Abstract
We modified microscopy for acid-fast bacilli to diagnose tuberculosis (TB) using small membrane filters (SMFs) after special processing and prefiltration. With the first specimen obtained from each of 335 persons suspected of having TB, the sensitivity of the new SMF method using fluorescence microscopy (FM) was 89% (95% confidence interval [CI]: 80%, 95%). This was significantly better (P = 0.0001) than the sensitivity of routine FM of centrifuged specimens of 60% (95% CI: 49%, 71%) or that of direct sputum smears of 56% (95% CI: 40%, 72%).
TEXT
The diagnosis of pulmonary tuberculosis (TB) in most of the world relies on microscopy of sputum smears for acid-fast bacilli (AFB). There are new diagnostic tests for TB, but most are relatively expensive. Although AFB microscopy has been castigated due to its poor sensitivity (5), most clinical laboratories in settings where TB is endemic use microscopes for a variety of purposes.
Little attention has been paid to improving the quality of the specimen viewed under the microscope beyond centrifugation. Although centrifugation improves the sensitivity of microscopy, some bacilli are lost in the supernatant (3) and there is a risk of aerosolization if the centrifuge is not adequately protected (4). Centrifuges are expensive, require electricity, and need maintenance. An alternative was developed almost 30 years ago by filtration of sputum digests through 25-mm-diameter polycarbonate membrane filters (PMFs) with 1.0-μm pores, improving the sensitivity of AFB microscopy to 80% (5). Now that 13-mm-diameter PMFs are commercially available, we hypothesized that the use of these small membrane filters (SMFs) could increase the sensitivity of AFB microscopy due to the exponential increase in the density of bacilli concentrated in a smaller area, given that the area of a circle equals π × r2.
Method development.
We pooled deidentified routine sputum specimens that were to be discarded by the clinical microbiology laboratory. Working sputum samples were spiked with Mycobacterium bovis BCG to 20,000 to 40,000 bacilli/ml and then digested with N-acetylcysteine at 25 mg/ml, mixed at 1:1 (vol/vol). These samples were then treated with 6% sodium hypochlorite at 4:1. One-milliliter samples of this mixture were filtered through 25- and 13-mm PMFs with 0.4-μm pores. In three experiments, there was a significant increase (1,000×) in the number of bacilli counted by fluorescence microscopy (FM) and the 13-mm PMFs, with mean numbers of bacilli per microscopic field of 0.04, 2.13, and 14.1 for the sputum smears and 25- and 13-mm PMFs, respectively (P = 0.03, repeated-measures analysis of variance).
Field trial.
The SMF method was then evaluated at the Núcleo de Doenças Infecciosas (NDI), Universidade Federal do Espírito Santo, Vitória, ES, Brazil, where a central microbiology laboratory receives specimens from six TB clinics. In pilot studies, we found that filtration of fresh sputum digests through SMFs with a 0.4-μm pore size was too slow and that some SMFs clogged. So we modified our method by adding 95% ethanol (5) and 1% Triton X detergent (6) to the bleach, using SMFs with an 0.8-μm pore size, and prefiltering the sputum digests through 25-mm polypropylene prefilters with 30-μm pores (Millipore). The SMFs were placed in syringe filter holders connected to a vacuum manifold (Fig. 1 and 2). After filtration, the SMFs were removed, placed on microscope slides, and dried on a slide warmer. Black SMFs were stained on the slides by the auramine method, and white SMFs were stained by the Kinyoun method.
Fig 1.
Assembly of small (13-mm) PMFs in stainless steel syringe filter holders.
Fig 2.
Pipetting of prefiltered sputum digests into 20-ml syringe barrels attached to syringe filter holders loaded with SMFs. The vacuum manifold is made of plastic pipe and valves at a cost of approximately $25. It is connected to a flask, and a vacuum is generated with a hand pump, so no electricity is needed.
We screened 410 persons suspected of having TB, and 75 were excluded because they were <18 years old, were unable to produce sputum, had a low suspicion of having TB according to our physician, or would not consent. After informed consent was obtained, specimens were collected from subjects not yet on treatment and were split for routine sputum smears and cultures versus the SMF method (2) (Fig. 3). Two experienced technicians, each blinded to the results of the other, examined sputum smears independently. Of the 335 patients enrolled, 21 were excluded from the analysis because of contaminated cultures. Most of the subjects were young to middle aged (mean age, 36.7 [standard deviation, 14.08] years), were male (77%), were HIV negative (87%; 3.6% positive, 9.5% missing), and had noncavitary chest radiographs (77%).
Fig 3.
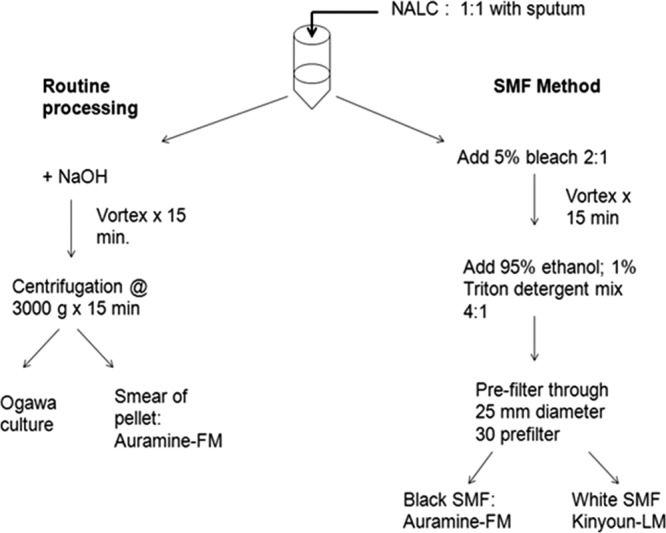
Schema of dividing and processing of sputum specimens.
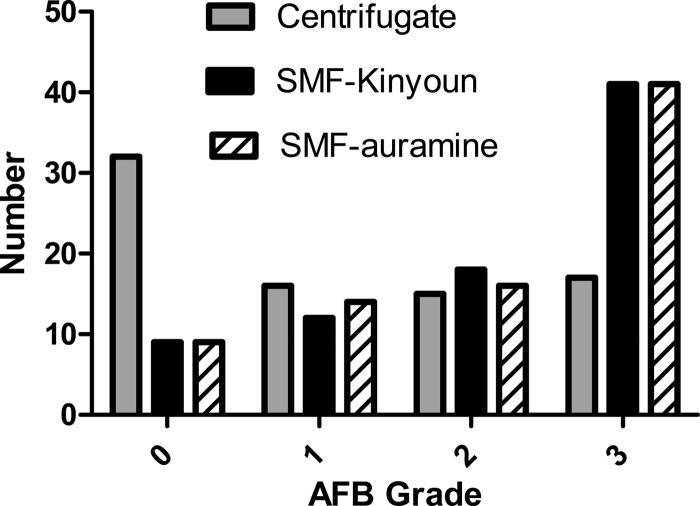
On microscopy of the SMFs, there was an increased density of bacilli (Fig. 4) and higher AFB sputum smear grades (Fig. 5). For sensitivity and specificity analyses, culture on Ogawa solid medium was considered the reference standard. Considering only the first specimen, the sensitivity of AFB microscopy was 89% using the SMFs with either light microscopy (LM) or FM, significantly better than the 56.1% of direct sputum smears or 60.2% of FM of centrifuged specimens (P < 0.0001; Tables 1 and 2). The specificity was 100% for the direct and centrifuged sputum smears and 99.6% for the SMF methods due to one AFB-positive, culture-negative case.
Fig 4.

AFB observed by LM of direct sputum smears (A), centrifuged specimens (B), and SMFs by the Kinyoun method. (C). Photographs were obtained with a magnification of ×1,000. All sputum smears were prepared from the same sample.
Fig 5.
Distribution of AFB microscopy grades among 80 culture-positive sputum specimens with complete data for all of the methods used in this study.
Table 1.
Results of AFB microscopy of first sputum specimens using direct sputum smears, centrifuged sputum smears, and SMFs
| Test and resulta | No. of specimens |
|
|---|---|---|
| Culture positive | Culture negative | |
| Direct sputum smear microscopy | ||
| Positive | 23 | 0 |
| Negative | 18 | 151 |
| Centrifuged sputum smear FM | ||
| Positive | 50 | 0 |
| Negative | 33 | 231 |
| SMF LM | ||
| Positive | 73 | 1 |
| Negative | 9 | 230 |
| SMF FM | ||
| Positive | 72 | 1 |
| Negative | 9 | 230 |
LM, LM using Kinyoun staining; FM, FM using auramine staining.
Table 2.
Comparison of results of AFB microscopy of first sputum specimens using direct smears, centrifuged smears, and SMFs for the diagnosis of pulmonary TB with Ogawa culture as the reference standard
| Methoda | % Sensitivity | % Specificity | % PPVb | % NPVc |
|---|---|---|---|---|
| Direct sputum smear microscopy | 56.1 (39.8, 71.5)d | 100.0 (97.6, 100) | 100 (85.2, 100) | 89.4 (83.7, 93.6) |
| Centrifuged sputum smear microscopy | 60.2 (48.9, 70.8) | 100.0 (98.4, 100) | 100 (92.9, 100) | 87.5 (82.9, 91.2) |
| SMF LM | 89.0 (80.2, 94.9) | 99.6 (97.6, 100) | 98.7 (92.7, 100) | 96.2 (93.0, 98.3) |
| SMF FM | 88.9 (80.0, 94.8) | 99.6 (97.6, 100) | 98.6 (92.6, 100) | 96.2 (93.0, 98.3) |
LM, LM using Kinyoun stain; FM, FM using auramine stain.
PPV, positive predictive value.
NPV, negative predictive value.
Values in parentheses are 95% CIs.
Among those with sputum smear-negative, culture-positive TB, the sensitivity of the SMF method using FM was 73% (24/33), similar to that of the Xpert MTB/RIF assay, although the latter confirms identification to the species level and rifampin resistance (1). As another approach to assess sensitivity in paucibacillary disease, we classified the 83 culture-positive patients as either “high CFU” (>200 CFU) or “low CFU” (1 to 200 CFU). In the high-CFU group, the sensitivities were 80% with both direct and centrifuged sputum smears and 100% for SMFs with either LM or FM. In the low-CFU group, the sensitivities were 19% with direct sputum smears, 32% with centrifuged specimens, and 73% with SMFs using either LM or FM (Table 3). Given this incremental gain in sensitivity of over 40%, the SMF method shows promise for improvement of the diagnosis of paucibacillary specimens by microscopy.
Table 3.
Sensitivities of AFB microscopy methods w low-CFU versus high-CFU cultures of first sputum specimens
| Method | % Sensitivity (95% CI) |
|
|---|---|---|
| Low CFUa | High CFUb | |
| Direct sputum smear microscopy | 19 (7, 43) | 80 (61, 91) |
| Centrifuged sputum smear FM | 32 (19, 49) | 80 (66, 89) |
| SMF Kinyoun stain LM | 73 (56, 85) | 100 |
| SMF auramine stain FM | 73 (56, 85) | 100 |
Low, 1 to 200 CFU; n = 34.
High, >200 CFU; n = 49.
In the subset of 55 subjects with two uncontaminated sputum specimens, the sensitivity of either LM or FM of SMFs (97%) was significantly greater than that of FM of centrifuged specimens (70%) (P = 0.001), using a reference standard of a positive culture for either specimen. The specificity of each method was 100%.
These data clearly demonstrate that the SMF method significantly improved the sensitivity of AFB microscopy in this setting. The retail cost of the prefilter and SMF was approximately $1.50 per test, and the method uses mostly reusable supplies and easily obtained reagents. It can be done without electricity, an advantage in many settings. It is sensitive and specific, and it is relatively user friendly, except for technical difficulties in removing the SMFs from the syringe filter holders for staining. This method can be implemented immediately, and it could potentially facilitate same-day diagnosis. Further studies are needed to assess costs, training, quality control, and work flow issues and to determine how the SMF method might be used most effectively in the diagnosis and management of TB.
ACKNOWLEDGMENTS
This study was funded by World Health Organization Special Programme for Research and Training in Tropical Diseases (TDR) grant A30499 and NDI intramural funds.
We thank the patients and staff of the TB clinics in the facilitation of this study. Kevin P. Fennelly thanks Nancy Reilly for her assistance in the preparation of IRB forms and consents; Linda Birnbaum for early technical assistance; and Jerrold Ellner, Robert Wallis, and Mark Hernandez for their support and mentorship.
Footnotes
Published ahead of print 14 March 2012
REFERENCES
- 1. Boehme CC, et al. 2010. Rapid molecular detection of tuberculosis and rifampin resistance. N. Engl. J. Med. 363:1005–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hadad DJ, et al. 2012. Evaluation of processing methods to equitably aliquot sputa for mycobacterial testing. J. Clin. Microbiol. 50:1440–1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Klein GC, Maltz M, Cummings MM, Fish CH. 1952. Efficacy of centrifugation as a method of concentrating tubercle bacilli. Am. J. Clin. Pathol. 22:581–585 [DOI] [PubMed] [Google Scholar]
- 4. Sewell DL. 1995. Laboratory-associated infections and biosafety. Clin. Microbiol. Rev. 8:389–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Smithwick RW, Stratigos CB. 1981. Acid-fast microscopy on polycarbonate membrane filter sputum sediments. J. Clin. Microbiol. 13:1109–1113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yazdankhah SP, Sorum H, Larsen HJ, Gogstad G. 2001. Rapid method for detection of gram-positive and -negative bacteria in milk from cows with moderate or severe clinical mastitis. J. Clin. Microbiol. 39:3228–3233 [DOI] [PMC free article] [PubMed] [Google Scholar]