Abstract
In the last few decades, the importance of selenium in human health has been the subject of numerous studies. It is believed that the physiological effects of selenium occur mainly through the function of selenoproteins, which incorporate selenium in the form of one or more selenocysteine residues. Recent advances in understanding the complex regulation of selenoprotein synthesis and functional characterization of several members of the selenoprotein family have contributed to an improved comprehension of the role(s) of selenium in human health and the great diversity of physiological pathways influenced by this trace element.
Selenium was discovered by the Swedish chemist Berzelius in 1817, but a biological role for this trace element remained unknown until 1957 when Schwarz and Foltz showed that selenium deficiency could cause necrotic liver degeneration (72). However, the first real understanding of the physiological basis for a selenium nutritional requirement did not occur until 1973, when it was shown that selenium was an essential component of mammalian enzymes like glutathione peroxidases (GPx) (29, 66). It is now well established that selenium plays an important biological role in living organisms, mostly through its incorporation in a family of proteins called selenoproteins. The main biological form of selenium is selenocysteine (Sec), a cysteine analog that is synthesized from a serine bound to tRNA (1). Sec is identical to cysteine except for the fact that, in place of sulfur, it contains a selenium atom, which is typically ionized at physiological pH (44). In several instances, replacement of Sec by cysteine in a selenoprotein has been shown to result in a dramatic decrease of enzymatic activity (32, 52), supporting the concept that the ionized selenium atom is critical for proper protein function.
The single unifying, and defining, feature of selenoproteins is the fact that they all include one or more Sec residues in their primary structure. To date, all selenoproteins with known functions, with the exception of selenoprotein P (see below), appear to have enzymatic activities in which the Sec residue is located at the catalytic site, where it likely participates in redox reactions (47). However, the amino acid sequences, enzymatic activities, tissue distribution of expression, and other molecular features of the different family members are extremely varied. Similarly, at the physiological level, these enzymes are involved in diverse metabolic and physiological functions ranging from antioxidant defense (6) to fertility (30), muscle development and function (65), thyroid hormone metabolism, and immune function (4). Consequently, the range of pathologies associated with primary or secondary defects of selenoprotein function is enormous, with no easily definable unifying feature to tie together this disparate group of phenotypes at the pathophysiological level.
Selenoprotein Biosynthesis
The incorporation of Sec, which is considered to be the 21st amino acid, occurs in a unique and peculiar way; in fact, the Sec codon is an in-frame UGA, which normally corresponds to a termination codon. The recognition of UGA as a Sec codon, instead of a translational stop signal, requires the presence of a stem loop sequence called SECIS (SEC insertion sequence), which typically resides several hundred to several thousand base pairs downstream of the UGA codon in the 3′ untranslated regions of eukaryotic selenoprotein transcripts. As shown in FIGURE 1, the process of Sec codon recognition and Sec insertion requires several trans-acting factors including tRNASec, a Sec-specific elongation factor, and SECIS-binding proteins (1, 11). Interestingly, the targeted deletion of tRNASec gene (Trsp) results in an embryonic lethal phenotype in mice (9). Since Trsp governs the production of all selenoproteins, this suggests that at least some selenoproteins are crucial for early embryonic development, and this idea is further supported by the observation that knockout of thioredoxin reductases (TrxRs) can also be embryonic lethal (Table 1) (18, 46).
FIGURE 1.
Schematic showing cis- and trans-acting elements required for selenocysteine incorporation into selenoproteins
Table 1.
Overview of human selenoprotein expression and function
| Protein | Gene | Transcript Expression | Protein Localization | Function | Knockout Phenotype |
|---|---|---|---|---|---|
| Glutathione peroxidases | |||||
| Cytosolic GPx (cGPx, GPx-1) | GPX1 | Ubiquitous | Cytosol | Antioxidant Catalyzes the reduction of H2O2 and various soluble organic peroxides |
No apparent phenotype in unchallenged mice. Survival decreased 8-fold compared with wild-type mice when exposed to lethal doses of pro-oxidant, more susceptible to myocarditis when infected with coxsackievirus (1, 11, 12). |
| Gastrointestinal GPx (GI-GPx, GPx-2) | GPX2 | Gastrointestinal tract | Cytosol | Antioxidant Catalyzes the reduction of various peroxides |
No apparent phenotype even after exposure to γ-radiation; double KO Gpx-1 and Gpx-2 present symptoms and histopathology consistent with inflammatory bowel disease (24, 25). |
| Plasma GPx (pGPx, GPx-3) | GPX3 | Kidney, plasma | Extracellular | Antioxidant Catalyzes the reduction of H2O2 and various soluble organic peroxides, its enzymatic activity is 10% of GPx1 |
n.d. |
| Phospholipid hydroperoxide GPx (PHGPx, GPx-4) | GPX4 | Various tissues including brain | Cytosol and membrane associated | Antioxidant Can directly reduce phospholipid and cholesterol hydroperoxides |
Homozygous mutant is embryonic lethal; heterozygous are fertile and seem normal but have increased sensitivity to oxidative stress (76). |
| Sperm nuclei GPx (snGPx) | GPX4 | Testis | Nucleus | Involved in sperm maturation and male fertility. Acts as a protamine thiol peroxidase responsible for disulfide cross-linking, it is necessary in chromatin condensation of spermatids. | |
| GPx-6 | GPX6 | n.d. | n.d. | n.d. | n.d. |
| Thioredoxin reductases | |||||
| Thioredoxin reductase 1 (TrxR1) | TXNRD1 | Ubiquitous | Cytosol, mitochondria | Catalyzes NADPH-dependent reduction of oxidized thioredoxin which is involved in various redox systems (ribonucleotide reductase essential for DNA synthesis, regulation of transcription factors, cell growth, etc.) | Ubiquitous disruption is embryonic lethal due to severe impairment of cell proliferation; mice with cardiac-specific disruption of the gene develop normally and seem healthy (43). |
| Thioredoxin reductase 2 (TrxR2, SelZf1, SelZf2) | TXNRD2 | Liver, kidney, heart | Mitochondria | Similar to TrxR1 | Ubiquitous disruption is embryonic lethal with smaller and severely anemic embryos showing increased apoptosis in liver; cardiac-specific disruption of the gene results in fatal dilated cardiomyopathy (17). |
| Thioredoxin reductase 3 (TrxR3, TGR) | TXNRD3 | Testis | Cytosol | Catalyzes the reduction of thioredoxin and glutathione; has a disulfide bond isomerization activity probably involved in spermatogenesis. | n.d. |
| Iodothyronine deiodinases | |||||
| Type 1 deiodinase (DIO1, IOD1, D1) | DI01 | Thyroid, liver, kidney, pituitary | Plasma membrane | Converts thyroid prohormone T4 to active hormone T3 by catalyzing the removal of iodine from T4 | Fertile and apparently healthy mice, serum level of T4 and rT3 were elevated, but TSH and T3 levels were unchanged. The metabolism and excretion of iodothyronines is markedly changed (63). |
| Type 2 deiodinase (DIO2, IOD2, D2) | DIO2 | Thyroid, brain, heart, intestine, skeletal muscle | Endoplasmic reticulum membrane | Similar to DIO1 | No gross phenotypic abnormality; serum T4 and TSH elevated by 40% and 100%, respectively, suggesting the pituitary gland has become resistant to plasma T4 feedback effect (62). |
| Type 3 deiodinase (DIO3, IOD3, D3) | DIO3 | Brain, placenta, skeletal muscle | Plasma membrane | Converts thyroid hormone T3 to inactive rT3 by catalyzing the removal of iodine from T3 | n.d. |
| Selenophosphate synthetase (SPS2) | SEPHS2 | Ubiquitous | Cytosol | Catalyzes the reaction of selenide with AMP producing selenophosphate, which provides selenium for the biosynthesis of selenocysteine | n.d. |
| Selenoprotein S (SelS, VIMP) | SEPS1 | n.d. | Endoplasmic reticulum | Influences inflammatory response | n.d. |
| Selenoprotein P (SEPP1, SelP) | SEPP1 | Ubiquitous (predominant in liver) | Secreted protein | May act as antioxidant and selenium transporter | Homozygous mutant mice are viable but, when fed a low-selenium diet, they lose weight and develop poor motor coordination. Males are infertile due to flagellar structural defects and show a 43% reduction of brain selenium. High-selenium diet does not restore male infertility but brings the brain selenium content to levels comparable to wild-type mice (35, 36). |
| Selenoprotein 15 kDa (Sel15) | SEP15* | Prostate, thyroid, parathyroid | Endoplasmic reticulum | Has thiol-disulfide isomerase activity, possibly involved in disulfide bond formation in the endoplasmic reticulum. Loss of heterozygosity at SEP15 locus is associated with cancer. | n.d. |
| Selenoprotein N (SelN) | SEPN1 | Ubiquitous | Endoplasmic reticulum membrane | Unknown, absence causes myopathy | n.d. |
| Selenoprotein X (SelX or SelR) | SEPX1 | Pancreas, liver, kidney, leukocytes | Cytosol | Methionine sulfoxide reductase | n.d. |
| Selenoprotein W (SelW) | SEPW1 | Skeletal muscle, heart, | Cytosol | n.d. | n.d. |
| Selenoprotein T (SelT) | SEPT1* | Ubiquitous (predominant in prostate) | n.d. | n.d. | n.d. |
| Selenoprotein H | SELH* | n.d. | Cytosol | n.d. | n.d. |
| Selenoprotein I | SELI* | n.d. | Cytosol | n.d. | n.d. |
| Selenoprotein K | SELK* | n.d. | Plasma membrane | n.d. | n.d. |
| Selenoprotein M | SELM* | n.d. | Perinuclear | Has thiol-disulfide isomerase activity, possibly involved in disulfide bond formation in the endoplasmic reticulum. | n.d. |
| Selenoprotein O | SELO* | n.d. | Cytosol | n.d. | n.d. |
| Selenoprotein V | SELV* | n.d. | Cytosol | n.d. | n.d. |
n.d., Not determined.
Gene symbol not yet approved by gene nomenclature consortium.
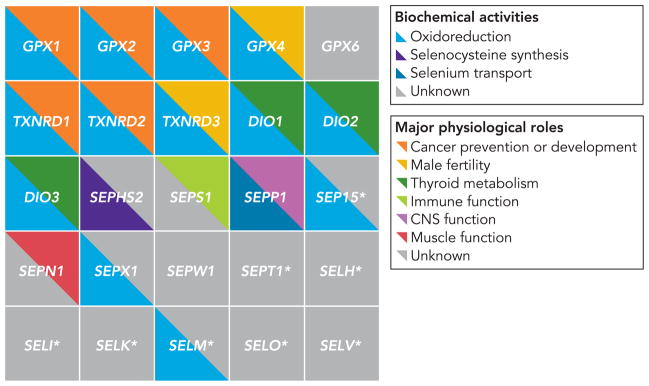
By taking advantage of the conserved secondary structure of SECIS elements, Kryukov et al. used a bioinformatic approach to scan the human genome for potential SECIS elements and then searched for open reading frames with in-frame UGA codons upstream of the SECIS elements (49). By using this method, the authors not only found the previously described selenoproteins but also identified seven new ones, bringing the total number of known human selenoproteins to 25 (Table 1 and FIGURE 2). With the aid of comparative analysis of rat, mouse, and other species’ genomes, the authors suggest that this number likely represents the entire human selenoproteome.
FIGURE 2. Biochemical and physiological roles of selenoproteins.
Major known activities and roles for the 25 selenoproteins identified in humans are indicated. Many proteins likely have multiple other functions not indicated here. Involvement in oxidoreduction reactions is a unifying feature of this protein family; however, there is great diversity in tissue distribution and physiological pathways where these reactions occur. The biochemical activities and/or physiological roles of more than half these proteins remain unknown. CNS, central nervous system.
Selenium supplementation has been suggested to have a preventive and sometimes therapeutic role in different diseases, such as cancer (17, 43), male infertility (74), viral infection (54), and immune system function (48). It is believed that this beneficial effect occurs mostly, but probably not exclusively, through the function of selenoproteins. In this review, we focus on diseases for which the direct involvement of individual selenoproteins has been documented or strongly suspected.
GPx and Disease
The GPxs are a family of closely related antioxidant enzymes encoded, in humans, by the GPX1 to GPX6 genes. With the exception of GPX5, all family members encode selenium-containing proteins (Table 1).
Viral infection
Nutritional deficiency of selenium has been associated with Keshan disease, a dilated cardiomyopathy formerly endemic in regions of China where selenium concentration in the soil is particularly low (33, 83). Remarkably, the simple use of selenium-fortified table salt by populations living in those regions resulted in a significant decrease in the incidence of this disease (14). Recent studies provide a better understanding of the role of selenium and GPxs in this disease. In fact, coxsackievirus has been isolated from blood and tissues of patients with Keshan disease and is now considered to be a cofactor in development of this cardiomyopathy (53). Mice infected with certain virulent strains of coxsackievirus develop a heart disease similar to the one found in Keshan disease. When mice fed with an adequate amount of selenium and vitamin E are infected with a benign strain of coxsackievirus, they do not develop any cardiomyopathy, whereas the infection of selenium-deficient mice with the same benign strain of virus results in a cardiomyopathy. GPX1 knockout mice infected with the benign strain of coxsackievirus also develop the disease, even when fed with adequate amounts of selenium. It appears that the absence of GPx-1 in these mice allows accumulation of mutations in the viral RNA genome that make them virulent (2). Thus the cardiomyopathy in Keshan disease patients is likely related to decreased activity of GPx-1, secondary to selenium deficiency, which allows the accumulation of coxsackievirus mutations, hence converting benign strains into cardiotoxic ones.
The effect of selenium on HIV-infected patients has also been the subject of numerous studies, with sometimes apparently contradictory results (20, 51, 76). The activation of HIV from its proviral state is stimulated by reactive oxygen species (ROS). Therefore, one could assume that boosting the activity of selenoproteins that have an antioxidant activity might be beneficial in stopping, or at least delaying, the progression of HIV infections. However, paradoxically, it has been shown that the introduction of a GPX1 expression construct in HIV-1-infected cells results in the faster appearance of cytopathic effects associated with HIV-1 infection (67). Reciprocally, decreasing the antioxidant activity results in reduced viral replication (67). It seems that antioxidants have a dual effect on the progression of HIV infection, depending on the stage of the viral cycle. At the beginning of an infection, the antioxidant activity of GPx-1 may prevent cells from undergoing apoptosis, thus facilitating the spread of an acute viral infection. Later in the disease course, by eliminating ROS, GPx-1 may suppress the activation of ROS-responsive transcription of viral genes, thus hampering virus production during the later chronic stages of infection.
Recently, it has been shown that HIV-1 potentially encodes a selenoprotein with significant homology to mammalian GPxs (79, 87). It has been suggested that viral selenoprotein synthesis may deprive the host of selenium and other components required for endogenous selenoenzyme production. Deficiencies of selenium and several other key amino acids likely play a role in the appearance of symptoms such as immune system collapse, greater susceptibility to cancer, myocardial infarction, muscle wasting, depression, diarrhea, psychosis, and dementia (31). Interestingly, despite the high mutagenic rate of HIV, the GPx sequence is well conserved among different strains of the virus, suggesting an important role of this selenoenzyme in viral infection. Indeed, the HIV-1 GPx provides an anti-apoptotic resistance to oxidative damage, which could enhance viral replication at the first stages of infection (16). In support of this hypothesis, it has been shown that molluscum contagiosum virus (MCV) also encodes a selenoprotein with homology to human GPx. Interestingly, Hela cells transfected with the gene encoding the viral selenoprotein showed increased resistance to ultraviolet- and peroxide-induced cell death. Given the fact that MCV replicates exclusively in epidermis, the expression of the viral selenoprotein seems to provide a clear advantage to MCV (75).
Cancer
Numerous epidemiological studies have reported an inverse correlation between selenium intake and incidence of different cancers (8, 10, 17, 23, 45, 61, 64, 80); however, the underlying molecular mechanism(s) remains elusive. Since most selenoproteins have been shown to have an antioxidant activity, one could assume that higher intake of selenium would lead to higher expression of selenoproteins, hence protecting DNA against oxidative damage.
GPx-1 is the first described selenoprotein and probably the best characterized. It is ubiquitously expressed and detoxifies hydrogen peroxides (6). Gpx-1 knockout mice show no apparent difference in growth or susceptibility to selenium deficiency compared with normal mice. However, when the mice are exposed to lethal doses of the pro-oxidants paraquat or diquat, the lifespan of knockout mice is reduced by eightfold compared with wild-type animals (13). Hu et al. (37–39) showed that loss of heterozygosity at GPX1 locus is a common event in the cancer of head and neck, breast, lung, and colon. The study of a GPX1 gene variant that results in a leucine or proline at codon 198 showed that the leucine allele is more frequently associated with breast cancer (38). Furthermore, MCF-7 cells transfected with GPX1 Pro198 constructs exhibited greater stimulation of GPx activity in response to increasing concentrations of selenium than those transfected with the Leu198 variant (38). Thus the authors suggest that the leucine variant of GPx-1 may be a risk or contributing factor to breast cancer development (38).
The protective effect of selenoproteins against cancer has been recently assessed in a mouse model (i6A−) with a mutation in SectRNA resulting in reduced selenoprotein expression (58). In particular, the expression of GPx-1 is dramatically decreased in the prostate of these animals (22). These mice were bred with a transgenic mouse (Tag) expressing the SV40 large T and small t oncogenes specifically in prostate, resulting in the development of cancer in that organ. The bigenic animals (i6A−/Tag) showed an accelerated development of lesions associated with prostate cancer progression (22), supporting the idea that GPx-1 possesses anti-oncogenic properties.
Male fertility and reproduction
To date, all selenoproteins with known functions have enzymatic activity. However, it is noteworthy that GPx-4, in addition to its enzymatic function, can also play a structural role (81). In spermatids, GPx-4 is soluble and has peroxidase activity. However, in mature spermatozoa, GPx-4 becomes insoluble and enzymatically inactive. This insoluble form of the protein apparently plays a structural role in the stability of the helicoidal form of mitochondria in the spermatozoan midpiece (81). A nuclear isoform of GPx-4 (snGPx), resulting from use of an alternative transcription start site (57), has been shown to play a role in the condensation of chromatin of mature spermatozoa by establishing links between thiol groups of protamines, which replace histones in chromatin of mature spermatozoa allowing a higher degree of chromatin condensation (63). Therefore, impairment of GPx-4 is highly suspected to result in male infertility. In support of this, Imai et al. (41, 42) observed a dramatic decrease in the expression of GPx-4 in spermatozoa of infertile men. In mice, complete Gpx4 knockout results in early embryonic lethality. The heterozygous Gpx-4 +/− mice, although viable and fertile, presented with increased sensitivity to oxidative stress produced by γ-irradiation, paraquat, tert-butylhydroperoxide, and hydrogen peroxide (84).
TrxRs and Disease
As their name implies, TrxRs reduce thioredoxins (Trxs), which are small, ubiquitous, redox-active peptides with a conserved catalytic site that undergoes reversible oxidation/reduction at two Cys residues. The Trx proteins provide reducing equivalents to various enzymes such as ribonucleotide reductase and thioredoxin peroxidase. They are also able to reduce key Cys residues in certain transcription factors, resulting in enhanced binding to DNA, hence influencing gene transcription (for review, see Refs. 59, 77). Perhaps not surprisingly, TrxRs are involved in a broad range of physiological and pathological pathways ranging from cancer (see below) to sperm maturation and male fertility (77).
Cancer development
Interestingly, it appears that TrxRs have a dual and contradictory effect on tumor development. In fact, the selenol group of TrxRs seems to function as sensors to detect the presence of ROS and trigger a signaling cascade leading to the transcription of genes encoding antioxidative proteins (78). Therefore, TrxR may have a protective effect before the development of cancer by preventing oxidative damage. However, once a tumor is established, TrxR activity may have a tumor-promoting role, since tumor development relies on a supply of deoxyribonucleotide, which depends on the Trx/TrxR system (7). It is noteworthy that several-fold-increased TrxR levels have been observed in tumor cells, and several anti-neoplastic agents such as carmustine, fotemustine, and cisplatin are effective inhibitors of mammalian TrxRs (3). Furthermore, mice injected with mouse lung carcinoma (LLC1) cells knocked down for Trx1 gene showed a dramatic reduction in tumor progression and metastasis compared with mice injected with LLC1 cells expressing Trx1 (86), suggesting that this selenoenzyme might be considered as a potential target for cancer therapy.
Iodothyronine Deiodinases and Thyroid Disease
Iodothyronine deiodinases (DIO1, DIO2, and DIO3) are a family of highly conserved integral membrane proteins involved in the thyroid hormone biosynthetic pathway. DIO1 and DIO3 are located in the plasma membrane, whereas DIO2 is located in the endoplasmic reticulum (ER) membrane. All three possess a Sec residue at their catalytic sites. DIO1 and DIO2 catalyze the removal of iodine from thyroid pro-hormone T4 and convert it to active hormone T3 (4). DIO3 catalyzes the removal of iodine from T3 and converts it to the inactive form rT3 (4). Although iodothyronine deiodinases are suspected to be involved in thyroid diseases, to date, no mutations have been reported in any of these genes. Disruption of the mouse Dio1 gene results in an increase of T4 and rT3 serum levels, whereas thyroid stimulating hormone (TSH) and T3 levels remain unchanged. These mice appear clinically normal, and the main phenotype consists of abnormalities of iodothyrinine metabolism and excretion (70). Dio2 knockout mice show significantly elevated serum levels of T4 and TSH; furthermore, the regulation of TSH expression seems to be resistant to T4 feedback (69).
It is noteworthy that a partial loss of function mutation in SECISBP2, encoding SECIS binding protein 2, which is required for the co-translational incorporation of selenium in all selenoproteins, results in a relatively mild disease presenting with abnormal thyroid hormone metabolism (24). Although activities of DIO2, GPx, and selenoproteion P were all decreased in serum and/or fibroblasts from these patients, the clinical picture of thyroid dysfunction likely relates specifically to loss of DIO activity. A hierarchy in the synthesis of selenoproteins has been postulated, meaning that different selenoproteins are not affected in the same way by selenium deficiency (5). Although the deficiency of SECISBP2 should theoretically affect the biosynthesis of all selenoproteins, the short half-life of DIO2 and the fact that the UGA codon is relatively distant from the SECIS element may result in enhanced sensitivity of this selenoprotein to reductions in SECISBP2 activity (24). In support of this is the observation that SECISBP2 could contribute to establishing the selenoprotein hierarchy by variability in binding to SECIS elements of different selenoproteins (55).
Selenoprotein S and Inflammatory Response
Selenoprotein S (SEPS1) is a resident protein of the ER membrane. It is involved in processing and removing misfolded proteins from the ER to the cytosol where they can be polyubiquitinated and degraded through proteasome complexes (85). A recent study of 13 SNPs in 570 individuals showed an association between SEPS1 polymorphisms and circulating levels of proinflammatory cytokines. In particular, the promoter polymorphism −105G>A significantly impairs SEPS1 expression after exposure to ER stress agents (19). Furthermore, the suppression of SEPS1 expression by RNA interference results in an increase of proinflammatory cytokines (19), confirming that polymorphisms affecting SEPS1 expression may account for some degree of genetic variation in the inflammatory response.
Selenoprotein P and Selenium Transport
Selenoprotein P (SEPP1) is unique among selenoproteins because, in humans, it incorporates 10 Sec residues. It is a secreted protein that contains about 50% of the selenium content of human plasma, and it appears to be important for transporting selenium to the brain (71). To date, no SEPP1 mutation has been reported in any human disease. Sepp1 knockout mice are viable, but when fed with a low-selenium diet, they lose weight and develop poor motor coordination (36). Interestingly, the low-selenium diet results in a 43% reduction of the brain selenium in SEPP1-deficient mice. However, feeding these mice with a high-selenium diet restores brain selenium to levels comparable to those of wild-type mice, suggesting that, although SEPP1 might play a role in transport of selenium to the brain, it is not the only source of selenium that the brain relies on (FIGURE 3). It has also been shown that SEPP1 might be involved in spermatogenesis since Sepp1 knockout male, but not female, mice are infertile (60). This seems to be due to flagellar structural defects, manifested by a hairpin-like bend formation at the midpiece-principal piece junction of spermatozoa, similar to defects found in wild-type mice fed with a low-selenium diet (60). Although the defect in these wild-type mice can be reverted with a normal selenium diet, higher doses of selenium have no effect on SEPP1 knockout mice phenotype and do not restore their fertility.
FIGURE 3. Selenoprotein P knockout mouse phenotypes.
Interactions between genotype and environmental factors are evident in Sepp1 knockout mice, whose severe phenotype can be ameliorated by nutritional supplementation with a high selenium diet.
Selenoprotein 15 kDa and Cancer
The 15-kDa selenoprotein (SEP15) gene product is an ER selenoprotein possibly involved in disulfide bond formation and protein folding (26). Two variants of SEP15, located within the SECIS element at positions 811 (T/C) and 1,125 (G/A) of the cDNA have been described (34, 50). Transcripts containing the T811/A1125 haplotype are more efficient in the incorporation of Sec at the UGA codon but are less responsive to increases of selenium availability (40). Interestingly, loss of heterozygosity has been observed at SEP15 in breast cancers and head and neck cancers, suggesting a role of this gene in tumor progression (21).
Selenoprotein N and Muscle Disease
With the exceptions of SEPS1 and SECISBP2, the involvement of selenoproteins or their biosynthesis in most diseases described above is secondary and, in some cases, simply results from variation of the activity of these selenoproteins according to the bioavailability of selenium. Selenoprotein N (SEPN1) was the first selenoprotein known to be mutated in a human genetic disease. Mutations of the SEPN1 gene were first described in congenital muscular dystrophy with spinal rigidity (56). Later, mutations in this gene were also found in three other related disorders: multiminicore myopathy (28), desmin-related myopathy with Mallory body-like inclusions (27), and congenital fiber-type disproportion myopathy (15). Although the histopathological findings are distinct, clinical reevaluation of patients with these diagnoses showed that they share essentially identical clinical features characterized by early weakness of axial and proximal muscles, scoliosis, and severe respiratory insufficiency. Therefore, these diseases may now be considered as a single clinical entity and are referred to as SEPN1-related myopathies. Each of these diagnoses is also associated with mutations in other genes, including the ryanodine receptor RYR1 (multiminicore myopathy), desmin DES (desmin-related myopathies), and alpha-actin ACTA1 (congenital fiber-type disproportion). Understanding any possible link(s) between these proteins, or their functions, and selenoprotein N may provide important insight into the molecular function of SEPN1, which is unknown. The protein is ubiquitously expressed and resides in the ER membrane (62). Hence, one can hypothesize that it may be involved in the maturation of other proteins involved in muscle development and/or function.
Conclusion and Discussion
Among the 25 selenoproteins identified in humans, less than half have been attributed a function, and mutations causing a human disease have been found only in SEPN1 and SEPS1. However, the involvement of selenoproteins in other human diseases affecting the brain (12, 73) and the immune and endocrine systems (4, 64) are suspected, and it is very likely that in the near future their molecular mechanisms will be unraveled. Several points have to be considered regarding the involvement of selenoproteins in human diseases. So far, selenoproteins have been considered primarily as enzymes because of the reactivity the Sec provides, but it is important to note that in some cases they may also play a structural role, as GPx-4 does in the mitochondria of spermatids. Furthermore, it is believed that selenoproteins can also affect cell-signaling molecules such as nuclear factor-κB (35) and hence influence important cellular functions such as gene transcription and cell growth.
The supranutritional intake of selenium has been suggested to have chemopreventive actions against cancer (43); however, this phenomenon may occur through multiple pathways, including ones that do not rely on selenoproteins themselves (25, 68). Although selenium almost certainly plays biological roles independently of selenoproteins, it is also clear that altering dietary levels of selenium can impact selenoprotein levels and their activity in specific organs and under certain relevant physiological conditions. Since the degree of saturation of selenoprotein activity with high intake of selenium has been measured for only a few enzymes, such as GPxs or TrxRs, it remains possible that other selenoproteins with unknown functions may be modulated if even higher intake of selenium is achieved (82), thus possibly explaining some of the effects of a high-selenium diet. Therefore, GPx activity may not be the best marker when selenium intake requirements are considered. It is a well-known fact that there is a hierarchy of selenium requirements among the selenoproteins, so selenium deficiency has differential effects on members of the selenoprotein family. Moreover, different tissues retain selenium to a variable extent under selenium-deficiency conditions. Thus elucidating the complex regulation of synthesis of the members of the selenoprotein family is essential for understanding the unique and unpredictable patterns of pathophysiology arising from dysfunction of each of these proteins.
Acknowledgments
The authors gratefully acknowledge support for work on selenoproteins and congenital myopathies by the Lee and Penny Anderson Family Foundation, the Joshua Frase Foundation, the Muscular Dystrophy Association, and National Institutes of Health Grants AR-44345 and NS-40828.
References
- 1.Allmang C, Krol A. Selenoprotein synthesis: UGA does not end the story. Biochimie. doi: 10.1016/j.biochi.2006.04.015. In press. [DOI] [PubMed] [Google Scholar]
- 2.Beck MA, Esworthy RS, Ho YS, Chu FF. Glutathione peroxidase protects mice from viral-induced myocarditis. FASEB J. 1998;12:1143–1149. doi: 10.1096/fasebj.12.12.1143. [DOI] [PubMed] [Google Scholar]
- 3.Becker K, Gromer S, Schirmer RH, Muller S. Thioredoxin reductase as a pathophysiological factor and drug target. Eur J Biochem. 2000;267:6118–6125. doi: 10.1046/j.1432-1327.2000.01703.x. [DOI] [PubMed] [Google Scholar]
- 4.Beckett GJ, Arthur JR. Selenium and endocrine systems. J Endocrinol. 2005;184:455–465. doi: 10.1677/joe.1.05971. [DOI] [PubMed] [Google Scholar]
- 5.Behne D, Hilmert H, Scheid S, Gessner H, Elger W. Evidence for specific selenium target tissues and new biologically important selenoproteins. Biochim Biophys Acta. 1988;966:12–21. doi: 10.1016/0304-4165(88)90123-7. [DOI] [PubMed] [Google Scholar]
- 6.Behne D, Kyriakopoulos A. Mammalian selenium-containing proteins. Annu Rev Nutr. 2001;21:453–473. doi: 10.1146/annurev.nutr.21.1.453. [DOI] [PubMed] [Google Scholar]
- 7.Biaglow JE, Miller RA. The thioredoxin reductase/thioredoxin system: novel redox targets for cancer therapy. Cancer Biol Ther. 2005;4:6–13. doi: 10.4161/cbt.4.1.1434. [DOI] [PubMed] [Google Scholar]
- 8.Bjorkhem-Bergman L, Torndal UB, Eken S, Nystrom C, Capitanio A, Larsen EH, Bjornstedt M, Eriksson LC. Selenium prevents tumor development in a rat model for chemical carcinogenesis. Carcinogenesis. 2005;26:125–131. doi: 10.1093/carcin/bgh290. [DOI] [PubMed] [Google Scholar]
- 9.Bosl MR, Takaku K, Oshima M, Nishimura S, Taketo MM. Early embryonic lethality caused by targeted disruption of the mouse selenocysteine tRNA gene (Trsp) Proc Natl Acad Sci USA. 1997;94:5531–5534. doi: 10.1073/pnas.94.11.5531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brenneisen P, Steinbrenner H, Sies H. Selenium, oxidative stress, and health aspects. Mol Aspects Med. 2005;26:256–267. doi: 10.1016/j.mam.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 11.Caban K, Copeland PR. Size matters: a view of selenocysteine incorporation from the ribo-some. Cell Mol Life Sci. 2006;63:73–81. doi: 10.1007/s00018-005-5402-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen J, Berry MJ. Selenium and selenoproteins in the brain and brain diseases. J Neurochem. 2003;86:1–12. doi: 10.1046/j.1471-4159.2003.01854.x. [DOI] [PubMed] [Google Scholar]
- 13.Cheng WH, Ho YS, Valentine BA, Ross DA, Combs GF, Jr, Lei XG. Cellular glutathione peroxidase is the mediator of body selenium to protect against paraquat lethality in transgenic mice. J Nutr. 1998;128:1070–1076. doi: 10.1093/jn/128.7.1070. [DOI] [PubMed] [Google Scholar]
- 14.Cheng YY, Qian PC. The effect of selenium-fortified table salt in the prevention of Keshan disease on a population of 1.05 million. Biomed Environ Sci. 1990;3:422–428. [PubMed] [Google Scholar]
- 15.Clarke NF, Kidson W, Quijano-Roy S, Estournet B, Ferreiro A, Guicheney P, Manson JI, Kornberg AJ, Shield LK, North KN. SEPN1: associated with congenital fiber-type disproportion and insulin resistance. Ann Neurol. 2005;59:546–552. doi: 10.1002/ana.20761. [DOI] [PubMed] [Google Scholar]
- 16.Cohen I, Boya P, Zhao L, Metivier D, Andreau K, Perfettini JL, Weaver JG, Badley A, Taylor EW, Kroemer G. Anti-apoptotic activity of the glutathione peroxidase homologue encoded by HIV-1. Apoptosis. 2004;9:181–192. doi: 10.1023/B:APPT.0000018800.87358.ba. [DOI] [PubMed] [Google Scholar]
- 17.Combs GF, Jr, Gray WP. Chemopreventive agents: selenium. Pharmacol Ther. 1998;79:179–192. doi: 10.1016/s0163-7258(98)00014-x. [DOI] [PubMed] [Google Scholar]
- 18.Conrad M, Jakupoglu C, Moreno SG, Lippl S, Banjac A, Schneider M, Beck H, Hatzopoulos AK, Just U, Sinowatz F, Schmahl W, Chien KR, Wurst W, Bornkamm GW, Brielmeier M. Essential role for mitochondrial thioredoxin reductase in hematopoiesis, heart development, and heart function. Mol Cell Biol. 2004;24:9414–9423. doi: 10.1128/MCB.24.21.9414-9423.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Curran JE, Jowett JB, Elliott KS, Gao Y, Gluschenko K, Wang J, Abel Azim DM, Cai G, Mahaney MC, Comuzzie AG, Dyer TD, Walder KR, Zimmet P, MacCluer JW, Collier GR, Kissebah AH, Blangero J. Genetic variation in selenoprotein S influences inflammatory response. Nat Genet. 2005;37:1234–1241. doi: 10.1038/ng1655. [DOI] [PubMed] [Google Scholar]
- 20.Diamond AM, Hu YJ, Mansur DB. Glutathione peroxidase and viral replication: implications for viral evolution and chemoprevention. Biofactors. 2001;14:205–210. doi: 10.1002/biof.5520140126. [DOI] [PubMed] [Google Scholar]
- 21.Diwadkar–Navsariwala V, Diamond AM. The link between selenium and chemoprevention: a case for selenoproteins. J Nutr. 2004;134:2899–2902. doi: 10.1093/jn/134.11.2899. [DOI] [PubMed] [Google Scholar]
- 22.Diwadkar-Navsariwala V, Prins GS, Swanson SM, Birch LA, Ray VH, Hedayat S, Lantvit DL, Diamond AM. Selenoprotein deficiency accelerates prostate carcinogenesis in a transgenic model. Proc Natl Acad Sci USA. 2006;103:8179–8184. doi: 10.1073/pnas.0508218103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duffield-Lillico AJ, Shureiqi I, Lippman SM. Can selenium prevent colorectal cancer? A signpost from epidemiology. J Natl Cancer Inst. 2004;96:1645–1647. doi: 10.1093/jnci/djh332. [DOI] [PubMed] [Google Scholar]
- 24.Dumitrescu AM, Liao XH, Abdullah MS, Lado–Abeal J, Majed FA, Moeller LC, Boran G, Schomburg L, Weiss RE, Refetoff S. Mutations in SECISBP2 result in abnormal thyroid hormone metabolism. Nat Genet. 2005;37:1247–1252. doi: 10.1038/ng1654. [DOI] [PubMed] [Google Scholar]
- 25.El-Bayoumy K, Sinha R. Molecular chemoprevention by selenium: a genomic approach. Mutat Res. 2005;591:224–236. doi: 10.1016/j.mrfmmm.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 26.Ferguson AD, Labunskyy VM, Fomenko DE, Arac D, Chelliah Y, Amezcua CA, Rizo J, Gladyshev VN, Deisenhofer J. NMR structures of the selenoproteins Sep15 and SelM reveal redox activity of new thioredoxin-like family. J Biol Chem. 2005;281:3636–3543. doi: 10.1074/jbc.M511386200. [DOI] [PubMed] [Google Scholar]
- 27.Ferreiro A, Ceuterick-de Groote C, Marks JJ, Goemans N, Schreiber G, Hanefeld F, Fardeau M, Martin JJ, Goebel HH, Richard P, Guicheney P, Bonnemann CG. Desmin-related myopathy with Mallory body-like inclusions is caused by mutations of the selenoprotein N gene. Ann Neurol. 2004;55:676–686. doi: 10.1002/ana.20077. [DOI] [PubMed] [Google Scholar]
- 28.Ferreiro A, Quijano-Roy S, Pichereau C, Moghadaszadeh B, Goemans N, Bonnemann C, Jungbluth H, Straub V, Villanova M, Leroy JP, Romero NB, Martin JJ, Muntoni F, Voit T, Estournet B, Richard P, Fardeau M, Guicheney P. Mutations of the selenoprotein N gene, which is implicated in rigid spine muscular dystrophy, cause the classical phenotype of multi-minicore disease: reassessing the nosology of early-onset myopathies. Am J Hum Genet. 2002;71:739–749. doi: 10.1086/342719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Flohe L, Gunzler WA, Schock HH. Glutathione peroxidase: a selenoenzyme. FEBS Lett. 1973;32:132–134. doi: 10.1016/0014-5793(73)80755-0. [DOI] [PubMed] [Google Scholar]
- 30.Foresta C, Flohe L, Garolla A, Roveri A, Ursini F, Maiorino M. Male fertility is linked to the selenoprotein phospholipid hydroperoxide glutathione peroxidase. Biol Reprod. 2002;67:967–971. doi: 10.1095/biolreprod.102.003822. [DOI] [PubMed] [Google Scholar]
- 31.Foster HD. How HIV-1 causes AIDS: implications for prevention and treatment. Med Hypotheses. 2004;62:549–553. doi: 10.1016/j.mehy.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 32.Gasdaska JR, Harney JW, Gasdaska PY, Powis G, Berry MJ. Regulation of human thioredoxin reductase expression and activity by 3′-untranslated region selenocysteine insertion sequence and mRNA instability elements. J Biol Chem. 1999;274:25379–25385. doi: 10.1074/jbc.274.36.25379. [DOI] [PubMed] [Google Scholar]
- 33.Ge K, Yang G. The epidemiology of selenium deficiency in the etiological study of endemic diseases in China. Am J Clin Nutr. 1993;57:259S–263S. doi: 10.1093/ajcn/57.2.259S. [DOI] [PubMed] [Google Scholar]
- 34.Gladyshev VN, Jeang KT, Wootton JC, Hatfield DL. A new human selenium-containing protein. Purification, characterization, and cDNA sequence. J Biol Chem. 1998;273:8910–8915. doi: 10.1074/jbc.273.15.8910. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Ueno Y, Okamoto T. Oxidoreductive regulation of nuclear factor kappa B. Involvement of a cellular reducing catalyst thioredoxin. J Biol Chem. 1993;268:11380–11388. [PubMed] [Google Scholar]
- 36.Hill KE, Zhou J, McMahan WJ, Motley AK, Atkins JF, Gesteland RF, Burk RF. Deletion of selenoprotein P alters distribution of selenium in the mouse. J Biol Chem. 2003;278:13640–13646. doi: 10.1074/jbc.M300755200. [DOI] [PubMed] [Google Scholar]
- 37.Hu Y, Benya RV, Carroll RE, Diamond AM. Allelic loss of the gene for the GPX1 selenium-containing protein is a common event in cancer. J Nutr. 2005;135:3021S–3024S. doi: 10.1093/jn/135.12.3021S. [DOI] [PubMed] [Google Scholar]
- 38.Hu YJ, Diamond AM. Role of glutathione peroxidase 1 in breast cancer: loss of heterozygosity and allelic differences in the response to selenium. Cancer Res. 2003;63:3347–3351. [PubMed] [Google Scholar]
- 39.Hu YJ, Dolan ME, Bae R, Yee H, Roy M, Glickman R, Kiremidjian-Schumacher L, Diamond AM. Allelic loss at the GPx-1 locus in cancer of the head and neck. Biol Trace Elem Res. 2004;101:97–106. doi: 10.1385/BTER:101:2:097. [DOI] [PubMed] [Google Scholar]
- 40.Hu YJ, Korotkov KV, Mehta R, Hatfield DL, Rotimi CN, Luke A, Prewitt TE, Cooper RS, Stock W, Vokes EE, Dolan ME, Gladyshev VN, Diamond AM. Distribution and functional consequences of nucleotide polymorphisms in the 3′-untranslated region of the human Sep15 gene. Cancer Res. 2001;61:2307–2310. [PubMed] [Google Scholar]
- 41.Imai H, Nakagawa Y. Biological significance of phospholipid hydroperoxide glutathione peroxidase (PHGPx, GPx4) in mammalian cells. Free Radic Biol Med. 2003;34:145–169. doi: 10.1016/s0891-5849(02)01197-8. [DOI] [PubMed] [Google Scholar]
- 42.Imai H, Suzuki K, Ishizaka K, Ichinose S, Oshima H, Okayasu I, Emoto K, Umeda M, Nakagawa Y. Failure of the expression of phospholipid hydroperoxide glutathione peroxidase in the spermatozoa of human infertile males. Biol Reprod. 2001;64:674–683. doi: 10.1095/biolreprod64.2.674. [DOI] [PubMed] [Google Scholar]
- 43.Irons R, Carlson BA, Hatfield DL, Davis CD. Both selenoproteins and low molecular weight selenocompounds reduce colon cancer risk in mice with genetically impaired selenoprotein expression. J Nutr. 2006;136:1311–1317. doi: 10.1093/jn/136.5.1311. [DOI] [PubMed] [Google Scholar]
- 44.Jacob C, Giles GI, Giles NM, Sies H. Sulfur and selenium: the role of oxidation state in protein structure and function. Angew Chem Int Ed Engl. 2003;42:4742–4758. doi: 10.1002/anie.200300573. [DOI] [PubMed] [Google Scholar]
- 45.Jacobs ET, Jiang R, Alberts DS, Greenberg ER, Gunter EW, Karagas MR, Lanza E, Ratnasinghe L, Reid ME, Schatzkin A, Smith-Warner SA, Wallace K, Martinez ME. Selenium and colorectal adenoma: results of a pooled analysis. J Natl Cancer Inst. 2004;96:1669–1675. doi: 10.1093/jnci/djh310. [DOI] [PubMed] [Google Scholar]
- 46.Jakupoglu C, Przemeck GK, Schneider M, Moreno SG, Mayr N, Hatzopoulos AK, de Angelis MH, Wurst W, Bornkamm GW, Brielmeier M, Conrad M. Cytoplasmic thioredoxin reductase is essential for embryogenesis but dispensable for cardiac development. Mol Cell Biol. 2005;25:1980–1988. doi: 10.1128/MCB.25.5.1980-1988.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Johansson L, Gafvelin G, Arner ES. Selenocysteine in proteins: properties and biotechnological use. Biochim Biophys Acta. 2005;1726:1–13. doi: 10.1016/j.bbagen.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 48.Kiremidjian-Schumacher L, Roy M. Effect of selenium on the immunocompetence of patients with head and neck cancer and on adoptive immunotherapy of early and established lesions. Biofactors. 2001;14:161–168. doi: 10.1002/biof.5520140121. [DOI] [PubMed] [Google Scholar]
- 49.Kryukov GV, Castellano S, Novoselov SV, Lobanov AV, Zehtab O, Guigo R, Gladyshev VN. Characterization of mammalian selenoproteomes. Science. 2003;300:1439–1443. doi: 10.1126/science.1083516. [DOI] [PubMed] [Google Scholar]
- 50.Kumaraswamy E, Korotkov KV, Diamond AM, Gladyshev VN, Hatfield DL. Genetic and functional analysis of mammalian Sep15 selenoprotein. Methods Enzymol. 2002;347:187–197. doi: 10.1016/s0076-6879(02)47017-6. [DOI] [PubMed] [Google Scholar]
- 51.Kupka R, Msamanga GI, Spiegelman D, Rifai N, Hunter DJ, Fawzi WW. Selenium levels in relation to morbidity and mortality among children born to HIV-infected mothers. Eur J Clin Nutr. 2005;59:1250–1258. doi: 10.1038/sj.ejcn.1602236. [DOI] [PubMed] [Google Scholar]
- 52.Lee SR, Bar-Noy S, Kwon J, Levine RL, Stadtman TC, Rhee SG. Mammalian thioredoxin reductase: oxidation of the C-terminal cysteine/selenocysteine active site forms a thioselenide, and replacement of selenium with sulfur markedly reduces catalytic activity. Proc Natl Acad Sci USA. 2000;97:2521–2526. doi: 10.1073/pnas.050579797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Levander OA, Beck MA. Interacting nutritional and infectious etiologies of Keshan disease. Insights from coxsackie virus B-induced myocarditis in mice deficient in selenium or vitamin E. Biol Trace Elem Res. 1997;56:5–21. doi: 10.1007/BF02778980. [DOI] [PubMed] [Google Scholar]
- 54.Look MP, Rockstroh JK, Rao GS, Kreuzer KA, Barton S, Lemoch H, Sudhop T, Hoch J, Stockinger K, Spengler U, Sauerbruch T. Serum selenium, plasma glutathione (GSH) and erythrocyte glutathione peroxidase (GSH-Px)-levels in asymptomatic versus symptomatic human immunodeficiency virus-1 (HIV-1)-infection. Eur J Clin Nutr. 1997;51:266–272. doi: 10.1038/sj.ejcn.1600401. [DOI] [PubMed] [Google Scholar]
- 55.Low SC, Grundner-Culemann E, Harney JW, Berry MJ. SECIS-SBP2 interactions dictate selenocysteine incorporation efficiency and selenoprotein hierarchy. EMBO J. 2000;19:6882–6890. doi: 10.1093/emboj/19.24.6882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moghadaszadeh B, Petit N, Jaillard C, Brockington M, Roy SQ, Merlini L, Romero N, Estournet B, Desguerre I, Chaigne D, Muntoni F, Topaloglu H, Guicheney P. Mutations in SEPN1 cause congenital muscular dystrophy with spinal rigidity and restrictive respiratory syndrome. Nat Genet. 2001;29:17–18. doi: 10.1038/ng713. [DOI] [PubMed] [Google Scholar]
- 57.Moreno SG, Laux G, Brielmeier M, Bornkamm GW, Conrad M. Testis-specific expression of the nuclear form of phospholipid hydroperoxide glutathione peroxidase (PHGPx) Biol Chem. 2003;384:635–643. doi: 10.1515/BC.2003.070. [DOI] [PubMed] [Google Scholar]
- 58.Moustafa ME, Kumaraswamy E, Zhong N, Rao M, Carlson BA, Hatfield DL. Models for assessing the role of selenoproteins in health. J Nutr. 2003;133:2494S–2496S. doi: 10.1093/jn/133.7.2494S. [DOI] [PubMed] [Google Scholar]
- 59.Mustacich D, Powis G. Thioredoxin reductase. Biochem J. 2000;346:1–8. [PMC free article] [PubMed] [Google Scholar]
- 60.Olson GE, Winfrey VP, Nagdas SK, Hill KE, Burk RF. Selenoprotein P is required for mouse sperm development. Biol Reprod. 2005;73:201–211. doi: 10.1095/biolreprod.105.040360. [DOI] [PubMed] [Google Scholar]
- 61.Patrick L. Selenium biochemistry and cancer: a review of the literature. Altern Med Rev. 2004;9:239–258. [PubMed] [Google Scholar]
- 62.Petit N, Lescure A, Rederstorff M, Krol A, Moghadaszadeh B, Wewer UM, Guicheney P. Selenoprotein N: an endoplasmic reticulum glycoprotein with an early developmental expression pattern. Hum Mol Genet. 2003;12:1045–1053. doi: 10.1093/hmg/ddg115. [DOI] [PubMed] [Google Scholar]
- 63.Pfeifer H, Conrad M, Roethlein D, Kyriakopoulos A, Brielmeier M, Bornkamm GW, Behne D. Identification of a specific sperm nuclei selenoenzyme necessary for protamine thiol cross-linking during sperm maturation. FASEB J. 2001;15:1236–1238. [PubMed] [Google Scholar]
- 64.Rayman MP, Rayman MP. The argument for increasing selenium intake. Proc Nutr Soc. 2002;61:203–215. doi: 10.1079/PNS2002153. [DOI] [PubMed] [Google Scholar]
- 65.Rederstorff M, Krol A, Lescure A. Understanding the importance of selenium and selenoproteins in muscle function. Cell Mol Life Sci. 2006;63:52–59. doi: 10.1007/s00018-005-5313-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG. Selenium: biochemical role as a component of glutathione peroxidase. Science. 1973;179:588–590. doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- 67.Sandstrom PA, Murray J, Folks TM, Diamond AM. Antioxidant defenses influence HIV-1 replication and associated cytopathic effects. Free Radic Biol Med. 1998;24:1485–1491. doi: 10.1016/s0891-5849(98)00023-9. [DOI] [PubMed] [Google Scholar]
- 68.Schlicht M, Matysiak B, Brodzeller T, Wen X, Liu H, Zhou G, Dhir R, Hessner MJ, Tonellato P, Suckow M, Pollard M, Datta MW. Cross-species global and subset gene expression profiling identifies genes involved in prostate cancer response to selenium. BMC Genomics. 2004;5:58. doi: 10.1186/1471-2164-5-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schneider MJ, Fiering SN, Pallud SE, Parlow AF, St Germain DL, Galton VA. Targeted disruption of the type 2 selenodeiodinase gene (DIO2) results in a phenotype of pituitary resistance to T4. Mol Endocrinol. 2001;15:2137–2148. doi: 10.1210/mend.15.12.0740. [DOI] [PubMed] [Google Scholar]
- 70.Schneider MJ, Fiering SN, Thai B, Wu SY, St Germain E, Parlow AF, St Germain DL, Galton VA. Targeted disruption of the type 1 selenodeiodinase gene (Dio1) results in marked changes in thyroid hormone economy in mice. Endocrinology. 2006;147:580–589. doi: 10.1210/en.2005-0739. [DOI] [PubMed] [Google Scholar]
- 71.Schomburg L, Schweizer U, Holtmann B, Flohe L, Sendtner M, Kohrle J. Gene disruption discloses role of selenoprotein P in selenium delivery to target tissues. Biochem J. 2003;370:397–402. doi: 10.1042/BJ20021853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schwarz K, Foltz CM. Selenium as an integral part of factor 3 against dietary necrotic liver degeneration. J Am Chem Soc. 1957;79:3292–3293. [PubMed] [Google Scholar]
- 73.Schweizer U, Brauer AU, Kohrle J, Nitsch R, Savaskan NE. Selenium and brain function: a poorly recognized liaison. Brain Res Brain Res Rev. 2004;45:164–178. doi: 10.1016/j.brainresrev.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 74.Scott R, MacPherson A, Yates RW, Hussain B, Dixon J. The effect of oral selenium supplementation on human sperm motility. Br J Urol. 1998;82:76–80. doi: 10.1046/j.1464-410x.1998.00683.x. [DOI] [PubMed] [Google Scholar]
- 75.Shisler JL, Senkevich TG, Berry MJ, Moss B. Ultraviolet-induced cell death blocked by a selenoprotein from a human dermatotropic poxvirus. Science. 1998;279:102–105. doi: 10.1126/science.279.5347.102. [DOI] [PubMed] [Google Scholar]
- 76.Singhal N, Austin J. A clinical review of micronutrients in HIV infection. J Int Assoc Physicians AIDS Care (Chic Ill) 2002;1:63–75. doi: 10.1177/154510970200100205. [DOI] [PubMed] [Google Scholar]
- 77.Su D, Novoselov SV, Sun QA, Moustafa ME, Zhou Y, Oko R, Hatfield DL, Gladyshev VN. Mammalian selenoprotein thioredoxin/glutathione reductase: roles in disulfide bond formation and sperm maturation. J Biol Chem. 2005;280:26491–26498. doi: 10.1074/jbc.M503638200. [DOI] [PubMed] [Google Scholar]
- 78.Sun QA, Wu Y, Zappacosta F, Jeang KT, Lee BJ, Hatfield DL, Gladyshev VN. Redox regulation of cell signaling by selenocysteine in mammalian thioredoxin reductases. J Biol Chem. 1999;274:24522–24530. doi: 10.1074/jbc.274.35.24522. [DOI] [PubMed] [Google Scholar]
- 79.Taylor EW, Bhat A, Nadimpalli RG, Zhang W, Kececioglu J. HIV-1 encodes a sequence overlapping env gp41 with highly significant similarity to selenium-dependent glutathione peroxidases. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;15:393–394. doi: 10.1097/00042560-199708150-00013. [DOI] [PubMed] [Google Scholar]
- 80.Taylor PR, Parnes HL, Lippman SM. Science peels the onion of selenium effects on prostate carcinogenesis. J Natl Cancer Inst. 2004;96:645–647. doi: 10.1093/jnci/djh147. [DOI] [PubMed] [Google Scholar]
- 81.Ursini F, Heim S, Kiess M, Maiorino M, Roveri A, Wissing J, Flohe L. Dual function of the selenoprotein PHGPx during sperm maturation. Science. 1999;285:1393–1396. doi: 10.1126/science.285.5432.1393. [DOI] [PubMed] [Google Scholar]
- 82.Xia Y, Hill KE, Byrne DW, Xu J, Burk RF. Effectiveness of selenium supplements in a low-selenium area of China. Am J Clin Nutr. 2005;81:829–834. doi: 10.1093/ajcn/81.4.829. [DOI] [PubMed] [Google Scholar]
- 83.Xia YM, Hill KE, Burk RF. Biochemical studies of a selenium-deficient population in China: measurement of selenium, glutathione peroxidase and other oxidant defense indices in blood. J Nutr. 1989;119:1318–1326. doi: 10.1093/jn/119.9.1318. [DOI] [PubMed] [Google Scholar]
- 84.Yant LJ, Ran Q, Rao L, Van Remmen H, Shibatani T, Belter JG, Motta L, Richardson A, Prolla TA. The selenoprotein GPX4 is essential for mouse development and protects from radiation and oxidative damage insults. Free Radic Biol Med. 2003;34:496–502. doi: 10.1016/s0891-5849(02)01360-6. [DOI] [PubMed] [Google Scholar]
- 85.Ye Y, Shibata Y, Yun C, Ron D, Rapoport TA. A membrane protein complex mediates retro-translocation from the ER lumen into the cytosol. Nature. 2004;429:841–847. doi: 10.1038/nature02656. [DOI] [PubMed] [Google Scholar]
- 86.Yoo MH, Xu XM, Carlson BA, Gladyshev VN, Hatfield DL. Thioredoxin reductase 1 deficiency reverses tumor phenotype and tumorigenicity of lung carcinoma cells. J Biol Chem. 2006;281:13005–13008. doi: 10.1074/jbc.C600012200. [DOI] [PubMed] [Google Scholar]
- 87.Zhao L, Cox AG, Ruzicka JA, Bhat AA, Zhang W, Taylor EW. Molecular modeling and in vitro activity of an HIV-1-encoded glutathione peroxidase. Proc Natl Acad Sci USA. 2000;97:6356–6361. doi: 10.1073/pnas.97.12.6356. [DOI] [PMC free article] [PubMed] [Google Scholar]