Abstract
Although isothiocyanates (ITC), which are found in cruciferous vegetables, have been shown to inhibit carcinogenesis in animal models and induce apoptosis and cell cycle arrest in tumor cells, the biochemical mechanisms of cell growth inhibition by these compounds are not fully understood. Studies have reported that ITC binding to intracellular proteins may be an important event for initiating apoptosis. Specific protein target(s) and molecular mechanisms for ITC have been investigated in human lung cancer A549 cells using proteomic tools. Cells were treated with various amounts (1–100 μmol/L) of radiolabeled phenethyl-ITC (PEITC) and sulforaphane (SFN) and the extracted proteins resolved using 2-dimensional gel electrophoresis. The results of mass spectrometric analyses suggested that tubulin may be an in vivo binding target for ITC. The binding of ITC to tubulin was associated with growth arrest. The proliferation of A549 cells was significantly reduced by ITC, with benzyl-ITC (BITC) having a greater relative activity than PEITC or SFN. Mitotic arrest and apoptosis as well as disruption of microtubule polymerization were induced in the order: BITC > PEITC > SFN. An analysis of tubulins isolated from BITC-treated A549 cells showed that Cys347, a conserved cysteine in all α-tubulin isoforms, was covalently modified by BITC. Taken together, these results suggest that tubulin is a binding target of ITC and that this interaction can lead to growth inhibition and apoptosis.
Introduction
Isothiocyanates (ITC)6 are a group of organosulfur compounds characteristic of a structurally similar functional group, –n = C = S, that exhibits promising cancer preventative potential (1, 2). Natural ITC, including benzyl-ITC (BITC), phenethyl-ITC (PEITC), and sulforaphane (SFN) are found in cruciferous vegetables such as broccoli, watercress, Brussels sprouts, cabbage, Japanese radish, and cauliflower. The ITC found in these vegetables largely contribute to their cancer chemopreventative benefits. In plant cells, ITC are usually stored in the form of glucosinolates (GS), which are responsible for the unique fragrance and sharp taste of cruciferous vegetables. GS can be converted to and released as active ITC when plant tissue is disintegrated or ground. The content of GS among cruciferous vegetables is determined by cultivating conditions and genotype. SFN is relatively abundant in broccoli and originates from glucoraphanin, whereas watercress contains more gluconasturtiin that is converted to PEITC. The conversion of GS into ITC is catalyzed by the enzyme myrosinase, a thio-glucosidase that is inactivated by heat during food preparation. Therefore, the absorbable ITC in the human diet are mostly derived from GS through a hydrolysis process modulated by microflora in the intestinal tract (3–5).
In animal studies, ITC have demonstrated anticarcinogenic activities throughout different stages of tumor growth, including initiation, promotion, and progression, suggesting that ITC have potential in both cancer prevention and therapeutics (6–11). More importantly, numerous studies have shown that ITC are well tolerated in a variety of animal models and that they act preferably toward cancer cells than noncancerous normal cells (12). Epidemiological studies also supported the association of increased dietary ITC intake with reduced lung cancer risk (6, 12, 13). Furthermore, both cell culture and animal studies indicated that ITC inhibited tumorigenesis and suppressed tumor cell proliferation by inducing apoptosis and cell cycle arrest (12, 14–22). A recent dietary study using MS to analyze serum obtained from individuals lacking glutathione S-transferase (GSH) activity has shown that eating cruciferous vegetables results in several changes within the circulating peptidome of these individuals (23). For example, a fragment of transthyretin and ZAG was found to be differentially abundant in individuals lacking glutathione S-transferase activity consuming diets high in cruciferous vegetables. Being able to detect differentially abundant proteins in circulation suggests that ITC have an important role in cell metabolism in vivo.
As electrophiles, ITC are likely to covalently bind to macromolecules such as DNA, RNA, and cysteine-containing proteins. Previous studies identified inhibitory interactions between ITC and enzymes such as cytochrome P450 and Kelch-like epoxycyclohexenone-associated protein-1 (24–26). Reactive oxygen species produced through ITC conjugation and subsequent GSH depletion were also suggested to induce apoptosis (15, 16). A number of signaling pathways were reported to be associated with ITC-induced apoptosis (6, 15, 27–31). In particular, one study found that activation of MAPK and activating protein-1 pathways were likely involved in apoptosis in ITC-treated mouse lungs (7, 8). However, direct evidence explaining how apoptosis can be initiated as a result of interactions between ITC and target proteins remains elusive. Recent progress in MS technologies has expedited the identification of post-translational modifications of proteins as well as covalent interactions between protein and small molecules that seemed difficult to elucidate using traditional protein technologies (32–34). By using proteomic approaches, Cross et al. (35) identified macrophage migration inhibitory factor as an ITC target. ITC covalently modify the N-terminal proline of MIF and abolish its catalytic tautomerase activity. Because MIF is associated with inflammatory responses, its inhibition by ITC may provide potential intervention and therapeutics for inflammatory diseases and cancer. As reactive electrophilic compounds, ITC are likely to covalently bind to and modify multiple cellular target proteins. Here, we summarized published data reporting on the identification of tubulin as a binding target of ITC using proteomic methods and discuss how tubulin modifications induced by ITC can lead to cell cycle arrest and apoptosis in lung cancer cells.
ITC Inhibit Cell Proliferation and Induce Apoptosis
In previous studies, we reported that culturing human non-small cell lung cancer A549 cells with an increased concentration of ITC (1–100 μmol/L) inhibited cell proliferation in a dose-dependent manner (22). The 50% inhibitory concentrations were 13.5, 18.3, and 43 μmol/L for BITC, PEITC, and SFN, respectively, whereas the PEITC structural analogue N-methyl phenethylamine did not inhibit cell proliferation. In a pharmacokinetic study using F344 rats, PEITC reached nearly 20 μmol/L in plasma a few hours after intake. Even 24 h after ingestion, 4 μmol/L of PEITC can still be detected (36). Thus, 5–20 μmol/L of ITC used in the cell-based studies is considered physiologically attainable. Besides dose, response to ITC also depends on cell type. Morphological analysis of A549 cells 24 h after treatment with ITC revealed an extensive bulge and irregular shrinkage in the plasma membrane, which are typical signs of apoptosis. These apoptotic-like morphological changes appeared after treatment with 10 μmol/L BITC and PEITC but required higher concentrations of SFN (30 μmol/L). These results were consistent with the relative potency of BITC, PEITC, and SFN in inhibiting the growth of A549 cells (BITC > PEITC > SFN). In addition, poly-(ADP-ribose) polymerase cleavage and stimulation of caspace-3 activity were seen after treatment of A549 cells with 20 μmol/L PEITC or 40 μmol/L SFN, respectively. Therefore, PEITC appeared to be a more potent inducer of apoptosis than SFN in A549 cells, because a higher concentration of SFN was required to produce the same effects of PEITC. Furthermore, time course experiments confirmed that ITC induced apoptosis in the order: BITC > PEITC > SFN (21, 22).
Tubulin Is a Binding Target for ITC
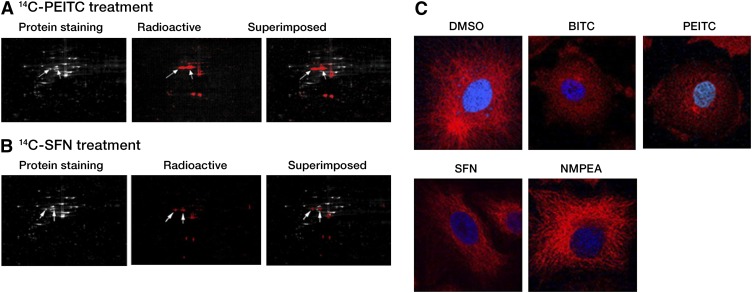
To better understand the molecular basis for ITC-induced apoptosis in lung cancer cells, DNA and RNA modifications were monitored in A549 cells treated with radiolabeled 14C-PEITC or 14C-SFN. Neither DNA nor RNA showed any detectable incorporation of radioactivity (<30 dpm in 200 μg DNA and <40 dpm in 400 μg RNA with a baseline radioactivity of ~20–50 dpm). Furthermore, no radioactivity was detected in calf thymus DNA incubated in vitro with 14C-PEITC or 14C-SFN (21). Because no detectable DNA- or RNA-ITC adducts were detected, we examined whether proteins were a target for ITC. A549 cells were incubated in the presence of 14C-PEITC and 14C-SFN. Proteins extracted from A549 cells were separated using 2-dimensional PAGE (Fig. 1A,B). Because the 14C was confined to the functional group of ITC, we predicted labeled proteins would be detectable upon formation of thiocarbamate links with ITC. Superimposing of the colloidal Coomassie blue stained gel image with the exposed X-ray film was used to determine the position of 14C-labeled proteins. The nonradioactive (i.e., Coomassie blue stained) protein spots were excised from the gel, digested with trypsin, and analyzed using matrix-assisted laser desorption/ionization time-of-flight MS using a Voyager 4700. Approximately 30 proteins were identified as potential targets of ITC. Both α- and β-tubulin peptides were detected in multiple spots (Table 1). Almost all known tubulin isoforms were identified, suggesting tubulin was a major binding target for ITC.
FIGURE 1.
ITC target tubulins and disrupt microtubule network. Images of A549 whole cell lysates separated using 2-dimensional PAGE after treatment with 20 μmol/L 14C-PEITC (A) and 14C-SFN (B) for 1 h. The total protein profiles are shown in the colloidal Coomassie blue-stained image. The 14C-ITC–bound proteins are shown in the radioactive image. The 2 images were superimposed to reveal the position of the ITC-modified proteins that were selected for MS identification. The arrows within all 3 images point to the protein spots identified as α- and β-tubulin. (C) The microtubule images of A549 cells revealed network disruption and degradation in cells treated with 5 μmol/L BITC and 5 μmol/L PEITC for 30 min and 1 h, respectively. The treatment with 10 μmol/L SFN for 4 h did not significantly influence the microtubule network and no detectable changes were observed in the microtubule network of cells treated with 10 μmol/L N-methyl phenethylamine for 4 h. Indirect immunofluorescent staining (red color) and 4′,6-diamidino-2-phenylindole (blue color) were used to visualize α-tubulin and the nuclei, respectively. Reproduced with permission (22). BITC, benzyl isothiocyanate; ITC, isothiocyanate; PEITC, phenethyl isothiocyanate; SFN, sulforaphane.
TABLE 1.
Tubulin peptides identified using MS (22)
| Protein name | NCBI access no. | Identified peptide sequence |
| Tubulin α1 chain | P68366 | K.DVNAAIAAIK.T |
| R.AVFVDLEPTVIDEIR.N | ||
| K.VGINYQPPTVVPGGDLAK.V | ||
| R.LISQIVSSITASLR.F | ||
| R.PTYTNLNR.L | ||
| K.EDAANNYAR.G | ||
| R.LSVDYGKK.S | ||
| Tubulin α2 chain | Q13748 | K.DVNAAIATIK.T |
| K.TIGGGDDSFNTFFSETGAGK.H | ||
| Tubulin α3 chain | Q71U36 | R.AVFVDLEPTVIDEVR.T |
| K.EIIDLVLDR.I | ||
| Tubulin β1 chain | Q9H4B7 | R.FPGQLNADLR.K |
| R.FPGQLNADLRK.L | ||
| Tubulin β2 chain | P07437 | R.ISVYYNEATGGK.Y |
| R.ALTVPELTQQVFDAK.N | ||
| K.NSSYFVEWIPNNVK.T | ||
| R.YLTVAAVFR.G | ||
| R.ISVYYNEATGGK.Y | ||
| Tubulin β2C chain | P68371 | R.INVYYNEATGGK.Y |
| Tubulin β3 chain | Q13509 | R.YLTVATVFR.G |
| K.MSSTFIGNSTAIQELFK.R | ||
| K.VREEYPDR.I | ||
| R.ISVYYNEASSHK.Y | ||
| β-Tubulin 4Q | Q8WZ78 | R.INVYYNEASGGR.Y |
ITC Hinder Tubulin Polymerization and Microtubule Formation
Polymerization is required to assemble functional tubulin networks. The influence of ITC on tubulin polymerization was analyzed in A549 cells. The treatment with 30 μmol/L BITC, PEITC, or SFN inhibited tubulin polymerization by 47, 33, and 10%, respectively, compared with treatment with vehicle DMSO or negative control N-methyl phenethylamine (data not shown). The effect of ITC on the intracellular microtubule network was analyzed using indirect immunofluorescence staining of α-tubulin (Fig. 1C). A fully developed network was observed in DMSO-treated A549 cells. Conversely, the microtubule network was disrupted following treatment with 5 μmol/L BITC for 30 min or equimolar levels of PEITC for 1 h. In contrast, the microtubule network remained nearly intact in the presence of 10 μmol/L SFN for 4 h. In the presence of BITC and PEITC, we observed a rounding of the cell shape, which was suggestive of collapsed microtubule network and abnormal cytoskeleton organization.
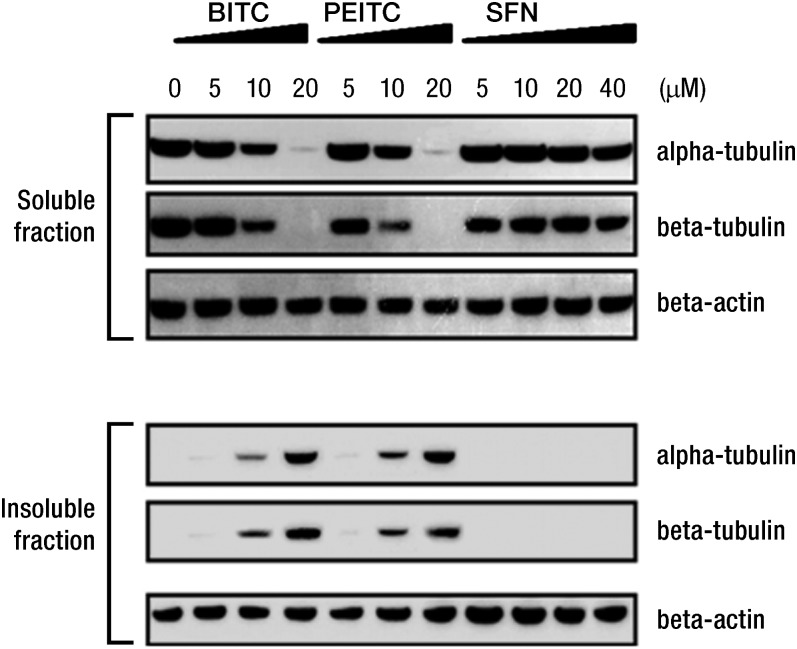
In parallel experiments, we observed that ITC induced changes in secondary and tertiary structures of tubulin (data not shown) (22). Following treatment of A549 cells with BITC or PEITC (5, 10, and 20 μmol/L) for 4 h, increasing amounts of α- and β-tubulins precipitates appeared in the insoluble fractions (insoluble in nonionic detergent lysis buffer but soluble in ionic detergent such as SDS) (Fig. 2). These results suggested that the binding of ITC to tubulins likely induced protein misfolding, leading to tubulin precipitation.
FIGURE 2.
Tubulin polymerization is inhibited by BITC, PEITC, and SFN. Treatment of A549 altered the amounts of α- and β-tubulin found in the soluble and insoluble fractions, as determined by Western blots. β-Actin was used as the loading control. Reproduced with permission from (22). BITC, benzyl isothiocyanate; PEITC, phenethyl isothiocyanate; SFN, sulforaphane.
ITC Covalently Bind to Thiol Groups in Tubulin
To examine the amino acids that in tubulin were targeted by ITC, purified tubulin was incubated with ITC in vitro, digested with trypsin, and analyzed using matrix-assisted laser desorption/ionization time of flight and nanospray reversed-phase liquid chromatography-tandem MS (RPLC-MS/MS). The results of these experiments showed that a cysteine-containing β-tubulin peptide, 298NMMAACDPR306 ([M+H]+ of m/z 1008.4) was covalently modified by ITC, generating a mass shift of +149, 163, and 177 Da when treated with BITC, PEITC, and SFN, respectively (22). To determine the numbers of thiol groups on tubulin that were modified following incubation with ITC, free thiols groups were quantified by measuring the absorbance at 410 nm after reaction with 5,5′-dithiobis (2-nitrobenzoic acid). The results revealed differential levels of tubulin thiol modification by BITC, PEITC, and SFN (Table 2). At concentrations of 80 and 160 μmol/L (ITC:tubulin cysteine ratio of 1:1 and 2:1), the numbers of thiols modified were 9.5 and 11.7 for BITC, 6.2 and 9.1 for PEITC, and 2.6 and 3.8 for SFN. Thus, the number of modified thiol groups was dependent on the relative concentrations of ITC and tubulin.
TABLE 2.
Number of thiols in tubulin modified by ITC compounds1
| Thiols modified/pair of tubulin, n |
||||||
| Free thiols/pair of tubulin, n | BITC (μmol/L) |
PEITC (μmol/L) |
SFN (μmol/L) |
|||
| 80 (1:1)2 | 160 (2:1) | 80 | 160 | 80 | 160 | |
| 14.7 ± 0.2 | 9.5 ± 0.2 | 11.7 ± 0.4 | 6.2 ± 0.3 | 9.1 ± 0.3 | 2.6 ± 0.1 | 3.8 ± 0.1 |
Values are mean ± SD, = 3. The tubulin cysteine concentration was 40 μmol/L. Adapted with permission (22). BITC, benzyl isothiocyanate; PEITC, phenethyl isothiocyanate; SFN, sulforaphane.
The stoichiometric ratio between compound and cysteines in tubulin.
An analysis of tubulin genes revealed that 20 cysteine residues are highly conserved (36). Most of the cysteines are folded and embedded within the protein structure with surrounding pockets accessible only to small molecules (37, 38). Excluding 6 cysteines that are possibly involved in the formation of disulfide bonds, the results suggest that the remaining 14 cysteines can be potentially modified by ITC (Table 2). Interestingly, the relative binding affinity of ITC toward cysteines (BITC > PEITC > SFN) was consistent with their ability to induce cell cycle arrest and apoptosis.
Because BITC showed the highest potency in inducing apoptotic changes in tubulin, both the soluble and insoluble fractions of the BITC-treated A549 cell lysates were analyzed using nano reversed-phase LC-MS/MS. The results showed that Cys347, a conserved residue in all α-tubulin isoforms (36), was covalently modified by BITC, as indicated by a mass shift of +149 Da (22). This modification was found only in tubulin from the insoluble cell lysate fraction, suggesting that it may relate to tubulin misfolding and aggregation induced by BITC. This result was in agreement with a previously reported observation that modifications of cysteines often result in loss of tubulin polymerization (39). MS analysis also showed that none of the 20 cysteines in α- and β-tubulin was modified by oxidation induced by BITC.
In conclusion, the data summarized in this article suggest that covalent modifications of tubulins may contribute to the antiproliferative and proapoptotic properties of ITC. The relative binding affinity of BITC, PEITC, and SFN for tubulins was in good agreement with their ability to induce cell growth arrest and apoptosis in A549 lung cancer cells. The ability of ITC to disrupt tubulin polymerization and microtubular network may contribute to their anticarcinogenic properties. The use of matrix-assisted laser desorption/ionization time of flight/MS/MS was necessary to identify specific cysteines residues modified by BITC. Taken together, the results from these studies support the concept that proteomic tools are necessary to identify protein targets of ITC and characterize their biochemical mechanisms of cell growth inhibition. Considering that tubulin was identified as a major target of ITC and that this protein is ubiquitously expressed in cells, it is quite probable that ITC modify proteins within noncancerous cells as well as cancerous cells. This hypothesis is highly relevant to epithelial cells of the colon and urogenital tract that are exposed to high levels of ITC (40).
Acknowledgments
Z.X., L.M., F-L.C., and T.D.V. contribute to preparing the manuscript. T.D.V. had primary responsibility for coordination of research and supervision of manuscript. All authors read and approved the final manuscript.
Footnotes
Published in a supplement to The Journal of Nutrition. Presented at the 2010 American Institute for Cancer Research Annual Conference held in Washington, DC, October 21–22, 2010. The conference was organized by the American Institute for Cancer Research. This work was supported by an Intergovernmetal Personnel Act from the Nutritional Sciences Research Group, Division of Cancer Prevention, National Cancer Institute, NIH to Donato F. Romagnolo, University of Arizona, Tucson. The views expressed in this publication are those of the authors and do not reflect the views or policies of the sponsors or the publisher, Editor, or Editorial Board of The Journal of Nutrition. The supplement coordinator for this supplement was Donato F. Romagnolo, University of Arizona, Tucson. Supplement Coordinator disclosures: D. F. Ramagnolo, no conflicts of interest. The supplement is the responsibility of the Guest Editor to whom the Editor of The Journal of Nutrition has delegated supervision of both technical conformity to the published regulations of The Journal of Nutrition and general oversight of the scientific merit of each article. The Guest Editor for this supplement was Harry D. Dawson, ARS/USDA. Guest Editor disclosure: H. D. Dawson, no conflicts of interest. Publication costs for this supplement were defrayed in part by the payment of page charges. This publication must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact. The opinions expressed in this publication are those of the authors and are not attributable to the sponsors or the publisher, Editor, or Editorial Board of The Journal of Nutrition.
Supported by the National Cancer Institute, NIH, under contract HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the United States Government.
Abbreviations used: BITC, benzyl isothiocyanate; GS, glucosinolate; ITC, isothiocyanate; LC, liquid chromatography; MIF, migration inhibitory factor; MS/MS, tandem MS; PEITC, phenethyl isothiocyanate; SFN, sulforaphane.
Literature Cited
- 1.Nakamura Y. Chemoprevention by isothiocyanates: molecular basis of apoptosis induction. Forum Nutr. 2009;61:170–81 [DOI] [PubMed] [Google Scholar]
- 2.Fahey JW, Zalcmann AT, Talalay P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry. 2001;56:5–51 [DOI] [PubMed] [Google Scholar]
- 3.Getahun SM, Chung FL. Conversion of glucosinolates to isothiocyanates in humans after ingestion of cooked watercress. Cancer Epidemiol Biomarkers Prev. 1999;8:447–51 [PubMed] [Google Scholar]
- 4.Shapiro TA, Fahey JW, Wade KL, Stephenson KK, Talalay P. Human metabolism and excretion of cancer chemoprotective glucosinolates and isothiocyanates of cruciferous vegetables. Cancer Epidemiol Biomarkers Prev. 1998;7:1091–100 [PubMed] [Google Scholar]
- 5.Fenwick GR, Heaney RK, Mullin WJ. Glucosinolates and their breakdown products in food and food plants. Crit Rev Food Sci Nutr. 1983;18:123–201 [DOI] [PubMed] [Google Scholar]
- 6.Jiao D, Smith TJ, Yang CS, Pittman B, Desai D, Amin S, Chung FL. Chemopreventive activity of thiol conjugates of isothiocyanates for lung tumorigenesis. Carcinogenesis. 1997;18:2143–7 [DOI] [PubMed] [Google Scholar]
- 7.Yang YM, Conaway CC, Chiao JW, Wang CX, Amin S, Whysner J, Dai W, Reinhardt J, Chung FL. Inhibition of benzo(a)pyrene-induced lung tumorigenesis in A/J mice by dietary N-acetylcysteine conjugates of benzyl and phenethyl isothiocyanates during the postinitiation phase is associated with activation of mitogen-activated protein kinases and p53 activity and induction of apoptosis. Cancer Res. 2002;62:2–7 [PubMed] [Google Scholar]
- 8.Yang YM, Jhanwar-Uniyal M, Schwartz J, Conaway CC, Halicka HD, Traganos F, Chung FL. N-acetylcysteine conjugate of phenethyl isothiocyanate enhances apoptosis in growth-stimulated human lung cells. Cancer Res. 2005;65:8538–47 [DOI] [PubMed] [Google Scholar]
- 9.Sticha KR, Kenney PM, Boysen G, Liang H, Su X, Wang M, Upadhyaya P, Hecht SS. Effects of benzyl isothiocyanate and phenethyl isothiocyanate on DNA adduct formation by a mixture of benzo[a]pyrene and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in A/J mouse lung. Carcinogenesis. 2002;23:1433–9 [DOI] [PubMed] [Google Scholar]
- 10.Tang L, Zhang Y. Dietary isothiocyanates inhibit the growth of human bladder carcinoma cells. J Nutr. 2004;134:2004–10 [DOI] [PubMed] [Google Scholar]
- 11.Tang L, Zhang Y. Isothiocyanates in the chemoprevention of bladder cancer. Curr Drug Metab. 2004;5:193–201 [DOI] [PubMed] [Google Scholar]
- 12.Conaway CC, Yang YM, Chung FL. Isothiocyanates as cancer chemopreventive agents: their biological activities and metabolism in rodents and humans. Curr Drug Metab. 2002;3:233–55 [DOI] [PubMed] [Google Scholar]
- 13.von Weymarn LB, Chun JA, Hollenberg PF. Effects of benzyl and phenethyl isothiocyanate on P450s 2A6 and 2A13: potential for chemoprevention in smokers. Carcinogenesis. 2006;27:782–90 [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y, Tang L, Gonzalez V. Selected isothiocyanates rapidly induce growth inhibition of cancer cells. Mol Cancer Ther. 2003;2:1045–52 [PubMed] [Google Scholar]
- 15.Zhang Y, Talalay P. Mechanism of differential potencies of isothiocyanates as inducers of anticarcinogenic Phase 2 enzymes. Cancer Res. 1998;58:4632–9 [PubMed] [Google Scholar]
- 16.Singh SV, Srivastava SK, Choi S, Lew KL, Antosiewicz J, Xiao D, Zeng Y, Watkins SC, Johnson CS, Trump DL, et al. Sulforaphane-induced cell death in human prostate cancer cells is initiated by reactive oxygen species. J Biol Chem. 2005;280:19911–24 [DOI] [PubMed] [Google Scholar]
- 17.Bonnesen C, Eggleston IM, Hayes JD. Dietary indoles and isothiocyanates that are generated from cruciferous vegetables can both stimulate apoptosis and confer protection against DNA damage in human colon cell lines. Cancer Res. 2001;61:6120–30 [PubMed] [Google Scholar]
- 18.Tang L, Zhang Y. Mitochondria are the primary target in isothiocyanate-induced apoptosis in human bladder cancer cells. Mol Cancer Ther. 2005;4:1250–9 [DOI] [PubMed] [Google Scholar]
- 19.Jakubikova J, Bao Y, Sedlak J. Isothiocyanates induce cell cycle arrest, apoptosis and mitochondrial potential depolarization in HL-60 and multidrug-resistant cell lines. Anticancer Res. 2005;25:3375–86 [PubMed] [Google Scholar]
- 20.Tseng E, Scott-Ramsay EA, Morris ME. Dietary organic isothiocyanates are cytotoxic in human breast cancer MCF-7 and mammary epithelial MCF-12A cell lines. Exp Biol Med (Maywood). 2004;229:835–42 [DOI] [PubMed] [Google Scholar]
- 21.Mi L, Wang X, Govind S, Hood BL, Veenstra TD, Conrads TP, Saha DT, Goldman R, Chung FL. The role of protein binding in induction of apoptosis by phenethyl isothiocyanate and sulforaphane in human non-small lung cancer cells. Cancer Res. 2007;67:6409–16 [DOI] [PubMed] [Google Scholar]
- 22.Mi L, Xiao Z, Hood BL, Dakshanamurthy S, Wang X, Govind S, Conrads TP, Veenstra TD, Chung FL. Covalent binding to tubulin by isothiocyanates. A mechanism of cell growth arrest and apoptosis. J Biol Chem. 2008;283:22136–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brauer HA, Libby TE, Mitchell BL, Li L, Chen C, Randolph TW, Yasui YY, Lampe JW, Lampe PD. Cruciferous vegetable supplementation in a controlled diet study alters the serum peptidome in a GSTM1-genotype dependant manner. Nutr J. 2011;10:11–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dinkova-Kostova AT, Holtzclaw WD, Cole RN, Itoh K, Wakabayashi N, Katoh Y, Yamamoto M, Talalay P. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc Natl Acad Sci USA. 2002;99:11908–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eggler AL, Liu G, Pezzuto JM, van Breemen RB, Mesecar AD. Modifying specific cysteines of the electrophile-sensing human Keap1 protein is insufficient to disrupt binding to the Nrf2 domain Neh2. Proc Natl Acad Sci USA. 2005;102:10070–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hong F, Freeman ML, Liebler DC. Identification of sensor cysteines in human Keap1 modified by the cancer chemopreventive agent sulforaphane. Chem Res Toxicol. 2005;18:1917–26 [DOI] [PubMed] [Google Scholar]
- 27.Fowke JH, Chung FL, Jin F, Qi D, Cai Q, Conaway C, Cheng JR, Shu XO, Gao YT, Zheng W. Urinary isothiocyanate levels, brassica, and human breast cancer. Cancer Res. 2003;63:3980–6 [PubMed] [Google Scholar]
- 28.Seow A, Yuan JM, Sun CL, Van Den Berg D, Lee HP, Yu MC. Dietary isothiocyanates, glutathione S-transferase polymorphisms and colorectal cancer risk in the Singapore Chinese Health Study. Carcinogenesis. 2002;23:2055–61 [DOI] [PubMed] [Google Scholar]
- 29.Xu K, Thornalley PJ. Signal transduction activated by the cancer chemopreventive isothiocyanates: cleavage of BID protein, tyrosine phosphorylation and activation of JNK. Br J Cancer. 2001;84:670–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keum YS, Jeong WS, Kong AN. Chemoprevention by isothiocyanates and their underlying molecular signaling mechanisms. Mutat Res. 2004;555:191–202 [DOI] [PubMed] [Google Scholar]
- 31.Xiao D, Choi S, Lee YJ, Singh SV. Role of mitogen-activated protein kinases in phenethyl isothiocyanate-induced apoptosis in human prostate cancer cells. Mol Carcinog. 2005;43:130–40 [DOI] [PubMed] [Google Scholar]
- 32.Yamakura F, Kawasaki H. Post-translational modifications of superoxide dismutase. Biochim Biophys Acta. 2010;1804:318–25 [DOI] [PubMed] [Google Scholar]
- 33.Zhao Y, Jensen ON. Modification-specific proteomics: strategies for characterization of post-translational modifications using enrichment techniques. Proteomics. 2009;9:4632–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rix U, Superti-Furga G. Target profiling of small molecules by chemical proteomics. Nat Chem Biol. 2009;5:616–24 [DOI] [PubMed] [Google Scholar]
- 35.Cross JV, Rady JM, Foss FW, Lyons CE, Macdonald TL, Templeton DJ. Nutrient isothiocyanates covalently modify and inhibit the inflammatory cytokine macrophage migration inhibitory factor (MIF). Biochem J. 2009;423:315–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Conaway CC, Jiao D, Kohri T, Liebes L, Chung FL. Disposition and pharmacokinetics of phenethyl isothiocyanate and 6-phenylhexyl isothiocyanate in F344 rats. Drug Metab Dispos. 1999;27:13–20 [PubMed] [Google Scholar]
- 37.Cowan NJ. Tubulin genes and the diversity of microtubule function. Oxf Surv Eukaryot Genes. 1984;1:36–60 [PubMed] [Google Scholar]
- 38.Löwe J, Li H, Downing KH, Nogales E. Refined structure of alpha beta-tubulin at 3.5 A resolution. J Mol Biol. 2001;313:1045–57 [DOI] [PubMed] [Google Scholar]
- 39.Britto PJ, Knipling L, McPhie P, Wolff J. Thiol-disulphide interchange in tubulin: kinetics and the effect on polymerization. Biochem J. 2005;389:549–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang G, Gao YT, Shu XO, Cai Q, Li GL, Li HL, Ji BT, Rothman N, Dyba M, Xiang YB, et al. Isothiocyanate exposure, glutathione S-transferase polymorphisms, and colorectal cancer risk. Am J Clin Nutr. 2010;91:704–11 [DOI] [PMC free article] [PubMed] [Google Scholar]