Abstract
We report a study on the formation of block liposomes (BLs) and nanotubes from membranes comprised of mixtures of membrane curvature-stabilizing multivalent cationic lipids MVL3(3+) and MVL5(5+) with neutral 1,2-dioleoyl-sn-glycero-3-phosphatidylcholine (DOPC). In conjunction with prior work on MVLBG2(16+), our experiments suggest that BL and nanotube formation is a general phenomenon in membranes containing multivalent lipids, thus enhancing the relevance of BLs for applications such as gene/drug storage and delivery or templating.
Keywords: block liposomes, lipid bilayer, curvature-stabilizing lipids, membrane curvature
Introduction
Recently, we reported the formation of a new class of chain-melted (liquid) liposomes, termed block liposomes (BLs)1, in mixtures of the highly charged curvature-stabilizing multivalent lipid MVLBG22 (headgroup charge +16 e) and the neutral lipid 1,2-dioleoyl-sn-glycero-3-phosphatidylcholine (DOPC) at ΦMVLBG2=0.1 in water with no added salt. Pure DOPC, in contrast, does not form BLs. Hallmarks of BLs are linked liposome shapes such as vesicles and micelles. In a BL, liposome shapes comprised of lipid bilayers such as spheres, pears, and tubes, but also micelles comprised of a lipid monolayer (in particular cylindrical micelles, also called nanorods in this work), are connected into one object. A detailed characterization of BLs by cryogenic transmission electron microscopy (cryo-TEM) led to a model for their formation that suggests an interplay of membrane undulations and concentration fluctuations of the two lipid components. These lead to the phase separation of MVLBG2 and DOPC, with MVLBG2 grouping into the regions of high membrane curvature, such as nanotubes and nanorods, and DOPC concentrating in the locally flat (zero curvature) regions, such as bilayer spheres of large radius frequently attached to the end of a nanotube/nanorod1.
Further investigations exposing BLs to varied pH and salt conditions identified both the high charge as well as the high membrane curvature provided by MVLBG2 (due to the large headgroup) as prerequisites for BL-formation3. In those experiments the extent of electrostatic screening was controlled by the salt content in the solution. Changes in pH altered the protonation of MVLBG2, thus changing its charge as well as the size of its headgroup, leading to various liposomal shape transitions dependent on the extent of alterations. Understanding the shape evolution of BLs under different environmental conditions is highly relevant to both applications and basic biological science: BLs are promising candidates for lipid-based gene and drug delivery4-7 and templating of other nanostructures such as wires and needles8-10, and many biological processes such as endocytosis, endoplasmatic reticulum-associated vesiculation, vesicle recycling and signaling take advantage of liposome shape changes11-16.
In this report we present a cryo-TEM study on the formation of block liposomes from membranes comprised of mixtures of the membrane curvature-stabilizing multivalent lipids, MVL317, MVL518 and MVLBG22,19 with charges of +3, +5, and +16 e, respectively, and DOPC. The chemical structures of these lipids can be found in the Supporting Information (Fig. S1). The work presented in this report focuses on cationic lipids because of their importance for nonviral delivery of nucleic acids such as DNA and siRNA. Both MVL3/DOPC and MVL5/DOPC lipid mixtures in water exhibit a regime of BL-formation with pronounced nanotube formation at ΦMVL3 = 0.1 and 0.3 and ΦMVL5 = 0.1. Our experiments thus suggest that the formation of BLs is a general phenomenon in membranes containing multivalent charged lipids, thus highlighting the relevance of BLs for applications such as gene/drug storage and delivery or templating.
Interestingly, new and unique features were found in the BLs formed by MVL3 and MVL5. Lipid mixtures of MVL3 and DOPC form more nanotubes and fewer nanorods, and the nanotubes often exhibit a pearling-like feature, where a nanotube widens into a “bubble” that narrows again to its original radius. MVL3-containing nanotubes also show branching as well as a reduced stiffness compared to the previously studied MVLBG2/DOPC nanotubes. Lipid mixtures of MVL5 and DOPC show a pronounced bundling of the nanotubes. Another key feature of this system is the formation of “spikes”, shorter nanorods budding off from a nanotube, representing a further step of curvature-driven phase separation. In this case, the nanotube is considered a region of lower curvature with respect to the nanorod, a region of extremely high curvature. This is quite remarkable, as the nanotube already possesses a fairly high membrane curvature.
Materials and Methods
Materials
DOPC and DOTAP were purchased from Avanti Polar Lipids. MVL3, MVL5 and MVLBG2 were synthesized as described2,17-19.
Lipid Solutions
Lipid stock solutions were prepared in chloroform/methanol (9:1, v/v). Lipid solutions were combined at the desired ratio of lipids and dried, first by a stream of nitrogen and subsequently in a vacuum for 8 to 12 hours. To the residue, high resistivity (18.2 MΩcm) water was added and the mixture incubated at 37 °C for at least 12 hours to give a final concentration of 10 mg/mL. The lipid solutions were stored at 4 °C until use. The lipids remained in their chain-melted liquid state due to their unsaturated lipid tails. This was confirmed by wide angle X-ray scattering experiments (data not shown).
Optical Microscopy
A Nikon Diaphot 300 inverted microscope equipped for epifluorescence and DIC and a SensiCamQE High Speed digital camera were used.
Cryo-TEM
The specimens were preserved in a layer of vitreous ice suspended over a holey carbon substrate. The holey carbon films consist of a thin layer of pure carbon fenestrated by 2 μm holes spaced 4 μm apart and suspended over 400 mesh copper grids20. The grids were cleaned prior to vitrification with a Solarus plasma cleaner (Gatan Inc.) using a 25% O2, 75% Ar mixture. The concentration of the sample solution was typically 5–10 mg/mL. Samples were vitrified by plunge freezing into liquid ethane using a Vitrobot (FEI Co.). Microscopy was carried out using a Tecnai F20 (FEI Co.) TEM at 120 keV with magnifications ranging from 29,000-280,000. Images were acquired at an underfocus of ~2.5 μm to a slow scan CCD camera (TVIPS GmbH) using the Leginon software system21.
Results and Discussion
BLs form in a very narrow composition interval around ΦMVLBG2=0.1 in the MVLBG2/DOPC system. Considering this and comparing headgroup geometries and charge, we first screened MVL3/DOPC and MVL5/DOPC mixtures of ΦMVL=0.1–0.5 with differential interference contrast (DIC) microscopy. We then investigated the samples with cryo-TEM. We found micrometer-scale tubular vesicles and BLs for ΦMVL=0.1 (see Fig. S2 and S3 in Supporting Information), and both of these mixtures were found to also form nanoscale BLs and nanotubes when investigated with cryo-TEM. In addition, cryo-TEM revealed BL and nanotube formation for the MVL3/DOPC system at ΦMVL3=0.3. No BLs were found for ΦMVL > 0.3.
Fig. 1 shows cryo-TEM images of BLs formed by a mixture of MVL3 and DOPC in water (ΦMVL3=0.1). In contrast to MVLBG2-containing BLs, where nanotubes and nanorods (cylindrical micelles) are very rigid with a persistence length of several micrometers, MVL3-containing nanotubes are very soft and bend easily. The white solid arrows point to the loci of highest bending. It is noteworthy that there is no preferred orientation of these nanotubes, confirming that the nanotubes are not induced by any flow effects during the sample preparation. Instead, this lipid mixture inherently forms nanotubes. The reduced stiffness of the nanotubes can be explained by the reduced charge of the lipid bilayer. MVL3 only has a charge of +3 e, whereas MVLBG2 has a charge of +16 e. Thus, the electrostatic contribution to the bending modulus K is strongly reduced22.
Figure 1.
Cryo-TEM images of MVL3/DOPC BLs (ΦMVL3=0.1). Both micrographs clearly show extensive bending of the flexible MVL3-containing nanotubes (white arrows point toward some of the regions where nanotubes are bent). Note the diversity in spatial orientation of the tubes, which confirms that the nanotubes are not formed by flow effects during sample preparation. Scale bar, 500 nm.
Further characteristic features of the MVL3/DOPC lipid mixture in water (ΦMVL3=0.1) are displayed in Fig. 2. Fig. 2 A shows the presence of nanorods (highlighted by white solid arrows). Although present, nanorods are not as abundant as they were in the MVLBG2/DOPC lipid mixture (ΦMVLBG2=0.1). This observation is consistent with the lower propensity of MVL3 to form micelles due to its smaller headgroup: although MVL3 has a conical molecular shape, the aspect ratio of this molecule is not as extreme as that of MVLBG2. Another key feature of the MVL3/DOPC system is branching of the nanotubes, as seen in Fig. 2 A (highlighted by a white dashed arrow).
Figure 2.
Cryo-TEM images of MVL3/DOPC BLs (ΦMVL3=0.1). (A) A dotted white arrow points to an example of a branched nanotube. White solid arrows point to the nanorods in the sample. (B) A group of nanotubes of different diameter, illustrating the broad distribution of radii of MVL3-containing nanotubes. The white solid arrow points to a nanorod. (C) A schematic drawing of a BL consisting of a sphere and a nanorod. Enlarged insets (a) and (b) show an illustration of the molecular arrangement of the charged (green) and neutral (white) lipid. Reprinted in part from ref. 1 with permission. Copyright 2008, American Chemical Society. Scale bar, 500nm.
The distribution of the diameters of MVL3-containing nanotubes is quite broad as demonstrated in Fig. 2 B. Fig. 2 C provides a schematic drawing of a BL consisting of a sphere and a nanorod as well as an illustration of the molecular organization (in the regions framed by rectangles) of the multivalent lipid (shown in green) and the neutral lipid (shown in white) as previously suggested for the MVLBG2/DOPC system. Note the high concentration of the charged lipid in the nanorod, where it stabilizes the high curvature.
Fig. 3 A depicts one of the unique hallmarks of the MVL3/DOPC lipid mixture in water (ΦMVL3=0.1)—the pearling instability. A pearling instability has previously been described in micrometer-scale tubular vesicles23 and was attributed to the increased tension in the lipid bilayer24. We hypothesize that lipid demixing in the nanotubes leads to a phase separation between charged MVL3 (localized mostly in the nanotubes) and neutral DOPC (localized mostly in the “bubbles”). In fact, the lipid demixing might be initialized by increased membrane tension due to the electrostatic repulsion among MVL3 molecules and their curvature stabilization efforts. In addition, the relatively low stiffness of the MVL3-containing membranes reduces the elastic energy cost of the structures resulting from pearling. Fig. 3 B shows a schematic drawing of a sphere-tube-sphere BL with illustrations of the suggested molecular organization in the nanotube and the tube-sphere transition regions. The transition from nanotube to a vesicle is highly reminiscent of the pearling instability described above. Similar features as described above for the MVL3/DOPC lipid mixture at ΦMVL3=0.1 were also found for ΦMVL3=0.3.
Figure 3.
Cryo-TEM image of MVL3/DOPC BLs (ΦMVL3=0.1). (A) This image is a collage of two separate sample views, thus expanding the observed area. The grey vertical line corresponds to the image boundary. White solid arrows point to the pearling instability occurring in this system. (B) A schematic drawing of a BL consisting of two spheres and a nanotube. Enlarged insets (a) and (b) show an illustration of the molecular arrangement of the charged (green) and neutral (white) lipid. Reprinted in part from ref. 1 with permission. Copyright 2008, American Chemical Society. Scale bar, 500 nm.
Screening of the phase behavior of MVL5/DOPC lipid mixtures in water showed BL and nanotube formation at ΦMVL5=0.1. As clearly visible in Fig. 4, this lipid mixture forms an abundance of long nanotubes and BLs. The length of the nanotubes can be well over 10 μm. The size of the spherical vesicular parts varies widely from tens to hundreds of micrometers. An expanded low magnification cryo-TEM image displaying a larger area of the sample can be found in the Supporting Information (Fig. S4).
Figure 4.
Low magnification cryo-TEM image of MVL5/DOPC BLs (ΦMVL5=0.1). This lipid mixture forms an abundance of long nanotubes and BLs. The length of the nanotubes can be well over 10 μm. The large circles in the image correspond to the holes in the carbon film on the copper grid. Scale bar, 2 μm.
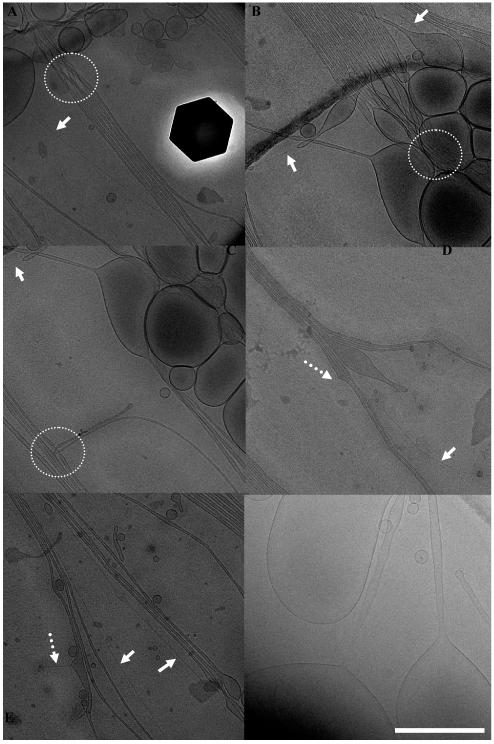
The MVL5/DOPC lipid mixture (ΦMVL5=0.1) also exhibits unique features not found in other BL-forming systems. Fig. 5 gives an overview of the key features observed in this system. Standard examples of BLs can be seen in Fig. 5 A (sphere–rod) and Fig. 5 F (sphere–tube). In addition, bundling of nanotubes is abundant in this system. Interestingly, the nanotubes in a bundle frequently cross each other and thus exchange their position in the bundle as shown in Fig. 5 A and B (areas highlighted by white dotted circles) or loop around each other as demonstrated in the example in Fig. 5 C (highlighted by white dotted circle). Finally, a novel feature that we term “spike” can be observed in Fig. 5 D and E (highlighted by white dotted arrows). These spikes are a remarkable feature fully consistent with the concept of BLs. It is a nanorod (cylindrical micelle) budding off perpendicularly from the nanotube side (rather than from the nanotube tip as observed previously1). Formation of this feature corresponds to a further curvature-induced phase separation within a nanotube, which itself is already a high-curvature object. In the spikes, the pentavalent MVL5 (essential for stabilizing the high curvature of nanotubes) continues to seek regions of even higher curvature, which can only be achieved by nanorod formation. The kinetics of this system have to be radically different from those of the MVLBG2/DOPC/water system, where the phase separation forming nanorods from nanotubes exclusively occurred at the tips of nanotubes. In contrast, the MVL5/DOPC/water system can nucleate a nanorod at any point along the whole length of a nanotube.
Figure 5.
Cryo-TEM images providing an overview of the most frequently observed features of MVL5/DOPC BLs (ΦMVL5=0.1). White dotted circles highlight the areas where nanotubes are entangled. White solid arrows point to nanorods. Note the sphere–rod BL in panel A. White dotted arrows point to a unique feature of this system: “spikes”, which are nanorods budding off from a nanotube. Pronounced bundling of nanotubes is observed in panels A-E. Scale bar, 500 nm.
As mentioned earlier, nanotube crossings and loops leading to complicated tubular entanglements are abundant in the MVL5/DOPC system. This is surprising considering the relatively high charge of the lipid bilayer, which should result in an electrostatic repulsive force. Fig. 6 A and B present numerous further examples of such entanglements (highlighted by white dotted circles). White solid arrows point to nanorods and a white dotted arrow to a spike, a feature introduced above. Due to the intermediate headgroup size of MVL5 (larger than MVL3, but smaller that MVLBG2), its propensity to form micelles is also intermediate and a fair number of lipid nanorods was found. Unfortunately, the presence of a vast number of long nanotubes (similarly to MVL3/DOPC lipid mixture) prevented us from quantifying the number of nanorods because they get harder to discern along their complete length, resulting in possible multiple counts of the same nanorod.
Figure 6.
Cryo-TEM images of MVL5/DOPC BLs (ΦMVL5=0.1). Pronounced bundling can be observed. White dotted circles highlight areas where nanotubes cross or an entanglement of the nanotubes occurs at quite large length scales. White solid arrows point to nanorods. Scale bar, 500 nm.
The large extent of nanotube bundling is further illustrated by Fig. 7, which is a superimposition of several high magnification cryo-TEM images (allowing us to review a larger sample area). A further expansion of this collage can be found in the Supporting Information (Fig. S5). The bundle shown in Fig. 7 consists of ≈20 nanotubes, which are in a surprising proximity considering their large positive charge. A possible explanation for such a tight bundling might be that the entanglements described above mechanically hold the bundle together at several locations along the bundle length. Another explanation might be a favorable surface interaction between the tubules based, for example, on surface charge fluctuations.
Figure 7.
Superimposition of several cryo-TEM images of a large MVL5/DOPC nanotube bundle (ΦMVL5=0.1). The bundling is maintained over more than 5 μm. See Fig. S5 in the Supporting Information for a further expanded view with one additional image. Scale bar, 500 nm.
Investigation of mixtures of monovalent DOTAP and DOPC in water failed to produce evidence of nanotube or block liposome formation. DOTAP carries a charge of +1 e (cf. Figure S1 in the Supporting Information) and its molecular shape can be described as cylindrical rather than conical (C0 ≈ 0). Therefore, the propensity to form micelles is very low. This suggests that formation of BLs and nanotubes requires a certain minimal head group size of the lipid or, in other words, a propensity for micelle formation and thus the ability to stabilize high membrane curvature in addition to charge.
A remarkable aspect of the observed BLs is the presence of regions with negative Gaussian curvature (saddle splay-type surfaces with C1C2 < 0, where C1 and C2 are the membrane principal curvatures) in membranes containing lipids with such large headgroups. Located, e.g., at the transition regions between tubes/rods and vesicles and at nanotube branching points, regions with C1C2 < 0 are a defining feature of BLs: without them, the constituting membrane shapes of a BL would form separate liposomes. Yet this combination of negative Gaussian curvature in structures formed from lipids that induce positive spontaneous curvature due to their large headgroup size (C0 > 0) is unprecedented.
The elastic energy of a lipid bilayer per unit area can be described by Helfrich’s relation25
| (1) |
where κ and κG are the membrane bending and the Gaussian elastic moduli, respectively, C = C1 + C2, and C0 = 2/R0 with the spontaneous radius R0. Accordingly, surfaces with negative Gaussian curvature are energetically favored if the Gaussian modulus is positive (κG > 0). The Gaussian modulus is related to C0 as
| (2) |
where δ is the membrane thickness and κG,Bilayer/Monolayer are the Gaussian moduli of lipid bilayer and monolayer, respectively. Negative values of C0 thus increase κG,Bilayer, favoring the formation of saddle splay surfaces26. This is reflected in the fact that lipid systems forming cubic phases (where C1C2 < 0) typically also exhibit an inverted hexagonal phase (where C < 0 and C1C2 = 0). In contrast, multivalent charged lipids have a conical molecular shape, giving rise to C0 > 0 and driving the formation of nanotubes and nanorods. It is thus remarkable that nanotubes and nanorods do not detach from spherical vesicles and/or each other, and the reason for this remains unclear. Possibly, an interaction between the multivalent and neutral lipid is essential for BL formation, because BLs do not form when the membranes contain only multivalent lipid. In addition, the electrostatic interaction plays a key role in BL formation3 and, therefore, needs to be considered in future theories. A theory of block liposome formation must also be able to predict the characteristic feature of BLs that a single object exhibits length scales varying by as much as two orders of magnitude (e.g., a diblock sphere–rod liposome may exhibit radii of curvature of < 10 nm (diameter of rod) and > 1000 nm (diameter of the spherical component)).
In a recent self-consistent field theory Greenall and Gompper have indeed found that mixtures of amphiphiles with different molecular shapes may form equilibrium block liposome phases27. Their starting point is amphiphiles, which separately form lamellar structures and spherical micelles similar to what is found in the mixtures which produce block liposomes: DOPC forms lamellar phases and small curvature vesicles while the highly cone shaped multivalent lipids form micelles1,28,29. Their theory shows that the presence of curvature forming amphiphiles in mixtures may lead to curvature induced lateral phase separation of amphiphiles and microphase separation of shapes leading to an equilibrium phase where vesicles connected by thread-like micelles exist at equilibrium in a narrow range of concentrations of the curvature forming amphiphile. This is precisely the hypothesis put forward in the original paper introducing the discovery of block liposomes1. The equilibrium structure described by Greenall and Gompper corresponds to a triblock (sphere-rod-sphere) liposome.
We caution that the Greenall-Gompper theory does not take into account electrostatics explicitly (i.e. the amphiphiles are charge neutral), which we know to be important. Thus, the way it fits into our experimental system is as follows. The shape of a lipid depends on the relative size of the headgroup area versus the tail area. There are two components to the headgroup size: steric and that due to charge (i.e. the Debye length3). The current theory considers the limit where the steric size alone makes the entire contribution and the headgroup is large enough to make a strong curvature generating lipid. In the experiments described here and earlier1,3,28 we have found that the added contribution of the charge to the “effective” headgroup size is needed to produce block liposomes. Thus, the theory may be applicable to the experimental system in that it effectively takes into account electrostatics by considering amphiphiles with large enough steric headgroups. The Greenall-Gompper model is an important starting point for a revised model where the charge of the curvature generating amphiphile and the counter-ions are explicitly taken into account.
Conclusions
In conclusions, our cryo-TEM studies have shown that mixtures of the multivalent cationic lipids MVL3 and MVL5 with neutral DOPC readily form nanotubes and block liposomes. In combination with studies of monovalent DOTAP, where no nanotubes or block liposomes were found, and previous investigations of the phase behavior of hexadecavalent MVLBG2, our data suggests that the formation of nanotubes and block liposomes is an inherent property of charged membranes formed by lipids with a propensity to form micelles, i.e., conical molecular shape (C0 > 0). This is of fundamental importance for the understanding of the shape evolution of liposome systems. The results described in this report should motivate future theories of block liposome formation in multicomponent charged membranes comprised of two lipid components with vastly varying shapes. We emphasize that future models, which may be based on the Greenall-Gompper theory, must incorporate the charged nature of the membrane, which experiments show to be an essential feature of block liposomes3. These new theories are required to understand BLs and might allow predicting nanotube formation in other, yet unstudied systems. The present study was motivated mainly by gene therapy and therefore focused on cationic lipids. However, we are aware of an example of brain derived, anionic curvature-generating lipids causing a spontaneous formation of lipid tubules30, which would expand our finding to anionic membranes as well.
Supplementary Material
Acknowledgement
We acknowledge support by NIH grant GM-59288, DOE BES DE-FG02-06ER46314 (lipid phase behavior), and NSF DMR-0803103. AZ is a Damon Runyon Fellow supported by the Damon Runyon Cancer Research Foundation (DRG 2040-10). The cryogenic transmission electron microscopy research was performed at the National Resource for Automated Molecular Microscopy, which is supported by the U. S. NIH National Center for Research Resources P41 program (RR17573). CRS acknowledges useful discussions with KAIST Faculty where he has a WCU (World Class University) Visiting Professor of Physics appointment supported by the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology No. R33-2008-000-10163-0.
Footnotes
Supporting Information Available: Description of materials and experimental methods; chemical structures of DOPC, DOTAP, MVL3, MVL5, and MVLBG2; differential-interference-contrast (DIC) optical micrographs of MVL3/DOPC and MVL5/DOPC mixtures; additional cryo-TEM images of block liposomes formed by MVL3 and MVL5.
References
- 1.Zidovska A, Ewert KK, Quispe J, Carragher B, Potter CS, Safinya CR. Block Liposomes from Curvature-Stabilizing Lipids: Connected Nanotubes, -rods and -spheres. Langmuir. 2009;25:2979–2985. doi: 10.1021/la8022375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ewert KK, Evans HM, Zidovska A, Bouxsein NF, Ahmad A, Safinya CR. A columnar phase of dendritic lipid-based cationic liposome-DNA complexes for gene delivery: Hexagonally ordered cylindrical micelles embedded in a DNA honeycomb lattice. J. Am. Chem. Soc. 2006;128:3998–4006. doi: 10.1021/ja055907h. [DOI] [PubMed] [Google Scholar]
- 3.Zidovska A, Ewert KK, Quispe J, Carragher B, Potter CS, Safinya CR. The effect of salt and pH on block liposomes studied by cryogenic transmission electron microscopy. Biochim. Biophys. Acta. 2009;1788:1869–1876. doi: 10.1016/j.bbamem.2009.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ewert K, Slack NL, Ahmad A, Evans HM, Lin AJ, Samuel CE, Safinya CR. Cationic lipid-DNA complexes for gene therapy: Understanding the relationship between complex structure and gene delivery pathways at the molecular level. Curr. Med. Chem. 2004;11:133–149. doi: 10.2174/0929867043456160. [DOI] [PubMed] [Google Scholar]
- 5.Ewert KK, Ahmad A, Evans HM, Safinya CR. Cationic lipid-DNA complexes for nonviral gene therapy: relating supramolecular structures to cellular pathways. Expert Opin. Biol. Ther. 2005;5:33–53. doi: 10.1517/14712598.5.1.33. [DOI] [PubMed] [Google Scholar]
- 6.Huang L, Hung M-C, Wagner E. Non-Viral Vectors for Gene Therapy. Elsevier; San Diego: 2005. [Google Scholar]
- 7.Mahato RI, Kim SW, Francis T. a., editors. Pharmaceutical Perspectives of Nucleic Acid-Based Therapeutics. London and New York: 2002. [Google Scholar]
- 8.Singh A, Wong EM, Schnur JM. Toward the rational control of nanoscale structures using chiral self-assembly: Diacetylenic phosphocholines. Langmuir. 2003;19:1888–1898. [Google Scholar]
- 9.Schnur JM. Lipid Tubules - a Paradigm for Molecularly Engineered Structures. Science. 1993;262:1669–1676. doi: 10.1126/science.262.5140.1669. [DOI] [PubMed] [Google Scholar]
- 10.Shimizu T, Masuda M, Minamikawa H. Supramolecular nanotube architectures based on amphiphilic molecules. Chem. Rev. 2005;105:1401–1443. doi: 10.1021/cr030072j. [DOI] [PubMed] [Google Scholar]
- 11.Alberts B. Molecular Biology of The Cell. Garland Science; New York: 2002. [Google Scholar]
- 12.Gallop JL, Butler PJG, McMahon HT. Endophilin and CtBP/BARS are not acyl transferases in endocytosis or Golgi fission. Nature. 2005;438:675–678. doi: 10.1038/nature04136. [DOI] [PubMed] [Google Scholar]
- 13.Gallop JL, McMahon HT. BAR domains and membrane curvature: bringing your curves to the BAR. Rafts and Traffic; Lipids: 2005. pp. 223–231. [DOI] [PubMed] [Google Scholar]
- 14.Hinshaw JE, Schmid SL. Dynamin Self-Assembles into Rings Suggesting a Mechanism for Coated Vesicle Budding. Nature. 1995;374:190–192. doi: 10.1038/374190a0. [DOI] [PubMed] [Google Scholar]
- 15.Roux A, Cuvelier D, Nassoy P, Prost J, Bassereau P, Goud B. Role of curvature and phase transition in lipid sorting and fission of membrane tubules. EMBO J. 2005;24:1537–1545. doi: 10.1038/sj.emboj.7600631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qi SY, Groves JT, Chakraborty AK. Synaptic pattern formation during cellular recognition. Proc. Natl. Acad. Sci. U.S.A. 2001;98:6548–6553. doi: 10.1073/pnas.111536798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahmad A, Evans HM, Ewert K, George CX, Samuel CE, Safinya CR. New multivalent cationic lipids reveal bell curve for transfection efficiency versus membrane charge density: lipid-DNA complexes for gene delivery. J. Gen. Med. 2005;7:739–748. doi: 10.1002/jgm.717. [DOI] [PubMed] [Google Scholar]
- 18.Ewert K, Ahmad A, Evans HM, Schmidt HW, Safinya CR. Efficient synthesis and cell-transfection properties of a new multivalent cationic lipid for nonviral gene delivery. J. Med. Chem. 2002;45:5023–5029. doi: 10.1021/jm020233w. [DOI] [PubMed] [Google Scholar]
- 19.Ewert KK, Evans HM, Bouxsein NF, Safinya CR. Dendritic cationic lipids with highly charged headgroups for efficient gene delivery. Bioconjugate Chem. 2006;17:877–888. doi: 10.1021/bc050310c. [DOI] [PubMed] [Google Scholar]
- 20.Quispe J, Damiano J, Mick SE, Nackashi DP, Fellmann D, Ajero TG, Carragher B, Potter CS. An improved holey carbon film for cryo-electron microscopy. Microsc. Microanal. 2007;13:365–371. doi: 10.1017/S1431927607070791. [DOI] [PubMed] [Google Scholar]
- 21.Suloway C, Pulokas J, Fellmann D, Cheng A, Guerra F, Quispe J, Stagg S, Potter CS, Carragher B. Automated molecular microscopy: The new Leginon system. J. Struct. Biol. 2005;151:41–60. doi: 10.1016/j.jsb.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 22.Winterhalter M, Helfrich W. Effect of Surface-Charge on the Curvature Elasticity of Membranes. J. Phys. Chem. 1988;92:6865–6867. [Google Scholar]
- 23.Barziv R, Moses E. Instability and Pearling States Produced in Tubular Membranes by Competition of Curvature and Tension. Phys. Rev. Lett. 1994;73:1392–1395. doi: 10.1103/PhysRevLett.73.1392. [DOI] [PubMed] [Google Scholar]
- 24.Goldstein RE, Nelson P, Powers T, Seifert U. Front propagation in the pearling instability of tubular vesicles. J. Phys. II. 1996;6:767–796. [Google Scholar]
- 25.Helfrich W. Elastic Properties of Lipid Bilayers - Theory and Possible Experiments. Z. Naturforsch., C: Biosci. 1973;C 28:693–703. doi: 10.1515/znc-1973-11-1209. [DOI] [PubMed] [Google Scholar]
- 26.Porte G. Lamellar phases and disordered phases of fluid bilayer membranes. J. Phys.: Condens. Matter. 1992;4:8649–8670. [Google Scholar]
- 27.Greenall MJ, Gompper G. Bilayers Connected by Threadlike Micelles in Amphiphilic Mixtures: A Self-Consistent Field Theory Study. Langmuir. 2011;27:3416–3423. doi: 10.1021/la200138b. [DOI] [PubMed] [Google Scholar]
- 28.Zidovska A, Ewert KK, Quispe J, Carragher B, Potter CS, Safinya CR. Methods in Enzymology Liposomes. 2009. Block Liposomes: Vesicles of Charged Lipids with Distinctly Shaped Nanoscale Sphere-, Pear-, Tube-, or Rod-Segments; pp. 111–128. Pt G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zidovska A, Evans HM, Ewert KK, Quispe J, Carragher B, Potter CS, Safinya CR. Liquid Crystalline Phases of Dendritic Lipid-DNA Self-Assemblies: Lamellar, Hexagonal, and DNA Bundles. J. Phys. Chem. B. 2009;113:3694–3703. doi: 10.1021/jp806863z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Akiyoshi K, Itaya A, Nomura SM, Ono N, Yoshikawa K. Induction of neuron-like tubes and liposome networks by cooperative effect of gangliosides and phospholipids. FEBS Lett. 2003;534:33–38. doi: 10.1016/s0014-5793(02)03743-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.