Abstract
In search of autoantigen presenting cells (APC) that prime pathogenic autoantibody-inducing T helper (Th) cells of lupus, we found that CD41+CD151+ cells in Lineage− (Lin−) CD117+ (c-Kit+) CX3CR1− splenocytes depleted of known APCs, were most proficient in presenting nuclear autoantigens from apoptotic cells to selectively induce autoimmune Th17 response in different lupus-prone mouse strains. The new APC have properties resembling Megakaryocyte– and/or bipotent Megakaryocyte/Erythroid–progenitors of bone marrow, hence they are called MM cells here. The MM APC produce requisite cytokines, but they require contact for optimal Th17 induction upon nucleosome feeding, and can induce Th17 only before undergoing differentiation to become c-Kit− CD41+ cells. The MM APC expand up to 10 fold in peripheral blood of lupus patients and 49 fold in spleens of lupus mice preceding disease activity; they accelerate lupus in vivo, and break tolerance in normal mice, inducing autoimmune Th17 cells. MM also cause Th17 skewing to foreign antigen in normal mice, without Th17-polarizing culture conditions. Several molecules in MM are targets for blocking autoimmunization. These studies advance our understanding of lupus pathogenesis and Th17 differentiation biology by characterizing a novel category of APC.
INTRODUCTION
Systemic lupus erythematosus (SLE or lupus) is the major systemic autoimmune disease with complex genetically determined effects on the immune system. MHC class II genes are major determinants of lupus susceptibility, underscoring the importance of autoantigen presentation by class II molecules to autoreactive T helper (Th) cells in initiating the disease (1). In lupus autoimmunity, pathogenic IgG autoantibodies that fix complement and bind FcγR on inflammatory cells, are produced with help from Th1 and Th17 cells that are specific for peptides from nucleosomes or ribonucleoproteins of apoptotic cells; and such Th cells also infiltrate vital organs (2–11). Macrophages (e.g. tingible body MΦ), and DCs are normally tolerant to apoptotic cell antigens (12), but they are activated to present such autoantigens after binding by FcγR to IgG immune complexes (IC) containing apoptotic cell derived DNA/RNA, which then dually stimulate via their TLR and FcγR (13–18). Hence, to generate the activating IC, IgG-switched autoantibodies have to be made first by T cell help. Moreover, B cells become efficient APC to Th cells pre-primed by other APC (19), and B cells can be stimulated by nuclear antigens synergistically via BCR and TLR after developing high affinity somatically hypermutated receptors with T cell help (20, 21), otherwise anti-DNA B cells are inactivated (22, 23). Thus, conventional APCs are essential for disease progression, but it is unknown who initially primes autoimmune Th cells. We fractionated spleen cells of lupus prone mice in search of such APC.
MATERIALS AND METHODS
Mice
NZB and SWR mice were purchased from The Jackson Laboratory (Bar Harbor, ME), to breed lupus-prone SNF1 hybrids2 (24). Female SNF1 mice, like BWF1, have high serum levels of IgG class anti-DNA and other anti-nuclear autoantibodies by 2 mo, and spontaneously begin to develop severe lupus nephritis by 5 mo age (25). The other lupus prone recombinant congenic strain B6.Sle (BcN/LmoJ or B6.Sle1,2,3) homozygous for the NZM2410 lupus susceptibility QTL (Sle1, Sle2, Sle3) on the normal C57BL/6J background (26), normal C57BL/6 (B6), and OTII Transgenic (B6.Cg-Tg(TcraTcrb)425Cbn/J) with T cells specific for ovalbumin peptide OVA323–339 on I-Ab, were from The Jackson Laboratory. Females were used and all studies were approved by IACUC.
Human Subjects
Ten patients in clinical remission (all females; age 27–63) fulfilling American College of Rheumatology revised criteria for SLE, (27) and six normal (healthy) subjects (five females and one male; aged 22–46) were studied, approved by IRB. Disease activity by Systemic Lupus Activity Measure or SLAM (5, 28, 29) ranged between 1 and 8; or that by Systemic Lupus Disease Activity Index (SLEDAI) ranged between 0 and 2 (30) for the patients. The lupus patients’ demographic including clinical and treatment status are shown in Table I.
Table I.
Lupus patient demographic: Clinical and treatment status of SLE patients who provided samples for this study.
| Patient Code | Age/Sex | SLEDAI | SLAM | Current Treatment |
|---|---|---|---|---|
| 1 | 54/F | 0 | Pred., HCQ | |
| 2 | 38/F | 0 | none | |
| 3 | 47/F | 2 | AZA, LEF | |
| 4 | 51/F | 4 | HCQ; SSZ | |
| 5 | 60/F | 8 | HCQ | |
| 6 | 27/F | 7 | MMF | |
| 7 | 40/F | 1 | HCQ; Vit D | |
| 8 | 50/F | 2 | HCQ | |
| 9 | 63/F | 3 | HCQ | |
| 10 | 34/F | 2 | HCQ |
SLE = systemic lupus erythematosus; SLEDAI = SLE disease activity index; SLAM = Systemic Lupus Activity Measure; Pred = Prednisone or Steroids; HCQ = Hydroxycholoquine (plaquenil); AZA = Azathioprine (imuran); LEF = Leflunomide (Arava); SSZ = Sulfasalazine; MMF = Mycophenolate Mofetil; Vit D = Vitamin D.
Cell isolation
CD90+, CD4+, CD8+, CD19+, γδ+, DX5+, CD117(c-Kit)+, CD11c+, CD11c+, Gr-1+, and FcεRI+ cells from spleens were purified by magnetic bead conjugated antibodies (Miltenyi Biotec, Auburn, CA). Lin−c-Kit+ pure cells were isolated using anti-CD117 beads after depletion twice of above markers. Mast cells were isolated by anti-FcεRI-FITC and anti-FITC beads. CD4− CD8− T cells were purified as before (10, 31). Cell subsets were >90% pure.
Enzyme-linked immunosorbent spot (ELISPOT) assay
ELISPOT plates were coated with capture antibodies to IFN-γ or IL-17 (BD Bioscience, San Diego, CA) as published (8, 32). Based on optimal cell ratios established from dose response curves, T cells (1 × 106) were cultured with APC subsets (0.25 × 106 each) from 5 mo-old SNF1 mice in the presence of nucleosomes or saline. Cytokine-expressing cells were detected at 24h for INF-γ; or 48h for IL-17. Lin− c-Kit+ cells cultured by themselves without nucleosomes or with nucleosomes (10–30 μg/ml) produced 30±5 and 34±6 ELISPOTs respectively. T cells plus Lin−c-Kit+ cells in co-culture without nucleosomes, produced 15 ± 8 spots, background; and so did T cells (1 × 106) co-cultured with low numbers (0.05 × 106) of Lin−c-Kit+ cells even in the presence of nucleosomes. Th17 response in OTII transgenic naïve T cells to ovalbumin peptide OVA323–339 were measured after 72h or 120h. All ELISPOT assays were performed without artificial polarizing conditions or PMA-Ionomycin. Nucleosomes were prepared as described (3, 4).
Transwell experiments
Since Transwell culture system (96-well plate) required bigger volume of culture, we used twice the number of cells than all other ELISPOT cultures. Lin−c-Kit+pure cells (0.5 × 106) were placed in transwell chambers separated by a 0.4-μm permeable membrane (Corning Costar, Cambridge, MA) from the culture of T cells (2 × 106/well) from 5 mo old in anti-IL-17 coated ELISPOT plates. After 48h of culture, IL-17 positive spots were analyzed. T cells and Lin−c-Kit+ cells without nucloesomes produced 23 ± 6 spots as background.
Blocking of Th17 responses by anti-MHC class II antibodies
T cells (1 × 106) were cultured with APC subsets (0.25 × 106 each) from 5 mo-old SNF1 mice in the presence of anti-MHC class II antibodies (clone M5/114 from eBioscience, San Diego, CA) or isotype control upon stimulation with nucleosomes in IL-17 ELISPOT plates. Anti-MHC class II antibodies were preincubated with APC for 1h before adding nucleosomes and T cells. Blocking of Th17 response to nucleosomes by anti-MHC class II antibodies was analyzed by comparing isotype control in the 48 hr ELISPOT assay.
Autoantibody quantitation
IgG class autoantibodies to ssDNA, dsDNA, histone and nucleosome (histone-DNA complex) were measured by ELISA (3, 4).
Helper Assay for IgG autoantibody production in vitro/ex vivo
To detect autoantibody-inducing help, CD90+ T (1 × 106 /well), or purified CD4+ T (0.25 × 106 /well) cells from spleens were co-cultured with B cells (1 × 106 /well) and APCs (0.25 × 106 /well) from 5 mo old SNF1 mice in 96 well for 7 days, in the presence of 0.3, 1, 3, and 10μg/ml nucleosomes. Culture supernatants were assayed for IgG antibodies (8, 33).
For testing inhibition in vitro, T, B and L−K+ MM cells from spleens of 5 mo old SNF1 were stimulated with nucleosomes (10μg/ml) in the presence of anti-CD151 (1–100μg/ml) or isotype control for 7 days and supernatants analyzed IgG autoantibodies.
For testing inhibitory effect in vivo, 5 mo SNF1 mice were injected with anti-CD41 or isotype control (R&D Systems Inc., Minneapolis, MN; details in Results section). Splenocytes of injected mice were stimulated with nucleosomes (3μg/ml) in culture for 7 days and then supernatant analyzed for IgG autoantibody production ex vivo.
Adoptive transfer and lupus nephritis
CD11b+cells were first isolated from CD90−CD19− splenocytes of 5 mo SNF1 mice; next CD11c+ cells were isolated from remaining CD90−CD19−CD11blow+splenocytes, and then CD117+cells were positively selected from Lin− cells. Isolated cells were pulsed with 30μg/ml nucleosomes for 1 h and then transferred (1 × 106 cells/mouse) I.V. into recipient mice 3 times at 2 wk interval. There were six recipient mice per group except for the saline injection controls where 15 animals were monitored (similar control batches were being used concurrently for other experiments). The recipients were monitored weekly for proteinurea by using albustix (VWR Scientific, Chicago, IL), as described (34), and sera collected a month later for IgG autoantibodies. Animals with persistent proteinurea greater than 100mg/dl for two weeks were sacrificed and nephritis graded as described (33, 34).
Gene Profiling Microarray and analysis
As detailed in text, spleen cells from 3 batches of three 5 mo SNF1 female mice were used each time to obtain three independent isolations of each type of APC population. RNA was purified using the RNeasy kit (Qiagen, Valencia, CA) from each APC after 6h pulse with nucleosomes (20μg/ml), and expression analysis in triplicate was performed at our Genomics Core Facility using Illumina Mouse WG-6 v2.0 Expression Beadchips, covering around 45,281 genes and expressed sequence tags, and then analyzed with our Bioinformatics Consulting Core. Before transformation and normalization, A/P call detection selected 20,890 out of 45,281 probes with P <0.01 having valid signals (35). For each pair: Lin−c-Kit+CX3CR1− versus Lin−c-Kit+ CX3CR1+; Lin−c-Kit+pure vs. Lin−c-Kit+CX3CR1+; Lin−c-Kit+CX3CR1− vs. DC; and Lin−c-Kit+pure vs. DC; differentially expressed genes were identified using an Analysis of Variance (ANOVA) model with empirical Bayesian variance estimation (36), yielding 1,619 genes on the basis of raw P-value < 0.01 and false discovery rate adjusted P-value < 0.05, and 1.5-fold change in expression level in at least one of the comparisons (Supplemental Fig. 3A); and out of these, 230 genes were significantly UP-regulated in ALL 4 pair comparisons (Supplemental Table I). Microarray data has been deposited in the GEO database under accession number “GSE36284” http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE36284.
Real time RT-PCR
To measure mRNAs for Th17 inducing cytokines in APC, or Th17 cell related molecules in responding T cells, Lin−c-Kit+pure or MM cells were incubated with nucleosomes, or T cells were stimulated by Lin−c-Kit+pure or MM cells with nucleosomes (20μg/ml) respectively for various time points. mRNAs for Th17 inducing cytokines in APC, or Th17 related molecules in responding T cells, were measured with primers as described (Applied Biosystems) (8).
Phagocytosis
Engulfment of non-carboxylated fluoresceinated latex beads (2.1μm; from Polyscience Warrington, PA), or CFSE (Invitrogen, Carlsbad, CA)–labeled and irradiated apoptotic thymocytes at various ratios, at 37°C for 6–72h by Lin−c-Kit+ or other APC were assessed by staining: with APC-anti-CD117 or PE-anti-CD11b before incubation with latex beads or apoptotic thymocytes for microscopy, but stained after the incubations for flow cytometry. Apoptotic thymocytes were prepared by γ-ray irradiation (3,000 rad) and stained with annexin V and PI after 6–12h to confirm apoptosis.
DQ-OVA endocytosis
BODIPY-conjugated DQ-ovalbumin (DQ-OVA, D-12053, Molecular Probes Inc., Eugene, OR) exhibits bright green fluorescence upon proteolytic processing. DQ-OVA (10μg/ml) was added to Lin−c-Kit+ cells (5 × 104 cells) and incubated at 4°C or 37°C for 20 min – 2h. Controls include 4°C with DQ-OVA or without DQ-OVA, and 37°C without DQ-OVA, according to manufacturer’s references. Cells were then washed and analyzed by flow cytometry.
Flow Cytometry
Cells were stained with Allophycocyanin -conjugated anti-CD117, FITC- or PE-anti-CD11b, FITC- or PE-anti-CD11c (BD Pharmingen), PE-Cy7-anti-Sca-1, FITC- or PE-anti-CD41, Percpcy5.5-anti-Gr-1 (eBioscience), PE-anti-CD151, (R&D Systems Inc.), PE- or Percpcy5.5-anti-CD150, Pacific blue-CD48 (Biolegend, San Diego, CA), or matched isotype controls at 4°C for 30 min; then acquired by LSR II with FACS Diva (Becton–Dickinson) at our IBC core facility, and analyzed using Flowjo (Tree Star Inc., Ashland, OR). For sorting, c-Kit+ cells were isolated from Lin− splenocytes by magnetic beads and then stained with FITC-anti-CD41 and Percpcy5.5-anti-CD150, PE-anti-CD151, Allophycocyanin -anti-CD105, PE-Cy7-anti-Sca-1, Pacific blue-anti-CD48 at 4°C for 30 min, and sorted by MoFlo (Dako Cytomation, Carpinteria CA) with Summit and FACS express 3 software (De Novo Software, Los Angeles, CA) at our RHLCCC core facility. For identification of MM cells in human PBMNC, PBMNC were prepared by Ficoll-paque (GE-healthcare Biosciences Corp., Piscataway, NJ) gradient of whole blood from lupus patients and buffy coats or whole blood from healthy donors. PBMNCs were stained with Alexafluo 700-anti-CD3, eFluo-anti-CD19, ECD-anti-CD14, Allophycocyanin -Cy7-anti-CD16, Allophycocyanin -anti-CD117, FITC-anti-CD41, and PE-anti-CD151 antibodies. After gating for CD3−CD19−CD14−CD16− fraction of cells in PBMNC, CD117+High CD41+ cells were gated from that fraction first, and all of them were CD151+. CD151Hi+ cells among CD117+High CD41+ cells were then gated.
Hematopoietic lineage potential
Sorted Lin−c-Kit+ cell subsets were cultured in MethoCult M3134 with 2% FBS for CFU, or IMDM with 10% FBS with mouse - SCF (50ng/ml), IL-3 (20ng/ml), TPO (40ng/ml), and EPO (20ng/ml) (R&D Systems; Stem Cell Technology), and then stained for CD42 and Ter 119 for megakaryocyte and erythrocytes by FACS and Giemsa (37).
Cell cloning by limiting dilution
Lin−c-Kit+ cells were cultured at a density of 100, 50, 10, 2 or 0.5 cell in RPMI with 10% FBS with SCF (50ng/ml). Culture supernatants of Lin−c-Kit+ cells were used with 1:1 fresh medium for expanding clones.
Statistical analysis
Chi squire (χ2) test, Log Rank test and the Student two-tailed t-test were used. Results are expressed as mean ± s.e.m.
RESULTS
Lin−c-Kit+pure cells almost exclusively induce Th17 responses to nuclear autoantigens without undergoing further differentiation
We depleted spleen cells of lupus-prone SNF1 mice of cells with mature lineage markers, including conventional APC (CD90+, CD3ε+, CD19+, B220+, CD11c+, CD11b+, CX3CR1+, NK1.1+, Gr-1+, Ter119+, and FcεRI+ mast cells-depleted) and then isolated pure CD117+ (c-Kit+ or K+) cells from the lineage− (Lin− or L−) cells (Supplemental Fig. 1A). These Lin−c-Kit+pure cells, which had morphology of hematopoietic progenitor cells, were almost the sole inducers of nuclear autoantigen-specific Th17 responses (Fig. 1A–C). CD11c+ DCs isolated before pulling out CD11b+ and CD117+ cells, thus including few CD11c+CD117low+ cells, induced mainly nucleosome-specific Th1 response, but very low Th17 (P = 0.003 – 0.00004 vs. Lin−c-Kit+pure cells). The Lin−c-Kit+pure cells presented autoantigens without differentiation into DC or macrophages, because they could process/present nucleosomes to induce Th17 response even after irradiation (3,000 rad), or culturing and cloning in media supplemented with stem cell factor (SCF, c-KitL) alone that prevented further differentiation (Fig. 1D–G). The ELISPOT assays to measure T cell responses in all of the above and subsequent experiments included background controls with APC alone, T cells alone and APC + T cells without nucleosomes. The Lin−c-Kit+pure cells cultured by themselves without nucleosomes or with nucleosomes (at 10–30 μg/ml) produced 30±5 and 34±6 ELISPOTs respectively, at background levels (representative examples shown later in Fig. 3A and 6C); and co-culture of these APCs with T cells without nucleosomes produced only a few background spots (15±8), as stated also in Figure legends and Methods. Moreover, nucleosome activated Lin−c-Kit+pure cells did not express IL-17 message by qPCR or microarray (Supplemental Table I).
FIGURE 1.
Lin−c-Kit+ cells purified from lupus spleens (L−K+pure) are the main inducers of Th17 response to nuclear autoantigens. (A–B) Isolated APC types (designated by color key) were co-cultured with splenic T cells from lupus-prone SNF1 at optimal ratios (Methods), and IFN-γ (A) and IL-17 (B) responses of the T cells to nucleosomes were measured at 24 and 48 hr respectively in ELISPOT assays (Y-axis), as shown by bars. Data (mean and s.e.m.) represent 5 independent experiments with 35 mice. Lin−c-Kit+ cells cultured by themselves without nucleosomes or with nucleosomes (at 10–30 μg/ml) produced 30±5 and 34±6 ELISPOTs respectively. Co-cultures of APC and T cells without nucleosomes produced 15 ± 8 background spots. (C) Gating c-Kit+ (CD117+) cells in Lin− splenocytes (left) and Giemsa staining of sorted CD117+cell (right). Scale bar =10 μm. (D) Irradiated Lin−c-Kit+ cells (APC) were cocultured with T cells and nucleosomes, or pulsed with nucleosomes for 1 h and then cocultured for IL-17 response of the T cells, as shown. (E) Lin−c-Kit+ pure cells in SCF only supplemented media did not differentiate into DCs. (F) Clone 5-4 derived by limiting dilution of above culture (E) has only CD117+CD11b− cells. (G) Clone 5-4 cocultured as APC with SNF1 T cells, induced only Th17 response to nucleosomes.
FIGURE 3.
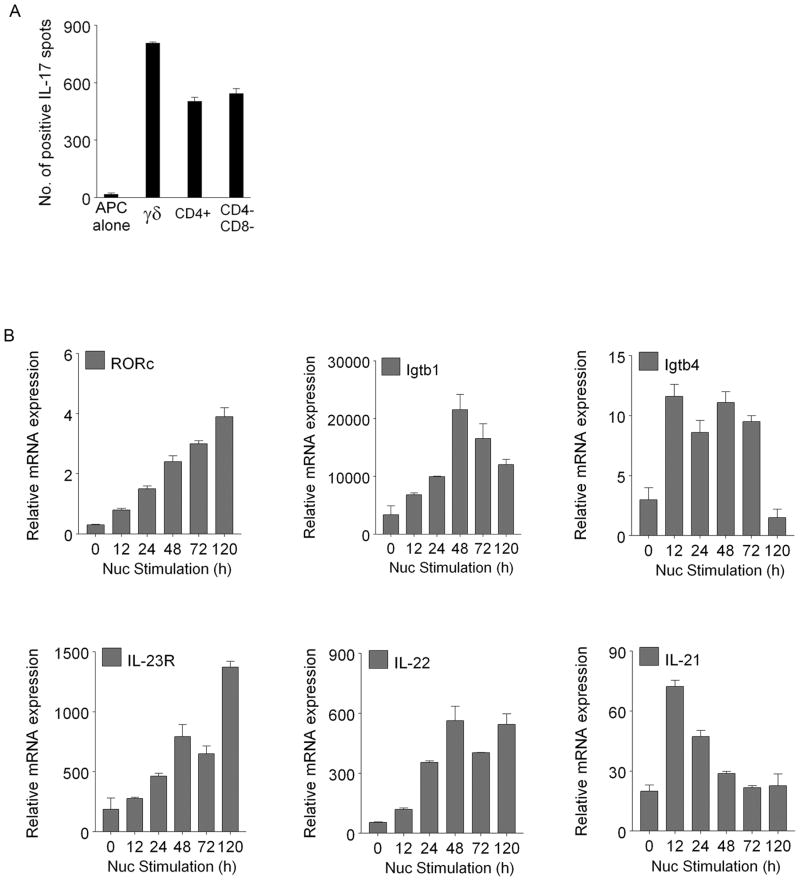
Lin−c-Kit+pure cells induce Th17 response by different subsets of T cells and induce molecules expressed by Th17 cells. (A) Lin−c-Kit+pure cells fed with nucleosomes (10 μg/ml) were co-cultured as APC with γδ+T cells, CD4+T cells, or CD4−CD8−T cell subsets fractionated from SNF1 splenocytes, and the T cells’ responses in IL-17 ELISPOT plates are shown, in addition to the IL-17spots produced by Lin−c-Kit+pure APC cultured by themselves in presence of nucleosomes (APC alone). The cells were isolated from 5 mo old female SNF1 mice. T cells and Lin−c-Kit+ cells cultured without nucleosomes produced 13 ± 6 spots as background. (B) Lin−c-Kit+pure cells and T cells from SNF1 mice were co-cultured in the presence of 20μg/ml nucleosomes, and then analyzed for relative expression of mRNA of RORγT, Igtb1, Igtb4, IL-23R, IL-22, and IL-21 by real time PCR at various time points. T cells alone or Lin−c-Kit+pure cell alone did not express these genes. Igtb1 and Igtb4 mean integrins β1 and β4. Mean ± s.e.m of 3 experiments are shown, n= 15 mice.
FIGURE 6.
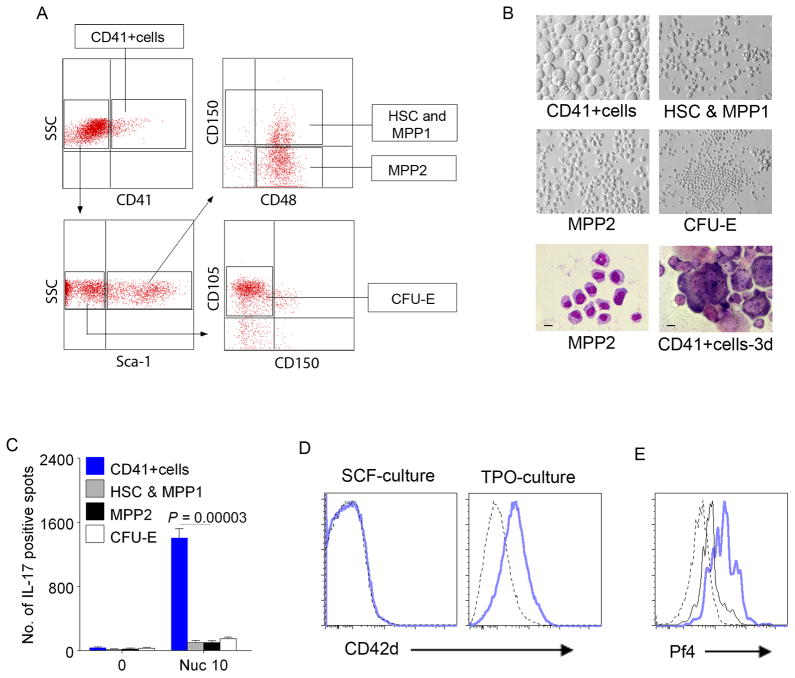
c-Kit+CD41+cells are the Th17 inducers in the Lin−c-Kit+CX3CR1− cell subset. (A) Isolated Lin−c-Kit+CX3CR1− cells were further sorted into CD41+cells, HSC (Sca-1+CD150+CD48−) and MPP1 (Sca-1+CD150+CD48+), MPP2 (Sca-1+CD150−CD48+), and CFU-E (Sca-1−CD150− FcγRIII/II− CD105+low/−). (B) c-Kit+CD41+cells differentiated into megakaryocytes in 3 day culture with IL-3, TPO, EPO and SCF (right bottom panel). Phase contrast of each subset on 3 day culture (top and middle panels) and Giemsa staining of MPP2 cells (left bottom. Bar = 10μm) and cultured CD41+cells (right bottom. Bar = 20μm). Culture conditions for determining hematopoietic lineage potential are in Methods. (C) Sorted c-Kit+CD41+cells (from A) exclusively induced Th17 response to nucleosomes when co-cultured with SNF1 T cells. The sorted subsets of Lin−c-Kit+CX3CR1− cells are designated by color key, and Th17 responses are shown by bars. Mean ± s.e.m. of 5 independent experiments. (D–E) Lin−c-Kit+pure cells differentiate into CD42+cells and produce platelet related molecule, Pf4. FACS shows Lin−c-Kit+pure cells differentiated into CD42+ cells in the presence of TPO but not in SCF alone (D). Nucleosomes feeding increased the production of Pf4 in Lin−c-Kit+pure cells (intracellular staining). PBS (black line) = 14.0% Pf4+ cells vs. Nucleosome (blue) = 55.4% Pf4+ cells in c-Kit+ (CD117+) cell gated population (E). Dotted line = isotype control.
Lin−c-Kit+pure cells express requisite cytokines but are also contact dependent for optimal Th17 induction
Freshly isolated or SCF-cultured Lin−c-Kit+pure cells from spleens of SNF1 mice, expressed mRNAs of Th17 inducing cytokines (38–41) upon incubation with LPS or Nucleosomes, which respectively increased IL-6 mRNA by 27- and 10-fold, TGF-β mRNA by 108- and 18-fold, TNF-α by 38-fold for both, IL-18 by 9- and 5-fold, and IL-1β mRNA by 126- and 90-fold, at 6h time point, but the levels of IL-23/IL-12 (p40), TGFβ-activating integrins (42), or TL1a (43) mRNAs did not increase (Fig. 2A–B, data not shown). Therefore, Lin−c-K+pure cells might also express other mediators facilitating expansion of pre-committed Th17 cells in addition to Th17 induction and priming (described below). Nucleosomes did not stimulate the Lin−c-K+pure cells due to any LPS contaminant, because adding Polymixin B or anti-TLR4 antibody did not inhibit Th17 response elicited by nucleosome-pulsed Lin−c-K+pure APC, and no differences in endotoxin levels were found between nucleosome preparation and PBS or culture media using a endotoxin detection kit (data not shown). Interestingly, transwell cultures showed freshly isolated Lin−c-Kit+pure cells are contact dependent for inducing nucleosome-specific Th17 response (Fig. 2C), indicating that these APC induce Th17 by secreted, and surface molecules working at close range.
FIGURE 2.
Lin−c-Kit+pure cells express Th17-inducing cytokines and are equipped with APC machinery. (A–B) Lin−c-Kit+ pure cells expressed mRNA for Th17 inducing cytokines on LPS stimulation (A) or on pulsing with nucleosomes (B). Note: Although levels of IL-6 and IL-18 mRNAs appear to be low, to accommodate all in the scale; the actual increases at 6hr time point were 27 fold (P = 0.000002) and 9 fold (P = 0.009) by LPS, and 10 fold (P = 0.00023) and 5 fold (P = 0.0015) by nucleosome pulse, respectively. (C) Lin−c-Kit+pure cells fed with nucleosomes were separated from co-cultured T cells by transwell membranes in IL-17 ELISPOT plates and the T cells’ responses are shown. (D–E) Lin−c-Kit+pure cells express MHC class II and CD86 (D), and their ability to induce Th17 response to nucleosomes was blocked by anti-MHC class II antibodies (E). (F) Lin−c-Kit+pure cells efficiently process ovalbumin (DQ-OVA). (G) Representative histograms of CFSE-labeled apoptotic cells engulfed (engulfment was confirmed by microscopy—Methods; similar to Supplemental Fig. 2, also see Fig. 4B). ▼ indicates apoptotic cell engulfed by CD11b+ (left panel) or Lin−c-Kit+pure cells (right panel). (H) % of apoptotic cell engulfed by cell subsets in FACS analysis. Mean ± s.e.m. of 5 separate experiments.
Lin−c-Kit+pure cells have APC machinery to efficiently process and present apoptotic cells and nuclear autoantigen particles
Like other APC, Lin−c-K+pure cells expressed MHC class II and costimulatory molecule CD86; and incubation with nucleosomes, or TLR ligands LPS and CpG, increased MHC class II on their surface. Anti-MHC class II antibodies blocked Lin−c-Kit+pure cell-induced Th17 responses significantly. The Lin−c-Kit+pure cells phagocytosed latex beads and apoptotic cells, and they also could process foreign antigen ovalbumin comparable to DC; and microarray showed they express Cathepsin G (Fig. 2D–H, Supplemental Fig. 2, Supplemental Table I).
Lin−c-Kit+pure cells play pathogenic role in development of lupus disease
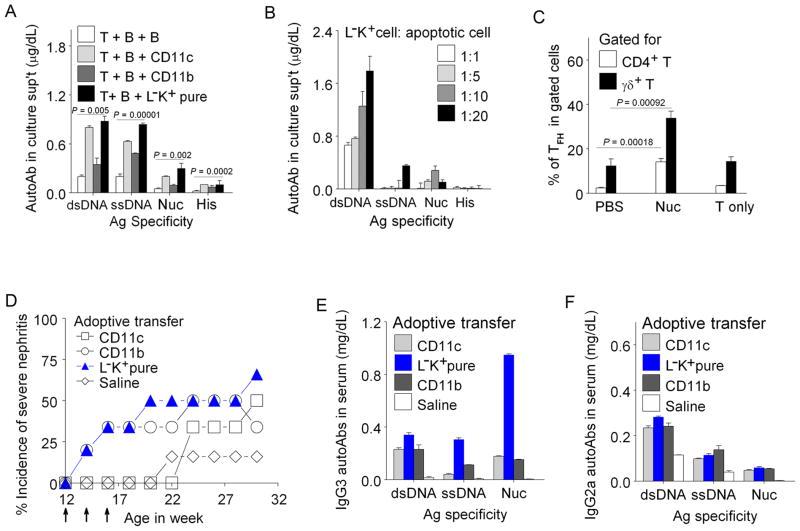
Upon apoptotic cell or nucleosome feeding, Lin−c-Kit+pure cells from female SNF1 mice induced Th17 cells belonging to different Th subsets that are known to induce pathogenic autoantibodies in lupus (10, 31), as detected by ELISPOT, and RORγt increase (44); and they also augmented help for autoantibody production along with expansion of Tfh cells (Fig. 3 and Fig. 4A–C). The CD4−CD8− Th17 cells induced by the Lin−c-Kit+pure APC (Fig. 3) could contain αβ and γδ TCR positive cells and NKT cells (10, 31, 45). To test pathogenic role in vivo, Lin−c-K+pure cells, CD11c+cells (CD11c+CD11b −/low+CD117 −/low+ DC), or CD11b+ cells (containing CD11clow+ DC subset and CD117 −/low+ cells) were pulsed with nucleosomes and then adoptively transferred I.V. into 12 wk female pre-nephritic SNF1 mice 3 times at 2 wk interval. Two weeks after the final transfer (18 wk age), 50% of recipients of Lin−c-Kit+pure cells and 30% CD11b+ cell-recipient mice developed severe nephritis while DC recipients or saline controls did not (Chi squire (χ2) test, P = 0.023; Log Rank Test, P < 0.05). Recipients of DCs started to manifest nephritis only from 8 weeks after the third transfer. The nucleosome pulsed, in vitro manipulated (activated) DC and CD11b+ cell preparations probably caused nephritis by inducing autoimmune Th1cells (as in Fig. 1) in vivo, consistent with other studies (46). Adoptive transfer of Lin−c-Kit+pure cells resulted in marked increase of pathogenic IgG3 anti-nucleosome autoantibodies (P = 0.00038), and also IgG2a anti-dsDNA (P = 0.00036), and IgG2b anti-nucleosome autoantibodies in sera of recipient mice (Fig. 4D–F, data not shown). Thus, nucleosome-pulsed Lin−c-Kit+pure cells are comparable to other professional APC in accelerating lupus disease with only a few transfers.
FIGURE 4.
Lin−c-Kit+pure cells increase production of IgG autoantibodies, expand Tfh cells and play pathogenic role in lupus disease. In vitro helper assay: (A) Lin−c-Kit+ cells (APC) increased production of IgG autoantibodies by lupus T and B cells upon nucleosome stimulation in 7-day helper assay (Methods), and (B) Lin−c-Kit+ cells also activated T cells and B cells to produce IgG autoantibodies in cocultures as shown after processing/presenting apoptotic thymocytes at different ratios. (C) % of Tfh cell expansion by Lin−c-Kit+cells. Three days after co-culturing Lin−c-Kit+cells and T cells with nucleosomes or PBS, cells were stained for ICOS, CXCR5 and PD-1 to identify Tfh cells. Lin−c-Kit+cells expanded Tfh cells in both CD4+ and γδT cell populations upon stimulation with nucleosomes, but Tfh cells were not expanded without APCs (T only). Mean ± s.e.m of 3 experiments, n= 15 mice. (D–F) Lin−c-Kit+pure cells accelerate severe lupus nephritis on adoptive transfer; and markedly increased pathogenic IgG autoantibody levels in sera of recipients (E, F). Arrows indicate the time of adoptive cell transfer. Number of mice/group = 6; except Saline control group = 15. CD11b = CD11b+ CD11c low or − CD117 low or − cells; L−K+cells = Lin−c-Kit+pure cells; Nuc: nucleosomes.
CX3CR1− c-Kit+cells differentially express genes related to megakaryocyte progenitors (MkP&MEP)
CD117 (c-Kit) is also expressed by macrophage/dendritic cell precursors (MDP), which also express the chemokine receptor CX3CR1 (47–49). Therefore, from T and B-cell depleted spleen cells, we sorted out CD117+CX3CR1−, CD117+CX3CR1+ (MDP), CD117−CX3CR1+, and CD117−CX3CR1− cell subsets, and then tested their abilities to induce Th1 and Th17 responses to nucleosomes. CD117+CX3CR1− cells were Lin− (Lin−c-Kit+CX3CR1− or L−K+Cx−), and they induced solely Th17 response whereas CD117−CX3CR1+cells induced only Th1 response, but CD117+CX3CR1+ (L−K+Cx+) cells induced high Th1 and low Th17 responses (Fig. 5A–C). The L−K+Cx− cells here are very similar to L−K+pure cells (Fig. 1), which are also CX3CR1−, although isolated in a different way. Therefore, we compared gene expression profiles of nucleosome-pulsed, Th17 inducing APC that were isolated in those two ways, namely purified Lin−c-Kit+ pure (L−K+pure) cells or the Lin−c-Kit+CX3CR1− (L−K+Cx−) cell subset, with Th1 inducing APCs, namely Lin−c-Kit+CX3CR1+ (L−K+Cx+) subset, or CD11c+CD11blowc-Kit− cells (DC). We purified CD11c+CD117− cells from total DC to compare (and contrast) with the former two cell preparations, because c-Kit+ (CD117+) DC that are present in total CD11c+ cells can induce nuclear autoantigen-specific Th17 cells, albeit to a much lower extent than the former (Fig. 1A–B, and Supplemental Fig. 1B–E). Spleen cells from 3 batches of three 5 mo SNF1 female mice per batch were used to perform three independent isolations of each cell subpopulation. By analyzing the functions of 230 genes that were significantly and differentially UP-regulated in all four pair comparisons (Methods; and microarray data in Supplemental Table I), the two Th17 inducing APC isolates, namely Lin−c-Kit+CX3CR1− (L−K+Cx−) cell subset or purified Lin−c-Kit+pure (L−K+pure) cells, highly expressed genes for transcription factors, Gfi-1B, GATA1, GATA2, NF-E2, Meis1, Tal1 (SCL); all important regulators of megakaryocytopoiesis found in megakaryocyte progenitor (MkP) or bipotent megakaryocyte-erythroid progenitor (MEP) cells (50, 51), as well as genes for other signature molecules expressed by megakaryocyte progenitors/platelet lineage, such as CD41, CD42, Pf4, CD151 and thrombopoietin (TPO) receptor (mpl, CD110) (Fig. 5D, Supplemental Fig. 3A, Supplemental Table I).
FIGURE 5.
Lin−c-Kit+CX3CR1− cell subset expresses megakaryocyte progenitor genes. (A) Flow cytometry of c-Kit+ (CD117+) cells for CX3CR1marker in CD90−CD19− splenocytes of SNF1 mice. (B–C) The subsets of c-Kit+ cells (APC) were isolated by sorting (designated by color key) and then analyzed for inducing IFN-γ (B) and IL-17 responses (C) in SNF1 T cells to nucleosomes. Mean ± s.e.m. of 5 independent experiments. (D) Microarray analysis (average of triplicates): Each row represents a gene and each column represents each APC type. L−K+Cx− = Lin−c-Kit+CX3CR1− cells; L−K+Cx+ = Lin−c-Kit+CX3CR1+ cells; DC = dendritic cells. Genes with highly significant changes (3–22 fold, P as low as 0.000000000044) are shown.
CD41+c-Kit+ cells in Lin−c-Kit+ CX3CR1− APC were responsible for inducing Th17 response to nucleosomes
We further fractionated Lin−c-Kit+CX3CR1− cells by sorting them to CD41+ cells, HSC (hematopoietic stem cell), MPP1(multipotent progenitor 1), MPP2, and CFU-E (Colony-Forming Unit-erythriod progenitor), (Fig. 6A, and Supplemental Fig. 3B), based on BM progenitor subsetting criteria (52, 53), and then analyzed their APC function in inducing Th17. Only the CD41+cells induced very strong Th17 responses to nucleosomes (Fig. 6C), and because these CD41+ Lin− c-Kit+CX3CR1− cells of lupus spleen contained MEP (PreMegE) and MkP by CFU assay, and could differentiate into megakaryocytes/platelets in the presence of TPO, we call them MM cells (MkP and MEP-like cells that are CD41+c-Kit+ and possessing APC machinery). Moreover, feeding the MM cells with nucleosomes resulted in increased production of platelet Pf4 (Fig. 6B–E, Supplemental Table I). Once the Lin−c-Kit+CX3CR1− subset’s CD41+ cells differentiated to become c-Kit−CD41+, they could not present nuclear autoantigens to induce Th17 cells (these cells would be included in the c-Kit−CX3CR1− cell subset of APC in Fig. 5 AC, and the cells in right lower quadrant of Fig. 7A, described below).
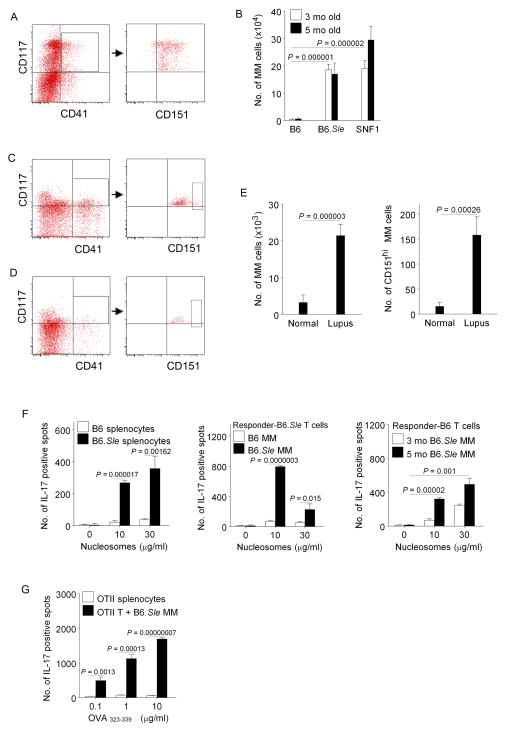
FIGURE 7.
c-Kit+CD151+CD41+cells are expanded in mice and humans with lupus. (A) FACS gating of c-Kit+ CD151+CD41+cells (MM)in Lin− splenocytes of 16wk old SNF1 mice. Most of c-Kit+CD41+cells belong to CD117+CD151+cell population. (B) c-Kit+CD41+CD151+ (MM) cell numbers per spleen of lupus-prone (nine SNF1 and six B6.Sle) and normal (six B6) mice. (C–D) FACS Gating of MM cells in lupus patient’s (C) and normal subject’s (D) PBMNC. From CD3−CD19−CD14−CD16− cells in PBMNC, CD117High + CD41+ cells were gated first (boxes), and all of them were CD151+. CD151High+ cells among CD117High + CD41+ cells are shown boxed in right panels. (E) Numbers of MM cells per 1 × 106 human PBMNC (10 lupus, and 6 normal subjects). CD117High +CD41+ with all CD151+ cells (left) and with CD151High+ cells (right). (F) B6.Sle splenocytes have augmented Th17 response to nucleosomes (left) like SNF1, and MM cells from B6.Sle, but not B6, induce Th17 response to nucleosomes in B6.Sle (center) as well as in B6 (right). (G) MM cells from B6.Sle induce ovalbumin peptide OVA323–339 -specific Th17 response in OTII transgenic normal B6 background mice, without artificial polarizing conditions. APC types are designated by key and Th17 responses shown as bars. Mean ± s.e.m. from 3 independent experiments.
Lin−c-Kit+CD151+CD41+ (MM) cells are expanded in different lupus prone mice and lupus patients
Among other surface markers indicated by microarray, all CD41+ cells in Lin−c-Kit+CX3CR1− subset (MM cells) highly expressed the tetraspanin CD151 (Fig. 5D, Fig 7A,C,D, Supplemental Table I). On the other hand, the c-Kit−CD41+ cells in the right lower quadrant of Fig. 7A, did not express CD151 in the mice or induce Th17 response to nucleosomes (11 ± 4 spots); and those cells would be included in all Lin− c-Kit− APC preparations, none of which were able to induce Th17 response (Figs. 1, 5 and 7). The MM cells in lupus-prone SNF1 spleens were increased up to 49 fold more (P = 0.00000001) than normal C57BL/6 (B6), increasing with age; but the SNF1 MM cell’s Th17-inducing ability were similar irrespective of age (Fig. 7B, data not shown). Similar to nucleosomes (8), whole apoptotic cells also stimulated Th17 response in splenocytes of lupus-prone SNF1 female mice (P = 0.0387 – 0.000136), but not in normal SWR, which have very few MM cells (data not shown). Another lupus-prone strain, B6.Sle also had up to 46 fold increase of MM (P = 0.00000021) as compared to B6. Even though measurement was feasible only in peripheral blood (not spleens), lupus patients (in clinical remission) also showed 4.8 fold increase in MM cells (P = 0.0000025), and 10.4 fold increase CD151+hi MM cells (P = 000026) gated for CD117High+ CD41+ cells in peripheral blood, as compared to normal humans (Fig. 7E). There was no correlation between frequency of MM cells and the clinical activity scores (SLEDAI or SLAM) in these patients (Table I).
MM cells break tolerance in normal mice and induce Th17 skewing in response to foreign antigens without polarizing culture conditions
The MM cells from B6.Sle induced Th17-skewed responses to nucleosomes like SNF1; and remarkably they could induce Th17 differentiation in naïve, normal B6 (Fig. 7F), without the polarizing culture conditions required by other APC types (54). However, cells of MM phenotype purified from large batches of B6 spleens lacked the ability to induce autoimmune Th17 (Fig. 7F). Remarkably, MM APC purified from B6.Sle mice could also elicit Th17-dominant response to foreign antigen, ovalbumin (OVA peptide323–339), in transgenic mice of normal B6 background’s T cells bearing receptors for OVA323–339, without artificial polarizing culture conditions or PMA Ionomycin stimulation. In contrast splenic APC of the OTII mice themselves failed to induce Th17 (Fig. 7G). Thus MM are Th17-inducing APC in general.
Molecular targets in MM APC for blocking autoimmune response
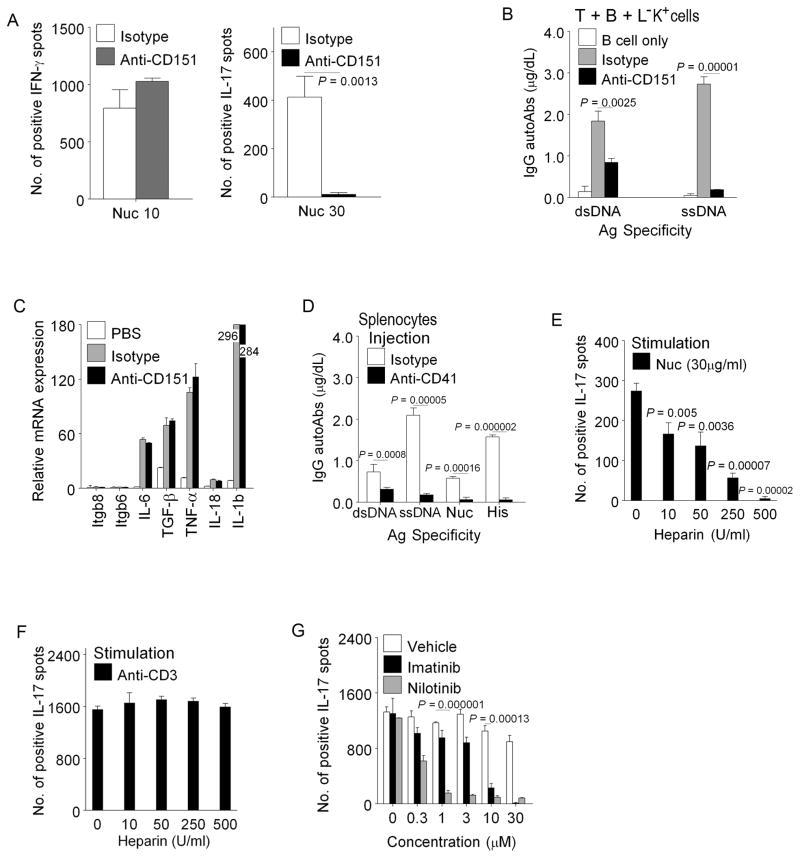
Lin−c-Kit+CX3CR1− cells expressed Megakaryocyte/platelet related molecules (Fig. 5D, Supplemental Table I). By qPCR and Flow Cytometry of differentially expressed gene products, we validated and narrowed down candidate molecules in MM cells, which could be tested to inhibit the c-Kit+ MM APC themselves from presenting autoantigens or producing known Th17-inducing cytokines, and/or interfere with close range (contact dependent) interactions necessary for inducing Th17 cells, after nucleosome pulsing. We also tested whether blocking of the select target molecule on MM cells pulsed with nucleosomes can suppress their ability to stimulate T cell helper function for B cells to produce IgG autoantibodies in co-culture using established methods (33, 34, 55). Among all those candidates tested (Fig. 5D), positive results are described here. Antibody to the tetraspanin CD151 blocked nucleosome stimulated anti-DNA autoantibody production, and induction of Th17 response by Lin−c-Kit+CX3CR1− APC, but not induction of Th1 response by Lin−c-Kit+CX3CR1+ APC subset (Fig. 8A–B); the former APC subset predominantly induces Th17 whereas the latter induces Th1 response to nucleosomes (Fig. 5B–C). CD151could stabilize interaction of MM cells by binding to integrin α4β1 and β4 on Th17 cells (56, 57), whose expression was markedly augmented in the T cells when co-cultured with MM cells pulsed with nucleosomes (Fig. 3B), and anti-CD151 did not inhibit MM APC directly (Fig. 8C). CD151 is also expressed by other cells and APC (Supplemental Fig. 3B, Supplemental Table I). We also obtained positive results by targeting CD41, the MM surface molecule (Fig. 5D, Supplemental Table I). CD41 is an integrin subunit expressed on MM cell membranes and hypothetically could augment Th17 induction by increasing adhesive interactions and cell surface signaling. The CD41 integrin subunit, alpha IIb forms a non-covalently linked complex with beta 3 to be expressed on cell surface (http://www.ncbi.nlm.nih.gov/gene/3674), and functions as a receptor for fibronectin and VWF. However, beta 3 integrin subunit message was not differentially increased in MM cells (Supplemental Table I). We injected lupus prone SNF1 mice I.P. with 1mg/Kg of anti-CD41 antibody (clone MWReg 30), or control IgG in 0.2 ml of PBS, followed by additional injections of 0.5 mg/Kg antibody per day for 6 days per protocol (58, 59). Frequency of splenic MM cells (CD41+CD117+CD151+) and nucleosome-specific Th17 cells; IgG autoantibody levels in sera were compared with controls, using described methods (8, 34). Surprisingly, the frequency of CD41+ CD117+ cells actually increased on anti-CD41 antibody treatment, probably due to a rapid compensatory response by BM to depletion of these cells. However, anti-CD41 injections caused a significant decrease of nucleosome-stimulated IgG autoantibody production ex vivo by the splenocytes of the treated mice after 7 days of anti-CD41 injections (Fig. 8D). In addition, we found that heparin, which binds strongly to Pf4 that is expressed by MM, inhibited MM-induced, nucleosome-specific Th17 response, but not anti-CD3 stimulated Th17 expansion (Fig. 8E-F, and Supplemental Table I). Finally, among the target molecules that tested positive in functional inhibition assays, was CD117 (c-Kit), the stem cell factor receptor expressed on MM cells, and c-Kit (CD117) inhibitors, Imatinib and Nilotinib suppressed MM APC-induced nucleosome-specific Th17 response (Fig. 8G).
FIGURE 8.
Certain agents block Th17 induction by MM (c-Kit+CD151+CD41+) cells. (A) Inhibition of nucleosome-specific Th17 response by anti-CD151: Lin−c-Kit+CX3CR+ (left) or Lin−c-Kit+CX3CR1− (right) APC subsets and T cells from SNF1 spleens were incubated with nucleosomes and CD151 antibody or isotype control, in IFN-γ and IL-17 response assays. The former subset induces Th1 and the latter induces Th17 (previously shown in Fig. 5 B–C). (B) Anti-CD151 antibody (100μg/ml) suppressed IgG autoantibody production in nucleosome-stimulated helper assay. T+B cell co-cultures without nucleosomes produce very little autoantibodies in these helper assays as previously shown in Fig. 4 A (T+B+B). (C) Blocking with anti-CD151 antibody did not affect mRNA expression of integrins (beta 6 and 8) and Th17 inducing cytokines by nucleosome pulsed Lin−c-Kit+pure cells. (D) Splenocytes from 4 mo old SNF1 mice injected with anti-CD41 antibody produced markedly reduced amount of IgG autoantibodies ex vivo in nucleosome stimulated cultures, as compared to isotype control injected mice. (E) Heparin inhibited Th17 response to nucleosomes presented by MM cells in ELISPOT assays. (F) Heparin could not inhibit Th17 response to anti-CD3 stimulation. (G) c-Kit inhibitors (Imatinib or Nilotinib) inhibited MM induced nucleosome-specific Th17 responses. Mean ± s.e.m. from 3 independent experiments. n= 15 mice.
DISCUSSION
The series of experiments here show that among Lin−c-Kit+ cells depleted of conventional APC and mast cells, the most efficient presentation of apoptotic cell nuclear antigens for inducing autoimmune Th17 cells is a property of the CD41+CD151+ subset of c-Kit+CX3CR1− cells, which could be made to differentiate into megakaryocyte/platelet and erythrocytes with appropriate growth factors (similar to MkP and MEP in BM), but they lost autoantigen-presenting and Th17-inducing ability upon differentiation to c-Kit−CD41+ cells. Thus we call these new type of APC resembling bone marrow MkP and MEP progenitor cells, as MM cells, because they are found in the lupus spleen with nuclear autoantigen presenting function, expressing previously unreported marker molecules in addition to possessing antigen processing machinery, and being capable of producing requisite Th17-inducing cytokines and co-stimulators. The MM cells lack receptors for Flt3 ligand and macrophage colony-stimulating factor (M-CSF-R) (data not shown), besides lacking CX3CR1; thus they do not have myeloid or dendritic cell-macrophage progenitor markers (47–49). Moreover, the earliest progenitor-like cells in the lupus splenic Lin−c-Kit+ cells that resemble HSC and MPP1 and MPP2 cells of BM (52, 53), did not have Th17 inducing capability. Another variety of Lin–c-Kit+ APC, totally different from MM here, are generated by deliberate stimulation with IL-25; they are MPP-like Th2 inducing cells residing in gut-associated lymphoid tissue; and they differentiate into macrophage and granulocyte lineages (60). Th17-inducing DC subsets have been detected in other systems (61, 62), but c-Kit stimulation of the DC or PMA and Ionomycin stimulation of the T cells was required to detect Th17 response in those cases in contrast to the Th17 inducing MM APC here. Moreover, Th17 inducing ability of c-Kit+ DCs was miniscule as compared to MM cells here under natural autoantigen presenting conditions (Fig. 1).
Notably, MM cells express megakaryocyte progenitor molecules that are also expressed by platelets (Fig. 5D, Supplementary Table I). Platelets have been implicated in lupus pathogenesis, by expressing CD40L, producing cytokines and participating in immune complex mediated inflammation (63, 64). However, the c-Kit+ MM APC from lupus subjects isolated by flow cytometry sorting or magnetic beads had the size and morphologic characteristic of nucleated mononuclear cells (Fig. 1, Supplemental Fig.1), which would exclude or gate out platelets, and we found here that only the MM APC could uptake and present nuclear autoantigens and induce Th17 cells in a class II dependent manner before undergoing further differentiation (Fig. 1 and 2). In a related issue, anti-CD41 injections that caused a significant decrease of nucleosome-stimulated IgG autoantibody production ex vivo by the splenocytes of the treated mice (Fig. 8D), would have also depleted platelets (58, 59), which could have helped autoimmune B cells by increased expression of CD40L. However, in lupus both autoimmune Th and B cells hyper-express CD40L due to intrinsic defects anyway (29, 65), and the cells from the treated animals here were tested for autoantibody production ex vivo in a 7 day assay.
Remarkably, Th17 induction and expansion by nucleosome-pulsed MM cells occurred without any Th17-polarizing culture conditions or PMA+Ionomycin stimulation that are generally used to detect Th17 cells in other systems. Moreover, contact was required for optimal autoimmune Th17 induction under these natural conditions indicating that MM cells, upon apoptotic cell or nucleosome feeding, require accessory molecules, such as CD151 working at close range for Th17 induction, in addition to producing known cytokines (IL-1, TGFβ, IL-6, TNF, as shown in Fig. 2A,B). The MM cells not only primed but also induced expansion of pre-committed nucleosome-specific Th17 cells which are increasingly seeded into the periphery of lupus mice probably due to inefficient deletion and autoreactive selection in the thymus (56, 66, 67).
Among the array of molecules expressed by MM (Supplementary Table I), we found that anti-CD151 blocked Th17 induction and autoantibody production (Fig. 8A–C). However, anti-CD151 did not inhibit the MM cells themselves (Fig. 8C). CD151could facilitate interaction of MM cells with Th17 cells by increasing adhesion. Indeed, Th17 cells express the CD151binding integrin α4β1(56), whose expression was markedly augmented in the T cells when co-cultured with MM cells pulsed with nucleosomes (Fig. 3B). Of course, CD151 or the other molecules on MM that could be targeted to inhibit Th17 induction, such as CD41, Pf4 and c-Kit (Fig. 8) are not uniquely specific for MM cells. Nevertheless, a combination of cytokines and surface molecules expressed by the MM cells make them potent Th17 inducers. Studies with the new type of Th17-inducing APC reveal new mechanisms for agents being beneficial in autoimmunity.
We have clearly shown that removal of Lin−c-Kit+CD41+CD151+ cells completely abrogates Th17 inducing ability (Fig. 6C); and conversely adoptive transfer of those cells fed with apoptotic nuclear antigens accelerate lupus disease (Fig. 4D). The preferential increase in IgG3 autoantibodies to nucleosomes by adoptive transfer of nucleosome-pulsed MM cells was striking, although pathogenic IgG2a and IgG2b autoantibody subclasses were also increased (Fig. 4E–F). IgG3 autoantibodies are especially pathogenic in lupus nephritis by forming cryoglobulin immune complex deposits (68, 69). Marginal zone B cells could also be involved in producing IgG3 anti-DNA autoantibodies in lupus (70), especially with the help of CD4−CD8− Th cells containing NKT cells (31, 45), which are induced by the MM cells (Fig. 3A). The expansion of MM cells preceding lupus disease activity and adoptive transfer studies (Fig. 7A–D, Fig. 4D–F) demonstrate their role in pathogenesis in the mouse models. The fact that MM cells are expanded in lupus patients in clinical “remission” confirms that such patients are not in true immunological remission, because they do have smoldering disease and harbor autoimmune Th cells (5, 30). The observations in this study advance our understanding of lupus autoimmunization and Th17 induction by characterizing a new type of APC.
Supplementary Material
Footnotes
Abbreviations: SNF1, (SWR x NZB)F1; MkP, Megakaryocyte progenitor; MEP, bipotent Megakaryocyte/Erythroid–progenitor; MM cells, APC resembling Megakaryocyte– and Megakaryocyte/Erythroid–progenitors; CX3CR1, CX3C chemokine receptor 1; HSC, hematopoietic stem cell; MPP, multipotent progenitor ; Sle, Systemic lupus erythematosus.
Three Supplemental Figures, and One Supplemental Table I showing Microarray data. Microarray data has been deposited in the GEO database under accession number “GSE36284” http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE36284
References
- 1.Barcellos LF, May SL, Ramsay PP, Quach HL, Lane JA, Nititham J, Noble JA, Taylor KE, Quach DL, Chung SA, Kelly JA, Moser KL, Behrens TW, Seldin MF, Thomson G, Harley JB, Gaffney PM, Criswell LA. High-density SNP screening of the major histocompatibility complex in systemic lupus erythematosus demonstrates strong evidence for independent susceptibility regions. PLoS Genet. 2009;5:e1000696. doi: 10.1371/journal.pgen.1000696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adams S, Leblanc P, Datta SK. Junctional region sequences of T-cell receptor b chain genes expressed by pathogenic anti-DNA autoantibody-inducing T helper cells from lupus mice: Possible selection by cationic autoantigens. Proc Natl Acad Sci USA. 1991;88:11271–11275. doi: 10.1073/pnas.88.24.11271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mohan C, Adams S, Stanik V, Datta SK. Nucleosome: A major immunogen for the pathogenic autoantibody-inducing T cells of lupus. J Exp Med. 1993;177:1367–1381. doi: 10.1084/jem.177.5.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaliyaperumal A, Mohan C, Wu W, Datta SK. Nucleosomal peptide epitopes for nephritis-inducing T helper cells of murine lupus. J Exp Med. 1996;183:2459–2469. doi: 10.1084/jem.183.6.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu L, Kaliyaperumal A, Boumpas DT, Datta SK. Major peptide autoepitopes for nucleosome-specific T cells of human lupus. J Clin Invest. 1999;104:345–355. doi: 10.1172/JCI6801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balomenos D, Rumold R, Theofilopoulos AN. Interferon-gamma is required for lupus-like disease and lymphoaccumulation in MRL-lpr mice. J Clin Invest. 1998;101:364–371. doi: 10.1172/JCI750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doreau A, Belot A, Bastid J, Riche B, Trescol-Biemont MC, Ranchin B, Fabien N, Cochat P, Pouteil-Noble C, Trolliet P, Durieu I, Tebib J, Kassai B, Ansieau S, Puisieux A, Eliaou JF, Bonnefoy-Berard N. Interleukin 17 acts in synergy with B cell-activating factor to influence B cell biology and the pathophysiology of systemic lupus erythematosus. Nat Immunol. 2009;10:778–785. doi: 10.1038/ni.1741. [DOI] [PubMed] [Google Scholar]
- 8.Kang HK, Liu M, Datta SK. Low-Dose Peptide Tolerance Therapy of Lupus Generates Plasmacytoid Dendritic Cells That Cause Expansion of Autoantigen-Specific Regulatory T Cells and Contraction of Inflammatory Th17 Cells (cover page Figure) J Immunol. 2007;178:7849–7858. doi: 10.4049/jimmunol.178.12.7849. [DOI] [PubMed] [Google Scholar]
- 9.Hsu HC, Yang P, Wang J, Wu Q, Myers R, Chen J, Yi J, Guentert T, Tousson A, Stanus AL, Le TV, Lorenz RG, Xu H, Kolls JK, Carter RH, Chaplin DD, Williams RW, Mountz JD. Interleukin 17-producing T helper cells and interleukin 17 orchestrate autoreactive germinal center development in autoimmune BXD2 mice. Nat Immunol. 2008;9:166–175. doi: 10.1038/ni1552. [DOI] [PubMed] [Google Scholar]
- 10.Crispin JC, Oukka M, Bayliss G, Cohen RA, Van Beek CA, Stillman IE, Kyttaris VC, Juang YT, Tsokos GC. Expanded double negative T cells in patients with systemic lupus erythematosus produce IL-17 and infiltrate the kidneys. J Immunol. 2008;181:8761–8766. doi: 10.4049/jimmunol.181.12.8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fu SM, Deshmukh US, Gaskin F. Pathogenesis of systemic lupus erythematosus revisited 2011: end organ resistance to damage, autoantibody initiation and diversification, and HLA-DR. J Autoimmun. 2011;37:104–112. doi: 10.1016/j.jaut.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 13.Leadbetter EA, I, Rifkin R, Hohlbaum AM, Beaudette BC, Shlomchik MJ, Marshak-Rothstein A. Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature. 2002;416:595–598. doi: 10.1038/416603a. [DOI] [PubMed] [Google Scholar]
- 14.Blanco P, Palucka AK, Gill M, Pascual V, Banchereau J. Induction of dendritic cell differentiation by IFN-alpha in systemic lupus erythematosus. Science. 2001;294:1540–1543. doi: 10.1126/science.1064890. [DOI] [PubMed] [Google Scholar]
- 15.Means TK, Latz E, Hayashi F, Murali MR, Golenbock DT, Luster AD. Human lupus autoantibody-DNA complexes activate DCs through cooperation of CD32 and TLR9. J Clin Invest. 2005;115:407–417. doi: 10.1172/JCI23025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bave U, Magnusson M, Eloranta ML, Perers A, Alm GV, Ronnblom L. Fc gamma RIIa is expressed on natural IFN-alpha-producing cells (plasmacytoid dendritic cells) and is required for the IFN-alpha production induced by apoptotic cells combined with lupus IgG. J Immunol. 2003;171:3296–3302. doi: 10.4049/jimmunol.171.6.3296. [DOI] [PubMed] [Google Scholar]
- 17.Niewold TB, Kelly JA, Flesch MH, Espinoza LR, Harley JB, Crow MK. Association of the IRF5 risk haplotype with high serum interferon-alpha activity in systemic lupus erythematosus patients. Arthritis Rheum. 2008;58:2481–2487. doi: 10.1002/art.23613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teichmann LL, Ols ML, Kashgarian M, Reizis B, Kaplan DH, Shlomchik MJ. Dendritic cells in lupus are not required for activation of T and B cells but promote their expansion, resulting in tissue damage. Immunity. 2010;33:967–978. doi: 10.1016/j.immuni.2010.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rock KL, Benacerraf B, Abbas AK. Antigen presentation by hapten-specific B lymphocytes. J Exp Med. 1984;160:1102–1113. doi: 10.1084/jem.160.4.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chaturvedi A, Dorward D, Pierce SK. The B cell receptor governs the subcellular location of Toll-like receptor 9 leading to hyperresponses to DNA-containing antigens. Immunity. 2008;28:799–809. doi: 10.1016/j.immuni.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu W, Meckel T, Tolar P, Sohn HW, Pierce SK. Antigen affinity discrimination is an intrinsic function of the B cell receptor. J Exp Med. 2010;207:1095–1111. doi: 10.1084/jem.20092123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benschop RJ, Aviszus K, Zhang X, Manser T, Cambier JC, Wysocki LJ. Activation and Anergy in Bone Marrow B Cells of a Novel Immunoglobulin Transgenic Mouse that Is Both Hapten Specific and Autoreactive. Immunity. 2001;14:33–43. doi: 10.1016/s1074-7613(01)00087-5. [DOI] [PubMed] [Google Scholar]
- 23.Coffey F, Liu X, Manser T. Primary development and participation in a foreign antigen-driven immune response of a chromatin-reactive B cell clonotype are not influenced by TLR9 or other MyD88-dependent TLRs. J Immunol. 2007;179:6663–6672. doi: 10.4049/jimmunol.179.10.6663. [DOI] [PubMed] [Google Scholar]
- 24.Datta SK, Schwartz RS. Genetics of expression of xenotropic virus and autoimmunity in NZB mice. Nature. 1976;263:412–415. doi: 10.1038/263412b0. [DOI] [PubMed] [Google Scholar]
- 25.Datta SK, Manny N, Andrzejewski C, Andre-Schwartz J, Schwartz RS. Genetic studies of autoimmunity and retrovirus expression in crosses of New Zealand Black mice. I. xenotropic virus. J Exp Med. 1978;147:854–871. doi: 10.1084/jem.147.3.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morel L, Croker BP, Blenman KR, Mohan C, Huang G, Gilkeson G, Wakeland EK. Genetic reconstitution of systemic lupus erythematosus immunopathology with polycongenic murine strains. Proc Natl Acad Sci U S A. 2000;97:6670–6675. doi: 10.1073/pnas.97.12.6670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, Schaller JG, Talal N, Winchester RJ. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 28.Liang MH, Socher SA, Larson MG, Schur PH. Reliability and validity of six systems for the clinical assessment of disease activity in systemic lupus erythematosus. Arthritis Rheum. 1989;32:1107–1118. doi: 10.1002/anr.1780320909. [DOI] [PubMed] [Google Scholar]
- 29.Desai-Mehta A, Lu L, Ramsey-Goldman R, Datta SK. Hyperexpression of CD40 ligand by B and T cells in human lupus and its role in pathogenic autoantibody production. J Clin Invest. 1996;97:2063–2073. doi: 10.1172/JCI118643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang L, Bertucci AM, Ramsey-Goldman R, Burt RK, Datta SK. Regulatory T cell (Treg) subsets return in patients with refractory lupus following stem cell transplantation, and TGF-b producing CD8+ Treg cells are associated with immunological remission of lupus. J Immunol. 2009;183:6346–6358. doi: 10.4049/jimmunol.0901773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rajagopalan S, Zordan T, Tsokos GC, Datta SK. Pathogenic anti-DNA autoantibody inducing T helper cell lines from patients with active lupus nephritis: Isolation of CD4−/CD8− T helper cell lines that express the gd T-cell receptor. Proc Natl Acad Sci USA. 1990;87:7020–7024. doi: 10.1073/pnas.87.18.7020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kang HK, Ecklund D, Liu M, Datta SK. Apigenin, a non-mutagenic dietary flavonoid, suppresses lupus by inhibiting autoantigen presentation for expansion of autoreactive Th1 and Th17 cells. Arthritis Res Ther. 2009;11:R59. doi: 10.1186/ar2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaliyaperumal A, Michaels MA, Datta SK. Naturally processed chromatin peptides reveal a major autoepitope that primes pathogenic T and B cells of lupus. J Immunol. 2002;168:2530–2537. doi: 10.4049/jimmunol.168.5.2530. [DOI] [PubMed] [Google Scholar]
- 34.Kang HK, Michaels MA, Berner BR, Datta SK. Very low-dose tolerance with nucleosomal peptides controls lupus and induces potent regulatory T cell subsets. J Immunol. 2005;174:3247–3255. doi: 10.4049/jimmunol.174.6.3247. [DOI] [PubMed] [Google Scholar]
- 35.Lin SM, Du P, Huber W, Kibbe WA. Model-based variance-stabilizing transformation for Illumina microarray data. Nucleic Acids Res. 2008;36:e11. doi: 10.1093/nar/gkm1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wettenhall JM, Smyth GK. limmaGUI: a graphical user interface for linear modeling of microarray data. Bioinformatics. 2004;20:3705–3706. doi: 10.1093/bioinformatics/bth449. [DOI] [PubMed] [Google Scholar]
- 37.Sunderkotter C, Nikolic T, Dillon MJ, Van Rooijen N, Stehling M, Drevets DA, Leenen PJ. Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response. J Immunol. 2004;172:4410–4417. doi: 10.4049/jimmunol.172.7.4410. [DOI] [PubMed] [Google Scholar]
- 38.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 39.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 40.Mangan PR, Harrington LE, O’Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 41.McGeachy MJ, Cua DJ. Th17 cell differentiation: the long and winding road. Immunity. 2008;28:445–453. doi: 10.1016/j.immuni.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 42.Melton AC, Bailey-Bucktrout SL, Travis MA, Fife BT, Bluestone JA, Sheppard D. Expression of alphavbeta8 integrin on dendritic cells regulates Th17 cell development and experimental autoimmune encephalomyelitis in mice. J Clin Invest. 2010;120:4436–4444. doi: 10.1172/JCI43786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pappu BP, Borodovsky A, Zheng TS, Yang X, Wu P, Dong X, Weng S, Browning B, Scott ML, Ma L, Su L, Tian Q, Schneider P, Flavell RA, Dong C, Burkly LC. TL1A-DR3 interaction regulates Th17 cell function and Th17-mediated autoimmune disease. J Exp Med. 2008;205:1049–1062. doi: 10.1084/jem.20071364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou L, Littman DR. Transcriptional regulatory networks in Th17 cell differentiation. Curr Opin Immunol. 2009;21:146–152. doi: 10.1016/j.coi.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zeng D, Lee MK, Tung J, Brendolan A, Strober S. Cutting edge: A role for CD1 in the pathogenesis of lupus in NZB/NZW mice. J Immunol. 2000;164:5000–5004. doi: 10.4049/jimmunol.164.10.5000. [DOI] [PubMed] [Google Scholar]
- 46.Okamoto A, Fujio K, van Rooijen N, Tsuno NH, Takahashi K, Tsurui H, Hirose S, Elkon KB, Yamamoto K. Splenic Phagocytes Promote Responses to Nucleosomes in (NZB x NZW) F1 Mice. J Immunol. 2008;181:5264–5271. doi: 10.4049/jimmunol.181.8.5264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fogg DK, Sibon C, Miled C, Jung S, Aucouturier P, Littman DR, Cumano A, Geissmann F. A clonogenic bone marrow progenitor specific for macrophages and dendritic cells. Science. 2006;311:83–87. doi: 10.1126/science.1117729. [DOI] [PubMed] [Google Scholar]
- 48.Onai N, Obata-Onai A, Schmid MA, Ohteki T, Jarrossay D, Manz MG. Identification of clonogenic common Flt3+M-CSFR+ plasmacytoid and conventional dendritic cell progenitors in mouse bone marrow. Nat Immunol. 2007;8:1207–1216. doi: 10.1038/ni1518. [DOI] [PubMed] [Google Scholar]
- 49.Waskow C, Liu K, Darrasse-Jeze G, Guermonprez P, Ginhoux F, Merad M, Shengelia T, Yao K, Nussenzweig M. The receptor tyrosine kinase Flt3 is required for dendritic cell development in peripheral lymphoid tissues. Nat Immunol. 2008;9:676–683. doi: 10.1038/ni.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tober J, Koniski A, McGrath KE, Vemishetti R, Emerson R, de Mesy-Bentley KK, Waugh R, Palis J. The megakaryocyte lineage originates from hemangioblast precursors and is an integral component both of primitive and of definitive hematopoiesis. Blood. 2007;109:1433–1441. doi: 10.1182/blood-2006-06-031898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Klimchenko O, Mori M, Distefano A, Langlois T, Larbret F, Lecluse Y, Feraud O, Vainchenker W, Norol F, Debili N. A common bipotent progenitor generates the erythroid and megakaryocyte lineages in embryonic stem cell-derived primitive hematopoiesis. Blood. 2009;114:1506–1517. doi: 10.1182/blood-2008-09-178863. [DOI] [PubMed] [Google Scholar]
- 52.Morita Y, Ema H, Nakauchi H. Heterogeneity and hierarchy within the most primitive hematopoietic stem cell compartment. J Exp Med. 2010;207:1173–1182. doi: 10.1084/jem.20091318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pronk CJ, Rossi DJ, Mansson R, Attema JL, Norddahl GL, Chan CK, Sigvardsson M, Weissman IL, Bryder D. Elucidation of the phenotypic, functional, and molecular topography of a myeloerythroid progenitor cell hierarchy. Cell Stem Cell. 2007;1:428–442. doi: 10.1016/j.stem.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 54.Dong C. TH17 cells in development: an updated view of their molecular identity and genetic programming. Nat Rev Immunol. 2008;8:337–348. doi: 10.1038/nri2295. [DOI] [PubMed] [Google Scholar]
- 55.Datta SK, Patel H, Berry D. Induction of a cationic shift in IgG anti-DNA autoantibodies. Role of T helper cells with classical and novel phenotypes in three murine models of lupus nephritis. J Exp Med. 1987;165:1252–1268. doi: 10.1084/jem.165.5.1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Marks BR, Nowyhed HN, Choi JY, Poholek AC, Odegard JM, Flavell RA, Craft J. Thymic self-reactivity selects natural interleukin 17-producing T cells that can regulate peripheral inflammation. Nat Immunol. 2009;10:1125–1132. doi: 10.1038/ni.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sterk LM, Geuijen CA, van den Berg JG, Claessen N, Weening JJ, Sonnenberg A. Association of the tetraspanin CD151 with the laminin-binding integrins alpha3beta1, alpha6beta1, alpha6beta4 and alpha7beta1 in cells in culture and in vivo. J Cell Sci. 2002;115:1161–1173. doi: 10.1242/jcs.115.6.1161. [DOI] [PubMed] [Google Scholar]
- 58.Marjon KD, Marnell LL, Mold C, Du Clos TW. Macrophages activated by C-reactive protein through Fc gamma RI transfer suppression of immune thrombocytopenia. J Immunol. 2009;182:1397–1403. doi: 10.4049/jimmunol.182.3.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nocito A, Georgiev P, Dahm F, Jochum W, Bader M, Graf R, Clavien PA. Platelets and platelet-derived serotonin promote tissue repair after normothermic hepatic ischemia in mice. Hepatology. 2007;45:369–376. doi: 10.1002/hep.21516. [DOI] [PubMed] [Google Scholar]
- 60.Saenz SA, Siracusa MC, Perrigoue JG, Spencer SP, Urban JF, Jr, Tocker JE, Budelsky AL, Kleinschek MA, Kastelein RA, Kambayashi T, Bhandoola A, Artis D. IL25 elicits a multipotent progenitor cell population that promotes T(H)2 cytokine responses. Nature. 2010;464:1362–1366. doi: 10.1038/nature08901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Krishnamoorthy N, Oriss TB, Paglia M, Fei M, Yarlagadda M, Vanhaesebroeck B, Ray A, Ray P. Activation of c-Kit in dendritic cells regulates T helper cell differentiation and allergic asthma. Nat Med. 2008;14:565–573. doi: 10.1038/nm1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bailey SL, Schreiner B, McMahon EJ, Miller SD. CNS myeloid DCs presenting endogenous myelin peptides ‘preferentially’ polarize CD4+ T(H)-17 cells in relapsing EAE. Nat Immunol. 2007;8:172–180. doi: 10.1038/ni1430. [DOI] [PubMed] [Google Scholar]
- 63.Duffau P, Seneschal J, Nicco C, Richez C, Lazaro E, Douchet I, Bordes C, Viallard JF, Goulvestre C, Pellegrin JL, Weil B, Moreau JF, Batteux F, Blanco P. Platelet CD154 potentiates interferon-alpha secretion by plasmacytoid dendritic cells in systemic lupus erythematosus. Sci Transl Med. 2010;2:47ra63. doi: 10.1126/scitranslmed.3001001. [DOI] [PubMed] [Google Scholar]
- 64.Lood C, Amisten S, Gullstrand B, Jonsen A, Allhorn M, Truedsson L, Sturfelt G, Erlinge D, Bengtsson AA. Platelet transcriptional profile and protein expression in patients with systemic lupus erythematosus: up-regulation of the type I interferon system is strongly associated with vascular disease. Blood. 2010;116:1951–1957. doi: 10.1182/blood-2010-03-274605. [DOI] [PubMed] [Google Scholar]
- 65.Yi Y, McNerney M, Datta SK. Regulatory defects in Cbl and mitogen-activated protein kinase (extracellular signal-related kinase) pathways cause persistent hyperexpression of CD40 ligand in human lupus T cells. J Immunol. 2000;165:6627–6634. doi: 10.4049/jimmunol.165.11.6627. [DOI] [PubMed] [Google Scholar]
- 66.Michaels MA, Kang HK, Kaliyaperumal A, Satyaraj E, Shi Y, Datta SK. A defect in deletion of nucleosome-specific autoimmune T cells in lupus-prone thymus: role of thymic dendritic cells. J Immunol. 2005;175:5857–5865. doi: 10.4049/jimmunol.175.9.5857. [DOI] [PubMed] [Google Scholar]
- 67.Xu Z, Cuda CM, Croker BP, Morel L. The NZM2410-derived lupus susceptibility locus Sle2c1 increases Th17 polarization and induces nephritis in fas-deficient mice. Arthritis Rheum. 2011;63:764–774. doi: 10.1002/art.30146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gavalchin J, Datta SK. The NZB x SWR model of lupus nephritis. II. Autoantibodies deposited in renal lesions show a restricted idiotypic diversity. J Immunol. 1987;138:138–148. [PubMed] [Google Scholar]
- 69.Baudino L, Azeredo da Silveira S, Nakata M, Izui S. Molecular and cellular basis for pathogenicity of autoantibodies: lessons from murine monoclonal autoantibodies. Springer Semin Immunopathol. 2006;28:175–184. doi: 10.1007/s00281-006-0037-0. [DOI] [PubMed] [Google Scholar]
- 70.Zhou Z, Niu H, Zheng YY, Morel L. Autoreactive marginal zone B cells enter the follicles and interact with CD4+ T cells in lupus-prone mice. BMC Immunol. 2011;12:7. doi: 10.1186/1471-2172-12-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.