SUMMARY
Studies have shown that glycosylphosphatidylinositols (GPIs) of Plasmodium faciparum activate macrophages mainly through TLR2- and to certain extent through TLR4-mediated signaling to induce proinflammatory cytokine production. However, the ability of parasite GPIs to activate DCs has not been reported. Here, we show that, parasite GPIs efficiently activate DCs through TLR2-mediated signaling mechanism and induce the production of TNF-α and IL-12. We also studied the role of scavenger receptor CD36 in P. falciparum GPI- and merozoite-induced cytokine responses by DCs. The results indicate that CD36 modulates the cytokine-inducing activity of the parasite GPIs by collaborating with TLR2 in DCs. Furthermore, our data reveal that CD36 modulates the activity of P. falciparum merozoites, likely by the contribution of phagocytosis-coupled CD36-mediated signaling to the signaling induced by merozoites. Altogether, these results contribute toward understanding of signaling mechanisms in malaria parasite-induced activation of the innate immune system.
Keywords: Plasmodium falciparum, Glycosylphosphatidylinositols, Merozoites, Dendritic cells, Toll-like receptors, CD36, Proinflammatory cytokines
INTRODUCTION
Plasmodium family of protozoan parasites causes malaria. Of several parasite species that can infect humans, Plasmodium falciparum is the most virulent and is responsible for the majority of deaths due to malaria (1–2). Proinflammatory responses produced during the initial stages of infections are critical for controlling parasite growth (3–6). However, excessive and prolonged production of pro-inflammatory mediators leads to pathogenesis (7–12). Unlike infections with other human malaria parasite species, P. falciparum infection is characterized by sequestration of infected erythrocytes in vascular capillaries of vital organs such as brain, liver and spleen (13,14). Production of high levels of inflammatory cytokines leads to up-regulated expression of endothelial cell surface adhesion molecules in the microvascular capillaries, augmented sequestration and infiltration of mononuclear cells. This in turn leads to excessive inflammation in organs by a positive feedback, causing tissue damage by cytotoxic lymphocytes, and organ dysfunction, causing cerebral and other organ-related fatal malaria illnesses (13–17).
P. falciparum glycosylphosphatidylinositols (GPIs) have been found to induce the production of pro-inflammatory cytokines such as TNF-α, IL-1 and IL-6 by macrophages, produce nitric oxide (NO) by endothelial cells, and up-regulate the expression of cell adhesion molecules on endothelial cell surfaces (15,18–20). Mice administered with parasite GPIs have been shown to develop malaria-like symptoms (21). Thus, P. falciparum GPI has been proposed as a malaria pathogenic factor (21,22). Although macrophages respond robustly to GPIs in vitro (20), during malaria infection, cytokine responses by macrophages are likely to be suppressed due to the ingestion of parasites in large amounts by phagocytosis, thereby impairing cell function and inducing apoptosis (23,24). Thus, dendritic cells (DCs) and NK cells appear to be the major sources of inflammatory cytokine responses during early stages of infection (3,25,26). Further, DCs play major roles in linking innate immune responses to adaptive immune responses by activating NK cells, inducing Th1/Th2 responses, which lead to the development of cell-mediated and humoral adaptive immunity (27,28). Therefore, it is important to study cytokine responses to the immunostimulatory components of parasites in DCs.
The scavenger receptor CD36 mediates the uptake of a variety of particulate ligands such as oxidized low-density lipoproteins, β-amyloid plaque, bacteria, and apoptotic cells, and involved in many disease processes including cardiovascular disease, Alzheimer’s disease, atherosclerosis and diabetes, and pathogenic infections, including malaria (29,30). The role of CD36 in these pathogenic processes has been attributed to its ability to bind to its ligands and participate in immune responses. Thus, it has been shown that CD36 functions as a co-receptor for certain TLR2-TLR6 ligands such as diacylated bacterial lipopeptide, diacylated lipomannans of Mycobacterium smegmatis, lipoteichoic acids of Staphylococcus aureus, and some synthetic diacylated lipopeptides (31). Recently, CD36 has also been reported to cooperate with TLR4-TLR6 in triggering respiratory burst to produce reactive oxygen species by macrophages in response to oxidized LDL, and in producing IL-1β by microglia in response to Alzheimer’s β-amyloid (32).
Previously, it has been shown that proinflammatory responses to P. falciparum GPIs by both mouse and human macrophages involve mainly TLR2- and to some extent TLR4-mediated signaling, leading to cytokine production (33). While WT mouse macrophages stimulated with parasite GPIs efficiently produced TNF-α in a dose-dependent manner, TNF-α production by macrophages deficient in TLR2 and TLR4 was reduced by ~80% and ~25%, respectively. Consistent with these results, human blood monocytes treated with anti-TLR2 monoclonal antibodies produced ~80% less TNF-α than untreated monocytes in response to GPIs. Further, treatment of cells with anti-TLR4 monoclonal antibodies caused 30–50% decrease in GPI-induced TNF-α production. Moreover, HEK cells transfected with human TLR2/TLR1 were efficiently activated in a GPI dose-dependent manner (33). Subsequent to these studies, it has been shown that CD36 modulates the activity of P. falciparum GPIs by cooperating with TLR2 (34). Furthermore, macrophages deficient in CD36 produced markedly decreased levels of TNF-α in response to parasite GPI stimulation, and CD36−/− mice infected with P. chabaudi chabaudi AS produced lower levels of TNF-α and exhibited higher parasitemia and higher mortality rates than wild type (WT) mice (35). Thus, these results indicated that CD36 modulates the activity of TLR2, contributing to the increased production of pro-inflammatory cytokine responses to malaria parasites and the ability of mice to control parasitemia (35). Similar to GPIs of P. falciparum, GPIs from other parasites, including those of Trypanosoma cruzi and mannosylated diacyl glycolipids of mycobacterial lipoarabinomannan, have been shown to activate macrophages through TLR2-mediated signaling (33,36–38). Thus, in all these studies, the observed cytokine responses by murine macrophages stimulated with P. falciparum GPIs and signaling mechanisms by mouse cells resembled those produced by human cells.
Furthermore, Pichyangkul et al. studied immune responses by plasmacytoid DCs (pDCs) isolated from human blood and by DCs obtained by the FLT3-differentaited mouse bone marrow cells that were mixture of pDCs and myeloid DCs (mDCs) to the soluble, buffer extracts of the late blood stage P. falciparum (39). They found that the soluble components of P. falciparum could activate both human pDCs and in vitro differentiated murine DCs in a TLR9-dependent manner and induced surface expression of costimulatory molecules and production of cytokines. They also found that although both human and murine cells produced cytokines, the nature of cytokines produced by human DCs were quite different than those produced by the murine cells. While human pDCs produced both IFN-α and IL-12, the murine cells produced IL-12 but not IFN-α. Subsequent studies in our laboratory demonstrated that, among various components released by the rupture of schizont stage P. falciparum-infected erythrocytes, merozoites (MZs) are the predominant immunostimulatory components (40). The P. falciparum MZs activate both human and mouse DCs by a similar mechanism, i.e., through the recognition of parasite DNA by TLR9. We also demonstrated that the uptake of parasite DNA involves complex formation with positively charged proteins, and that a histone DNA complex (nucleosomes) is the physiological ligand (40,41). Thus, the results of above studies indicated that although there is some difference in the types of cytokines produced by human and mouse DCs in response to P. falciparum, the overall parasite factors and host receptors interaction mechanisms are similar as indicated by the efficient activation of both human and mouse TLR2 and TLR9 by P. falciparum GPIs and DNA, respectively (33,40). Thus, studies using murine immune cells and P. facliparum components are relevant in understanding the mechanisms of parasite-host interactions involved in human malaria, although the results in some cases need to be interpreted cautiously.
In the present study, we assessed the activity and TLR recognition specificity of P. falciparum GPIs in DCs. We also determined the roles of CD36 in P. falciparum GPI- and MZ-induced proinflammatory cytokine production by DCs. The results of these studies are presented here.
MATERIALS AND METHODS
Reagents
The standard TLR ligands were purchased from the following sources: FSL-1 and Pam3CSK4 from Microcollections (Tübingen, Germany); CpG ODN-1826 from Coley Pharmaceutical (Kanata, ON, Canada). Salmonella minnesota Re595 strain LPS (catalog# L9764) from Sigma-Aldrich (St. Louis, MO). The cell culture reagents: DMEM, RPMI 1640 medium, and penicillin/streptomycin solution were from Invitrogen (Carlsbad, CA); fetal bovine serum (FBS) was from Atlanta Biologicals (Lawrenceville, GA); sodium pyruvate, 2-mercaptoethanol, 4-aminobenzoic acid, gentamycin, and MEM non-essential amino acids from Sigma-Aldrich (St. Louis, MO). Human blood and plasma for P. falciparum culturing were obtained from The Blood Bank, Hershey Medical Center Hospital, Hershey, PA. Duoset ELISA kits for measuring TNF-α and IL-12p40 were from R & D Systems (Minneapolis, MN). Cell trace™ CFSE cell-staining kit was from Molecolur Probes, Inc. (Eugene, OR). Anti-mouse CD16/32 (clone 93), FITC- and APC-labeled anti-mouse CD11c (clone 418N), and APC-labeled anti-mouse MHC class II (I-A/I-E) (clone M5/114.15.2) monoclonal antibodies were purchased from eBioscience (San Diego, CA). PE-labeled anti-mouse CD11b antibody (clone M1/70) was from BD Biosciences (San Jose, CA).
Mice
The TLR2−/−, TLR4−/−, TLR9−/− and MyD88−/− mice (all in C57BL/6J background) were provided by Dr. Shizuo Akira, Research Institute for Microbial Diseases, Osaka University, Japan. The WT C57BL/6J mice were purchased from Jackson Laboratories. CD36−/− mice (C57BL/6J strain) were provided by Dr. Maria Febbraio from the Lerner Research Institute, Cleveland Clinic, Cleveland, OH. Mice were bred and maintained in a pathogen-free facility according to the guidelines of the Pennsylvania State University College of Medicine.
Ethics statement
The use of human blood and plasma for parasite culturing and of mice were approved, respectively, by the Institutional Review Board and the Institutional Animal Care and Use Committee of the Hershey Medical Center, Pennsylvania State University College of Medicine, Hershey, PA.
Parasite culturing
P. falciparum parasites (3D7 strain) were cultured using O-positive human erythrocytes in RPMI 1640 medium containing 10% human O-positive plasma and 50 μg/ml gentamycin under 90% nitrogen, 5% oxygen and 5% carbon dioxide (42). The cultures were routinely tested for mycoplasma contamination using PCR mycoplasma test kit from Stratagene (La Jolla, CA).
Isolation and purification of P. falciparum merozoites
P. falciparum merozoites (MZs) released into the culture medium were isolated by percoll gradient centrifugation of cell culture harvests and purified by magnetic-activated cell sorting (MACS) as described previously (40). Briefly, the synchronized parasite cultures (20–30% parasitemia) at the schizont stages were diluted to 0.1–0.2% hematocrit. When the majority of the schizonts were burst, the released MZs (under this condition most MZs could not invade due to the limited availability of erythrocytes and remain in the culture medium) and food vacuoles were pelleted along with infected erythrocytes by centrifugation at 2,500 g for 15 min after removing uninfected and infected erythrocytes by centrifuging at 250 g for 5 min. The pellets were re-suspended in RPMI 1640 medium and overlaid on the top of step-wise layers of 30%, 45% and 60% percoll cushions to remove accompanying erythrocytes and debris. After centrifuging at 2,500 g for 15 min, MZs on the top of 30% percoll were collected. The MZs were further purified by passing through magnetic LS columns (Miltenyi Biotec, Auburn, CA) under magnetic field to separate from precipitated food vacuole or hemozoin. The column effluents were centrifuged at 2,500 g and MZ pellets collected. Purity of MZs preparation was estimated by making a thin smears of isolated MZs on glass slides and stained with Giemsa.
Isolation of GPIs from P. falciparum
The parasite cultures at the late trophozoite and schizont stages (20–30% parasitemia) were harvested and the cell pellets (10–12 ml) containing red blood cells and infected red blood cells were lysed with 0.05% saponin as described previously (21). The released parasites were collected and purified by suspending in PBS and centrifugation on 5% BSA cushions; yield of parasite pellets ranged between 2 and 2.5 ml/10–12 culture harvests. GPIs were isolated from the parasite pellets as described previously (21). Briefly, the purified parasite preparations from several batches were pooled to obtain 10 ml packed wet parasite pellet, lyophilized, and extracted 3–4 times with 50 ml of chloroform/methanol (2:1, v/v) to remove neutral and nonglycosylated lipids. The GPIs in the parasite residue were then extracted 3–4 times with 50 ml of chloroform/water/methanol (10:3:3, v/v/v) and the extract was dried, dissolved in aqueous 1-butanol, and partitioned between water and water-saturated 1-butanol. The GPI-containing water-saturated 1-butanol phase was dried and the GPIs in the residue was extracted with 80% aqueous 1-propanol and dried. The GPIs were further purified by HPLC using a C4 reversed phase Supelcosil LC-304 column (4.6 × 250 mm, 5 μm particle size; Supelco). The GPIs bound to the column were eluted at a flow rate of 0.5 ml/min with a linear gradient of 20–60% aqueous 1-propanol containing 0.1% trifluroacetic acid over a period of 80 min and held for 30 min (21). The GPI containing fractions were pooled and lyophilized. All GPI isolation and purification procedures were performed using high quality solvents, sterile water and buffers, and thoroughly washed and siliconized glassware to avoid loss due to surface adsorption.
Isolation of P. falciparum polynucleosomes
Soluble polynucleosomes were prepared as described previously (41). Briefly, the infected erythrocytes at the late trophozoite and schizont stages were treated with 0.05% saponin and the released parasites were pelleted and lysed to obtain nuclear material. Proteins bound to nuclear material were removed by extracting with 0.3 M KCl followed by 0.6 M KCl containing 10% glycerol. The chromatin pellet was suspended in 0.65 M NaCl and 0.3 M sucrose solution and sonicated to obtain soluble polynucleosomes.
Preparation of DCs from bone morrow cells by differentiation with FLT3 ligand or GM-CSF
Bone marrow cells from WT and CD36−/−, TLR2−/−, TLR4−/−, TLR9−/− and MyD88−/− mice were cultured for 7 to 8 days in complete DMEM (DMEM supplemented with 10% FBS, 1% non-essential amino acids, 1% penicillin-streptomycin, 1 mM sodium pyruvate and 50 μM β-mercaptoethanol) containing 15% of culture supernatant from B16 cells that expressing retrovirus-coded FLT3 ligand (40). Bone marrow cells from WT and CD36−/− mice were also cultured in complete DMEM containing 10% of conditioned medium from GM-CSF producing X63 cells (43). The DCs obtained by differentiation with FLT3 ligand and GM-CSF were designated as FL-DCs and GM-DCs, respectively.
Cell stimulation and analysis of cytokines
DCs (1 × 105/well) were plated in 96-well plates and stimulated with the indicated doses of GPIs coated on gold particles (33), MZs or polynucleosomes in 200 μl of complete DMEM. DCs were also stimulated with uncoated gold particles and standard TLR ligands: FSL-1 (TLR2 ligand, 10 μM), Pam3CSK4 (TLR2 ligand, 10 ng/ml), LPS (TLR4 ligand, 100 ng/ml) or CpG ODN-1826 (TLR9 ligand, 2 μg/ml) as controls. After 24 h, the culture supernatants were collected and cytokine levels measured by ELISA (20).
Flow cytometry analysis
FL-DCs (1 × 106/well) were cultured in 24-well plated in the presence of MZs or CpG in 1 ml of complete DMEM. After 24 h, cells were harvested and stained with anti-mouse CD11c, CD11b and MHC class II antibodies. The stained cells were acquired using FACSCalibur and the results were analyzed by using CellQuest software (BD Biosciences; San Jose, CA). DCs cultured in parallel without stimulation were similarly analyzed as control.
Analysis of MZ uptake by DCs
MZs were labeled with CFSE as described previously (44). Briefly, freshly isolated MZs were suspended in PBS, pH 7.2, and stained at 37°C with 2 μM CFSE. After 10 min, two volumes of fetal bovine serum was added, incubated at 37°C for 5 min, and washed two times with complete DMEM. The CFSE-stained MZs were added to FL-DCs (0.5 × 106) in 24-well plates in 0.5 ml complete DMEM. After incubating at 37°C for 2 h, cells were stained with APC-labeled anti-mouse CD11c antibody, and the MZ uptake was analyzed by flow cytometry.
Statistical analysis
The data were plotted as mean values ± SD. Statistical analysis of data was performed by Student’s t tests. One-way ANOVA followed by the Newman-Keuls test were used for comparing cytokine responses from different pairs of samples. GraphPad prism software version 3.0 was used for the analysis. p values <0.05 were considered statistically significant.
RESULTS AND DISCUSSION
DCs stimulated with P. falciparum GPIs efficiently produce pro-inflammatory cytokines
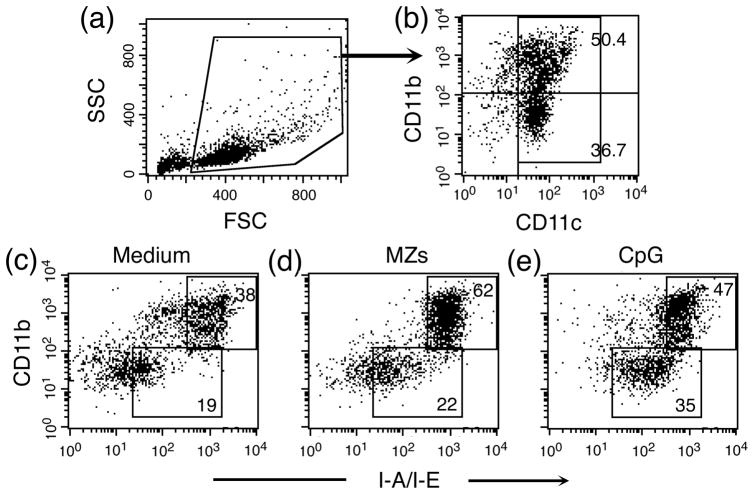
Previously, we and others showed that P. falciparum GPIs activate mouse bone marrow cell-derived macrophages, peritoneal macrophages, and human monocytes to induce the production of inflammatory mediators, including TNF-α, IL-12, IL-6 and nitric oxide, in a dose-dependent manner (20,35). Even though, macrophages responds to GPIs stimulation under in vitro condition, macrophages may not be the major cytokine producing cells in vivo as they can ingest large amounts of parasites by phagocytosis; accumulation of undigested hemozoin and other parasite materials suppresses their immune responses (23,24). However, studies have shown that DCs from infected mice are functional and are the major source of inflammatory cytokines at early stage of infection (25,26). Therefore, in the present study, we assessed the ability of parasite GPIs to activate FL-DCs and GM-DCs obtained, respectively, by FLT3 ligand-and GM-CSF-induced differentiation of mouse bone marrow cells. Most GM-DCs exhibited myeloid DC-like features with characteristic dendrites. In the case of FL-DCs, while some cells showed dendrites, albeit shorter compared to GM-DCs, others exhibited plasmocytoid DC-type morphology with smooth surface under light microscopy. Analysis of cell surface markers by flow cytometry showed that FL-DCs used in this study are a mixture of ~58% myeloid DCs (CD11C+CD11b+) and ~42% plasmacytoid DCs (CD11c+CD11b−); both DC subtypes expressed MHC-II molecules and the expression of MHC-II significantly increased upon stimulation with MZs (Fig. 1). GM-DCs were found to be 100% myeloid-like DCs (CD11c+CD11b+), most of which expressing MHC-II (data not shown).
Figure 1. FL-DCs are mixtures of myeloid and plasmacytoid DCs and both populations express MHC class II molecule on their cell surface.
FL-DCs were either unstimulated (control) or stimulated separately with MZs and CpG for 24 h, stained with anti-mouse CD11c, CD11b, and MHC-II (I-A/I-E) monoclonal antibodies, and analyzed by flow cytometry. (a and b) Cells were selected by side scattering (SSC) and forward scattering (FSC) in panel a and analyzed for CD11b and CD11c expression (b). The percentage of myeloid DCs (CD11c+CD11b+) and plasmacytoid DCs (CD11c+CD11b−) in total gated cells are indicated. (c, d and e) The gated total CD11c+ cells in panel b were further analyzed by flow cytometry for the expression of MHC-II molecules. Shown are the dot-blots of flow cytometry data, indicating percent of MHC class II positive myeloid and plasmacytoid DCs in gated total DCs. The experiments were repeated three times and data from a representative experiment is shown.
The FL-DCs and GM-DCs were stimulated in parallel with GPIs coated onto gold particles to mimic the properties of membrane-bound GPIs and with various standard TLR ligands, namely CpG (recognized by TLR9), LPS (TLR4) and Pam3CSK4 (TLR2-TLR1 dimer) and FSL-1 (TLR2-TLR6 dimer). While untreated FL-DCs and GM-DCs and those treated with uncoated control gold particles produced either low level or no cytokines, both cell types efficiently produced cytokines in response to GPIs and to standard ligands (Figs. 2 and 3). As in the case of macrophages (33), the cytokine responses to GPIs were dose dependent. Between FL-DCs and GM-DCs, GPIs more efficiently induced cytokine production by the former cells than by the latter cells. This is evident by the fact that 100–200 μg/ml GPIs was sufficient to produce saturated level of cytokine secretion by FL-DCs, whereas 400 μg/ml or higher concentration of GPIs was needed for GM-DCs to produce saturated level of cytokines. Furthermore, we observed that GM-DCs exhibit much higher levels of phagocytic activity than FL-DCs, as evidenced by a large number of gold particle accumulation in GM-DCs (not shown), presumably in the lysosomes. Therefore, in GM-DCs the efficient uptake of gold particles resulted in the faster removal of GPI-coated beads, thereby decreasing the interaction of GPIs with the receptor and thus requiring higher concentrations of GPI-coated beads for optimal cytokine production. Alternatively, ingestion of a large number of gold particles and the resulting heavy load of gold particles in the lysosomes may cause inhibition of the overall cell function, producing lower levels of cytokines.
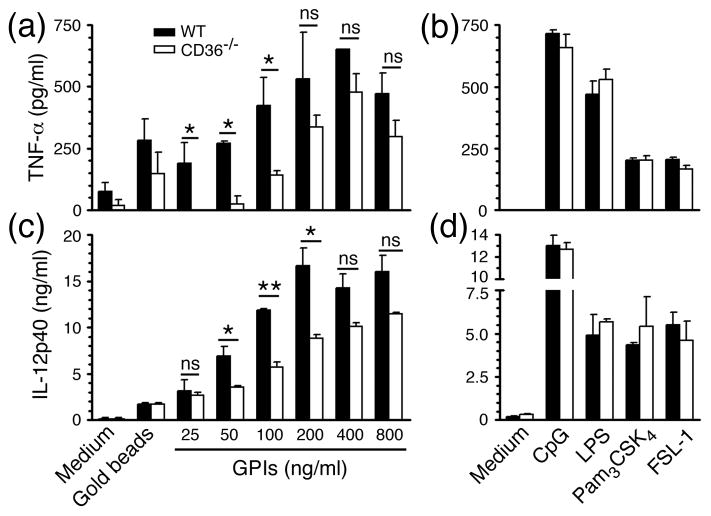
Figure 2. CD36 regulates P. falciparum GPI-induced TNF-α and IL-12 production by DCs.
FL-DCs (1 × 105 cells/well) derived from bone marrow cells of WT and CD36−/− mice were plated in 96-well plates and stimulated with indicated doses of GPIs coated on gold particles in 200 μl of complete medium. The TNF-α (a and b) and IL-12 (c and d) secreted into the culture medium were measured by ELISA. DCs stimulated with uncoated gold particles or TLR ligands: CpG (2 μg/ml), LPS (100 ng/ml), Pam3CSK4 (10 ng/ml) or FSL-1 (10 μM) were analyzed as controls. Experiment was done three times, each in duplicates. Mean values ± SD are plotted. *, p <0.05; **, p <0.01; ns, p >0.05.
Figure 3. CD36 modulates P. falciparum GPI-induced TNF-α and IL-12 production by GM-DCs.
TNF-α (a and b) and IL-12 (c and d) produced by WT and CD36 deficient GM-DCs (1 × 105 cells/well) in 200 μl of complete medium stimulated with different doses of GPIs coated on gold particles. Cells stimulated with uncoated gold particles or TLR ligands: CpG (2 μg/ml), LPS (100 ng/ml), Pam3CSK4 (10 ng/ml) or FSL-1 (10 μM) were analyzed as controls. Experiment was done three times, each in duplicates. Shown are data from a representative experiment. Mean values ± SD are plotted. *, p <0.05; **, p <0.01; ns, p >0.05.
CD36 modulates P. falciparum GPI-induced inflammatory responses by DCs
Recently, Patel et al. have shown that macrophages deficient in CD36 produce reduced levels of TNF-α than WT macrophages in response to P. falciparum GPIs and, that CD36−/− mice infected with P. chabaudi chabaudi AS exhibit higher parasitemia and higher mortality rates than infected WT mice (35). Given that DCs are likely to be the major source of initial cytokine responses in malaria infection, we examined the role of CD36 in GPI-induced production of inflammatory cytokines by DCs. FL-DCs and GM-DCs deficient in CD36 stimulated with GPIs produced significantly decreased levels of TNF-α and IL-12 in a dose dependent manner than the corresponding WT DCs (see Figs. 2 and 3). However, in the case of standard TLR ligands, both FL-DCs and GM-DCs that are deficient in CD36 produced similar levels of cytokines as that produced by WT DCs. These results indicated that CD36 also plays an important role in the GPI-induced production of inflammatory cytokines by DCs. Here, it should be noted that the GPIs that were presented as molecules on the surface of gold beads to mimic physiologic cell surface presentation are not only recognized by TLR2 but also are taken by cells through phagocytic mechanism, wherein CD36 can modulate GPI-induced TLR2-dependent signaling. In contrast, the standard ligands presented as soluble molecules were likely entered cells by pinocytosis. Therefore, it appears likely that modulation of TLR-dependent GPI activity by CD36 is at least partly dependent on convergence of TLR-mediated signaling with that induced by CD36/phagocytosis. From our results it is clear that, at higher doses of GPIs, the differences in cytokine production by WT and CD36 deficient DCs became statistically non-significant (Figs. 2 and 3). This is likely because when the combined signaling by TLR and CD36/phagocytosis becomes saturated in WT DCs, the TLR-mediated activity of GPIs in CD36 deficient DCs is still increasing in a concentration-dependent manner. Therefore, under such circumstance, it is expected that kinetically the differences in signaling out put by the two cell types becomes less and less significant up to a point where they become equal.
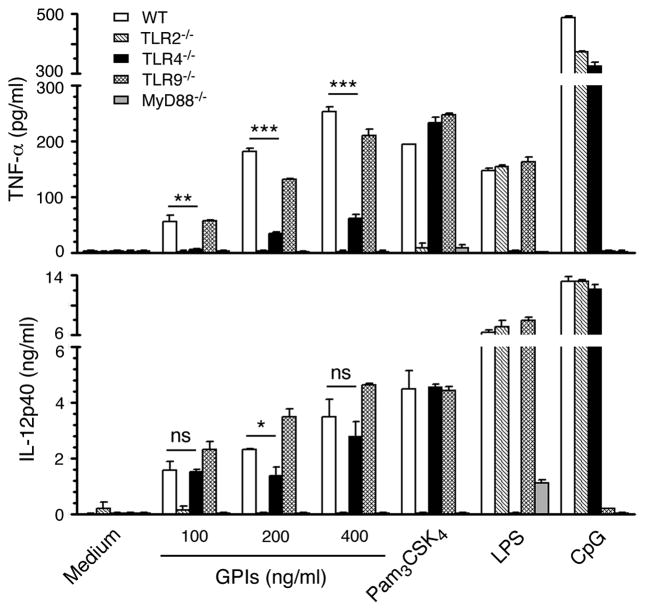
TLR2 and MyD88-mediate P. falciparum GPI-induced activation of DCs
Previous studies have shown that P. falciparum GPIs activate both mouse and human macrophages mainly through the recognition by TLR2 and to certain extent through TLR4 to initiate downstream production of inflammatory mediators, including TNF-α, IL-12, IL-6 and nitric oxide (20,35). Further, we have reported that both mouse and human TLR2-TLR1 and TLR2-TLR6 dimers recognize the parasite GPIs, although the former recognizes GPI more efficiently than the latter (45). Here, we tested stimulatory activity of GPIs using FL-DCs derived from the bone marrow cells of WT and TLR2−/−, TLR4−/−, TLR9−/− and MyD88−/− mice. GPIs efficiently activated WT DCs and DCs deficient in TLR9, producing similar levels of TNF-α and IL-12 (Fig. 4). DCs deficient in TLR2 produced little or no cytokines. Consistent with these results, cytokine production by DCs lacking MyD88 was completely absent. These data demonstrated that, unlike macrophages, GPIs activate DCs almost exclusively through TLR2 and MyD88. However, DCs deficient in TLR4 produced significantly lower levels of cytokines than WT DCs. The reason for this unexpected observation is unclear, but is specific to parasite GPIs as the activity of standard TLR2 ligand, Pam3CSK4, was normal in DCs deficient in TLR4. In any event, these above results taken together with those observed regarding the modulation of GPI activity by CD36 (see Figs 2 and 3) indicate that CD36 plays an important role in the GPI-induced production of inflammatory cytokines by DCs, likely by cooperating with TLR2. Furthermore, the results are consistent with the previous reports that CD36 cooperates with TLR2 in recognizing certain TLR2 ligands such as diacylated lipopeptides and glycolipids, and bacterial lipotoichoic acid, and that macrophages deficient in CD36 produce significantly reduced levels of TNF-α (31,32,34).
Figure 4. P. falciparum GPIs induce cytokine production mainly through TLR2-mediated signaling.
FL-DCs derived from bone marrow cells of WT mice and TLR2−/−, TLR4−/−, TLR9−/− and MyD88−/− mice were plated in 96-well plates (1 × 105 cells/well) and stimulated with indicated doses of GPIs coated gold particles in 200 μl of complete medium. Pam3CSK4 (TLR2 ligand, 10 ng/ml), LPS (TLR4 ligand, 100 ng/ml), and CpG (TLR9 ligand, 2 μg/ml) were used as control stimulants. The TNF-α and IL-12 secreted into the culture medium were analyzed using ELISA. The experiment was repeated two times and the mean values ± SD from one of the experiments are plotted. *, p <0.05; **, p <0.01; ***p <0.001; ns, p >0.05.
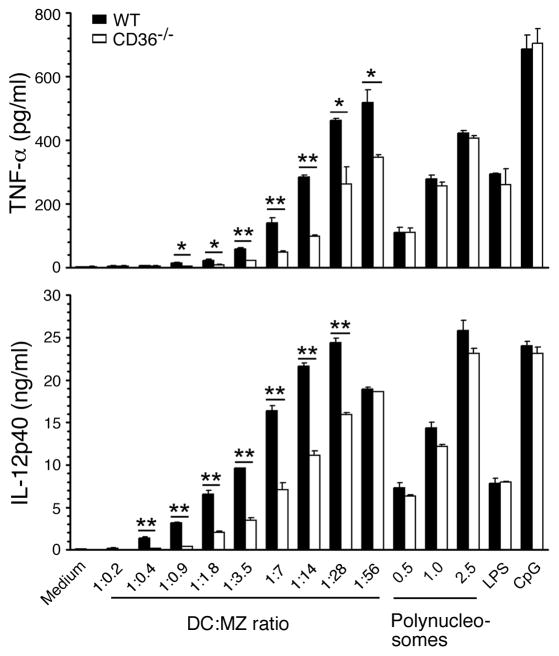
CD36 modulates MZ-induced inflammatory responses by DCs
We recently showed that nucleosomes isolated from P. falciparum MZs and the parasite-infected erythrocytes activate DCs through TLR9-mediated signaling, leading to efficient induction of TNF-α and IL-12 production (41). To determine whether CD36 modulates the cytokine-inducing activity of P. falciparum polynucleosomes and MZs, we examined the cytokine responses by WT FL-DCs and FL-DCs deficient in CD36. When stimulated with soluble polynucleosomes, both WT DCs and CD36-deficient DCs efficiently produced cytokines and the levels of cytokines produced were essentially comparable (Fig. 5). In contrast, when cells were stimulated with MZs, DCs deficient in CD36 produced significantly decreased levels of TNF-α and IL-12, suggesting that CD36 plays a role in the uptake of MZs and/or modulation of MZ’s stimulatory activity. To determine which of these processes contributes to decreased cytokine production by CD36 deficient DCs, we analyzed uptake of MZs by flow cytometry and the uptake by WT and CD36 deficient FL-DCs was comparable at all doses tested (Fig. 6). Therefore, the higher levels of cytokine production by WT DCs than CD36 deficient DCs appear to be due to modulation of MZ’s stimulatory activity by CD36 but not due to the decreased levels of phagocytosis. The inability of CD36 to modulate the polynucleosome-induced cytokine production is likely due to the fact that the polynucleosomes used were soluble components, which were likely taken up by the cells through pinocytosis rather than by phagocytosis. When consider together, these two data argue that modulation of the activity of MZs by CD36 is also dependent on phagocytosis. Therefore, it appears that phagocytic process is involved in the initiation of CD36-associated signaling contributes to MZ-mediated signaling.
Figure 5. CD36 regulates P. falciparum merozoite-induced pro-inflammatory cytokine production by DCs.
FL-DCs derived from WT and CD36−/− mice were plated into 96 well plates (1 × 105 cells per well) and stimulated with MZs (1 to 280 × 105 MZs/ml) to obtain the indicated ratios of DCs to MZs or polynucleosomes (0.5–2.5 μg/ml DNA content) in 200 μl of culture medium. TNF-α and IL-12 released into the culture medium were analyzed by ELISA. FL-DCs similarly stimulated with LPS (100 ng/ml) or CpG (2 μg/ml) were analyzed as controls. Data are representative of three independent experiments. Mean values ± SD are plotted. *, p <0.05; **, p <0.01.
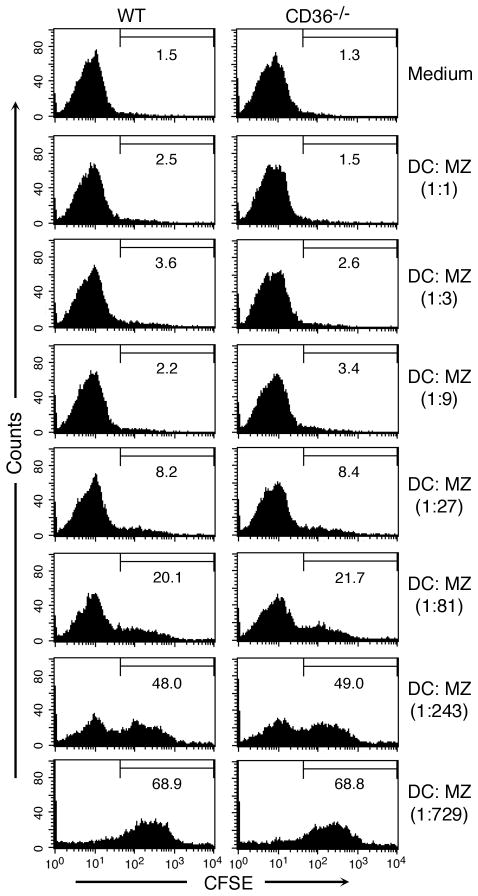
Figure 6. The levels of phagocytic uptake of MZs by WT and CD36 deficient FL-DCs are similar.
WT and CD36 deficient FL-DCs were incubated with CFSE-labeled MZs at 37°C. After 2 h, cells were stained with anti-mouse CD11c antibody and analyzed by flow cytometry. The percent CFSE-positive cells (indicative of the extent of MZ uptake) is shown in the histograms. The experiment was performed two times, and the data from a representative experiment is shown.
At higher MZ doses (DC to MZ ratio of 1:56), essentially there was no difference in the levels of IL-12 production by WT DCs and by CD36 deficient DCs even though the level of TNF-α production by the latter cells is significantly lower than that by the former cells (Fig. 6). We previously showed that the signaling threshold required for the maximal induction of TNF-α by DCs is much higher than that required for the maximal production of IL-12 (44). Further, at higher MZ doses, the differences in cytokine production by WT and CD36 deficient DCs was statistically non-significant (Fig. 5). This is likely because, as explained above for GPIs, at high MZ doses, while the co-operative signaling by TLR and CD36/phagocytosis is saturated in WT DCs, MZ/DNA-mediated activity is increasing in CD36 deficient DCs. Therefore, kinetically, it is expected that the differences between the two cell types becomes gradually less significant, eventually becoming equal.
Overall, the results of the present study demonstrate that P. falciparum GPIs activate DCs through TLR2-mediated signaling. Further, the TLR2-dependent activity of GPIs in DCs is modulated by CD36. These results agree with the previously reported cooperation of CD36 with signaling by several TLR2 and TLR4 ligands (31). However, the P. falciparum MZs were unable to exhibit TLR2-dependent activity of GPIs, even though GPIs are expected to be present in the plasma membranes of MZs. The likely reason is that the levels of GPIs on plasma membrane are not sufficient to reach signaling threshold required for cytokine production.
The results of the present study also suggest that CD36 modulates the activity of P. falciparum MZs. Previous studies have shown that CD36-mediated binding and uptake of various particulate ligands contributes to the TLR ligand-mediated cell signaling and cytokine production (31,32,34). Thus, it is possible that CD36, being a scavenge receptor for diverse types of ligands, can also bind P. falciparum MZs and present it to the pattern recognition receptor, thereby modulating the MZ activity. Although CD36 is known to bind P. falciparum infected erythrocytes by interacting with P. falciparum erythrocyte membrane protein 1 (PfEMP1) (46), recent studies have shown that CD36 binds P. berghei-infected erythrocytes, which do not express the homologues of PfEMP1, and sequester them in the lungs and adipocyte tissues (47). Also, it has been reported that phagocytosis process itself modulates TLR-mediated activity of ligands (48). Thus, it is possible that CD36 modulates both phagocytosis and the activity of TLRs. Further studies are underway to determine the mechanisms by which CD36 regulates the activity of P. falciparum merozoites.
Acknowledgments
We thank:Dr. Shizuo Akira, Research Institute for Microbial Diseases, Osaka University, Japan, for providing TLR and MyD88 knockout mice; Dr. Maria Febbraio, Lerner Research Institute, Cleveland Clinic, Cleveland, OH for providing CD36 knockout mice; Dr. Glenn Dranoff, Dana-Farber Cancer Institute, Harvard University Medical School for giving FLT3 ligand-expressing B16 cell line; Dr. Christopher Norbury, Department of Microbiology and Immunology, Hershey Medical Center, Hershey, for giving GM-CSF producing X63 melanoma cell line. This work is supported by Grant AI41139 from National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Footnotes
Conflict of interest disclosure: None
References
- 1.Snow RW, Guerra CA, Noor AM, Myint HY, Hay SI. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature. 2005;434:214–217. doi: 10.1038/nature03342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Guidelines for the Treatment of Malaria. 2. Vol. 2010. World Health Organization; Geneva: Feb 5, 2011. Available at http://www.who.int/malaria/publications/atoz/9789241547925/en/index.html. [Google Scholar]
- 3.Artavanis-Tsakonas K, Eleme K, McQueen KL, et al. Innate immune response to malaria: rapid induction of IFN-γ from human NK cells by live Plasmodium falciparum-infected erythrocytes. J Immunol. 2002;169:2956–2963. doi: 10.4049/jimmunol.169.6.2956. [DOI] [PubMed] [Google Scholar]
- 4.Su Z, Stevenson MM. Central role of endogenous gamma interferon in protective immunity against blood-stage Plasmodium chabaudi AS infection. Infect Immun. 2000;68:4399–4406. doi: 10.1128/iai.68.8.4399-4406.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jacobs P, Radzioch D, Stevenson MM. A Th1-associated increase in tumor necrosis factor alpha expression in the spleen correlates with resistance to blood-stage malaria in mice. Infect Immun. 1996;64:535–541. doi: 10.1128/iai.64.2.535-541.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stevenson MM, Tam MF, Wolf SF, Sher A. IL-12-induced protection against blood-stage Plasmodium chabaudi AS requires IFN-gamma and TNF-alpha and occurs via a nitric oxide-dependent mechanism. J Immunol. 1995;155:2545–2556. [PubMed] [Google Scholar]
- 7.Dodoo D, Omer FM, Todd J, Akanmori BD, Koram KA, Riley EM. Absolute levels and ratios of pro-inflammatory and anti-inflammatory cytokine production in vitro predict clinical immunity to Plasmodium falciparum malaria. J Infect Dis. 2002;185:971–979. doi: 10.1086/339408. [DOI] [PubMed] [Google Scholar]
- 8.Lyke KE, Burges R, Cissoko Y, et al. Serum levels of the proinflammatory cytokines interleukin-1β (IL-1β), IL-6, IL-8, IL-10, TNF-α, and IL-12(p70) in Malian children with severe Plasmodium falciparum malaria and matched uncomplicated malaria or healthy controls. Infect Immun. 2004;72:5630–5637. doi: 10.1128/IAI.72.10.5630-5637.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perkins DJ, Weinberg JB, Kremsner PG. Reduced interleukin-12 and transforming growth factor-beta1 in severe childhood malaria: relationship of cytokine balance with disease severity. J Infect Dis. 2000;182:988–992. doi: 10.1086/315762. [DOI] [PubMed] [Google Scholar]
- 10.Grau GE, Fajardo LF, Piguet PF, Allet B, Lambert PH, Vassalli P. Tumor necrosis factor (cachectin) as an essential mediator in murine cerebral malaria. Science. 1987;237:1210–1212. doi: 10.1126/science.3306918. [DOI] [PubMed] [Google Scholar]
- 11.Karunaweera ND, Grau GE, Gamage P, Carter R, Mendis KN. Dynamics of fever and serum levels of tumor necrosis factor are closely associated during clinical paroxysms in Plasmodium vivax malaria. Proc Natl Acad Sci USA. 1992;89:3200–3203. doi: 10.1073/pnas.89.8.3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Kossodo S, Grau GE. Profiles of cytokine production in relation with susceptibility to cerebral malaria. J Immunol. 1993;151:4811–4820. [PubMed] [Google Scholar]
- 13.Van Der Heyde HC, Nolan J, Combes V, Gramaglia I, Grau GE. A unified hypothesis for the genesis of cerebral malaria: sequestration, inflammation and hemostasis leading to microcirculatory dysfunction. Trends Parasitol. 2006;22:503–508. doi: 10.1016/j.pt.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 14.Patnaik JK, Das BS, Mishra SK, et al. Vascular clogging, mononuclear cell margination, and enhanced vascular permeability in the pathogenesis of human cerebral malaria. Am J Trop Med Hyg. 1994;51:642–647. [PubMed] [Google Scholar]
- 15.Schofield L, Novakovic S, Gerold P, Schwarz RT, McConville MJ, Tachado SD. Glycosylphosphatidylinositol toxin of Plasmodium up-regulates ICAM-1, VCAM-1, and E-selectin expression in vascular endothelial cells and increases leukocyte and parasite cytoadherence via protein tyrosine kinase-dependent signal transduction. J Immunol. 1996;156:1886–1896. [PubMed] [Google Scholar]
- 16.Turner GD, Morrison H, Jones M, et al. An immunohistochemical study of the pathology of fatal malaria. Evidence for widespread endothelial activation and a potential role for intercellular adhesion molecule-1 in cerebral sequestration. Am J Pathol. 1994;145:1057–1069. [PMC free article] [PubMed] [Google Scholar]
- 17.Ockenhouse CF, Tegoshi T, Maeno Y, et al. Human vascular endothelial cell adhesion receptors for Plasmodium falciparum-infected erythrocytes: roles for endothelial leukocyte adhesion molecule 1 and vascular cell adhesion molecule 1. J Exp Med. 1992;176:1183–1189. doi: 10.1084/jem.176.4.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schofield L, Hackett F. Signal transduction in host cells by a glycosylphosphatidylinositol toxin of malaria parasites. J Exp Med. 1993;177:145–153. doi: 10.1084/jem.177.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tachado SD, Gerold P, McConville MJ, et al. Glycosylphosphatidylinositol toxin of Plasmodium induces nitric oxide synthase expression in macrophages and vascular endothelial cells by a protein tyrosine kinase-dependent and protein kinase C-dependent signaling pathway. J Immunol. 1996;156:1897–1907. [PubMed] [Google Scholar]
- 20.Zhu J, Krishnegowda G, Gowda DC. Induction of proinflammatory responses in macrophages by the glycosylphosphatidylinositols of Plasmodium falciparum: the requirement of extracellular signal-regulated kinase, p38, c-Jun N-terminal kinase and NF-κB pathways for the expression of proinflammatory cytokines and nitric oxide. J Biol Chem. 2005;280:8617–8627. doi: 10.1074/jbc.M413539200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Naik RS, Branch OH, Woods AS, et al. Glycosylphosphatidylinositol anchors of Plasmodium falciparum: Molecular characterization and naturally elicited antibody response that may provide immunity to malaria pathogenesis. J Exp Med. 2000;192:1563–1576. doi: 10.1084/jem.192.11.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schofield L, Tachado SD. Regulation of host cell function by glycosylphosphatidylinositols of the parasitic protozoa. Immunol Cell Biol. 1996;74:555–563. doi: 10.1038/icb.1996.89. [DOI] [PubMed] [Google Scholar]
- 23.Schwarzer E, Turrini F, Ulliers D, Giribaldi G, Ginsburg H, Arese P. Impairment of macrophage functions after ingestion of Plasmodium falciparum-infected erythrocytes or isolated malarial pigment. J Exp Med. 1992;176:1033–1041. doi: 10.1084/jem.176.4.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwarzer E, Alessio M, Ulliers D, Arese P. Phagocytosis of the malarial pigment, hemozoin, impairs expression of major histocompatibility complex class II antigen, CD54, and CD11c in human monocytes. Infect Immun. 1998;66:1601–1606. doi: 10.1128/iai.66.4.1601-1606.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bruna-Romero O, Rodriguez A. Dendritic cells can initiate protective immune responses against malaria. Infect Immun. 2001;69:5173–5176. doi: 10.1128/IAI.69.8.5173-5176.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gowda NM, Wu X, Gowda DC. TLR9 and MyD88 are crucial for the development of protective immunity to malaria. J Immunol. 2011 doi: 10.4049/jimmunol.1102143. Accepted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilson NS, Villadangos JA. Regulation of antigen presentation and cross-presentation in the dendritic cell network: facts, hypothesis, and immunological implications. Adv Immunol. 2005;86:241–305. doi: 10.1016/S0065-2776(04)86007-3. [DOI] [PubMed] [Google Scholar]
- 28.Steinman RM, Hemmi H. Dendritic cells: translating innate to adaptive immunity. Curr Top Microbiol Immunol. 2006;311:17–58. doi: 10.1007/3-540-32636-7_2. [DOI] [PubMed] [Google Scholar]
- 29.Febbraio M, Hajjar DP, Silverstein RL. CD36: a class B scavenger receptor involved in angiogenesis, atherosclerosis, inflammation, and lipid metabolism. J Clin Invest. 2001;108:785–791. doi: 10.1172/JCI14006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Urban BC, Ferguson DJ, Pain A, et al. Plasmodium falciparum-infected erythrocytes modulates the maturation of dendritic cells. Nature. 1999;400:73–77. doi: 10.1038/21900. [DOI] [PubMed] [Google Scholar]
- 31.Hoebe K, Georgel P, Rutschmann S, et al. CD36 is a sensor of diacylglycerides. Nature. 2005;433:523–527. doi: 10.1038/nature03253. [DOI] [PubMed] [Google Scholar]
- 32.Stewart CR, Stuart LM, Wilkinson K, van Gils JM, Deng J. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat Immunol. 2009;11:155–161. doi: 10.1038/ni.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krishnegowda G, Hajjar AM, Zhu J, et al. Induction of proinflammatory responses in macrophages by the glycosylphosphatidylinositols of Plasmodium falciparum: cell signaling receptors, glycosylphosphatidylinositol (GPI) structural requirement, and regulation of GPI activity. J Biol Chem. 2005;280:8606–8616. doi: 10.1074/jbc.M413541200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Erdman LK, Cosio G, Helmers AJ, Gowda DC, Grinstein S, Kain KC. CD36 and TLR interactions in inflammation and phagocytosis: implications for malaria. J Immunol. 2009;183:6452–6459. doi: 10.4049/jimmunol.0901374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patel SN, Lu Z, Ayi K, Serghides L, Gowda DC, Kain KC. Disruption of CD36 impairs cytokine response to Plasmodium falciparum glycosylphosphatidylinositol and confers susceptibility to severe and fatal malaria in vivo. J Immunol. 2007;178:3954–3961. doi: 10.4049/jimmunol.178.6.3954. [DOI] [PubMed] [Google Scholar]
- 36.Camargo MM, Almeida IC, Pereira ME, Ferguson MA, Travassos LR, Gazzinelli RT. Glycosylphosphatidylinositol-anchored mucin-like glycoproteins isolated from Trypanosoma cruzi trypomastigotes initiate the synthesis of proinflammatory cytokines by macrophages. J Immunol. 1997;158:5890–5901. [PubMed] [Google Scholar]
- 37.Oliveira AC, Peixoto JR, de Arruda LB, et al. Expression of functional TLR4 confers proinflammatory responsiveness to Trypanosoma cruzi glycoinositolphospholipids and higher resistance to infection with T. cruzi. J Immunol. 2004;173:5688–5696. doi: 10.4049/jimmunol.173.9.5688. [DOI] [PubMed] [Google Scholar]
- 38.Tapping RI, Tobias PS. Mycobacterial lipoarabinomannan mediates physical interactions between TLR1 and TLR2 to induce signaling. J Endotoxin Res. 2003;9:264–268. doi: 10.1179/096805103225001477. [DOI] [PubMed] [Google Scholar]
- 39.Pichyangkul S, Yongvanitchit K, Kum-arb U, et al. Malaria blood stage parasites activate human plasmacytoid dendritic cells and murine dendritic cells through a Toll-like receptor 9-dependent pathway. J Immunol. 2004;172:4926–4933. doi: 10.4049/jimmunol.172.8.4926. [DOI] [PubMed] [Google Scholar]
- 40.Wu X, Gowda NM, Kumar S, Gowda DC. Protein-DNA complex is the exclusive malaria parasite component that activates dendritic cells and triggers innate immune responses. J Immunol. 2010;184:4338–4348. doi: 10.4049/jimmunol.0903824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gowda NM, Wu X, Gowda DC. The nucleosome (histone-DNA complex) is the TLR9-specific immunostimulatory component of Plasmodium falciparum that activates DCs. PLoS One. 2011;6:e20398. doi: 10.1371/journal.pone.0020398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alkhalil A, Achur RN, Valiyaveettil M, Ockenhouse CF, Gowda DC. Structural requirements for the adherence of Plasmodium falciparum-infected erythrocytes to chondroitin sulfate proteoglycans of human placenta. J Biol Chem. 2000;275:40357–40364. doi: 10.1074/jbc.M006399200. [DOI] [PubMed] [Google Scholar]
- 43.Inaba K, Inaba M, Romani N, et al. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu X, Gowda NM, Gowda DC. Plasmodium falciparum: differential merozoite dose requirements for maximal production of various inflammatory cytokines. Exp Parasitol. 2011;127:202–207. doi: 10.1016/j.exppara.2010.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhu J, Krishnegowda G, Li G, Gowda DC. Proinflammatory responses by glycosylphosphatidylinositols (GPIs) of Plasmodium falciparum are mainly mediated through the recognition of TLR2/TLR1. Exp Parasitol. 2011;128:205–211. doi: 10.1016/j.exppara.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baruch DI, Gormely JA, Ma C, Howard RJ, Pasloske BL. Plasmodium falciparum erythrocyte membrane protein 1 is a parasitized erythrocyte receptor for adherence to CD36, thrombospondin, and intercellular adhesion molecule 1. Proc Natl Acad Sci USA. 1996;93:3497–5302. doi: 10.1073/pnas.93.8.3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Franke-Fayard B, Janse CJ, Cunha-Rodrigues M, et al. Murine malaria parasite sequestration: CD36 is the major receptor, but cerebral pathology is unlinked to sequestration. Proc Natl Acad Sci USA. 2005;102:11468–11473. doi: 10.1073/pnas.0503386102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Underhill DM, Gantner B. Integration of Toll-like receptor and phagocytic signaling for tailored immunity. Microbes Infect. 2004;6:1368–1373. doi: 10.1016/j.micinf.2004.08.016. [DOI] [PubMed] [Google Scholar]