Abstract
Streptococcus pneumoniae pneumolysin (PLY) is a virulence factor that causes toxic effects contributing to pneumococcal pneumonia. To date, deriving a PLY candidate vaccine with the appropriate detoxification and immune profile has been challenging. A pneumolysin protein that is appropriately detoxified and that retains its immunogenicity is a desirable vaccine candidate. In this study, we assessed the protective efficacy of our novel PlyD1 detoxified PLY variant and investigated its underlying mechanism of protection. Results have shown that PlyD1 immunization protected mice against lethal intranasal (i.n.) challenge with pneumococci and lung injury mediated by PLY challenge. Protection was associated with PlyD1-specific IgG titers and in vitro neutralization titers. Pretreatment of PLY with PlyD1-specific rat polyclonal antiserum prior to i.n. delivery of toxin reduced PLY-mediated lung lesions, interleukin-6 (IL-6) production, and neutrophil infiltration into lungs, indicating that protection from lung lesions induced by PLY is antibody mediated. Preincubation of PLY with a neutralizing monoclonal PLY antibody also specifically reduced the cytotoxic effects of PLY after i.n. inoculation in comparison to nonneutralizing monoclonal antibodies. These results indicate that the induction of neutralizing antibodies against PLY can contribute to protection against bacterial pneumonia by preventing the development of PLY-induced lung lesions and inflammation. Our detoxified PlyD1 antigen elicits such PLY neutralizing antibodies, thus serving as a candidate vaccine antigen for the prevention of pneumococcal pneumonia.
INTRODUCTION
Streptococcus pneumoniae is the leading bacterial pathogen responsible for community-acquired pneumonia, which can frequently progress to more invasive diseases, including meningitis or septicemia. Currently, protein-polysaccharide conjugate and capsular polysaccharide vaccines have limitations since they confer a restricted serotype-specific coverage. Consequently, efforts are under way to develop alternative pneumococcal protein-based vaccines that have a broader coverage and offer longer-lasting protection (27).
Pneumolysin (PLY) is an important pneumococcal virulence factor that has a variety of toxic effects in vivo (6, 23). The toxicity of PLY is associated with its ability to induce pores in cholesterol-containing membranes (10, 12, 36). The protein is highly conserved in both amino acid sequence and antigenicity among clinical isolates (16), thus satisfying some basic criteria for its use as a vaccine antigen. Pneumococcal ply deletion mutants were shown to have a reduced virulence in mice compared to wild-type bacteria, indicating that PLY contributes to disease progression (2, 4). Indeed, at sublytic concentrations, intranasal (i.n.) delivery of PLY alone to mice can induce apoptosis in pulmonary epithelial and endothelial cells, stimulate upregulation of proinflammatory cytokines such as interleukin-6 (IL-6) and keratinocyte-derived chemokine (KC), and cause neutrophil infiltration (3, 24, 33, 35). Collectively, these events culminate in considerable lung damage and contribute to the development of pneumonia (23).
Due to inherent cytolytic properties, the vaccine potential of PLY has been evaluated in the form of reduced-toxicity pneumolysin mutant derivatives (18, 32). The most commonly used mutant, PdB, contains a single amino acid substitution of Trp433Phe (32). PLY mutant proteins have been tested in in vivo sepsis models using various mouse strains, various S. pneumoniae serotypes, and various routes of immunization. Overall, these studies indicate that vaccination with reduced-toxicity variants of PLY proteins can prolong the survival of mice compared to survival of placebo control groups (1, 18, 21, 22, 27–29, 31). In the pneumonia model, immunization of mice with PdB generated a significant decrease in pneumococcal lung burden in infected mice compared to immunization with a placebo control (5). It has also been observed that enhanced protection against a wide variety of strains was possible when PdB was used in combination with other pneumococcal proteins such as PspA, PspC, and PsaA (22, 27–29).
While the PdB mutant is a promising vaccine candidate, it possesses a low level of hemolytic activity (18, 19). Furthermore, a study in rats was performed by Dortant et al. (9) in which increasing dosages of PdB (PLY W433F) and PdBD (PLY D384N W433F) were administered intravenously in order to determine a possible reduction in toxicity of PdB and PdBD based on the estimated 50% lethal dose (LD50). The authors found that, in vivo, PdB and PdBD retained approximately 10% of the toxicity of pneumolysin, a higher level than was expected on the basis of in vitro hemolytic activity.
For this reason, we developed a highly detoxified pneumolysin mutant designated PlyD1 (30). A significant advantage of PlyD1 is that it was designed to possess a dual mechanism of detoxification. Two key mutations engineered into PLY to generate PlyD1 were T65C and G293C. Mutation G293C alone was shown to eliminate the hemolytic activity of PLY. In addition, the combination of T65C and G293C was shown to introduce a disulfide bond between domain 1 and domain 3 of PLY, which is expected to prevent the transitioning of PLY from the prepore to the pore-forming conformation. From a vaccine safety perspective, such built-in redundancy in detoxification mechanisms is highly desirable.
Using this mutant, we show that vaccination of mice with PlyD1 significantly protects against lethal i.n. pneumococcal infection. In addition, we demonstrate that PlyD1 vaccination significantly reduces lung damage caused by the toxin alone and that protection is mediated by neutralizing anti-PLY antibodies that inhibit the cytolytic activities of PLY in vivo.
MATERIALS AND METHODS
Mice.
Female, 6- to 9-week-old BALB/c or CBA/CaJ mice (Charles River or Jackson Laboratories, respectively) were maintained in accordance with procedures approved by the Canadian Council of Animal Care and the Sanofi Pasteur Animal Health Care Committee.
S. pneumoniae antigen proteins.
Recombinant PLY was produced from Escherichia coli containing the entire gene sequence of wild-type pneumolysin from strain R36A; the protein was column purified. PlyD1, a highly detoxified PLY variant, was generated by site-directed mutagenesis and differs from the wild-type form by three amino acid substitutions of T65C, G293C, and C428A. Recombinant PlyD1 protein was expressed in E. coli as soluble protein and column purified (30).
Antibodies.
Nonneutralizing (clones 1-5-3, 24-10-9, and 40-8-1) and neutralizing (clone 44-10-2) monoclonal anti-PLY antibodies, as well as a monoclonal antibody (MAb) specific for a different pneumococcal protein (negative control), were obtained from hybridomas from commercial custom MAb generation services (BioGenes). The neutralizing capability of these MAbs was determined using a hemolysis inhibition (HI) assay similar to that described below (see Table S2 in the supplemental material). Polyclonal antibodies were raised in rats against an irrelevant control and PlyD1. Rats received three subcutaneous injections of 10 μg of recombinant protein together with Freund's complete adjuvant in the first injection and incomplete Freund's adjuvant in the subsequent injections.
Immunization of mice.
PlyD1 formulations were prepared by adsorbing the protein onto aluminum hydroxide adjuvant (2% Alhydrogel; Brenntag Biosector, Frederikssund, Denmark) at a final elemental aluminum concentration of 0.56 mg/ml in 10 mM Tris buffered-saline (TBS) and 2 mM phosphate-buffered saline (PBS), pH 7.4. CBA/CaJ mice were immunized intramuscularly with either 2 mM phosphate-treated aluminum hydroxide adjuvant (AlOOH) (65 μg/dose) in TBS alone, which served as a negative control, or PlyD1 formulated in 2 mM AlOOH in TBS. Mice were immunized at 0, 3, and 6 weeks with recombinant PlyD1 antigen at various doses (10, 5, 2.5, 1, 0.5, 0.25, 0.125, or 0.06 μg). Sera were collected from the mice by retro-orbital bleeding 2 to 4 days prior to the first immunization and 3 weeks after the third immunization and analyzed individually for PLY-specific antibodies by enzyme-linked immunosorbent assay (ELISA). Unless indicated otherwise in the figure legends, sera were analyzed individually by the hemolysis inhibition (HI) assay to determine neutralizing antibody titers against PLY following immunization.
Bacterial challenge.
Following immunization, CBA/CaJ mice were challenged intranasally with S. pneumoniae strain 14453 serotype 6B (1.5 × 106 CFU per dose, approximately 30 times the LD50). Frozen challenge stock was diluted in phosphate-buffered saline (14190-144; Gibco) to the desired target dose prior to i.n. challenge. Following the challenge, mice were monitored daily for health status. All surviving mice were euthanized at 14 days postchallenge. A Wilcoxon test was used to determine if there was a significant delay to death between the immunized group(s) and the placebo control.
PLY challenge.
For pneumolysin toxicity studies, BALB/c and CBA/CaJ mice were inoculated intranasally either with a dose of 5 μg of PLY alone or PLY coincubated with antibodies (polyclonal or monoclonal) for 1 h at room temperature (RT) or with a PBS–15% glycerol control. The total volume given was 100 μl per inoculation.
ELISA.
PLY-specific IgG antibodies in mouse serum were quantified by ELISA. Ninety-six-well microplates (Nunc Maxisorp) were coated overnight at RT with 50 μl/well PLY at 2.0 μg/ml. Plates were washed with 0.1% Tween 20 (Sigma) in PBS, blocked for 1 h at RT with 100 μl/well of 1% bovine serum albumin (BSA; Sigma) in PBS, and then washed prior to the addition of 100 μl/well mouse serum diluted in 0.1% BSA–0.1% Tween 20 in PBS. One serum of known anti-PLY IgG titer was used as a standard, and another was used as an internal control. For detection, horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG(H+L) (Jackson ImmunoResearch) was added and incubated at RT for 1 h, followed by 3,3′,5,5′-tetramethylbenzidine (TMB; Sigma) added for 15 min at RT. The reaction was stopped by the addition of 50 μl/well 2.0 N sulfuric acid (VWR), and the optical densities (ODs) were read on a 96-well spectrophotometer at a wavelength of 450 nm to 540 nm. Anti-PLY IgG was calculated in ELISA units (EU)/ml by referencing OD values of the standard serum.
Inhibition of hemolysis assay.
The PLY-neutralizing ability of antibodies was tested using the HI assay. In 96-well round-bottom plates, serum samples were serially diluted 2-fold in PBS to a final volume of 25 μl. Full-length PLY protein was diluted to a concentration of 1.1 μg/ml, and 25 μl of diluted PLY was added to each well. Sheep red blood cells (SRBC) were diluted to 1% in PBS, and a volume of 50 μl was added to each well. The 96-well plate was incubated at 37°C for 60 min without shaking and then centrifuged for 10 min at 1,000 rpm, and 80 μl of supernatant was transferred from each well to a 96-well flat-bottom plate. For each well, the absorbance at 414 nm was read using a SpectraMax 384 Plus plate reader/spectrophotometer (Molecular Devices, CA) using SoftMax Pro GxP software, version 4.7.1. The average value of duplicate absorbance readings was plotted against serum titer values. Control wells containing nonlysed 1% SRBC were used to determine the average maximum absorbance value at 414 nm. The hemolytic titer was defined as the reciprocal dilution of serum that corresponded to a 50% drop in the maximum absorbance value at 414 nm.
The hemolysis inhibition titers of matched preimmune and postimmune pairs of sera were compared, and the fold increase in inhibitory activity was determined. An anti-PLY serum with a known inhibitory titer was used as a positive control, and wells containing no serum were used as negative controls.
BAL fluid collection and cell lineage stain.
Bronchoalveolar lavage (BAL) fluid from mice was collected by intratracheal instillation of three flushes of PBS (0.7 ml each), which were pooled and centrifuged (3 min at 1,400 rpm). The cell-free supernatant was assessed for cytokines or antibody titers, while the cell pellet was resuspended in 0.25 ml of PBS. An aliquot of the resuspended cells was counted using a hemacytometer, and the remaining cells were applied to microscope slides using a Cytospin apparatus centrifuged at RT for 3 min at 500 rpm. The slides were then subjected to Kwik-Diff cell lineage differential staining (ThermoShandon, Pittsburgh, PA).
Cytokine detection.
Cytokine levels in cell-free supernatant collected during BAL fluid harvest or from serum were analyzed via a Milliplex Map kit (Millipore) for detection of mouse cytokines/chemokines, according to the manufacturer's instructions.
Scoring of lung lesions.
Lungs from challenged mice were isolated after 24 h, and four sections were taken from four different lung lobes from each mouse. Thin sections were stained with hematoxylin and eosin (H&E). In blinded studies, each lung section was monitored for three phenotypes indicative of damage or injury, which included the following: (i) edema (swelling around blood vessels or airways), (ii) hemorrhage (fluid and/or blood leakage into alveolar spaces), and (iii) disruption of alveolar architecture/tissue organization. For each lobe examined, a score was given for each phenotype, similar to a previous methodology (3), as follows: a value of 2 for severe phenotype, 1 for a light to moderate phenotype, and 0 for no damage. A total lung damage score was obtained for each mouse by combining all the scores obtained for each phenotype for each of its four lobes. Mice that died shortly after inoculation (<6 h) were not scored as their death may have been caused by handling stress during inoculation.
For each mouse the score per lung lobe was obtained by dividing the total lung damage score by the number of lung lobes examined in a particular mouse. Mice that died following intranasal inoculation (greater than 6 h postchallenge) were assigned a score per lung lobe of 6.
Data analysis.
Data were analyzed by either Excel or SAS, version 9.13, software, the latter via a mixed model format using log10-transformed data.
RESULTS
PlyD1 immunization delayed time to death in mice following serotype 6B challenge.
We have generated a novel, recombinant, and genetically detoxified PLY variant, named PlyD1, that contains three amino acid substitutions of T65C, G293C, and C428A. PlyD1 is nontoxic as it exhibits substantially decreased hemolytic activity compared to full-length recombinant PLY and to the detoxified PLY variant PdB. In the investigation of Oloo et al., PdB exhibited a residual hemolytic activity of 1% while PlyD1 had less than 0.001% activity compared to that of PLY (30).
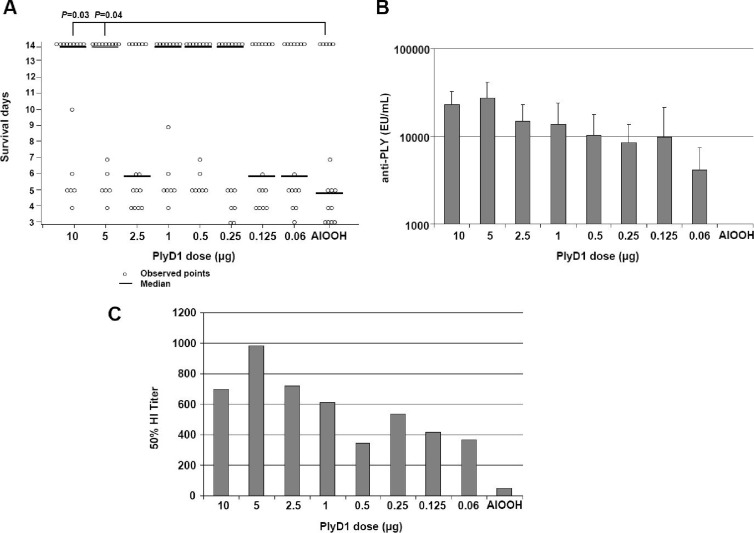
In order to assess the protective efficacy of PlyD1 following intranasal challenge with S. pneumoniae, two independent studies were carried out in mice as described in Materials and Methods. Results showed that PlyD1 immunization at the higher doses (5 and 10 μg/dose) significantly delayed the progression to death in CBA/CaJ mice following lethal challenge with S. pneumoniae strain 14453 (Fig. 1A). At the lower doses tested (2.5, 1, 0.5, 0.25, 0.125, and 0.06 μg/dose), the median survival levels were not significantly different from those of the placebo control group, indicating that these PlyD1 doses were too low to elicit protective immune responses. Antibody responses to PLY were detected in all PlyD1-immunized mice. IgG titers increased gradually with increasing doses of antigen in a dose-specific manner (P < 0.001) (Fig. 1B).
Fig 1.
PlyD1 immunization confers protection to mice against intranasal pneumococcal challenge. (A) CBA/CaJ mice were immunized with increasing doses of PlyD1 (0.06 μg to 10 μg) as described in Materials and Methods. Three weeks following the last immunization, mice were challenged with S. pneumoniae strain 14453 serotype 6B at a lethal dose of 1.5 × 106 CFU per mouse, and survival was monitored for 2 weeks. The P value shows a significant difference between the placebo control group (AlOOH) and the indicated test group. Circles indicate data points for individual mice. (B) Serum antibody titers to PLY for the above mice, as determined by ELISA. The control group received aluminum hydroxide adjuvant alone. (C) Hemolytic inhibition conferred by pooled anti-PlyD1 sera for the above groups on PLY-mediated hemolysis of sheep red blood cells.
The ability of antisera from immunized mice to inhibit hemolysis of sheep red blood cells by PLY was tested using a hemolysis inhibition (HI) assay following the third immunization. The sera from each group were pooled; therefore, a statistical analysis on these samples was not performed. Nevertheless, results indicated that the highest HI titers against PLY were generated in mice immunized with PlyD1 at a protective dose of 5 μg (Fig. 1C).
Taken together, these results indicate that PlyD1-immunized mice that are protected against bacterial challenge produce high levels of hemolysis-neutralizing antibodies.
Development of the in vivo PLY challenge model.
To determine the mechanism of protection following PlyD1 immunization, we first developed an in vivo PLY intranasal (i.n.) challenge model. This allowed us to monitor any host lung tissue damage and immunological responses induced by PLY alone following toxin delivery into mouse lungs. In doing so, we were able to more clearly address how immunization with PlyD1 provided specific protection against PLY in the lung system.
Both CBA/CaJ and BALB/c mice were used in the development of the model. Mice were inoculated i.n. with either 0, 0.5, 1, 2.5, or 5 μg of PLY per dose and monitored for the presence of lung lesions, IL-6 production, and neutrophil infiltration in the lungs as these were previously identified as the main toxic effects associated with i.n. challenge with PLY (3, 33). We observed that the higher dosage of PLY (5 μg/dose) used in our studies resulted in significant increases in levels of IL-6 detected in the bronchoalveolar (BAL) fluid and serum as early as 4 h postchallenge. Neutrophil infiltration in lung tissue was also detected between 6 and 12 h postchallenge (data not shown). Examination of lung sections from mice receiving i.n. PLY challenge (2.5 and 5 μg doses) at both 24 and 48 h revealed obvious lung tissue damage, characterized by the presence of hemorrhage, edema, and substantial disruption of the alveolar architecture, as previously reported (14, 17). Control mice and mice that received lower concentrations of PLY did not exhibit the above characteristic phenotypes. Since an i.n. dose of 5 μg of PLY provided more consistency in the lung injury phenotypes observed, it was selected as our standard challenge dose in order to reduce variability in the model. Both mouse strains (CBA/CaJ and BALB/c) generated similar phenotypes following PLY i.n. challenge; however, since BALB/c mice exhibited more resistance to the effects of pneumococcal challenge and since a comparison between bacterial and PLY challenge was desired, CBA/CaJ mice were selected for these studies.
Overall, our in vivo PLY challenge model involving i.n. PLY inoculation of mice displayed several disease phenotypes that are characteristic of pneumococcal lung infection and are in accordance with findings of other studies (33).
PlyD1 immunization can inhibit lung lesions and delay death in mice following intranasal challenge with purified PLY.
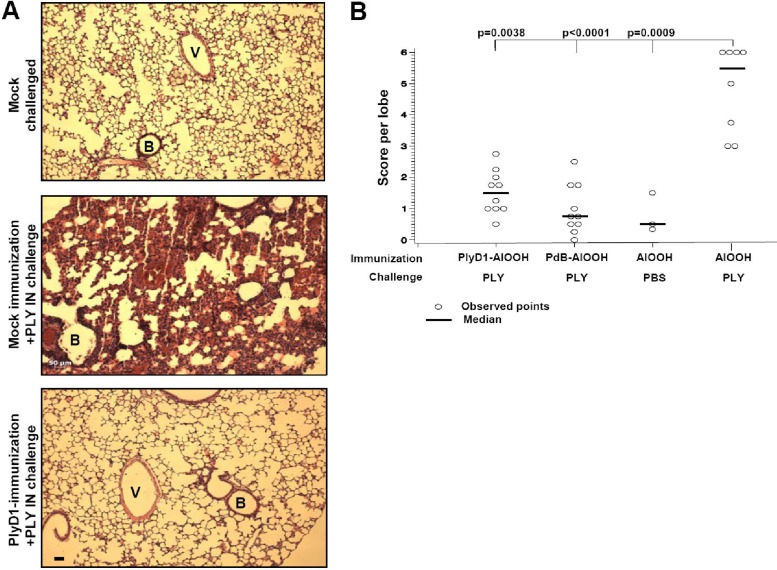
We next investigated whether immunization with PlyD1 could protect mice against the lung damage induced by i.n. challenge with PLY. Three independent studies were conducted on CBA/CaJ mouse groups immunized with PlyD1 (10 μg/dose) or mock immunized and subsequently challenged i.n. with or without PLY. Figure 2A shows lung sections of representative mice that were either PlyD1 (Fig. 2A, bottom panel) or mock immunized (Fig. 2A, middle panel) and then challenged with PLY or mock challenged (Fig. 2A, top panel). In each of the three separate studies, all mice that were mock immunized and challenged with PLY (n = 35 total) either died between 6 and 24 h postchallenge or displayed considerable damage to their lungs, as characterized by hemorrhage, disruption of the alveolar space and alveolar walls, and/or severe edema (Fig. 2A, middle panel). In contrast, mice immunized with PlyD1 at 10 μg/dose (n = 35 from three studies) (see Table S1 in the supplemental material for a summary) and challenged with PLY (Fig. 2A, bottom) had no damage or only limited damage to their lungs, which appeared similar to lung tissue from mock-challenged mice (Fig. 2A, top two panels). Results shown in Fig. 2B demonstrate significantly less lung damage in both PlyD1- and PdB-immunized mice (n = 10 mice/group) compared to the placebo control group that was challenged intranasally with PLY (P = 0.0038 and P < 0.0001, respectively). Furthermore, there was no statistical difference between PlyD1- and PdB-immunized mice in terms of protection from PLY-induced lung damage (P = 0.355, Wilcoxon test). Hence, immunization of mice with PlyD1 provided significant protection from i.n. PLY challenge, which was also comparable to PdB immunization.
Fig 2.
PlyD1 immunization inhibits lung lesions in mice challenged intranasally with PLY. (A) H&E-stained lung sections from representative mice that were PlyD1 or mock immunized and then challenged with PLY at 5 μg/dose or mock challenged. The top panel shows a lung section from a control mouse mock challenged with PBS–15% glycerol i.n. The middle panel shows a typical lung section from a mock-immunized mouse challenged i.n. with PLY. The bottom panel shows a typical lung section from a mouse immunized with PlyD1 at 10 μg/dose and challenged with PLY. V, blood vessel; B, bronchiole. All images are at the same magnification. Scale bar, 50 μm. (B) Immunization with PlyD1 reduces PLY-mediated lung damage in mice. In this representative study, mice were immunized with 10 μg/dose of PlyD1, PdB, or AlOOH alone and challenged with PLY or PBS–15% glycerol. Lung damage was assessed using a histopathology scoring system as indicated in Materials and Methods. The P value indicates a significant difference between the placebo control group challenged with PLY and each test group. Circles indicate data points for individual mice.
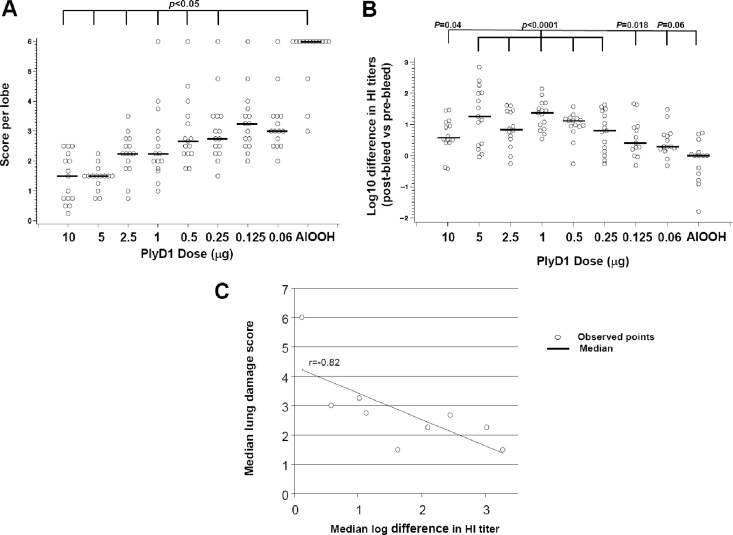
To investigate any association between PlyD1 immunization dosage, protection from PLY-induced lung damage, and functional antibody titers, CBA/CaJ mice (n = 15/group) immunized with increasing amounts of PlyD1 (from 0.06 to 10 μg/dose) and then challenged i.n. with PLY were examined (Fig. 3). All PlyD1-immunized groups exhibited significantly less lung damage than the mock-immunized control group, except for mice receiving the two lowest doses of PlyD1 (0.06 and 0.125 μg/dose). The 5 μg/dose offered the optimum protection against PLY-induced lung damage (Fig. 3A) and generated a high HI titer (Fig. 3B). Since both lung damage scoring and functional antibody analysis were performed on each mouse, a correlation between in vivo and in vitro neutralization of PLY was evaluated using the Spearman correlation coefficient. Results showed a significant inverse correlation (r = −0.82) between the two (Fig. 3C), suggesting that PlyD1-mediated protection from lung damage is dependent on hemolysis-neutralizing antibodies.
Fig 3.
High neutralizing antibody titers against PlyD1 correlate with reduced lung damage induced by PLY. (A) Immunization of mice with PlyD1 provides dose-dependent protection to mouse lungs following i.n. PLY challenge. Mice were immunized with increasing doses of PlyD1 (0.06 to 10 μg/dose) and challenged with PLY at 5 μg/dose. Lung damage was scored as indicated in Materials and Methods. Circles indicate data points for individual mice. (B) Hemolysis inhibition titers of individual sera taken from the above mice immunized with increasing doses of PlyD1 (0.06 to 10 μg/dose). The HI titers are represented as a ratio between postbleed and prebleed sera. The P value indicates a significant difference between the placebo control group and each test group. Circles indicate data points for individual mice. (C) Correlation between the neutralizing HI titers and protection from lung damage. Using the Spearman correlation coefficient, a significant correlation (r = −0.82) is observed.
PlyD1-mediated inhibition of lung damage is initiated by hemolysis-neutralizing antibodies against PLY.
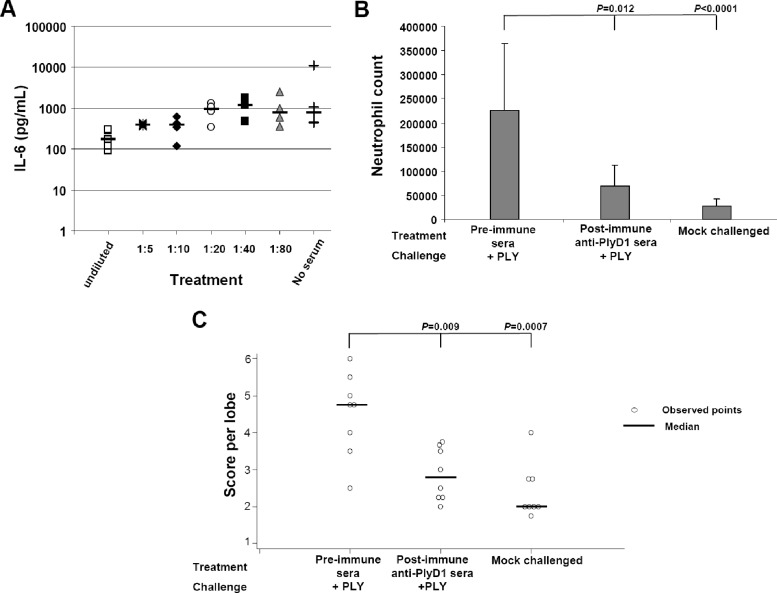
The above results suggested a role for the antibody response in conferring protection against i.n. PLY challenge. To determine whether pretreatment of PLY with anti-PlyD1 antiserum could reduce the proinflammatory response that accompanied PLY i.n. delivery of the toxin, we preincubated PLY with rat anti-PlyD1 polyclonal serum prior to inoculation of mice. Preincubation of PLY with increasing concentrations of rat PlyD1 antiserum (from 1:80 to undiluted serum) led to a dose-dependent decline in serum IL-6 levels (P < 0.0001) following i.n. delivery of the toxin (Fig. 4A). This pretreatment of PLY with polyclonal anti-PlyD1 serum could also significantly reduce neutrophil infiltration and inhibit lung damage compared to preimmune serum (P = 0.012 and 0.009, respectively) (Fig. 4B and C).
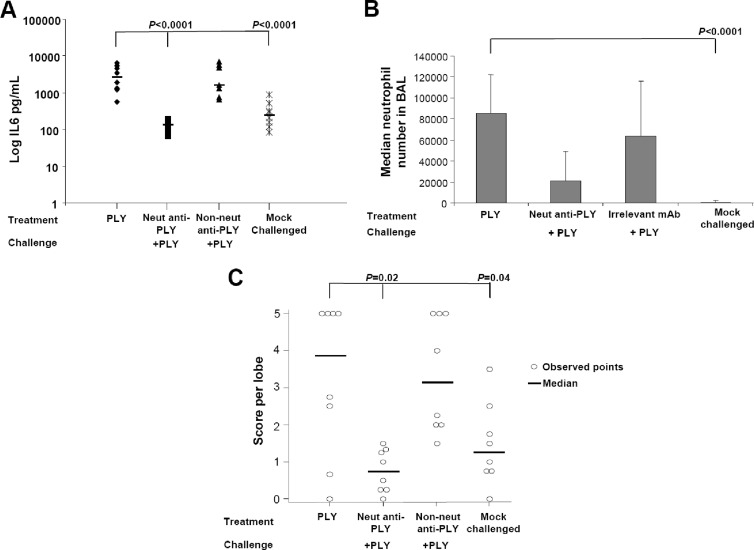
Fig 4.
PlyD1-specific antisera reduced inflammatory and toxic effects associated with PLY i.n. challenge. (A) Pretreatment of PLY (5 μg/dose) with increasing concentrations of rat PlyD1 antiserum reduces the serum IL-6 levels normally associated with PLY i.n. challenge. (B) Preincubation of PLY with undiluted rat PlyD1 antiserum prior to i.n. challenge with the toxin reduces neutrophil infiltration into the lung, as assessed by neutrophil cell counts in BAL fluid. As a negative control, neutrophil counts were also determined in mock-challenged mice that received only PBS–15% glycerol i.n. The P value indicated on top of the figure refers to a comparison between the preimmune serum treatment and each test group. (C) Preincubation of PLY with undiluted rat PlyD1 antiserum prior to i.n. challenge with the toxin reduces PLY-mediated lung damage. PLY was preincubated with either rat preimmune serum or postimmune anti-PlyD1 serum. Mice were challenged i.n. with either of these mixtures, and the histopathology of lung sections was scored and compared to that of mock-challenged mice. The P value indicates a significant difference between the preimmune sera and each test group. Circles indicate data points for individual mice.
To specifically show that antibodies were responsible for conferring protection, we tested anti-PLY monoclonal antibodies (MAbs) in a similar manner as above. A neutralizing anti-PLY MAb (clone 44-10-2) and a pool of nonneutralizing MAbs (clones 1-5-3, 24-10-9, and 40-8-1) were used. Preincubation of PLY with the neutralizing MAb prior to i.n. inoculation of mice significantly reduced the serum IL-6 levels, neutrophil infiltration, and lung damage compared to mice receiving untreated PLY or PLY preincubated with a pool of the three nonneutralizing MAbs (Fig. 5A, B, and C). These results indicate that neutralizing anti-PLY antibodies have the ability to effectively prevent inflammation and PLY-mediated damage to the lungs in challenged mice.
Fig 5.
Monoclonal anti-PLY neutralizing antibodies reduced in vivo PLY-mediated inflammation and toxicity. (A) Serum IL-6 levels in mice challenged i.n. with untreated PLY or PLY preincubated with either a neutralizing MAb (20 μg) or a pool of nonneutralizing anti-PLY MAbs (20 μg each). As a negative control, IL-6 levels were also determined in mock-challenged mice that received only PBS–15% glycerol i.n. (B) Neutrophil infiltration into the BAL fluid of mice challenged i.n. with untreated PLY or PLY pretreated with a neutralizing MAb or an irrelevant control. Mock-challenged mice received PBS–15% glycerol i.n. (C) Mouse lung damage scores after i.n. challenge with untreated PLY or PLY pretreated with neutralizing monoclonal antibodies. As a negative control, lung damage was also determined in mock-challenged mice that received only PBS–15% glycerol i.n. The P value refers to a significant difference between the PLY-challenged group and each indicated test group. Circles indicate data points for individual mice.
DISCUSSION
Pneumolysin is a key virulence factor of S. pneumoniae that has a conserved amino acid sequence (16) and represents an important vaccine target against pneumonia-associated diseases caused by S. pneumoniae. Due to the lytic activity of PLY, several reduced-toxicity derivatives have been evaluated preclinically for their immunogenicity and protective capacity. Vaccination with some of these PLY derivatives has been shown to delay the onset of disease (1, 8, 18, 27), albeit via an uncertain mechanism(s). In some cases these PLY derivatives such as PdB still retained residual hemolytic activity (9). Efforts have therefore been made to increase the safety profile of PLY derivatives while maintaining their immunogenicity. We report here on a highly detoxified PLY derivative, PlyD1, and show that this mutant elicits a high level of immunity that confers protection in mice against virulent pneumococcus challenge. Furthermore, by utilizing a PLY challenge model, we have demonstrated that this protection is mediated by neutralizing antibodies.
The contribution of PLY to pneumococcal pneumonia has been previously described, using models in which PLY or PLY-negative mutants were administered to mice or rats. Feldman et al. (11) reported that histological changes in rat lungs induced by administration of PLY into the apical lobe bronchus resembled changes induced by intrapulmonary administration of pneumococcal bacteria. The in vivo role of PLY in the early pathogenesis of pneumococcal pneumonia was confirmed by Rubins et al. (34). Following endotracheal delivery of a PLY-negative serotype 2 mutant or its parent strain into mouse lungs, the authors observed that the wild-type strain induced greater damage to the alveolar-capillary barrier, and the important role of PLY in acute lung damage, intra-alveolar bacterial growth, and invasion of lung interstitium was demonstrated. Inflammatory responses induced by intranasal administration of PLY into mouse lungs was examined by Rijneveld et al. (33) and included an increased influx of neutrophils and increased production of IL-6 and CXC chemokines. Reduced neutrophil influx and chemokine production were observed following intranasal delivery of PLY mutants. The direct effect of PLY on inducing vascular leakage and pulmonary edema in murine lung injury has also been described in detail (20, 24, 38).
We used a murine intranasal challenge model in order to assess vaccine-mediated protection against PLY-induced lung damage independently of deleterious effects caused by other pneumococcal components. We observed that intranasal administration of PLY resulted in upregulation of the proinflammatory cytokine IL-6, hemorrhaging, edema, and neutrophil infiltration in lung tissue and disrupted alveolar structure. These observations were in accordance with those of previous studies in which PLY was administered to mice or rats (11, 33).
Our findings indicated that immunization with PlyD1 conferred significant protection against challenge with either live pneumococci or PLY (Spearman correlation coefficient, r = 0.97). To our knowledge, this is the first evidence demonstrating that protection from lung injury directly caused by PLY can be achieved through active immunization. More importantly, the reduction in lung damage may be the underlying mechanism by which a pneumolysin-derivative vaccine protects mice against i.n. pneumococcal challenge.
Since the anti-PlyD1 antibody response detected by ELISA (Fig. 1B) in immunized mice increased with the antigen dose (P < 0.001), we investigated whether protection against PLY-induced lung damage was antibody mediated. As observed with PlyD1 immunization, preincubation of PLY with polyclonal anti-PlyD1 antiserum reduced lung tissue damage and inflammatory effects normally associated with i.n. PLY challenge. Our studies are in accordance with others where rabbit anti-PLY IgG delivered into the tail vein of mice was shown to be protective against i.n. pneumococcal challenge (14). Purified human anti-PLY IgG has also been shown to be passively protective in mice; however, those studies examined intraperitoneal challenge, not i.n. challenge, by bacteria (26).
Our observations suggest that PlyD1-specific antibodies protect in vivo by neutralizing the toxic activities of PLY. Indeed, the anti-PLY monoclonal antibody described here, known to inhibit the hemolytic activity of PLY in vitro (see Table S2 in the supplemental material), was also able to confer protection in vivo by reducing tissue damage, proinflammatory IL-6 response, and neutrophil infiltration in the lungs (Fig. 5), similar to polyclonal anti-PlyD1 serum (Fig. 4). Anti-PLY monoclonal antibodies (PLY4, PLY5, PLY7, and PLY9) previously generated by J. de los Toyos et al. (7) were also tested in our model (see Fig. S1 and Table S2 in the supplemental material). Incubation of PLY with the MAb PLY4, which has a strong neutralizing ability in vitro and in vivo (7, 13), significantly reduced IL-6 production (similarly observed with our neutralizing 44-10-2 MAb) compared to incubation with the nonneutralizing MAb PLY9 or PLY treatment alone. Monoclonal antibodies PLY5 and PLY7 had limited neutralizing activity in vivo, which confirmed previous findings described in Garcia-Suarez et al. (13). Overall, our results treating PLY toxin with neutralizing MAb prior to i.n. inoculation are in accordance with studies using anti-PLY MAbs to passively protect mice against i.n. challenge with live pneumococci (13). Taken together, these results indicate that antibodies may play an important role in reducing inflammation and damage in the lungs by neutralizing PLY presented during pneumococcal infection.
Interestingly, the induction of IL-6 following PLY challenge was always accompanied by lung damage. In our studies, neutralizing MAbs to PLY were able to reduce both IL-6 production (4 h postchallenge) and lung damage (24 h postchallenge) caused by the toxin. These results suggest that IL-6 production is linked to lung damage caused by PLY. Indeed, it has been shown by Maus et al. (24) that lung damage is caused by the direct cytotoxic effects of PLY rather than indirectly through the chronic induction of inflammation in the BAL fluid due to the intranasal instillation of PLY. Conceivably, neutralization of PLY in vivo would inhibit its ability to cause pore formation in host cells, resulting in reduced IL-6 production normally acting as an early danger signal indicating damaged tissue (33, 37).
Our results indicate that following immunization with PlyD1, circulating antibodies generated against PlyD1 can migrate into the lungs to confer protection against PLY-mediated damage. Indeed, we have been able to detect anti-PlyD1 antibodies in the BAL fluid of immunized mice (see Fig. S2 in the supplemental material). Conceivably, the reduction in inflammation and lung tissue damage due to the protective effects of PLY neutralizing antibodies would limit or delay the ability of bacteria to enter the bloodstream and cause septicemia and death. In fact Berry et al. (4) have shown that pneumococcal PLY deletion mutant strains have reduced virulence, leading to increased survival of mice and decreased bacterial load in several organs following intraperitoneal challenge.
Based on the demonstration here that neutralizing antibodies are sufficient to impair the cytotoxic and inflammatory effects of PLY in vivo, it is conceivable that monitoring neutralizing antibody activity can be used as an approach to assess vaccine efficacy.
Overall, we have shown that a novel, highly detoxified PLY variant, PlyD1, can serve as a target vaccine antigen against pneumococcal pneumonia. Such a vaccine may be effective in reducing chronic inflammation and disease severity typically observed in infants with S. pneumoniae-related pneumonia (25). Furthermore, considering that patients with acute pneumococcal infections have lower antibodies against PLY than healthy individuals (15), our data support the use of the pneumolysin-derivative PlyD1 as a vaccine component aimed at increasing pneumolysin-specific immunity and, as a consequence, reducing acute pneumococcal infections and disease.
Supplementary Material
Footnotes
Published ahead of print 2 April 2012
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1. Alexander JE, et al. 1994. Immunization of mice with pneumolysin toxoid confers a significant degree of protection against at least nine serotypes of Streptococcus pneumoniae. Infect. Immun. 62:5683–5688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Benton KA, Everson MP, Briles DE. 1995. A pneumolysin-negative mutant of Streptococcus pneumoniae causes chronic bacteremia rather than acute sepsis in mice. Infect. Immun. 63:448–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bergeron Y, et al. 1998. Cytokine kinetics and other host factors in response to pneumococcal pulmonary infection in mice. Infect. Immun. 66:912–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Berry AM, Ogunniyi AD, Miller DC, Paton JC. 1999. Comparative virulence of Streptococcus pneumoniae strains with insertion-duplication, point, and deletion mutations in the pneumolysin gene. Infect. Immun. 67:981–985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Briles DE, et al. 2003. Immunizations with pneumococcal surface protein A and pneumolysin are protective against pneumonia in a murine model of pulmonary infection with Streptococcus pneumoniae. J. Infect. Dis. 188:339–348 [DOI] [PubMed] [Google Scholar]
- 6. Cockeran R, Anderson R, Feldman C. 2002. The role of pneumolysin in the pathogenesis of Streptococcus pneumoniae infection. Curr. Opin. Infect. Dis. 15:235–239 [DOI] [PubMed] [Google Scholar]
- 7. de los Toyos J, et al. 1996. Functional analysis of pneumolysin by use of monoclonal antibodies. Infect. Immun. 64:480–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Denoel P, et al. 2011. A protein-based pneumococcal vaccine protects rhesus macaques from pneumonia after experimental infection with Streptococcus pneumoniae. Vaccine 29:5495–5501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dortant PM, et al. 2000. Toxicity of pneumolysin and rDNA derived pneumolysin mutants in rats. RIVM report 124000001. National Instituted of Public Health and the Environment, Bilthoven, the Netherlands [Google Scholar]
- 10. El-Rachkidy RG, Davies NW, Andrew PW. 2008. Pneumolysin generates multiple conductance pores in the membrane of nucleated cells. Biochem. Biophys. Res. Commun. 368:786–792 [DOI] [PubMed] [Google Scholar]
- 11. Feldman C, et al. 1991. Pneumolysin induces the salient histologic features of pneumococcal infection in the rat lung in vivo. Am. J. Respir. Cell Mol. Biol. 5:416–423 [DOI] [PubMed] [Google Scholar]
- 12. Fortsch C, et al. 2011. Changes in astrocyte shape induced by sublytic concentrations of the cholesterol-dependent cytolysin pneumolysin still require pore-forming capacity. Toxins (Basel) 3:43–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Garcia-Suarez MM, et al. 2004. Protection against pneumococcal pneumonia in mice by monoclonal antibodies to pneumolysin. Infect. Immun. 72:4534–4540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Garcia-Suarez MM, et al. 2007. The role of pneumolysin in mediating lung damage in a lethal pneumococcal pneumonia murine model. Respir. Res. 8:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Huo Z, et al. 2004. Antibody response to pneumolysin and to pneumococcal capsular polysaccharide in healthy individuals and Streptococcus pneumoniae infected patients. Vaccine 22:1157–1161 [DOI] [PubMed] [Google Scholar]
- 16. Jefferies JM, et al. 2010. Identification of novel pneumolysin alleles from paediatric carriage isolates of Streptococcus pneumoniae. J. Med. Microbiol. 59:808–814 [DOI] [PubMed] [Google Scholar]
- 17. Kerr AR, et al. 2002. Role of inflammatory mediators in resistance and susceptibility to pneumococcal infection. Infect. Immun. 70:1547–1557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kirkham LA, et al. 2006. Construction and immunological characterization of a novel nontoxic protective pneumolysin mutant for use in future pneumococcal vaccines. Infect. Immun. 74:586–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Korchev YE, et al. 1998. A conserved tryptophan in pneumolysin is a determinant of the characteristics of channels formed by pneumolysin in cells and planar lipid bilayers. Biochem. J. 329:571–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lim JH, et al. 2007. Tumor suppressor CYLD regulates acute lung injury in lethal Streptococcus pneumoniae infections. Immunity 27:349–360 [DOI] [PubMed] [Google Scholar]
- 21. Lock RA, Paton JC, Hansman D. 1988. Comparative efficacy of pneumococcal neuraminidase and pneumolysin as immunogens protective against Streptococcus pneumoniae. Microb. Pathog. 5:461–467 [DOI] [PubMed] [Google Scholar]
- 22. Lu YJ, Forte S, Thompson CM, Anderson PW, Malley R. 2009. Protection against pneumococcal colonization and fatal pneumonia by a trivalent conjugate of a fusion protein with the cell wall polysaccharide. Infect. Immun. 77:2076–2083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Marriott HM, Mitchell TJ, Dockrell DH. 2008. Pneumolysin: a double-edged sword during the host-pathogen interaction. Curr. Mol. Med. 8:497–509 [DOI] [PubMed] [Google Scholar]
- 24. Maus UA, et al. 2004. Pneumolysin-induced lung injury is independent of leukocyte trafficking into the alveolar space. J. Immunol. 173:1307–1312 [DOI] [PubMed] [Google Scholar]
- 25. Michelow IC, et al. 2004. Epidemiology and clinical characteristics of community-acquired pneumonia in hospitalized children. Pediatrics 113:701–707 [DOI] [PubMed] [Google Scholar]
- 26. Musher DM, Phan HM, Baughn RE. 2001. Protection against bacteremic pneumococcal infection by antibody to pneumolysin. J. Infect. Dis. 183:827–830 [DOI] [PubMed] [Google Scholar]
- 27. Ogunniyi AD, Folland RL, Briles DE, Hollingshead SK, Paton JC. 2000. Immunization of mice with combinations of pneumococcal virulence proteins elicits enhanced protection against challenge with Streptococcus pneumoniae. Infect. Immun. 68:3028–3033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ogunniyi AD, Grabowicz M, Briles DE, Cook J, Paton JC. 2007. Development of a vaccine against invasive pneumococcal disease based on combinations of virulence proteins of Streptococcus pneumoniae. Infect. Immun. 75:350–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ogunniyi AD, Woodrow MC, Poolman JT, Paton JC. 2001. Protection against Streptococcus pneumoniae elicited by immunization with pneumolysin and CbpA. Infect. Immun. 69:5997–6003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Oloo EO, Yethon JA, Ochs MM, Carpick B, Oomen R. 2011. Structure-guided antigen engineering yields pneumolysin mutants suitable for vaccination against pneumococcal disease. J. Biol. Chem. 286:12133–12140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Paton JC, Lock RA, Hansman DJ. 1983. Effect of immunization with pneumolysin on survival time of mice challenged with Streptococcus pneumoniae. Infect. Immun. 40:548–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Paton JC, et al. 1991. Purification and immunogenicity of genetically obtained pneumolysin toxoids and their conjugation to Streptococcus pneumoniae type 19F polysaccharide. Infect. Immun. 59:2297–2304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rijneveld AW, et al. 2002. Roles of interleukin-6 and macrophage inflammatory protein-2 in pneumolysin-induced lung inflammation in mice. J. Infect. Dis. 185:123–126 [DOI] [PubMed] [Google Scholar]
- 34. Rubins JB, et al. 1995. Dual function of pneumolysin in the early pathogenesis of murine pneumococcal pneumonia. J. Clin. Invest. 95:142–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rubins JB, et al. 1993. Toxicity of pneumolysin to pulmonary alveolar epithelial cells. Infect. Immun. 61:1352–1358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tilley SJ, Orlova EV, Gilbert RJ, Andrew PW, Saibil HR. 2005. Structural basis of pore formation by the bacterial toxin pneumolysin. Cell 121:247–256 [DOI] [PubMed] [Google Scholar]
- 37. Watanabe H, et al. 2008. Danger signaling through the inflammasome acts as a master switch between tolerance and sensitization. J. Immunol. 180:5826–5832 [DOI] [PubMed] [Google Scholar]
- 38. Witzenrath M, et al. 2006. Role of pneumolysin for the development of acute lung injury in pneumococcal pneumonia. Crit. Care Med. 34:1947–1954 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.