Abstract
Styrene oxide isomerase (SOI) is involved in peripheral styrene catabolism of bacteria and converts styrene oxide to phenylacetaldehyde. Here, we report on the identification, enrichment, and biochemical characterization of a novel representative from the actinobacterium Rhodococcus opacus 1CP. The enzyme, which is strongly induced during growth on styrene, was shown to be membrane integrated, and a convenient procedure was developed to highly enrich the protein in active form from the wild-type host. A specific activity of about 370 U mg−1 represents the highest activity reported for this enzyme class so far. This, in combination with a wide pH and temperature tolerance, the independence from cofactors, and the ability to convert a spectrum of substituted styrene oxides, makes a biocatalytic application imaginable. First, semipreparative conversions were performed from which up to 760 μmol of the pure phenylacetaldehyde could be obtained from 130 U of enriched SOI. Product concentrations of up to 76 mM were achieved. However, due to the high chemical reactivity of the aldehyde function, SOI was shown to be the subject of an irreversible product inhibition. A half-life of 15 min was determined at a phenylacetaldehyde concentration of about 55 mM, indicating substantial limitations of applicability and the need to modify the process.
INTRODUCTION
Members of the bacterial genera Pseudomonas, Xanthobacter, Corynebacterium, and Rhodococcus, as well as various fungi, have been reported to utilize styrene under aerobic conditions as a sole energy and carbon source (reviewed in references 23, 25, and 39). The pathway of side chain oxygenation was shown to be frequently used in that respect (Fig. 1); it comprised a styrene monooxygenase (SMO; a two-component enzyme encoded by styA/styB), a styrene oxide isomerase (SOI; encoded by styC), and a phenylacetaldehyde dehydrogenase (PAD; encoded by styD). These enzymes consecutively convert the alkenylbenzene to styrene oxide, phenylacetaldehyde, and phenylacetic acid, respectively (2, 3, 12, 14, 24). After activation by coenzyme A, the latter intermediate is funneled into the tricarboxylic acid cycle (3, 27, 28, 37).
Fig 1.

Upper pathway of styrene degradation via side chain oxygenation. Styrene is converted by styrene monooxygenase (SMO) to styrene oxide, which undergoes isomerization by styrene oxide isomerase (SOI) to phenylacetaldehyde. The latter compound is oxidized by phenylacetaldehyde dehydrogenase (PAD) to phenylacetic acid.
Whereas the initial SMO has received considerable attention due to its biotechnological relevance in the preparation of enantiopure (S)-styrene oxide (16, 29, 30, 31, 32, 33, 35; reviewed in references 22 and 39), less is known about the following enzyme SOI (EC 5.3.99.7) transforming an epoxide into an aldehyde (Fig. 1).
Studies on biochemical properties of SOIs have been limited to the alphaproteobacterium Xanthobacter sp. strain 124X (11), the gammaproteobacterium Pseudomonas putida S12 (21), and the Gram-positive actinobacterium Corynebacterium sp. strain AC-5 (14). In several pseudomonads and, more recently, in Rhodococcus sp. strain ST-5, SOI genes (styC) were found to be located in the styrene-catabolic gene cluster [sty(SR)ABCD] (2, 26, 33, 41, 42).
Recently, we reported on the identification and biochemical characterization of a novel styrene monooxygenase system, StyA1/StyA2B, from the nocardioform actinobacterium Rhodococcus opacus 1CP, consisting of a styrene monooxygenase (StyA1) and another monooxygenase fused to a flavin-oxidoreductase (StyA2B). This system differs from the typical two-component styrene monooxygenases (StyA/StyB) of pseudomonads by gene fusion as well as by the kinetics and efficiency of styrene epoxidation (22, 38, 40). Moreover, styA1 and styA2B were not found to be part of a styrene-catabolic operon (styABCD), which raised questions about the existence of a complete styrene-catabolic pathway in strain 1CP. Considering the low styrene-oxygenating activity of StyA1/StyA2B as well as the low growth rate of strain 1CP on styrene (38, 40), the consumption of this hydrocarbon could be related to a kind of fortuitous metabolism by unspecific pathways.
As an attempt to identify further enzymes of peripheral styrene metabolism and in order to illuminate the physiological role of StyA1 and StyA2B, biomass of strain 1CP was analyzed for enzyme activities of side chain oxygenation. SOI activity was found to be specifically induced during growth on styrene. In order to characterize this enzyme in respect to its biochemical properties, an enrichment procedure was developed and evidence was provided for an integral membrane localization of SOI. A high specific activity, the insensitivity toward various chemical treatments and conditions, and the independence from additional cofactors suggested further investigation of the applicability of the enzyme in the preparation of pure phenylacetaldehyde, an important ingredient and building block for the personal care and pharmaceutical industries (13). In addition, the purification procedure described was successfully adapted to the isomerase from Pseudomonas fluorescens ST and should be applicable as a general strategy to obtain highly enriched SOIs.
MATERIALS AND METHODS
Chemicals and enzymes.
Standard chemicals; detergents; bromo-, fluoro-, and unsubstituted styrene oxide; phenylacetaldehyde; and substituted phenylacetic acids were purchased from Sigma-Aldrich (Steinheim, Germany) or Carl Roth (Karlsruhe, Germany) in the highest available purity. 3-Chloro-, 4-chloro-, and 4-methylstyrene oxide were synthesized as described elsewhere (38).
Bacterial strains and culture conditions.
Rhodococcus opacus 1CP had originally been isolated using 2,4-dichlorophenol as the sole source of energy and carbon (10) and was available from the strain collection of the institute. For preservation, strain 1CP was kept on mineral medium plates (7) in the presence of 5 mM benzoate. In order to inoculate liquid cultures growing on styrene as the sole carbon source, strain 1CP was streaked out on solid mineral medium. Plates were then incubated at room temperature in a 5-liter desiccator in which an evaporating aliquot of 50 μl styrene was provided.
To investigate the influence of substrates on SOI and PAD expression, 100-ml cultures of strain 1CP were grown in baffled 1-liter Erlenmeyer flasks at 30°C under constant shaking (120 rpm) on mineral medium and in the presence of one of the following carbon sources: styrene (total, 250 μmol; 2- to 3-μl aliquots added through an evaporation adapter); styrene oxide (total, 130 μmol; 1- to 2-μl aliquots added through an evaporation adapter); phenylacetaldehyde (total, 0.8 mmol; added in 80- to 160-μmol portions); and phenylacetic acid (total, 3 mmol; added in 500-μmol portions).
Higher growth rates of strain 1CP for the purpose of SOI preparation were later obtained by feeding liquid cultures (100 and 300 ml) with 5 mM phenylacetic acid and 0.1% (wt/vol) yeast extract prior to appending an additional induction phase with styrene. To obtain higher cell yields for SOI isolation, fed-batch cultivation of strain 1CP was performed in a 5-liter biofermentor (ED/ES5; B. Braun Biotech AG, Melsungen, Germany) containing 4 to 5 liters mineral medium. Again, rapid biomass growth was achieved by using phenylacetic acid as the initial substrate. In total, 90 mmol of this carbon source was added in 12-mmol portions (temperature, 30°C; 600 rpm; pH 7.0 to 7.5; aeration, 2 standard liters per minute [SLPM]), yielding an optical density at 546 nm (OD546) of 8 to 9. After substrate depletion, small aliquots (25 to 100 μl) of pure liquid styrene were added to the culture in order to induce the expression of styrene-catabolic enzymes. The airflow was temporarily stopped after each substrate addition until the aliquot was completely metabolized as indicated by a stagnation of the online-measured oxygen consumption. The airflow then was readjusted to 2 to 5 SLPM for 30 min, and another styrene aliquot was added. After a total of 10.5 to 13.1 mmol (1.2 to 1.5 ml) styrene had been added within 48 to 72 h, cells were harvested by centrifugation (5,000 × g, 20 min, 4°C) and biomass was stored at −80°C.
Pseudomonas fluorescens ST (DSM 6290) has been isolated as a styrene-degrading bacterium (1) and was available from the DSMZ (German Collection of Microorganisms and Cell Cultures, Braunschweig, Germany). For strain preservation, it was kept either on LB medium plates at 4°C or, similarly to strain 1CP, on mineral medium with styrene as carbon source. Liquid cultures (300 ml) of strain ST were incubated as described for strain 1CP.
Partial purification of the SOIs from R. opacus 1CP and P. fluorescens ST.
All purification steps were performed at 4°C. Freshly thawed biomass of styrene-grown strain 1CP (7 ml) was adjusted to a final volume of 8 ml by the addition of 25 mM phosphate buffer (pH 7.2), and an appropriate amount of DNase I (56 U) was added to degrade nucleic acids. Cells were disrupted by four passages through a French press at a 40,000-lb/in2 cell pressure. The obtained suspension contained the membrane fraction with SOI activity and was used for the determination of specific induction of that enzyme. Whole cells and cell wall debris were removed by centrifugation at 20,000 × g (20 min).
For further purification, cell-free supernatant was subjected to high-speed centrifugation at 100,000 × g (12 h) in order to separate the major extent of SOI-containing cell membranes. After removal of the cytosolic supernatant, the opaque red-to-brown pellet was sequentially washed with 10 ml high-salt buffer (25 mM phosphate buffer, pH 7.2; 1.0 M NaCl) and 10 ml low-salt buffer (12 mM phosphate buffer, pH 7.2) in order to remove peripheral membrane proteins. The resuspended membrane fraction was centrifuged at 100,000 × g in both cases for 1 to 2 h. The washed pellet was resuspended in 1 ml 25 mM phosphate buffer (pH 7.2), subjected to SOI activity determination, and used for further biochemical studies.
In order to solubilize and to remove integral membrane proteins from the above membrane preparation, the pellet was resuspended and incubated for 1 h in a solubilization buffer consisting of 16 mM phosphate buffer (pH 7.2), 1% 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS), and 3.5 M guanidine hydrochloride (GdnHCl). After another centrifugation step (100,000 × g, 4 h), SOI activity was found exclusively in the pellet.
SOI from Pseudomonas fluorescens ST was enriched in a procedure similar to that described for strain 1CP.
Enzyme assays and protein quantification.
Styrene oxide isomerase activity was routinely determined by gas chromatographic (GC) analysis of the reaction product phenylacetaldehyde using styrene oxide as the substrate. Reaction mixtures (total volume, 1.8 ml) contained 90 μmol phosphate buffer (pH 7.2) and 1.8 μmol racemic styrene oxide (100 mM stock solution in methanol) and were placed in continuously shaken 2-ml high-pressure liquid chromatography (HPLC) vials sealed with polytetrafluoroethylene (PTFE)-coated septa. Reaction was started at room temperature (21 to 22°C) by the addition of 2 to 3 μl SOI-containing sample. Aliquots of 125 μl were taken by means of a glass syringe, transferred into an 1.5-ml Eppendorf tube, and immediately quenched by the addition of 10 μl 85% phosphoric acid. Unconsumed styrene oxide is rapidly hydrolyzed under these conditions into readily water-soluble 1-phenylethane-1,2-diol, and phenylacetaldehyde can be extracted from the acidified mixture by an organic solvent. For that purpose, 125 μl n-hexane was added 2 min after addition of the phosphoric acid and the mixture was vigorously shaken for 2 min at a 30-Hz frequency in a swing mill (MM-200; Retsch, Germany). After a brief centrifugation (16,000 × g, 30 s), the organic head phase was removed and subjected to GC analysis using a Packard Model 437 gas chromatograph (Packard, Downers Grove, IL) and a glass column (1-m length and 2-mm inside diameter [i.d.]) packed with 10% SE-30 on Chromosorb W. Separation of phenylacetaldehyde (retention time, 1.6 min) was achieved under isothermal conditions (140°C) with a carrier gas flow of 25 ml min−1 N2 and an injector and detector temperature of 250°C.
In order to investigate the enantioselectivity of SOI, the above-described assay was modified by applying similar amounts of pure (S)- and (R)-styrene oxide instead of racemic epoxide.
For characterization of factors affecting isomerase stability, SOI preparations were preincubated at various temperatures (21 to 70°C, 30-min duration), pH values (4 to 9, 15-h duration), and GdnHCl concentrations (0 to 5.3 M, 5-h duration) or were stored over weeks at different temperatures (2 to 3°C or −20°C). These preparations afterwards were subjected to the above-described standard assay.
The temperature dependence and pH dependence of SOI activity were determined with the above-described standard assay by changing the incubation temperature (21 to 70°C) or the pH (4 to 9).
PAD activity was determined by photometric detection of NADH formation (λ = 340 nm; ε = 6.220 mM−1 cm−1) during transformation of phenylacetaldehyde. Reaction assay mixtures (total volume, 1.0 ml) in quartz cuvettes contained 50 μmol phosphate buffer (pH 7.2), 2 μmol NAD, and 0.1 μmol phenylacetaldehyde (100 mM in methanol) and were started by addition of crude cell extract. Blank reaction and NADH ablation were considered, and alcohol dehydrogenase activity could be excluded.
Protein concentrations were determined according to the method of Bradford (5) using a commercial protein assay reagent (Bio-Rad) and bovine serum albumin for calibration.
Determination of SOI substrate tolerance.
The activity of SOI toward substituted styrene oxides was determined indirectly by means of an HPLC-based quantification of substituted phenylacetic acids, which were obtained quantitatively by chemical oxidation of the enzymatically formed phenylacetaldehydes. For this purpose, 200-μl samples from the above-described routine SOI assay in which styrene oxide was substituted by 3-chlorostyrene oxide, 4-chlorostyrene oxide, 4-methylstyrene oxide, 4-fluorostyrene oxide, or 4-bromostyrene oxide were oxidized by addition of 10 μl 1.0 M sulfuric acid and 3.5 μl 0.09 M potassium permanganate. After 16 h of incubation at room temperature, excessive permanganate was quenched by addition of 20 μl 2.0 M NaOH, and after separation of MnO2 by centrifugation, 10 μl of the supernatant was directly subjected to HPLC analysis.
Reversed-phase HPLC (RP-HPLC) was performed on a Dionex instrument (P680 pump, UVD340S diode array detector [DAD], Gina 50 autosampler) using a Vertex column (125-mm length, 4-mm i.d.) packed with Eurospher C18 (5-μm particle size, 100-Å pore size; Knauer, Germany). Forty percent (vol/vol) and 50% (vol/vol) methanol (MeOH; 0.02% [wt/vol] phosphoric acid) was used as the mobile phase in an isocratic mode at a flow rate of 0.7 ml min−1. The indicated net retention volumes were obtained: phenylacetic acid, 5.5 ml (40% MeOH); 3-chlorophenylacetic acid, 6.4 ml (50%); 4-chlorophenylacetic acid, 6.6 ml (50%); 4-methylphenylacetic acid, 5.7 ml (50%). Obtained peaks were compared to authentic reference compounds in regard to retention and UV spectrum (200 to 300 nm).
Discontinuous SDS-PAGE.
In order to further enrich SOI as well as to identify the corresponding protein band(s), disc SDS-PAGE was performed with application of two variants of sample treatment, under native and under fully denaturing conditions.
For that purpose, an SOI-containing membrane fraction was treated with nondenaturing sample buffer (0.4% [wt/vol] SDS) at room temperature and subjected to SDS-PAGE using 10 or 15% slab gels and 0.1% SDS (17). In order to localize SOI, lanes were afterwards sliced into uniform pieces of about 50 to 70 mg each from which SOI activity was detected by means of the standard assay. Reaction mixtures to which the slugs were added instead of a liquid sample were constantly shaken overnight at room temperature in order to allow substrate and product to migrate through the gel. Acidification, extraction, and GC analysis of the organic phase were performed as described for the standard assay.
Knowledge of the SOI localization in the native gel allowed excision of a corresponding region from a neighboring duplicate lane. Complete denaturation was now achieved in these samples by incubation of gel slugs in the presence of 4% SDS and 10% mercaptoethanol at 40°C (45 min). After a second SDS-PAGE basically under conditions similar to those described above, gels were stained with Coomassie brilliant blue R-250 (8, 9, 19) and by an additional silver-based procedure (15, 20), if necessary. An unstained protein molecular mass marker (14.4 to 116 kDa; Fermentas) was used for size determination.
Preparative approach for SOI-catalyzed phenylacetaldehyde formation.
In 20-ml glass vials, equipped with a magnetic stirrer and a PTFE-coated lid, 25 μl (33 U), 50 μl (65 U), or 100 μl (130 U) of SOI preparation was suspended in 10 ml 25 mM phosphate buffer (pH 7.2). Under constant stirring (1,000 to 1,400 rpm), 1 mmol or 2.6 mmol pure racemic styrene oxide was added as a single dose and in the form of multiple aliquots of increasing size (0.1 to 0.6 mmol), respectively. Mixtures were allowed to react up to 60 min, and 20-μl samples were quenched by addition of 10 μl 85% phosphoric acid. Phenylacetaldehyde (net retention volume, 2.7 ml) was quantified from the hydrolyzed mixtures by RP-HPLC using 50% (vol/vol) methanol (0.7 ml min−1) and a stationary phase as described for the phenylacetic acids.
Determination of the susceptibility of SOI to product inhibition.
In order to determine the extent and reversibility of product inhibition of SOI, 5 μl of enzyme preparation (0.5 U) was suspended in 50 μl 25 mM phosphate buffer (pH 7.2) containing finally 5% (vol/vol) methanol. Addition of 0 to 90 mM phenylacetaldehyde (stock solutions in methanol) was followed by a 15-min incubation at room temperature under constant shaking. Afterwards, SOI was separated by centrifugation (16,000 × g, 30 s). The obtained pellet was washed with 25 mM phosphate buffer (pH 7.2) two times and resuspended in 5 μl 25 mM phosphate buffer (pH 7.2). Residual SOI activity was determined by GC analysis as described above.
RESULTS AND DISCUSSION
Detection of SOI and PAD activity in R. opacus 1CP.
The ability of the nocardioform actinobacterium R. opacus 1CP to utilize styrene as sole carbon source points to the existence of a complete degradation pathway in that microorganism. Because of the recently identified styrene monooxygenases StyA1/StyA2B (38, 40), styrene-grown biomass was tested for other enzymes of peripheral styrene degradation via side chain oxygenation. In the subsequent experiments, crude extract of styrene-grown strain 1CP was shown to convert styrene oxide to phenylacetaldehyde, indicating the presence of a specific epoxidase (EC 5.3.99.7). Moreover, phenylacetaldehyde could be further converted by the same crude extract to phenylacetic acid at the expense of NADH, a conversion for which an aldehyde dehydrogenase (EC 1.2.1.39) is responsible.
The highest specific SOI activity (5.12 U mg−1 = 100%) was induced in crude cell extract of styrene-grown cells, whereas growth on styrene oxide and phenylacetaldehyde led to drastically lower relative activities of 24 and 16%, respectively. No measurable SOI was found in glucose- and phenylacetic acid-grown 1CP biomass (Table 1).
Table 1.
Comparison of specific SOI activities after growth of R. opacus 1CP, P. putida CA-3, and Xanthobacter sp. strain 124X on various substrates
The expression of the NAD-dependent PAD seemed to be constitutive, since styrene- and glucose-grown biomass yielded similar specific activities (0.02 U mg−1).
Available data on the expression of styrene-catabolic enzymes are currently limited to P. putida CA-3 (24) and Xanthobacter sp. strain 124X (11). Interestingly, styrene oxide and phenylacetaldehyde were both shown to induce the highest specific SOI activity rates in strain CA-3, indicating differences in regulation from R. opacus 1CP. This hypothesis is strengthened by the report on a specific induction of PAD by styrene in strain CA-3 (24).
Differences were also found from the SOI expression in Xanthobacter sp. strain 124X, which was reported to be induced by phenylacetic acid to a considerable extent. However, the available data are incomplete and hardly allow comparison.
Partial enrichment of SOI from R. opacus 1CP.
Prolonged high-speed centrifugation of a cell crude extract of styrene-grown strain 1CP was accompanied with a continuous decrease of SOI activity and gave a first hint of a localization of this enzyme in the membrane fraction. Of the initial SOI activity measured after cell disruption in the presence of undisrupted whole cells and larger particles of cell debris, only 49% could be separated as a red opaque pellet by fractionated centrifugation at low speed (20,000 × g, 20 min) and high speed (100,000 × g, 12 h) (Table 2). The considerable loss of 51% of initial activity does not indicate enzyme inactivation but can be attributed to an incomplete isolation of the smallest membrane particles and supports the use of ultracentrifugation for a higher quantitative yield. Incubation of the pellet under various ionic strengths was performed in order to differentiate between membrane-associated proteins and those integrated in the phospholipid bilayer. Localization of SOI was found to be unaffected by this treatment, and the removal of associated proteins led to an overall enrichment of this protein by a factor of 11. This purification stage of SOI, which yet appears low, nevertheless corresponds to a specific activity of 99.6 U mg−1, which exceeds that of all other previously reported SOIs (compared to those in references 11, 14, and 24). Since this preparation did not contain activities which interfered noticeably with the preparation of phenylacetaldehyde, it was used for the biotransformation approaches described below. Integral membrane localization of SOI made attempts at pseudosolubilization necessary in order to achieve further enrichment. For that purpose, the membrane fraction was incubated in the presence of 0.05 to 2% (wt/vol) concentrations of various detergents (Triton X-100, Tween 20, SDS, CHAPS), the chaotrope GdnHCl, and combinations thereof (1 to 2% [wt/vol] CHAPS with 1.5 to 3.5 M GdnHCl). None of the chosen conditions was suited to bring about SOI activity in solution. However, a combination of 1% CHAPS and 3.5 M GdnHCl was found to be very effective in solubilizing other integral proteins and in achieving an additional enrichment. After incubation and centrifugation, specific SOI activities of up to 370 U mg−1 were measured, representing a 42-fold enrichment. The complete purification is summarized in Table 2.
Table 2.
Enrichment of the SOI from R. opacus 1CPb
| Purification step | Total protein (mg) | Total activity (U) | Sp act (U mg−1) | Purification (fold) | Yield (%) |
|---|---|---|---|---|---|
| Crude cell extract | 139 | 1240 | 8.9 | 1 | 100 |
| Cell extract | 103 | 1050 | 10.2 | 1.1 | 85.3 |
| Membrane fraction | 23.1 | 424 | 18.4 | 2.1 | 34.3 |
| Pellet after removal of peripheral proteins | 5.3 | 352 | 99.6 | 11.2 | 28.4 |
| Solubilization approacha | 0.6 | 235 | 375 | 42.1 | 19 |
1% CHAPS, 3.5 M GdnHCl.
Details on the enrichment and activity determination are given in Materials and Methods. Shown activity values are averages of two measurements. The data were confirmed by an independently performed enrichment experiment which revealed a similar degree of purified SOI.
A similar procedure was performed on the SOI of P. fluorescens ST, and a 28-fold enrichment (313 U mg−1) was achieved after treatment with CHAPS and GdnHCl. Because of the shared feature of membrane localization, the procedure might be of general applicability to purification of SOIs from different microorganisms.
Three strongly hydrophobic regions could be identified in the amino acid sequences of Pseudomonas fluorescens ST (2), Pseudomonas sp. strain Y2 (42), and Rhodococcus sp. strain ST-5 (41) by searching for transmembrane helices (data not shown; predicted with DAS transmembrane prediction server [6]). Although the SOI sequence from strain 1CP is not available yet, a similar motif is likely and would explain the behavior of this enzyme during enrichment.
Further enrichment of styrene oxide isomerase from strain 1CP by gel electrophoresis and evidence for a size similar to that of SOIs from Pseudomonas.
The unusually high stability of SOI toward chaotropes and detergents allowed further enrichment of the enzyme by discontinuous gel electrophoresis in order to determine quaternary structure and subunit size. After treatment of the SOI preparation with a low SDS concentration (0.4% wt/vol) without heating, native isomerase migrated through the gel as a broad band from which activity could be detected. The smear-like mobility may be attributed to the size range of membrane particles into which the enzyme is integrated and from which leaching failed.
In order to achieve a sharper separation of the SOI, gel plugs with isomerase activity were treated under strongly denaturing conditions (4% SDS, 10% mercaptoethanol, 40°C for 45 min) and subjected to a second SDS-PAGE. After sequential staining with Coomassie blue and silver, a single protein band of approximately 18 kDa in mass could be identified whose intensity was shown to be consistent with SOI activity of the sample applied (Fig. 2A).
Fig 2.

Detection and size determination of the 1CP SOI by disc SDS-PAGE applying SOI active gel slugs (A) and membrane fractions of differently induced biomasses of strain 1CP and P. fluorescens ST (B). (Lanes A1 to A3) SOI active gel slices obtained from disc SDS-PAGE of an 11-fold SOI enrichment using nondenaturing sample treatment (0.4% SDS) were denatured (4% SDS, 10% mercaptoethanol, 45 min, 40°C) and subjected to a second disc SDS-PAGE. (Lane A4) A 42-fold enrichment of the 1CP SOI obtained from CHAPS/GdnHCl treatment was preincubated with TFA and subjected to denatured SDS-PAGE. Gels were consecutively stained with Coomassie blue and silver. (B) SDS-PAGE of enriched integral membrane fractions of styrene-grown strain 1CP (1CP+; specific SOI activity, 99.6 U mg−1), phenylacetic acid-grown strain 1CP (1CP−; no measurable SOI activity), and styrene-grown P. fluorescens ST (ST+; specific SOI activity, 28.4 U mg−1). Membrane samples were denatured (4% SDS, 10% mercaptoethanol, 45 min, 40°C), and the gel was finally stained with Coomassie blue. Arrowheads indicate an SOI-related protein band. Lanes M, unstained protein molecular mass markers (Fermentas).
Correlation of this protein band with SOI expression was also shown by comparison of the integral membrane fractions obtained from styrene-grown (99.6 U mg−1 SOI activity) and phenylacetic acid-grown (<0.01 U mg−1 SOI activity) biomass (Fig. 2B). A similar SOI expression pattern in the presence of the two substrates was observed for StyC of P. fluorescens ST, which could be allocated from the gel on the basis of its calculated size of 18.1 kDa (2).
Recently, Toda and Itoh published the sequence of styC as part of a styABCD operon from Rhodococcus sp. ST-5 (41). The protein shared 69.5% identical amino acid positions with the SOI of Pseudomonas sp. strain Y2 (accession no. CAA04002); its size of 18.2 kDa corresponds well to the molecular mass determined for SOI from strain 1CP.
Remarkable stability of membrane-bound SOI of R. opacus 1CP toward denaturation.
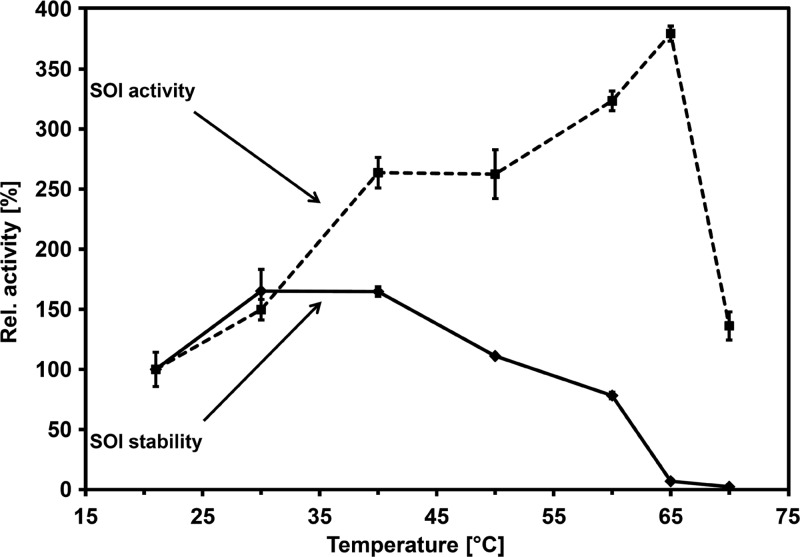
Probably due to its localization as an integral membrane protein, SOI of strain 1CP turned out to be a protein with above-average stability. A 50°C heat treatment (30 min) of a partially purified SOI preparation (99.6 U ml−1) did not lead to a significant loss of activity toward styrene, and the activity maximum of the protein was determined to be around 65°C (Fig. 3). Taking both maxima into consideration, 40°C might be an optimal temperature for application of the protein to achieve both pronounced specific activity and sufficient stability.
Fig 3.
Temperature dependence of SOI stability and activity. The stability (solid line) of SOI activity is expressed as percentage of initial activity (100% = 91.7 U mg−1) and was assessed by determination after heat treatment at 21°C to 70°C of the partially purified SOI preparation for 30 min. Isomerase activity (broken line) was determined in a similar temperature range and is relative to the specific activity at room temperature (100% = 84.6 U mg−1). Data points represent the means of duplicate measurements, and standard error bars are given.
It is noticeable that heat treatment of the SOI of strain 1CP led to permanent enzyme activation. Reproducibility was shown by a duplicated experiment in which thermal incubation of an enriched SOI preparation at 40°C for 30 min was accompanied by a relative increase to 165% and 201% of the initial specific activity, respectively. This effect might be attributed to a rearrangement of the membrane bilayer, of the protein here, or of both. A similar activation to 222% was determined for the SOI of P. fluorescens ST.
The effect of pH on enzyme activity and stability was investigated in phosphate buffer (25 mM). Both activity and stability optima were found to be localized at pH 7.0 (Fig. 4).
Fig 4.
pH dependence of SOI stability and activity. Partially purified SOI was incubated at a certain pH for 15 h, and the stability (solid line) of SOI is expressed as percentage of activity relative to a 15-h incubation at pH 7.0 (100% = 93.1 U mg−1). pH dependence of activity (broken line) was determined by means of the standard assay and phosphate buffer in a pH range of 4.6 to 9.1. Obtained activities are relative to the activity at pH 7.3 (100% = 88.9 U mg−1). Data points represent the means of duplicate measurements, and standard error bars are given.
Probably the most remarkable behavior of the SOI is its pronounced stability toward surface-active agents (CHAPS and SDS) and the chaotrope GdnHCl. Enriched SOI of strain 1CP (99.6 U ml−1) tolerated a 30-min incubation in the presence of up to 0.3% (wt/vol) SDS and 2% (wt/vol) CHAPS without measurable loss of activity. Incubation for a similar period in the presence of 3.5 M GdnHCl led to only slight inactivation of about 11% (±4%), whereas only 47% (±5%) of the initial activity could be restored after treatment with 5.3 M chaotrope. This robustness indicates either a high permanent stability of the tertiary and quaternary protein structure or a pronounced ability of the SOI to refold in such a structure after being partially unfolded. The robustness toward otherwise denaturing conditions allowed the enrichment of the active enzyme by a modified SDS-PAGE method as described above.
It should be mentioned that apart from a certain degree of inactivation, GdnHCl/CHAPS treatment leads to a modification of the SOI preparation. During discontinuous SDS-PAGE, GdnHCl/CHAPS-treated SOI preparations migrated only to a small extent into the stacking gel and were concentrated on the bottom of the sample slugs. In fact, SOI activity from those separations was primarily found in the stacking gel. Aggregation of SOI or SOI-containing membrane particles by hydrophobic interactions is a likely explanation, which was confirmed by the observation that membrane particles showed agglutination after treatment with GdnHCl/CHAPS.
Incubation with trifluoroacetic acid (TFA) restored the migration behavior of the SOI preparation and resulted in a broad band in the separating gel with the expected size after Coomassie blue and silver staining (Fig. 2A). However, activity was irreversibly lost by this treatment.
Preparations of the SOI-containing membrane fraction of strain 1CP could be stored for months at −20°C without detectable loss of activity. Storage at 2 to 3°C for 10 weeks caused a decrease of 30% of the initial activity after 10 weeks. SOI preparations which had been subjected to GdnHCl/CHAPS treatment were less stable and showed complete inactivation after a few weeks of storage at −20°C.
Activity of SOI toward substituted styrene oxides.
Conversion of racemic styrene oxide by the SOI of strain 1CP yielded phenylacetaldehyde as the sole product. A Meinwald rearrangement has been proposed by Miyamoto and coworkers as a likely mechanism (18, 21) and comprises the following steps: (i) enzymatic protonation of the oxirane oxygen, (ii) ring opening to a benzyl cation intermediate, (iii) enzymatic deprotonation into an enol, and (iv) conversion of the enol to an aldehyde by keto-enol tautomerization. The absence of detectable amounts of acetophenone in the reaction catalyzed by the SOI of strain 1CP is in accordance with that mechanism.
4-Methylstyrene oxide and the halogenated derivatives 3-chloro-, 4-chloro-, 4-bromo-, and 4-fluorostyrene oxide proved to be substrates for the enzyme, although they were converted at considerably lower relative rates than was the parental compound (Table 3).
Table 3.
Comparison of the substrate specificity of the SOI from R. opacus 1CP to that of P. putida S12 and Corynebacterium sp. strain AC-5 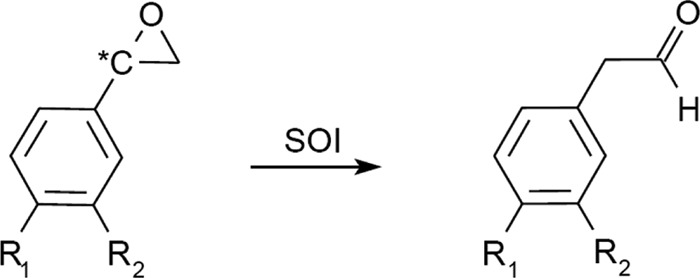
| Substrate | Substitution | Relative SOI activity (%) |
||
|---|---|---|---|---|
| R. opacus 1CPa | P. putida S12 (21) | Corynebacterium sp. strain AC-5 (14) | ||
| (R,S)-Styrene oxide | R1 = H, R2 = H | 100b | 100b | NDd |
| (R,S)-4-Bromostyrene oxide | R1 = Br, R2 = H | 2.2b | ND | ND |
| (R,S)-4-Chlorostyrene oxide | R1 = Cl, R2 = H | 2.6b | 3.7b | ND |
| (R,S)-3-Chlorostyrene oxide | R1 = H, R2 = Cl | 11.7b | ND | ND |
| (R,S)-4-Fluorostyrene oxide | R1 = F, R2 = H | 17.9b | ND | ND |
| (R,S)-4-Methylstyrene oxide | R1 = CH3, R2 = H | 77.3b | 113b | ND |
| (S)-Styrene oxide | R1 = H, R2 = H | 100c | 100c | 100c |
| (R)-Styrene oxide | R1 = H, R2 = H | 45.0c | 49.0c | 51.0c |
Shown data are averages of two measurements.
Relative to SOI activity toward (R,S)-styrene oxide.
Relative to SOI activity toward (S)-styrene oxide.
ND, not determined.
Comparison of relative activities and taking into consideration the van der Waals radii of corresponding parasubstituents of styrene (100% relative activity, H = 120 pm), 4-methylstyrene oxide (77.3%, CH3 = 200 pm), 4-fluorostyrene (17.9%, F = 147 pm), 4-chlorostyrene oxide (2.6%, Cl = 177 pm), and 4-bromostyrene oxide (2.2%, Br = 192 pm) (4, 34), hydrophilic binding effects as well as steric factors seem to determine the reaction rate. Stabilization of the proposed benzyl cation intermediate by the resonance effect in the presence of inductive electron-pushing substituents could also be important in that respect (21).
In contrast to the recombinant SOI from P. putida strain S12, which was reported to convert 4-methylstyrene oxide with a higher reaction rate (113%) than that for the parental compound (100%) (21), styrene oxide was shown to be the best substrate for SOI of strain 1CP under the experimental conditions used (Table 3).
Studies on the stereoselectivity of SOI were restricted to the wild-type enzyme from Corynebacterium sp. strain AC-5 (14) and the recombinant SOI of P. putida S12 (21). A 2-fold-higher reaction rate for (S)-styrene oxide than for the (R)-enantiomer indicated a distinct specificity. However, at least for the parental compound this would be far too low for biotechnological purposes (14). An almost similar preference was shown in this study for the SOI of strain 1CP (Table 3). This preference makes sense in the metabolic context, since almost all SMOs described so far convert styrene to (S)-styrene oxide in a way that is highly enantioselective. This is also true for StyA1/StyA2B from R. opacus 1CP, which yields (S)-styrene oxide with an enantiomeric excess of >94% (40).
Preparative application of SOI to produce phenylacetaldehyde(s).
The high specific activity even of a partially purified enzyme preparation, the simplicity of the enrichment procedure, high stability toward denaturing agents and during long-term storage, independence from cosubstrates, and a broad substrate specificity predispose styrene oxide isomerase from R. opacus 1CP to be a biocatalyst for the preparation of pure phenylacetaldehyde(s). This is especially true since chemical methods for the preparation of those compounds are frequently accompanied by considerable side effects or a high expenditure of energy (e.g., see reference 43).
In order to evaluate the capability to convert styrene oxide into the aldehyde under conditions of substrate saturation, 1.0 mmol of racemic styrene oxide was incubated in 10 ml phosphate buffer with 33 U of SOI preparation from strain 1CP. Only 40.6% ± 0.9% of the epoxide was converted during the first 30 min of incubation into the aldehyde (Fig. 5). A similar inhibition-like phenomenon was observed when 65 and 130 units of SOI were applied. However, reactions were now completed within 15 min and phenylacetaldehyde yields increased to 64.5% ± 4.1% and 76.6% ± 1.1%, respectively. Calculation and comparison of the corresponding product/biocatalyst ratios of 12.3 ± 0.3 μmol U−1 (for 33 U), 9.9 ± 0.6 μmol U−1 (for 65 U), and 5.9 ± 0.1 μmol U−1 (for 130 U) indicated a nonlinear dependence between formed product and applied enzyme, which makes it ineffective to achieve complete conversion by increasing the amount of biocatalyst. Disregarding inactivation, a final product concentration of 76 mM phenylacetaldehyde could be reached by applying 130 U of 1CP SOI.
Fig 5.
Preparation of phenylacetaldehyde from styrene oxide by enriched SOI. Each assay mixture contained 1 mmol of racemic styrene oxide and various amounts of enriched SOI in 10 ml phosphate buffer (pH 7.0). Quenched samples were analyzed for phenylacetaldehyde by RP-HPLC. Data points represent the means of duplicate measurements, and standard error bars are given.
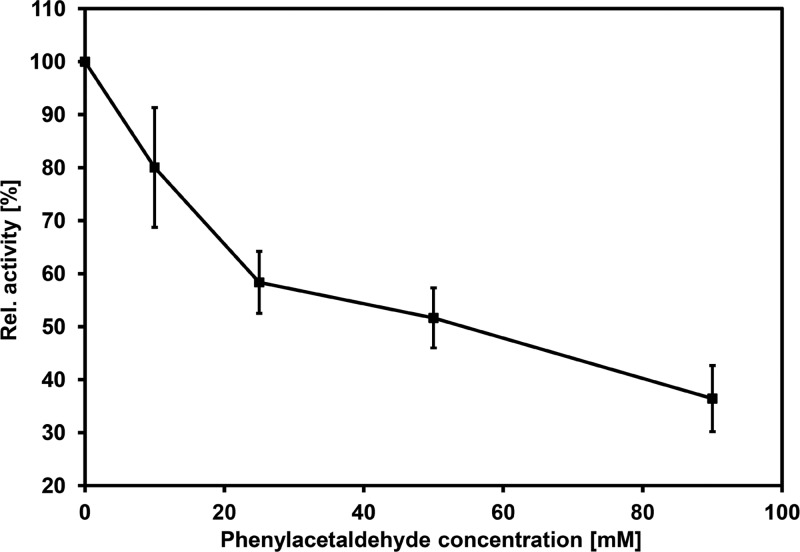
Presumed product inhibition was investigated by incubating SOI in the presence of 0 to 90 mM phenylacetaldehyde (Fig. 6), followed by activity determination.
Fig 6.
Irreversible inhibition of SOI activity by phenylacetaldehyde. Equal amounts of SOI were incubated in the presence of 0 to 90 mM phenylacetaldehyde for 15 min under constant shaking. Incubation mixtures were centrifuged, the SOI-containing pellet was washed several times with phosphate buffer, and residual SOI activity was determined and referred to the initial activity (100% = 16.0 U mg−1). Data points represent the means of quadruplicate measurements, and standard error bars are given.
Irreversibility of enzyme inhibition was evident since collection of inactivated enzyme by centrifugation, removal of bound phenylacetaldehyde by two washing steps, and finally incubation in the presence of fresh medium and styrene oxide could not restore measurable activity. Moreover, inhibition could be clearly attributed to phenylacetaldehyde since an addition of styrene oxide to the transformation mixture in small aliquots of 0.1 to 0.6 mmol did not significantly prevent this process (data not shown). Covalent modification(s) of nucleophilic residues of the enzyme is likely, since it is well known that aldehydes may react with proteins. This property is utilized in chemical disinfection and for (cross-)linking of proteins (36).
During the whole catalytic process, no detectable by-product occurred and the obtained phenylacetaldehyde was contaminated only with excessive styrene oxide. The latter can rapidly be transformed into 1-phenylethane-1,2-diol through acid-catalyzed hydrolysis by acidification of the reaction mixture with phosphoric acid. Afterwards, the more volatile aldehyde can easily be separated from the diol by distillation under reduced pressure.
Conclusions.
Together with the previously found styrene monooxygenase StyA1/StyA2B, the detection of a styrene-inducible SOI and a constitutive PAD in R. opacus 1CP provides further evidence for catabolism of that alkenylbenzene through side chain oxygenation. The localization of SOI within the membrane fraction was a prerequisite for a high enrichment of that enzyme from strain 1CP by a convenient and simple method. A more general applicability to SOIs from other bacterial species might be indicated by a successful adaptation to P. fluorescens ST, and purification from wild-type strains could be of some interest since recombinant expression of membrane proteins is frequently associated with obstacles.
In addition to the newly found SOI from strain 1CP, only three SOIs, those from P. putida S12 (21), Corynebacterium sp. strain AC-5 (14), and Xanthobacter sp. 124X (11), have been characterized to some extent. Available sequence data for all representatives indicated three transmembrane domains, and the obviously general property of membrane localization might explain the remarkable stability of the SOI from strain 1CP toward denaturing agents as well as a distinct enzyme activation by heat treatment.
The substrate specificity of the SOI of strain 1CP toward various substituted styrene oxides indicated a relatively broad substrate tolerance, which was found to be similar to that of the recombinant isomerase from P. putida S12 (21). Thus, SOIs should be applicable for the preparation of a number of different aldehydes. However, clear evidence was provided for an irreversible type of inhibition which is obviously caused by the reactive aldehyde. Strategies to decrease the product concentration during biotransformation are necessary in order to achieve long-term stability of the process.
ACKNOWLEDGMENTS
We thank Anika Riedel for assistance in the determination of substrate tolerance.
This project was supported by a fellowship from the Deutsche Bundesstiftung Umwelt.
Footnotes
Published ahead of print 13 April 2012
REFERENCES
- 1. Baggi G, Boga MM, Catelani D, Galli E, Treccani V. 1983. Styrene catabolism by a strain of Pseudomonas fluorescens. Syst. Appl. Microbiol. 4:141–147 [DOI] [PubMed] [Google Scholar]
- 2. Beltrametti F, et al. 1997. Sequencing and functional analysis of styrene catabolism genes from Pseudomonas fluorescens ST. Appl. Environ. Microbiol. 63:2232–2239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bestetti G, et al. 2004. Characterization of styrene catabolic pathway in Pseudomonas fluorescens ST. Int. Biodeterior. Biodegrad. 54:183–187 [Google Scholar]
- 4. Bondi A. 1964. Van der Waals volumes and radii. J. Phys. Chem. 68:441–451 [Google Scholar]
- 5. Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248–254 [DOI] [PubMed] [Google Scholar]
- 6. Cserzö M, Wallin E, Simon I, von Heijne G, Elofsson A. 1997. Prediction of transmembrane alpha-helices in prokaryotic membrane proteins: the dense alignment surface method. Protein Eng. 10:673–676 [DOI] [PubMed] [Google Scholar]
- 7. Dorn E, Hellwig M, Reineke W, Knackmuss H-J. 1974. Isolation and characterization of a 3-chlorobenzoate-degrading pseudomonad. Arch. Microbiol. 99:61–70 [DOI] [PubMed] [Google Scholar]
- 8. Dunbar BS, Kimura H, Timmons TM. 1990. Protein analysis using two-dimensional polyacrylamide gel electrophoresis. Methods Enzymol. 182:441–477 [DOI] [PubMed] [Google Scholar]
- 9. Garfin DE. 1990. One-dimensional gel electrophoresis. Methods Enzymol. 182:425–441 [DOI] [PubMed] [Google Scholar]
- 10. Gorlatov SN, Maltseva OV, Shevchenko VI, Golovleva LA. 1989. Degradation of chlorophenols by a culture of Rhodococcus erythropolis. Mikrobiologiia 58:802–806 [Google Scholar]
- 11. Hartmans S, Smits JP, van der Werf MJ, Volkering F, de Bont JAM. 1989. Metabolism of styrene oxide and 2-phenylethanol in the styrene-degrading Xanthobacter strain 124X. Microbiology 55:2850–2855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hartmans S, van der Werf MJ, de Bont JAM. 1990. Bacterial degradation of styrene involving a novel flavin adenine dinucleotide-dependent styrene monooxygenase. Appl. Environ. Microbiol. 56:1347–1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hölderich WH, Barsnick U. 2001. Rearrangement of epoxides, p 217–231 In Sheldon SA, van Bekkum H. (ed), Fine chemicals through heterogeneous catalysis. Wiley-VCH, Weinheim, Germany [Google Scholar]
- 14. Itoh N, Hayashi K, Okada K, Ito T, Mizuguchi N. 1997. Characterization of styrene oxide isomerase, a key enzyme of styrene and styrene oxide metabolism in Corynebacterium sp. Biosci. Biotechnol. Biochem. 61:2058–2062 [DOI] [PubMed] [Google Scholar]
- 15. Kaschabek SR. 1995. Chemische Synthese von Metaboliten des mikrobiellen Chloraromatenabbaus und Untersuchung der Substratspezifität der Maleylacetat-Reduktase aus Pseudomonas sp. Stamm B13. Dissertation Bergische Universität Gesamthochschule Wuppertal, Wuppertal, Germany [Google Scholar]
- 16. Kuhn D, Kholiq MA, Heinzle E, Bühler B, Schmid A. 2010. Intensification and economic and ecological assessment of a biocatalytic oxyfunctionalization process. Green Chem. 12:815–827 [Google Scholar]
- 17. Laemmli UK. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685 [DOI] [PubMed] [Google Scholar]
- 18. Meinwald J, Labana SS, Chadha MSJ. 1963. Peracid reactions. III. The oxidation of bicyclo[2.2.1]heptadiene. J. Am. Chem. Soc. 85:582–585 [Google Scholar]
- 19. Merril CR. 1990. Gel-staining techniques. Methods Enzymol. 182:477–488 [DOI] [PubMed] [Google Scholar]
- 20. Merril CR, Goldman D, Sedman SA, Ebert MH. 1981. Ultrasensitive stain for proteins in polyacrylamide gels shows regional variation in cerebrospinal fluid proteins. Science 211:1437–1438 [DOI] [PubMed] [Google Scholar]
- 21. Miyamoto K, Okuro K, Ohta H. 2007. Substrate specificity and reaction mechanism of recombinant styrene oxide isomerase from Pseudomonas putida S12. Tetrahedron Lett. 48:3255–3257 [Google Scholar]
- 22. Montersino S, Tischler D, Gassner GT, van Berkel WJH. 2011. Catalytic and structural features of flavoprotein hydroxylases and epoxidases. Adv. Synth. Catal. 353:2301–2319 [Google Scholar]
- 23. Mooney A, Ward PG, O'Connor KE. 2006. Microbial degradation of styrene: biochemistry, molecular genetics, and perspectives for biotechnological applications. Appl. Microbiol. Biotechnol. 72:1–10 [DOI] [PubMed] [Google Scholar]
- 24. O'Connor K, Buckley CM, Hartmans S, Dobson ADW. 1995. Possible regulatory role for nonaromatic carbon sources in styrene degradation by Pseudomonas putida CA-3. Appl. Environ. Microbiol. 61:544–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. O'Leary ND, O'Connor KE, Dobson ADW. 2002. Biochemistry, genetics and physiology of microbial styrene degradation. FEMS Microbiol. Rev. 26:403–417 [DOI] [PubMed] [Google Scholar]
- 26. O'Leary ND, O'Connor KE, Duetz W, Dobson ADW. 2001. Transcriptional regulation of styrene degradation in Pseudomonas putida CA-3. Microbiology 147:973–979 [DOI] [PubMed] [Google Scholar]
- 27. Olivera ER, et al. 1994. Catabolism of aromatics in Pseudomonas putida U. Formal demonstration that phenylacetic acid and 4-hydroxyphenylacetic acid are catabolized by two unrelated pathways. Eur. J. Biochem. 221:375–381 [DOI] [PubMed] [Google Scholar]
- 28. Olivera ER, et al. 1998. Molecular characterization of the phenylacetic acid catabolic pathway in Pseudomonas putida U: the phenylacetyl-CoA catabolon. Proc. Natl. Acad. Sci. U. S. A. 95:6419–6424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Otto K, Hofstetter K, Roethlisberger M, Witholt B, Schmid A. 2004. Biochemical characterization of StyAB from Pseudomonas sp. strain VLB120 as a two-component flavin-diffusible monooxygenase. J. Bacteriol. 186:5292–5302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Panke S, de Lorenzo V, Kaiser A, Witholt B, Wubbolts MG. 1999. Engineering of a stable whole-cell biocatalyst capable of (S)-styrene oxide formation for continuous two-liquid-phase applications. Appl. Environ. Microbiol. 65:5619–5623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Panke S, Held M, Wubbolts MG, Witholt B, Schmid A. 2002. Pilot-scale production of (S)-styrene oxide from styrene by recombinant Escherichia coli synthesizing styrene monooxygenase. Biotechnol. Bioeng. 80:33–41 [DOI] [PubMed] [Google Scholar]
- 32. Panke S, Meyer A, Huber CM, Witholt B, Wubbolts MG. 1999. An alkane-responsive expression system for the production of fine chemicals. Appl. Environ. Microbiol. 65:2324–2332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Panke S, Witholt B, Schmid A, Wubbolts MG. 1998. Towards a biocatalyst for (S)-styrene oxide production: characterization of the styrene degradation pathway of Pseudomonas sp. strain VLB120. Appl. Environ. Microbiol. 64:2032–2043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pauling L. 1960. The nature of the chemical bond, 3rd ed Cornell University Press, Ithaca, NY [Google Scholar]
- 35. Schmid A, Hofstetter K, Feiten H-J, Hollmann F, Witholt B. 2001. Integrated biocatalytic synthesis on gram scale: the highly enantioselective preparation of chiral oxiranes with styrene monooxygenase. Adv. Synth. Catal. 343:732–737 [Google Scholar]
- 36. Sheldon RA. 2007. Enzyme immobilization: the quest for optimum performance. Adv. Synth. Catal. 349:1289–1307 [Google Scholar]
- 37. Teufel R, et al. 2010. Bacterial phenylalanine and phenylacetate catabolic pathway revealed. Proc. Natl. Acad. Sci. U. S. A. 107:14390–14395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tischler D, et al. 2009. Identification of a novel self-sufficient styrene monooxygenase from Rhodococcus opacus 1CP. J. Bacteriol. 191:4996–5009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tischler D, Kaschabek SR. 2012. Microbiological styrene degradation: from basics to biotechnology, p 67–99 In Singh SN. (ed), Microbial degradation of xenobiotics, environmental science and engineering. Springer-Verlag, Heidelberg, Germany [Google Scholar]
- 40. Tischler D, et al. 2010. StyA1 and StyA2B from Rhodococcus opacus 1CP: a multifunctional styrene monooxygenase system. J. Bacteriol. 192:5220–5227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Toda H, Itoh N. 2012. Isolation and characterization of styrene metabolism genes from styrene-assimilating soil bacteria Rhodococcus sp. ST-5 and ST-10. J. Biosci. Bioeng. 113:12–19 [DOI] [PubMed] [Google Scholar]
- 42. Velasco A, Alonso S, García JL, Perera J, Díaz E. 1998. Genetic and functional analysis of the styrene catabolic cluster of Pseudomonas sp. strain Y2. J. Bacteriol. 180:1063–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Watson JM. January 1975. Thermolysis of styrene oxide. US patent 3,860,614