Abstract
α4βδ GABAA receptors (GABARs) have low CNS expression, but their expression is increased by 48 h exposure to the neurosteroid THP (3α-OH-5α[β]-pregnan-20-one). THP also increases the efficacy of δ-containing GABARs acutely, where GABA is a partial agonist. Thus, we examined effects of THP (100 nM) and full GABA agonists at α4β2δ (gaboxadol, 10 μM, and β-alanine, 10 μM – 1 mM), on surface expression of α4β2δ. To this end, we used an α4 construct tagged with a 3XFLAG (F) epitope or measured expression of native α4 and δ. HEK-293 cells or cultured hippocampal neurons were transfected with α4Fβ2δ and treated 24 h later with GABA agonists, THP, GABA plus THP or vehicle (0.01% DMSO) for 0.5 h – 48 h. Immunocytochemistry was performed under both non-permeabilized and permeabilized conditions to detect surface and intracellular labeling, respectively, using confocal microscopy. The high efficacy agonists and GABA (1 or 10μM) plus THP increased α4β2δ surface expression up to 3-fold after 48 h, an effect first seen by 0.5 h. This effect was not dependent upon the polarity of GABAergic current, although expression was increased by KCC2. Intracellular labeling was decreased while functional expression was confirmed by whole cell patch clamp recordings of responses to GABA agonists. GABA plus THP treatment did not alter the rate of receptor removal from the surface membrane, suggesting that THP-induced α4β2δ expression is likely via receptor insertion. Surface expression of α4β2δ was decreased by rottlerin (10 μM), suggesting a role for PKC- δ. These results suggest that trafficking of α4β2δ GABARs is regulated by high efficacy states.
Keywords: α4, δ, GABAA receptor, trafficking, pregnanolone, β-alanine, KCC2
1. Introduction
The ligand-gated GABAA receptor (GABAR) is responsible for most inhibition in the CNS (Olsen and Sieghart, 2009). This receptor is a pentameric membrane protein which gates a Cl− conductance, and is generally of the form 2α, 2β and 1γ (Chang et al., 1990) although many subtypes exist from a pool of 6α, 3β, 3γ, δ, ε, π, ρ and θ. The remarkable diversity of the properties associated with the various receptor subtypes provides a basis for the selective expression of particular subtypes in a region or function-specific manner. In particular, receptors containing the δ subunit have relatively low expression in the CNS (Pirker et al., 2000; Wisden et al., 1992) but display a high degree of plasticity (Shen and Smith, 2009). These receptors express at extrasynaptic locations (Wei et al., 2003) and generate a tonic current (Stell and Mody, 2002) in response to ambient levels of GABA (~1 μM) (Wu et al., 2001). The tonic inhibitory current has been shown to generate more current (i.e., total charge transfer) than the phasic inhibitory synaptic current (Bai et al., 2000), suggesting that regulating the tonic inhibitory current may be an efficient mechanism to reduce neuronal excitability.
One trigger for altered expression of α4βδ GABAR is exposure to the neurosteroid THP (3α-OH-5[α]β-pregnan-20-one or [allo]pregnanolone), a metabolite of the ovarian steroid progesterone (Compagnone and Mellon, 2000) which can also be formed directly in hippocampal pyramidal cells from cholesterol (Agis-Balboa et al., 2006). Levels of this steroid fluctuate across the ovarian cycle, pregnancy and at the onset of puberty (Compagnone and Mellon, 2000; Shen et al., 2007) and are also increased by sustained stress (Girdler et al., 2001; Higashi et al., 2005; Purdy et al., 1991). In vivo administration of this steroid to female rats increases hippocampal expression of α4 and δ subunits by 2 to 3-fold above control levels after 48 h (Shen et al., 2005), accompanied by increases in the tonic inhibitory current. The naturally occurring fluctuations in this steroid also alter expression of α4βδ GABAR in a number of CNS sites, including CA1 hippocampus, dentate gyrus and the midbrain central grey (Lovick et al., 2005; Maguire et al., 2005; Maguire and Mody, 2009; Sanna et al., 2009; Shen et al., 2007; Shen et al., 2010). Alterations in expression of this receptor by fluctuating steroid levels, either endogenous or exogenously administered (Smith et al., 2006), can be associated with alterations in anxiety behavior, panic responses and seizure susceptibility, suggesting that this receptor may play a role in certain neuropsychological pathologies. Despite this in vivo evidence of neurosteroid regulation of α4βδ GABAR expression, however, little is known of the cellular mechanisms which underlie these changes in expression. Although recent studies have shown that brainderived neurotrophic factor (BDNF) (Joshi and Kapur, 2009) and protein kinase C (PKC)-induced phosphorylation (Abramian et al., 2010) increase δ and α4 expression, respectively, the mechanism by which THP alters surface expression of these receptors is not known.
Recent studies have shown that GABA can increase trafficking of α1β2γ2 GABARs to the cell membrane (Eshaq et al., 2010). α4βδ GABARs have a unique pharmacological profile, however, different from α1β2γ2 GABARs. Although they have a high sensitivity to GABA (EC50=0.5 μM)(Brown et al., 2002), GABA is a partial agonist at these receptors (Bianchi and Macdonald, 2003; Zheleznova et al., 2008), unlike its effect at α1β2γ2 where it acts as a full agonist. However, δ-containing GABARs are the most sensitive target for THP (Belelli et al., 2002) and the related steroid THDOC (3α,21-dihydroxy-5α-pregnan-20-one) (Brown et al., 2002; Wohlfarth et al., 2002), which are positive modulators at physiological concentrations. These steroids increase receptor efficacy (Bianchi and Macdonald, 2003; Zheleznova et al., 2008), producing current greater than the maximal GABA-gated current by increasing long duration receptor channel openings. A number of high efficacy agonists for α4βδ GABARs have been reported, which include both synthetic (THIP or gaboxadol) (Brown et al., 2002) and endogenous (β-alanine (Bianchi and Macdonald, 2003) and taurine (Jia et al., 2008)) compounds. Thus, we initially tested the effect of THP in combination with GABA on cell surface expression of a FLAG-tagged α4 construct transfected with β2 and δ in HEK-293 cells and cultured hippocampal neurons. We assessed receptor trafficking by employing a high expression CMV promoter and assessed surface receptor expression under non-permeabilized conditions following expression of intracellular protein (Eshaq et al., 2010). This 3XFLAG tag on the C-terminus of α4 produces a highly visible signal when targeted with monoclonal anti-FLAG antibodies and a fluorescent secondary antibody (Hernan et al., 2000). Functional receptor expression was assessed with whole cell patch clamp recordings from transfected cells. These findings were compared with those obtained with high efficacy agonists and GABA itself in their effect on trafficking of α4βδ GABARs to the cell surface in order to determine whether steroid effects on expression of this receptor are due to increases in receptor efficacy.
Regulation of cell surface expression of α4βδ GABAR protein may either be due to an increase in receptor insertion or a reduction in receptor internalization and degradation. Recent studies have suggested that δ-containing GABARs have a greater stability in the membrane than γ2-containing GABARs, with a τ1/2 for internalization of hours versus minutes, respectively (Joshi and Kapur, 2009). Thus, regulation of receptor insertion rate may be a more likely mechanism for increasing cell surface expression. Our findings suggest that conditions which increase receptor efficacy increase expression of α4βδ GABARs, regardless of whether the steroid was present. These increases in receptor expression appear to be due to increased receptor insertion.
2. Results
2.1 The α4(3XFLAG)β2δ GABAR displays functional expression in HEK-293 Cells
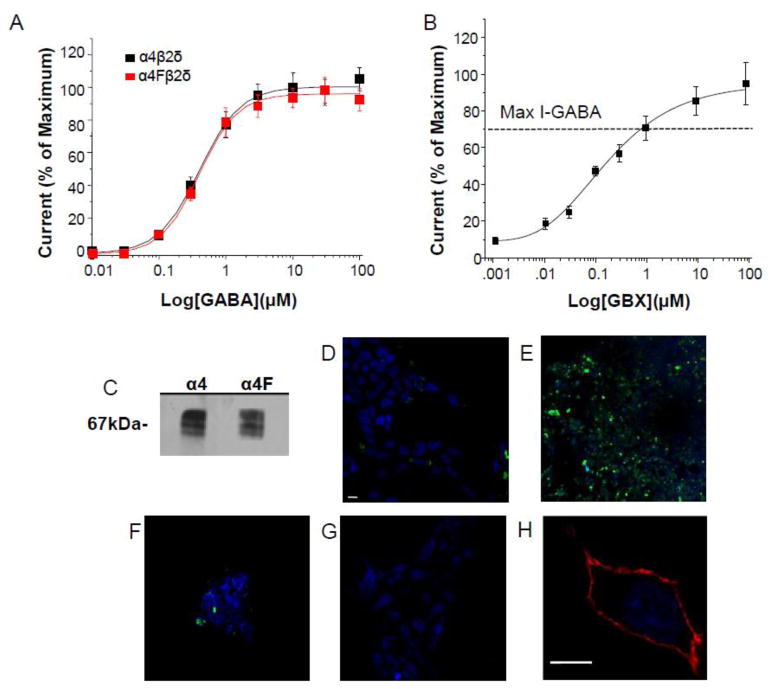
We initially characterized our novel 3XFLAG-tagged α4 (α4F) subunit via electrophysiological techniques comparing the GABA responses of α4F to untagged α4 co-transfected with β2 and δ cDNAs (Fig. 1A). To this end, whole cell voltage clamp recordings were used to determine responses to GABA (0.01 – 100 μM) of the two constructs expressed in HEK-293 cells in the presence of 1 μM ZnCl which inhibits current from binary receptors (Meera et al., 2011). In fact, concentration-response curves for α4β2δ and α4Fβ2δ were very similar with an EC50 (mean, 95% confidence limits) of 0.40 (0.125, 1.27) μM and 0.42 (0.122, 1.44) μM and Hill coefficients of 1.36 ± 0.15 and 1.38 ± 0.13, respectively (Fig. 1A). α4Fβ2δ also generated a maximum response to gaboxadol which was 30% greater than the maximal response to GABA (Fig. 1B), with an EC50 of 127 (28.3, 570) nM, consistent with other reports (Meera et al., 2011).
Figure 1. Characterization of α4[3XFLAG]β2δ expression.
Concentration-response curves for GABA (A, 10 nM – 100 μM) or the GABA agonist gaboxadol (B, GBX, 30 nM – 1 mM) constructed from whole cell current responses recorded with patch clamp techniques from HEK-293 cells transfected with β2, δ and α4 or α4[3XFLAG] (α4F). A, Both α4Fβ2δ and α4β2δ yielded similar GABA concentration-response curves. (EC50: α4β2δ, 0.4 μM (.125, 1.27); α4Fβ2δ, 0.42 (0.122, 1.44) μM), (n=4 cells/point) (B) α4Fβ2δ also yielded a maximum GBX-generated current larger than GABA in the presence of 1 μM ZnCl2, signifying incorporation of the δ subunit (EC50, 127 (28.3, 570) nM), (n=6 cells/point). Dashed line, response to a saturating concentration of GABA (determined in A). C, Representative Western blot showing identical band detection (67 kDa) using anti-FLAG (left lane) or anti- α4 (right lane) antibodies performed on crude membranes obtained from recombinant α4Fβ2δ expressed in HEK-293 cells. D–G, Specificity of FLAG detection: HEK cells transfected with α4, β2 and δ (D) or α4F, β2 and δ (E), α4F and β2 (F) or α4F and δ (G) cDNA. Cell surface α4F was labeled by immunofluorescence using an anti-FLAG (M2) antibody and imaged by confocal microscopy (Alexa-488 IgG, secondary antibody). Antibody recognition of the FLAG receptor is distinct and bright in the confocal image at 40X W-Immersol for α4Fβ2δ (E), with lower levels of detection for α4Fβ2 (F), but not detectable in D and G. H, Membrane stain, wheat germ agglutinin (WGA)-Alexa-546 (red), 63X, untransfected HEK-293 cell. Nuclear cell stain, DAPI (blue). Scale bar, 10 μm. (All cells incubated with GABA (10 μM) + THP (100 nM) to ensure high levels of expression.) D–H, Representative of 3 experiments.
Western blot analysis of HEK-293 cells transfected with α4F with β2 and δ cDNAs was also performed to determine if the anti-FLAG antibody recognizes α4. To this end, membrane preparations were probed with either an anti-α4 or anti-FLAG antibody and revealed a band at 67kDa, the characteristic molecular size of α4 (Fig. 1C), in both cases.
Detectable specific immunoreactivity using mouse anti-FLAG antibody was only observed with α4Fβ2δ, but not with untagged α4β2δ (Fig. 1D,E) suggesting that it is specific for the FLAG tag. In contrast to α4β2δ, expression of the binary receptors α4β2 and α4δ was barely detectable visually (Fig. 1E, F). Staining with WGA (wheat-germ agglutinin) of un-fixed, non-permeabilized, untransfected HEK-293 cells verifies that the integrity of the cell was not compromised during the staining procedure (Fig. 1H).
We also performed a control experiment to determine the rate of intracellular acculumation of α4Fβ2δ protein after transfection. FLAG expression was detectable intracellularly at 24 h, but not at 3 h, after transfection. The protein was localized outside of the nucleus but not yet on the cell surface suggesting that this is the time required for transcription and translation (not shown).
2.2 The neurosteroid THP increases surface expression of α4Fβ2δ in HEK-293 cells when co-administered with GABA
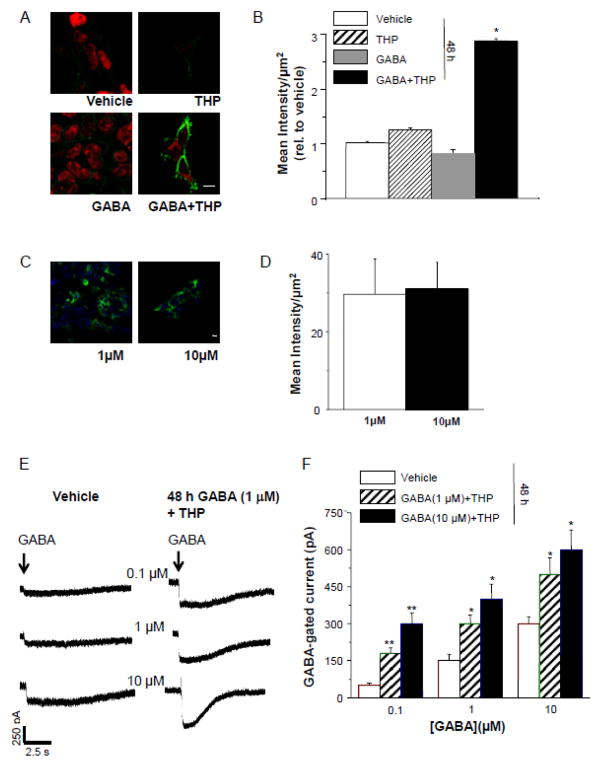
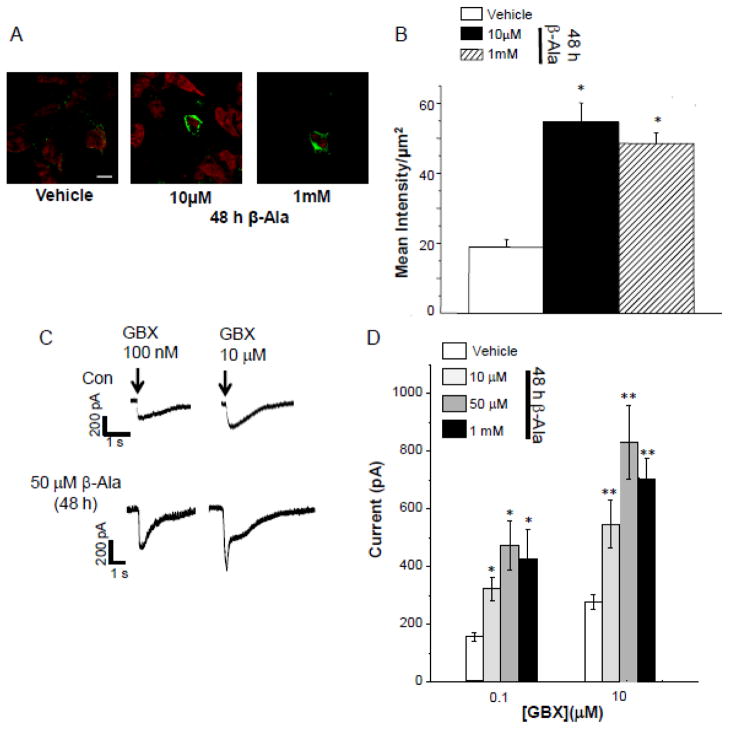
48 h administration of THP has been shown to increase expression of α4β2δ in vivo (Shen et al., 2005). Therefore, we tested whether this steroid, co-administered with GABA at concentrations of either 1 or 10 μM, would also increase expression of α4Fβ2δ in an in vitro expression system. To this end, we treated HEK-293 cells 24 h after transfection with 100 nM THP and/or GABA (1 or 10 μM) or vehicle alone (0.01% DMSO) for 48 h. In fact, GABA plus THP increased surface expression of α4Fβ2δ by 3- fold (166%, P<0.05) compared to expression levels in vehicle-treated cells (Fig. 2 A, B). In contrast, expression levels after treatment of HEK-293 cells with GABA or THP alone did not increase receptor expression. In a separate experiment, expression levels of the receptor were compared in cells treated either with 1 μM or 10 μM GABA in addition to 100 nM THP for 48 h. Surface expression of α4Fβ2δ in the two groups was not significantly different (Fig. 2 C, D). Both conditions resulted in functional receptor expression, as revealed by whole cell patch clamp recordings. Mean values for GABA (100 nM)-gated current were 3.5-fold greater after GABA (1 μM) plus THP treatment and 5-fold greater after GABA (10 μM) plus THP treatment compared to vehicle treatment (P<0.05) (Fig. 2 E, F). In this case, 48 h treatment with 10 μM GABA produced a significantly greater GABA response (P<0.001) than 48 h treatment with 1 μM GABA. However, at higher concentrations of acutely applied GABA, the two 48 h treatment paradigms both produced significantly greater responses than observed in vehicle-treated cells, but which were not significantly different from each other.
Figure 2. GABA plus THP increases surface expression of α4Fβ2δ in HEK-293 cells.
A, B, 48 h administration of GABA (10 μM), THP (100 nM), GABA+THP or vehicle (0.01% DMSO) to HEK-293 cells transfected with α4F, β2 and δ cDNA. Only GABA+THP resulted in high levels of visible fluorescence to α4F (2° Antibody, Alexa-488 IgG, green). Cell stain, TO-PRO-3 (red). A, Representative confocal images (40X), scale bar, 10 μm; B, averaged data. ANOVA, F(3,44) = 64, P<0.0001. *P<0.05 vs. other groups. C, D, Comparison of immunostaining after 48 h treatment with THP + GABA (1 μM, left, or 10 μM, right). C, Representative images; scale bar, 10 μm. D, Averaged data (n=3). E, F, Whole cell current responses to GABA (0.1, 1.0, 10 μM) after 48 h treatment with THP + GABA (1 or 10 μM). E, Representative traces. Arrow, onset of GABA application (~400 ms). F, Averaged data. ANOVA, 100 nM GABA, F(2,24) = 12, P = 0.0002, 1 μM GABA, F(2,24) = 9.7, P = 0.0007, 10 μM GABA, F(2,24) = 4.31, P = 0.025. *P<0.05 vs. Vehicle; **P<0.05 vs. both other groups.
2.3. Time-course of the surface expression of α4Fβ2δ following GABA plus THP exposure
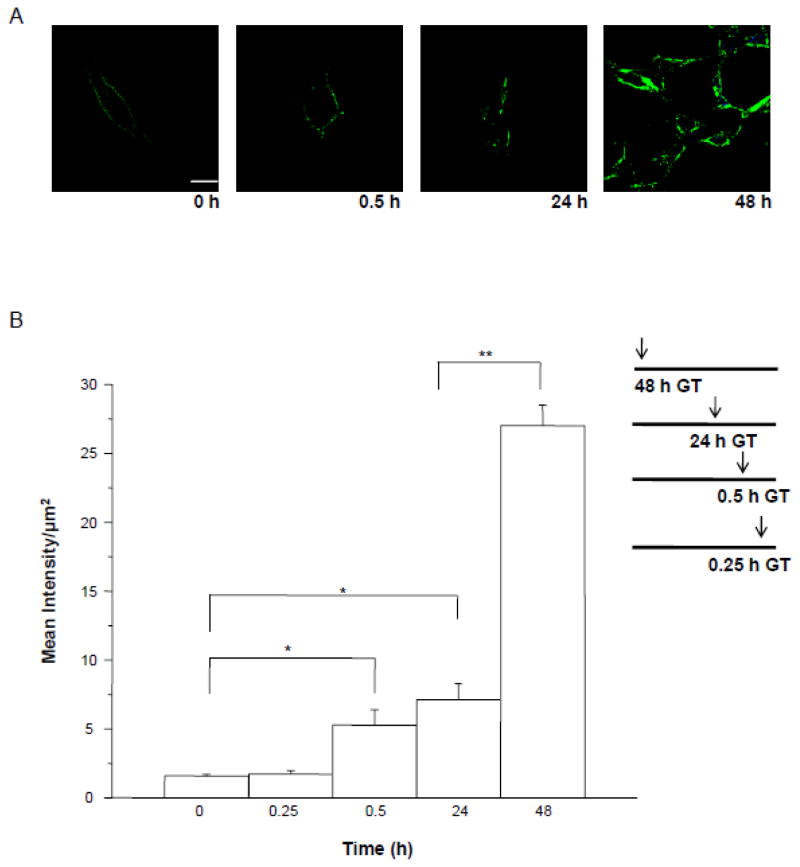
Our lab has previously shown that significant increases in expression levels of α4 and δ are not detected in the CA1 hippocampus until 48–72 h of in vivo exposure to THP (Shen et al., 2005). To confirm that these increases are at the surface membrane and that they do not occur with shorter exposure times we examined the time-course of detectable expression of α4Fβ2δ expression following treatment of transfected HEK- 293 cells with GABA (10 μM) plus THP (100 nM). Briefly, 24 h after transfection, cells were incubated for an additional 48 h with GABA plus THP or vehicle added for varying lengths of time: 0.25, 0.5, 24 or 48 h, which all terminated at the end of the 48 h period. At this time, cells were harvested, followed by immunostaining under non-permeabilized conditions. Across this 48 h period, the highest levels of receptor expression were noted at 48 h of GABA plus THP treatment, which produced nearly a 10-fold increase in FLAG immunostaining compared to vehicle (P<0.05) (Fig. 3). However, significant increases (250%, P<0.05) in surface expression of α4Fβ2δ first occurred after 30 min of GABA plus THP treatment compared to vehicle (Fig. 3B).
Figure 3. Time-course of the effect of GABA plus THP on surface expression of α4Fβ2δ in HEK-293 cells.
A, Representative confocal images (63X) show an increase in surface expression first detectable after 0.5 h exposure of HEK cells to GABA (10 μM) + THP (100 nM), Anti-FLAG, Alexa-488 F(ab′)2 (green); cell stain, DAPI (blue). Scale bar, 10 μm. B, Averaged data. Peak levels of expression are achieved at 48 h treatment. All timed treatments terminated at the conclusion of the 48 h incubation period (timeline inset, GT, GABA plus THP). Vehicle, 0.01% DMSO. ANOVA, F(4,20) = 71.4, P<0.0001. *P< 0.05 vs. 0 h, 0.25 h; **P<0.05 vs. other groups.
2.4. Full agonists increase surface expression of α4Fβ2δ
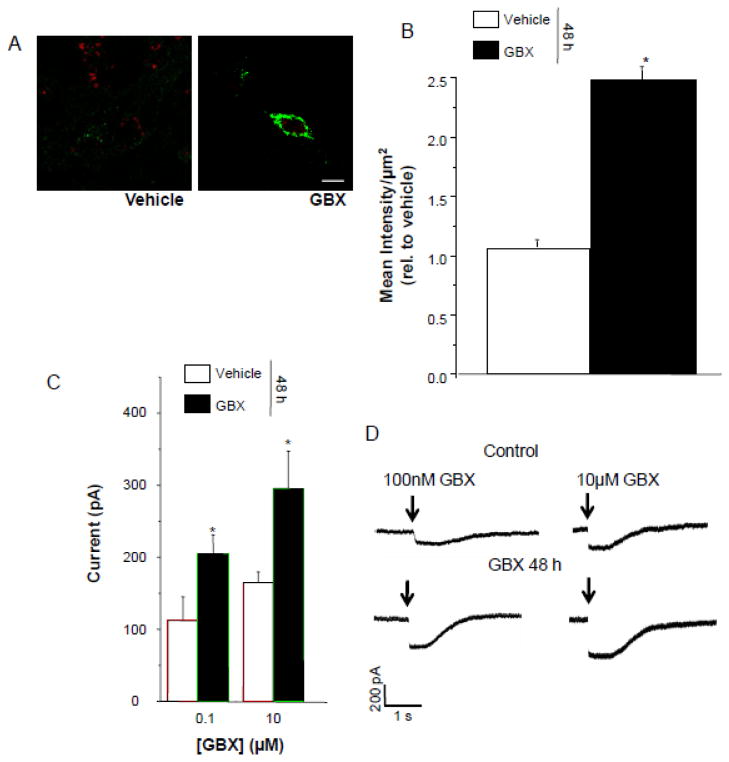
Neurosteroids such as THDOC, a steroid related to THP, increase the efficacy of GABA current gated by δ-containing GABAR (Bianchi and Macdonald, 2003; Zheleznova et al., 2008), where GABA itself is a partial agonist (Brown et al., 2002). Therefore, we tested whether agonists with increased efficacy at α4β2δ, gaboxadol (Brown et al., 2002) and β-alanine (Bianchi and Macdonald, 2003), would also increase expression of the receptor. HEK-293 cells transfected with α4Fβ2δ were treated with vehicle (0.01% DMSO), 10 μM gaboxadol, or β-alanine (10 μM – 1 mM) for 48 h and analyzed immunocytochemically and electrophysiologically for α4Fβ2δ expression. 48 h treatment of cells with either gaboxadol or β-alanine increased cell surface expression by approximately two-fold (Fig. 4,5, A,B) compared to vehicle treatment (P<0.05). This increase in FLAG labeling was representative of functional receptors because the peak current gated by acute application of gaboxadol (100 nM and 10 μM) in whole-cell patch clamp recordings was significantly greater(P<0.05) by 2- to 3-fold after 48 h treatment with gaboxadol or β-alanine compared to current recorded from vehicle-treated controls (P<0.05, Figs. 4,5 C,D).
Figure 4. Gaboxadol upregulates surface expression of α4Fβ2δ in HEK-293 cells.
48 h treatment with 10 μM gaboxadol (GBX) increases surface expression of α4Fβ2δ significantly compared to vehicle (0.01% DMSO) A, Representative confocal images (63X) of α4FLAG immunolabeling; B, averaged data. 2° Antibody, Alexa-488 IgG; cell stain, TO-PRO-3; scale bar, 10 μm. t(22) = 6.6; *P<0.0001 vs. vehicle. C, D, Increased expression was correlated with increased whole cell current response to acutely applied GBX. C, Averaged data. D, Representative currents; Arrow, onset of agonist application. t(7) = 2; *P<0.05 vs. vehicle groups.
Figure 5. β-Alanine upregulates surface expression of α4Fβ2δ in HEK-293 cells.
48 h treatment with β-alanine (β-Ala, 10 μM, 50 μM, 1 mM) increases surface expression of α4Fβ2δ compared to vehicle (0.01% DMSO). A, Representative confocal images (63X); B, Averaged data. 2° Antibody, Alexa-488 F(ab′)2 (green); cell stain, TOPRO- 3; scale bar, 10μm. ANOVA, F(2,24) = 27, P<0.0001. *P<0.05 vs. vehicle. C, D, Increased expression was correlated with increased whole cell current responses to acutely applied gaboxadol (GBX). C, Representative currents; Arrow, onset of agonist application; D, Averaged data. ANOVA, 100 nM GBX, F(3,31) = 6.4, P = 0.0017; 10 μM GBX, F(3,31) = 6.3, P = 0.0018. *P<0.05 vs. vehicle groups.
2.5. Levels of α4Fβ2δ in the ER decrease after exposure to GABA plus THP or gaboxadol
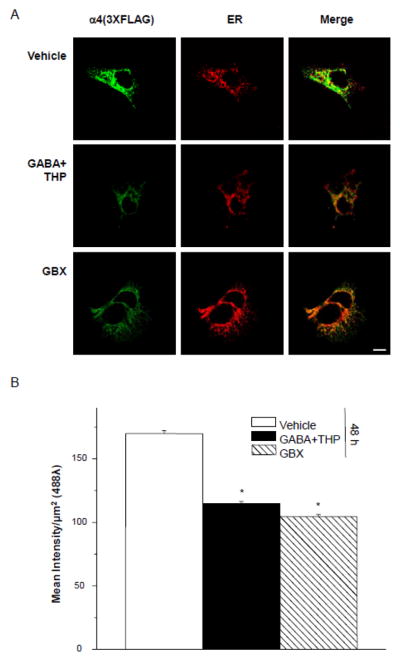
To address the possibility that the observed increases in cell surface receptor expression were due to trafficking of receptor to the surface in the ER pathway we measured intracellular levels of α4Fβ2δ in response to 48 h treatment of transfected HEK-293 cells with GABA plus THP or gaboxadol compared to vehicle. Calnexin was used as a marker for the ER. As predicted, the intracellular detection of α4F with an anti-FLAG antibody was ~80% greater for vehicle-treated cells than for GABA plus THP or gaboxadol- treated cells (P<0.05, Fig. 6). These results are inversely correlated with results from the cell surface expression study and suggest that 48 h treatment with GABA plus THP or gaboxadol promotes α4Fβ2δ trafficking out of the ER to the cell surface.
Figure 6. ER localization of α4F decreases with 48 h of agonist exposure.
A, Left to right, Representative confocal images (40X) of intracellular α4 FLAG labeling (2° Antibody, Alexa-488 IgG, green), ER labeling (calnexin, 546λ, red) and merged images (merge). The internal FLAG signal was obtained in permeabilized conditions after 48 h treatment with (top to bottom): vehicle (0.01% DMSO), GABA (10 μM) +THP (100 nM) or gaboxadol (GBX, 10 μM). Scale bar, 10 μm. B, Averaged data. GABA+THP and GBX treatments result in significantly lower mean intensity of FLAG labeling than vehicle. ANOVA, F(2,6) = 58, P = 0.0001. *P<0.05 vs. vehicle.
2.6. Surface expression of α4Fβ2δ increases in neurons after treatment with gaboxadol or GABA plus THP
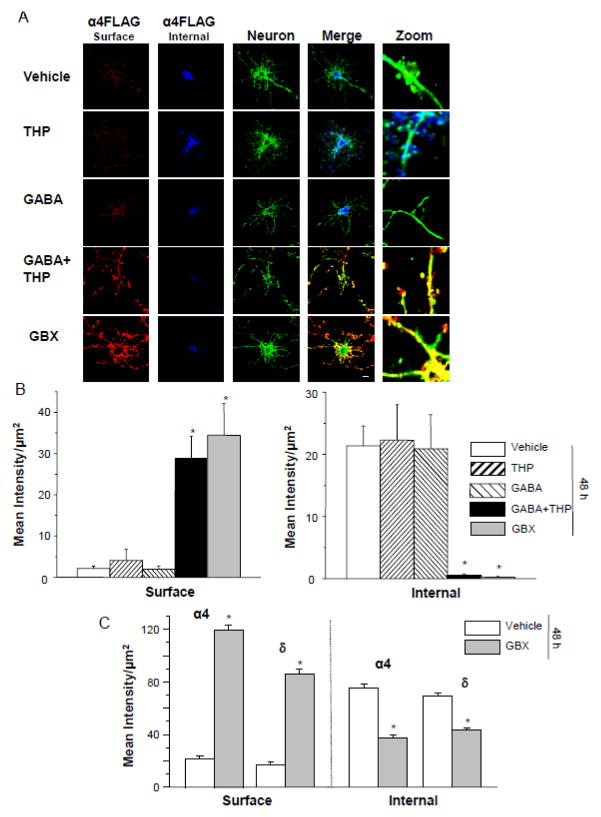
Dissociated hippocampal cells were transfected with α4F, β2 and δ cDNA after 8 d in culture and treated with either gaboxadol or GABA plus THP for 48 h. After administration of the primary antibody to probe for surface FLAG expression, neurons were permeabilized and fixed, and intracellular FLAG labeling was investigated in the same neurons. The findings in neurons substantiate the results in HEK-293 cells showing a 30- to 35-fold increase in surface labeling after 48 h treatment with gaboxadol or GABA plus THP (P<0.05, Fig. 7). In contrast, surface α4F labeling was almost undetectable in neurons treated with GABA or THP alone or vehicle. However, intracellular levels decreased as surface levels increased, such that intracellular FLAG labeling was highest in vehicle, GABA or THP-treated cells but almost undetectable in GABA plus THP and gaboxadol-treated cells (P<0.05). In general, intracellular staining appeared to be localized to the cell soma, while surface staining was localized to the processes of the neurons rather than the somatic region. Zoomed images of merged channels show the level of surface and intracellular α4Fβ2δ receptor (Fig. 7).
Figure 7. Expression of transfected α4Fβ2δ and native α4 and δ subunits in cultured hippocampal neurons.
A,B, Cultured neurons (8 d) were transfected with α4F, β2 and δ (48 h). A, Left to right, Representative confocal images (63X) of α4 FLAG labeling from the surface (546 λ, red, non-permeabilized) and internal (350 λ, blue, permeabilized) sites; neuron, F-actin labeling with phalloidin (488 λ green), merge, merged images; zoom, representative zoomed images. Immunostaining performed after 48 h treatment with (top to bottom) vehicle (0.01% DMSO), THP (100 nM), GABA (10 μM), GABA + THP or gaboxadol (GBX, 10 μM). Both GABA + THP and GBX treatments produced highly visible surface α4FLAG labeling, which was nearly undetectable in the other groups. Internal labeling was greatest in THP, GABA and vehicle-treated groups. Scale bar, 10 μm. B, Averaged data. ANOVA, Surface, F(4,40) = 15.2, P<0.0001; Internal, F(4,40) = 7.24, P = 0.0002. *P<0.05 vs. THP, GABA and vehicle groups. C, Untransfected neurons treated with GBX or vehicle for 48 h. Averaged data for surface and internal expression of native α4 and δ expression. Surface α4, t(36)=23.3; surface δ, t(36)=17.1; internal α4, t(36)=9.38; internal δ, t(36)=10.69; *P<0.0001 vs. vehicle.
2.7. Surface expression of native α4 and δ in neurons after gaboxadol treatment
To determine that overexpression of the subunits was not responsible for the increase in surface expression of α4Fβ2δ that we observed with high efficacy states, we immunostained untransfected, dissociated hippocampal neurons using primary antibodies for α4 and δ. Surface immunostaining for both α4 and δ increased 6-fold and 4-fold, respectively, after 48 h gaboxadol exposure (Fig. 7 C; P<0.0001). In contrast, internal immunostaining was 60–100% greater for vehicle-treated neurons than observed after 48h treatment with gaboxadol (P<0.0001). These findings suggest that high efficacy states increase surface expression of native α4 and δ GABAR subunits in a manner similar to the tagged, transfected receptor.
2.8. Acute gaboxadol responses of neurons treated 48 h with gaboxadol
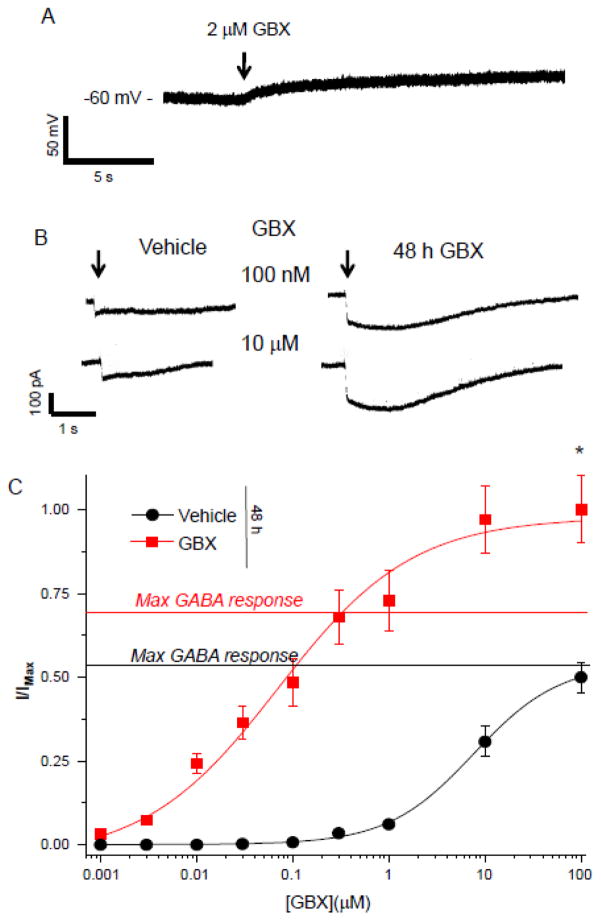
Neurons transfected with α4Fβ2δ were first tested for the polarity of GABA-gated current 48 h after transfection. To this end, we recorded the response of neurons to 2 μM gaboxadol using tight seal, cell-attached techniques (Perkins, 2006). The use of a high resistance seal (>1 GΩ) permits assessment of the polarity of the GABAergic current (Perkins, 2006). Gaboxadol produced an upward deflection (Fig. 8A), suggesting that GABA is depolarizing in these neurons. Neurons that were treated for 48 h with gaboxadol produced a significantly greater response to acutely applied gaboxadol across a concentration range, where the maximum response to gaboxadol was 2-fold greater than observed in vehicle-treated neurons (P<0.0001) and the EC50 was reduced by almost 100-fold (75 (22.6, 248.2) nM vs. 6 (1.71, 21) μM, Vehicle, P<0.05), consistent with an increase in δ-containing GABARs which have a higher sensitivity to gaboxadol than other GABAR sub-types (Brown et al., 2002; Meera et al., 2011). In addition, the ratio of the maximal gaboxadol response: the maximal GABA response was increased in 48 h gaboxadol-treated neurons (1.42 vs. 0.97, Vehicle, P<0.05). Taken together, these findings support the immunocytochemistry data and suggest that 48 h exposure to gaboxadol increases the expression of functional α4Fβ2δ receptors in neurons as it does in HEK-293 cells.
Figure 8. 48 h Gaboxadol treatment increases gaboxadol responsiveness of cultured neurons.
A, Representative trace, tight seal, cell-attached current clamp recording of a cultured neuron (8 d) transfected with α4F, β2 and δ (48 h). Gaboxadol (GBX) produced an upward deflection, reflecting a depolarizing response. (Representative of 5 cells.) B, Representative currents, whole cell voltage clamp recordings of responses to acutely applied GBX (100 nM, 10 μM). Neurons were treated with vehicle or 10 μM GBX for 48 h. C, Concentration-response curves of averaged data. 48 h GBX treatment increased the maximum response and shifted the curve to the left (EC50, Con, 6.0 (1.71, 21) μM; GBX, 0.075 (.023, .248) μM). Dashed line, maximum GABA response indicated for each group determined with the response to a saturating concentration of GABA (100 μM). t(19) = 4.8; *P<0.0001.
2.9. Reversal of the polarity of GABAergic current does not prevent increases in surface expression of α4Fβ2δ by 48 h gaboxadol treatment
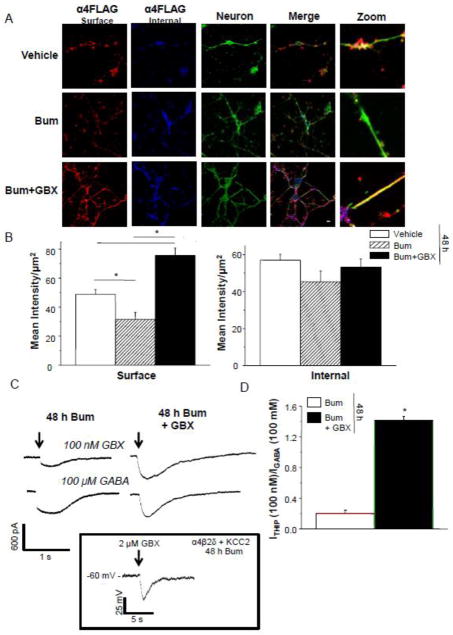
Because GABAergic current is hyperpolarizing in mature hippocampal pyramidal cells (Rivera et al., 1999), we created conditions to reverse the polarity of GABAergic current from depolarizing to hyperpolarizing. To this end, we co-transfected cDNA for KCC2 along with α4F, β2 and δ 48 h before testing. KCC2 is the K+-Cl− co-transporter which produces hyperpolarizing GABAergic current because it maintains a low intracellular Cl− concentration by extruding Cl− (Payne, 1997). In addition, we incubated neurons for 48 h with 10 μM bumetanide which blocks NKCC1 (Payne, 1997), the Na+-K+-Cl− cotransporter which is responsible for the depolarizing nature of GABAergic current in immature neurons (Rivera et al., 1999). This procedure successfully reversed the polarity of Cl− current, assessed by the voltage change in response to 2 μM gaboxadol, recorded with tight seal, cell-attached techniques in current clamp mode (Perkins, 2006), as described above. In this case the voltage response was a downward deflection (Fig. 9 Inset), reflecting a hyperpolarizing response to the GABA agonist. Interestingly, co-transfection of KCC2 with α4Fβ2δ produced highly visible surface FLAG labeling, suggesting increased expression compared to neurons without KCC2. Administration of bumetanide to reverse the polarity of GABAergic current significantly reduced surface labeling by about 30% (P<0.05, Fig. 9) compared to vehicle.
Figure 9. Reversal of the polarity of GABAergic current does not prevent gaboxadol effects on α4Fβ2δ expression.
Cultured neurons (8 d) were transfected with KCC2 in addition to GABAR subunits α4F, β2 and δ and treated with vehicle or 10 μM bumetanide (Bum) for 48 h to block NKCC1 activity. A, Left to right, representative confocal images (63X) of α4 FLAG labeling from the surface (546 λ, red, nonpermeabilized) and internal (350 λ, blue, permeabilized) sites; neuron, phalloidin (488 λ, green), merge, merged images; zoom, representative zoomed images. Immunostaining performed after 48 h treatment with (top to bottom) vehicle (0.01% DMSO), Bum (10 μM), Bum + gaboxadol (GBX, 10 μM); scale bar, 10 μm. 48 h GBX increased surface fluorescence intensity compared to vehicle. B, Averaged data for surface (left, ANOVA, F(2,33) = 83, P<0.0001) and internal (right, ANOVA, F(2,33) = 1.82, P = 1.82) staining. *P<0.05 vs. vehicle. C, Representative traces, whole cell voltage clamp recordings of responses to acutely applied GBX (100 nM) and GABA (100 μM), arrows. D, Averaged data, expressed as a ratio of the response to GBX relative to the response to GABA (IGBX/IGABA). 48 h GBX treated cells exhibited a greater IGBX/IGABA suggesting increased expression of δ-containing GABAR. t(8) = 12.2; *P<0.0001. Inset, Representative trace, tight seal, cell-attached current clamp recording. GBX (2 μM) produced a downward deflection, reflecting a hyperpolarizing response. (Representative of 5 cells).
Under conditions of hyperpolarizing GABAergic responses, 48 h treatment of neurons with gaboxadol significantly increased cell surface expression (Fig. 9B,C) more than 2.5-fold compared to bumetanide-treated neurons (Fig. 9B). Intracellular staining intensity was not significantly different between groups, but the localization of staining differed (Fig. 9A). The gaboxadol treated group exhibited highly visible staining localized to both the soma and processes, whereas immunolabeling in the other groups was mostly localized to the soma.
The increased surface expression of α4Fβ2δ observed after 48 h treatment with gaboxadol was also associated with an increased response of neurons to 100 nM gaboxadol (Fig. 9), which at this concentration is selective for δ-containing GABAR (Meera et al., 2011). Neurons were recorded using whole cell voltage clamp techniques in response to acutely applied 100 nM gaboxadol and 100 μM GABA, and the responses expressed as a ratio (Igaboxadol/IGABA) to reflect the population of δ-containing GABAR out of the total population. The Igaboxadol/IGABA was 7-fold greater after 48 h gaboxadol treatment than in vehicle-treated neurons (Fig. 9), suggesting a significant (P<0.0001) increase in functional α4Fβ2δ receptors. These findings suggest that 48 h exposure to the high efficacy agonist gaboxadol increases expression of α4Fβ2δ in neurons regardless of whether GABAergic current is depolarizing or hyperpolarizing.
2.10. Effect of GABA plus THP treatment on the rate of removal of α4Fβ2δ GABARs from the surface membrane
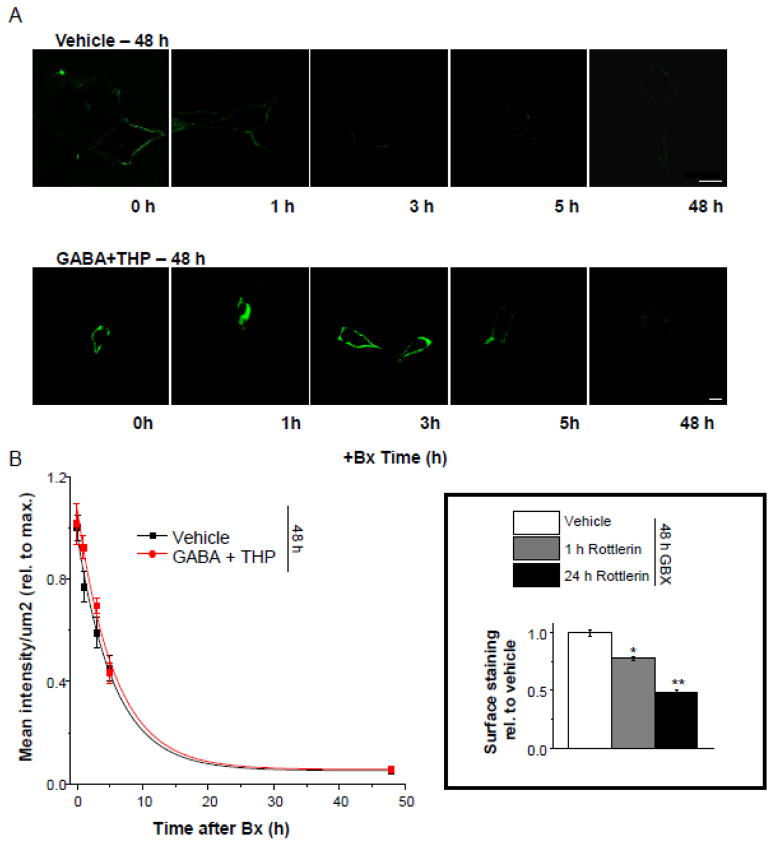
Increased expression of α4β2δ GABARs induced by GABA plus THP treatment couId be due either to increases in the rate of insertion of receptors into the membrane (Connolly et al., 1996; Kittler et al., 2000) or to decreases in the rate of receptor removal from the membrane via endocytosis. Therefore, we examined the rate of receptor removal from the surface membrane in transfected HEK-293 cells treated either with GABA plus THP or vehicle for 48 h. To this end, we blocked receptor insertion for varying lengths of time (1, 3, 5 or 48 h) with application of botulinum toxin b (5 nM) (Schenk et al., 2003) and assessed surface labeling of α4F using the immunofluorescence techniques described above. Results were plotted as a function of surface labeling versus time of exposure to botulinum toxin b, and the curve fitted with a single exponential decay function.
The time constant for the decrease in surface labeling of α4F after blockade of receptor insertion was similar for both vehicle and GABA plus THP-treated cells (τ, 5.7 ± 0.64 h, Vehicle; τ, 5.71 ± 0.89 h, GABA + THP; t(8) = 0.05, P = 0.9613). These results suggest that the rate of receptor removal (likely involving endocytosis) is not altered by GABA+THP treatment, thereby suggesting that receptor removal is not the underlying mechanism for the increase in surface expression of the α4Fβ2δ receptor produced by 48 h exposure to GABA+THP, but rather is likely due to an increased rate of receptor insertion into the cell membrane.
2.11. Rottlerin decreases α4FLAG surface labeling
Because recent reports have suggested that α4βδ GABARs co-localize with protein kinase C-δ (PKC- δ) (Messing et al., 2007), we tested its role in trafficking of α4Fβδ GABARs with the use of rottlerin, which inhibits PKC- δ activity (Chew et al., 2011), administered for 1 or 48 h to HEK-293 cells transfected with α4β2δ and treated with gaboxadol for 48 h. This PKC- δ inhibitor significantly (P<0.05) reduced α4F surface labeling by 1 h after administration, reaching peak reductions after 48 h, when surface labeling was decreased by more than 50% compared with gaboxadol treatment alone (P<0.05). These findings suggest that PKC- δ plays a role in gaboxadol-mediated surface trafficking of α4β2δ GABARs
3. Discussion
This study shows that cell surface expression of α4Fβδ GABARs is increased by GABAR modulators and agonists which increase the efficacy of this receptor. 48 h exposure of HEK-293 cells or neurons to the neurosteroid THP along with GABA or to the GABA agonists gaboxadol and β-alanine produced significant increases in expression of α4Fβ2δ GABARs, which were tightly correlated with increases in current gated by GABA agonists. Expression of native α4 and δ GABAR subunits was also increased by gaboxadol, suggesting that the effect of this high efficacy agonist does not depend upon overexpression of the receptor. In contrast, 48 h exposure to GABA alone, a partial agonist at these receptors, failed to increase cell surface expression of α4β2δ GABARs. The mechanism for this steroid-induced increase in receptor expression is most likely via insertion of receptors in the cell membrane because the rate of disappearance of the receptor was not decreased when receptor insertion was blocked.
3.1. Relevance of GABA concentration
The 10 μM concentration of GABA used produces maximal current at α4β2δ GABARs, which allowed us to directly test increases in efficacy with THP. However, THP used in combination with GABA at a 1 μM concentration was also effective and produced similar effects on receptor expression as 10 μM GABA. The 1 μM concentration of GABA is also physiologically relevant as it is believed to represent ambient levels of GABA (Wu et al., 2001) which would come into contact with extrasynaptic α4βδ GABARs. Subtle increases in gaboxadol-gated current were seen, however, after treatment with GABA(10 μM) plus THP compared to GABA(1μM) plus THP suggesting that there may be some effect of GABA concentration on the expression of this receptor.
3.2. Time course of THP effects on α4Fβ2δ expression
Our findings suggest that cell surface expression of α4Fβ2δ is significantly increased by 30 min, but reaches peak levels 48 h after treatment with GABA plus THP. This is likely a peak effect because our earlier studies revealed that levels of native α4- containing receptors are maximal after 48 h exposure to THP or its parent compound progesterone, as determined by both in vivo and in vitro studies in hippocampus and cultured neuroblastoma cells, respectively (Gulinello et al., 2001; Shen et al., 2005; Zhou and Smith, 2007; Zhou and Smith, 2009). Rapid effects of neurosteroids on α4βδ GABAR expression have also been reported, however, where 30 min exposure to the related steroid THDOC increases tonic current in dentate gyrus granule cells, which is mediated by increased expression of α4βδ GABARs (Maguire and Mody, 2007). Interestingly, withdrawal from THP also increases expression of α4βδ GABARs (Griffiths and Lovick, 2005; Shen et al., 2007; Shen et al., 2010; Smith et al., 2006; Sundstrom-Poromaa et al., 2002), suggesting that the regulation of expression of this receptor is complex but highly responsive to the presence of neurosteroids.
3.3. High efficacy GABA agonists
In addition to GABA plus THP, two GABA agonists which have high efficacy at δ-containing GABARs, gaboxadol and β-alanine (Bianchi and Macdonald, 2003; Brown et al., 2002) produced significant increases in α4β2δ expression. In contrast to gaboxadol, which is synthetic, β-alanine is an endogenous transmitter (Mathers et al., 2009; Tiedje et al., 2010). β-Alanine is produced in the brain either as a by-product of the reaction pyruvate to L-alanine or as the product of deamination and carboxylation of the pyrimidine uracil (Tiedje et al., 2010). It is released in areas such as hippocampus by a Na+-dependent transport mechanism (Tiedje et al., 2010). Average reported levels of this amino acid are in an effective range to increase expression of α4β2δ GABARs (30 – 80 μM) (Martin del Rio et al., 1977; Tiedje et al., 2010), but are highly variable and regulated by GABA transporter activity (Tiedje et al., 2010). Levels of this amino acid can also be increased by oxidative stress and hypoxia (Saransaari and Oja, 1999). Interestingly, brain trauma and excitotoxic insults also increase α4βδ expression (Mtchedlishvili et al., 2010; Santhakumar et al., 2010), where β-alanine could serve as a possible mechanism. The fact that highly efficacious agonists also increase α4β2δ expression suggests that the effect of THP results from increased receptor efficacy rather than a steroid-specific effect.
Our findings in HEK-293 cells were substantiated in cultured neurons, further suggesting that efficacious compounds increase cell surface expression of α4β2δ GABARs. It is especially noteworthy that findings are similar in both systems because HEK-293 cells do not have specific neuronal chaperone or anchoring proteins, and thus suggest that neuron-specific proteins are not required for trafficking of the receptor to the cell surface.
3.4. Tagged versus native α4 expression
The high efficacy agonist gaboxadol significantly increased expression of both tagged and native α4 GABAR subunits suggesting that its effect is not an artifact of overexpression of the receptor. The magnitude of the increase was greater for the tagged receptor, which may simply reflect a difference in antibody affinity. Gaboxadolmediated increases in native α4 and δ expression likely reflects expression of the full α4βδ receptor based on our earlier in vivo studies showing increased expression of native α4βδ receptors in response to 48 h THP treatment (Shen et al., 2005). Expression of the untagged α4 subunit was increased ~40% more by 48 h gaboxadol treatment than was δ, however, which may suggest the formation of native α4β 2 GABARs because α4 can co-express with either 2 or δ (Sur et al., 1999). Alternatively, the difference in apparent expression level may be the result of reduced affinity of the δ primary antibody. Interestingly, internal expression of both α4 and δ was higher in vehicle-treated than in gaboxadol-treated neurons, suggesting that rates of transcription and translation of these subunits are high in the absence of high efficacy states. This finding suggests that regulation of receptor expression appears to be mediated via trafficking to the surface membrane.
3.5. Functional expression of α4βδ
Functional expression of α4βδ GABARs was confirmed in both cell types with whole cell patch clamp electrophysiology. 48 h treatment with GABA plus THP or the high efficacy agonists yielded a greater response to the GABA agonist gaboxadol, which has greater potency and efficacy at α4β2δ GABARs compared to other receptor subtypes (Brown et al., 2002; Meera et al., 2011). In both cell types transfected with α4β2δ, the response to 100 nM gaboxadol, a concentration selective for δ-containing GABARs (Meera et al., 2011), was significantly greater following 48 h gaboxadol treatment compared to control. In the neuronal population, unlike the HEK-293 cells, the heterogeneous native GABAR population would also contribute to the recorded current. Thus, for the neurons, the data was presented as the ratio of the response to this low dose relative to the response to a high dose of GABA, thereby reflecting the relative ratio of δ-containing GABARs out of the total GABAR population. This was also suggested by the lower EC50 for gaboxadol observed after 48 h gaboxadol treatment compared to vehicle treatment, which would reflect a larger population of δ-containing GABARs (Brown et al., 2002; Meera et al., 2011).
3.6. Use of the 3X FLAG-tagged α4 construct
In this study, we established the successful use of a 3X FLAG-tagged α4 GABAR subunit as a reporter for surface expression studies in HEK-293 cells and hippocampal neuronal cultures. Use of α4 and α4(3XFLAG) in conjunction with β2 and δ resulted in similar GABA concentration-response curves, thus confirming that the 3XFLAG tag does not interfere with receptor function and produces a strong signal with minimal background. Although other labs have reported expression of the binary α4β3 (Meera et al., 2011), they generate an EC50 which is significantly greater than that of α4β3δ (~0.4 μM), suggesting that we are measuring α4β2δ rather than α4β2. Furthermore, the α4Fβ2δ yielded a maximal gaboxadol-generated current greater than the maximal GABA-gated current in the presence of 1 μM ZnCl2 (Meera et al., 2011), also confirming the expression of δ-containing GABAR distinct from dimers such as α4β2.
3.7. Potential mechanism for THP-induced α4βδ expression
Our results suggest that GABA plus THP treatment likely increased surface expression of α4β2δ by increasing the rate of receptor insertion into the membrane. Blockade of receptor insertion with botulinum toxin b resulted in rates of removal of α4F signal from the surface which were nearly identical for cells treated either with vehicle or GABA plus THP. If GABA plus THP treatment were increasing α4β2δ expression by decreasing receptor endocytosis, then the rate of receptor decline in the presence of botulinum toxin should have been reduced compared to vehicle treatment. Our findings are also consistent with reports showing that the stability of δ-containing GABARs in the membrane is on the order of hours rather than minutes, as it is for γ2-containing GABARs (Joshi and Kapur, 2009; Thomas et al., 2005).
The 48 h timecourse necessary for maximal receptor expression in the presence of high efficacy agonists/modulators suggests multiple processes are required. Recent reports suggest that surface expression of δ can be increased to peak levels by brainderived neurotrophic factor (BDNF) by 6 h after administration (Joshi and Kapur, 2009). In contrast, however, many transfection studies report that expression of δ-containing GABAR takes longer than for other GABAR subtypes (Wallner et al., 2003) and results in reduced currents. In our study, we waited an initial 24 h in order for protein synthesis to occur in order to directly assess receptor trafficking distinct from transcription or translation.
Our findings suggest a similar timecourse for decreases in ER labeling as observed for increases in cell surface labeling. Others have found that associated proteins in the ER, such as PLIC-1/2, allow assembled receptor to traffic out of the ERgolgi complex to the cell membrane (Jacob et al., 2008). Although these are findings for the α1β2γ2 synaptic receptor, similar mechanisms may apply to extrasynaptic targeting.
3.8. Possible mechanisms which initiate α4βδ trafficking
The initial trigger that allows high efficacy agonists/modulators to initiate receptor trafficking from the ER to the cell membrane is not known. Receptor efficacy has been correlated with conformational changes in a variety of receptor types. In glycine receptors, which are members of the cys-loop family of ionotropic receptors to which GABARs also belong, binding of a high efficacy agonist produces conformational changes in the loop 2 region of the receptor leading to a fast transition to a pre-open “flip” state (Pless and Lynch, 2009), while GABA modulators have been shown to produce allosteric changes in the GABA binding site (Sancar and Czajkowski, 2011). Thus, similar types of conformational changes in the α4β2δ could be the initial step, leading to activation of intracellular pathways that would influence receptor trafficking.
High efficacy states would also lead to increases in Cl− flux at those receptors which initially reach the cell membrane that could directly act to regulate trafficking. Our findings in neurons suggest that gaboxadol is effective at increasing α4βδ expression regardless of the direction of Cl− flux. However, our data also surprisingly show that cotransfection of KCC2 along with α4β2δ increases receptor expression to levels seen with high efficacy agonists. Use of bumetanide to block endogenous NKCC1 activity reduced receptor expression, suggesting the possibility that spontaneous inward current through α4β2δ, which occurs in homologous receptors (Hadley and Amin, 2007), may play a role in receptor expression. Cl− flux may result in binding of Cl− to putative modulatory sites, which have been identified in α4 (i.e., Arg 353) (Shen et al., 2007), as well as in other receptor systems (Chen et al., 2006).
Alternatively, GABA agonists and THP may enter the intracellular space where they may act as chaperone molecules. The latter possibility is supported by findings from a recent study showing that GABA increases cell surface expression of a mutant α1β2γ2 GABAR which is retained in the ER, while facilitation of surface expression of wild-type receptors was prevented by brefeldin A, a compound which blocks trafficking in the early secretory pathway (Eshaq et al., 2010).
3.9. Role of phosphorylation
Recent studies have established that phosphorylation of α4 at residue Ser 443 in the TM3–TM4 intracellular loop increases cell surface expression of the α4β2δ receptor (Abramian et al., 2010), while earlier reports noted that PKC-δ expression patterns in the CNS overlapped with expression patterns of α4 and δ (Choi et al., 2008). Indeed, out findings are consistent with a role for PKC-δ, which is highly expressed in hippocampal neurons and HEK-293 cells (Choi et al., 2008), because rottlerin prevented the increase in cell surface expression observed with GABA plus THP treatment. Rottlerin causes potent and highly selective inhibition of PKC-δ with an IC50 of 3–6 μM, an effect 5–10 fold more potent than for PKC-α or PKC-β and nearly 13 to 33 fold more potent than for PKC-ε, χ, or γ (Gschwendt et al., 1994). Rottlerin can affect other kinases such as CAM kinase II (Parmer et al., 1997), but these kinases are not expressed in HEK-293 cells. One recent report (Soltoff, 2007) has suggested that rottlerin can uncouple mitochondrial oxidation, but other studies have not confirmed this effect (Wermuth et al., 2011). In contrast, recent studies (Chew et al., 2011; Wermuth et al., 2011) have shown similar effects of rottlerin and siRNA on PKC- δ effects, suggesting that rottlerin is an effective blocker of this PKC isoform. Therefore, it is likely that phosphorylation plays a role in surface expression of α4β2δ GABAR.
3.10. α4 promoter
Unlike receptor trafficking, transcriptional regulation of α4 expression has been well studied. The α4 promoter has been identified (Ma et al., 2004) and contains multiple transcription sites, suggesting that transcription of this subunit may be regulated by multiple mechanisms. BDNF can activate the promoter via early growth factor-3 (egr3) (Roberts et al., 2005; Roberts et al., 2006), while other studies have shown that it can be regulated by heat shock factor 1 (Pignataro et al., 2007).
3.11. Behavioral and clinical implications
Fluctuations in THP levels at puberty, across the ovarian cycle and pregnancy alter expression of the α4βδ GABAR in the limbic system (Lovick et al., 2005; Maguire et al., 2005; Maguire and Mody, 2009; Sanna et al., 2009; Shen et al., 2007). This receptor has been implicated in a number of neuropsychiatric disorders, including rodent models of premenstrual syndrome (Smith et al., 2006), post-partum dysphoria (Maguire and Mody, 2009), catamenial epilepsy (Maguire et al., 2005), as well as panic disorder (Lovick, 2000) and pubertal mood swings (Shen et al., 2007). In addition, recent genetic studies have suggested a correlation between α4 and/or δ expression with other psychoaffective disorders, including major depression and schizophrenia (Damgaard et al., 2011; Feng et al., 2010). Therefore, understanding the mechanisms which regulate expression of this receptor may suggest novel therapeutic strategies for these disorders.
4. Experimental Procedures
4.1. Cell culture
4.1.1. HEK-293 Cells
Human embryonic kidney (HEK) 293 cells (ATCC, Manassas, VA) were grown in Dulbecco’s Modified Eagle’s Medium (DMEM/F-12, Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS, Sigma, St. Louis, MO), penicillin (100IU/ml) and streptomycin (100μg/ml) (Invitrogen, Carlsbad, CA) on MatTek glass bottom dishes (MatTek Corp, Ashland, MA) at 37°C in a humidified incubation chamber (5% CO2, 95% O2).
4.1.2. Neurons
Embryonic day 18 (E18) dissociated rat hippocampal cells were removed from timed pregnant Sprague-Dawley rats (Hilltop, Scottdale, PA) following an established protocol (Banker and Goslin, 1998, Brewer, 1999, Brewer, 1997, Brewer and Cotman, 1989) and plated on poly-D-lysine (Sigma, St. Louis, MO) coated glass coverslips. The cells were fed with Neurobasal media (Gibco BRL, Grand Island, NY) supplemented with B-27 and 0.5 mM L-glutamine (Gibco BRL) and grown at 37°C in a humidified incubation chamber (5% CO2, 95% O2) for 8 days. The media was replenished every 2 days. 10 μM of cytosine arabinofuranoside (Sigma) was added after 3 days in culture for 24 h to inhibit non-neuronal cell proliferation. Animal use was in accordance with EC Directive 86/609/EEC for animal experiments
4.2. cDNA
cDNA for GABAR subunits mouse α4 (N.L. Harrison, Columbia U., New York), rat β2 (J. Bracamontes, Washington U, St. Louis) and human δ (K. Wafford, Merck, Sharp and Dohme, UK), and rat KCC2 (J. Payne, U.C. Davis) were used for all studies. (Mouse, rat and human cDNA sequences for β2 are nearly identical.) The expression vector was pcDNA3.1.
4.3. 3XFLAG Constructs
We used a 3XFLAG epitope (FLAG sequence, DYKDDDDK) at the C terminus of the GABAR α4 subunit for immunocytochemical detection. Briefly, α4 fusion constructs were made using sub-cloning polymerase chain reaction (PCR, Phusion high-fidelity PCR kit, Finnzymes, Dharmicon, Lafayette, CO) to add restriction sites (Not1 and BAMH1, New England Biolabs (NEB, Ipswich, MA)) for sub-cloning, to add a Kozak consensus sequence to facilitate protein translation and to remove the stop codon. The PAGE purified PCR primers (Integrated DNA Technologies, Carlsbad, CA) were: 5′-tag tat gcg gcc gc gcc acc acc atg gtt tct gtc cag aag gta ccc gcg (forward) and 5′-gat tgc gga tcc cat tag act ttc tga ttt ctc cat gg (reverse). After the PCR reaction, the DNA was precipitated with Na-Acetate- ethanol, digested with the appropriate restriction enzymes (NotI and BAMHI) and run on a 0.6% agarose gel. Sub-cloning to the p3XFLAG-CMV- 14 expression vector (Sigma, St. Louis, MO) followed standard procedures using the Min-Elute PCR Purification Kit and Quick Ligation Kits (NEB, Ipswich, MA). Successful cloning was verified by double stranded DNA sequencing (Genewiz, S. Plainfield, NJ). The p3XFLAG vector uses a CMV promoter rather than the endogenous α4 promoter. Previous studies have shown that use of the 3XFLAG reporter produces a greater signal:noise ratio than use of a single FLAG reporter (Hernan et al., 2000).
4.4. Transfection
Cells were transfected with α4 or α4(3xFLAG, F), β2 and δ cDNA (1:1:1; α4(F):β2:δ) using the Nucleofector method (Amaxa/Lonza, Walkersville, MD) with reagents and protocols optimal for either HEK-293 cells or cultured neurons. (Transfection of 1:1:0.1 yielded no GABA-gated current when recorded with whole cell patch clamp procedures; therefore, this transfection ratio was not used.) In some cases, neurons were also transfected with rat KCC2. Cell Nuceofector solution was used for HEK-293 cells, and Neuron Nucleofector solution was used for dissociated hippocampal cells. A total of 5 μg of cDNA was used per 100 μl of Nucleofector transfection reagent. For electrophysiology experiments, both HEK-293 and dissociated hippocampal cells were additionally co-transfected with 2 μg eGFP cDNA (Amaxa/Lonza) for detection of transfected cells under fluorescence microscopy. To determine transfection efficiency a subset of cells for immunocytochemical analysis were transfected with only eGFP cDNA (2 μg). The transfection efficiency was 70–80% and did not vary across treatments. The final surface density of plated HEK-293 cells was 10,000 cells/plate and approximately 3×105 neurons/cover slip.
4.5. Drug Delivery
All drugs were made in sterile double distilled water (ddH20) containing 1% DMSO (100x) unless otherwise noted. Vehicle conditions are cells that were supplied with ddH20 and DMSO to a final amount of 0.01% per 3ml of media. After 24 h of transfection, media was removed from cells and replaced with the indicated drug for the length of 48 h unless otherwise noted. In the case of time-course experiments, agonists, modulators and/or secondary drugs (botulinum toxin b) were added for varying durations such that all time-based groups were harvested at the end of the 48 h drug incubation period. This procedure ensured that all groups had equivalent time for protein synthesis and that tissue for immumocytochemistry could be processed in parallel. Final concentrations were as follows: GABA (1 and 10 μM), gaboxadol (10 μM), β-alanine (10 μM, 50 μM and 1 mM), THP (100 nM) and botulimum toxin B (5 nM). All drugs except for THP (Steraloids, Newport, RI) and botulinum toxin b (List Biological Laboratories, Inc., Campbell, CA) were from Sigma Chemical Co. (St. Louis, MO). We used an incubation period with a maximal timebase of 48 h because previous findings in our lab established that, in vivo, the α4 and δ subunits were increased only at 48 h and did not change further at 72 h (Zhou and Smith, 2009).
Immunocytochemistry
4.6. Non-Permeabilized Cells (Surface Staining)
4.6.1. HEK-293 Cells
Cells were probed using an adapted immunocytochemistry protocol for cell surface expression in non-permeable conditions in which all steps are performed on ice with washes using ice cold HBS (Eshaq et al., 2010). Briefly, the live cells were incubated with mouse (Ms) monoclonal anti-FLAG M2 primary antibody (1:50–1:100) (Sigma, St. Louis, MO) followed by incubation with goat (Gt) anti-mouse Alexa Fluor 488 IgG (1:200) or F(ab′)2 (1:500) secondary antibody (Molecular Probes, Grand Island, NY). The cells were then fixed with 4% paraformaldehyde and stained by either DAPI (1:1000), a nuclear stain used as a cell marker, or permeabilized with 0.1% Triton X- 100, and incubated with TO-PRO 3 (1:1000) a cell and nuclear marker (Molecular Probes). Because the use of the IgG secondary antibody increased the measured mean intensity above values measured when F(ab′)2 secondary antibody was used, we expressed the change in immunofluorescence for these experiments as a ratio, relative to vehicle control.
To verify the selectivity of membrane staining and structural stability, the cells were incubated with Wheat Germ Agglutinin (WGA) conjugated to Alexa-594 (Invitrogen, Carlsbad, CA) for the length of time of the non-permeable conditions followed by DAPI (Fig. 3H).
4.6.2. Neurons
Immunofluoresence was detected with monoclonal Rb anti-FLAG primary antibody (clone SIG1–25) (Sigma, St. Louis, MO) (1:50) overnight at 4°C. Native subunits were probed with polyclonal Gt α4 (Santa Cruz Biotechnology, Inc, Santa Cruz, CA) (1:1000) and polyclonal Rb δ (Novus Biologicals, Littleton, CO) (1:1000) primary antibodies. The following day, Gt anti-rabbit IgG Alexa-546 (1:100), anti-goat IgG Alexa-568 (1:500), or anti-rabbit F(ab′)2 Alexa-568 (1:500) secondary antibody was added on ice, the cells were fixed with 4% paraformaldehyde and incubated with anti-phalloidin antibody conjugated to Alexa-488 (Invitrogen) (1:500), which binds to F-actin and was used as a neurite marker (Cuitino et al., 2010, Farias et al., 2009). The cells were mounted with ProLong® Gold Antifade Reagent (Invitrogen) on Superfrost®; Plus Microscope slides (Fisher Scientific).
4.7. Permeabilized Cells (Intracellular Staining)
4.7.1. HEK-293 Cells
Intracellular staining was performed under permeabilized conditions at room temperature. Cells were fixed with 4% paraformaldehyde/4% sucrose and permeabilized with 0.1% Triton X-100. The cells were then blocked with 10% BSA prior to probing with Ms monoclonal anti-FLAG M2 primary antibody (1:100) (Sigma, St. Louis, MO) and Rb anti-calnexin antibody (1:500) (AbCam, Cambridge, MA) (a marker of the endoplasmic reticulum (ER)). The cells were then incubated with Gt anti-Ms IgG Alexa-488 secondary antibody (1:200) and Gt anti-Rb IgG Alexa-546 secondary antibody (1:100) (Molecular Probes, Grand Island, NY).
4.7.2. Neurons
Dissociated hippocampal cell cultures were stained using the permeabilized method after undergoing probing under non-permeabilized conditions (see methods above). Briefly, after cells were probed with monoclonal Rb anti-FLAG primary antibody (clone SIG1–25, Sigma, St. Louis, MO) and Gt anti-rabbit IgG Alexa-546 secondary antibody for cell surface FLAG staining, cells were fixed with 4% paraformaldehyde and permeabilized with 0.1% Triton X-100 at room temperature. The cells were blocked with 10% BSA and then probed with Ms monoclonal anti-FLAG M2 primary antibody (1:100), and Alexa-350 secondary antibody (1:500) to detect internal staining. Native subunits were probed with the same protocol except using polyclonal Gt α4 (1:1000) and polyclonal Rb δ (1:1000) primary antibodies, and anti-goat IgG Alexa-350 and antirabbit F(ab′)2 Alexa-350 secondary antibodies. The anti-phalloidin antibody conjugated to Alexa-488 (Invitrogen, Carlsbad, CA) (1:500) was added followed by mounting of cover slips to Superfrost®; Plus Microscope slides using ProLong® Gold Antifade Reagent.
4.8. Imaging
All images were captured on the Zeiss 710 or 510 inverted confocal microscope at 63X oil or 40X as single image (Microscopy Core of NYU Langone Medical Center, NY, NY). Analyses of images were performed with the Image J program from NIH (Daniel et al., 2010, Cuitino et al., 2010) using the ROI (Region of Interest) manager in the Zen 2008 Light Edition program. Representative cells were chosen for analysis. The intensity of the fluorescent pixels around the circumference of the cell was calculated as the integrated density/total area (mean intensity/μm2) after determination of a threshold intensity and subtraction of background.
4.9. Determination of the rate of receptor removal from the membrane surface
In order to compare the rate of removal of surface expressing α4Fβ2δ GABARs under different treatment conditions, transfected HEK-293 cells treated with vehicle or GABA plus THP for 48 h were also treated with botulinum toxin b (5 nM) for varying lengths of time (1, 3, 5 or 48 h) to block insertion of newly formed receptors. These treatments were timed such that all cells were harvested at the same time so that tissue could be processed in parallel using immunofluorescence as described above. Surface immunofluoresence intensity of α4F was plotted as a function of time of exposure to botulinum toxin b for both treatment groups. Analysis of the rate of receptor removal was accomplished with the least squares fit to the exponential decay function, y = A1^exp(−x/τ) + y0, where A1 is the amplitude, y0 is the offset and τ is the decay time constant (Origin 8.5.1, Microcal, Piscataway, NJ).
4.10. Electrophysiology
4.10.1. Pharmacological tests
Currents were recorded in response to GABA or gaboxadol at room temperature (21–22°C) at a holding potential of −50 mV using whole cell voltage clamp techniques on a Nikon Diaphot inverted microscope. The bath solution contained (in mM): NaCl 120, CsCl 5, CaCl2 2, MgCl2 1, Hepes 10 and glucose 25, pH 7.4, 320 mOsmol. Patch pipets (filament-capillary tubes, Sutter Instruments, Novato, CA) were fabricated from borosilicate glass using a Flaming-Brown puller to yield open tip resistances of 3 – 5 MΩ. The pipet solution contained (in mM): N-methyl-D-glucamine chloride 120, Cs4, BAPTA 5 (Calbiochem, San Diego, CA), Mg-ATP 5, and an ATP regeneration system (20 mM Tris phosphocreatine and creatine kinase). Currents were recorded using an Axopatch 1D amplifier (Axon Instruments, Union city, CA) filtered at 2 kHz (four-pole Bessel filter) and detected at 10 kHz (pClamp 8.2).
Recordings were carried out after incubating the cells in agonist-free bath solution for 1 h to remove any agonist present during the 48 h incubation. Cells were recorded in the presence of 1 μM ZnCl2 to block current from binary receptors which may have formed (Meera et al., 2011). Agonist delivery was accomplished with a solenoid-controlled micropipette array 50 μm from the cell to deliver agonist for approximately 400–500 ms exposure times with 200–250 ms onset of application (Smith et al., 1998). Analysis of peak current was accomplished with pClamp 10.1 (Axon Instruments, Union City, CA) and Origin (Microcal, Piscataway, NJ) software packages. In all cases, 2–3 current traces were averaged for each agonist or agonist + THP group. Mean values were plotted as a semi-logarithmic function as a percentage of the maximal current generated by gaboxadol or GABA (In/IMAX × 100), where In is the current for each concentration of agonist, and IMAX is the maximal current generated either by gaboxadol or GABA. The curve was fitted by the least squares method as a sigmoidal function using the logistic equation, I = A2 + (A1+A2)(1+x/x0)^p, where I is the current for the indicated concentration x, A1 is the minimum current, A2 is the maximal current at a saturating agonist concentration, x is the concentration of agonist, x0 is the EC50 (concentration of agonist needed to produce a response that is 50% of the maximal response) and p is the Hill coefficient.
4.10.2. Determination of the polarity of GABAergic current
In order to determine whether the current gated by GABAR expressed in cultured neurons was hyperpolarizing or depolarizing, we used high resistance (>1 GΩ, cell attached recordings in current clamp mode (Mason et al., 2005; Perkins, 2006). This technique does not disturb the intracellular Cl− milieu, and the high resistance of the pipet seal permits assessment of the voltage change produced by the small amounts of current generated by a GABA agonist when the cell is at its resting membrane potential with no current passing through the pipet (150 mM NaCl). In this manner we recorded the voltage response to 2 μM gaboxadol, applied with a solenoid-controlled pipet (described under the electrophysiology methods). This technique has been used successfully (Shen et al., 2007) to determine the polarity of GABAergic current. Neurons were tested after 8 d in culture, and 48 h after transfection of α4, β2 and δ cDNA. In some cases, neurons were also transfected with KCC2 and treated for 48 h with 10 μM bumetanide to block the Na-Cl− co-transporter NKCC1 (Payne, 1997).
4.11. Statistical Analysis
Values for the EC50 are shown as the mean with the 95% confidence limits indicated in pararentheses using Origin (OriginLab, Northampton, MA). The 95% t-based confidence interval was calculated using the standard error of the mean for the log EC50 and the critical value of the t distribution with the appropriate degrees of freedom. Other values are expressed as the mean ± SEM using Origin. Standard one-way ANOVA and Tukey’s tests were used to evaluate differences between >2 groups, while the Student’s t-test was used to determine the significance of data when comparing 2 groups, with significant differences established when P<0.05 using Origin. Each experiment was repeated at least 3 individual times for a sample size (n) of 3 or higher where indicated (Cumming et al., 2007). For HEK-293 cells, images were captured of three cells per plate in the glass bottom area of the dish for an n of 1. For neurons, an n of 1 was taken from pixel counts from 3 individual regions on one neuron. Three neurons were chosen per slide. Percentage calculations were taken from mean intensities as:
4.12. Western blot
HEK-293 cells were transfected with α4 or α4(3XFLAG) plus β2 and δ cDNAs. Cells were harvested 48 h after transfection, dissociated from the bottom of the culture dish and used to obtain crude membrane preparations. Membranes (20 μg of protein) were electrophoresed on a NuPAGE Bis-Tris 4–12% gradient gel (Invitrogen, Carlsbad, CA). Wet gel transfer to nitrocellulose membranes (Invitrogen, Carlsbad, CA) was followed by blocking of the membrane with 2% BSA. Rb anti-α4 (1:1,000) (Affinity Bioreagents, Golden, CO) or Ms anti-FLAG primary antibody (1:1,000) (Sigma, St. Louis, MO) was used for overnight incubation at 4°C. After secondary probing with either Gt anti-Rb (α4) (1:10,000) (Chemicon International, Temecula, CA) or Gt anti-Ms (FLAG) (1:30,000) (Novus Biologicals, Littleton, CO) conjugated to HRP (Horse Radish Peroxidase) for 1 h at room temperature, a chemiluminescent assay was performed using SuperSignal West Femto Maximum Sensitivity Substrate (Thermo Scientific, Pittsburgh, PA). Protein bands were visualized on Amersham Hyperfilm ECL (GE Healthcare, Piscataway, NJ). α4 was detected as a 67 kDa band (Smith et al., 1998).
Supplementary Material
Figure 10. The rate of removal of surface α4Fβ2δ expression after 48 h gaboxadol treatment.
Transfected HEK-293 cells were treated with vehicle or gaboxadol (GBX, 10 μM) for 48 h. Where indicated, botulinum toxin b (Bx, 5 nM) was added to block receptor insertion across a time-course (1, 3, 5, or 48 h). A, Representative confocal images (63X) of α4 FLAG staining (488 λ, green) after treatment with Bx for the indicated time periods, administered alone (top) or in addition to 48 h GBX (bottom). scale bars, 10 μm. B, Averaged data. The rate of disappearance of α4F labeling is plotted as a function of time (n=5 cells per time point). The plot was best fit to a single exponential decay function, which was not altered by GBX treatment. Inset, Effects of rottlerin (10 μM), a PKC- δ inhibitor, on α4FLAG surface labeling after 48 h treatment with gaboxadol. ANOVA, F(2,9) = 8.3, P = 0.0091; *P<0.05 vs. Vehicle; **P<0.05 vs. 1 h GBX.
Highlights.
Surface expression of α4FLAGβ2δ is increased by high efficacy states.
Internalized α4FLAGβ2δ is decreased by high efficacy states.
α4FLAGβ2δ expression is not dependent on the polarity of GABAergic current.
α4FLAGβ2δ expression is dependent upon PKC- δ.
THP increases α4FLAGβ2δ via increased receptor insertion rather than endocytosis.
Acknowledgments
The authors thank NL Leidenheimer for the immunocytochemistry protocol as well as Q H Gong and Y Deng (Microscopy Core of NYU Langone Medical Center, NY, NY) for helpful technical assistance. This work was supported by NIH grants DA09618 and AA12958 to SSS.
Abbreviations
- α4F
α4FLAG (3xFLAG tag)
- BDNF
Brain-derived neurotrophic factor
- DMSO
dimethyl sulfoxide
- FLAG
DYKDDDDK epitope
- GABAR
GABAA receptor
- GBX
Gaboxadol, 4,5,6,7-tetrahydroisoxazolo(5,4-c)pyridin-3-ol, THIP
- HEK-293
human embryonic kidney cell-293
- PKC
protein kinase C
- THP
3α-OH-5α[β]-pregnan-20-one, [allo]pregnanolone
- WGA
wheat germ agglutinin
Footnotes
Conflict of interest statement
We have no conflicts of financial interest in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Abramian AM, Comenencia-Ortiz E, Vithlani M, Tretter EV, Sieghart W, Davies PA, Moss SJ. Protein kinase C phosphorylation regulates membrane insertion of GABAA receptor subtypes that mediate tonic inhibition. J Biol Chem. 2010;285:41795–417805. doi: 10.1074/jbc.M110.149229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agis-Balboa RC, Pinna G, Zhubi A, Maloku E, Veldic M, Costa E, Guidotti A. Characterization of brain neurons that express enzymes mediating neurosteroid biosynthesis. Proc Natl Acad Sci. 2006;103:14602–14607. doi: 10.1073/pnas.0606544103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai D, Zhu G, Pennefather P, Jackson MF, Macdonald JF, Orser BA. Distinct functional and pharmacological properties of tonic and quantal inhibitory postsynaptic currents mediated by gamma-aminobutyric acid(A) receptors in hippocampal neurons. Molec Pharmac. 2000;59:814–824. doi: 10.1124/mol.59.4.814. [DOI] [PubMed] [Google Scholar]
- Belelli D, Casula A, Ling A, Lambert JJ. The influence of subunit composition on the interaction of neurosteroids with GABA(A) receptors. Neuropharm. 2002;43:651–661. doi: 10.1016/s0028-3908(02)00172-7. [DOI] [PubMed] [Google Scholar]
- Bianchi MT, Macdonald RL. Neurosteroids shift partial agonist activation of GABA(A) receptor channels from low- to high-efficacy gating patterns. J Neurosci. 2003;23:10934–10943. doi: 10.1523/JNEUROSCI.23-34-10934.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown N, Kerby J, Bonnert TP, Whiting PJ, Wafford KA. Pharmacological characterization of a novel cell line expressing human α4β3δ GABA(A) receptors. Br J Pharmacol. 2002;136:965–974. doi: 10.1038/sj.bjp.0704795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y, Wang R, Barot S, Weiss DS. Stoichiometry of a recombinant GABA-A receptor. J Neurosci. 1990;16:534–541. doi: 10.1523/JNEUROSCI.16-17-05415.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Talley EM, Patel N, Gomis A, McIntire WE, Dong B, Viana F, Garrison JC, Bayliss DA. Inhibition of a background potassium channel by Gq protein alpha-subunits. Proc Natl Acad Sci. 2006;103:3422–3427. doi: 10.1073/pnas.0507710103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew YC, Adhikary G, Wilson GM, Reece EA, Eckert RL. Protein kinase C (PKC) delta suppresses keratinocyte proliferation by increasing p21(Cip1) level by a KLF4 transcription factor-dependent mechanism. J Biol Chem. 2011;286:28772–28782. doi: 10.1074/jbc.M110.205245. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- Choi DS, et al. Protein kinase C delta regulates ethanol intoxication and enhancement of GABA-stimulated tonic current. J Neurosci. 2008;28:11890–11899. doi: 10.1523/JNEUROSCI.3156-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compagnone NA, Mellon SH. Neurosteroids: Biosynthesis and Function of These Novel Neuromodulators. Frontiers in Neuroendocrinology. 2000;21:1–56. doi: 10.1006/frne.1999.0188. [DOI] [PubMed] [Google Scholar]
- Connolly CN, Krishek BJ, McDonald BJ, Smart TG, Moss SJ. Assembly and cell surface expression of heteromeric and homomeric gamma-aminobutyric acid type A receptors. J Biol Chem. 1996;271:89–96. doi: 10.1074/jbc.271.1.89. [DOI] [PubMed] [Google Scholar]
- Damgaard T, Plath N, Neill JC, Hansen SL. Extrasynaptic GABAA receptor activation reverses recognition memory deficits in an animal model of schizophrenia. Psychopharmacology (Berl) 2011;214:403–413. doi: 10.1007/s00213-010-2039-9. [DOI] [PubMed] [Google Scholar]
- Eshaq RS, Stahl LD, Stone R, 2nd, Smith SS, Robinson LC, Leidenheimer NJ. GABA acts as a ligand chaperone in the early secretory pathway to promote cell surface expression of GABA-A receptors. Brain Res. 2010;1346:1–13. doi: 10.1016/j.brainres.2010.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, et al. Association of the GABRD gene and childhood-onset mood disorders. Genes Brain Behav. 2010;9:668–672. doi: 10.1111/j.1601-183X.2010.00598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girdler SS, Straneva PA, Light KC, Pedersen CA, Morrow AL. Allopregnanolone levels and reactivity to mental stress in premenstrual dysphoric disorder. Biol Psych. 2001;49:788–797. doi: 10.1016/s0006-3223(00)01044-1. [DOI] [PubMed] [Google Scholar]
- Griffiths J, Lovick T. Withdrawal from progesterone increases expression of α1, β2 and δ GABA(A) receptor subunits in neurons in the periaqueductal gray matter in female Wistar rats. J Comp Neurol. 2005;486:89–97. doi: 10.1002/cne.20540. [DOI] [PubMed] [Google Scholar]
- Gschwendt M, Muller HJ, Kielbassa K, Zang R, Kittstein W, Rincke G, Marks F. Rottlerin, a novel protein kinase inhibitor. Biochem Biophys Res Commun. 1994;199:93–98. doi: 10.1006/bbrc.1994.1199. [DOI] [PubMed] [Google Scholar]
- Gulinello M, Gong QH, Li X, Smith SS. Short-term exposure to a neuroactive steroid increases α4 GABAA receptor subunit levels in association with increased anxiety. Brain Res. 2001;910:55–66. doi: 10.1016/s0006-8993(01)02565-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadley SH, Amin J. Rat α6β2δ GABAA receptors exhibit two distinct and separable agonist affinities. J Physiol. 2007;581:1001–1018. doi: 10.1113/jphysiol.2007.132886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernan R, Heuermann K, Brizzard B. Multiple epitope tagging of expressed proteins for enhanced detection. Biotechniques. 2000;28:789–793. doi: 10.2144/00284pf01. [DOI] [PubMed] [Google Scholar]
- Higashi T, Takido N, Shimada K. Studies on neurosteroids XVII. Analysis of stress-induced changes in neurosteroid levels in rat brains using liquid chromatography-electron capture atmosperic pressure chemical ionization-mass spectrometry. Steroids. 2005;70:1–11. doi: 10.1016/j.steroids.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Jacob TC, Moss SJ, Jurd R. GABA-A receptor trafficking and its role in the dynamic modulation of neuronal inhibition. Nat Rev Neurosci. 2008;9:331–343. doi: 10.1038/nrn2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia F, Yue M, Cjandra D, Keramides A, Goldstein PA, Homanics GE, Harrison NL. Taurine is a potent activator of extrasynaptic GABA(A) receptors in the thalamus. J Neurosci. 2008;28:106–115. doi: 10.1523/JNEUROSCI.3996-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi S, Kapur J. Slow intracellular accumulation of GABA(A) receptor delta subunit is modulated by brain-derived neurotrophic factor. Neuroscience. 2009;164:507–519. doi: 10.1016/j.neuroscience.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittler JT, Wang J, Connolly CN, Vicini S, Smart TG, Moss SJ. Analysis of GABAA receptor assembly in mammalian cell lines and hippocampal neurons using gamma 2 subunit green fluorescent protein chimeras. Mol Cell Neurosci. 2000;16:440–452. doi: 10.1006/mcne.2000.0882. [DOI] [PubMed] [Google Scholar]
- Lovick T. Panic disorder: a malfunction of multiple transmitter control systems within the midbrain periaqueductal gray matter? Neuroscientist. 2000;6:48–59. [Google Scholar]
- Lovick TA, Griffiths JL, Dunn SM, Martin IL. Changes in GABA(A) receptor subunit expression in the midbrain during the oestrous cycle in Wistar rats. Neuroscience. 2005;131:397–405. doi: 10.1016/j.neuroscience.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Ma L, Song L, Radoi GE, Harrison NL. Transcriptional regulation of the mouse gene encoding the alpha[4] subunit of the GABA-A receptor. J Biol Chem. 2004;279:40451–40461. doi: 10.1074/jbc.M406827200. [DOI] [PubMed] [Google Scholar]
- Maguire JL, Mody I. Neurosteroid synthesis-mediated regulation of GABA(A) receptors: relevance to the ovarian cycle and stress. J Neurosci. 2007;27:2155–2162. doi: 10.1523/JNEUROSCI.4945-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire JL, Mody I. GABA(A)R plasticity during pregnancy: relevance to postpartum depression. Neuron. 2009;59:207–213. doi: 10.1016/j.neuron.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire JL, Stell BM, Rafizadeh M, Mody I. Ovarian cycle-linked changes in GABA(A) receptors mediating tonic inhibition alter seizure susceptibility and anxiety. Nat Neurosci. 2005;8:797–804. doi: 10.1038/nn1469. [DOI] [PubMed] [Google Scholar]
- Martin del Rio R, Orensanz Munoz LM, DeFeudis FV. Contents of beta-alanine and gamma-Aminobutyric acid in regions of rat CNS. Exp Brain Res. 1977;28:225–227. doi: 10.1007/BF00235704. [DOI] [PubMed] [Google Scholar]
- Mason MJ, Simpson AK, Mahaut-Smith MP, Robinson HP. The interpretation of current-clamp recordings in the cell-attached patch-clamp configuration. Biophys J. 2005;88:739–750. doi: 10.1529/biophysj.104.049866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathers DA, McCarthy SM, Cooke JE, Ghavanini AA, Puil E. Effects of the beta-amino acid antagonist TAG on thalamocortical inhibition. Neuropharmacology. 2009;56:1097–1105. doi: 10.1016/j.neuropharm.2009.03.008. [DOI] [PubMed] [Google Scholar]
- Meera P, Wallner M, Otis T. Molecular basis for the high THIP/gaboxadol sensitivity of extrasynaptic GABA-A receptors. J Neurophysiology. 2011;106:2057–2011. doi: 10.1152/jn.00450.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing RO, Choi DS, Wei W, Kharazia VN, Deitchman JK, Lesscher HMB, Mody I. Protein kinase C-delta regulates GABA-mediated tonic inhibition and motor response to ethanol. Alc Clin Exp Res. 2007;31 (Suppl):296A. [Google Scholar]
- Mtchedlishvili Z, Lepsveridze E, Xu H, Kharlamov EA, Lu B, Kelly KM. Increase of GABA-A receptor-mediated tonic inhibtion in dentate granule cells after traumatic brain injury. Neurobiol Dis. 2010;38:464–475. doi: 10.1016/j.nbd.2010.03.012. [DOI] [PubMed] [Google Scholar]
- Olsen RW, Sieghart W. GABA A receptors: subtypes provide diversity of function and pharmacology. Neuropharmacology. 2009;56:141–148. doi: 10.1016/j.neuropharm.2008.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmer TG, Ward MD, Hait WN. Effects of rottlerin, an inhibitor of calmodulin-dependent protein kinase III, on cellular proliferation, viability, and cell cycle distribution in malignant glioma cells. Cell Growth Differ. 1997;8:327–334. [PubMed] [Google Scholar]
- Payne JA. Functional characterization of the neuronal-specific K-Cl cotransporter: implications for [K+]o regulation. Am J Physiol. 1997;273:C1516–C1525. doi: 10.1152/ajpcell.1997.273.5.C1516. [DOI] [PubMed] [Google Scholar]
- Perkins KL. Cell-attached voltage-clamp and current-clamp recording and stimulation techniques in brain slices. J Neurosci Methods. 2006;154:1–18. doi: 10.1016/j.jneumeth.2006.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignataro L, Miller AN, Ma L, Midha S, Protiva P, Herrera DG, Harrison NL. Alcohol regulates gene expression in neurons via activation of heat shock factor 1. J Neurosci. 2007;27:12957–12966. doi: 10.1523/JNEUROSCI.4142-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GABA(A) receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience. 2000;101:815–850. doi: 10.1016/s0306-4522(00)00442-5. [DOI] [PubMed] [Google Scholar]
- Pless SA, Lynch JW. Magnitude of a conformational change in the glycine receptor â1-â2 loop is correlated with agonist efficacy. J Biol Chem. 2009;284:27370– 27376. doi: 10.1074/jbc.M109.048405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purdy RH, Morrow AL, Moore PH, Jr, Paul SM. Stress-induced elevations of gamma-aminobutyric acid type A receptor-active steroids in the rat. Proc Natl Acad Sci. 1991;88:4553–4557. doi: 10.1073/pnas.88.10.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera C, Voipio J, Payne JA, Ruusuvuori E, Lahtinen H, Lamsa K, Saarma M, Kaila K. The K-Cl cotransporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature. 1999;397:251–255. doi: 10.1038/16697. [DOI] [PubMed] [Google Scholar]
- Roberts DS, Hu Y, Lund IV, Brooks-Kayal AR, Russek SJ. Brain-derived neurotrophic factor (BDNF)-induced synthesis of early growth response factor 3 (Egr3) controls the levels of type A GABA receptor alpha4 subunits in hippocampal neurons. J Biol Chem. 2006;281:29431–29435. doi: 10.1074/jbc.C600167200. [DOI] [PubMed] [Google Scholar]
- Roberts DS, Raol YH, Bandyopadhyay S, Lund IV, Budreck EC, Passini MA, Wolfe JH, Brooks-Kayal AR, Russek SJ. Egr3 stimulation of GABARA4 promoter activity as a mechanism for seizure-induced up-regulation of GABA(A) receptor alpha4 subunit expression. Proc Natl Acad Sci. 2005;102:11894– 11899. doi: 10.1073/pnas.0501434102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancar F, Czajkowski C. Allosteric modulators induce distinct movements at the GABA-binidng site interface of the GABA-A receptor. Neuropharm. 2011;60:520–528. doi: 10.1016/j.neuropharm.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanna E, Mostallino MC, Murru L, Carta M, Talani G, Zucca S, Mura ML, Maciocco E, Biggio G. Changes in expression and function of extrasynaptic GABA-A receptors in the rat hippocampus during pregnancy and after delivery. J Neurosci. 2009;29:1755–1765. doi: 10.1523/JNEUROSCI.3684-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santhakumar V, Jones RT, Mody I. Developmental regulation and neuroprotective effects of striatal tonic GABA-A currents. Neuroscience. 2010;167:644–655. doi: 10.1016/j.neuroscience.2010.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saransaari P, Oja SS. Beta-alanine release from the adult and developing hippocampus is enhanced by ionotropic glutamate receptor agonists and celldamaging conditions. Neurochem Res. 1999;24:407–414. doi: 10.1023/a:1020941818168. [DOI] [PubMed] [Google Scholar]
- Schenk U, Verderio C, Benfenati F, Matteoli M. Regulated delivery of AMPA receptor subunits to the presynaptic membrane. EMBO J. 2003;22:558–568. doi: 10.1093/emboj/cdg059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Gong QH, Aoki C, Yuan M, Ruderman Y, Dattilo M, Williams K, Smith SS. Reversal of neurosteroid effects at α4β2δ GABA-A receptors triggers anxiety at puberty. Nat Neurosci. 2007;10:469–477. doi: 10.1038/nn1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Gong QH, Yuan M, Smith SS. Short-term steroid treatment increases delta GABA-A receptor subunit expression in rat CA1 hippocampus: pharmacological and behavioral effects. Neuropharmacology. 2005;49:573–586. doi: 10.1016/j.neuropharm.2005.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Sabaliauskas N, Sherpa A, Fenton AA, Stelzer A, Aoki C, Smith SS. A critical role for α4βδ GABA-A receptors in shaping learning deficits at puberty in mice. Science. 2010;327:1515–1518. doi: 10.1126/science.1184245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Smith S. Plasticity of the alpha4-beta-delta GABA-A receptor. Biochem Soc Trans. 2009;37:1378–1384. doi: 10.1042/BST0371378. [DOI] [PubMed] [Google Scholar]
- Smith SS, Gong QH, Hsu FC, Markowitz RS, ffrench-Mullen JMH, Li X. GABAA receptor α4 subunit suppression prevents withdrawal properties of an endogenous steroid. Nature. 1998;392:926–929. doi: 10.1038/31948. [DOI] [PubMed] [Google Scholar]
- Smith SS, Ruderman Y, Frye CA, Homanics GE, Yuan M. Steroid withdrawal in the mouse results in anxiogenic effects of 3alpha, 5alpha-THP: A possible model of premenstrual dysphoric disorder. Psychopharmacology. 2006;186:323–333. doi: 10.1007/s00213-005-0168-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltoff SP. Rottlerin: an inappropriate and ineffective inhibitor of PKCdelta. Trends Pharmacol Sci. 2007;28:453–458. doi: 10.1016/j.tips.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Stell BM, Mody I. Receptors with different affinities mediate phasic and tonic GABA(A) conductances in hippocampal neurons. J Neurosci. 2002;22:RC223. doi: 10.1523/JNEUROSCI.22-10-j0003.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundstrom-Poromaa I, Smith DH, Gong Q, Sabado TN, Li X, Light A, Wiedmann M, Williams K, Smith S. Hormonally regulated α4β2δ GABA-A receptors are a target for alcohol. Nat Neurosci. 2002;5:721–722. doi: 10.1038/nn888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sur C, Farrar SJ, Kerby J, Whiting PJ, Atack JR, McKernan RM. Preferential coassembly of alpha4 and delta subunits of the gamma-aminobutyric acidA receptor in rat thalamus. Mol Pharmacol. 1999;56:110–115. doi: 10.1124/mol.56.1.110. [DOI] [PubMed] [Google Scholar]
- Thomas P, Mortensen M, Hosie AM, Smart TG. Dynamic mobility of functional GABA-A receptors at inhibitory syanpses. Nat Neurosci. 2005;8:889–897. doi: 10.1038/nn1483. [DOI] [PubMed] [Google Scholar]
- Tiedje KE, Stevens K, Barnes S, Weaver DF. Beta-alanine as a small molecule neurotransmitter. Neurochem Int. 2010;57:177–188. doi: 10.1016/j.neuint.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Wallner M, Hanchar HJ, Olsen RW. Ethanol enhances alpha 4 beta 3 delta and alpha 6 beta 3 delta gamma-aminobutyric acid type A receptors at low concentrations known to affect humans. Proc Natl Acad Sci. 2003;100:15218–15223. doi: 10.1073/pnas.2435171100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Zhang N, Peng Z, Houser CR, Mody I. Perisynaptic localization of delta subunit-containing GABA(A) receptors and their activation by GABA spillover in the mouse dentate gyrus. J Neurosci. 2003;23:10650–10661. doi: 10.1523/JNEUROSCI.23-33-10650.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wermuth PJ, Addya S, Jimenez SA. Effect of Protein Kinase C delta (PKCdelta) Inhibition on the Transcriptome of Normal and Systemic Sclerosis Human Dermal Fibroblasts In Vitro. PLoS One. 2011;6:e27110. doi: 10.1371/journal.pone.0027110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisden W, Laurie DJ, Monyer H, Seeburg P. The distribution of 13 GABA–A receptor subunit mRNAs in the rat brain. I. Telencephalon, diencephalon, mesencephalon. J Neurosci. 1992;12:1040–1062. doi: 10.1523/JNEUROSCI.12-03-01040.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlfarth KM, Bianchi MT, Macdonald RL. Enhanced neurosteroid potentiation of ternary GABA(A) receptors containing the delta subunit. J Neurosci. 2002;22:1541–1549. doi: 10.1523/JNEUROSCI.22-05-01541.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Wang W, Richerson G. GABA transaminase inhibition induces spontaneous and enhances depolarization-evoked GABA efflux via reversal of the GABA transporter. J Neurosci. 2001;21:2630–2639. doi: 10.1523/JNEUROSCI.21-08-02630.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheleznova N, Sedelnikova A, Weiss DS. Alpha1-beta2-delta, a silent GABAA receptor: recruitment by tracazolate and neurosteroids. Brit J Pharmacol. 2008;153:1062–1071. doi: 10.1038/sj.bjp.0707665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Smith SS. Steroid requirements for regulation of the alpha-4 subunit of the GABA-A receptor in an in vitro model. Neurosci Lett. 2007;411:61–66. doi: 10.1016/j.neulet.2006.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Smith SS. Expression levels of the alpha4 subunit of the GABA(A) receptor in differentiated neuroblastoma cells are correlated with GABA-gated current. Neuropharm. 2009;56:1041–1053. doi: 10.1016/j.neuropharm.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.