Abstract
Low vitamin B-6 status, based on plasma concentrations of pyridoxal-5-phosphate (PLP), has been identified in inflammatory diseases, including cardiovascular disease, rheumatoid arthritis, inflammatory bowel disease, and diabetes. Our objective was to examine the association between plasma PLP and multiple markers of inflammation in a community-based cohort [n = 2229 participants (55% women, mean age 61 ± 9 y)]. We created an overall inflammation score (IS) as the sum of standardized values of 13 individual inflammatory markers. Multivariable-adjusted regression analysis was used to assess the associations between the IS and plasma PLP. Geometric mean plasma PLP concentrations were lower in the highest tertile category of IS relative to the lowest (61 vs. 80 nmol/L; P-trend < 0.0001). Similarly, the prevalence of PLP insufficiency was significantly higher for participants in the highest compared with the lowest tertiles for IS categories. These relationships persisted after accounting for vitamin B-6 intake. Also, there were significant inverse relationships between plasma PLP and 4 IS based on functionally related markers, including acute phase reactants, cytokines, adhesion molecules, and oxidative stress. In addition, secondary analyses revealed that many of the individual inflammatory markers were inversely associated with plasma PLP after adjusting for plasma C-reactive protein concentration. This study, in combination with past findings, further supports our hypothesis that inflammation is associated with a functional deficiency of vitamin B-6. We discuss 2 possible roles for PLP in the inflammatory process, including tryptophan metabolism and serine hydroxymethyltransferase activity.
Introduction
Low vitamin B-6 status, marked by low concentrations of plasma pyridoxal-5′-phosphate (PLP)10, has been identified as a risk factor for cardiovascular disease (CVD) morbidity and mortality, including myocardial infarction (1), atherosclerosis (2), and stroke (3). Patients with confirmed CVD often present with lower PLP plasma concentrations compared with healthy controls (1, 4, 5). Low plasma PLP is also seen in patients with rheumatoid arthritis (6–9), inflammatory bowel disease (10, 11), and diabetes (12, 13).
Inflammation is generally recognized as a contributing factor to the development of CVD (14). For instance, C-reactive protein (CRP), an acute phase protein synthesized and secreted by the liver in response to proinflammatory cytokines (15), is a powerful indicator of future CVD risk (16).
A recent analysis of >2500 participants in the 2003–2004 NHANES found an inverse relationship between vitamin B-6 status and serum CRP concentrations, which remained significant after adjusting for vitamin B-6 intake, supplement use, and homocysteine levels among other covariates (17). This large population study confirmed previous findings of homocysteine-independent inverse associations between plasma PLP and CRP (18–20) and other markers of inflammation (10, 19).
The present study was undertaken to further understand the basis of the associations between vitamin B-6 and inflammation by examining the relationship between vitamin B-6 status and overall inflammation, functional indicators of inflammation, and individual biomarkers of inflammation.
Participants and Methods
Participants
The design and selection criteria of the Framingham Offspring Study have been described elsewhere (21). Men and women recruited in 1971 have been examined every 3–8 y since. For this cross-sectional analysis of the relations between vitamin B-6 status and inflammatory biomarkers, data were used from the seventh examination, which took place from 1998 to 2001. Participants underwent routine physical examination, medical history, and laboratory assessments. Participants were excluded from the current study if they did not have valid dietary intake information (n = 134), were missing data on inflammatory biomarkers (n = 1014; excluding TNFα, which was measured on a subset of the cohort), or were missing data on other covariates (n = 162). Of the 3539 members of the cohort who participated in the seventh study examination, data on 2229 men and women were available for analysis. The Framingham Heart Study protocol is reviewed annually by the Boston University Medical Center Institutional Review Board and all participants signed written informed consent.
Plasma PLP
Vitamin B-6 status was assessed by plasma PLP concentrations. Fasting blood samples were collected at the seventh examination. Plasma PLP was assayed by the tyrosine decarboxylase apoenzyme method (22).
Inflammatory biomarkers
Single measurements of plasma CRP were made using a high-sensitivity assay while the following inflammatory biomarkers were measured in duplicate from fasting blood samples taken during the seventh examination cycle (1998–2001) using commercially available enzyme-linked immunoassay kits11: plasma cluster of differentiation 40 ligand (CD40L), plasma P-selectin, plasma osteoprotegerin, plasma TNFα, plasma TNF receptor 2 (TNFR-2), serum soluble intercellular adhesion molecular-1 (ICAM-1), serum IL-6, serum monocyte chemotactic protein-1 (MCP-1), serum myeloperoxidase, plasma lysosomal phospholipase A2 (LPL-A2) mass and activity, and urinary isoprostanes indexed to urinary creatinine. Plasma fibrinogen was measured in duplicate using the clot-time method of Clauss (23) with Diagnostica Stago reagents.
Using the aforementioned markers of inflammation, we developed 2 types of scores to represent inflammation: a score representative of overall inflammation and scores based on markers that are thought to be functionally interrelated (24). The individual marker values were first standardized as Z-scores and then summed to compute the different scores. The overall inflammation score (IS) is the sum of the standardized values of all of the inflammatory biomarkers, including CRP, fibrinogen, IL-6, TNFα, TNFR-2, osteoprotegerin, P-selectin, CD40L, ICAM-1, MCP-1, myeloperoxidase, LPL-A2 mass, LPL-A2 activity, and isoprostanes indexed to creatinine. IS-acute phase reactants included CRP and fibrinogen, 2 acute phase proteins. IS-cytokines included IL-6, TNFα, TNFR-2, and osteoprotegerin. IS-selectins included P-selectin and CD40L. IS-oxidative stress included the biomarkers myeloperoxidase, LPL-A2 mass, LPL-A2 activity, and isoprostanes indexed to creatinine. ICAM-1 and MCP-1 are 2 biomarkers not associated with the above-mentioned functional categories and thus their associations with plasma PLP were assessed individually.
Covariates
Covariates used in these analyses included age; sex; BMI (kg/m2); self-reported cigarette smoking and nonsteroidal antiinflammatory drug (NSAID) use; vitamin B-6, protein, and energy intakes; multivitamin supplement use; and circulating concentrations of folate, vitamin B-12, homocysteine, total cholesterol, and creatinine. Nutrient intakes from food and supplements were assessed using the Harvard FFQ (25). Questionnaires were considered invalid and data were excluded from analysis if a participant reported an energy intake of <2.51 MJ/d (600 kcal/d) or >16.74 MJ/d (4000 kcal/d) for women and >17.58 MJ/d (4200 kcal/d) for men or if the participant left >12 items on the FFQ blank. Fasting blood samples were collected at each examination and frozen at −80°C for future analysis. Circulating concentrations of plasma homocysteine were determined by HPLC with fluorimetric detection (26). Plasma folate was measured by a microbial assay (Lactobacillus casei) in a 96-well plate (27). Plasma vitamin B-12 was measured with a (Magic) RIA kit from Ciba-Corning. Plasma cholesterol concentrations were measured (28). Creatinine levels were measured in serum and urine from fasting participants by the modified Jaffé method (29)
Statistical analyses
The statistical analyses were performed with SAS (version 9.1; SAS Institute).
Participant characteristics.
We examined participant characteristics, including nutrient intakes, plasma/serum concentrations of nutrients, and a number of demographic and lifestyle factors by tertile category of plasma PLP. Least square means (95% CI), geometric means (95% CI), and proportions were generated using SAS PROC GLM. A P-trend was determined as the P value of the regression coefficient for the independent variable (i.e., inflammatory marker) of interest derived by entering this variable into the model as a continuous variable. P < 0.05 was considered significant.
Primary analyses.
To assess associations between biomarkers of inflammation and plasma PLP concentrations, we used multivariable regression analysis to calculate geometric mean plasma PLP (95% CI) across tertile categories of inflammatory biomarkers. In our multivariable regression analysis, plasma PLP, normalized by natural log transformation, was used as a continuous dependent variable and the inflammation indices, divided into tertile categories, were used as independent variables. Covariates included: age; sex; BMI; plasma homocysteine, folic acid, and vitamin B-12; serum creatinine and total cholesterol; vitamin B-6, protein, and energy intakes; multivitamin supplement and NSAID use; and current smoking status.
Additionally, to examine the interrelationship among vitamin B-6 intake, inflammatory status, and plasma PLP concentrations, we constructed a graph presenting the geometric means of plasma PLP adjusted for the abovementioned covariates and stratified by tertile categories of inflammatory status as indicated by IS and tertile categories of vitamin B-6 intake from diet and supplements.
Secondary analyses.
In a secondary analysis, we examined the prevalence (95% CI) of inadequate PLP [plasma PLP < 20 nmol/L (30)] across tertile categories of the overall IS. We also assessed associations between individual biomarkers of inflammation and plasma PLP concentrations using multivariable regression analysis, where plasma PLP was used as a continuous dependent variable and the individual biomarkers of inflammation, divided into tertile categories, were used as independent variables. Each biomarker was entered into the models one at a time. Where noted, natural log-transformed CRP was entered into the model as a continuous covariate to determine whether the relationship between plasma PLP concentrations and individual biomarkers persisted after accounting for CRP. Other covariates included: age; sex; BMI; plasma homocysteine, folic acid, and vitamin B-12; serum creatinine and total cholesterol; vitamin B-6, protein, and energy intakes; multivitamin supplement and NSAID use; and current smoking status.
Results
Characteristics of the population.
There was no association between sex or age and plasma PLP (Table 1). However, BMI was lower with increasing plasma PLP tertile categories. Similarly, the percentage of cigarette smokers and the prevalence of both diabetes and CVD were lower with increasing plasma PLP tertile categories. Homocysteine and 2 indicators of inflammation (CRP and IS) were also lower with increasing tertile categories of plasma PLP. On the other hand, the percentage of participants taking multivitamin supplements was higher with increasing tertile categories of plasma PLP. Additionally, the intakes of energy, protein, and vitamin B-6 were higher with increasing tertile categories of plasma PLP as were plasma folate and vitamin B-12 in these unadjusted analyses.
TABLE 1.
Characteristics of participants of the Framingham Offspring Study seventh examination by PLP tertile category1
| Plasma PLP tertile categories, nmol/L |
||||
| Characteristic | 1 35 (34, 36) | 2 69 (67, 71) | 3 177 (173, 181) | P-trend2 |
| Participants, n | 743 | 743 | 743 | |
| Male, % | 42 | 48 | 44 | 0.35 |
| Age, y | 61 (61, 62) | 61 (61, 62) | 61 (60, 62) | 0.87 |
| BMI, kg/m2 | 29 (29, 30) | 28 (27, 28) | 27 (27, 28) | <0.001 |
| Multivitamin supplement use, % yes | 21 | 52 | 82 | <0.001 |
| NSAID use, % yes | 10 | 11 | 11 | 0.56 |
| Cigarette use, % yes | 21 | 10 | 8 | <0.001 |
| Diabetes, % yes | 10 | 7 | 6 | 0.001 |
| CVD, % yes | 14 | 13 | 11 | 0.1 |
| Energy intake, kcal/d | 1770 (1720, 1810) | 1820 (1780, 1870) | 1870 (1830, 1910) | <0.001 |
| Protein intake, g/d | 76.3 (74.2, 78.4) | 77.9 (75.8, 80.0) | 82.5 (80.4, 84.6) | <0.001 |
| Vitamin B-6 intake, g/d | 2.7 (0.9, 4.5) | 5.2 (3.4, 6.9) | 18.6 (16.8, 20.3) | <0.001 |
| Plasma cholesterol, mmol/L | 199 (196, 201) | 201 (198, 203) | 201 (198, 203) | 0.21 |
| Plasma homocysteine, μmol/L | 9.3 [9.0, 9.6] | 8.3 [8.0, 8.6] | 8.0 [7.7, 8.2] | <0.001 |
| Plasma folate, nmol/L | 12.3 [11.8, 12.7] | 16.4 [15.8, 17.0] | 21.1 [20.3, 21.9] | <0.001 |
| Plasma vitamin B-12, pmol/L | 340 [331, 349] | 407 [397, 418] | 473 [461, 486] | <0.001 |
| Serum creatinine, μmol/L | 94.6 (91.9, 96.4) | 94.6 (92.8, 97.2) | 95.5 (92.8, 97.2) | 0.60 |
| Plasma C-reactive protein, mg/L | 3.1 [2.9, 3.4] | 2.1 [1.9, 2.3] | 1.8 [1.6, 1.9] | <0.001 |
| IS3 | 1.7 (1.3, 2.1) | −0.2 (−0.6, 0.2) | −1.1 (−1.5, −0.7) | <0.001 |
Values are mean (95% CI), geometric mean [95% CI], or percent. CD40L, CD40 ligand; CVD, cardiovascular disease; ICAM-1, intracellular adhesion molecule-1; IS, inflammation score; LPLP-A2, lipoprotein phospholipase A2; NSAID, nonsteroidal antiinflammatory drug; PLP, pyridoxal-5-phosphate; TNFR-2, TNF receptor-2.
value of the regression coefficient for the independent variable
Summed Z-scores of: CRP, fibrinogen, IL-6, TNFα, TNFR-2, osteoprotegerin, P-selectin, CD40L, ICAM-1, MCP-1, myeloperoxidase, LPLP-A2 mass, LPLP-A2 activity, and isoprostanes.
Relationship between inflammation and plasma PLP concentrations.
In multivariable-adjusted regression analysis, we found that geometric mean plasma PLP concentrations were significantly inversely associated with tertile category of the IS, including IS, IS-acute phase reactants, IS-cytokines, and IS-oxidative stress (Table 2). For IS, the mean plasma PLP concentrations of individuals in the highest tertile category of inflammation were nearly 25% less than the mean plasma PLP concentrations for those in the lowest tertile category
TABLE 2.
Associations between PLP concentration and inflammation indices in participants of the Framingham Offspring Study seventh examination1
| Plasma PLP, nmol/L |
||||
| Tertile category of inflammation index |
||||
| Inflammation index | 1 | 2 | 3 | P-trend2 |
| IS3 | 80 (74, 86) | 71 (67, 76) | 61 (57, 65) | <0.001 |
| n | 621 | 621 | 621 | |
| IS-acute phase reactants3 | 76 (71, 80) | 68 (64, 72) | 61 (58, 65) | <0.001 |
| n | 605 | 641 | 617 | |
| IS-cytokines3 | 76 (71, 81) | 71 (67, 76) | 61 (57, 65) | <0.001 |
| n | 621 | 621 | 621 | |
| IS-oxidative stress3 | 73 (69, 78) | 70 (66, 75) | 63 (59, 66) | <0.001 |
| n | 743 | 743 | 743 | |
| IS-selectins3 | 69 (65, 74) | 69 (65, 73) | 66 (62, 70) | 0.08 |
| n | 743 | 743 | 743 | |
| ICAM-13 | 72 (68, 77) | 70 (66, 74) | 64 (61, 69) | <0.001 |
| n | 743 | 743 | 743 | |
| MCP-13 | 66 (62, 70) | 70 (66, 74) | 67 (63, 71) | 0.50 |
| n | 743 | 743 | 743 | |
Values are geometric mean (95% CI) from participants with valid dietary intake data who were not missing data on inflammatory biomarkers or other covariates stratified by tertile category of inflammation indices and adjusted for sex, age, BMI, circulating homocysteine, folate, vitamin B-12, creatinine, and total cholesterol, vitamin B-6, protein, and energy intakes, and NSAID, cigarette, and multivitamin use. CD40L, CD40 ligand; ICAM-1, intracellular adhesion molecule-1; IS, inflammation score; LPLP-A2, lipoprotein phospholipase A2; NSAID, nonsteroidal antiinflammatory drug; PLP, pyridoxal-5-phosphate; TNFR-2, TNF receptor-2.
value of the regression coefficient for the independent variable (i.e., IS or biomarker) derived by entering this variable into the model as a continuous variable.
Summed Z-scores of: CRP, fibrinogen, IL-6, TNFα, TNFR-2,osteoprotegerin, P-selectin, CD40L, ICAM-1, MCP-1, myeloperoxidase, LPLP-A2 mass, LPLP-A2 activity, and isoprostanes; IS- acute phase reactants = summed Z-scores of: CRP and fibrinogen, IS- Cytokines = summed Z-scores of: IL-6, TNFα, TNFR-2, and osteoprotegerin, IS-oxidative stress = summed Z-scores of: myeloperoxidase, LPLP-A2 mass, LPLP- A2 activity, and isoprostanes, IS-Selectins = summed Z-scores of: P-selectin and CD40L.
In our secondary analyses, we observed a significant trend toward increasing vitamin B-6 inadequacy (plasma PLP < 20 nmol/L) with increasing tertile categories of inflammation (Supplemental Fig. 1). The prevalence of low plasma PLP nearly doubled with increasing categories of inflammation. We also found lower mean plasma PLP concentrations in the higher tertile categories of individual inflammatory biomarkers, including ICAM (Table 2), CRP, fibrinogen, IL-6, lipoprotein phospholipase A2 (LPLP-A2) activity, TNFR-2, and TNFα (Supplemental Table 1). These relationships remained significant after additional adjustment for CRP (Supplemental Table 2). Of note, there was no inverse relationship between plasma folate and vitamin B-12, 2 other B-vitamins involved in homocysteine metabolism and overall inflammation (results not shown).
The analyses were rerun excluding participants with prevalent CVD and diabetes (n = 401) and these exclusions did not substantively affect observations (Supplemental Table 3).
Relationships among inflammation, vitamin B-6 intake, and plasma PLP concentration.
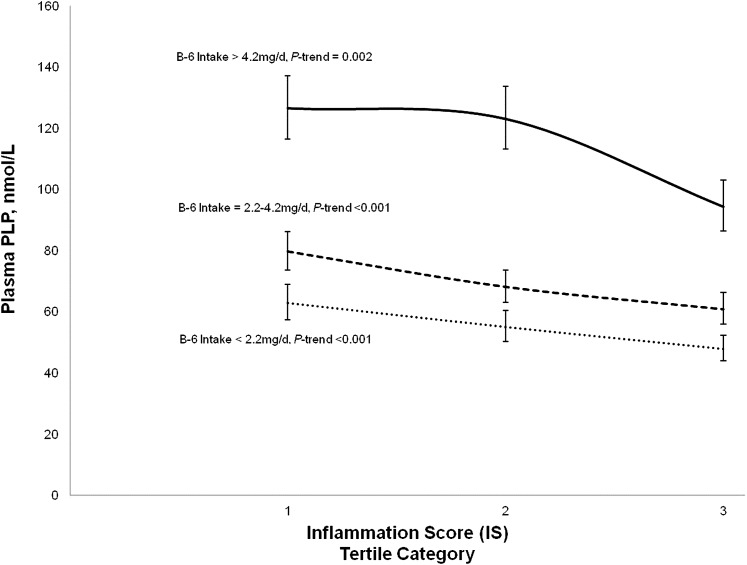
Those with the highest intake of vitamin B-6 had increased concentrations of plasma PLP (Fig. 1). Yet, regardless of vitamin B-6 intake, those with the greatest degree of inflammation had significantly lower plasma PLP concentrations than their low inflammation counterparts. Additionally, there was no significant interaction between inflammation and vitamin B-6 intake when comparing participants with the highest tertile compared with the lower 2 tertile categories of vitamin B-6 intake category.
FIGURE 1.
Decrease in plasma PLP with higher IS at each level of vitamin B-6 intake in 1863 participants of the seventh examination of the Framingham Offspring Study. The IS is the sum of Z-scores of CRP, fibrinogen, IL-6, TNFα, TNFR-2, osteoprotegerin, P-selectin, CD40L, ICAM-1, MCP-1, myeloperoxidase, LPLP-A2 mass, LPLP-A2 activity, and isoprostanes. Geometric means (95%CI) of plasma PLP (nmol/L) from participants of the seventh examination (n = 1863) adjusted for sex, age, BMI, plasma homocysteine, folate, vitamin B-12, creatinine, total cholesterol, protein intake, energy intake, NSAID use, and cigarette use. P-trend for the relation between plasma PLP and IS stratified by vitamin B-6 intake was derived by entering IS into the model as a continuous rather than categorical variable. n = 631 for vitamin B-6 intake >4.2 mg/d, n = 610 for vitamin B-6 intake between 2.2 and 4.2 mg/d, and n = 622 for vitamin B-6 intake <2.2 mg/d. CD40L, CD40 ligand; ICAM-1, intracellular adhesion molecule-1; IS, inflammation score; LPLP-A2, lipoprotein phospholipase A2; MCP-1, monocyte chemoattractant protein-1; NSAID, nonsteroidal antiinflammatory drug; PLP, pyridoxal-5-phosphate; TNFR-2, TNF receptor-2.
Discussion
In this comprehensive study of the relations between vitamin B-6 status and inflammation, we have shown that overall inflammation is inversely associated with plasma PLP concentrations and that the prevalence of vitamin B-6 inadequacy increases with inflammation. The unique feature of this study is the demonstration of the inverse correlation of plasma PLP with multiple markers of inflammation, including an overall IS and scores representing functional groups. Such results expand on previous findings that showed inverse relationships between plasma PLP and inflammatory diseases (6, 10, 31) and/or individual biomarkers of inflammation, including CRP (17, 19, 32), IL-6 (19), α1-antichymotripsin (33), thus revealing a nearly universal relationship between vitamin B-6 and numerous aspects of inflammation. These relationships persisted after accounting for relevant covariates, including homocysteine and vitamin B-6 intake. Additionally, there was no inverse relationship between plasma folate and vitamin B-12 and overall inflammation, indicating the inverse association between vitamin B-6 and inflammation did not extend to other B vitamins.
The mechanisms that underlie the associations between plasma PLP and markers of inflammation remain to be determined. An attractive hypothesis is that the low plasma PLP is a reflection of mobilization of this coenzyme into inflammatory sites. Because of the cross-sectional nature of the analyses presented in this paper, we cannot determine causality in the relationship between PLP and inflammation. However, Figure 1 illustrates that even among participants with the highest intake of vitamin B-6, there is a significant trend of decreasing plasma PLP with higher tertile categories of inflammation. This finding suggests that inflammation alters plasma PLP status. Further support for the interpretation that the inflammation-PLP association is secondary to the mobilization of PLP to inflammatory sites derives from a number of studies that showed that vitamin B-6 supplementation was without effect on inflammatory markers despite the improvement in plasma PLP levels (31, 34, 35). Furthermore, some data suggest that inflammation-associated changes in PLP resemble those of CRP, because they reflect an acute phase response. In inflammatory bowel disease, low PLP was seen only in patients with active disease, whereas those in remission had normal plasma PLP levels without any apparent increase in vitamin B-6 intake (10). Similarly, Bates et al. (33) found that low plasma PLP among elderly during winter months was not coincident with lower vitamin B-6 intakes, but rather the concentrations were inversely associated with makers of the acute phase response and possibly the occurrence of winter illnesses.
The targets for PLP involvement during inflammation remain to be determined. However, the fact that associations with PLP include a large number of inflammatory markers, as presented in this study, strongly implies that its interaction with these targets precede the formation of these markers. Whether the association of PLP is obligatory to the formation of these inflammatory markers or merely coincidental is of potential importance.
A potential site of PLP involvement is the degradation of tryptophan. The kynurenine pathway, which is responsible for over 95% of tryptophan degradation, utilizes many PLP-dependent enzymes (36). Indoleamine 2,3-dioxygenase (37), one of the enzymes that catalyzes the initial rate-limiting step in the conversion of tryptophan to kynurenine, is upregulated by inflammatory stimuli (38). Kynurenine is further degraded by PLP-dependent enzymes to a number of metabolites (36). It is quite possible that the induction of indoleamine 2,3-dioxygenase in response to inflammatory stimuli will result in the mobilization of plasma PLP for use in the degradation of kynurenine formed by the action of this enzyme. Thus, the relations between plasma PLP and inflammation may be a passive consequence of its pivotal role in tryptophan metabolism.
A more active potential role for PLP in the inflammatory process is as a cofactor of serine hydroxymethyltransferase, an enzyme essential for 1-carbon metabolism (39). Serine hydroxymethyltransferase activity is increased in lymphocytes stimulated to proliferate and the addition of 4-deoxypyridoxine, an inhibitor of certain PLP-dependent enzymes, to lymphocyte cultures has been shown to inhibit mitogen-stimulated serine hydroxymethyltransferase activity and cause a decrease in cell proliferation as well as the production of the cytokines IL-1b, IL-2 and the expression of the cytokine receptor, IL-2 receptor (40). This observation suggests that PLP is required for serine hydroxymethyltransferase activity and thus plays a role in serine hydroxymethyltransferase-stimulated inflammation.
Strengths and limitations.
Strengths of the present study include the community-based cohort design with systematic assessment of biomarkers and vitamin intake and levels, the strict laboratory quality control used to measure the outcome and exposure variables as well as clinical covariates, and the large cohort of men and women data available for analysis. Limitations include the observational nature of these associations, preventing any inference regarding a causal connection. The inclusion of participants without consideration for illness has strengths and limitations. Although their inclusion enhances the pool of individuals with inflammation, it is also possible that in primary analyses where the presence of chronic diseases was not controlled for, their inclusion introduces confounding. In secondary analyses where participants with diabetes and known CVD were excluded, the inverse association between vitamin B-6 status and inflammation remained significant. The multiple statistical tests conducted raise the possibility of false-positive findings, particularly for the individual biomarkers. However, given the strength of the findings, the majority of the associations remain significant even after accounting for multiple testing. Finally, the generalizability of these findings to other populations is unclear. First, participants of the seventh examination of the Framingham Offspring Study had higher median intakes of vitamin B-6 [3.1 mg/d from foods and supplements compared with 1.6 mg/d among supplement users who participated in the 1999–2000 NHANES (41), a population representative of the U.S. population]. The implications of the findings presented in this paper may be far greater among a population whose vitamin B-6 intakes are substantially lower, because increased inflammation may result in severe vitamin B-6 inadequacy. Second, the participants were largely white and of European ancestry, so the generalizability to other ethnicities is limited.
In conclusion, because of the strong inverse relationship between inflammation and plasma PLP in this large community-based population and the prevalence of people with inflammatory conditions, as well as those with inflammatory/immune related conditions, further studies on the mechanism of the relationship between PLP and inflammation are clearly warranted. This phenomenon would be better examined in experimental models in which one can either induce inflammation and examine the impact on vitamin B-6 status or modify vitamin B-6 status and examine the impact on inflammation.
Supplementary Material
Acknowledgments
P.F.J., J.S., and Y.B. designed the research; L.S. performed statistical analyses; E.J.B. and J.D.F. provided essential materials and critical revisions of the manuscript; L.S., R.R., M.O., P.F.J., and J.S. wrote the paper; and L.S., P.F.J., and J.S. had responsibility for final content. All authors read and approved the final manuscript.
Footnotes
Supported in part by a gift from Pharmavit and by USDA agreement nos. 58-1950-7-707 and 51520-008-04S. Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the view of the USDA. It was also supported by grants 1RO1 HL64753, R01 HL076784, and 1 R01 AG028321 and a Framingham Heart Study core contract NO1-HC251-95.
Supplemental Tables 1–3 and Supplemental Figure 1 are available from the “Online Supporting Material” link in the online posting of the article and from the same link in the online table of contents at http://jn.nutrition.org.
Abbreviations used: CD40L, CD40 ligand; CVD, cardiovascular disease; ICAM-1, intracellular adhesion molecule-1; IS, inflammation score, summed Z-scores of: CRP, fibrinogen, IL-6, TNFα, TNFR-2, osteoprotegerin, P-selectin, CD40L, ICAM-1, MCP-1, myeloperoxidase, lipoprotein phospholipase A2 mass, lipoprotein phospholipase A2 activity, and isoprostanes; IS-acute phase reactants, summed Z-scores of CRP and fibrinogen; IS-cytokines, summed Z-scores of: IL-6, TNFα, TNFR-2, and osteoprotegerin; IS-oxidative Stress, summed Z-scores of: myeloperoxidase, lipoprotein phospholipase A2 mass, lipoprotein phospholipase A2 activity, and isoprostanes; IS-selectins, summed Z-scores of P-selectin and CD40L; LPLP-A2, lipoprotein phospholipase A2; NSAID, nonsteroidal antiinflammatory drug; PLP, pyridoxal-5-phosphate; TNFR-2, TNF receptor-2.
CD40L: Bender MedSystems, Inc; plasma P-selectin: R&D Systems, Inc.; plasma osteoprotegerin: BioMedica Gesellschaft mbH, distributed by ALPCO Diagnostics; plasma TNFα, plasma TNFR-2, serum soluble ICAM-1, serum IL-6, and serum MCP-1: R&D systems; serum myeloperoxidase: Oxis International, Inc.; urinary isoprostanes: Cayman Chemical, Inc.; plasma LPL-A2 mass and activity: GlaxoSmithKline, distributed by diaDexus.
Literature Cited
- 1.Verhoef P, Stampfer M, Buring J, Gaziano J, Allen R, Stabler S, Reynolds RD, Kok FJ, Hennekens C, Willett WC. Homocysteine metabolism and risk of myocardial infarction: relation with vitamins B6, B12, and folate. Am J Epidemiol. 1996;143:845–59 [DOI] [PubMed] [Google Scholar]
- 2.Siri PW, Verhoef P, Kok FJ. Vitamins B6, B12, and folate: association with plasma total homocysteine and risk of coronary atherosclerosis. J Am Coll Nutr. 1998;17:435–41 [DOI] [PubMed] [Google Scholar]
- 3.Kelly PJ, Shih VE, Kistler JP, Barron M, Lee H, Mandell R, Furie KL. Low vitamin B6 but not homocyst(e)ine is associated with increased risk of stroke and transient ischemic attack in the era of folic acid grain fortification. Stroke. 2003;34:e51–4 [DOI] [PubMed] [Google Scholar]
- 4.Vermaak WJ, Barnard HC, Potgieter GM, Marx JD. Plasma pyridoxal-5′-phosphate levels in myocardial infarction. S Afr Med J. 1986;70:195–6 [PubMed] [Google Scholar]
- 5.Kok FJ, Schrijver J, Hofman A, Witteman J, Kruyssen D, Remme WJ, Low Vitamin HAV. B6 status in patients with acute myocardial infarction. Am J Cardiol. 1989;63:513–6 [DOI] [PubMed] [Google Scholar]
- 6.Roubenoff R, Selhub J, Nadeau MR, Cannon JG, Freeman LM, Dinarello CA, Rosenberg IH. Abnormal vitamin B6 status in rheumatoid cachexia. Arthritis Rheum. 1995;38:105–9 [DOI] [PubMed] [Google Scholar]
- 7.Chiang E-PSD, Selhub J, Dallal G, Wang Y-C, Roubenoff R. Inflammation causes tissue-specific depletion of vitamin B6. Arthritis Res Ther. 2005;7:R1254–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bekpinar S, Kocak H, Unlucerci Y, Genc S, Akdag-Kose A, Gogus F. The evaluation of C-reactive protein, homocysteine and vitamin B6 concentrations in Behcet and rheumatoid arthritis disease. Clin Chim Acta. 2003;329:143–5 [DOI] [PubMed] [Google Scholar]
- 9.Woolf K, Manore MM. Elevated plasma homocysteine and low vitamin B-6 status in nonsupplementing older women with rheumatoid arthritis. J Am Diet Assoc. 2008;108:443–53, discussion 54 [DOI] [PubMed] [Google Scholar]
- 10.Saibeni S, Cattaneo M, Vecchi M, Zighetti L, Lecchi A, Lombardi R, Meucci G, Spina L, de Franchis R. Low vitamin B6 plasma levels, a risk factor for thrombosis in IBS: role of inflammation and correlation with acute phase reactants. Am J Gastroenterol. 2003;98:112–7 [DOI] [PubMed] [Google Scholar]
- 11.Kuroki F, Iida M, Tominaga M, Matsumoto T, Hirakawa K, Sugiyama S, Fujishima M. Multiple vitamin status in Crohn's disease. Correlation with disease activity. Dig Dis Sci. 1993;38:1614–8 [DOI] [PubMed] [Google Scholar]
- 12.Davis RE, Calder JS, Curnow DH. Serum pyridoxal and folate concentrations in diabetics. Pathology. 1976;8:151–6 [DOI] [PubMed] [Google Scholar]
- 13.Wilson RG, Davis RE. Serum pyridoxal concentrations in children with diabetes mellitus. Pathology. 1977;9:95–8 [DOI] [PubMed] [Google Scholar]
- 14.Ross R. Atherosclerosis: an inflammatory disease. N Engl J Med. 1999;340:115–26 [DOI] [PubMed] [Google Scholar]
- 15.Powell LJ. C-reactive protein: a review. Am J Med Technol. 1979;45:138–42 [PubMed] [Google Scholar]
- 16.de Ferranti S, Rifai N. C-reactive protein and cardiovascular disease: a review of risk prediction and interventions. Clin Chim Acta. 2002;317:1–15 [DOI] [PubMed] [Google Scholar]
- 17.Morris MS, Sakakeeny L, Jacques PF, Picciano MF, Selhub J. Vitamin B-6 intake is inversely related to, and the requirement is affected by inflammation status. J Nutr. 2010;140:103–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kelly PJ, Kistler JP, Shih VE, Mandell R, Atassi N, Barron M, Lee H, Silveira S, Furie KL. Inflammation, homocysteine, and vitamin B6 status after ischemic stroke. Stroke. 2004;35:12–5 [DOI] [PubMed] [Google Scholar]
- 19.Gori A, Sofi F, Corsi A, Gazzini A, Sestini I, Lauretani F, Bandinelli S, Gensini G, Ferruci L, Abbate R. Predictors of vitamin B6 and folate concentrations in older persons: The InCHIANTI Study. Clin Chem. 2006;52:1318–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Friso S, Jacques PF, Wilson PW, Rosenberg IH, Selhub J. Low circulating vitamin B(6) is associated with elevation of the inflammation marker C-reactive protein independently of plasma homocysteine levels. Circulation. 2001;103:2788–91 [DOI] [PubMed] [Google Scholar]
- 21.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham Offspring Study. Am J Epidemiol. 1979;110:281–90 [DOI] [PubMed] [Google Scholar]
- 22.Camp VM, Chipponi J, Faraj BA. Radioenzymatic assay for direct measurement of plasma pyridoxal 5′- phosphate. Clin Chem. 1983;29:642–4 [PubMed] [Google Scholar]
- 23.Clauss A. Rapid physiological coagulation method in determination of fibrinogen.. Acta Haematol. 1957;17:237–46 [DOI] [PubMed] [Google Scholar]
- 24.Schnabel RB, Lunetta KL, Larson MG, Dupuis J, Lipinska I, Rong J, Chen M-H, Zhao Z, Yamamoto JF, Meigs JB, et al. The relation of genetic and environmental factors to systemic inflammatory biomarker concentrations. Circ Cardiovasc Genet. 2009;2:229–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, Hennekens CH, Speizer FE. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122:51–65 [DOI] [PubMed] [Google Scholar]
- 26.Araki A, Sako Y. Determination of free and total homocysteine in human plasma by high-performance liquid chromatography with fluorescence detection. J Chromatogr. 1987;422:43–52 [DOI] [PubMed] [Google Scholar]
- 27.Tamura T, Freeberg LE, Cornwell PE. Inhibition of EDTA of growth of Lactobacillus casei in the folate microbiological assay and its reversal by added manganese or iron. Clin Chem. 1990;36:1993 [PubMed] [Google Scholar]
- 28.McNamara JR, Schaefer EJ. Automated enzymatic standardized lipid analyses for plasma and lipoprotein fractions. Clin Chim Acta. 1987;166:1–8 [DOI] [PubMed] [Google Scholar]
- 29.Jaffe M. gber den Niederschlag welchem Pikrins@ure in normalem Harn erzeugt and hber eine neue Reaktion des Kreatinins. Z Phys Chem. 1886;10:391–400 [Google Scholar]
- 30.Food and Nutrition Board, Institute of Medicine Dietary reference intakes for thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin, and choline. A report of the Standing Committee on the Scientific Evaluation of Dietary Reference Intakes and its Panel on Folate, Other B Vitamins, and Choline and Subcommittee on Upper Reference Levels of Nutrients. Washington, DC: National Academies Press; 1998 [PubMed] [Google Scholar]
- 31.Chiang EP Selhub J, Bagley PJ, Dallal G, Roubenoff R. Pyridoxine supplementation corrects vitamin B6 deficiency but does not improve inflammation in patients with rheumatoid arthritis. Arthritis Res Ther. 2005;7:R1404–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shen J, Lai C-Q, Mattei J, Ordovas JM, Tucker KL. Association of vitamin B-6 status with inflammation, oxidative stress, and chronic inflammatory conditions: the Boston Puerto Rican Health Study. Am J Clin Nutr. 2010;91:337–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bates CJ, Pentieva KD, Prentice A, Mansoor MA, Finch S. Plasma pyridoxal phosphate and pyridoxic acid and their relationship to plasma homocysteine in a representative sample of British men and women aged 65 years and over. Br J Nutr. 1999;81:191–201 [PubMed] [Google Scholar]
- 34.Schumacher HR, Bernhart FW, Gyorgy P. Vitamin B6 levels in rheumatoid arthritis: effect of treatment. Am J Clin Nutr. 1975;28:1200–3 [DOI] [PubMed] [Google Scholar]
- 35.Bleie Ø, Semb AG, Grundt H, Nordrehaug JE, Vollset SE, Ueland P, Nilsen DW, Bakken AM, Refsum H, Nygard OK. Homocysteine-lowering therapy does not affect inflammatory markers of athersclerosis in patients with stable coronary artery disease. J Intern Med. 2007;262:244–53 [DOI] [PubMed] [Google Scholar]
- 36.Keszthelyi D, Troost FJ, Masclee AA. Understanding the role of tryptophan and serotonin metabolism in gastrointestinal function. Neurogastroenterol Motil. 2009;21:1239–49 [DOI] [PubMed] [Google Scholar]
- 37.Papaspyridonos M, Smith A, Burnand KG, Taylor P, Padayachee S, Suckling KE, James CH, Greaves DR, Patel L. Novel candidate genes in unstable areas of human atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 2006;26:1837–44 [DOI] [PubMed] [Google Scholar]
- 38.King NJ, Thomas SR. Molecules in focus: indoleamine 2,3-dioxygenase. Int J Biochem Cell Biol. 2007;39:2167–72 [DOI] [PubMed] [Google Scholar]
- 39.Schirch L. Serine hydroxymethyltransferase. Adv Enzymol Relat Areas Mol Biol. 1982;53:83–112 [DOI] [PubMed] [Google Scholar]
- 40.Trakatellis A, Exindari M, Scountzou J, Koliakos G, Christodoulou D, Malissiovas N, Antoniadis A, Pohlyzoni Th. Effect of pyridoxine deficiency on immunological phenomena. Postgrad Med J. 1992;68:S70–7 [PubMed] [Google Scholar]
- 41.Ervin RB, Wright JD, Wang CY, Kennedy-Stephenson J. Dietary intake of selected vitamins for the United States population: 1999–2000. Adv Data. 2004:1–4 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.