The structure of 3-methyladenine DNA glycosylase I in complex with 3-methyladenine is reported.
Keywords: 3-methyladenine DNA glycosylase I, fluorescence measurements, ITC, DNA repair, recognition
Abstract
The removal of chemically damaged DNA bases such as 3-methyladenine (3-MeA) is an essential process in all living organisms and is catalyzed by the enzyme 3-MeA DNA glycosylase I. A key question is how the enzyme selectively recognizes the alkylated 3-MeA over the much more abundant adenine. The crystal structures of native and Y16F-mutant 3-MeA DNA glycosylase I from Staphylococcus aureus in complex with 3-MeA are reported to 1.8 and 2.2 Å resolution, respectively. Isothermal titration calorimetry shows that protonation of 3-MeA decreases its binding affinity, confirming previous fluorescence studies that show that charge–charge recognition is not critical for the selection of 3-MeA over adenine. It is hypothesized that the hydrogen-bonding pattern of Glu38 and Tyr16 of 3-MeA DNA glycosylase I with a particular tautomer unique to 3-MeA contributes to recognition and selection.
1. Introduction
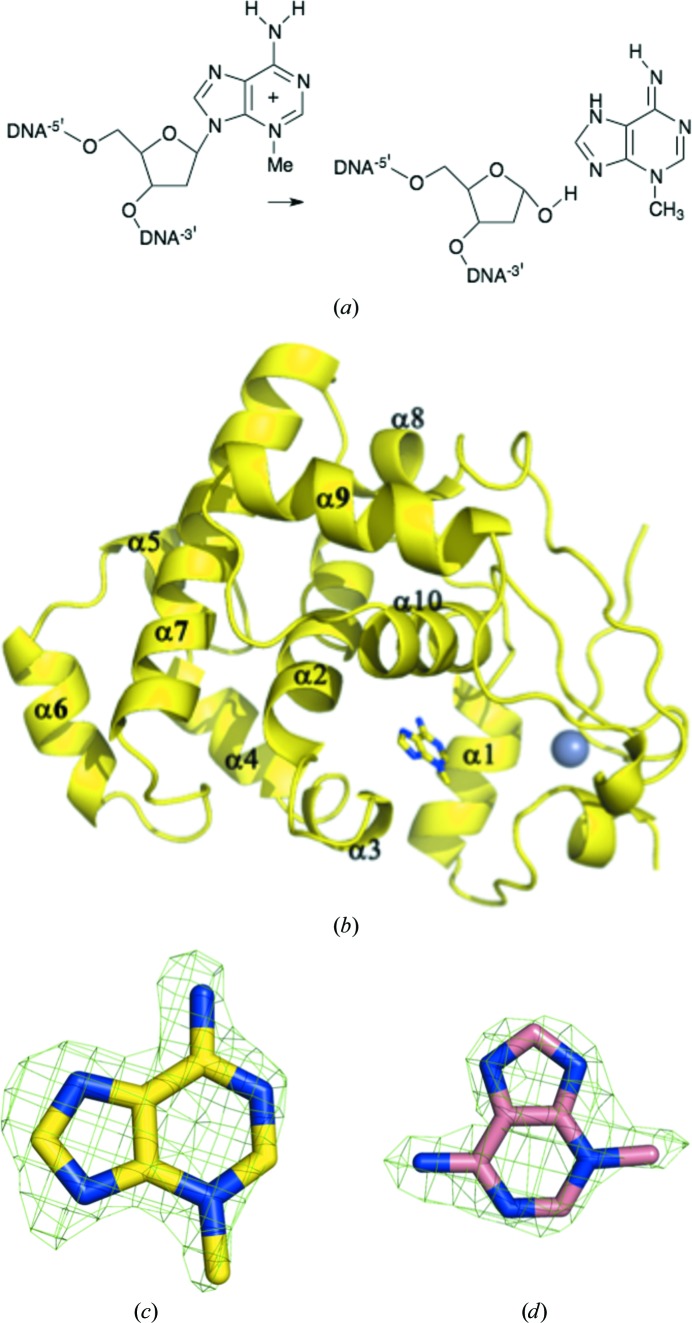
Bacterial 3-methyladenine DNA glycosylase I (TAG; Forsyth et al., 2002 ▶; Ji et al., 2001 ▶) is ubiquitous in eubacteria (Supplementary Fig. S11; Drohat et al., 2002 ▶) but shows no sequence or structural similarity to mammalian 3-methyladenine DNA glycosylase (AAG; Lau et al., 2000 ▶). TAG belongs to the alkylpurine DNA glycosylase superfamily and hydrolyzes the N9–C1′ glycosylic bond between a 3-methyladenosine (3-MeA) nucleobase lesion and the deoxyribose ring (Riazuddin & Lindahl, 1978 ▶; Bjelland et al., 1993 ▶; Fig. 1 ▶ a). 3-Methylation of adenine does not influence base pairing (Sedgwick et al., 2007 ▶); rather, the methyl group blocks replication by interfering with the interactions of DNA polymerase (Sedgwick et al., 2007 ▶; Engelward et al., 1996 ▶). Like the 8-oxoguanylate DNA glycosylases MutM and hOGG1 (Banerjee et al., 2005 ▶, 2006 ▶; Banerjee & Verdine, 2006 ▶; Blainey et al., 2006 ▶), TAG is thought to slide along the duplex until it encounters a lesion. TAG binds flipped-out 3-MeA and then cleaves the damaged base from the ribose. TAG from Staphylococcus aureus shares around 40% amino-acid sequence identity with the structurally characterized TAG enzymes from Salmonella typhi (Metz et al., 2007 ▶) and Escherichia coli (Drohat et al., 2002 ▶). The crystal structure of the S. typhi enzyme complexed with 3-MeA and abasic DNA (Metz et al., 2007 ▶) and an NMR structure of the E. coli enzyme complexed with 3-MeA (Cao et al., 2003 ▶) have been reported. Two absolutely conserved residues, Tyr16 and Glu38, were identified to form hydrogen bonds with 3-MeA and Trp46 stacks with 3-MeA (Cao et al., 2003 ▶; Metz et al., 2007 ▶). The methyl group does not appear to make extensive contacts. The crystal structure of the apo S. aureus enzyme has been reported (Oke et al., 2010 ▶). We wished to probe the basis of the discrimination between adenine and 3-MeA in the S. aureus enzyme.
Figure 1.
(a) The reaction catalyzed by TAG. (b) TAG is mainly α-helical; a structural zinc ion (grey sphere) is a found in all homologues of the enzyme. 3-MeA is shown in stick representation, with C atoms coloured yellow, N atoms coloured blue and O atoms coloured red. (c) Difference F o − F c electron density contoured at 3σ for 3-MeA in the active site of TAG. (d) Difference F o − F c electron density contoured at 3σ for 3-MeA in the active site of Y16F-mutant TAG; C atoms are coloured pink. 3-MeA binds in a different orientation in the Y16F mutant.
2. Materials and methods
2.1. Protein production
Native and mutant protein were purified as described by Oke et al. (2010 ▶). Y16F and E38Q mutations were introduced using QuikChange (Stratagene); primers are listed in Table 1 ▶.
Table 1. Macromolecule-production information.
The following primers were used to create the mutations: Y16F, 5-GTACTAAAGATCCAGTCTACTTAAACTTTCATGATCATGTATGGG-3 and 5-CCCATACATGATCATGAAAGTTTAAGTAGACTGGATCTTTAGTAC-3; E38Q, 5-GCAAGGCATTGTTTAAACTTTTAGCATTACAGTCACAACATGCTGGG-3 and 5-CCCAGCATGTTGTGACTGTAATGCTAAAAGTTTAAACAATGCCTTGC-3. Mutation sites are shown in bold.
| Source organism | S. aureus strain MSSA476 |
| Expression vector | pHis-TEV |
| Expression host | E. coli |
| Complete amino-acid sequence of the construct produced | GAMNECAFGTKDPVYLNYHDHVWGQPLYDSKALFKLLALESQHAGLSWLTILKKKEAYEEAFYDFEPEKVAQMTAQDIDR LMTFPNIVHHRKKLEAIVNQAQGYLKIEQAYGSFSKFLWSYVNGKPKDLQYEHASDRITVDDTATQLSKDLKQYGFKFLGPVTVFSFLEAAGLYDAHLKDCPSKPKHN |
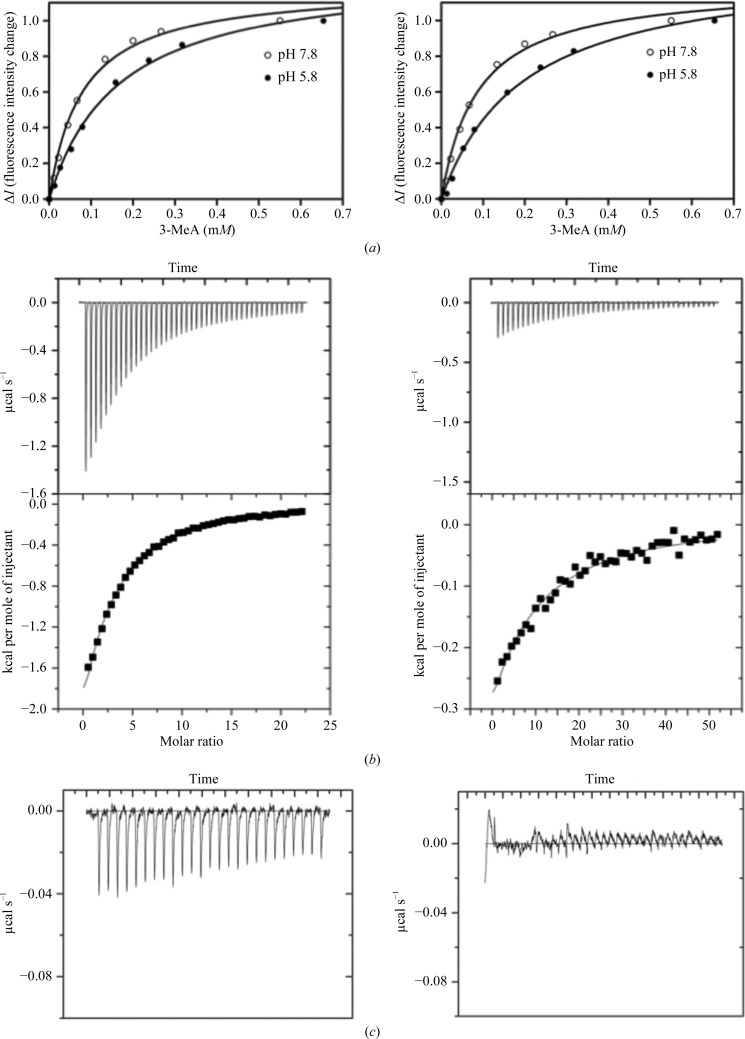
Fluorescence binding measurements were performed as described by Cao et al. (2003 ▶) and Drohat et al. (2002 ▶). 2 µM TAG was titrated with 10–650 µM 3-MeA or adenine in 20 mM phosphate buffer pH 7.8 and 5.8; Figs. 2 ▶ a and 2 ▶ b). Isothermal titration calorimetry (ITC) experiments were carried out using a VP-ITC device (MicroCal) in the same buffer. 5 mM 3-MeA or 1.5 mM adenine solution was injected at 298 K into a sample cell containing ∼1.4 ml protein solution at 30–40 µM. Each titration consisted of a first 1 µl injection followed by up to 25 subsequent 10 µl injections or 48 subsequent 5 µl injections of the ligand as indicated. Calorimetric data were analyzed using the MicroCal ORIGIN software, fixing the stoichiometry as N = 1 (Figs. 2 ▶ c and 2 ▶ d; Supplementary Table S11).
Figure 2.
(a) Measurement of the binding of 3-MeA to S. aureus TAG using intrinsic fluorescence quenching at pH 5.8 (K d = 165 µM) and pH 7.8 (K d = 78 µM); the results are similar to those previously reported for the E. coli enzyme (Cao et al., 2003 ▶). (b) Fluorescence quenching of 3-MeA with E38Q-mutant S. aureus TAG at pH 5.8 and 7.8. The small reduction in the binding constant was inconsistent with structural and previous functional data (Cao et al., 2003 ▶). This indicated that the fluorescence was unreliable for the S. aureus enzyme. (c) ITC measurement of the binding of 3-MeA to S. aureus TAG at pH 7.8 (K d = 220 µM) and pH 5.8 (K d = 470 µM). Adenosine does not bind. (d) ITC measurement of the binding of 3-MeA to Y16F-mutant (K d = 1.2 mM; left) and E38Q-mutant (no binding; right) S. aureus TAG at pH 7.8. 1 cal = 4.186 kJ.
2.2. Crystallization
Sitting-drop vapour-diffusion crystallization trials (1 µl protein solution plus 1 µl precipitant solution) were set up using a Cartesian Honeybee nanodrop crystallization robot which was integrated in a Hamilton-Thermo Rhombix system. The 3-MeA complexes of native and Y16F TAG were obtained by incubating TAG with 10 mM 3-MeA for 6 h before crystallization at 277 K. The complex crystals grew using a precipitant solution consisting of 0.1 M Tris–HCl pH 8.5, 1.8 M ammonium sulfate, 0.2 M Li2SO4 at 293 K as thin plates and grew to full size (0.2 × 0.2 × < 0.05 mm) in two to three weeks. Cryoprotectant solution was made by supplementing the crystallization precipitant solution with 20% glycerol. Crystals were mounted in Hampton Research cryoloops and rapidly cooled to 100 K prior to data collection.
2.3. Data collection and processing
Data for the native TAG–3-MeA complex were collected from a single crystal using 0.2° oscillations at a wavelength of 0.933 Å (ESRF beamline ID14-2) and were reduced using XDS (Kabsch, 2010 ▶). Data were collected from a single crystal of the Y16F TAG–3-MeA complex using an in-house Rigaku MicroMax-007 HF rotating-anode generator and Saturn 944 CCD detector. Data were reduced using HKL-2000 (Otwinowski & Minor, 1997 ▶) and POINTLESS (Evans, 2006 ▶; Potterton et al., 2003 ▶; Winn et al., 2011 ▶). Full details are given in Table 2 ▶. The E38Q mutant was also crystallized, but as no 3-MeA was located in the active site the structure is not described here; however, the structure has been deposited (PDB entry 4ai4).
Table 2. Data-collection and processing statistics.
Values in parentheses are for the last shell.
| Protein | Native, 3-MeA complex | Y16F, 3-MeA complex |
|---|---|---|
| Diffraction source | ESRF beamline ID14-2 | Rotating anode |
| Wavelength () | 0.933 | 1.54 |
| Temperature (K) | 100 | 100 |
| Detector | ADSC Quantum 4 CCD | Saturn CCD |
| Crystal-to-detector distance (mm) | 203 | 55 |
| Rotation range per image () | 0.2 | 0.5 |
| Total rotation range () | 108 | 180 |
| Exposure time per image (s) | 5 | 5 |
| Space group | C2 | C2 |
| Unit-cell parameters | ||
| a, b, c () | 73.00, 78.59, 179.81 | 72.3, 78.8, 179.3 |
| , , () | 90, 90.56, 90 | 90, 90.5, 90 |
| Mosaicity () | 0.3 | 0.56 |
| Resolution range () | 29.601.80 (1.851.80) | 502.2 (2.282.20) |
| Total No. of reflections | 341926 | 118143 |
| No. of unique reflections | 92544 (5876) | 47714 (3209) |
| Completeness (%) | 98.4 (91.6) | 95.5 (89.1) |
| Redundancy | 3.7 (3.1) | 2.6 (2.3) |
| I/(I) | 17.50 (3.9) | 28.2 (10.9) |
| R r.i.m. † | 0.059 (0.292) | 0.04 (0.11) |
| Overall B factor from Wilson plot (2) | 18 | 24.2 |
Estimated R r.i.m. = R merge[N/(N 1)]1/2, where N is the data multiplicity.
2.4. Structure solution and refinement
The structures were solved with Phaser (McCoy et al., 2007 ▶) using the native apo structure (Oke et al., 2010 ▶; PDB entry 2jg6) as a search model. As the complex crystals grew in a different space group to the native crystals, a new free set of reflections was assigned for refinement. All structures were refined with REFMAC v.5.6.0117 (Murshudov et al., 2011 ▶); manual intervention employed Coot (Emsley & Cowtan, 2004 ▶). 3-MeA was added to the models when the F o − F c density was clear (Figs. 1 ▶ c and 1 ▶ d). MolProbity (Chen et al., 2010 ▶) was used for structure validation and Ramachandran analysis. TLS parameters were used in refinement. TLS groups were assigned using the TLSMD server (Painter & Merritt, 2006 ▶). Details of the refinement are given in Table 3 ▶.
Table 3. Structure refinement.
Values in parentheses are for the last shell.
| Protein | Native, 3-MeA complex (PDB entry 4aia) | Y16F, 3-MeA complex (PDB entry 4ai5) |
|---|---|---|
| Resolution range () | 28.191.80 (1.8471.800) | 179.292.22 (2.2762.218) |
| Completeness (%) | 98.2 | 95.3 |
| cutoff | 0 | 0 |
| No. of reflections, working set | 87884 (5568) | 45350 (3043) |
| No. of reflections, test set | 4654 (308) | 2364 (166) |
| Final R cryst | 0.179 (0.233) | 0.183 (0.193) |
| Final R free | 0.218 (0.289) | 0.216 (0.244) |
| No. of non-H atoms | ||
| Protein | 7598 | 7602 |
| Ion | 25 | 25 |
| Ligand | 55 | 55 |
| Water | 927 | 486 |
| Total | 8605 | 8168 |
| R.m.s. deviations | ||
| Bonds () | 0.009 | 0.015 |
| Angles () | 1.189 | 1.550 |
| Average B factors (2) | ||
| Protein | 22.2 | 21.7 |
| Ion | 29.9 | 29.2 |
| Ligand | 15.4 | 17.3 |
| Water | 25.5 | 22.1 |
| Ramachandran plot | ||
| Favoured regions (%) | 98.5 | 98.4 |
| Additionally allowed (%) | 1.4 | 1.5 |
3. Results and discussion
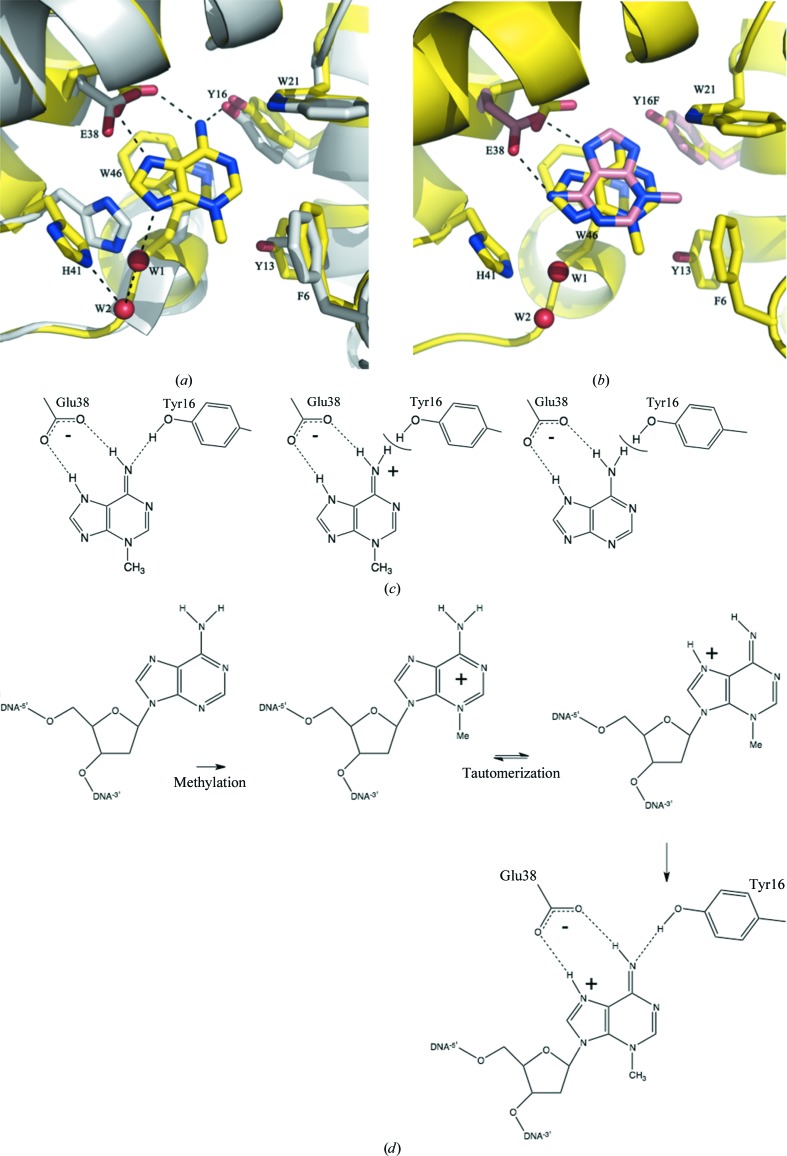
The structure of the S. aureus TAG–3-MeA complex was determined to 1.8 Å resolution and that of the Y16F TAG–3-MeA complex to 2.22 Å resolution. The structure of the native 3-MeA complex is very similar to the crystal structure of the S. typhi TAG–3-MeA–abasic DNA complex (Metz et al., 2007 ▶) and the NMR structure of the E. coli TAG–3-MeA complex (Cao et al., 2003 ▶). Relative to apo TAG (Oke et al., 2010 ▶), Glu38 has rotated to make 2.7 Å contacts with the exocyclic N atom and N7 of 3-MeA. Tyr16 moves to make a 2.8 Å contact with the exocyclic N atom of 3-MeA (Fig. 3 ▶ a). Trp46 stacks with the bound purine ring of 3-MeA, while Phe6, Tyr13 and Tyr21 make edge-on contacts. His41 rotates 80° to create space for 3-MeA to bind. The Y16F-mutant complex revealed that 3-MeA adopts a different orientation, although it preserves a bidentate hydrogen bond to Glu38 and a stacking interaction with Trp46 (Fig. 3 ▶ b). This conformation is unlikely to be physiologically relevant, as it would require a very different orientation of the DNA to that observed in the S. typhi complex (Metz et al., 2007 ▶). Using a fluorescence assay, we measured 3-MeA binding (Fig. 2 ▶ a), obtaining a similar result at pH 7.8 (K d = 78 µM) to that for the E. coli enzyme at pH 7.5 (K d = 42 µM; Cao et al., 2003 ▶). However, the assay is flawed for the S. aureus enzyme as the E38Q mutant gave the same result as for the native protein (Fig. 2 ▶ b), which is physically unreasonable. ITC (Figs. 2 ▶ c and 2 ▶ d) showed clear differences between the native and mutant S. aureus enzymes (Y16F, K d = 1.2 mM; E38Q, no binding) and gave K d values of 220 µM at pH 7.8 and 471 µM at pH 5.8 for the native enzyme. We did not detect adenine binding.
Figure 3.
(a) Structure of the 3-MeA–TAG complex (C atoms, yellow; N atoms, blue; O atoms, red) showing the key interactions. The apo structure is shown with C atoms in white. (b) Structure of the 3-MeA–Y16F TAG complex (C atoms shown in pink); the 3-MeA ring adopts a different orientation in the mutant. The 3-MeA in the native protein is also shown. (c) The most common tautomer of 3-MeA could be recognized by a specific hydrogen-bond arrangement of Tyr16 and Glu38. The predominant tautomer of protonated 3-MeA and adenosine would not match this hydrogen-bonding arrangement. (d) DNA damage leads to formation of the positively charged tautomer that is optimal for recognition by TAG; in addition, the highly electron-deficient ring would interact favourably with the TAG active site.
3-Methyldeoxyadenosine is positively charged in DNA, whilst deoxyadenosine is neutral; simple charge–charge recognition was therefore the original explanation for the specificity of TAG (Labahn et al., 1996 ▶; Lau et al., 2000 ▶; Hollis et al., 2000 ▶). However, it has been shown that E. coli TAG binds 3-MeA but not adenine and binds protonated 3-MeA (pH 5.7) more weakly than neutral 3-MeA (pH 7.5) (Cao et al., 2003 ▶; Drohat et al., 2002 ▶), establishing that charge–charge recognition is not the sole explanation (Cao et al., 2003 ▶). We suggest that a particular hydrogen-bond pattern contributes to the selection of a specific but favoured (Sharma & Lee, 2002 ▶) neutral tautomer of 3-MeA (Fig. 3 ▶ c) that is not available to adenosine (Fig. 3 ▶ c) and that is disfavoured for protonated 3-MeA (Fig. 3 ▶ c). Our hypothesis implies that there is an energetic penalty in reorganizing the hydrogen-bond network around Tyr16 to avoid a van der Waals clash (Fig. 3 ▶ c). In DNA, 3-methyldeoxyadenosine can adopt a tautomer that has the same hydrogen arrangement as neutral 3-MeA and has positive charge (Fig. 3 ▶ d), which is favoured at the active site (Metz et al., 2007 ▶). A clash of H atoms was observed between the amide of His136 and the amino group of adenine in human AAG and is used to preferentially select the damaged purine base (O’Brien & Ellenberger, 2004 ▶). Higher resolution data or neutron diffraction are required to further test the hypothesis for the TAG enzyme.
Supplementary Material
Supporting information file. DOI: 10.1107/S1744309112016363/gx5204sup1.pdf
PDB reference: 3-methyladenine DNA glycosylase I–3-MeA complex, 4aia
PDB reference: Y16F mutant, 4ai5
Acknowledgments
The work was funded by the BBSRC SPoRT initiative (BB_BBS/B/14426).
Footnotes
Supplementary material has been deposited in the IUCr electronic archive (Reference: GX5204).
References
- Banerjee, A., Santos, W. L. & Verdine, G. L. (2006). Science, 311, 1153–1157. [DOI] [PubMed]
- Banerjee, A. & Verdine, G. L. (2006). Proc. Natl Acad. Sci. USA, 103, 15020–15025. [DOI] [PMC free article] [PubMed]
- Banerjee, A., Yang, W., Karplus, M. & Verdine, G. L. (2005). Nature (London), 434, 612–618. [DOI] [PubMed]
- Bjelland, S., Bjørås, M. & Seeberg, E. (1993). Nucleic Acids Res. 21, 2045–2049. [DOI] [PMC free article] [PubMed]
- Blainey, P. C., van Oijen, A. M., Banerjee, A., Verdine, G. L. & Xie, X. S. (2006). Proc. Natl Acad. Sci. USA, 103, 5752–5757. [DOI] [PMC free article] [PubMed]
- Cao, C., Kwon, K., Jiang, Y. L., Drohat, A. C. & Stivers, J. T. (2003). J. Biol. Chem. 278, 48012–48020. [DOI] [PubMed]
- Chen, V. B., Arendall, W. B., Headd, J. J., Keedy, D. A., Immormino, R. M., Kapral, G. J., Murray, L. W., Richardson, J. S. & Richardson, D. C. (2010). Acta Cryst. D66, 12–21. [DOI] [PMC free article] [PubMed]
- Drohat, A. C., Kwon, K., Krosky, D. J. & Stivers, J. T. (2002). Nature Struct. Biol. 9, 659–664. [DOI] [PubMed]
- Emsley, P. & Cowtan, K. (2004). Acta Cryst. D60, 2126–2132. [DOI] [PubMed]
- Engelward, B. P., Dreslin, A., Christensen, J., Huszar, D., Kurahara, C. & Samson, L. (1996). EMBO J. 15, 945–952. [PMC free article] [PubMed]
- Evans, P. (2006). Acta Cryst. D62, 72–82. [DOI] [PubMed]
- Forsyth, R. A. et al. (2002). Mol. Microbiol. 43, 1387–1400. [DOI] [PubMed]
- Hollis, T., Ichikawa, Y. & Ellenberger, T. (2000). EMBO J. 19, 758–766. [DOI] [PMC free article] [PubMed]
- Ji, Y., Zhang, B., Van Horn, S. F., Warren, P., Woodnutt, G., Burnham, M. K. & Rosenberg, M. (2001). Science, 293, 2266–2269. [DOI] [PubMed]
- Kabsch, W. (2010). Acta Cryst. D66, 125–132. [DOI] [PMC free article] [PubMed]
- Labahn, J., Schärer, O. D., Long, A., Ezaz-Nikpay, K., Verdine, G. L. & Ellenberger, T. E. (1996). Cell, 86, 321–329. [DOI] [PubMed]
- Lau, A. Y., Wyatt, M. D., Glassner, B. J., Samson, L. D. & Ellenberger, T. (2000). Proc. Natl Acad. Sci. USA, 97, 13573–13578. [DOI] [PMC free article] [PubMed]
- McCoy, A. J., Grosse-Kunstleve, R. W., Adams, P. D., Winn, M. D., Storoni, L. C. & Read, R. J. (2007). J. Appl. Cryst. 40, 658–674. [DOI] [PMC free article] [PubMed]
- Metz, A. H., Hollis, T. & Eichman, B. F. (2007). EMBO J. 26, 2411–2420.
- Murshudov, G. N., Skubák, P., Lebedev, A. A., Pannu, N. S., Steiner, R. A., Nicholls, R. A., Winn, M. D., Long, F. & Vagin, A. A. (2011). Acta Cryst. D67, 355–367. [DOI] [PMC free article] [PubMed]
- O’Brien, P. J. & Ellenberger, T. (2004). J. Biol. Chem. 279, 9750–9757. [DOI] [PubMed]
- Oke, M. et al. (2010). J. Struct. Funct. Genomics, 11, 167–180. [DOI] [PMC free article] [PubMed]
- Otwinowski, Z. & Minor, W. (1997). Methods Enzymol. 276, 307–326. [DOI] [PubMed]
- Painter, J. & Merritt, E. A. (2006). J. Appl. Cryst. 39, 109–111.
- Potterton, E., Briggs, P., Turkenburg, M. & Dodson, E. (2003). Acta Cryst. D59, 1131–1137. [DOI] [PubMed]
- Riazuddin, S. & Lindahl, T. (1978). Biochemistry, 17, 2110–2118. [DOI] [PubMed]
- Sedgwick, B., Bates, P. A., Paik, J., Jacobs, S. C. & Lindahl, T. (2007). DNA Repair, 6, 429–442. [DOI] [PubMed]
- Sharma, S. & Lee, J. K. (2002). J. Org. Chem. 67, 8360–8365. [DOI] [PubMed]
- Winn, M. D. et al. (2011). Acta Cryst. D67, 235–242.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information file. DOI: 10.1107/S1744309112016363/gx5204sup1.pdf
PDB reference: 3-methyladenine DNA glycosylase I–3-MeA complex, 4aia
PDB reference: Y16F mutant, 4ai5