Abstract
A simple and fast method of lipid analysis of isolated intact mitochondria by means of MALDI-TOF mass spectrometry is described. Mitochondria isolated from bovine heart and yeast have been employed to set up and validate the new method of lipid analysis. The mitochondrial suspension is directly applied over the target and, after drying, covered by a thin layer of the 9-aminoacridine matrix solution. The lipid profiles acquired with this procedure contain all peaks previously obtained by analyzing the lipid extracts of isolated mitochondria by TLC and/or mass spectrometry. The novel procedure allows the quick, simple, precise, and accurate analysis of membrane lipids, utilizing only a tiny amount of isolated organelle; it has also been tested with intact membranes of the bacterium Paracoccus denitrificans for its evolutionary link to present-day mitochondria. The method is of general validity for the lipid analysis of other cell fractions and isolated organelles.
Keywords: cardiolipin, heart, Paracoccus denitrificans, yeast, 9-aminoacridine, matrix-assisted laser desorption/ionization time-of-flight mass spectrometry
Historically lipid profiling of biological samples has been achieved with preliminary isolation of tissues, cells, membranes or organelles, followed by lipid extraction and purification with chromatographic techniques, such as HPLC or (HP)TLC (1, 2), and by lipid characterization and/or quantitation with gas chromatography (GC) (3), GC-mass spectrometry (MS) (4), fast atom bombardment (FAB)-MS (5–7), ESI-MS (8–12), and/or NMR analyses (13).
In the last decade, the capabilities of MALDI time-of-flight (TOF)/MS in lipid analysis have been largely demonstrated for the analysis of lipid extracts from different biological materials (14–18), but the most promising advantage of the MALDI-TOF/MS technique turned out to be the possibility to perform lipid analysis avoiding extraction and/or separation steps.
Recent advances in MALDI-TOF/MS techniques of lipid analysis have led to the direct coupling of HPTLC-MALDI (19–22), as well as to the direct analysis of tissue slices with the MALDI-MSI (23–25). In both cases, a plate containing the sample is coated with the matrix solution (silica plate or tissue on microscope glass plate, respectively), attached onto the MALDI target, and directly scanned with the laser to obtain a fast MS analysis of the lipids on the plate. Both these methods allow a fast and convenient lipid analysis, skipping extraction steps that are usually required for these kinds of analyses.
In 2010 we developed a protocol that allows the direct lipid analysis by MALDI mass spectrometry in intact archaeal membranes without prior extraction/separation steps: lyophilized archaeal membranes were ground with 9-aminoacridine (9-AA) as dry matrix, and the powder mixture was crushed in a mechanical die press to form a thin pellet; small pieces of the pellet were then attached onto the MALDI target, and the archaeal membrane lipid profile was directly achieved (26).
Another new protocol for the direct lipid fingerprinting with MALDI-TOF/MS was published by Ferreira et al. in 2010 (27). They report a direct lipid analysis of a single embryo and oocyte by MALDI-TOF/MS: the biological sample is moisturized with the matrix solution, and lipid profiles are directly obtained.
Results contained in the above reports suggest that MALDI-TOF/MS can be successfully used for the direct analysis of intact biological samples, allowing a fast and detailed lipidomic analysis without artifacts due to the extraction/separation steps.
Here we show that the direct MALDI-TOF/MS lipid analysis of intact mitochondria, isolated from heart tissue, and yeasts give rise to lipid profiles similar to those previously obtained from the analyses of the corresponding mitochondrial lipid extracts. In addition, we have also analyzed intact bacterial membranes of Paracoccus denitrificans, whose components and functional organization closely resemble the electron transport chain of the inner mitochondrial membrane. We focus on the presence of cardiolipin (CL), the signature lipid of mitochondria, and show the great potential of our technique for the qualitative and quantitative detection of a lipid that is tightly bound to membrane proteins and might be difficult to completely recover in lipid extracts.
Preliminary results of this investigation have been presented at the 52nd International Conference on the Bioscience of Lipids (ICBL), Warsaw, Poland (28).
EXPERIMENTAL PROCEDURES
Materials
9-Aminoacridine hemihydrate was purchased from Acros Organics (Morris Plains, NJ). The following commercial glycerophospholipids (used as standards) were purchased from Avanti Polar Lipids Inc. (Alabaster, AL): 1,1’,2,2’-tetratetrade-can-oyl cardio-lipin; 1,1’,2,2’-tetra-(9Z-octadecenoyl) cardiolipin; 1,2-ditetradecanoyl-sn-glycero-3-phosphate; 1,2-ditetradecanoyl-sn-glycero-3-phospho-(1’-rac-glycerol); 1,2-ditetradecanoyl-sn-glycero-3-phospho-L-serine; and 1,2-di-(9Z-hexadecenoyl)-sn-glycero-3-phosphoethanola-mine. All organic solvents used in extraction and MS analysis were commercially distilled and of the highest available purity and were purchased from Sigma Aldrich, J. T. Baker (Avantor Performance Materials, Center Valley, PA), or C. Erba, Reagenti S.p.A. [Arese (MI), IT].
Yeast strains, growth conditions, and mitochondria isolation
The following yeast S. cerevisiae strains were used for this study: FGY3 (Mat a, ura3–52, lys2–801, ade2–101, trp1Δ1, his3Δ200, leu2Δ1), which served as wild-type, and strains carrying null mutations in the genes CRD1 (29) and TAZ1 (30). Cells were grown in YPGE (3% glycerol, 1% ethanol) at 30°C, and mitochondria were isolated as described by Daum et al. (31). Total mitochondrial protein was determined by Bradford reagent (Bio-Rad) with BSA as the standard.
Isolation of mitochondria from bovine heart
Mitochondria were prepared from bovine heart as previously described (32).
Paracoccus denitrificans growth conditions and membrane isolation
Cells of Paracoccus denitrificans were grown on succinate medium, and its membranes were isolated as previously described (33).
Preparation of mitochondria samples for MALDI-TOF/MS lipid analysis
The procedure for sample preparation is analogous to the sandwich method, commonly used in MALDI/TOF-MS. The different mitochondrial suspensions were all diluted to 0.1 μg/μl of total mitochondrial protein concentration, determined by Bradford assay. Then 1 μl of mitochondrial suspension was spotted onto the MALDI target. After water evaporation, a thin layer (0.25 μl) of matrix solution (9-AA, 30 mg/ml in 2-propanol/acetonitrile, 60/40, v/v) is then spotted on the dried sample. After the evaporation of the matrix solvent, the samples are ready to be directly analyzed with MALDI-TOF/MS.
To quantify the CL content in the samples, 10 μl of solution of internal standard (IS), tetra-myristoyl-CL (14:0)4, 64 μM in chloroform, were mixed with 90 μl of the matrix solution. Finally 0.25 μl of the mixed matrix solution, containing 1.6 pmol of the internal standard, were directly spotted on the dried sample as above. Assuming that all the CL species have the same capability to ionize, the picomoles of CL species present in the sample were calculated by determining the ratio of the peak area of the CL molecular species of interest to the internal standard; this ratio was then multiplied by the picomoles of internal standard present on the target.
Preparation of mitochondrial lipid extracts for MALDI-TOF/MS lipid analysis
Total lipids were extracted using the Bligh and Dyer method as previously described (34); the extracts were carefully dried under N2 before weighing and then dissolved in chloroform at the final concentration of 10 mg/ml. From a wild-type (WT) yeast mitochondrial suspension, containing 1 mg of total protein, 0.3 mg of total lipids were extracted.
To quantify the CL content, the lipid extract solution (10 mg/ml in CHCl3) was first mixed 1:1 with a solution of IS CL (14:0)4 0.4 mM in CHCl3, obtaining a solution that was 5 mg/ml of total yeast mitochondrial lipids and 0.2 mM of IS CL (14:0)4. Then, as previously described by Sun et al. (14), 20 μl of the resulting solution were diluted in 180 μl of 2-propanol/acetoneitrile (60/40, v/v), then 10 μl of the diluted solution was mixed with 10 μl of matrix solution (9-AA, 10 mg/ml in 2-propanol/acetonitrile, 60/40, v/v). The resulting lipids-matrix solution was then spotted onto the MALDI target in droplets of 0.35 μl and analyzed as described below.
MALDI-TOF mass spectrometry
MALDI-TOF mass spectra of “intact” samples were generally acquired on a Bruker Microflex LRF mass spectrometer, whereas post-source decay (PSD) spectra were acquired on a Bruker Autoflex mass spectrometer (Bruker Daltonics, Bremen, Germany). The systems utilize a pulsed nitrogen laser, emitting at 337 nm, the extraction voltage was 20 kV, and gated matrix suppression was applied to prevent detector saturation. For each mass spectrum, 999 single laser shots (sum of 3 × 333) were averaged. The laser fluence was kept about 5% above threshold to have a good signal-to-noise ratio. All spectra were acquired in reflector mode using the delayed pulsed extraction; only spectra acquired in negative ion mode are shown in this study. Peaks areas, spectral mass resolutions, and signal-to-noise ratios were determined by the software for the instrument “Flex Analysis 3.3.65” (Bruker Daltonics).
A mix containing 1,1’,2,2’-tetratetradecanoyl cardiolipin; 1,1’,2,2’-tetra-(9Z-octadecenoyl) cardiolipin; 1,2-ditetradecanoyl-sn-glycero-3-phosphate; 1,2-ditetradecanoyl-sn-glycero-3-phospho-(1’-rac-glycerol); 1,2-ditetradecanoyl-sn-glycero-3-phospho-L-serine; and 1,2-di-(9Z-hexadecenoyl)-sn-glycero-3-phosphoethanolamine was always spotted next to the sample as external standard, and an external calibration was performed before each measurement. The mass range of the authentic standards is 590–1450 amu.
RESULTS
MALDI-TOF/MS lipid profiles of intact mitochondria, isolated from bovine heart and yeast, and of intact membranes of the bacterium Paracoccus denitrificans were recorded.
Mitochondria isolated from bovine heart
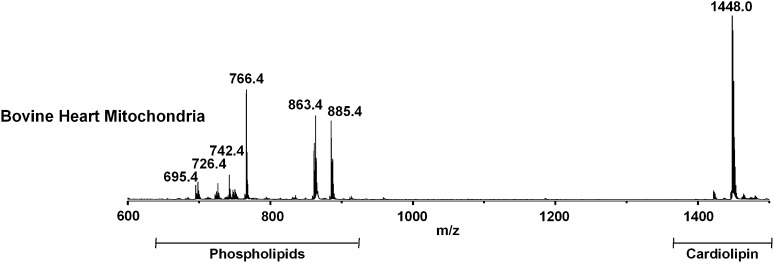
The mass spectrum of lipids of intact bovine heart mitochondria (BHM), acquired in negative ion mode and using 9-AA as matrix is shown in Fig. 1. The signals in the spectra attributable to the negative [M-H]- molecular ions of phospholipids, sphingolipids, and glycolipids are collected in Table 1. The peaks can be grouped into three main m/z ranges: i) m/z 650–1000, attributable to major phospholipids; ii) m/z 800–1400, to glycosphingolipids and lysocardiolipins; and iii) m/z 1350–1500, to CLs.
Fig. 1.
Negative MALDI-TOF/MS lipid profile of intact bovine heart mitochondria acquired using 9-AA as matrix. The m/z signals corresponding to the different lipid components are (from the left): PA 36:4, [M-H]- at 695.4; PS 32:4, [M-H]- at 726.4; PE 36:2, [M-H]- at 742.4; PE 38:4, [M-H]- at 766.4; PI 36:1, [M-H]- at 863.4; PI 38:4, [M-H]- at 885.4; CL (18:2)4, [M-H]- at 1448.0. A detailed list of detected peaks is shown in Table 1.
TABLE 1.
Assignments of m/z values detected in the negative ion mode MALDI-TOF mass spectra of intact bovine heart mitochondria using 9-AA as matrix
| m/zValue | Assignment [M-H]- |
| 695.4 | PA 36:4 |
| 698.4 | PS 30:4 |
| 722.4 | PS 32:6 |
| 724.4 | PS 32:5 |
| 726.4 | PS 32:4 |
| 738.4 | PE 36:4 |
| 740.4 | PE 36:3 |
| 742.4 | PE 36:2 |
| 747.4 | PG 34:1 |
| 750.4 | PS 34:6 |
| 764.4 | PE 38:5 |
| 766.4 | PE 38:4 |
| 792.4 | PE 40:5 |
| 794.4 | PE 40:4 |
| 796.4 | PE 40:3 |
| 798.4 | PE 40:2 |
| 831.4 | PI 34:3 |
| 833.4 | PI 34:2 |
| 835.4 | PI 34:1 |
| 859.4 | PI 36:3 |
| 861.4 | PI 36:2 |
| 863.4 | PI 36:1 |
| 883.4 | PI 38:5 |
| 885.4 | PI 38:4 |
| 887.4 | PI 38:3 |
| 911.4 | PI 40:5 |
| 913.4 | PI 40:4 |
| 1185.6 | MLCL 54:6 |
| 1422.0 | CL 70:7 |
| 1436.0 | CL 70:0 |
| 1446.0 | CL 72:9 |
| 1448.0 | CL 72:8a |
| 1450.0 | CL 72:7 |
| 1452.0 | CL 72:6 |
| 1462.0 | CL 72:1 |
| 1464.0 | CL 72:0 |
| 1474.0 | CL 74:9 |
| 1476.0 | CL 74:8 |
| 1478.0 | CL 74:7 |
| 1480.0 | CL 74:6 |
| 1482.0 | CL 74:5 |
| 1494.0 | CL 75:6 |
| 1496.0 | CL 75:5 |
| 1510.1 | CL 76:5 |
| 1512.1 | CL 76:4 |
The chain length of the cardiolipin peak at m/z 1448.0 has been assigned by PSD analysis of present study (see spectrum in supplementary Fig. I).
In the range of major phospholipids (Fig. 1), the peak at m/z 695.4 can be attributed to phosphatidic acid (PA) 36:4; the peaks at m/z 742.4 and 766.4 are attributable to phosphatidylethanolamine (PE) 36:2 and 38:4; the peak at m/z 747.4 is assigned to phosphatidylglycerol (PG) 34:1; the peaks at m/z 698.4, 726.4, and 750.4 are assigned to phosphatidylserine (PS) 30:4, 32:4, and 34:6; and various species of phosphatidylinositol (PI) are present at m/z 835.4 (34:1), 861.4 (36:2), 863.5 (36:3), 885.4 (38:4), 887.7 (38:3), 911.3 (40:5), and 913.4 (40:4). A detailed list, including minor peaks, is available in Table 1.
In the cardiolipin range, a major peak at m/z 1448.0 is present, which corresponds to the CL having four 18:2 chains, as confirmed by PSD analysis (see supplementary Fig. I). In addition to this major CL 72:8, other CLs are present (see details in Table 1).
In the lyso-CL range, only a very small peak at m/z 1185.6 can be attributed to monolysocardiolipin (MLCL) 54:6, arising from the CL 72:8.
Mitochondria isolated from WT yeast and CL mutants
Mitochondria isolated from yeast WT cells and CL null mutants (crd1Δ and taz1Δ) were analyzed with the new method.
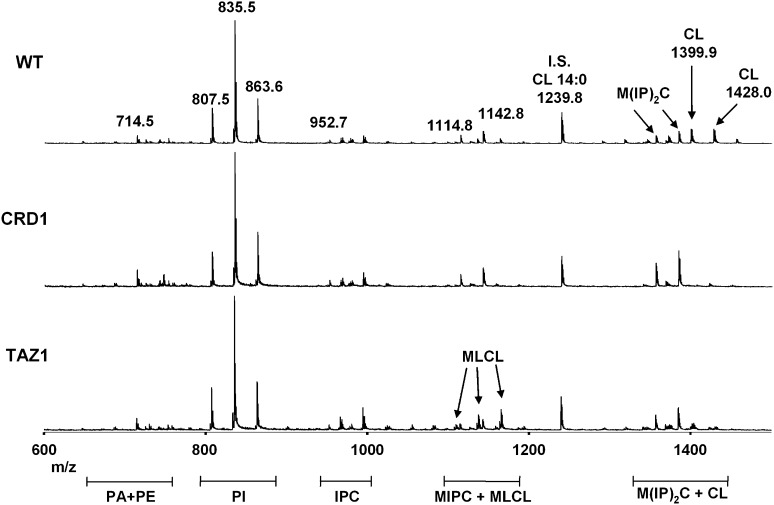
The MALDI-TOF/MS profiles of the three different mitochondrial preparations are shown in Fig. 2.
Fig. 2.
Negative MALDI-TOF/MS lipid profiles of intact yeast mitochondria (WT, crd1Δ, and taz1Δ null mutants) acquired using 9-AA as matrix. The m/z signals corresponding to the different lipid components are (from the left): PE 34:2, [M-H]- at 714.5; PI 32:1, [M-H]- at 807.5; PI 34:1, [M-H]- at 835.5; PI 36:1, [M-H]- at 863.6; IPC (t18:0/26:0-C), [M-H]- at 952.7; MIPC (t18:0/26:0-C), [M-H]- at 1114.8; MIPC (t20:0/26:0-C), [M-H]- at 1142.8; CL 68:4, [M-H]- at 1399.9; CL 70:4, [M-H]- at 1428.0. A detailed list of detected peaks is shown in Tables 2 and 3. Tetra-myristoyl-CL (14:0)4, peak at m/z 1239.8, was used as internal standard (IS) to quantify the CL content in yeast mitochondria.
The signals in the spectra attributable to the nega-tive [M-H]- molecular ions of yeast mitochondrial lipids are collected in Table 2 (phospholipids) and Table 3 (glycosphin-golipids).
TABLE 2.
Assignments of m/z values detected in the negative ion mode MALDI-TOF mass spectra of intact yeast mitochondria (WT, crd1Δ and taz1Δ mutants) using 9-AA as matrix, attributable to phospholipids
| m/z Value | Assignment [M-H]- |
| 619.2 | PA 30:0 |
| 643.4 | PA 32:2 |
| 645.4 | PA 32:1 |
| 647.3 | PA 32:0 |
| 663.1 | PG 28:1 |
| 671.5 | PA 34:2 |
| 673.5 | PA 34:1 |
| 686.5 | PE 32:2 |
| 688.5 | PE 32:1 |
| 701.1 | PA 36:1 |
| 703.1 | PA 36:0 |
| 714.5 | PE 34:2 |
| 716.5 | PE 34:1 |
| 730.4 | PS 32:2 |
| 732.5 | PS 32:1 |
| 742.5 | PE 36:2 |
| 745.5 | PG 34:2 (only crd1Δ) |
| 747.5 | PG 34:1 (only crd1Δ) |
| 758.5 | PS 34:1 |
| 760.5 | PS 34:0 |
| 775.6 | PG 36:1 (only crd1Δ) |
| 777.6 | PG 36:0 (only crd1Δ) |
| 779.5 | PI 30:1 |
| 781.5 | PI 30:0 |
| 784.1 | PE 36:3 |
| 786.1 | PE 36:2 |
| 805.5 | PI 32:2 |
| 807.5 | PI 32:1 |
| 821.5 | PI 33:1 |
| 833.5 | PI 34:2 |
| 835.5 | PI 34:1a |
| 854.5 | PE 44:2 |
| 861.5 | PI 36:2 |
| 863.5 | PI 36:1 |
| 1107.7 | MLCL 48:3 |
| 1109.8 | MLCL 48:2 |
| 1135.8 | MLCL 50:3 only WT and taz1Δ |
| 1137.8 | MLCL 50:2 only taz1Δ |
| 1163.8 | MLCL 52:3 only WT and taz1Δ |
| 1165.8 | MLCL 52:2 only taz1Δ |
| 1191.8 | MLCL 54:3 only WT and taz1Δ |
| 1193.8 | MLCL 54:2 only taz1Δ |
| 1343.9 | CL 64:4 only WT and taz1Δ (more in WT) |
| 1345.9 | CL 64:3 only taz1Δ |
| 1347.9 | CL 64:2 only taz1Δ |
| 1371.9 | CL 66:4 only WT and taz1Δ (more in WT) |
| 1373.9 | CL 66:3 only taz1Δ |
| 1375.9 | CL 66:2 only taz1Δ |
| 1399.9 | CL 68:4a only WT and taz1Δ (more in WT) |
| 1401.9 | CL 68: 3 only taz1Δ |
| 1403.9 | CL 68: 2 only taz1Δ |
| 1428.0 | CL 70:4a only WT and taz1Δ (more in WT) |
| 1430.0 | CL 70:3 only taz1Δ |
| 1432.9 | CL 70:2 only taz1Δ |
| 1456.0 | CL 72:4 |
Chains of peaks at m/z 835.5, 1399.9, and 1428.0 were assigned by PSD analyses.
TABLE 3.
Assignments of m/z values detected in the negative ion mode MALDI-TOF mass spectra of intact yeast mitochondria (WT, crd1Δ and taz1Δ mutants) using 9-AA as matrix, attributable to glycosphingolipids
| m/zValue | Assignment [M-H]- | Reference |
| 936.5 | IPC (t18:0/26:0-B) | 45-46b, 43c |
| 952.7 | IPC (t18:0/26:0-C) | 45-46b, 43c |
| 964.5 | IPC (t20:0/26:0-B) | 45-46b, 43c |
| 968.5 | IPC (t18:0/26:0-D) | 45-46b, 43c |
| 980.5 | IPC (t20:0/26:0-C) | 45-46b, 43c |
| 1098.6 | MIPC (t18:0/26:0-B) | 45b, 43c |
| 1114.8 | MIPC (t18:0/26:0-C)a | 45b, 43c |
| 1126.8 | MIPC (t20:0/26:0-B) | 45b, 43c |
| 1130.8 | MIPC (t18:0/26:0-D) | 45b |
| 1142.8 | MIPC (t20:0/26:0-C)a | 45b, 43c |
| 1340.8 | M(IP)2C (t18:0/26:0-B) | 43d, 45d |
| 1356.8 | M(IP)2C (t18:0/26:0-C)a | 43d, 45d |
| 1368.9 | M(IP)2C (t20:0/26:0-B) | 45d |
| 1384.9 | M(IP)2C (t20:0/26:0-C)a | 45d |
Glycosphingolipids peaks have been assigned to their molecular species according to the past literature (43-46). Number of hydroxyl groups in the fatty-acyl moiety as in Guan and Wenk (43): B = 0, C = 1, D = 2.
PSD analyses of the peaks at m/z 1384.9, 1356.8, 1142.8, 1114.8 were performed.
Based on precursor ion scanning (PIS) or multiple reaction monitoring (MRM) data achieved on the identical [M-H]- observed in yeast Saccharomyces cerevisiae.
PIS or MRM data achieved on the correspondent [M+H]+ observed in yeast Saccharomyces cerevisiae.
PIS or MRM data achieved on the correspondent [M-2H]2- observed in yeast Saccharomyces cerevisiae.
The peaks can be grouped in three main m/z ranges: i) m/z 650–1000, attributable to major phospholipids; ii) m/z 800–1400, to glycosphingolipids and lysocardiolipins; and iii) m/z 1350–1500, to CLs.
In the range of major phospholipids (Fig. 2), the small peaks at m/z 647.3 and 671.5 can be attributed to phosphatidic acid (PA) 32:0 and 34:2; the peak at m/z 714.5 is attributable to phosphatidylethanolamine (PE) 34:2; and various species of phosphatidylinositol (PI) are present at m/z 807.6, 835.6 and 863.6 corresponding to PI 32:1, 34:1 and 36:1 respectively. The peak of PI at m/z 835.6 was also investigated by PSD analysis; results indicated the presence of 18:1 and 16:0 chains (data not shown).
In the range of glycosphingolipids, a number of peaks are present. The peaks m/z 900–1000 are attributable to the inositolphosphoceramides (IPC), with a long chain base of 18 or 20 carbons, different level of hydroxylation, and linked fatty acyl residues with chain length of 26 carbons. The peaks at m/z 1114.8 and 1142.8 corresponds to mannosyl-inositolphosphoceramides (MIPC); larger mannosyl-diinositolphosphoceramide [M(IP)2C] molecules can be also seen from m/z 1340 to 1420 mixed with the cardiolipin peaks.. Notably, due to the lack of cardiolipin peaks in the lipid profile of the crd1Δ mutant, the glycopshingolipid peaks can be easily distinguished by comparing lipids of wild-type and mutant strains. However, the peaks of two MIPCs and two M(IP)2Cs were analyzed by PSD, confirming their structural identification (only two examples of these analyses are shown in spectra in supplementary Fig. II-A, B). A detailed list of glycosphingolipid assignments can be seen in Table 3.
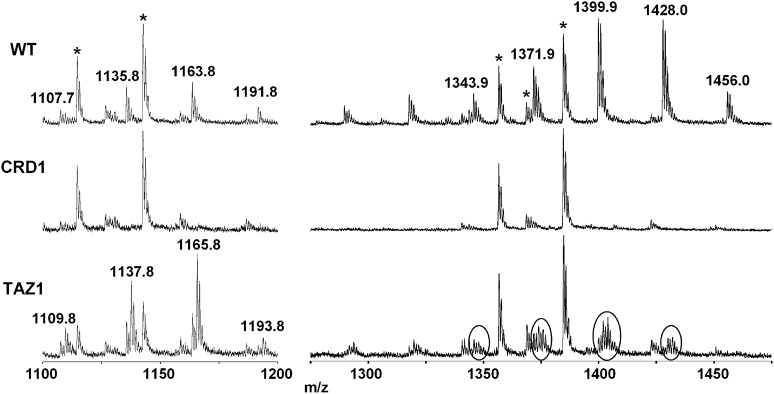
Fig. 3 shows two enlargements in the MLCLs and CLs ranges, respectively.
Fig. 3.
Enlargement of the negative MALDI-TOF/MS lipid profiles of intact yeast mitochondria (WT, crd1Δ and taz1Δ mutants) acquired using 9-AA as matrix, focus on yeast mitochondrial MLCLs and CLs. Peaks marked with an asterisk are attributable to inositolceramides (glycosphingolipids). The m/z signals corresponding to MLCLs and CLs with different chain lengths are (from the left): MLCL 48:3, [M-H]- at 1107.7; MLCL 50:3, [M-H]- at 1135.8; MLCL 52:3, [M-H]- at 1163.8; MLCL 54:3, [M-H]- at 1191.8; CL 64:4, [M-H]- at 1343.9; CL 66:4, [M-H]- at 1371.9; CL 68:4, [M-H]- at 1399.9; CL 70:4, [M-H]- at 1428.0; CL 72:4, [M-H]- at 1456.0. In the mitochondria from the crd1Δ mutant, neither MLCL nor CL is present. In the mitochondria from the taz1Δ mutant, a lower amount of CL and a higher amount of MLCL are present compared with the wild-type, and both MLCL and CL are present with unusual saturated fatty acyl chains.
In the lyso-CL range, peaks at m/z 1107.7, 1135.8, 1163.8, and 1191.8, can be attributed to MLCL 48:3, 50:3, 52:3, and 54:3, respectively.
In the CLs range, a large number of peaks are present, attributable to CLs of different chain lengths. Major peaks at m/z 1343.9, 1371.9, 1399.9, 1428.0. and 1456.0 are attributed to CL 64:4, 66:4, 68:4, 70:4, and 72:4, respectively. Again, PSD analyses of the two peaks at 1399.9 and 1428.0 confirmed assignments based on literature (spectra are available in supplementary Fig. III).
As previously reported in taz1Δ mutant, an aberrant CL-acyl composition occurs (35, 36). Circles in the taz1Δ mutant mass spectrum in Fig. 3 show the presence of CL molecular species containing saturated fatty acids, probably 16:0 and 18:0 instead of 16:1 and 18:1, as reported in the past literature (35, 36). CL molecular species 64:4 at m/z 1343.9, 66:4 at m/z 1371.9, 68:4 at m/z 1399.9, 70:4 at m/z 1428.0, and 72:4 at m/z 1456.0 are the most abundant in the wild-type. In contrast, CL molecular species with unsaturated fatty acids markedly decrease in the taz1Δ mutant, whereas the more saturated CLs, indicated by circles in the Fig. 3, are predominantly present. As a consequence, the acyl composition of the MLCLs species changes similarly in the taz1Δ mutant; peaks at m/z 1109.8, 1137.8, 1165.8 and 1193.8 are attributable to MLCL 48:2, 50:2, 52:2 and 54:2 respectively. For details see Table 2.
These data confirm the accumulation of MLCL and the decrease in unsaturated fatty acyl CL species in the taz1Δ yeast mutant (35).
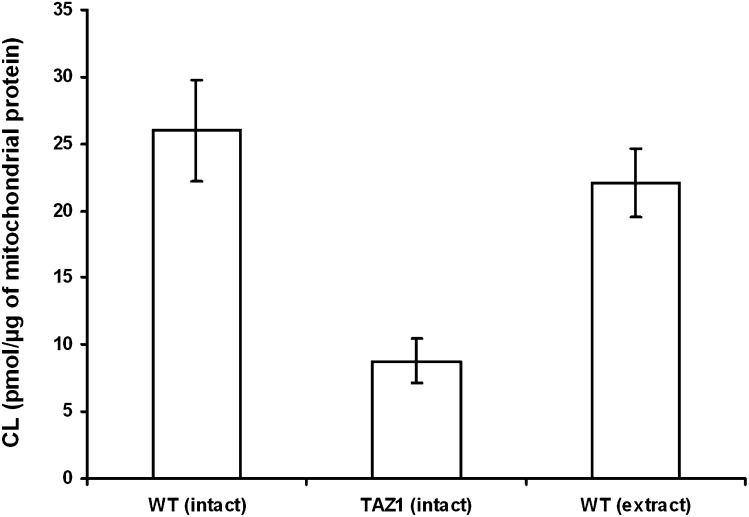
We quantified the CL content in the mitochondria of wild-type and taz1Δ mutant yeast, using the tetra-myristoyl-CL as an internal standard (Fig. 4). At the same time, one aliquot of wild-type mitochondria was extracted, and the internal standard was mixed to equilibrium to quantitate CL in the lipid extract and compare results. The CL content in the “intact” wild-type mitochondria was 26.0 (±3.8 SD) picomoles per microgram of total mitochondrial proteins, whereas CL in the lipid extract of the same mitochondrial preparation was 22.1 (±2.6 SD) picomoles per microgram of total mitochondrial proteins, indicating that results obtained with the two different methods are clearly comparable. In addition, the CL content in the “intact” taz1Δ mutant mitochondria was 8.8 (±1.7 SD) picomoles per microgram of total mitochondrial proteins. Therefore, using the “intact” method, the CL content in taz1Δ mutant was found to be 33.8% (±11.5 SD) of the wild-type. In the crd1Δ mutant, the CL is absent or present in traces, but notably some minor peaks attributable to molecular species of its precursor PG are present at m/z 745.5 (34:2), 747.5 (34:1), 775.6 (36:1), and 777.6 (36:0). For details, see Table 2; in addition, a scheme of the CL biosynthesis pathways in yeast is shown in supplementary Fig. IV.
Fig. 4.
Quantitation of CL content in yeast mitochondria. To quantify the CL content, the ratio of the peak area of the CL molecular species of interest to the internal standard CL (14:0)4 was determined and multiplied by the picomoles of internal standard present on the target. We have analyzed one preparation of each strain and collected 5–7 spectra per strain. Error bars represent SD. The CL content in the “intact” wild-type yeast mitochondria is 26.0 (±3.8 SD) picomoles per microgram of total mitochondrial proteins. The CL content in the “intact” taz1Δ mutant yeast mitochondria is 8.8 (±1.7 SD) picomoles per microgram of total mitochondrial proteins. The CL content in the lipid extract of wild-type yeast mitochondria is 22.1 (±2.6 SD) picomoles per microgram of total mitochondrial proteins.
A comparison of lipid profiles of the WT yeast intact mitochondria and the WT yeast mitochondrial lipid extract is shown in supplementary Fig. V. The peaks of cardiolipins, lysocardiolipins, and complex glycosphingolipids are smaller in the lipid extract than in the lipid profile of intact membranes, suggesting that these components, as expected, are partially lost during the extraction process. Further analysis of lipids associated with the remaining heterogeneous protein pellet after the lipid extraction revealed that the reduced and/or missing peaks in the lipid extract are better visible and/or enriched in this material. All together, above results show that the complete extraction of all lipid species is difficult to obtain.
Membranes isolated from Paracoccus denitrificans
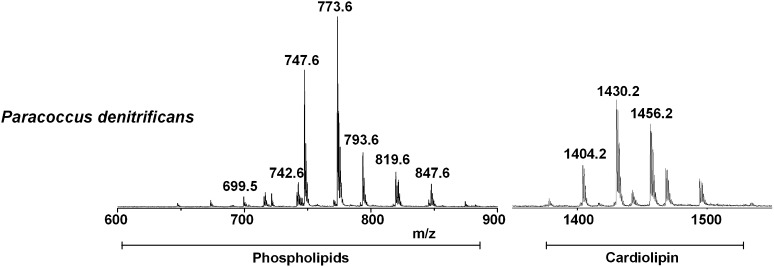
The validity of the new method was finally tested with intact membranes of Paracoccus denitrificans, a bacterium of close evolutionary relationship to mitochondria. Fig. 5 shows the MALDI-TOF mass spectrum of the intact membranes of Paracoccus denitrificans, acquired in the negative ion mode using 9-AA as matrix. A detailed list of signals in the spectra attributable to the negative [M-H]- molecular ions of phospholipids (PL) is presented in Table 4. The peaks can be grouped into two main m/z ranges: i) m/z 600–900, attributable to PLs and ii) m/z 1350–1450, to CLs. The m/z range 900–1350 was omitted because only some unidentified peaks were present. The CL m/z range was enlarged four times in the y axis to emphasize the CL signals pattern.
Fig. 5.
Negative MALDI-TOF/MS lipid profiles of intact membranes of Paracoccus denitrificans acquired using 9-AA as matrix. The m/z signals corresponding to the different lipid components are (from the left): PA 36:2, [M-H]- at 699.5; PE 36:2, [M-H]- at 742.6; PG 34:1, [M-H]- at 747.6; PG 36:2, [M-H]- at 773.6; PG 38:6, [M-H]- at 793.6; PG 40:5, [M-H]- at 819.6; PG 42:7, [M-H]- at 847.6; CL 68:2, [M-H]- at 1404.2; CL 70:3, [M-H]- at 1430.2; CL 72:4, [M-H]- at 1456.2. A detailed list of detected peaks is shown in Table 4.
TABLE 4.
Assignments of m/z values detected in the negative ion mode MALDI-TOF mass spectra of intact membranes of Paracoccus denitrificans using 9-AA as matrix
| m/zValue | Assignment [M-H]- |
| 647.3 | PA 32:0 |
| 673.5 | PA 34:1 |
| 699.5 | PA 36:2 |
| 715.6 | PG 32:3 |
| 716.6 | PE 34:1 |
| 721.6 | PG 32:0 |
| 741.6 | PG 34:4 |
| 742.6 | PE 36:2 |
| 744.6 | PE 36:1 |
| 745.6 | PG 34:2 |
| 747.6 | PG 34:1 |
| 773.6 | PG 36:2 |
| 1404.2 | CL 68:2 |
| 1430.2 | CL 70:3 |
| 1456.2 | CL 72:4 |
In the range of PLs, the peak at m/z 699.5 can be attributed to PA 36:2; the peak at m/z 742.6 is attributable to PE 36:2; and various species of PG are present at m/z 747.6 (34:1) and 773.6 (36:2).
In the CL range, a large number of peaks are present, attributable to CLs of different chain lengths. Major peaks at m/z 1404.2, 1430.2, and 1456.2 are attributed to CL 68:2, 70:3, and 72:4, respectively. A detailed list, including minor peaks, is available in Table 4. Results are comparable with and update the past literature (37).
DISCUSSION
Lipid isolation is, beyond doubt, a big and controversial chapter in lipidomics: the manipulation processes of biological samples used to perform lipid extraction and lipid separation techniques can introduce artifacts and hamper the accurate determination of the proportion of various lipid components in the biomembranes. For this reason, a two-step extraction method is often needed to recover almost all the lipid (80–99% of relatively apolar and 74–95% of polar lipids) that are present in a biological sample (38). If preparative TLC is used for lipid purification, a likely source of error can be represented by partial loss of sample during the extraction of lipids from the silica bands (39).
It has already pointed out that MALDI-TOF/MS is an important analytical technique in the emerging field of the single-cell metabolomics (40–43): the need to preserve the original metabolome during sample processing is a crucial aspect in studies of biological dynamics, and it is often a very difficult task because of the presence of enzymes in the sample and the fast metabolic turnover rates.
Here, we have presented a method of direct MALDI-TOF/MS analysis of lipids in intact mitochondria and bacterial membranes. A small drop of suspension of mitochondrial membranes or bacterial cells was deposited on the MALDI target and dried at room temperature, and then the sample was covered with a droplet of matrix (9-AA) solution and directly scanned with the MALDI laser to desorb and analyze the lipid content.
This study offers additional evidence that, as previously demonstrated by Schiller et al. (15–18), MALDI-TOF/MS can provide data comparable to other modern MS methods, but in a faster and more convenient way.
Our method promises not only fast and quick lipid analysis but also the possibility to extend the knowledge on mitochondrial lipidome previously studied by ESI-MS lipid extract analyses in many publications (38, 44–47).
It is indeed in line with a recent study in which ESI-MS, another “soft ionization” MS technique, has been used for direct analysis of intact membrane proteins: ATPases/synthases from T. termophilus and Enterococcus hirae have been analyzed as intact membrane domains and with soluble subunit interactions preserved in the vacuum during the MS analysis. This approach has brought about new insights in the regulatory effect of lipids bound to these transmembrane proteins (48).
Data reported show that all negatively charged membrane lipids can be detected by means of this method; phosphatidylcholine species can also be detected in intact membranes with 9-AA as matrix in the positive ion mode (not shown). Worthy of note, yeast inositol-containing ceramides, including inositolphosphoceramide, mannosyl-inositolphosphoceramide, and mannosyl-diinositolphosphoceramide, can be detected also.
Finally, the great potential of our technique is that it easily allows focusing on the signature lipid of mitochondria cardiolipin, a lipid component that is tightly bound to membrane proteins and that is often difficult to recover in lipid extracts.
Supplementary Material
Footnotes
Abbreviations:
- BHM
- bovine heart mitochondria
- CL
- cardiolipin
- IPC
- inositolphosphoceramide
- IS
- internal standard
- MALDI-TOF/MS
- MALDI time-of-flight mass spectrometry
- MIPC
- mannosyl-inositolphosphoceramide
- M(IP)2C
- mannosyl-diinositolphosphoceramide
- MLCL
- monolysocardiolipin
- PA
- phosphatidic acid
- PE
- phosphatidylethanolamine
- PG
- phosphatidylglycerol
- PI
- phosphatidylinositol
- PL
- phospholipid
- PS
- phosphatidylserine
- PSD
- post-source decay
- WT
- wild-type
- 9-AA
- 9-aminoacridine
This work was supported by Laboratory Sens&Micro LAB Project POFESR 2007–2013, code no. 15, of Apulia Region, Italy, and Ministero della Difesa Italiano Contract no. 1999 (A. Corcelli); and by DFG SFB472 and CEF-MC Project EXC 115 (B. Ludwig).
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of five figures.
REFERENCES
- 1.Guichardant M., Lagarde M. 1983. Phospholipid analysis and fatty acid content in platelets by the combination of high-performance liquid chromatography and glass capillary gas-liquid chromatography. J. Chromatogr. 275: 400–406 [DOI] [PubMed] [Google Scholar]
- 2.Fuchs B., Süß R., Teuber K., Eibisch M., Schiller J. 2011. Lipid analysis by thin-layer chromatography – a review of the current state. J. Chromatogr. A. 1218: 2754–2774 [DOI] [PubMed] [Google Scholar]
- 3.Seewald M., Eichinger H. M. 1989. Separation of major phospholipid classes by high-performance liquid chromatography and subsequent analysis of phospholipid-bound fatty acids using gas chromatography. J. Chromatogr. 469: 271–280 [DOI] [PubMed] [Google Scholar]
- 4.Viswanathan C. V. 1974. Coupled gas chromatography-mass spectrometry in the separation and characterization of polar lipids. J. Chromatogr. 98: 105–128 [DOI] [PubMed] [Google Scholar]
- 5.Lehmann W. D., Kessler M. 1983. Fatty acid profiling of phospholipids by field desorption and fast atom bombardment mass spectrometry. Chem. Phys. Lipids. 32: 123–135 [DOI] [PubMed] [Google Scholar]
- 6.Påhlsson P., Nilsson B. 1988. Fast atom bombardment-mass spectrometry of glycosphingolipids extracted from thin-layer chromatography plates. Anal. Biochem. 168: 115–120 [DOI] [PubMed] [Google Scholar]
- 7.Matsubara T., Hayashi A. 1991. FAB/mass spectrometry of lipids. Prog. Lipid Res. 30: 301–322 [DOI] [PubMed] [Google Scholar]
- 8.Brügger B., Erben G., Sandhoff R., Wieland F. T., Lehmann W. D. 1997. Quantitative analysis of biological membrane lipids at the low picomole level by nano-electrospray ionization tandem mass spectrometry. Proc. Natl. Acad. Sci. USA. 94: 2339–2344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Han X., Gross R. W. 2003. Global analysis of cellular lipidomes directly from crude extracts of biological samples by ESI mass spectrometry: a bridge to lipidomics. J. Lipid Res. 44: 1071–1079 [DOI] [PubMed] [Google Scholar]
- 10.Han X., Gross R. W. 2005. Shotgun lipidomics: multidimensional MS analysis of cellular lipidomes. Expert Rev. Proteomics. 2: 253–264 [DOI] [PubMed] [Google Scholar]
- 11.Schwudke D., Oegema J., Burton L., Entchev E., Hannich J. T., Ejsing C. S., Kurzchalia T., Shevchenko A. 2006. Lipid scanning by multiple precursor and neutral loss scanning driven by the data-dependent acquisition. Anal. Chem. 78: 585–595 [DOI] [PubMed] [Google Scholar]
- 12.Schwudke D., Liebisch G., Herzog R., Schmitz G., Shevchenko A. 2007. Shotgun lipidomics by tandem mass spectrometry under data-dependent acquisition control. Methods Enzymol. 433: 175–191 [DOI] [PubMed] [Google Scholar]
- 13.Meneses P., Glonek T. 1988. High resolution 31P NMR of extracted phospholipids. J. Lipid Res. 29: 679–689 [PubMed] [Google Scholar]
- 14.Sun G., Yang K., Zhao Z., Guan S., Han X., Gross R. W. 2008. Matrix-assisted laser desorption/ionization time-of-flight mass spectrometric analysis of cellular glycerophospholipids enabled by multiplexed solvent dependent analyte-matrix interactions. Anal. Chem. 80: 7576–7585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schiller J., Süß R., Arnhold J., Fuchs B., Leßig J., Muller M., Petkovic M., Spalteholz H., Zschörnig O., Arnold K. 2004. Matrix-assisted laser desorption and ionization time-of-flight (MALDI-TOF) mass spectrometry in lipid and phospholipid research. Prog. Lipid Res. 43: 449–488 [DOI] [PubMed] [Google Scholar]
- 16.Schiller J., Süß R., Fuchs B., Muller M., Zschörnig O., Arnold K. 2007. MALDI-TOF MS in lipidomics. Front. Biosci. 12: 2568–2579 [DOI] [PubMed] [Google Scholar]
- 17.Fuchs B., Schiller J. 2008. MALDI-TOF MS analysis of lipids from cells, tissues and body fluids. Subcell. Biochem. 49: 541–565 [DOI] [PubMed] [Google Scholar]
- 18.Fuchs B., Süß R., Schiller J. 2010. An update of MALDI-TOF mass spectrometry in lipid research. Prog. Lipid Res. 49: 450–475 [DOI] [PubMed] [Google Scholar]
- 19.Fuchs B., Schiller J., Süß R., Schürenberg M., Suckau D. 2007. A direct and simple method of coupling matrix-assisted laser de-sorption ionization time-of-flight mass spectrometry (MALDI-TOF MS) to thin-layer chromatography (TLC) for the analysis of phospholipids from egg yolk. Anal. Bioanal. Chem. 389: 827–834 [DOI] [PubMed] [Google Scholar]
- 20.Fuchs B., Nimptsch A., Süß R., Schiller J. 2008. Analysis of brain lipids by directly coupled matrix-assisted laser desorption ionization time-of-flight mass spectrometry and high-performance thin-layer chromatography. J. AOAC Int. 91: 1227–1236 [PubMed] [Google Scholar]
- 21.Fuchs B., Schiller J., Süß R., Zscharnack. A. Bader M., Müller P., Schürenberg M., Becker M., Suckau D. 2008. Analysis of stem cell lipids by offline HPTLC-MALDI-TOF MS. Anal. Bioanal. Chem. 392: 849–860 [DOI] [PubMed] [Google Scholar]
- 22.Angelini R., Corral P., Lopalco P., Ventosa A., Corcelli A. 2012. Novel ether lipid cardiolipin in archaeal membranes of extreme haloalkaliphiles. Biochim. Biophys. Acta. 1818: 1365–1373 [DOI] [PubMed] [Google Scholar]
- 23.Murphy R. C., Hankin J. A., Barkley R. M. 2009. Imaging of lipid species by MALDI mass spectrometry. J. Lipid Res. 50: S317–S322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berry K. A., Hankin J. A., Barkley R. M., Spraggins J. M., Caprioli R. M., Murphy R. C. 2011. MALDI imaging of lipid biochemistry in tissues by mass spectrometry. Chem. Rev. 111: 6491–6512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murphy R. C., Henkin J. A., Barkley R. M., Zemski Berry K. A. 2011. MALDI imaging of lipids after matrix sublimation/deposition. Biochim. Biophys. Acta. 1811: 970–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Angelini R., Babudri F., Lobasso S., Corcelli A. 2010. MALDI-TOF/MS analysis of archaebacterial lipids in lyophilized membranes dry-mixed with 9-aminoacridine. J. Lipid Res. 51: 2818–2825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferreira C. R., Saraiva S. A., Catharino R. R., Garcia J. S., Gozzo F. C., Sanvido G. B., Santos L. F., Lo Turco E. G., Pontes J. H., Basso A. C., et al. 2010. Single embryo and oocyte lipid fingerprinting by mass spectrometry. J. Lipid Res. 51: 1218–1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Angelini R., Lopalco P., Lobasso S., Corcelli A. 2011. Direct MALDI-TOF/MS analyses of cardiolipin in intact membranes. Chem. Phys. Lipids. 164: S46 [Google Scholar]
- 29.Jiang F., Rizavi H. S., Greenberg M. L. 1997. Cardiolipin is not essential for the growth of Saccharomyces cerevisiae on fermentable or non-fermentable carbon sources. Mol. Microbiol. 26: 481–491 [DOI] [PubMed] [Google Scholar]
- 30.Ma L., Vaz F. M., Gu Z., Wanders R. J., Greenberg M. L. 2004. The human TAZ gene complements mitochondrial dysfunction in the yeast taz1Δ mutant. Implications for Barth syndrome. J. Biol. Chem. 279: 44394–44399 [DOI] [PubMed] [Google Scholar]
- 31.Daum G., Bohni P. C., Schatz G. 1982. Import of proteins into mitochondria. Cytochrome b2 and cytochrome c peroxidase are located in the intermembrane space of yeast mitochondria. J. Biol. Chem. 257: 13028–13033 [PubMed] [Google Scholar]
- 32.Low H., Vallin I. 1963. Succinate-linked diphosphopyridine nucleotide reduction in submitochondrial particles. Biochim. Biophys. Acta. 69: 361–374 [DOI] [PubMed] [Google Scholar]
- 33.Ludwig B. 1986. Cytochrome c oxydase from Paracoccus denitrificans. Methods Enzymol. 126: 153–159 [DOI] [PubMed] [Google Scholar]
- 34.Corcelli A., Saponetti M. S., Zaccagnino P., Lopalco P., Mastrodonato M., Liquori G. E., Lorusso M. 2010. Mitochondria isolated in nearly isotonic KCl buffer: focus on cardiolipin and organelle morphology. Biochim. Biophys. Acta. 1798: 681–687 [DOI] [PubMed] [Google Scholar]
- 35.Gu Z., Valianpour F., Chen S., Vaz F. M., Hakkaart G. A., Wanders R. J., Greenberg M. L. 2004. Aberrant cardiolipin metabolism in the yeast taz1 mutant: a model for Barth Syndrome. Mol. Microbiol. 51: 149–158 [DOI] [PubMed] [Google Scholar]
- 36.Schlame M., Ren M., Xu Y., Greenberg M. L., Haller Y. 2005. Molecular symmetry in mitochondrial cardiolipins. Chem. Phys. Lipids. 138: 38–49 [DOI] [PubMed] [Google Scholar]
- 37.Wilkinson B. J., Morman M. R., White D. C. 1972. Phospholipid composition and metabolism of Micrococcus denitrificans. J. Bacteriol. 112: 1288–1294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ejsing C. S., Sampaio J. L., Surendranath V., Duchoslav E., Ekroos K., Klemm R. W., Simons K., Shevchenko A. 2009. Global analysis of the yeast lipidome by quantitative shotgun mass spectrometry. Proc. Natl. Acad. Sci. USA. 106: 2136–2141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kates M. 2010. Techniques of Lipidology: Isolation, Analysis and Identification of Lipids. 3rd revised edition. Newport Somerville Innovation Ltd., Ottawa, Canada. [Google Scholar]
- 40.Li L., Garden R. W., Sweedler J. V. 2000. Single-cell MALDI: a new tool for direct peptide profiling. Trends Biotechnol. 18: 151–160 [DOI] [PubMed] [Google Scholar]
- 41.Amantonico A., Urban P. L., Fagerer S. R., Balabin R. M., Zenobi R. 2010. Single-cell MALDI-MS as an analytical tool for studying intrapopulation metabolic heterogeneity of unicellular organisms. Anal. Chem. 82: 7394–7400 [DOI] [PubMed] [Google Scholar]
- 42.Amantonico A., Urban P. L., Zenobi R. 2010. Analytical techniques for single-cell metabolomics: state of the art and trends. Anal. Bioanal. Chem. 398: 2493–2504 [DOI] [PubMed] [Google Scholar]
- 43.Heinemann M., Zenobi R. 2011. Single cell metabolomics. Curr. Opin. Biotechnol. 22: 26–31 [DOI] [PubMed] [Google Scholar]
- 44.Guan X. L., Wenk M. R. 2006. Mass spectrometry-based profiling of phospholipids and sphingolipids in extracts from Saccharomyces cerevisiae. Yeast. 23: 465–477 [DOI] [PubMed] [Google Scholar]
- 45.Guan X. L., Riezman L., Wenk M. R., Riezman H. 2010. Yeast lipid analysis and quantification by mass spectrometry. Methods Enzymol. 470: 369–391 [DOI] [PubMed] [Google Scholar]
- 46.Ejsing C. S., Moehring T., Bahr U., Duchoslav E., Michael K., Simons K., Shevchenko A. 2006. Collision-induced dissociation pathways of yeast sphingolipids and their molecular profiling in total lipid extracts: a study by quadrupole TOF and linear ion trap-orbitrap mass spectrometry. J. Mass Spectrom. 41: 372–389 [DOI] [PubMed] [Google Scholar]
- 47.Shui G., Guan X. L., Low C. P., Chua G. H., Goh J. S. Y., Yang H., Wenk M. R. 2010. Toward one step analysis of cellular lipidomes using liquid chromatography coupled with mass spectrometry: application to Saccharomyces cerevisiae and Schizosaccharomyces pombe lipidomics. Mol. Biosyst. 6: 1008–1017 [DOI] [PubMed] [Google Scholar]
- 48.Zhou M., Morgner N., Barrera N. P., Politis A., Isaacson S. C., Matak-Vinković D., Murata T., Bernal R. A., Stock D., Robinson C. V. 2011. Mass spectrometry of intact V-type ATPases reveals bound lipids and the effects of nucleotide binding. Science. 334: 380–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.