Abstract
In this paper, one-step air plasma treatment is successfully used for poly(dimethylsiloxane)(PDMS)-plastic chip bonding. The technique is green, cheap, and requires no other reagent other than air. Hydrocarbon plastics: polystyrene (PS), cyclic olefin copolymer (COC), and polypropylene (PP) have all been successfully bonded to PDMS irreversibly. The corresponding compressed air resistances are measured to be around 500 kPa for PDMS-PS, PDMS-COC, and PDMS-PP hybrid chips. The bondings are also of good quality even after storage under different temperatures and subject to solutions from acid to base.
INTRODUCTION
Poly(dimethylsiloxane) (PDMS) is one of the most popular materials in microfluidics for its biocompatibility, optical transparency, simplicity for chip fabrication, and low cost. It has been widely applied to various areas of microfluidics and even nanofluidics. However, in many situations pure PDMS chip might not suffice the requirement due to its elastomeric origin. Hybrid structure is a good choice.
Traditional glass or Si/SiO2 based solid materials can be bonded to PDMS merely by plasma based surface hydroxylation,1 which has dominated the early days of hybrid chips. However, they might not be a good candidate for ultrathin layer or multilayer chips since they are crispy and difficult to be processed.2 In comparison, techniques for processing plastics boom in recent years and have increasingly been applied to microfluidics3 and nanofluidics.4 Plastics are advantageous not only because they are easier for processing or cheaper but also many of them are of high quality in terms of optical transparency, biochemical compatibility, electrical isolation, and/or resistance to the water for long term usage.
Among the plastics applicable to micro-nanofluidics, the family of the hydrocarbon plastics deserves special attention. They are characterized by long term resistance to water and stability in hot water and, thus, are qualified for reuse by autoclave treatment. Also, they are composed of no hetero-element beside C and H, which provides them a low background for different chemical groups. This is especially important if on chip chemical reactions or analysis are required. So hydrocarbon polymers such as polystyrene (PS), cyclic olefin copolymer (COC), and polypropylene (PP) are increasingly integrated to the microfluidic chips. The most outstanding material among them might be COC, which holds qualities that may take the place of other plastics in most circumstances for chip fabrication. Besides the qualities mentioned above, with optical transparency comparable to glass, it is widely adaptable for applications from optical monitoring to x-ray analysis. PS is also of high quality for optical transparency and biocompatibility. PP is the most widely applied material as water resistant heat stable containers. Considering this, it is figured that bonding PDMS to hydrocarbon plastics is valuable.
Many pioneer researchers have realized the potential value and have tried to achieve irreversible hybrid bonding by means of thin-adhesive layer,5, 6, 7 chemical molecular level gluing,8, 9, 10, 11 or plasma treatment.2 Detailed summarization of these techniques has been published in several papers.11, 12
The first two methods are formerly applied as alternative methods for thermal plastics bonding, where the original thermal bonding techniques usually require delicate pressure and temperature control with expertise. The pressure might sweep away delicate patterns on PDMS and even cause clasped channel structures.13 The thin-adhesive layer method is achieved by applying the adhesive to the bonding surface for a thin adhesive membrane. By doing this, the requirement for heating and pressing is alleviated. However, since the adhesive layer is liquid before curing, it might pollute the channel or even block the delicate structures if they are too small in size. Arayanarakool et al.5 listed the values of glue layer thickness based on different bonding techniques. The minimum thickness for the gluing layers is more than 1 μm. This cannot satisfy the accuracy requirement for submicro-structure bondings. Thus, the chemical molecular level gluing strategy is developed afterwards.
The chemical molecular level gluing strategy modifies only the surface chemical functional group to thickness of several molecule layers. Usually at least two steps are required: the surface of the plastics would be (1) activated and then (2) grafted by wet chemistry with one type of molecule, which can be chemically bonded to the other type of molecule that is grafted or induced on PDMS surface.
Compared to the chemical gluing technique, direct plasma bonding is much simpler, which requires only one step for material pre-treatment. Early researches have pointed out that it is possible to bond plastics to PDMS by means of oxygen plasma.1 However, further researches concluded that the bonding would delaminate upon extended periods of exposure to moisture.2 By substituting part of the oxygen by inert gas argon, PDMS-COC/PS hybrid structures are successfully bonded.2 The highest quality is 120 kPa for burst pressure, realized by 1:2 argon-oxygen plasma with heating at 60 °C and less than 1 lb pressure over night. Although the bonding quality cannot meet the requirement for all the applications in microfluidics, it is valuable to point out that by applying a second kind of gas to the plasma system, the bonding quality for the substrates can be improved. So it is highly possible that suitable substituent gas might improve the quality for the PDMS-plastic irreversible bonding.
In this paper, air plasma based one-step bonding technique is proved applicable for high quality PDMS-PS, PDMS-COC, and PDMS-PP hybrid chip bonding at room temperature. The bonding condition is optimized by scanning the power of the plasma. Compared with the results from oxygen plasma and nitrogen plasma, air plasma shows synergic effect between oxygen and nitrogen, which is better than the individual gas plasma. In addition, the air plasma bonding is cheaper than oxygen plasma, simpler for operation and would cause the least surface deformation for the chips than the other bonding techniques. Due to its simplicity and free of chemical reagents, this technique is also adaptable for reel to reel bonding based mass production. To examine the quality and durability of the bonding, various characterization tests are carried out. The test results have proved the hybrid chips are of high quality: (1) they can survive large air pressures over 500 kPa; (2) they can be stored at different temperatures from −20 °C to 50 °C for over a week; and (3) they can also be stored in deionized water at room temperature for over a month.
EXPERIMENT
Chemicals and materials
Sylgard 184 elastomer base and curing agent for PDMS were purchased from Dow Corning (Midland, MI). PS (NEST Biotech Co., Ltd. China), PP (ChiXiang Industrial Co, Ltd., China), and COC (Topas, Ticona in USA) were applied in the experiment. The COC sheet is homemade from COC particles by melting and molding. SG-2506 borosilicate glass (with 145 nm thick chrome film and 570 nm thick positive S-1805 type photoresist, Changsha Shaoguang Chrome Blank Co. Ltd) was applied as the initial glass wafer for PDMS mold-copy based chip fabrication.
Apparatus and instruments
DT-02 cold plasma generator from OPS Plasma Technology Co., Ltd (Suzhou, China) was applied to bond the PDMS-plastic hybrid chips. Atomic force microscope (AFM) 5500 is from Agilent Technologies, Inc., USA. The contact angle (CA) was tested using OCA30 system produced by Dataphysics Instrument Gmb of German. The x-ray photon spectroscopy (XPS) was carried out with PHI5000 VersaProbe, produced by ULVAC-PHI, Inc., Japan. Microfluidic pump (Model TS-2A, Longer pump Corp., Baoding, China) was applied for liquid manipulation. TL70000 optical system mounted with Olympus DP71 cooled CCD camera were applied for chip image recording, results of which were analyzed by the Image-Pro Plus (IPP) 6.0 software.
Microchannel fabrication techniques
The microchannel was patterned on the PDMS by soft lithography technique. 10:1 PDMS base and curing agent were mixed to fill the glass mold, degassed, and cured at 80 °C for 60 min before use. The glass mold was fabricated by microlithography methods detailed in the supporting information.16
Bonding techniques
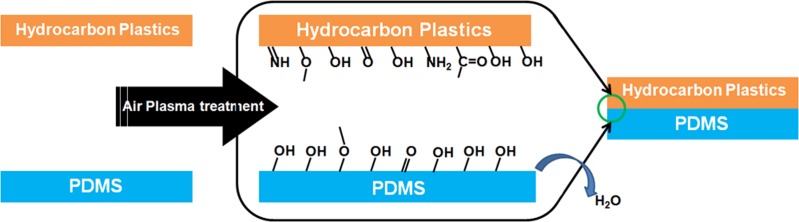
Before plasma bonding, the plastic sheets were immersed to ethanol and subject to 10 min sonification. Then they were dried at room temperature. The PDMS sheets were used after peeling off the mold, which requires no further cleaning. The air plasma bonding is sketched in Scheme ch1. The treatment condition was set as follows: 10 Pa for the background pressure, 14 sccm for the gas flow rate, 40 s for the balancing time before power on, and 30 s for the plasma treatment time. The power of the treatment was screened for different materials ranging from 100 to 300 W. The typical treatment power for PS, COC, and PP are 150, 150, and 250 W respectively. Both PDMS and the hydrocarbon plastic were inserted into the plasma container at the same time and put together for bonding immediately after the plasma treatment.
Figure .
Illustration for the one step air plasma bonding.
Bonding quality characterization
Delamination test for bond strength
Manual delamination was carried out to check the bonding quality for the PS-PDMS, COC-PDMS, and PP-PDMS hybrid chips. The contacting area of the PDMS and the plastics is 1 × 1 cm for all the tests.
Compressed air resistance test
20 mL of air was compressed by syringe pump with a constant speed of 15 mL/min, which was linked to the prefabricated chip channel by polytetrafluoroethylene (PTFE) tubes. The chip is 50 μm for depth, 900 μm for width, and 35 mm for length. The other side of the channel was linked to the gas pressure sensor (MPX5700A from Freescale Semiconductor, Inc., USA). All the connecting junctions were sealed with silicate seal gel or epoxy glue. The resulting pressure-time relationship was recorded by Keithley Model 4200-SCS Semiconductor Characterization System. The compressed air resistance was applied to monitor the bonding strength. At least three tests were carried out for each type of bonding. The same tests were also carried out for PS-PDMS hybrid chip after storage at −20, 4, 25, and 50 °C over one week.
Leakage test
Leakage test was applied using the LSP02-1B mode syringe pump, with the injection speed of 0.5, 1, 2, 5, 10, and 15 ml/min tested for each sample. The channel holds the geometry of 50 μm for depth, 900 μm for width, and 35 mm for length. PTFE tube was used to link the on-chip silicone tube to the external environment. Red ink solution was applied to visualize the flushing flow. The typical movie for the test under the maximum flow speed was recorded.
Durability test
Deionised water, 1 M HCl, and 1 M NaOH were injected into different channels of the PDMS-plastic hybrid chip. The channel holds the geometry of 50 μm for depth, 400 μm for width, and 35 mm for length. Then the inlets were sealed with silicate seal gel or placed in box with saturated humidity to prevent solution evaporation. They were then stored at room temperature (25 °C) for weeks and were examined for leakage each day.
RESULTS AND DISCUSSION
Plasma power optimization
Cold plasma surface treatment is a green and relatively convenient technique for chip bonding. By modulating the power of the plasma, it is possible to selectively destroy and rebuild the chemical bonds of the gas and of the substrate materials. Considering this, we tried to screen the plasma power for the optimum condition for PDMS-plastic bonding. The bonding effects of the hybrid plastic-PDMS chips under the power from 100 to 300 W are examined by manual delamination test within 5 min after sticking them together. The recorded bonding qualities by air plasma under different conditions are posted in Table S1.16 The results imply that the optimum bonding power for PS-PDMS, COC-PDMS, and PP-PDMS are 150, 150, and 250 W, respectively. Under the optimum bonding power, the plastics and the PDMS are strongly bonded.
For comparison, the bonding quality of the oxygen plasma treatment and nitrogen plasma treatment for the three types of hybrid structures are also explored. The results are summarized in Tables S2 and S3.16 No high quality bonding is observed for PS and PP. Irreversible bonding for COC can be generated only at much higher power by oxygen or nitrogen gas plasma than by the air plasma. So within the experiment condition, the bonding quality of the air plasma is better than the oxygen plasma and the nitrogen plasma. This phenomenon implies the existence of synergic effect between the oxygen and the nitrogen during the air plasma.
Typically, the O2 plasma treatment would generate −OH groups and their free radical forms on the substrate, sometimes with a small portion of −C=O groups.14 However, the N2 plasma usually gives rise to (R1, R2)C=NH imine groups and their free radical forms of chemical groups on the substrate.14 Their combination can make a synergic contribution to the total process.14 To confirm the existence of the synergic effect, argon-oxygen (1:4 volume) plasma is taken to bond the PDMS-plastic hybrid chip under optimum condition (Table S4).16 Here argon is inert for plasma treatment, which would have no synergic effect with oxygen. As expected, no reversible bonding is generated under such circumstances, which confirms that the function of nitrogen in the chip bonding is far beyond diluting the oxygen gas.
Next, we changed the ratio between the volume of nitrogen and oxygen. When nitrogen-oxygen (1:4) plasma is applied (Table S5),16 irreversible bonding can be achieved for PS, COC, and PP-PDMS hybrid chips. Comparing the bonding effect with that of pure oxygen plasma and pure nitrogen plasma, it can be deduced that the combination of oxygen and nitrogen can significantly increase the quality of the bonding, indicating the existence of synergic effects. It also indicates that the PDMS-plastic irreversible bonding is not so sensitive to gas composition change. Comparing this result with that under the nitrogen-oxygen ratios of 1:1 (Table S6)16 and ∼4:1 (air, Table S1)16 situation, it can be further deduced that increasing the oxygen content in the presence of nitrogen resulted in a widened bonding power range. However, since air is cheap and easy to adopt for irreversible chip bonding, the following research is focused on the air plasma bonding.
SURFACE CHARACTERIZATION
Contact angle test
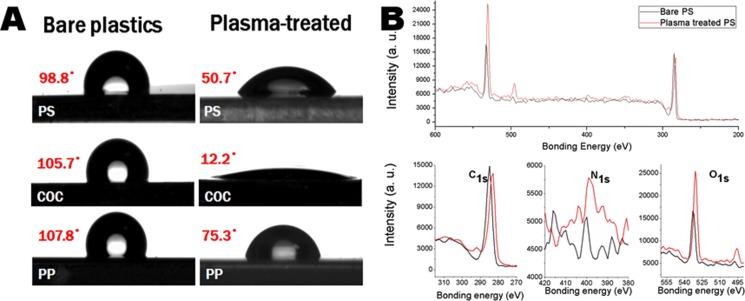
To visualize the surface chemistry transformation of the plastics due to the air plasma treatment, the contact angle test is taken. The results in Fig. 1A show that the hydrophobic surfaces of the plastics are all turned to hydrophilic upon plasma treatment. They imply that hydrophilic chemical groups have been formed on the plastic substrates.
Figure 1.
Surface characterization of the air plasma treated plastics. (A) Contact angle test. (B) XPS analysis of the surface element of PS before (black line) and after (red line) air plasma treatment.
XPS test
To better understand the surface chemistry change of the substrate in the optimized condition, XPS is taken to characterize the surface element composition change. Quantitative data are posted in Table S7.16 Taking PS for example (Fig. 1B), the increment is 0.24% for N and 11.31% for O, while the C1s peak decreased a little. Before the plasma treatment, the surface absorption of the oxygen and nitrogen might be contributed to the content of O and N element. Industrial additives also may have been a source to the O and N element on the surface of the native plastic sheets. Since the composition of PS is a fixed value, it can be deduced that the increment of the N1s and O1s peaks come from contribution from the excited air plasma. The slight decrease of the C1s peak might be caused by the introduction of the O and N element to the plastic substrate, which double confirmed that the air plasma has modified the surface chemistry of the plastics. Because the increment for N is far less than O, it implies that the bonding mechanism might be laid heavily on reaction between the −O containing groups. It also implies that the synergic effect between nitrogen and oxygen might follow a mechanism where the nitrogen tends to help to increase the reactive −O on the substrate for bonding.
Based on the XPS results for PS, it’s reasonable to deduce that the surface modification should also occur to the COC and PP at their optimized condition because their optimum plasma excitation power is equal or higher than the PS. As expected, both PP and COC show increased percentage for O, minor increased percentage for N, but decrement for the percentage for C. It is reasonable for the PS and COC to share a similar optimum condition (150 W), because they all have carbon-carbon double bonds. While for PP, it sparsely has carbon-carbon double bonds but is rich in carbon-carbon single bonds. The later bond requires much higher energy to be destroyed by plasma excitation.
Surface deformation
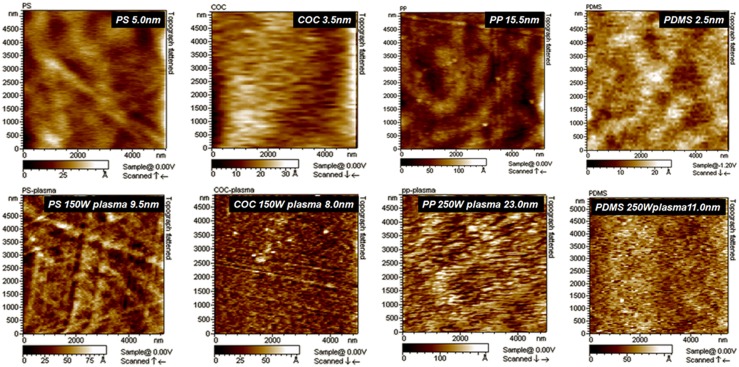
Low degree of surface deformation is of vital importance if the micro-nano structures are to be included on the surface of the bonding. To quantify the degree of surface deformation caused by surface plasma treatment, AFM is taken to measure the surface roughness of the plastics and PDMS before and after air plasma treatment. The results are posted in Fig. 2. The surface roughness of bare PS, COC, PP, and PDMS are 5.0, 3.5, 15.5, and 2.5 nm, respectively. After plasma treatment, the values increased to 9.5, 8.0, 23.0, and 11.0 nm, respectively. The increments of the surface roughness are all less than 10 nm, indicating the influence of the plasma treatment is within the nano level. So the technique might be applicable to bond micro and submicro structures.
Figure 2.
AFM test for surface roughness of bare and plasma treated PS, COC, PP, and PDMS.
BONDING QUALITY CHARACTERIZATION
Delamination test
The results of delamination test are the most direct evidences for the success of irreversible PDMS-plastics bonding. The images of the manually delaminated PS-PDMS, COC-PDMS, and the PP-PDMS hybrid structures are posted in Fig. 3. They show that the interfaces are completely merged with each other. Upon peeling, only the PDMS side of the structure is broken, resulting in fractured cross sections. The images confirmed that the plastic-PDMS hybrids are successfully bonded after the one-step air plasma treatment at room temperature.
Figure 3.
Optical images for the delaminated PDMS-plastic hybrids.
Compressed air resistance test
The quality of the irreversible bonding is examined by the compressed air resistance test. In the test, air is pumped to channel of the hybrid chip to examine that the bonding is uniform and the sealing is airtight. The strength of the bonding can be reflected by the value of the maximum pressure the chip can withstand.
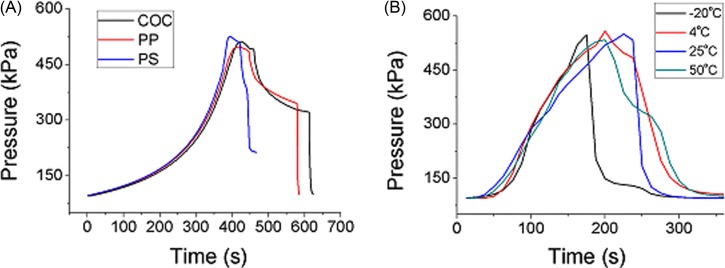
To generate the maximum pressure, the syringes are sealed by epoxy glue and linked to the silicon connector by PTFE tubes. The time serial increment of the pressure in the hybrid channels are recorded as shown in Fig. 4A. At room temperature the PS-PDMS, COC-PDMS, and the PP-PDMS hybrid channels can withstand pressures over 500 kPa. They are comparable to the bonding qualities reported with other techniques. Since the pressure generated inside a microchannel for microfluidics is usually no higher than 50 psi (Ref. 15) (equal to 345 kPa), the results mean the proposed bonding technique is universally applicable for microfluidic chip fabrications.
Figure 4.
Burst curve for measurement of the air pressure resistance for the channels (A) at room temperature, tested shortly after bonding by syringe pump. (B) PS-PDMS chips under storage at different temperatures for 1 week. The pressure is added by manual pressing.
For many specific applications, chips may be stored at extreme temperatures; thus, the quality of the bonding is also tested after storage of the hybrid chips at different temperatures for long time. Fig. 4B is the corresponding resistance curves of the PS-PDMS hybrid channels stored at −20, 4, 25, and 50 °C over one week. The results show there is merely any degradation for the quality of the strength of the hybrid bondings. The maximum pressures the chip can withstand are all around 500 kPa.
Leakage test
Although the compressed air resistance test can qualitatively give the data for the bonding strength, it is not representing the real working state of the chips. Considering this, leakage test is introduced because it well mimicked the liquid generated pressure that is encountered in real applications. In the leakage test, the red colour ink is injected into the channel by syringe pump at a steady speed as shown in Fig. 5. The speed increased gradually from time to time, until it reached the maximum speed the pump can provide. The results are satisfying that all three types of hybrid chips can withstand the flow rate of 15 mL/min, which is the working limit of the pump applied. The corresponding movie for the leakage test is appended in Movies S1, S2, and S3.16 By calculating the pressure distribution along the channel, the maximum pressure the chip may endure at 15 mL/min is 360 kPa (Fig. S2).16 It is estimated the volume of liquid flowed through the channel within 1 min is over 9000 times the volume of the microfluidic channel.
Figure 5.
Maximum pressure resistance to the liquid flow. The scale bar is 7 mm. The maximum pressure at 15 mL/min is 360 kPa.
Durability test
The durability test here tried to reflect the performance of the hybrid channel upon filling with different solutions. As shown in Fig. S3,16 solutions of 1 M HCl, deionized water, and 1 M NaOH are filled into the channel and sealed for constant inspection for leakage. The leakage of the chip is not observable within 3 days after exposure to 1 M HCl. And no observable leakage is found during one week for 1 M NaOH and one month for water. This proved that the hybrid chip is sealed with good quality.
CONCLUSION
In this paper, irreversible PDMS-plastic bonding for PS, COC, and PP are achieved by one step air plasma treatment. The XPS results proved that both O and N have modified the surface chemistry of the plastics after the air plasma treatment. AFM surface characterization further confirmed that this bonding technique would lead to surface deformation to less than 10 nm and thus is applicable in chip fabrications extended to sub micro scale. Various bond quality tests are taken to check the applicability of the hybrid plastic-PDMS structures. They give out solid data to support that the one-step bonding is successful and of high quality.
ACKNOWLEDGMENTS
The financial support from the National Natural Science Foundation (Nos. 20890020 and 21025522) and the National Natural Science Foundation (No. 20821063) of China are gratefully acknowledged.
References
- Duffy D. C., McDonald J. C., Schueller O. J. A., and Whitesides G. M., Anal. Chem. 70(23 ), 4974 (1998). 10.1021/ac980656z [DOI] [PubMed] [Google Scholar]
- Mehta G., Lee J., Cha W., Tung Y.-C., Linderman J. J., and Takayama S., Anal. Chem. 81(10 ), 3714 (2009). 10.1021/ac802178u [DOI] [PubMed] [Google Scholar]
- Becker H. and Locascio L. E., Talanta 56(2 ), 267 (2002). 10.1016/S0039-9140(01)00594-X [DOI] [PubMed] [Google Scholar]
- Chantiwas R., Park S., Soper S. A., Kim B. C., Takayama S., Sunkara V., Hwang H., and Cho Y.-K., Chem. Soc. Rev. 40(7 ), 3677 (2011). 10.1039/c0cs00138d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arayanarakool R., Gae S. L., and v. d. Berg A., Lab Chip 10(16 ), 2115 (2010). 10.1039/c004436a [DOI] [PubMed] [Google Scholar]
- Im S. G., Bong K. W., Lee C.-H., Doyle P. S., and Gleason K. K., Lab Chip 9(3 ), 411 (2009). 10.1039/b812121d [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Yamada M., and Seki M., Sens. Actuators B 148(1 ), 323 (2010). 10.1016/j.snb.2010.04.018 [DOI] [Google Scholar]
- Aran K., Sasso L. A., Kamdar N., and Zahn J. D., Lab Chip 10(5 ), 548 (2010). 10.1039/b924816a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. S. and Ram R. J., Lab Chip 9(11 ), 1618 (2009). 10.1039/b820924c [DOI] [PubMed] [Google Scholar]
- Ogilvie I. R. G., Sieben V. J., Cortese B., Mowlem M. C., and Morgan H., Lab Chip 11(14 ), 2455 (2011). 10.1039/c1lc20069k [DOI] [PubMed] [Google Scholar]
- Sunkara V., Park D.-K., Hwang H., Chantiwas R., Soper S. A., and Cho Y.-K., Lab Chip 11(5 ), 962 (2011). 10.1039/c0lc00272k [DOI] [PubMed] [Google Scholar]
- Rezai P., Selvaganapathy P. R., and Rwohl G., J. Micromech. Microeng. 21(6 ), 065024 (2011). 10.1088/0960-1317/21/6/065024 [DOI] [Google Scholar]
- Park S. M., Huh Y. S., Craighead H. G., and Erickson D., PNAS 106(37 ), 15549 (2009) 10.1073/pnas.0904004106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridman A., Plasma Chemistry (Cambridge University Press, 2008), p. 639. [Google Scholar]
- Tang L. and Lee N. Y., Lab Chip 10(10 ), 1274 (2010). 10.1039/b924753j [DOI] [PubMed] [Google Scholar]
- See supplementary material at http://dx.doi.org/10.1063/1.3694251 for Tables S1, S2, S3, and S4, Figs. S1 and S2, and Movies S1, S2, and S3.