6.24.E-45 | −0.89 | 2.86.E-103 | 1.27.E-97 | 2.73.E-92 | −0.86 |
| 8 | rs3802266 | ILMN_2184966 | ZHX2 | 8.18.E-44 | 3.63.E-38 | 2.17.E-36 | −1.89 | 2.13.E-56 | 9.47.E-51 | 2.64.E-47 | −1.99 | 4.35.E-101 | 1.93.E-95 | 3.06.E-90 | −1.94 |
| 19 | rs260462 | ILMN_2052079 | ZNF544 | 7.19.E-54 | 3.19.E-48 | 3.15.E-45 | −1.21 | 1.75.E-47 | 7.79.E-42 | 2.60.E-39 | −1.12 | 8.26.E-101 | 3.67.E-95 | 4.38.E-90 | −1.16 |
| 17 | rs2525574 | ILMN_2369018 | EVI2A | 2.04.E-48 | 9.05.E-43 | 1.08.E-40 | 1.81 | 8.82.E-49 | 3.92.E-43 | 1.61.E-40 | 1.79 | 1.52.E-99 | 6.73.E-94 | 4.88.E-89 | 1.81 |
| 7 | rs6955367 | ILMN_1723984 | PILRB | 1.58.E-52 | 6.99.E-47 | 4.02.E-44 | 1.21 | 8.75.E-49 | 3.89.E-43 | 1.66.E-40 | 0.99 | 4.80.E-97 | 2.13.E-91 | 1.23.E-86 | 1.08 |
| 7 | rs7313 | ILMN_2400759 | CPVL | 2.40.E-42 | 1.06.E-36 | 5.15.E-35 | −0.88 | 2.13.E-49 | 9.48.E-44 | 5.07.E-41 | −1.01 | 5.00.E-92 | 2.22.E-86 | 5.96.E-82 | −0.96 |
| 11 | rs7124057 | ILMN_2262288 | EEF1G | 6.28.E-50 | 2.78.E-44 | 6.10.E-42 | −1.03 | 1.54.E-41 | 6.83.E-36 | 4.95.E-34 | −0.74 | 2.64.E-91 | 1.17.E-85 | 2.67.E-81 | −0.90 |
| 10 | rs2182513 | ILMN_1689177 | PPAPDC1A | 4.60.E-35 | 2.04.E-29 | 2.09.E-28 | 0.87 | 1.25.E-52 | 5.55.E-47 | 4.30.E-44 | 0.82 | 1.08.E-87 | 4.79.E-82 | 6.93.E-78 | 0.84 |
| 6 | rs2975033 | ILMN_2130441 | HLA-H | 8.19.E-42 | 3.63.E-36 | 1.60.E-34 | 1.53 | 8.13.E-42 | 3.61.E-36 | 2.83.E-34 | 1.39 | 3.53.E-84 | 1.57.E-78 | 1.73.E-74 | 1.45 |
| 5 | rs11738432 | ILMN_2093720 | THG1L | 5.52.E-45 | 2.45.E-39 | 1.84.E-37 | −0.94 | 3.78.E-39 | 1.68.E-33 | 9.18.E-32 | −0.81 | 8.06.E-84 | 3.58.E-78 | 3.49.E-74 | −0.86 |
| 15 | rs12912744 | ILMN_1691772 | ZSCAN29 | 1.25.E-35 | 5.53.E-30 | 7.32.E-29 | −0.52 | 4.47.E-47 | 1.99.E-41 | 5.78.E-39 | −0.59 | 3.47.E-83 | 1.54.E-77 | 1.33.E-73 | −0.56 |
| 22 | rs136564 | ILMN_1809147 | FAM118A | 1.79.E-35 | 7.93.E-30 | 8.98.E-29 | 0.88 | 1.58.E-44 | 7.02.E-39 | 7.45.E-37 | 0.82 | 8.44.E-81 | 3.74.E-75 | 2.57.E-71 | 0.85 |
| 17 | rs8070454 | ILMN_2388272 | MED24 | 1.77.E-33 | 7.82.E-28 | 5.89.E-27 | −1.98 | 7.10.E-46 | 3.15.E-40 | 7.11.E-38 | −2.05 | 3.51.E-80 | 1.56.E-74 | 9.58.E-71 | −2.03 |
| 20 | rs2387976 | ILMN_2064132 | NANP | 3.88.E-38 | 1.72.E-32 | 3.69.E-31 | −0.57 | 1.42.E-40 | 6.29.E-35 | 3.68.E-33 | −0.51 | 9.58.E-79 | 4.25.E-73 | 1.93.E-69 | −0.54 |
| 7 | rs10239340 | ILMN_2312606 | IRF5 | 5.84.E-33 | 2.59.E-27 | 1.66.E-26 | 0.81 | 7.47.E-44 | 3.32.E-38 | 3.05.E-36 | 0.82 | 1.52.E-76 | 6.73.E-71 | 2.31.E-67 | 0.81 |
| 6 | rs2395943 | ILMN_1683279 | PEX6 | 7.06.E-35 | 3.13.E-29 | 2.93.E-28 | −0.39 | 1.90.E-41 | 8.43.E-36 | 5.68.E-34 | −0.40 | 3.63.E-76 | 1.61.E-70 | 5.06.E-67 | −0.39 |
| 13 | rs1046028 | ILMN_2390162 | PHF11 | 1.08.E-34 | 4.79.E-29 | 4.28.E-28 | 0.37 | 1.26.E-40 | 5.61.E-35 | 3.52.E-33 | 0.33 | 1.07.E-75 | 4.73.E-70 | 1.36.E-66 | 0.35 |
| 17 | rs1981997 | ILMN_1710903 | MAPT | 1.89.E-37 | 8.38.E-32 | 1.48.E-30 | −0.51 | 2.55.E-33 | 1.13.E-27 | 1.20.E-26 | −0.44 | 4.16.E-71 | 1.85.E-65 | 3.88.E-62 | −0.48 |
| 9 | rs2240913 | ILMN_1811048 | GPR107 | 4.16.E-34 | 1.84.E-28 | 1.51.E-27 | 0.62 | 3.74.E-36 | 1.66.E-30 | 4.81.E-29 | 0.69 | 2.76.E-69 | 1.23.E-63 | 2.39.E-60 | 0.66 |
| 16 | rs11649236 | ILMN_2201966 | N4BP1 | 1.99.E-39 | 8.82.E-34 | 2.59.E-32 | −1.70 | 2.71.E-32 | 1.20.E-26 | 1.10.E-25 | −1.48 | 5.69.E-69 | 2.53.E-63 | 4.37.E-60 | −1.57 |
| 10 | rs7909832 | ILMN_1795336 | PTER | 3.28.E-33 | 1.45.E-27 | 1.01.E-26 | −0.70 | 6.43.E-35 | 2.86.E-29 | 5.23.E-28 | −0.81 | 1.70.E-68 | 7.54.E-63 | 1.04.E-59 | −0.75 |
| 5 | rs11134054 | ILMN_1789419 | EXOC3 | 1.45.E-35 | 6.43.E-30 | 8.07.E-29 | −0.54 | 1.07.E-33 | 4.77.E-28 | 5.40.E-27 | −0.59 | 4.40.E-68 | 1.95.E-62 | 2.52.E-59 | −0.57 |
| 19 | rs10405576 | ILMN_2074477 | GPR4 | 2.49.E-32 | 1.10.E-26 | 6.57.E-26 | −1.35 | 4.86.E-34 | 2.16.E-28 | 3.04.E-27 | −1.27 | 5.76.E-68 | 2.56.E-62 | 3.09.E-59 | −1.32 |
| 6 | rs198834 | ILMN_2075334 | HIST1H4C | 1.98.E-29 | 8.78.E-24 | 2.23.E-23 | −0.42 | 8.82.E-38 | 3.92.E-32 | 1.60.E-30 | −0.44 | 4.76.E-67 | 2.11.E-61 | 2.05.E-58 | −0.43 |
| 21 | rs6517526 | ILMN_2296011 | BRWD1 | 3.00.E-30 | 1.33.E-24 | 4.50.E-24 | −0.71 | 6.10.E-37 | 2.71.E-31 | 9.69.E-30 | −0.88 | 6.31.E-67 | 2.80.E-61 | 2.51.E-58 | −0.80 |
| 22 | rs737950 | ILMN_1697286 | SF3A1 | 2.77.E-30 | 1.23.E-24 | 4.36.E-24 | 0.42 | 3.78.E-34 | 1.68.E-28 | 2.52.E-27 | 0.45 | 2.05.E-66 | 9.11.E-61 | 7.72.E-58 | 0.44 |
| 1 | rs1763601 | ILMN_2364072 | CLCNKA | 1.06.E-36 | 4.69.E-31 | 7.35.E-30 | 1.30 | 8.25.E-31 | 3.66.E-25 | 1.99.E-24 | 1.14 | 3.49.E-66 | 1.55.E-60 | 1.24.E-57 | 1.22 |
| 12 | rs10878255 | ILMN_2183938 | LEMD3 | 1.25.E-29 | 5.54.E-24 | 1.55.E-23 | −0.68 | 9.20.E-36 | 4.09.E-30 | 9.31.E-29 | −0.56 | 2.61.E-65 | 1.16.E-59 | 7.91.E-57 | −0.62 |
| 19 | rs4806187 | ILMN_1655637 | UPK1A | 6.89.E-27 | 3.05.E-21 | 4.54.E-21 | −0.99 | 5.73.E-38 | 2.55.E-32 | 1.15.E-30 | −1.05 | 2.82.E-65 | 1.25.E-59 | 8.11.E-57 | −1.04 |
| 11 | rs2848630 | ILMN_1765332 | TIMM10 | 4.66.E-36 | 2.07.E-30 | 3.05.E-29 | −0.62 | 1.18.E-28 | 5.23.E-23 | 1.84.E-22 | −0.42 | 3.40.E-65 | 1.51.E-59 | 9.32.E-57 | −0.53 |
| 17 | rs962800 | ILMN_2401641 | ALDH3A2 | 7.84.E-28 | 3.48.E-22 | 6.56.E-22 | 0.44 | 4.61.E-38 | 2.05.E-32 | 9.84.E-31 | 0.49 | 3.31.E-64 | 1.47.E-58 | 8.21.E-56 | 0.46 |
There are 2,980 cerebellar cisSNP/transcript associations with q<0.05 both in the ADs and non–ADs. Some of these top associations are shown. Only one cisSNP/transcript pair is selected for depiction. The chromosome (CHR), SNP, Probe, Gene Symbol (SYMBOL) of these associations are depicted. The uncorrected (P), Bonferroni-corrected (PBonf) P, q values, and Beta coefficients of association are shown for the Non–ADs, ADs and combined (All) analyses. Regression coefficients are based on the SNP minor allele using an additive model.
To assess the genetic component contributing to gene expression variability, we estimated intraclass correlation coefficients (ICC) [31] in the 15 samples measured in replicate on 5–6 different plates and 2–3 different days. Between-subject variance accounted for a median of 60% of total probe expression variance (Supplementary Table 4 in Dataset S1; Figure S4). The 746 probes for the top 2,980 cerebellar cisSNP associations had higher between-subject variance (median = 78%).
Using multivariable linear regression, we next estimated the percent variation in cerebellar probe expression levels due to the “best” cisSNP for each transcript after accounting for technical and biological covariates. We found that the “best” cisSNP explained a median of ∼3% of the expression variation. For the top 746 probes, the “best” cisSNPs accounted for a median of 18% of the expression variance (Table 2, Supplementary Table 5 in Dataset S1).
Table 2. Variance of cerebellar probe expression levels due to technical, biological, and cisSNP effects.
| Probe | Symbol | Raw_Variance | R2technical | addR2covariates | adjR2covariates | addR2best-SNP | adjR2best-SNP | Best-cis-SNP |
| ILMN_1651745 | TMEM25 | 1.894 | 0.08 | 0.004 | 0.005 | 0.784 | 0.852 | rs11552421 |
| ILMN_1718932 | MTRR | 0.864 | 0.05 | 0.004 | 0.005 | 0.768 | 0.809 | rs3776455 |
| ILMN_1694711 | MAD2L1BP | 1.169 | 0.154 | 0.007 | 0.008 | 0.642 | 0.763 | rs1096699 |
| ILMN_1730477 | TAS2R43 | 1.212 | 0.372 | 0.001 | 0.002 | 0.477 | 0.76 | rs2708389 |
| ILMN_2345908 | DDX11 | 0.798 | 0.029 | 0.003 | 0.003 | 0.732 | 0.755 | rs4031375 |
| ILMN_1807798 | ATP5G2 | 0.722 | 0.156 | 0.009 | 0.011 | 0.61 | 0.722 | rs1971762 |
| ILMN_2052079 | ZNF544 | 0.983 | 0.055 | 0.003 | 0.003 | 0.675 | 0.714 | rs260462 |
| ILMN_2369018 | EVI2A | 2.208 | 0.043 | 0.005 | 0.005 | 0.68 | 0.711 | rs2525574 |
| ILMN_2184966 | ZHX2 | 2.816 | 0.11 | 0.012 | 0.013 | 0.629 | 0.706 | rs3802266 |
| ILMN_2376667 | POFUT2 | 0.503 | 0.076 | 0.032 | 0.035 | 0.649 | 0.703 | rs2838859 |
| ILMN_1723984 | PILRB | 0.671 | 0.253 | 0.006 | 0.008 | 0.519 | 0.695 | rs6955367 |
| ILMN_2400759 | CPVL | 0.644 | 0.062 | 0.013 | 0.014 | 0.631 | 0.672 | rs7313 |
| ILMN_2262288 | EEF1G | 0.615 | 0.107 | 0.025 | 0.028 | 0.592 | 0.663 | rs7124057 |
| ILMN_1689177 | PPAPDC1A | 0.519 | 0.138 | 0.006 | 0.007 | 0.57 | 0.661 | rs2182513 |
| ILMN_2093720 | THG1L | 0.621 | 0.208 | 0.003 | 0.004 | 0.512 | 0.647 | rs11738432 |
| ILMN_1691772 | ZSCAN29 | 0.19 | 0.14 | 0.011 | 0.013 | 0.548 | 0.638 | rs12912744 |
| ILMN_1809147 | FAM118A | 0.282 | 0.07 | 0.002 | 0.002 | 0.589 | 0.634 | rs104664 |
| ILMN_2130441 | HLA-H | 1.386 | 0.092 | 0.033 | 0.037 | 0.573 | 0.628 | rs2975033 |
| ILMN_2388272 | MED24 | 3.036 | 0.043 | 0.007 | 0.007 | 0.597 | 0.624 | rs8070454 |
| ILMN_2064132 | NANP | 0.283 | 0.194 | 0.005 | 0.006 | 0.502 | 0.62 | rs2387976 |
| ILMN_1683279 | PEX6 | 0.164 | 0.245 | 0.006 | 0.008 | 0.46 | 0.609 | rs2395943 |
| ILMN_2312606 | IRF5 | 0.662 | 0.181 | 0.005 | 0.006 | 0.496 | 0.606 | rs10239340 |
| ILMN_2390162 | PHF11 | 0.117 | 0.165 | 0.015 | 0.018 | 0.501 | 0.601 | rs1046028 |
| ILMN_1710903 | MAPT | 0.118 | 0.052 | 0.01 | 0.011 | 0.552 | 0.584 | rs1981997 |
| ILMN_1811048 | GPR107 | 0.519 | 0.354 | 0.002 | 0.004 | 0.374 | 0.58 | rs2240913 |
| ILMN_2201966 | N4BP1 | 1.97 | 0.029 | 0.005 | 0.006 | 0.555 | 0.572 | rs11649236 |
| ILMN_1795336 | PTER | 0.591 | 0.143 | 0.012 | 0.014 | 0.486 | 0.567 | rs7909832 |
| ILMN_1789419 | EXOC3 | 0.214 | 0.097 | 0.012 | 0.014 | 0.508 | 0.563 | rs11134054 |
| ILMN_2364072 | CLCNKA | 1.356 | 0.044 | 0.009 | 0.01 | 0.535 | 0.562 | rs1763601 |
| ILMN_2075334 | HIST1H4C | 0.213 | 0.296 | 0.007 | 0.01 | 0.395 | 0.561 | rs198834 |
| ILMN_2296011 | BRWD1 | 0.821 | 0.413 | 0.005 | 0.009 | 0.328 | 0.559 | rs6517526 |
| ILMN_2074477 | GPR4 | 1.342 | 0.058 | 0.016 | 0.017 | 0.526 | 0.558 | rs10405576 |
| ILMN_1697286 | SF3A1 | 0.168 | 0.359 | 0.009 | 0.014 | 0.356 | 0.555 | rs737950 |
| ILMN_1765332 | TIMM10 | 0.164 | 0.079 | 0.005 | 0.006 | 0.507 | 0.551 | rs2848630 |
| ILMN_2401641 | ALDH3A2 | 0.22 | 0.142 | 0.012 | 0.014 | 0.472 | 0.543 | rs2108971 |
| ILMN_2183938 | LEMD3 | 0.335 | 0.084 | 0.026 | 0.029 | 0.495 | 0.541 | rs10878255 |
| ILMN_2198408 | MFF | 0.153 | 0.26 | 0.002 | 0.003 | 0.399 | 0.54 | rs7560053 |
| ILMN_1728199 | POLE | 0.113 | 0.175 | 0.006 | 0.007 | 0.446 | 0.538 | rs4883627 |
| ILMN_1655637 | UPK1A | 1.044 | 0.131 | 0.023 | 0.026 | 0.467 | 0.538 | rs4806187 |
| ILMN_2209027 | RPS26 | 0.238 | 0.146 | 0.002 | 0.002 | 0.457 | 0.535 | rs10876864 |
Results from some of the top probes are depicted. Only one probe is selected per gene for depiction. R2technical = variance due to technical variables only (i.e. plates, RIN). addR2covariates = added proportion of variance due to biological covariates (i.e. age, sex, ApoE4 dose), adjR2covariates = addR2covariates adjusted for technical variance, addR2best-SNP = proportion of variance due to the best cisSNP, adjR2best-SNP = addR2best-SNP adjusted for technical variance.
The top 2,980 cerebellar eGWAS associations were followed up in the temporal cortex validation study. We found that 2,685 top cerebellar cisSNP/transcript associations could be tested in the temporal cortex (2,387 unique cisSNPs, 677 unique probes and 625 unique genes) (Figure 1, Table 3, Supplementary Table 6 in Dataset S1). A total of 2,089 of these (1,888 unique cisSNPs, 502 unique probes and 471 unique genes) were significant after study-wide Bonferroni corrections, many of which had effect sizes showing remarkable similarity to those from the cerebellar eGWAS (Pearson's correlation coefficient = 0.94, p<0.0001).
Table 3. Validation of top cerebellar cisSNP/transcript associations in the temporal cortex.
| Cerebellar eGWAS | Temporal Cortex Validation | |||||||||
| CHR | SNP | PROBE | SYMBOL | ALL_P | ALL_PBonf | ALL_Q | ALL_BETA | ALL_P | ALL_PBonf-study | ALL_BETA |
| 5 | rs3776455 | ILMN_1718932 | MTRR | 6.37E-133 | 2.83E-127 | 7.34E-120 | 1.20 | 5.15E-133 | 1.38E-129 | 1.23 |
| 12 | rs10843881 | ILMN_2345908 | DDX11 | 1.48E-112 | 6.57E-107 | 2.39E-100 | −1.06 | 1.81E-113 | 4.87E-110 | −1.16 |
| 12 | rs1971762 | ILMN_1807798 | ATP5G2 | 1.10E-104 | 4.87E-99 | 1.64E-93 | 1.01 | 7.83E-107 | 2.10E-103 | 1.15 |
| 21 | rs2838859 | ILMN_2376667 | POFUT2 | 2.86E-103 | 1.27E-97 | 2.73E-92 | −0.86 | 4.15E-115 | 1.11E-111 | −0.84 |
| 8 | rs3802266 | ILMN_2184966 | ZHX2 | 4.35E-101 | 1.93E-95 | 3.06E-90 | −1.94 | 1.11E-104 | 2.97E-101 | −2.35 |
| 19 | rs260462 | ILMN_2052079 | ZNF544 | 8.26E-101 | 3.67E-95 | 4.38E-90 | −1.16 | 7.00E-129 | 1.88E-125 | −1.30 |
| 17 | rs2525574 | ILMN_2369018 | EVI2A | 1.52E-99 | 6.73E-94 | 4.88E-89 | 1.81 | 4.56E-89 | 1.22E-85 | 1.67 |
| 7 | rs6955367 | ILMN_1723984 | PILRB | 4.80E-97 | 2.13E-91 | 1.23E-86 | 1.08 | 1.70E-141 | 4.56E-138 | 1.56 |
| 7 | rs7313 | ILMN_2400759 | CPVL | 5.00E-92 | 2.22E-86 | 5.96E-82 | −0.96 | 5.76E-71 | 1.55E-67 | −1.34 |
| 11 | rs7124057 | ILMN_2262288 | EEF1G | 2.64E-91 | 1.17E-85 | 2.67E-81 | −0.90 | 1.49E-91 | 4.01E-88 | −0.70 |
| 10 | rs2182513 | ILMN_1689177 | PPAPDC1A | 1.08E-87 | 4.79E-82 | 6.93E-78 | 0.84 | 8.07E-01 | NS | 0.01 |
| 5 | rs11738432 | ILMN_2093720 | THG1L | 8.06E-84 | 3.58E-78 | 3.49E-74 | −0.86 | 1.16E-100 | 3.12E-97 | −1.09 |
| 15 | rs12912744 | ILMN_1691772 | ZSCAN29 | 3.47E-83 | 1.54E-77 | 1.33E-73 | −0.56 | 6.18E-96 | 1.66E-92 | −0.63 |
| 22 | rs136564 | ILMN_1809147 | FAM118A | 8.44E-81 | 3.74E-75 | 2.57E-71 | 0.85 | 7.87E-57 | 2.11E-53 | 0.68 |
| 17 | rs8070454 | ILMN_2388272 | MED24 | 3.51E-80 | 1.56E-74 | 9.58E-71 | −2.03 | 3.97E-85 | 1.06E-81 | −2.16 |
| 20 | rs2387976 | ILMN_2064132 | NANP | 9.58E-79 | 4.25E-73 | 1.93E-69 | −0.54 | 1.97E-87 | 5.29E-84 | −0.65 |
| 7 | rs10239340 | ILMN_2312606 | IRF5 | 1.52E-76 | 6.73E-71 | 2.31E-67 | 0.81 | 3.76E-90 | 1.01E-86 | 0.92 |
| 6 | rs2395943 | ILMN_1683279 | PEX6 | 3.63E-76 | 1.61E-70 | 5.06E-67 | −0.39 | 1.64E-69 | 4.40E-66 | −0.46 |
| 13 | rs1046028 | ILMN_2390162 | PHF11 | 1.07E-75 | 4.73E-70 | 1.36E-66 | 0.35 | 5.38E-83 | 1.44E-79 | 0.38 |
| 17 | rs1981997 | ILMN_1710903 | MAPT | 4.16E-71 | 1.85E-65 | 3.88E-62 | −0.48 | 2.42E-44 | 6.48E-41 | −0.51 |
| 9 | rs2240913 | ILMN_1811048 | GPR107 | 2.76E-69 | 1.23E-63 | 2.39E-60 | 0.66 | 2.11E-80 | 5.66E-77 | 0.95 |
| 16 | rs11649236 | ILMN_2201966 | N4BP1 | 5.69E-69 | 2.53E-63 | 4.37E-60 | −1.57 | 1.95E-85 | 5.25E-82 | −1.44 |
| 10 | rs7909832 | ILMN_1795336 | PTER | 1.70E-68 | 7.54E-63 | 1.04E-59 | −0.75 | 1.24E-84 | 3.32E-81 | −1.00 |
| 5 | rs11134054 | ILMN_1789419 | EXOC3 | 4.40E-68 | 1.95E-62 | 2.52E-59 | −0.57 | 1.51E-78 | 4.06E-75 | −0.60 |
| 19 | rs10405576 | ILMN_2074477 | GPR4 | 5.76E-68 | 2.56E-62 | 3.09E-59 | −1.32 | 5.20E-62 | 1.39E-58 | −1.53 |
| 6 | rs198834 | ILMN_2075334 | HIST1H4C | 4.76E-67 | 2.11E-61 | 2.05E-58 | −0.43 | 1.25E-51 | 3.35E-48 | −0.52 |
| 22 | rs737950 | ILMN_1697286 | SF3A1 | 2.05E-66 | 9.11E-61 | 7.72E-58 | 0.44 | 1.01E-71 | 2.71E-68 | 0.46 |
| 1 | rs1763601 | ILMN_2364072 | CLCNKA | 3.49E-66 | 1.55E-60 | 1.24E-57 | 1.22 | 6.22E-50 | 1.67E-46 | 1.02 |
| 12 | rs10878255 | ILMN_2183938 | LEMD3 | 2.61E-65 | 1.16E-59 | 7.91E-57 | −0.62 | 1.73E-69 | 4.65E-66 | −0.78 |
| 19 | rs4806187 | ILMN_1655637 | UPK1A | 2.82E-65 | 1.25E-59 | 8.11E-57 | −1.04 | 3.82E-39 | 1.02E-35 | −0.72 |
| 11 | rs2848630 | ILMN_1765332 | TIMM10 | 3.40E-65 | 1.51E-59 | 9.32E-57 | −0.53 | 2.04E-68 | 5.48E-65 | −0.45 |
| 17 | rs962800 | ILMN_2401641 | ALDH3A2 | 3.31E-64 | 1.47E-58 | 8.21E-56 | 0.46 | 4.26E-76 | 1.14E-72 | 0.44 |
| 2 | rs7560053 | ILMN_2198408 | MFF | 6.41E-63 | 2.85E-57 | 1.38E-54 | 0.37 | 5.20E-71 | 1.40E-67 | 0.30 |
| 12 | rs10876864 | ILMN_2209027 | RPS26 | 6.36E-62 | 2.82E-56 | 1.06E-53 | 0.49 | 3.01E-64 | 8.07E-61 | 0.57 |
| 12 | rs4883627 | ILMN_1728199 | POLE | 8.61E-62 | 3.82E-56 | 1.36E-53 | 0.33 | 3.95E-19 | 1.06E-15 | 0.22 |
| 6 | rs2191651 | ILMN_1694100 | PRIM2 | 1.55E-61 | 6.88E-56 | 2.26E-53 | −0.59 | 5.06E-57 | 1.36E-53 | −0.57 |
| 10 | rs9527 | ILMN_2151056 | C10orf32 | 2.90E-61 | 1.29E-55 | 3.90E-53 | −0.46 | 1.27E-65 | 3.42E-62 | −0.45 |
Of the 2,980 top cisSNP/transcript associations, 2,685 existed in the temporal cortex replication study. Some of these top associations are shown. Only one cisSNP/transcript pair is selected for depiction. The chromosome (CHR), SNP, Probe, Gene Symbol (SYMBOL) of these associations are depicted. The uncorrected (P), genome-wide (PBonf) and study-wide Bonferroni-corrected (PBonf-study) P values, Beta coefficient of association are shown for the combined (All) analyses in the cerebellar eGWAS and the temporal cortex replication study. Regression coefficients are based on the SNP minor allele using an additive model.
The top cerebellar eGWAS results were also compared to published liver [23] and brain [24], [25] eGWAS and overlap was identified for 4–11% of the top transcripts from these published studies (Text S1) Using HapMap2 genotypes, all transcripts and association threshold p<1.0E-4 in our eGWAS, we determined that 24–32% of the top transcripts from the published eGWAS overlapped with ours.
We used the cerebellar eGWAS as the discovery analysis and the temporal cortex eGWAS as the validation; since our goal is to identify significant cisSNP associations while minimizing any confounding factors due to pathology and given the fact that half of our subjects had pathologic AD, in which cerebellum is relatively unaffected whereas temporal cortex is one of the first affected brain regions. Nonetheless, we have also used temporal cortex as the discovery set and cerebellum as the validation, with remarkably similar results (Text S1, Supplementary Tables 7 and 8 in Dataset S1).
Enrichment of brain cisSNPs among human disease-associated SNPs
To examine whether the brain eGWAS approach identified variants implicated in human diseases/traits, we linked the 2,596 top cerebellar eGWAS cisSNPs to the “Catalog of Published GWAS” [26], which compiles weekly search results from all published GWAS of ≥100,000 SNPs where associations of p≤1.0E-05 are reported. We identified 47 cisSNPs that were also associated with 36 diseases/traits (Table 4, Supplementary Table 9 in Dataset S1). This represents a 2.4-fold enrichment of significant cerebellar cisSNPs amongst disease/trait associated SNPs, which is significant (p<10−6) based on simulations adjusted for minor allele frequencies [3] (Text S1).
Table 4. Examples of top cerebellar eGWAS cisSNPs also associated with complex diseases/traits.
| Cerebellar eGWAS | Disease GWAS | |||||||||||
| CHR | SNP | MinorAllele | PROBE | SYMBOL | ALL_P | ALL BETA | PUBMED ID | Disease/Trait | Reported Gene(s) | Strongest SNP-Risk Allele | p-Value | OR or beta |
| 7 | rs4132601 | C | ILMN_1676575 | IKZF1 | 1.63.E-21 | −0.36 | 19684604 | Acute lymphoblastic leukemia (childhood) | IKZF1 | rs4132601-C | 1.00E-19 | 1.69 |
| 19 | rs260461 | A | ILMN_2052079 | ZNF544 | 3.10.E-16 | 0.73 | 18821565 | Attention deficit hyperactivity disorder | ZNF544 | rs260461-? | 8.00E-06 | NR |
| 11 | rs10838738 | G | ILMN_2195462 | C1QTNF4 | 1.48.E-09 | −0.18 | 19079261 | Body mass index | MTCH2 | rs10838738-G | 5.00E-09 | 0.07 |
| 2 | rs13015714 | C | ILMN_1781700 | IL18R1 | 8.30.E-24 | 0.55 | 18311140 | Celiac disease | IL1RL1,IL18R1,IL18RAP, SLC9A4 | rs13015714-C | 4.00E-09 | 1.28 |
| 2 | rs13015714 | C | ILMN_2313672 | IL1RL1 | 1.22.E-13 | 0.84 | 18311140 | Celiac disease | IL1RL1,IL18R1,IL18RAP, SLC9A4 | rs13015714-C | 4.00E-09 | 1.28 |
| 7 | rs2252521 | A | ILMN_2400759 | CPVL | 1.86.E-18 | 0.55 | 19734545 | Cognitive performance | CPVL | rs2252521-? | 5.00E-06 | NR |
| 14 | rs4444235 | G | ILMN_1740900 | BMP4 | 3.20.E-18 | −0.27 | 19011631 | Colorectal cancer | BMP4 | rs4444235-C | 8.00E-10 | 1.11 |
| 15 | rs748404 | G | ILMN_1691772 | ZSCAN29 | 2.29.E-40 | −0.42 | 19654303 | Lung cancer | TGM5 | rs748404-? | 1.00E-06 | 1.15 |
| 6 | rs499818 | A | ILMN_1661622 | TBC1D7 | 1.32.E-15 | 0.17 | 17903304 | Major CVD | Intergenic | rs499818-? | 7.00E-06 | NR |
| 17 | rs6565681 | A | ILMN_1749722 | RNF213 | 2.10.E-22 | −0.38 | 21048783 | Moyamoya disease | RNF213 | rs6565681-A | 2.00E-08 | 4.82 |
| 6 | rs2517713 | C | ILMN_2130441 | HLA-H | 8.27.E-15 | −0.66 | 19664746 | Nasopharyngeal carcinoma | HLA-A | rs2517713-A | 4.00E-20 | 1.88 |
| 6 | rs3129055 | G | ILMN_2203729 | HCG4 | 1.90.E-14 | −0.7 | 19664746 | Nasopharyngeal carcinoma | HLA-F | rs3129055-G | 7.00E-11 | 1.51 |
| 10 | rs1561570 | G | ILMN_2381899 | OPTN | 1.51.E-11 | 0.07 | 20436471 | Paget's disease | OPTN | rs1561570-? | 6.00E-13 | 1.54 |
| 17 | rs8070723 | G | ILMN_1710903 | MAPT | 7.02.E-69 | −0.47 | 21044948 | Parkinson's disease | MAPT | rs8070723-? | 7.00E-12 | 1.3 |
| 17 | rs11012 | A | ILMN_2393693 | LRRC37A4 | 1.69.E-33 | −0.52 | 20070850 | Parkinson's disease | PLEKHM1, MAPT, IMP5 | rs11012-T | 6.00E-08 | 1.43 |
| 17 | rs11012 | A | ILMN_2286783 | LRRC37A4 | 1.64.E-37 | −0.65 | 20070850 | Parkinson's disease | PLEKHM1, MAPT, IMP5 | rs11012-T | 6.00E-08 | 1.43 |
| 4 | rs6532197 | G | ILMN_1660114 | MMRN1 | 4.86.E-12 | 0.52 | 19915575 | Parkinson's disease | MMRN1 | rs6532197-G | 1.00E-07 | 1.32 |
| 11 | rs538147 | A | ILMN_1772208 | CCDC88B | 3.92.E-22 | −0.27 | 21399635 | Primary biliary cirrhosis | RPS6KA4 | rs538147-G | 2.00E-10 | 1.23 |
| 2 | rs13385191 | G | ILMN_1660275 | C2orf43 | 7.01.E-22 | −0.26 | 20676098 | Prostate cancer | C2orf43 | rs13385191-G | 8.00E-08 | 1.15 |
| 5 | rs27524 | A | ILMN_2336220 | ERAP1 | 9.60.E-17 | 0.3 | 20953190 | Psoriasis | ERAP1 | rs27524-A | 3.00E-11 | 1.13 |
| 20 | rs1008953 | A | ILMN_1756590 | SYS1 | 4.15.E-10 | −0.09 | 20953189 | Psoriasis | SDC4 | rs1008953-C | 1.00E-07 | 1.14 |
| 7 | rs4728142 | A | ILMN_2312606 | IRF5 | 2.30.E-24 | −0.52 | 19838193 | Systemic lupus erythematosus | IRF5 | rs4728142-A | 8.00E-19 | 1.43 |
| 6 | rs2301271 | A | ILMN_1700428 | HLA-DOB | 1.27.E-11 | 0.38 | 21408207 | Systemic lupus erythematosus | HLA-DQA2 | rs2301271-T | 2.00E-12 | 1.47 |
| 11 | rs4963128 | A | ILMN_2349061 | IRF7 | 6.84.E-21 | −0.27 | 18204446 | Systemic lupus erythematosus | KIAA1542 | rs4963128-? | 3.00E-10 | 1.28 |
| 11 | rs4963128 | A | ILMN_2349061 | IRF7 | 6.84.E-21 | −0.27 | 21408207 | Systemic lupus erythematosus | KIAA1542 | rs4963128-? | 4.00E-06 | 1.33 |
| 12 | rs1701704 | C | ILMN_2209027 | RPS26 | 1.31.E-39 | 0.42 | 18198356 | Type 1 diabetes | RAB5B, SUOX, IKZF4, ERBB3, CDK2 | rs1701704-C | 9.00E-10 | 1.25 |
| 12 | rs3764021 | A | ILMN_1782729 | CLECL1 | 7.67.E-22 | −0.39 | 17554300 | Type 1 diabetes | NR | rs3764021-C | 5.00E-08 | 1.57 |
| 15 | rs8042680 | A | ILMN_2395932 | UNC45A | 1.84.E-13 | −0.26 | 20581827 | Type 2 diabetes | PRC1 | rs8042680-A | 2.00E-10 | 1.07 |
| 7 | rs4728142 | A | ILMN_2312606 | IRF5 | 2.30.E-24 | −0.52 | 21297633 | Ulcerative colitis | IRF5, TNPO3 | rs4728142-A | 2.00E-08 | 1.07 |
| 9 | rs4077515 | A | ILMN_1811301 | INPP5E | 2.74.E-13 | −0.12 | 20228799 | Ulcerative colitis | CARD9 | rs4077515-C | 5.00E-08 | 1.14 |
The top 2,980 cerebellar eGWAS cisSNPs were compared to the “Catalog of Published GWAS” (www.genome.gov/gwastudies). Some of the resulting 60 common associations are reported. The chromosome (CHR), SNP, eGWAS Minor Allele, Probe, Gene Symbol (SYMBOL) of these associations are depicted. The uncorrected (P) and Beta coefficient of associations are shown for the combined (All) analyses of the cerebellar eGWAS. Regression coefficients are based on the SNP minor allele using an additive model. The information for the complex disease/trait GWAS was downloaded from their website accessed on 04/23/2011. The disease/trait associated SNPs shown are the strongest SNPs depicted in the disease/trait GWAS. The associating allele (Strongest SNP-Risk Allele), p-value, OR or beta for the strongest disease/trait SNPs are shown.
Among the 36 diseases/traits associating with top cerebellar cisSNPs were central nervous system (CNS)-related conditions including Parkinson's disease (PD), Moyamoya disease, cognitive performance and attention-deficit hyperactivity disorder (ADHD). We both identified novel cisSNP/transcript associations and confirmed some previously reported ones. We found novel associations between rs6532197, which confers increased risk of PD [32], and higher brain levels of MMRN1 (cerebellar eGWAS p = pCer = 4.86×10−12; temporal cortex eGWAS p = pTCx = 4.57×10−9). MMRN1 encodes for multimerin and was found to be in a region of duplication/triplication with SNCA (encoding α-synuclein), a well-established risk gene in PD [33]. We found no significant cisSNP/SNCA level associations. These results suggest that MMRN1 may deserve further investigations as an additional PD risk gene.
Another example of a cisSNP which associates with human disease risk is rs8070723, the minor allele of which is associated with reduced risk of PD [32] and reduced brain MAPT levels (pCer = 3.36×10−7–7.02×10−69; pTCx = 9.03×10−4–8.61×10−44). Rs11012 minor allele, which confers increased risk of PD [34], showed association with lower brain LRRC37A4 levels (pCer = 1.69×10−33; pTCx = 3.378E−20). MAPT region variants were previously identified to associate with brain levels of MAPT and LRRC37A4 in neurologically normal subjects [27], [32], in a MAPT haplotype H1/H2-dependent manner [27]. Indeed, rs8070723 is in tight linkage disequilibrium with rs1052553 (r2 = 0.95, D′ = 0.97), the major allele of which marks the MAPT-H1 haplotype and associates with higher brain MAPT levels [24].
Many top cerebellar cisSNPs also associate with non–CNS diseases/traits (Supplementary Table 9 in Dataset S1). IRF5 cisSNP rs4728142 is associated with both cerebellar IRF5 levels and risk of systemic lupus erythematosus (SLE) [35]. Previously, IRF variants were shown to influence IRF splicing and expression as well as SLE risk [36], [37]. Interestingly, rs4728142 is also associated with ulcerative colitis (UC) [38] where both IRF5 and TNPO3 are reported as candidate genes. Given its influence on IRF5, but not TNPO3 expression levels, rs4728142 most likely marks IRF5, but not TNPO3 as the candidate UC risk gene.
Our approach to identify human disease-associated SNPs amongst the 2,596 top cerebellar eGWAS cisSNPs may be overly conservative, given our selection criteria to only include transcripts that are detectable in >75% of the subjects and only those cisSNPs that are significant in both independent cohorts (ADs and non–ADs). Furthermore, given that our eGWAS genotyping platform consisted of ∼300 K SNPs, it is plausible that transcript associations with SNPs from the “Catalog of Published GWAS” [26] may be missed if those SNPs did not exist in our platform. To address these issues, we repeated the cerebellar and temporal cortex eGWAS, without restrictions for transcript detection rates and using genotypes imputed to HapMap2 (>2 million SNPs). Comparison of the eGWAS associations with p<1.0E-4 to the “Catalog of Published GWAS” identified 392 unique cerebellar cisSNPs that also associate with 189 human diseases/traits; and 339 such temporal cortex cisSNPs associating with 167 diseases/traits (Text S1, Supplementary Tables 10 and 11 in Dataset S1). Amongst the associations identified by this less stringent approach were those for brain levels of CLU [39], [40], CR1 [40] and GAB2 [41] which were identified as risk loci in GWAS of Alzheimer's disease.
We also performed comparisons of the eGWAS results from the ADs and non–ADs separately to determine whether there were any results unique to these diagnostic groups (Text S1, Supplementary Tables 12, 13, 14, 15 in Dataset S1). Although 13–25% of the disease/trait associations were with cisSNPs that were unique to ADs or non–ADs, all but a few of these could also be identified in the combined analysis of all subjects. There were only 2–7 human diseases/traits with cisSNP associations that were detectable just in ADs or non–ADs, but not the combined group.
Of these unique cisSNP, those that associate with cerebellar levels of C9orf72 in non–ADs are interesting, as these variants were previously identified in GWAS of amyotrophic lateral sclerosis (ALS), where C9orf72 was one of the candidate genes at the disease locus [42], [43]. This gene was recently identified as the most common cause of familial ALS, with a repeat expansion leading to loss of an alternatively spliced transcript [44], [45]. These results further support the utility of the combined eGWAS and disease GWAS approaches in the potential identification of disease genes with modified transcript levels as the plausible disease mechanism.
Identification of brain cisSNPs among PSP GWAS loci
In a recent PSP GWAS [27], four loci near MAPT, STX6, EIF2AK3, and MOBP conferred significant risk, in addition to three suggestive loci at 1q41 intergenic locus, BMS1 and SLCO1A2. We assessed these seven strongest PSP risk loci in our eGWAS in the ADs, non–ADs and combined datasets, as well as the PSP subset of non–ADs (Table 5, Supplementary Table 16 in Dataset S1). We found novel, significant rs11568563 minor allele associations with reduced brain SLCO1A2 levels (pCer = 2.33×10−8; pTCx = 4.36×10−2–9.14×10−18), which confers increased PSP risk [27]. SLCO1A2 encodes solute carrier organic anion transporter family member 1a2 and is a drug transporter into the CNS [46]. Fine-mapping of the SLCO1A2 region revealed rs11568563 to be the strongest cisSNP influencing brain levels of this gene (Figure S5). This SNP was also identified as the top PSP-associating variant at this locus [27]. All other cisSNPs that associate with brain SLCO1A2 levels have weaker effects that appear to be due to their LD with s11568563, which is a missense coding mutation within SLCO1A2. Whether rs11568563 is merely tagging the functional variant(s) regulating levels of SLCO1A2 or coding changes also influence expressed transcript levels require further investigations. Additionally, MAPT/rs242557 minor allele increased PSP risk [27] and brain MAPT levels (pCer = 9.78×10−3–8.8×10−13, pTCx = 1.1×10−8). MAPT/rs8070723 minor allele associated with lower brain MAPT levels in our eGWAS, decreased PSP risk [27], similar to a PD GWAS [32]. We also found nominally significant increases in brain MOBP levels (pCer = 2.13×10−2–1.71×10−7; pTCx = 1.55×10−2–1.57×10−6) with rs1768208, which increases PSP risk [27].
Table 5. PSP GWAS cisSNP/transcript associations in the cerebellar and temporal cortex.
| ALL CER | PSP CER | AD CER | ALL TCX | PSP TCX | AD TCX | PSP GWASb | |||||||||||||
| CHR | SNP | PROBE | Symbol | BETA | P | BETA | P | BETA | P | BETA | P | BETA | P | BETA | P | Gene | OR | CI | P |
| 17 | rs8070723 | ILMN_1710903 | MAPT | −0.473 | 7.02E-69 | −0.428 | 1.38E-08 | −0.429 | 1.49E-31 | −0.498 | 8.61E-44 | −0.276 | 9.05E-02 | −0.495 | 3.12E-37 | MAPT | 5.11 | 4.43–5.91 | 1.5×10−118 |
| 12 | rs11568563 | ILMN_2381020 | SLCO1A2 | −0.397 | 2.33E-08 | −0.371 | 3.62E-03 | −0.438 | 1.98E-05 | −0.730 | 9.14E-18 | −0.644 | 3.89E-05 | −0.715 | 1.23E-08 | SLCO1A2 | 0.68 | 0.59–0.78 | 7.0×10−8 |
| 17 | rs242557 | ILMN_1710903 | MAPT | 0.183 | 8.80E-13 | 0.087 | 1.66E-02 | 0.179 | 2.39E-06 | 0.181 | 1.10E-08 | −0.033 | 6.65E-01 | 0.251 | 4.03E-10 | MAPT | 0.51 | 0.48–0.55 | 2.7×10−71 |
| 3 | rs1768208 | ILMN_2298464 | MOBP | 0.363 | 1.71E-07 | 0.342 | 1.24E-02 | 0.334 | 3.70E-04 | 0.325 | 1.57E-06 | 0.342 | 2.33E-02 | 0.305 | 1.23E-03 | MOBP | 0.73 | 0.67–0.78 | 5.3×10−17 |
| 3 | rs1768208 | ILMN_2414962 | MOBP | 0.243 | 1.17E-04 | 0.220 | 8.02E-02 | 0.204 | 1.25E-02 | 0.180 | 1.76E-05 | 0.238 | 1.74E-02 | 0.137 | 8.18E-03 | MOBP | 0.73 | 0.67–0.78 | 5.3×10−17 |
| 17 | rs8070723 | ILMN_2298727 | MAPT | −0.173 | 3.36E-07 | −0.063 | 5.91E-01 | −0.161 | 8.40E-04 | −0.137 | 9.03E-04 | −0.073 | 6.15E-01 | −0.211 | 8.67E-05 | MAPT | 5.11 | 4.43–5.91 | 1.5×10−118 |
| 3 | rs1768208 | ILMN_1768947 | MOBP | 0.143 | 7.57E-03 | 0.079 | 4.55E-01 | 0.159 | 1.72E-02 | 0.107 | 3.03E-03 | 0.199 | 1.83E-02 | 0.067 | 1.38E-01 | MOBP | 0.73 | 0.67–0.78 | 5.3×10−17 |
| 1 | rs1411478 | ILMN_2157951 | STX6 | −0.005 | 6.41E-01 | −0.031 | 1.32E-01 | −0.002 | 8.97E-01 | 0.027 | 1.17E-02 | −0.003 | 8.90E-01 | 0.041 | 8.50E-03 | STX6 | 0.79 | 0.73–0.84 | 3.5×10-11 |
| 3 | rs1768208 | ILMN_1750271 | MOBP | 0.074 | 2.13E-02 | 0.064 | 2.84E-01 | 0.073 | 8.86E-02 | 0.046 | 1.55E-02 | 0.082 | 1.03E-01 | 0.045 | 4.29E-02 | MOBP | 0.73 | 0.67–0.78 | 5.3×10−17 |
| 12 | rs11568563 | ILMN_1656097 | SLCO1A2 | −0.077 | 2.41E-01 | −0.027 | 8.14E-01 | −0.117 | 2.12E-01 | −0.117 | 4.36E-02 | −0.061 | 6.08E-01 | −0.064 | 4.22E-01 | SLCO1A2 | 0.68 | 0.59–0.78 | 7.0×10−8 |
| 17 | rs242557 | ILMN_2310814 | MAPT | 0.002 | 8.27E-01 | −0.032 | 9.13E-02 | 0.008 | 6.19E-01 | −0.026 | 1.09E-01 | −0.054 | 3.49E-01 | −0.022 | 9.33E-02 | MAPT | 0.51 | 0.48–0.55 | 2.7×10−71 |
| 17 | rs8070723 | ILMN_2200636 | KIAA1267 | 0.021 | 1.14E-01 | 0.046 | 2.87E-01 | 0.036 | 4.04E-02 | −0.016 | 1.70E-01 | −0.025 | 5.80E-01 | −0.026 | 1.02E-01 | MAPT | 5.11 | 4.43–5.91 | 1.5×10−118 |
| 17 | rs8070723 | ILMN_1665311 | STH | 0.044 | 1.79E-02 | 0.114 | 7.56E-02 | 0.026 | 3.05E-01 | 0.051 | 1.86E-01 | 0.188 | 2.59E-01 | −0.022 | 6.24E-01 | MAPT | 5.11 | 4.43–5.91 | 1.5×10−118 |
| 17 | rs242557 | ILMN_2298727 | MAPT | 0.071 | 9.78E-03 | 0.086 | 9.40E-02 | 0.075 | 8.56E-02 | 0.041 | 2.17E-01 | −0.054 | 4.26E-01 | 0.110 | 2.26E-02 | MAPT | 0.51 | 0.48–0.55 | 2.7×10−71 |
| 1 | rs1411478 | ILMN_2167416 | MR1 | 0.045 | 4.27E-02 | 0.092 | 4.48E-02 | 0.015 | 6.02E-01 | 0.029 | 2.24E-01 | −0.002 | 9.66E-01 | 0.077 | 1.20E-02 | STX6 | 0.79 | 0.73–0.84 | 3.5×10−11 |
| 17 | rs8070723 | ILMN_2310814 | MAPT | −0.007 | 5.77E-01 | −0.028 | 5.12E-01 | 0.007 | 6.94E-01 | 0.018 | 3.65E-01 | 0.039 | 7.52E-01 | 0.024 | 1.03E-01 | MAPT | 5.11 | 4.43–5.91 | 1.5×10−118 |
| 17 | rs242557 | ILMN_2200636 | KIAA1267 | −0.012 | 2.59E-01 | −0.022 | 2.56E-01 | −0.019 | 2.23E-01 | 0.008 | 3.83E-01 | 0.030 | 1.50E-01 | −0.003 | 8.08E-01 | MAPT | 0.51 | 0.48–0.55 | 2.7×10−71 |
| 17 | rs242557 | ILMN_1665311 | STH | −0.027 | 6.59E-02 | −0.020 | 4.88E-01 | −0.017 | 4.42E-01 | −0.024 | 4.28E-01 | −0.082 | 2.91E-01 | 0.027 | 4.89E-01 | MAPT | 0.51 | 0.48–0.55 | 2.7×10−71 |
| 1 | rs1411478 | ILMN_1721833 | IER5 | −0.041 | 2.12E-01 | −0.074 | 2.56E-01 | NA | NA | 0.016 | 4.76E-01 | 0.025 | 5.73E-01 | 0.000 | 9.95E-01 | STX6 | 0.79 | 0.73–0.84 | 3.5×10−11 |
| 10 | rs2142991 | ILMN_1772713 | BMS1 | 0.001 | 9.36E-01 | −0.007 | 8.18E-01 | 0.006 | 7.26E-01 | 0.003 | 8.02E-01 | 0.028 | 2.30E-01 | −0.014 | 4.56E-01 | BMS1 | 1.3 | 1.18–1.44 | 3.2×10−7 |
| 3 | rs1768208 | ILMN_1781231 | SLC25A38 | 0.004 | 7.76E-01 | −0.020 | 4.33E-01 | 0.025 | 1.47E-01 | 0.002 | 9.02E-01 | 0.001 | 9.78E-01 | 0.019 | 2.84E-01 | MOBP | 0.73 | 0.67–0.78 | 5.3×10−17 |
| 2 | rs7571971 | ILMN_1714809 | RPIA | −0.035 | 7.03E-02 | −0.071 | 9.57E-02 | NA | NA | 0.002 | 9.21E-01 | 0.027 | 5.19E-01 | 0.008 | 7.68E-01 | EIF2AK3 | 0.75 | 0.70–0.82 | 4.2×10−13 |
| 3 | rs1768208 | ILMN_2411723 | RPSA | −0.025 | 6.92E-02 | −0.057 | 3.77E-02 | −0.034 | 8.72E-02 | 0.000 | 9.66E-01 | −0.008 | 7.29E-01 | −0.001 | 9.41E-01 | MOBP | 0.73 | 0.67–0.78 | 5.3×10−17 |
Seven SNPs near six PSP candidate risk genes from a recent PSP GWAS [27] were tested for transcript associations in cis in our cerebellar (CER) eGWAS and temporal cortex replications. The chromosome (CHR), SNP, Probe, Gene Symbol (SYMBOL) of these associations are depicted. The uncorrected (P) and Beta coefficient of association are shown for the combined (All), PSP, AD analyses in the cerebellar eGWAS and the temporal cortex replication study. Regression coefficients are based on the SNP minor allele using an additive model. NA: Not available. a. Though a SNP is present in the sequence of probe ILMN_2298727 (rs73314997), this is essentially monomorphic in the eGWAS subjects. b. The odds ratios (OR), confidence intervals and P values of disease risk association from the PSP GWAS are also shown using results from Supplementary Table 2 in Dataset S1 of this study [27].
The recent PSP GWAS by Hoglinger et al. [27] included eQTL analysis for the significant loci using brain expression levels from 387 subjects without clinical neurologic diseases. In addition to associations between MAPT locus cisSNPs with brain MAPT and LRRC37A4 levels, they also detected signals for the nearby ARL17A and PLEKHM1 genes, neither of which were detectable in our eGWAS. They also identified cisSNP associations with brain MOBP levels but even stronger influence on the nearby SLC25A38 levels. We did not identify significant cisSNP/SLC25A38 brain expression associations. Although some of the significant probes for MOBP and MAPT harbor variants within their probe sequence, which may potentially confound associations with expression levels, these genes had other significant probes without any sequence variants (Text S1).
Most non–AD subjects in our study had pathologic diagnosis of PSP (nCer = 98, nTCx = 107, Supplementary Table 2 in Dataset S1). We assessed the 2,980 top cerebellar cisSNP/transcript associations in this subset, and found that most results were consistent with the ADs (Supplementary Tables 17 and 18 in Dataset S1).
Enrichment of brain cisSNPs in the AD GWAS from ADGC
To investigate whether any of the significant brain cisSNPs may influence risk of AD, we compared our eGWAS results to the AD risk associations from the large AD GWAS conducted by ADGC [28]. We obtained results of meta-analyses for the ADGC Stage 1+2 cohort (11,840 LOAD vs. 10,931 controls) [28] and investigated those SNPs with suggestive AD risk association in this dataset (pmeta<10−3). To ensure uniform comparison between our eGWAS and the ADGC GWAS, we assessed results from >2 million SNPs for each study using SNPs genome-wide imputed to HapMap phase 2 (release 22). There were 77,126 cerebellar (63,652 unique SNPs, 2,338 unique genes) and 68,172 temporal cortex (57,922 unique SNPs and 2,201 unique genes) cisSNP/transcript associations significant at q<0.05 representing a clear excess (Figure S6). There were 380 cisSNPs that were significant for the cerebellar transcript associations and also had suggestive AD risk associations (2.9-fold enrichment), 432 such temporal cortex cisSNPs (3.3-fold enrichment) and 356 cisSNPs significant in both the cerebellum and temporal cortex (2.7-fold enrichment, p<10−6 for all three analyses) (Figure 1, Supplementary Tables 19 and 20 in Dataset S1).
MAPT and LRRC37A4 cisSNPs, implicated in PSP [27] and PD [32] GWAS and which significantly influenced brain levels of these genes also had suggestive AD risk associations (pmeta = 8.82×10−4–1.53×10−5). CisSNP alleles associating with lower brain MAPT levels were associated with lower AD risk, similar to PD [32] and PSP [27] GWAS, which may suggest a common mechanism for these neurodegenerative diseases. ABCA7, identified recently as a novel LOAD risk locus [28], [47], had significant cerebellar cisSNPs. Further investigations of the other genes with evidence of brain transcript and AD risk association is warranted to understand their role in AD (Text S1).
To ensure that we did not miss any associations due to the stringent eGWAS criteria that we applied, we repeated the analyses using no restrictions for transcript detection rates and eGWAS p value threshold of p<1.0E-4. We also investigated cisSNPs identified in AD and non–AD brains, both separately, and jointly, given that some cisSNP associations may be unique to one group. We compared these eGWAS results to the ADGC GWAS as described above (Supplementary Tables 21, 22, 23, 24, 25, 26 in Dataset S1). Using cerebellar and temporal cortex eGWAS from all subjects, 561 and 488 unique transcripts with cisSNPs that yield suggestive AD risk associations were identified, respectively. There were 259–312 such transcripts identified in each AD or non–AD eGWAS, with >50% overlap between the two diagnostic groups' results, although many of these results could be identified in the eGWAS of combined samples. About 7–10% of the transcripts could only be identified in just ADs or non–ADs, but not the combined eGWAS. Amongst such unique transcripts were CLU and BIN1, which reside at the LOAD GWAS loci [39], [40], [48] and associate with cisSNPs in the cerebellum of non–ADs. Detailed analyses of the CLU locus cisSNP/transcript associations are in-press [49].
Discussion
In a large eQTL study on 773 brain samples from ∼400 autopsied subjects, we demonstrate significant contribution of genetic factors to human brain gene expression, reliably detected across different brain regions and pathologies. There is significant enrichment of brain cisSNPs amongst disease-associated variants, advocating gene expression changes as a mechanism for the first time for certain genes implicated in human diseases, including PSP (SLCO1A2), PD (MMRN1), Paget's disease (OPTN) while replicating others (e.g. PD/MAPT, SLE/UC/IRF5). MAPT cisSNPs associating with PSP, PD and AD risk highlight potential common mechanisms for these neurodegenerative diseases.
The reported results have several important implications for the genetics of human brain gene expression: First, despite technical challenges of gene expression measurements in post-mortem brain tissue [50], ∼70% of the transcriptome can be reliably detected in >75% of the subjects across two brain regions and different disease pathologies. Second, although there is significant contribution from technical covariates, genetic factors account for a substantial proportion of the variance in brain gene expression levels. We estimate that genetic factors explain an average 3% (range: 0–85%) of the variance in human cerebellar gene expression overall, and 18% (range: 8–85%) of the variance for the top cis-regulated transcripts. These estimates show remarkable similarity to those from other eQTL studies, such as a large, family-based lymphocyte eQTL, where cis eQTLs had an overall median effect size of 1.8% and significant eQTLs accounted for 24.6% of the variance in expression [16]. Similarly, significant cisSNPs explained 2–90% of expression variance in a liver eGWAS [23].
Third, there is remarkable replication of significant cisSNP associations across different brain regions and underlying tissue pathologies. Indeed, the 2,980 top cerebellar cisSNP/transcript associations represent 58% and 68%of all significant associations in the ADs and non–ADs. Since >50% of the non–ADs were comprised of subjects with PSP, we also conducted a separate analysis of this pathologically distinct group of non–ADs and again determined that many of the top cisSNPs were also significant in the PSPs despite the small sample size (n = 98). Importantly, most of the cisSNPs had highly similar effect sizes in the ADs, non–ADs and PSP subset of non–ADs. Furthermore, 78% of the top cerebellar cis-associations were also significant in the temporal cortex. Cerebellum is a relatively unaffected region in AD, whereas temporal cortex is typically one of the first areas to harbor neuropathology [51]. It is not inherently evident whether the unaffected or affected tissue regions would be most suitable for eQTL studies. Whereas unaffected regions would have the advantage of minimizing confounding on expression measurements from pathology (such as inflammation and cell death), affected regions may be more relevant for disease-associated eQTL mapping. The substantial overlap in significant cisSNP associations between different brain regions and disease types in our study implies that sample size may be the most critical element of successful eQTL mapping. In other words, analysis of expression data collected in different tissue regions and diseases, provided there is careful statistical control, could greatly enhance power to detect eQTLs. Nevertheless, there may be important eQTLs that are specific to brain region and disease.
It is not obvious whether the cisSNP that display similar effects in different brain regions and different disease types would have relevance to human disease. The top 2,596 cerebellar cisSNPs that are significant in both ADs and non–ADs, and many of which are also significant in the temporal cortex are also enriched for variants implicated in human disease, including CNS disease, such as PD and PSP. Thus, the fourth implication of our study is that it may be possible to map disease-associated variants using eQTL studies conducted in unaffected tissue or unaffected subjects. In addition to providing a general characterization of the genetics of brain gene expression, this study successfully replicated many previously published cisSNP associations, such as rs8070723/ MAPT level, rs11012/ LRRC37A4 level associations, both of which were implicated in PD. We found novel brain expression level associations for transcripts implicated in disease, including rs11568563 association with SLCO1A2, recently identified in a PSP GWAS. The disease-associating cisSNP associations identified in this study were not restricted to CNS diseases, but also included non–CNS diseases, such as SLE, where we replicated the previously published rs4728142/ IRF5 level associations.
These findings imply that many disease-associated cisSNPs can influence gene expression idependently of tissue/region/pathology, and be mapped reliably in tissue which is unaffected, not disease-related or from unaffected subjects. Indeed, our findings are consistent with a study of lymphoblastoid cell lines from subjects affected and unaffected with asthma, where Dixon et al. [12] found no differences between asthmatics and non-asthmatics. Furthermore, they detected significant transcript level associations with SNPs that also associate with asthma. Emilsson et al. [20] performed eQTL mapping in both blood and adipose tissue and determined that >50% of significant adipose tissue cisSNPs were also significant in blood. This is similar to the overlap we detected for cerebellum and temporal cortex, though two brain regions are more likely to have similar eQTL profiles than two different tissues.
Although many cisSNP effects can be detected in many different tissue types and disease conditions as shown here and by others [12], [20], there conceivably exist expression variants which exert their effects in a tissue or disease-specific manner. For example in the eQTL comparing blood and adipose tissue, Emilsson et al. [20] also found that more transcripts from adipose tissue had significant correlations with obesity-related traits. In reality, both scenarios may be at play, such that some expression variants have more ubiquitous effects, whereas others may need tissue/cell/region/disease specific factors to exert their influence on gene expression. Indeed, many of the CNS disease related cisSNP associations in our brain eGWAS could not be identified in our comparison to a liver eGWAS [23] or an existing database for a LCL eGWAS [12], suggesting that disease-relevant tissue may be necessary to detect effects of certain cisSNPs, and highlighting the value of this brain eGWAS for CNS traits/conditions.
Despite the enrichment of our samples with tissue from AD subjects and our use of both cerebellar and temporal cortex tissue, we did not identify strong transcript associations for some of the top genes recently implicated in AD risk in large LOAD GWAS studies [28], [39], [40], [47], [48]. This could be because the AD risk variants in these genes exert their effects via mechanisms other than influencing transcript levels, namely changes in protein conformation. If so, even the negative results from an eGWAS could be informative in guiding the future deep-sequencing efforts which should focus on coding rather than non-coding, functional regions. Alternative explanations include technical shortcomings, such as inability to measure all transcript species, measurements of global rather than cell-specific gene expression, not including all tested disease-associated variants in our genotyping platform. We also need to consider that the top genes nearest the strongest variants from the LOAD GWAS may not be actual disease genes. These loci require further investigations to account for this possibility. Additionally, our criteria for selection of the top cisSNPs, requiring significance in both ADs and non–ADs, might be too stringent, thereby leading to some false negative results. Finally, it may be possible to identify additional disease-related expression variants by focusing on those that have differential influence in disease vs. non-disease tissue, although this was not a focus of analysis in this study. Given that our non–AD tissue also consisted of subjects with other neurodegenerative diseases, there may be more similarities with the AD tissue, making it more difficult to detect variants with differential disease-related expression-associations in our current study. Nevertheless, we did find associations with cisSNPs for ABCA7, a novel AD risk locus gene [28], [47] and MAPT [52], [53], [24], [54] implicated in AD.
It is important to emphasize that although the identification of transcript level associations provides another layer of confidence for disease-associating variants and genes, it is entirely possible that a variant in an LD region encompassing multiple genes, could be marking a functional disease variant in one gene and an expression variant in another gene. Thus, although highly useful in conjunction with disease association studies, eGWAS should be seen as a guide rather than ultimate evidence in disease-mapping efforts. Similarly, absence of eGWAS associations for a disease-associated variant should not be seen as contradictory evidence, but rather raise the possibility of alternative functional mechanisms for that variant.
Despite the wealth of information our study provides, we acknowledge several shortcomings. First, our non–ADs were not normal controls but often had other brain pathologies. It will be necessary to seek replication of these findings or novel cisSNP/transcript associations in normal brain tissue, as well. Second, we only focused on single SNP associations. The preliminary observations from our eGWAS findings suggest that multiple independent variants may affect brain expression levels of some genes, whereas others might be under the influence of a single strong variant. Finally, like any association study, it is not clear whether the cisSNPs identified in our eGWAS are themselves the functional SNPs or simply in LD with un-genotyped regulatory variants. Future studies focusing on analysis of haplotypes, SNPxSNP interactions, novel variant discovery and functional in-vitro studies testing effects of multiple variants are required to dissect the genetic variation underlying brain gene expression levels.
In summary, this cerebellar eGWAS study and the temporal cortex validations provide insight about the genetics of brain gene expression, a framework to guide future studies with respect to tissue/region/disease choice in eQTL studies, examples about the utility of this approach in gene mapping, replication of some known transcript associations and evidence for novel transcript associations in human disease. Combined eGWAS-disease GWAS approach may provide complementary information in mapping human disease and enable identification of functional variants that may not be possible by either approach alone.
The complete set of results from the brain eGWAS can be accessed at the National Institute on Aging Genetics of Alzheimer's Disease Data Storage (NIAGADS) website at http://alois.med.upenn.edu/niagads/. Questions about the dataset can be addressed to the corresponding author of this manuscript (taner.nilufer@mayo.edu).
Methods
Subjects
All subjects were participants in the published Mayo LOAD GWAS [29] as part of the autopsy-based series (AUT_60–80). All subjects had neuropathologic evaluation by DWD. All ADs had definite diagnosis according to the NINCDS-ADRDA criteria [55] and had Braak scores of ≥4.0. All non–ADs had Braak scores of ≤2.5, and many had brain pathology unrelated to AD (Supplementary Tables 1 and 2 in Dataset S1). Three-hundred forty subjects had measurements in both cerebellum and temporal cortex. This study was approved by the appropriate institutional review board.
Expression genome-wide association study (eGWAS)
RNA extraction and gene expression measurements
Total RNA was extracted from frozen brain samples using the Ambion RNAqueous kit according to the manufacturer's instructions. The quantity and quality of the RNA samples were determined by the Agilent 2100 Bioanalyzer using the Agilent RNA 6000 Nano Chip. Transcript levels were measured using the Whole Genome DASL assay (Illumina, San Diego, CA). Probe annotations were done based on NCBI Ref Seq, Build 36.2. The RNA samples were randomized across the chips and plates using a stratified approach to ensure balance with respect to diagnosis, age, gender, RINs and APOE genotype. Replicate samples were utilized for QC and also for intra-class coefficient (ICC) estimations. Raw probe level mRNA expression data were exported from GenomeStudio software (Illumina Inc.) for preprocessing with background correction, variance stabilizing transformation, quantile normalization and probe filtering using the lumi package of BioConductor [56], [57] (Text S1). A probe with detectable signal in >75% of the samples was regarded as informative and used in subsequent analyses, although we also did supplementary analyses without imposing any restrictions based on probe detection levels. The number of informative probes differed slightly between the AD, non–AD and combined groups (Figure S7).
Genome-wide genotyping
Genotypes were generated using Illumina's HumanHap300-Duo Genotyping BeadChips and analyzed with an Illumina BeadLab Station (Illumina, San Diego, CA) at the Mayo Clinic Genotyping Shared Resource according to the manufacturer's protocols. The LOAD GWAS QC methods were previously published [29] (Text S1).
Statistical methods for eGWAS
Linear regression analysis to test for cisSNP/transcript associations were done in PLINK [58]. Preprocessed probe transcript levels were utilized as endophenotypes. Each probe was assessed separately, even though one gene may have multiple probes. CisSNPs localized to ±100 kb flanking region of the gene targeted by the probe of interest, mapped according to NCBI Build 36, were assessed for transcript level associations, using an additive model, with the minor allele dosage (0, 1, 2) as the independent variable, and APOE ε4 dosage (0, 1, 2), age at death, gender, PCR plate, RIN, (RIN-RINmean)2 as covariates. The cerebellum and temporal cortex expression levels were analyzed separately. The ADs and non–ADs were analyzed both separately and jointly. The joint analyses included diagnosis as an additional covariate (AD = 1, non–AD = 0). We also ran analyses including the top 10 eigenvectors from EIGENSTRAT, and compared eGWAS results to those excluding the eigenvectors (Text S1, Figure S8, Supplementary Table 27 in Dataset S1) [59].
Q values used for multiple testing corrections are based on false-discovery rates [30] and were corrected for genomic inflation of significance (Text S1). In addition, permutations (pperm-WY) and Bonferroni adjustment were used for comparison of correction strategies. Permutation p values were obtained by shuffling the endophenotype, while maintaining the covariate structure, 10,000 times and applying the Westfall and Young [60] resampling-style stepdown approach to account for correlations between probes.
Variance of gene expression
To assess the genetic contribution to the variance in human cerebellar gene expression, we first determined between-subject variance, as a percentage of the total variance in probe expression, using ICC [31] for 15 samples measured in replicate on 5–6 different plates and 2–3 different days.
Using multivariable linear regression models, we then calculated the proportion of variance in cerebellar gene expression levels that were explained by technical effects (PCR plate, RIN, (RIN-RINmean)2), biological covariates (APOE ε4 dosage, age at death, gender) and the “best” cisSNP for each probe. These analyses were carried out on the combined dataset consisting of cerebellar expression measurements from 374 subjects and 15,283 probes with at least one cisSNP (Text S1).
Replication of top cerebellar eGWAS hits in the temporal cortex
We identified 2,980 cisSNP/transcript associations (2,596 unique SNPs, 746 unique probes and 686 unique genes) that achieved genome-wide significance within both the ADs and non–ADs analyses with q values<0.05. All 2,980 cisSNP/transcript associations achieved genome-wide significance with q<0.05 and pBonf<0.05 in the combined ADs+non–ADs analysis. We sought validation of these hits in the temporal cortex of 399 subjects who had WG-DASL whole transcriptome measurements and whole-genome genotypes. RNA extractions, QC, WG-DASL measurements, transcript level detections and association analyses were performed for these temporal cortex samples, in the same manner as that for the cerebellar samples. After appropriate QC, 2,685 of the 2,980 top cerebellar cisSNP/transcript associations remained detectable among the temporal cortex results (2,387 unique SNPs, 677 unique probes and 625 unique genes).
Comparison of cerebellar eGWAS results with other published complex disease and trait GWAS
To determine whether the cerebellar eGWAS captured variants implicated in complex diseases/traits, we compared the top 2,980 cerebellar eGWAS cisSNPs with the top disease/trait associated SNPs in the “Catalog of Published GWAS” [26], curated by the National Human Genome Research Institute (www.genome.gov/gwastudies). This catalog compiles weekly search results from all published GWAS of ≥100,000 SNPs where associations of p≤1.0E-05 are reported. The catalog accessed on 04/23/2011 had 5,272 entries. We restricted our search to those entries where the “SNPs” column had only one SNP with an rs number. Thus, haplotypes and variants without rs numbers were excluded. There were 5,101 entries after this exclusion, comprised of 4,248 unique SNPs and 433 unique diseases. One SNP may associate with >1 disease/trait and each disease/trait may have ≥1 associating SNP. This list was linked to the 2,980 top cerebellar cisSNPs by common rs numbers.
To assess whether the number of observed cisSNPs that have both significant cerebellar eGWAS and disease/trait associations represent a significant enrichment, we performed simulations while adjusting for cisSNP minor allele frequencies, as previously reported [3]. We performed 1 million simulations and adjusted for the minor allele frequencies of all the tested cisSNPs in 10 bins from 0–0.05 to 0.45–0.50. Using the total number of cisSNPs that are both transcript and disease/trait associating for each simulation, we obtained an empirical p value and an estimate of fold-enrichment.
Cerebellar eGWAS results were also compared to other published eGWAS results from a human liver [23] and two human brain [24], [25] studies. The methods and results are depicted in Text S1.
Alzheimer's Disease Genetics Consortium (ADGC) meta-analyses
To determine whether any of the cisSNPs significant at q<0.05 influenced risk of AD, we obtained meta-analyses results from the ADGC [28]. The cohorts that are assessed by ADGC, as well as the methodological details of the meta-analyses are described in detail in a recent publication [28]. Briefly, the meta-analyses of the ADGC dataset results reported here (Supplementary Tables 17 and 18 in Dataset S1) are generated from the combined analyses of stage 1 and stage 2 cohorts (Text S1), with detailed descriptions provided elsewhere [28]. Stage 1 cohorts are comprised of 8,309 LOAD cases and 7,366 cognitively normal elder controls. Stage 2 has 3,531 LOAD vs. 3,565 control subjects. Each cohort was tested for AD risk association using a logistic regression approach, assuming an additive model and adjusting for age, sex, APOE ε4 dosage and principal components from EIGENSTRAT [59]. The meta-analyses results were generated using the inverse variance method implemented in the software package METAL [61].
Supporting Information
This file includes Supplementary Tables 1–27. The individual supplementary table legends are included in the first tab of this file.
(XLS)
Q-Q-Plots: Q-Q plots of observed (y-axis) versus expected (X-axis) −log(p) values of association for all cisSNP/transcript associations in the combined cerebellar 374 samples obtained before (a,b) and after (c,d) inflation-adjustments. Q-Q plots for all data points (a, c), as well as those that are in the lower, left hand corner (b,d) are shown. The data in b and d account reflect the association results, where there should be no deviations from the expected (i.e. null hypothesis of no association).
(PDF)
Venn diagram of significant cerebellar cisSNP/transcript associations: Q values<0.05 in the ADs, non–ADs and combined (All) analyses. Notably, 2,980 cis-SNP/transcript associations are significant both in the ADs and non–ADs.
(PDF)
Box Plots of some top cisSNP/transcript associations in the non–AD (a), AD (b) and combined groups (c): The SNP genotypes are shown on the X-axis with the genotype counts in parentheses. Variance stabilizing transformed (VST) expression levels are on the Y-axis. The bottom and top of a box represent the lower and upper quartiles, respectively. The band near the middle of the box is the median. The ends of the whiskers depict the most extreme observations still within 1.5 inter quartile range of the corresponding quartile. Any data not included between the whiskers are plotted as dots.
(PDF)
Histogram of intra-class coefficients (ICC) for the cerebellar probe expressions. Using 15 replicate samples, ICC, which is the between-subject variance, as a percentage of the total variance in probe expression, was estimated for 17,121 probes.
(JPG)
Data plots of SNPs tested for association with expression levels of SLCO1A2 in the Temporal Cortex and Cerebellum. Forty-six SNPs were tested for association of SLCO1A2 levels in the Cerebellum (Blue lines) and Temporal Cortex (Pink lines). P-values were transformed using −log10 and are plotted against the position of each SNP along the chromosome (Kbp). Genes found within the locus boundaries are shown from the UCSC genome browser (http://genome.ucsc.edu/). The LD across the locus is represented by a plot generated with Haploview, using data from the Mayo GWAS. The top eSNP in this study, rs11568563, is highlighted on the p-value plot by red squares and a red box around the SNP in the list of rs numbers. This is also the top PSP-associating SNP at this locus in Hoglinger et al. (Nat Genet, 2011) [27].
(PDF)
Q-Q-Plots for cerebellar and temporal cortex cisSNP/transcript associations with the HapMap phase 2 imputed genotypes: Q-Q plots of observed (y-axis) versus expected (X-axis) −log(p) values of association for all cisSNP/transcript associations in the combined dataset obtained before (a) and after (b) genomic inflation-adjustments, as discussed in the text. Also shown are the Q-Q plots for the temporal cortex associations in the combined dataset obtained obtained before (c) and after (d) inflation-adjustments.
(PDF)
Venn diagram of detectable cerebellar probes. Venn diagram of cerebellar probes detectable in ≥75% of subjects in the AD (AD), non–AD (CON) and combined (All) analyses. Notably, 13,349 probes were detectable in all 374 subjects.
(PDF)
Scatterplots of −log10 p values for eGWAS associations with and without inclusion of eigenvectors. Transformed P-values of a) Cerebellar and b) Temporal Cortex eGWAS cisSNP/transcript associations from models including (y-axis) and excluding (x-axis) the top 10 eigenvectors are plotted. A linear line demonstrating the null hypothesis of no deviation of the results between the two datasets is also shown. The results are displayed for those SNPs with a Hardy-Weinberg P-value>1.0E-07 and a probe detection threshold >75%.
(JPG)
Supplementary Results, Methods and References.
(DOC)
Acknowledgments
ADGC Authors
Liana G. Apostolova, MD,1 Steven E. Arnold, MD,2 Clinton T. Baldwin, PhD,3 Robert Barber, PhD,4 Michael M. Barmada, PhD,5 Thomas Beach, MD, PhD,6 Gary W. Beecham, PhD,7,8 Duane Beekly, BS,9 David A. Bennett, MD,10,11 Eileen H. Bigio, MD,12 Thomas D. Bird, MD,13 Deborah Blacker, MD,14,15 Bradley F. Boeve, MD,16 James D. Bowen, MD,17 Adam Boxer, MD, PhD,18 James R. Burke, MD, PhD,19 Jacqueline Buros, BS,3 Joseph D. Buxbaum, PhD,20,21,22 Nigel J. Cairns, PhD, FRCPath,23 Laura B. Cantwell, MPH,24 Chuanhai Cao, PhD,25 Chris S. Carlson, PhD,26 Regina M. Carney, MD,27 Steven L. Carroll, MD, PhD,28 Helena C. Chui, MD,29 David G. Clark, MD,30 Jason Corneveaux, BS,31 Carl W. Cotman, PhD,32 Paul K. Crane, MD, MPH,33 Carlos Cruchaga, PhD,34 Jeffrey L. Cummings, MD,1 Philip L. De Jager, MD, PhD,35,36 Charles DeCarli, MD,37 Steven T. DeKosky, MD,38 F. Yesim Demirci, MD,5 Ramon Diaz-Arrastia, MD, PhD,39 Malcolm Dick, PhD,32 Beth A. Dombroski, PhD,24 Ranjan Duara, MD,40 William G. Ellis, MD,41 Denis Evans, MD,42 Kelley M. Faber, MS,114 Kenneth B. Fallon, MD,28 Martin R. Farlow, MD,43 Steven Ferris, PhD,44 Tatiana M. Foroud, PhD,114 Matthew P. Frosch, MD, PhD,45 Douglas R. Galasko, MD,46 Paul J. Gallins, MS,7 Mary Ganguli, MD,47 Marla Gearing, PhD,48,49 Daniel H. Geschwind, MD, PhD,50 Bernardino Ghetti, MD,51 John R. Gilbert, PhD,7,8 Sid Gilman, MD, FRCP,52 Bruno Giordani, PhD,53 Jonathan D. Glass, MD,54 Alison M. Goate, D.Phil,34 Robert C. Green, MD,3,55,56 John H. Growdon, MD,57 Hakon Hakonarson, MD, PhD,58 Ronald L. Hamilton, MD,59 John Hardy, PhD,60 Lindy E. Harrell, MD, PhD,30 Elizabeth Head, PhD,61 Lawrence S. Honig, MD, PhD,62 Matthew J. Huentelman, PhD,31 Christine M. Hulette , MD,63 Bradley T. Hyman, MD, PhD,57 Gail P. Jarvik, MD, PhD,64,65 Gregory A. Jicha, MD, PhD,66 Lee-Way Jin, MD, PhD,41 Nancy Johnson, PhD,67 Gyungah Jun, PhD,68,3,69 M. Ilyas Kamboh, PhD,5,70 Jason Karlawish, MD,71 Anna Karydas, BA,18 John S.K. Kauwe, PhD,72 Jeffrey A. Kaye, MD,73,74 Ronald Kim, MD,75 Edward H. Koo, MD,46 Neil W. Kowall, MD,55,76 Patricia Kramer, PhD,77,73 Walter A. Kukull, PhD,78 James J. Lah, MD, PhD,54 Eric B. Larson, MD, MPH,79 Allan I. Levey, MD, PhD,54 Andrew P. Lieberman, MD, PhD,80 Oscar L. Lopez, MD,70 Kathryn L. Lunetta, PhD,68 Wendy J. Mack, PhD,81 Daniel C. Marson, JD, PhD,30 Eden R. Martin, PhD,7,8 Frank Martiniuk, PhD,82 Deborah C. Mash, PhD,83 Eliezer Masliah, MD,46,84 Wayne C. McCormick, MD, MPH,33 Susan M .McCurry, PhD,85 Andrew N. McDavid, BA26 Ann C. McKee, MD,55,76 Marsel Mesulam, MD,86 ,87 Bruce L. Miller, MD,18 Carol A. Miller, MD,88 Joshua W. Miller, PhD,41 Thomas J. Montine, MD, PhD,89 John C. Morris, MD,23,90 Amanda J. Myers, PhD,91 Adam C. Naj, PhD,7 Petra Nowotny, PhD,34 Joseph E. Parisi, MD,92,93 Daniel P. Perl, MD,94 Elaine Peskind, MD,95 Wayne W. Poon, PhD,32 Huntington Potter, PhD,25 Joseph F. Quinn , MD,73 Ashok Raj, MD,25 Ruchita A. Rajbhandary, MPH,7 Murray Raskind, MD,95 Eric M. Reiman, MD,31,96,97,98 Barry Reisberg, MD,44,99 Christiane Reitz, MD, PhD,100,101,102 John M. Ringman, MD,1 Erik D. Roberson, MD, PhD,30 Ekaterina Rogaeva, PhD,103 Roger N. Rosenberg, MD,39 Mary Sano, PhD,21 Andrew J. Saykin, PsyD,33,104 Julie A. Schneider, MD,105,10 Lon S. Schneider, MD,29,106 William Seeley, MD,18 Michael L. Shelanski, MD, PhD,107 Michael A. Slifer, MD, PhD,7,8 Charles D. Smith, MD,66 Joshua A. Sonnen, MD,89 Salvatore Spina, MD,51 Peter St George-Hyslop, MD, FRCP,103,108 Robert A. Stern, PhD,55 Rudolph E. Tanzi, PhD,57 John Q. Trojanowski, MD, PhD,24 Juan C. Troncoso, MD,109 Debby W. Tsuang, MD,95 Vivianna M. Van Deerlin, MD, PhD,24 Badri Narayan Vardarajan , MS,3 Harry V. Vinters, MD,1,110 Jean Paul Vonsattel, MD,111 Li-San Wang, PhD,24 Sandra Weintraub, PhD,86,87 Kathleen A. Welsh-Bohmer, PhD,19,112 Jennifer Williamson, MS,62 Randall L. Woltjer, MD, PhD113
ADGC Author Affiliations
1Department of Neurology, University of California Los Angeles, Los Angeles, California; 2Department of Psychiatry, University of Pennsylvania Perelman School of Medicine, Philadelphia, Pennsylvania; 3Department of Medicine (Genetics Program), Boston University, Boston, Massachusetts; 4Department of Pharmacology and Neuroscience, University of Texas Southwestern, Fort Worth, Texas; 5Department of Human Genetics, University of Pittsburgh, Pittsburgh, Pennsylvania; 6Civin Laboratory for Neuropathology, Banner Sun Health Research Institute, Phoenix, Arizona; 7The John P. Hussman Institute for Human Genomics, University of Miami, Miami, Florida; 8Dr. John T. Macdonald Foundation Department of Human Genetics, University of Miami, Miami, Florida; 9National Alzheimer's Coordinating Center, University of Washington, Seattle, Washington; 10Department of Neurological Sciences, Rush University Medical Center, Chicago, Illinois; 11Rush Alzheimer's Disease Center, Rush University Medical Center, Chicago, Illinois; 12Department of Pathology, Northwestern University, Chicago, Illinois; 13Department of Neurology, University of Washington, Seattle, Washington; 14Department of Epidemiology, Harvard School of Public Health, Boston, Massachusetts; 15Department of Psychiatry, Massachusetts General Hospital/Harvard Medical School, Boston, Massachusetts; 16Department of Neurology, Mayo Clinic, Rochester, Minnesota; 17Swedish Medical Center, Seattle, Washington; 18Department of Neurology, University of California San Francisco, San Fransisco, California; 19Department of Medicine, Duke University, Durham, North Carolina; 20Department of Neuroscience, Mount Sinai School of Medicine, New York, New York; 21Department of Psychiatry, Mount Sinai School of Medicine, New York, New York; 22Departments of Genetics and Genomic Sciences, Mount Sinai School of Medicine, New York, New York; 23Department of Pathology and Immunology, Washington University , St. Louis, Missouri; 24Department of Pathology and Laboratory Medicine, University of Pennsylvania Perelman School of Medicine, Philadelphia, Pennsylvania; 25Byrd Alzheimer Institute, University of Southern Florida Health, Tampa, Florida; 26Fred Hutchinson Cancer Research Center, Seattle, Washington; 27Department of Psychiatry, Vanderbilt University, Nashville, Tennessee; 28Department of Pathology, University of Alabama at Birmingham, Birmingham, Alabama; 29Department of Neurology, University of Southern California, Los Angeles, California; 30Department of Neurology, University of Alabama at Birmingham, Birmingham, Alabama; 31Neurogenomics Division, Translational Genomics Research Institute, Phoenix, Arizona; 32Institute for Memory Impairments and Neurological Disorders, University of California Irvine, Irvine, California; 33Department of Medicine, University of Washington, Seattle, Washington; 34Department of Psychiatry and Hope Center Program on Protein Aggregation and Neurodegeneration, Washington University School of Medicine, St. Louis, Missouri; 35Program in Translational NeuroPsychiatric Genomics, Department of Neurology, Brigham and Women's Hospital, Boston, Massachusetts; 36Program in Medical and Population Genetics, Broad Institute, Cambridge, Massachusetts; 37Department of Neurology, University of California Davis , Sacramento, California; 38University of Virginia School of Medicine, Charlottesville, Virginia; 39Department of Neurology, University of Texas Southwestern, Dallas, Texas; 40Wien Center for Alzheimer's Disease and Memory Disorders, Mount Sinai Medical Center, Miami Beach, Florida; 41Department of Pathology and Laboratory Medicine, University of California Davis , Sacramento, California; 42Rush Institute for Healthy Aging, Department of Internal Medicine, Rush University Medical Center, Chicago, Illinois; 43Department of Neurology, Indiana University, Indianapolis, Indiana; 44Department of Psychiatry, New York University, New York, New York; 45C.S. Kubik Laboratory for Neuropathology, Massachusetts General Hospital, Charlestown, Massachusetts; 46Department of Neurosciences, University of California San Diego, La Jolla, California; 47Department of Psychiatry, University of Pittsburgh, Pittsburgh, Pennsylvania; 48Department of Pathology and Laboratory Medicine, Emory University, Atlanta, Georgia; 49Emory Alzheimer's Disease Center, Emory University, Atlanta, Georgia; 50Neurogenetics Program, University of California Los Angeles, Los Angeles, California; 51Department of Pathology and Laboratory Medicine, Indiana University, Indianapolis, Indiana; 52Department of Neurology, University of Michigan , Ann Arbor, Michigan; 53Department of Psychiatry, University of Michigan , Ann Arbor, Michigan; 54Department of Neurology, Emory University, Atlanta, Georgia; 55Department of Neurology, Boston University, Boston, Massachusetts; 56Department of Epidemiology, Boston University, Boston, Massachusetts; 57Department of Neurology, Massachusetts General Hospital/Harvard Medical School, Boston, Massachusetts; 58Center for Applied Genomics, Children's Hospital of Philadelphia, Philadelphia, Pennsylvania; 59Department of Pathology (Neuropathology), University of Pittsburgh, Pittsburgh, Pennsylvania; 60Institute of Neurology, University College London, Queen Square, London; 61Department of Molecular and Biomedical Pharmacology, University of California Irvine, Irvine, California; 62Taub Institute on Alzheimer's Disease and the Aging Brain, Department of Neurology, Columbia University , New York, New York; 63Department of Pathology, Duke University, Durham, North Carolina; 64Department of Genome Sciences, University of Washington, Seattle, Washington; 65Department of Medicine (Medical Genetics), University of Washington, Seattle, Washington; 66Department of Neurology, University of Kentucky , Lexington, Kentucky; 67Department of Psychiatry and Behavioral Sciences, Northwestern University, Chicago, Illinois; 68Department of Biostatistics, Boston University, Boston, Massachusetts; 69Department of Ophthalmology, Boston University, Boston, Massachusetts; 70University of Pittsburgh Alheimer's Disease Research Center, Pittsburgh, Pennsylvania; 71Department of Medicine, University of Pennsylvania Perelman School of Medicine, Philadelphia, Pennsylvania; 72Department of Biology, Brigham Young University, Provo, Utah; 73Department of Neurology, Oregon Health & Science University, Portland, Oregon; 74Department of Biomedical Engineering , Oregon Health & Science University, Portland, Oregon; 75Department of Pathology and Laboratory Medicine, University of California Irvine, Irvine, California; 76Department of Pathology, Boston University, Boston, Massachusetts; 77Department of Molecular & Medical Genetics, Oregon Health & Science University, Portland, Oregon; 78Department of Epidemiology, University of Washington, Seattle, Washington; 79Group Health Research Institute, Seattle, Washington; 80Department of Pathology, University of Michigan , Ann Arbor, Michigan; 81Department of Preventive Medicine, University of Southern California, Los Angeles, California; 82Department of Medicine - Pulmonary, New York University, New York, New York; 83Department of Neurology, University of Miami, Miami, Florida; 84Department of Pathology, University of California San Diego, La Jolla, California; 85School of Nursing Northwest Research Group on Aging, University of Washington, Seattle, Washington; 86Alzheimer's Disease Center, Northwestern University, Chicago, Illinois; 87Cognitive Neurology, Northwestern University, Chicago, Illinois; 88Department of Pathology, University of Southern California, Los Angeles, California; 89Department of Pathology, University of Washington, Seattle, Washington; 90Department of Neurology, Washington University , St. Louis, Missouri; 91Department of Psychiatry & Behavioral Sciences, University of Miami, Miami, Florida; 92Department of Anatomic Pathology, Mayo Clinic, Rochester, Minnesota; 93Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, Minnesota; 94Department of Pathology, Mount Sinai School of Medicine, New York, New York; 95Department of Psychiatry and Behavioral Sciences, University of Washington, Seattle, Washington; 96Department of Psychiatry, University of Arizona, Phoenix, Arizona; 97Arizona Alzheimer's Consortium, Phoenix, Arizona; 98Banner Alzheimer's Institute, Phoenix, Arizona; 99Alzheimer's Disease Center, New York University, New York, New York; 100Taub Institute on Alzheimer's Disease and the Aging Brain, Columbia University , New York, New York; 101Gertrude H. Sergievsky Center, Columbia University , New York, New York; 102Department of Neurology, Columbia University , New York, New York; 103Tanz Centre for Research in Neurodegenerative Disease, University of Toronto, Toronto, Ontario; 104Department of Radiology and Imaging Sciences, Indiana University, Indianapolis, Indiana; 105Department of Pathology (Neuropathology), Rush University Medical Center, Chicago, Illinois; 106Department of Psychiatry, University of Southern California, Los Angeles, California; 107Department of Pathology, Columbia University , New York, New York; 108Cambridge Institute for Medical Research and Department of Clinical Neurosciences, University of Cambridge, Cambridge, Massachusetts; 109Department of Pathology, Johns Hopkins University, Baltimore, Maryland; 110Department of Pathology & Laboratory Medicine, University of California Los Angeles, Los Angeles, California; 111Taub Institute on Alzheimer's Disease and the Aging Brain, Department of Pathology, Columbia University , New York, New York; 112Department of Psychiatry & Behavioral Sciences, Duke University, Durham, North Carolina; 113Department of Pathology , Oregon Health & Science University, Portland, Oregon, 114Department of Medical and Molecular Genetics, Indiana University School of Medicine, Indianapolis, Indiana.
Footnotes
NR Graff-Radford has served as a consultant to Codman and received grant support from Elan Pharmaceutical Research, Pfizer Pharmaceuticals, Medivation, and Forrest. RC Petersen has been a consultant to GE Healthcare and Elan Pharmaceuticals, has served on a data safety monitoring committee for Pfizer and Janssen Alzheimer Immunotherapy, and has provided a CME lecture for Novartis. ADGC Authors: T.D.B. received licensing fees from and is on the speaker's bureau of Athena Diagnostics. M.R.F. receives research funding from BristolMyersSquibb Company, Danone Research, Elan Pharmaceuticals, Eli Lilly, Novartis Pharmaceuticals, OctaPharma AG, Pfizer, and Sonexa Therapeutics; receives honoraria as scientific consultant from Accera, Astellas Pharma US Inc., Baxter, Bayer Pharmaceuticals Corporation, BristolMyersSquibb, Eisai Medical Research, GE Healthcare, Medavante, Medivation, Merck, Novartis Pharmaceuticals, Pfizer, Prana Biotechnology, QR Pharma, The sanofi-aventis Group, and Toyama Chemical; and is speaker for Eisai Medical Research, Forest Laboratories, Pfizer, and Novartis Pharmaceuticals. A.M.G. has research funding from AstraZeneca, Pfizer and Genentech, and has received remuneration for giving talks at Pfizer and Genentech. R.C.P. is on the Safety Monitory Committee of Pfizer (Wyeth) and a consultant to the Safety Monitoring Committee at Janssen Alzheimer's Immunotherapy Program (Elan), to Elan Pharmaceuticals, and to GE Healthcare. R.E.T. is a consultant to Eisai, Japan, in the area of Alzheimer's genetics and a shareholder in, and consultant to, Pathway Genomics, San Diego, CA.
Support for this research was provided by the National Institutes of Health grants: National Institute on Aging (R01 032990 to NET and R01 AG018023 to NR Graff-Radford and SG Younkin); Mayo Alzheimer's Disease Research Center: (P50 AG016574 to RC Petersen, DW Dickson, NR Graff-Radford, SG Younkin, and N Ertekin-Taner); Mayo Alzheimer's Disease Patient Registry: (U01 AG006576 to RC Petersen); National Institute on Aging (AG025711, AG017216, AG003949 to DW Dickson). This project was also generously supported by the Robert and Clarice Smith and Abigail Van Buren Alzheimer's Disease Research Program (to RC Petersen, DW Dickson, NR Graff-Radford, and SG Younkin), and by the Palumbo Professorship in Alzheimer's Disease Research (to SG Younkin). N Ertekin-Taner is the recipient of National Institutes of Health (KL2 RR024151) and Siragusa Foundation grants. ADGC Authors: The National Institutes of Health, National Institute on Aging (NIH-NIA) supported this work through the following grants: ADGC, U01 AG032984, RC2 AG036528; NACC, U01 AG016976; NCRAD, U24 AG021886; NIA LOAD, U24 AG026395, U24 AG026390; Banner Sun Health Research Institute, U24 NS072026, P30 AG19610 Arizona Department of Health Services contract 211002, Arizona Biomedical Research Commission contracts 4001, 0011, 05-901 and 100, Michael J. Fox Foundation for Parkinson's Research; Boston University, P30 AG013846, R01 HG02213, K24 AG027841, U01 AG10483, R01 CA129769, R01 MH080295, R01 AG009029, R01 AG017173, R01 AG025259; Columbia University, P50 AG008702, R37 AG015473; Duke University, P30 AG028377; Emory University, AG025688; Group Health UO1 AG06781; Indiana University, P30 AG10133; Johns Hopkins University, P50 AG005146, R01 AG020688; Massachusetts General Hospital, P50 AG005134; Mayo Clinic, P50 AG016574; Mount Sinai School of Medicine, P50 AG005138, P01 AG002219; New York University, P30 AG08051, MO1RR00096, and UL1 RR029893; Northwestern University, P30 AG013854; Oregon Health and Science University, P30 AG008017, R01 AG026916; Rush University, P30 AG010161, R01 AG019085, R01 AG15819, R01 AG17917, R01 AG30146; University of Alabama at Birmingham, P50 AG016582, UL1RR02777; University of Arizona/TGEN, P30 AG019610, R01 AG031581, R01 NS059873; University of California, Davis, P30 AG010129; University of California, Irvine, P50 AG016573, P50, P50 AG016575, P50 AG016576, P50 AG016577; University of California, Los Angeles, P50 AG016570; University of California, San Diego, P50 AG005131; University of California, San Francisco, P50 AG023501, P01 AG019724; University of Kentucky, P30 AG028383; University of Michigan, P50 AG008671; University of Pennsylvania, P30 AG010124, AG17586; University of Pittsburgh, P50 AG005133, AG030653; University of Southern California, P50 AG005142; University of Texas Southwestern, P30 AG012300; University of Miami, R01 AG027944, AG010491, AG027944, AG021547, AG019757; University of Washington, P50 AG005136, UO1 HG004610; Vanderbilt University, R01 AG019085; and Washington University, P50 AG005681, P01 AG03991. ADNI Funding for ADNI is through the Northern California Institute for Research and Education by grants from Abbott, AstraZeneca AB, Bayer Schering Pharma AG, Bristol-Myers Squibb, Eisai Global Clinical Development, Elan, Genentech, GE Healthcare, GlaxoSmithKline, Innogenetics, Johnson and Johnson, Eli Lilly, Medpace, Merck, Novartis AG, Pfizer Inc, F. Hoffman-La Roche, Schering-Plough, Synarc, Alzheimer's Association, Alzheimer's Drug Discovery Foundation, the Dana Foundation, and by the National Institute of Biomedical Imaging and Bioengineering and NIA grants U01 AG024904, RC2 AG036535, K01 AG030514. We thank Creighton Phelps, Marcelle Morrison-Bogorad, and Marilyn Miller from NIA who are ex-officio ADGC members. Support was also from the Alzheimer's Association (LAF, IIRG-08-89720; MP-V, IIRG-05-14147) and the Veterans Affairs Administration. P.S.G.-H. is supported by Wellcome Trust, Howard Hughes Medical Institute, and the Canadian Institute of Health Research. Additional acknowledgements for TGen data: Many data and biomaterials were collected from several National Institute on Aging (NIA) and National Alzheimer's Coordinating Center (NACC, grant #U01 AG016976) funded sites. Amanda J. Myers, PhD (University of Miami, Department of Psychiatry) and John A. Hardy, PhD (Reta Lila Weston Institute, University College London) collected and prepared the series. Marcelle Morrison-Bogorad, PhD., Tony Phelps, PhD, and Walter Kukull PhD are thanked for helping to co-ordinate this collection. The directors, pathologist and technicians involved include: National Institute on Aging: Ruth Seemann, John Hopkins Alzheimer's Disease Research Center (NIA grant # AG05146): Juan C. Troncoso, MD, Dr. Olga Pletnikova, University of California, Los Angeles (NIA grant # P50 AG16570): Harry Vinters, MD, Justine Pomakian, The Kathleen Price Bryan Brain Bank, Duke University Medical Center (NIA grant #AG05128, NINDS grant # NS39764, NIMH MH60451 also funded by Glaxo Smith Kline): Christine Hulette, MD, Director, John F. Ervin, Stanford University: Dikran Horoupian, MD, Ahmad Salehi, MD, PhD, New York Brain Bank, Taub Institute, Columbia University (NYBB): Jean Paul Vonsattel, MD, Katerina Mancevska, Massachusetts Alzheimer's Disease Research Center (P50 AG005134): E. Tessa Hedley-Whyte, MD, MP Frosch, MD, Karlotta Fitch, University of Michigan (NIH grant P50-AG08671): Dr. Roger Albin, Lisa Bain, Eszter Gombosi, University of Kentucky (NIH #AG05144): William Markesbery, MD, Sonya Anderson, Mayo Clinic, Jacksonville: Dennis W. Dickson, MD, Natalie Thomas, University of Southern California: Caroll A. Miller, MD, Jenny Tang, M.S., Dimitri Diaz, Washington University, St Louis Alzheimer's Disease Research Center (NIH #P50AG05681): Dan McKeel, MD, John C. Morris, MD, Eugene Johnson, Jr., PhD, Virginia Buckles, PhD, Deborah Carter, University of Washington, Seattle (NIH #P50 AG05136): Thomas Montine, MD, PhD, Aimee Schantz, MEd., University of Pennsylvania School of Medicine, Alzheimer's Disease Research Center: John Q. Trojanowski, MD, Virginia M Lee, MD, Vivianna Van Deerlin, MD, Terry Schuck, Boston University Alzheimer's Disease Research Center (NIH grant P30-AG13846): Ann C. McKee, Carol Kubilus Sun Health Research Institute, Arizona (NIA #P30 AG19610): Joseph Rogers, PhD, Thomas G. Beach, MD, PhD, Lucia I. Sue Emory University: Bruce H. Wainer, MD, PhD, Marla Gearing, PhD, University of Texas, Southwestern Medical School: Charles L. White, III, M.D., Roger Rosenberg, Marilyn Howell, Joan Reisch, University of California, Davis: William Ellis, MD, Mary Ann Jarvis, Rush University Medical Center, Rush Alzheimer's Disease Center (NIH #AG10161): David A. Bennett, M.D. Julie A. Schneider, MD, MS, Karen Skish, MS, PA (ASCP)MT, Wayne T Longman, University of Miami/NPF Brain Endowment Bank: Deborah C. Mash, MD, Margaret J Basile, Mitsuko Tanaka Oregon Health and Science University: Randy Wotljer, PhD. Additional tissues include samples from the following sites: Newcastle Brain Tissue Resource (funding via the Medical Research Council, local NHS trusts and Newcastle University): C. M. Morris, MD, Ian G. McKeith, Robert H. Perry MRC London Brain Bank for Neurodegenerative Diseases (funding via the Medical Research Council): Simon Lovestone, Md PhD, Safa Al-Sarraj. MD, Claire Troakes, South West Dementia Brain Bank (funding via numerous sources including the Higher Education Funding Council for England (HEFCE), Alzheimer's Research Trust (ART), BRACE as well as North Bristol NHS Trust Research and Innovation Department and DeNDRoN): Seth Love, MD, Patrick Kehoe, PhD, Laura Palmer, The Netherlands Brain Bank (funding via numerous sources including Stichting MS Research, Brain Net Europe, Hersenstichting Nederland Breinbrekend Werk, International Parkinson Fonds, Internationale Stiching Alzheimer Onderzoek): Inge Huitinga, MD, Marleen Rademaker, Michiel Kooreman, Institut de Neuropatologia, Servei Anatomia Patologica, Universitat de Barcelona: Isidre Ferrer Abizanda, MD, PhD, Susana Casas Boluda.
References
- 1.Ertekin-Taner N. Gene expression endophenotypes: a novel approach for gene discovery in Alzheimer's disease. Mol Neurodegener. 2011;6:31. doi: 10.1186/1750-1326-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murphy A, Chu JH, Xu M, Carey VJ, Lazarus R, et al. Mapping of numerous disease-associated expression polymorphisms in primary peripheral blood CD4+ lymphocytes. Hum Mol Genet. 2010;19:4745–4757. doi: 10.1093/hmg/ddq392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nicolae DL, Gamazon E, Zhang W, Duan S, Dolan ME, et al. Trait-associated SNPs are more likely to be eQTLs: annotation to enhance discovery from GWAS. PLoS Genet. 2010;6:e1000888. doi: 10.1371/journal.pgen.1000888. doi: 10.1371/journal.pgen.1000888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schadt EE, Monks SA, Drake TA, Lusis AJ, Che N, et al. Genetics of gene expression surveyed in maize, mouse and man. Nature. 2003;422:297–302. doi: 10.1038/nature01434. [DOI] [PubMed] [Google Scholar]
- 5.Cheung VG, Conlin LK, Weber TM, Arcaro M, Jen KY, et al. Natural variation in human gene expression assessed in lymphoblastoid cells. Nat Genet. 2003;33:422–425. doi: 10.1038/ng1094. [DOI] [PubMed] [Google Scholar]
- 6.Morley M, Molony CM, Weber TM, Devlin JL, Ewens KG, et al. Genetic analysis of genome-wide variation in human gene expression. Nature. 2004;430:743–747. doi: 10.1038/nature02797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Monks SA, Leonardson A, Zhu H, Cundiff P, Pietrusiak P, et al. Genetic inheritance of gene expression in human cell lines. Am J Hum Genet. 2004;75:1094–1105. doi: 10.1086/426461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheung VG, Spielman RS, Ewens KG, Weber TM, Morley M, et al. Mapping determinants of human gene expression by regional and genome-wide association. Nature. 2005;437:1365–1369. doi: 10.1038/nature04244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stranger BE, Forrest MS, Clark AG, Minichiello MJ, Deutsch S, et al. Genome-wide associations of gene expression variation in humans. PLoS Genet. 2005;1:e78. doi: 10.1371/journal.pgen.0010078. doi: 10.1371/journal.pgen.0010078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stranger BE, Nica AC, Forrest MS, Dimas A, Bird CP, et al. Population genomics of human gene expression. Nat Genet. 2007;39:1217–1224. doi: 10.1038/ng2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stranger BE, Forrest MS, Dunning M, Ingle CE, Beazley C, et al. Relative impact of nucleotide and copy number variation on gene expression phenotypes. Science. 2007;315:848–853. doi: 10.1126/science.1136678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dixon AL, Liang L, Moffatt MF, Chen W, Heath S, et al. A genome-wide association study of global gene expression. Nat Genet. 2007;39:1202–1207. doi: 10.1038/ng2109. [DOI] [PubMed] [Google Scholar]
- 13.Dausset J, Cann H, Cohen D, Lathrop M, Lalouel JM, et al. Centre d'etude du polymorphisme humain (CEPH): collaborative genetic mapping of the human genome. Genomics. 1990;6:575–577. doi: 10.1016/0888-7543(90)90491-c. [DOI] [PubMed] [Google Scholar]
- 14.A haplotype map of the human genome. Nature. 2005;437:1299–1320. doi: 10.1038/nature04226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stranger BE, Forrest MS, Clark AG, Minichiello MJ, Deutsch S, et al. Genome-wide associations of gene expression variation in humans. PLoS Genet. 2005;1:e78. doi: 10.1371/journal.pgen.0010078. doi: 10.1371/journal.pgen.0010078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goring HH, Curran JE, Johnson MP, Dyer TD, Charlesworth J, et al. Discovery of expression QTLs using large-scale transcriptional profiling in human lymphocytes. Nat Genet. 2007;39:1208–1216. doi: 10.1038/ng2119. [DOI] [PubMed] [Google Scholar]
- 17.Rotival M, Zeller T, Wild PS, Maouche S, Szymczak S, et al. Integrating genome-wide genetic variations and monocyte expression data reveals trans-regulated gene modules in humans. PLoS Genet. 2011;7:e1002367. doi: 10.1371/journal.pgen.1002367. doi: 10.1371/journal.pgen.1002367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dimas AS, Deutsch S, Stranger BE, Montgomery SB, Borel C, et al. Common regulatory variation impacts gene expression in a cell type-dependent manner. Science. 2009;325:1246–1250. doi: 10.1126/science.1174148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ding J, Gudjonsson JE, Liang L, Stuart PE, Li Y, et al. Gene expression in skin and lymphoblastoid cells: Refined statistical method reveals extensive overlap in cis-eQTL signals. Am J Hum Genet. 2010;87:779–789. doi: 10.1016/j.ajhg.2010.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Emilsson V, Thorleifsson G, Zhang B, Leonardson AS, Zink F, et al. Genetics of gene expression and its effect on disease. Nature. 2008;452:423–428. doi: 10.1038/nature06758. [DOI] [PubMed] [Google Scholar]
- 21.Zhong H, Beaulaurier J, Lum PY, Molony C, Yang X, et al. Liver and adipose expression associated SNPs are enriched for association to type 2 diabetes. PLoS Genet. 2010;6:e1000932. doi: 10.1371/journal.pgen.1000932. doi: 10.1371/journal.pgen.1000932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grundberg E, Adoue V, Kwan T, Ge B, Duan QL, et al. Global analysis of the impact of environmental perturbation on cis-regulation of gene expression. PLoS Genet. 2011;7:e1001279. doi: 10.1371/journal.pgen.1001279. doi: 10.1371/journal.ppat.1001279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schadt EE, Molony C, Chudin E, Hao K, Yang X, et al. Mapping the genetic architecture of gene expression in human liver. PLoS Biol. 2008;6:e107. doi: 10.1371/journal.pbio.0060107. doi: 10.1371/journal.pbio.0060107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Myers AJ, Gibbs JR, Webster JA, Rohrer K, Zhao A, et al. A survey of genetic human cortical gene expression. Nat Genet. 2007;39:1494–1499. doi: 10.1038/ng.2007.16. [DOI] [PubMed] [Google Scholar]
- 25.Webster JA, Gibbs JR, Clarke J, Ray M, Zhang W, et al. Genetic control of human brain transcript expression in Alzheimer disease. Am J Hum Genet. 2009;84:445–458. doi: 10.1016/j.ajhg.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hindorff LA, Sethupathy P, Junkins HA, Ramos EM, Mehta JP, et al. Potential etiologic and functional implications of genome-wide association loci for human diseases and traits. Proc Natl Acad Sci U S A. 2009;106:9362–9367. doi: 10.1073/pnas.0903103106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoglinger GU, Melhem NM, Dickson DW, Sleiman PM, Wang LS, et al. Identification of common variants influencing risk of the tauopathy progressive supranuclear palsy. Nat Genet. 2011;43:699–705. doi: 10.1038/ng.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Naj AC, Jun G, Beecham GW, Wang LS, Vardarajan BN, et al. Common variants at MS4A4/MS4A6E, CD2AP, CD33 and EPHA1 are associated with late-onset Alzheimer's disease. Nat Genet. 2011;43:436–441. doi: 10.1038/ng.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carrasquillo MM, Zou F, Pankratz VS, Wilcox SL, Ma L, et al. Genetic variation in PCDH11X is associated with susceptibility to late-onset Alzheimer's disease. Nat Genet. 2009;41:192–198. doi: 10.1038/ng.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Storey JD, Tibshirani R. Statistical significance for genomewide studies. Proc Natl Acad Sci U S A. 2003;100:9440–9445. doi: 10.1073/pnas.1530509100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barry WT, Kernagis DN, Dressman HK, Griffis RJ, Hunter JD, et al. Intratumor heterogeneity and precision of microarray-based predictors of breast cancer biology and clinical outcome. J Clin Oncol. 2010;28:2198–2206. doi: 10.1200/JCO.2009.26.7245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simon-Sanchez J, Schulte C, Bras JM, Sharma M, Gibbs JR, et al. Genome-wide association study reveals genetic risk underlying Parkinson's disease. Nat Genet. 2009;41:1308–1312. doi: 10.1038/ng.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fuchs J, Nilsson C, Kachergus J, Munz M, Larsson EM, et al. Phenotypic variation in a large Swedish pedigree due to SNCA duplication and triplication. Neurology. 2007;68:916–922. doi: 10.1212/01.wnl.0000254458.17630.c5. [DOI] [PubMed] [Google Scholar]
- 34.Edwards TL, Scott WK, Almonte C, Burt A, Powell EH, et al. Genome-wide association study confirms SNPs in SNCA and the MAPT region as common risk factors for Parkinson disease. Ann Hum Genet. 2010;74:97–109. doi: 10.1111/j.1469-1809.2009.00560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Han JW, Zheng HF, Cui Y, Sun LD, Ye DQ, et al. Genome-wide association study in a Chinese Han population identifies nine new susceptibility loci for systemic lupus erythematosus. Nat Genet. 2009;41:1234–1237. doi: 10.1038/ng.472. [DOI] [PubMed] [Google Scholar]
- 36.Graham RR, Kozyrev SV, Baechler EC, Reddy MV, Plenge RM, et al. A common haplotype of interferon regulatory factor 5 (IRF5) regulates splicing and expression and is associated with increased risk of systemic lupus erythematosus. Nat Genet. 2006;38:550–555. doi: 10.1038/ng1782. [DOI] [PubMed] [Google Scholar]
- 37.Graham RR, Kyogoku C, Sigurdsson S, Vlasova IA, Davies LR, et al. Three functional variants of IFN regulatory factor 5 (IRF5) define risk and protective haplotypes for human lupus. Proc Natl Acad Sci U S A. 2007;104:6758–6763. doi: 10.1073/pnas.0701266104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anderson CA, Boucher G, Lees CW, Franke A, D'Amato M, et al. Meta-analysis identifies 29 additional ulcerative colitis risk loci, increasing the number of confirmed associations to 47. Nat Genet. 2011;43:246–252. doi: 10.1038/ng.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harold D, Abraham R, Hollingworth P, Sims R, Gerrish A, et al. Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer's disease. Nat Genet. 2009 doi: 10.1038/ng.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lambert JC, Heath S, Even G, Campion D, Sleegers K, et al. Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer's disease. Nat Genet. 2009;41:1094–1099. doi: 10.1038/ng.439. [DOI] [PubMed] [Google Scholar]
- 41.Reiman EM, Webster JA, Myers AJ, Hardy J, Dunckley T, et al. GAB2 alleles modify Alzheimer's risk in APOE epsilon4 carriers. Neuron. 2007;54:713–720. doi: 10.1016/j.neuron.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Es MA, Veldink JH, Saris CG, Blauw HM, van Vught PW, et al. Genome-wide association study identifies 19p13.3 (UNC13A) and 9p21.2 as susceptibility loci for sporadic amyotrophic lateral sclerosis. Nat Genet. 2009;41:1083–1087. doi: 10.1038/ng.442. [DOI] [PubMed] [Google Scholar]
- 43.Laaksovirta H, Peuralinna T, Schymick JC, Scholz SW, Lai SL, et al. Chromosome 9p21 in amyotrophic lateral sclerosis in Finland: a genome-wide association study. Lancet Neurol. 2010;9:978–985. doi: 10.1016/S1474-4422(10)70184-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dejesus-Hernandez M, Mackenzie IR, Boeve BF, Boxer AL, Baker M, et al. Expanded GGGGCC Hexanucleotide Repeat in Noncoding Region of C9ORF72 Causes Chromosome 9p-Linked FTD and ALS. Neuron. 2011;72:245–256. doi: 10.1016/j.neuron.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Renton AE, Majounie E, Waite A, Simon-Sanchez J, Rollinson S, et al. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron. 2011;72:257–268. doi: 10.1016/j.neuron.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee W, Glaeser H, Smith LH, Roberts RL, Moeckel GW, et al. Polymorphisms in human organic anion-transporting polypeptide 1A2 (OATP1A2): implications for altered drug disposition and central nervous system drug entry. J Biol Chem. 2005;280:9610–9617. doi: 10.1074/jbc.M411092200. [DOI] [PubMed] [Google Scholar]
- 47.Hollingworth P, Harold D, Sims R, Gerrish A, Lambert JC, et al. Common variants at ABCA7, MS4A6A/MS4A4E, EPHA1, CD33 and CD2AP are associated with Alzheimer's disease. Nat Genet. 2011;43:429–435. doi: 10.1038/ng.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Seshadri S, Fitzpatrick AL, Ikram MA, DeStefano AL, Gudnason V, et al. Genome-wide analysis of genetic loci associated with Alzheimer disease. JAMA. 2010;303:1832–1840. doi: 10.1001/jama.2010.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Allen M, Zou F, Chai HS, Younkin CS, Crook J, et al. Novel late-onset Alzheimer's disease loci variants associate with brain gene expression. Neurology. 2012 doi: 10.1212/WNL.0b013e3182605801. in-press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Atz M, Walsh D, Cartagena P, Li J, Evans S, et al. Methodological considerations for gene expression profiling of human brain. J Neurosci Methods. 2007;163:295–309. doi: 10.1016/j.jneumeth.2007.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol (Berl) 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 52.Caffrey TM, Joachim C, Paracchini S, Esiri MM, Wade-Martins R. Haplotype-specific expression of exon 10 at the human MAPT locus. Hum Mol Genet. 2006;15:3529–3537. doi: 10.1093/hmg/ddl429. [DOI] [PubMed] [Google Scholar]
- 53.Caffrey TM, Joachim C, Wade-Martins R. Haplotype-specific expression of the N-terminal exons 2 and 3 at the human MAPT locus. Neurobiol Aging. 2008;29:1923–1929. doi: 10.1016/j.neurobiolaging.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Myers AJ, Pittman AM, Zhao AS, Rohrer K, Kaleem M, et al. The MAPT H1c risk haplotype is associated with increased expression of tau and especially of 4 repeat containing transcripts. Neurobiol Dis. 2007;25:561–570. doi: 10.1016/j.nbd.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 55.McKhann G, Drachman D, Folstein M, Katzman R, Price D, et al. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 56.Du P, Kibbe WA, Lin SM. lumi: a pipeline for processing Illumina microarray. Bioinformatics. 2008;24:1547–1548. doi: 10.1093/bioinformatics/btn224. [DOI] [PubMed] [Google Scholar]
- 57.Lin SM, Du P, Huber W, Kibbe WA. Model-based variance-stabilizing transformation for Illumina microarray data. Nucleic Acids Res. 2008;36:e11. doi: 10.1093/nar/gkm1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, et al. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet. 2006;38:904–909. doi: 10.1038/ng1847. [DOI] [PubMed] [Google Scholar]
- 60.Westfall PH, Zaykin DV, Young SS. Multiple tests for genetic effects in association studies. Methods Mol Biol. 2002;184:143–168. doi: 10.1385/1-59259-242-2:143. [DOI] [PubMed] [Google Scholar]
- 61.Willer CJ, Li Y, Abecasis GR. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics. 2010;26:2190–2191. doi: 10.1093/bioinformatics/btq340. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This file includes Supplementary Tables 1–27. The individual supplementary table legends are included in the first tab of this file.
(XLS)
Q-Q-Plots: Q-Q plots of observed (y-axis) versus expected (X-axis) −log(p) values of association for all cisSNP/transcript associations in the combined cerebellar 374 samples obtained before (a,b) and after (c,d) inflation-adjustments. Q-Q plots for all data points (a, c), as well as those that are in the lower, left hand corner (b,d) are shown. The data in b and d account reflect the association results, where there should be no deviations from the expected (i.e. null hypothesis of no association).
(PDF)
Venn diagram of significant cerebellar cisSNP/transcript associations: Q values<0.05 in the ADs, non–ADs and combined (All) analyses. Notably, 2,980 cis-SNP/transcript associations are significant both in the ADs and non–ADs.
(PDF)
Box Plots of some top cisSNP/transcript associations in the non–AD (a), AD (b) and combined groups (c): The SNP genotypes are shown on the X-axis with the genotype counts in parentheses. Variance stabilizing transformed (VST) expression levels are on the Y-axis. The bottom and top of a box represent the lower and upper quartiles, respectively. The band near the middle of the box is the median. The ends of the whiskers depict the most extreme observations still within 1.5 inter quartile range of the corresponding quartile. Any data not included between the whiskers are plotted as dots.
(PDF)
Histogram of intra-class coefficients (ICC) for the cerebellar probe expressions. Using 15 replicate samples, ICC, which is the between-subject variance, as a percentage of the total variance in probe expression, was estimated for 17,121 probes.
(JPG)
Data plots of SNPs tested for association with expression levels of SLCO1A2 in the Temporal Cortex and Cerebellum. Forty-six SNPs were tested for association of SLCO1A2 levels in the Cerebellum (Blue lines) and Temporal Cortex (Pink lines). P-values were transformed using −log10 and are plotted against the position of each SNP along the chromosome (Kbp). Genes found within the locus boundaries are shown from the UCSC genome browser (http://genome.ucsc.edu/). The LD across the locus is represented by a plot generated with Haploview, using data from the Mayo GWAS. The top eSNP in this study, rs11568563, is highlighted on the p-value plot by red squares and a red box around the SNP in the list of rs numbers. This is also the top PSP-associating SNP at this locus in Hoglinger et al. (Nat Genet, 2011) [27].
(PDF)
Q-Q-Plots for cerebellar and temporal cortex cisSNP/transcript associations with the HapMap phase 2 imputed genotypes: Q-Q plots of observed (y-axis) versus expected (X-axis) −log(p) values of association for all cisSNP/transcript associations in the combined dataset obtained before (a) and after (b) genomic inflation-adjustments, as discussed in the text. Also shown are the Q-Q plots for the temporal cortex associations in the combined dataset obtained obtained before (c) and after (d) inflation-adjustments.
(PDF)
Venn diagram of detectable cerebellar probes. Venn diagram of cerebellar probes detectable in ≥75% of subjects in the AD (AD), non–AD (CON) and combined (All) analyses. Notably, 13,349 probes were detectable in all 374 subjects.
(PDF)
Scatterplots of −log10 p values for eGWAS associations with and without inclusion of eigenvectors. Transformed P-values of a) Cerebellar and b) Temporal Cortex eGWAS cisSNP/transcript associations from models including (y-axis) and excluding (x-axis) the top 10 eigenvectors are plotted. A linear line demonstrating the null hypothesis of no deviation of the results between the two datasets is also shown. The results are displayed for those SNPs with a Hardy-Weinberg P-value>1.0E-07 and a probe detection threshold >75%.
(JPG)
Supplementary Results, Methods and References.
(DOC)