Summary
Bioluminescence is widespread among many different types of marine organisms. Metazoans contain two types of luminescence production, bacteriogenic (symbiotic with bacteria) or autogenic, via the production of a luminous secretion or the intrinsic properties of luminous cells. Several species in two families of squids, the Loliginidae and the Sepiolidae (Mollusca: Cephalopoda) harbor bacteriogenic light organs that are found central in the mantle cavity. These light organs are exceptional in function, that is, the morphology and the complexity suggests that the organ has evolved to enhance and direct light emission from bacteria that are harbored inside. Although light organs are widespread among taxa within the Sepiolidae, the origin and development of this important feature is not well studied. We compared light organ morphology from several closely related taxa within the Sepiolidae and combined molecular phylogenetic data using four loci (nuclear ribosomal 28S rRNA and the mitochondrial cytochrome c oxidase subunit I and 12S and 16S rRNA) to determine whether this character was an ancestral trait repeatedly lost among both families or whether it evolved independently as an adaptation to the pelagic and benthic lifestyles. By comparing other closely related extant taxa that do not contain symbiotic light organs, we hypothesized that the ancestral state of sepiolid light organs most likely evolved from part of a separate accessory gland open to the environment that allowed colonization of bacteria to occur and further specialize in the eventual development of the modern light organ.
Introduction
Symbiotic (bacteriogenic) light organs associated with the ink sac and intestine are known in two representatives of the decabrachian families: Sepiolidae (order Sepiolida) and Loliginidae (order Teuthida). These luminescent organs are used for the production of light ventral to the squid mantle cavity, otherwise known as counter-illumination (Young 1977). A similar organ exists in Chtenopteryx, a very peculiar teuthid squid that is thought to be closely related to the Loliginidae (Young 1991). Among the oceanic teuthid squids living at epi- and mesopelagic levels, so-called anal light organs of similar overall aspects occur but apparently never house symbiotic luminous bacteria (Herring 1977).
Basic components of bacteriogenic light organs in the Sepiolidae and Loliginidae are (a) the crypts formed from ectodermic invaginations during embryonic development, ultimately housing the luminescent bacteria (Montgomery and McFall-Ngai 1993); (b) the mesodermally derived lens tissue covering the ventrolateral surface located on either side around the pore leading into the duct of the light organ (Montgomery and McFall-Ngai 1992; Weis et al. 1993); and (c) the mesodermally derived reflector formed by the ventral lining of the ink sac (Meyer 1906). These tissues, when fully developed, are poised to receive luminous bacteria from the environment within the first few hours after hatching (McFall-Ngai and Ruby 1991). The colonization of the light organ complex is highly specific; that is, only symbiotically competent strains of bacteria from the genera Vibrio (V. fischeri or V. logei) or Photobacterium (P. leiognathi) can colonize the light organ of host squids (Fidopiastis et al. 1998; Nishiguchi 2002a,b). Once the light organ contains the symbiotic bacteria, it is fully functional for emission of luminescence.
Naef (1923) previously hypothesized on the likely homologous evolutionarily derived anatomical relationship of bacterial light organs with the so-called accessory nidamental glands (ANG). However, several variants raise new questions regarding the respective morphogenesis of these two complexes. In Semirossia these variants range from a bilateral through a unilateral connection to ANG, or no connection to ANG, to a connection with supposedly ANG-homologous structures in males (Boletzky 1970). Given this, we hope to elucidate the possible origin(s) of bacteriogenic light organs by comparing morphological similarities among representative genera. To that end, we also examine the phylogenetic relationships among closely related taxa with and without bacteriogenic light organs. We ultimately hope to shed light on the morphological diversification and functional co-option of this dynamic complex among the Cephalopoda.
Methods
Morphology
Serial sections (7 μm) of Semirossia spp. and Sepiola spp. were obtained by sectioning paraffin-embedded specimens that were preserved in 70% ethanol after fixation in 10% formalin (Semirossia spp., the original specimens described in Boletzky 1970) or Bouin's fixative (Sepiola spp.). Standard methods of histological staining (Azan, Masson's trichrome, Prolabo, Paris, France) and mounting (Entellan, Merck, Darmstadt, Germany) were used. The sections were observed under a light microscope (Leitz, Wetzlar, Germany) at 25 × objective magnification, and sections of embryonic stages of Sepiola ligulata were drawn using a camera lucida (Leitz drawing arm).
DNA isolation, sequencing, and phylogenetic analyses
All squids used in the phylogenetic analyses had been previously collected at various localities or tissues were donated from various sources (Table 1). Approximately 25 mg of squid tissue was removed from either gill or mantle of each animal. DNA was then isolated from the tissue using the Qiagen DNAeasy Isolation kit (Qiagen, Valencia, CA, USA). Once the DNA was isolated, 1–10 ng was used for polymerase chain reaction (PCR) amplifications. For the analysis, four different loci were used: a partial sequence from the mitochondrial 12S rRNA, 16S rRNA, and the entire cytochrome c oxidase subunit I locus (COI), and a portion of the nuclear 28S rRNA. Each primer set was used to amplify each of the four loci (Table 2A). These loci were chosen specifically for resolving relationships between sepiolid taxa because previous analyses have shown that they provide the most resolution for this family of squids (Nishiguchi et al. 1998; Nishiguchi 2001). All PCR amplifications were performed in two thermocyclers (Perkin Elmer 9700, Applied Biosystems, Foster City, CA, USA; and an MJ Research Dyad, Waltham, MA, USA). Programs used to amplify the four loci were very similar with only slight variations in annealing temperature and number of cycles (Table 2B). Some variation shown in Table 2B for the 28S rRNA locus is due to the fact that some species were more difficult to amplify than others (thus, the annealing temperature was decreased and number of cycles was increased for those species). Each of the 50-μl PCR reactions contained 1–10 ng of DNA template, 0.2 μM of each primer (forward and reverse), 2.5 mM of magnesium chloride, 200 μM of each dNTP, 1 × buffer (10mM Tris-HCl, pH 9.0, 50 mM KCl, and 0.1% Triton X-100), and 0.2 units of Taq Polymerase. Promega Taq polymerase was used for most of samples except those that were difficult to amplify. Those samples included Amplitaq ®DNA Polymerase (Perkin Elmer, Foster City, CA, USA) in the PCR reactions. The resulting PCR products were purified with GeneClean II DNA purification kit (Bio 101, Carlsbad, CA, USA). Samples were presequenced using Applied Biosystems Big Dye v.3.1 (Foster City, CA, USA) and excess fluorescent dNTPs removed with Edge spin columns or plates (Gaithersburg, MD, USA). All samples sequenced for this study were run on an Applied Biosystems 3100 automated capillary sequencer. The resulting forward and reverse complete DNA sequence of each sample was then combined and edited using Sequencher v. 4.1 (Gene Codes™, Ann Arbor, MI, USA).
Table 1. Squid species and loci used in this study.
| Species | Collection Site/Date | Loci | Accession Numbers |
|---|---|---|---|
| Sepiadarium kochi | Tosa Bay, Japan/7-2002 | 12S, 16S, COI, 28S | AY293652, AY293678, AY293726, AY293702 |
| Spirula spirula | Atlantic Ocean/4-2002 | 12S, 16S, COI, 28S | AY293631, AY293659, AY293709, AY293683 |
| Rondeletiola minor | Banyuls-sur-mer, France/7-2002 | 12S, 16S, COI, 28S | AY293651, AY293677, AY293725, AY293686 |
| Sepia elegans | Banyuls-sur-mer, France/7-2001 | 12S, 16S, COI, 28S | AY293633, AY293657, AY293707, AY293687 |
| Chtenopteryx sicula | Hokusei Maru, Japan/1996 | 12S, 16S, COI, 28S | AY293630, AY293660, AY293705, AY293698 |
| Idiosepius pygmaeus | Sydney, Australia/4-2000 | 12S, 16S, COI, 28S | AY293634, AY293658, AY293708, AY293684 |
| Loligo pealei | 16S, COI, 28S | AF110079, AF120629, AF120568 | |
| Loligo bleekeri | 12S | AB009838 | |
| Photololigo noctiluca | Sydney/2-1995 | 12S, 16S, COI, 28S | AY293632, AY293656, AY293706, AY293685 |
| Sepiolina nipponensis | Tosa, Bay, Japan/7-2002 | 12S, 16S, COI, 28S | AY293653, AY293679, AY293727, AY293682 |
| Rossia bipillata | Tokyo Bay, Japan/7-1999 | 12S, 16S, | AY293650, AY293676 |
| Rossia pacifica | Los Angeles, CA/12-1994 | COI | AF035705 |
| Semirossia tenera | Gulf of St. Lawrence, Canada/9-2003 | 12S, 16S, COI | AY426434, AY426435, AY426436 |
| Stoloteuthis leucoptera | Smithsonian collection ABL9402.19.27 | 12S, 16S, COI, 28S | AY293655, AY293681, AF000068, AY293704 |
| Heteroteuthis hawaiiensis | Hokusei Maru/1996 | 12S, 16S, COI, 28S | AY293654, AY293680, AY293728, AY293703 |
| Euprymna morsei | Tosa, Bay, Japan/7-2002 | 12S, 16S, COI, 28S | AY293635, AY293661, AY293710, AY293691 |
| Euprymna tasmanica | Melbourne, Australia/4-2001 | 12S, 16S, COI, 28S | AY293638, AY293664, AY293713, AY293699 |
| Euprymna scolopes | Paiko, Honolulu, HI/10-2002 | 12S, 16S, COI, 28S | AY293637, AY293663, AY293712, AY293688 |
| Euprymna berryi | Tosa Bay, Japan/7-2002 | 12S, 16S, COI, 28S | AY293636, AY293662, AY293711, AY293689 |
| Euprymna hyllebergi | Gulf of Thailand, Thailand/3-2001 | 12S, 16S, COI, 28S | AY293639, AY293665, AY293714, AY293690 |
| Sepietta oweniana | Banyuls-sur-mer, France/7-2001 | 12S, 16S, COI | AY293649, AY293675, AY293724 |
| Sepietta neglecta | Banyuls-sur-mer, France/7-1999 | 12S, 16S, COI, 28S | AY293647, AY293673, AY293722, AY293696 |
| Sepietta obscura | Banyuls-sur-mer, France/9-1998 | 12S, 16S, COI, 28S | AY293648, AY293674, AY293723, AY293695 |
| Sepiola birostrata | Tosa, Bay, Japan/9-2002 | 12S, 16S, COI, 28S | AY293640, AY293666, AY293715, AY293694 |
| Sepiola ligulata | Banyuls-sur-mer, France/7-2002 | 12S, 16S, COI, 28S | AY293642, AY293668, AY293717, AY293692 |
| Sepiola atlantica | Vigo, Spain/7-2001 | 12S, 16S, COI, 28S | AY293646, AY293672, AY293721, AY293693 |
| Sepiola rondeleti | Banyuls-sur-mer, France/7-2001 | 12S, 16S, COI | AY293645, AY293671, AY293720 |
| Sepiola affinis | Banyuls-sur-mer, France/8-2001 | 12S, 16S, COI, 28S | AY293641, AY293667, AY293716, AY293697 |
| Sepiola intermedia | Banyuls-sur-mer, France/7-2001 | 12S, 16S, COI, 28S | AY293643, AY293669, AY293718, AY293700 |
| Sepiola robusta | Banyuls-sur-mer, France/9-1995 | 12S, 16S, COI, 28S | AY293644, AY293670, AY293719, AY293701 |
Table 2.
| Table 2A. Primer sequences used for PCR amplification and sequencing | ||
|---|---|---|
|
| ||
| Primer | Primer Sequence 5′-3′ | PCR Product Size |
| 12Sai | AAACTAGGATTAGATACCCTATTA T | ∼400 base pairs |
| 12Sbi | AAGAGCGACGGGCGATGTGT | |
| 16Sa | CGCCTGTTTATCAAAAACAT | ∼400 base pairs |
| 16Sb | CTCCGGTTTGAACTCAGATCA | |
| COIF | TAAACTTCAGGGTGACCAAAAAATCA | ∼ 600 base pairs |
| COIR | GGTCAACAAATCATAAAGATATTGG | |
| 28Sa | GACCCGTCTTGAAACACGGA | ∼ 600 base pairs |
| 28Sb | TCGGAAACCAGCTAC | |
| Table 2B. PCR amplification profiles | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Loci | Number of Cycles | Hotstart | Denaturation | Annealing | Extension | Final Extension |
| 12S | 25 | 94°C for 2 min | 94°C for 15 sec | 42°C for 30 sec | 72°C for 30 sec | 72°C for 7 min |
| 16S | 25 | 94°C for 2 min | 94°C for 15 sec | 42°C for 30 sec | 72°C for 30 sec | 72°C for 7 min |
| CO1 | 25 | 94°C for 2 min | 94°C for 15 sec | 36°C for 30 sec | 72°C for 30 sec | 72°C for 7 min |
| 28S | 25–30 | 94°C for 2 min | 94°C for 15 sec | 40–49°C for 30 sec | 72°C for 30 sec | 72°C for 7 min |
Phylogenetic analysis
Sequence data were analyzed using the direct optimization method described by Wheeler (1996) and implemented in the computer program POY (Wheeler et al. 2002). The four molecular partitions were analyzed independently and combined directly, with all characters weighted equally (transitions/transversion/indel costs) without regard to source. These data sets are referred to as 12S (12S rRNA data set alone), 16S (16S data set alone), COI (COI data set alone), 28S (28S rRNA data set alone), and molecular (12S+16S+COI+28S). The tree search strategy adopted combined spr and tbr branch swapping on the best of 50 random addition replicates (-random 50), holding 100 trees per round (-maxtrees 100) and performing one round of tree-fusing (Goloboff 1999). The commands -slop 5 and -checkslop 10 were used. These commands are intended to check all cladogram lengths that are within “n” tenths of a percent of the current minimum value. A slop value of 10 would check all cladograms found within 1% of the minimum tree length. This option slows down the search but is less affected by the heuristics of the tree length calculation shortcuts. Nodal support was evaluated via jackknifing for 1000 replicates. The “implied alignments” obtained with POY were checked with PAUP*4b (Swofford 2002) and identical tree lengths obtained.
Results
Comparative morphology of bacteriogenic light organs
The three components of bacteriogenic light organs mentioned in our introduction show varying patterns of arrangement and overall outline depending on genera and species. The ontogenetic origin of the light organ can best be visualized by observing the invagination of the first pair of crypts in an embryo of, for example, Sepiola ligulata (Fig. 1). This invagination appears at the earliest conceivable stage of morphogenesis. Indeed, the small volume of embryonic tissue available at this site virtually excludes the achievement of an ectodermic invagination by stage XIV (Fig. 1A). The considerable enlargement of the mesodermic cell mass lying on either side of the hindgut–ink sac complex, occurring between stage XIV and XV, then provides the physical prerequisite for a rearrangement of the mesodermic cells that must give way to the invaginating ectoderm to form the first pair of crypts. The blind ends of these crypts come to lie close to the flexure between the tubular hindgut and the ink sac rudiment, which begins to form secondary diverticula (Fig. 1B). When placing emphasis on this obvious morphogenetic constraint on the onset of crypt formation, one has to recall that the (originally tubular) ink sac rudiment is an integral part of the mesendodermic midgut complex, forming a diverticulum of the prospective hindgut (Boletzky 1988). In other words, at that stage there is no invagination other than the first crypts. Additional crypts are subsequently formed by similar invaginations (Foster et al. 2002).
Fig. 1.
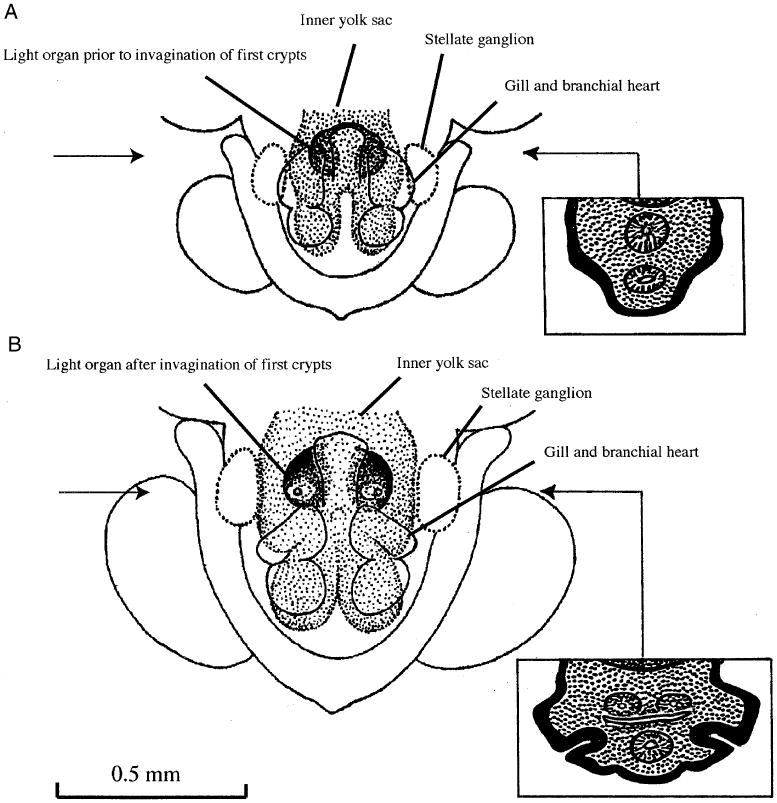
The mantle complex of Sepiola ligulata at embryonic stage XIV (A) and XV (B), reconstructed from histological cross-sections (staging according to Naef 1928). Semi-schematic representations of an enlarged (twice the main figure) reference section for each stage are inserted on the right side; their exact position is marked with an arrow. The thick black line (drawn to scale) indicates the variable thickness of the ectoderm before (A) and after (B) the invagination of the first pair of light organ crypts. The light stippling in these sections represents the mesodermic complex surrounding the endodermic derivatives (intestine [below] and ink gland [above]). Note that the invagination of the first crypt “points at” the area between the intestine and the ink gland that already displays branching tubules. The dark stippling at the top of each section represents part of the inner yolk sac.
The bilateral symmetry of the whole complex is barely recognizable in the sepiolid genera Rondeletiola and Sepiolina, where light organs are rounded and fused. Fusion of the two parts of the light organ across the middle plane was also observed in several specimens of Semirossia. Strongly differentiated surface structures involving the anterior and posterior ends of the lens on either side exist in the sepiolid subfamily Sepiolinae (Foster et al. 2002), in contrast to the round form observed in Rondeletiola (Naef 1923), Sepiolina, and Semirossia (Boletzky 1970). The pore leading into the united ducts of the crypts on either side is surrounded by an elevated rim that forms a very distinct papilla, especially in Semirossia (Boletzky 1970). In some individuals among representatives of this genus, the papilla was found to be elongated into a short tube (male) or into a long tube that opens into the ANG (female). In one female S. tenera such a tube was observed on one side, whereas on the other side the light organ pore opened to the organ surface independently of the ANG (Boletzky 1970).
Morphogenesis of bacteriogenic light organs and of “ANGs”
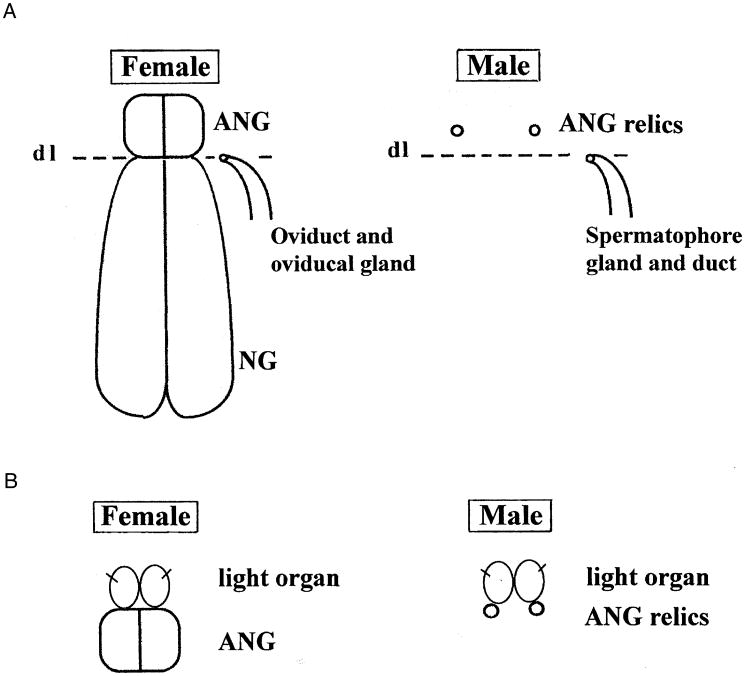
Differentiation of sepiolid light organs is initiated by the serial invagination of ectodermic zones (Fig. 1) that form paired crypts, which become arranged in a bilaterally symmetrical organ, the pores of each side being pulled together (one definitive pore on each side; Foster et al. 2002). The so-called ANGs are formed by similar but more numerous ectodermic invaginations (Lemaire 1972) forming long tube-shaped crypts, which later take up nonluminous bacteria (Bloodgood 1977; Kaufman et al. 1998). In females, the ANGs are closely associated with the actual nidamental glands (Fig 2). In males, they remain rudimentary or (more generally) are lacking altogether (the question remains unanswered for the “epirenal bodies” in Neorossia, cf. Boletzky 1971).
Fig. 2.
(A) A semi-schematic representation of nidamental glands (NG) and sexual ducts lying below the broken line (DL 5 dividing line between two likely morphogenetic fields) and of accessory nidamental glands (ANG) or corresponding relics, which are distinct from the former and lie above the broken line. (B) A semi-schematic representation of a conceivable derivation of light organ crypts from the accessory nidamental gland (ANG) or corresponding structures.
Phylogenetic analyses
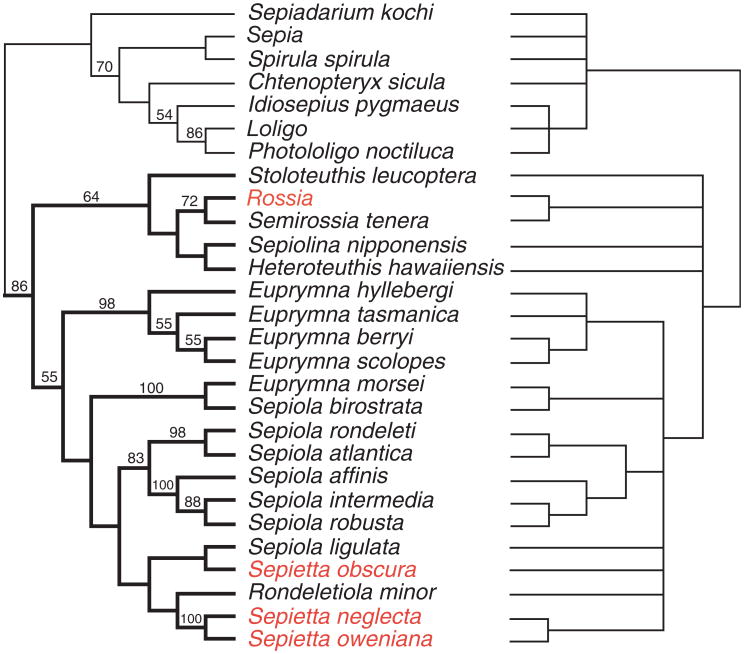
The phylogenetic analysis of all data analyzed in combination suggests that the parameter set that minimizes incongruence among partitions is that which assumes equal weights for all transformations (ILD 5 0.0242). A single tree of length 4046 was found in 65% of the replicates (Fig. 3, left); the strict consensus of the four parameter sets analyzed is also shown (Fig. 3, right). These trees clearly show monophyly of the family Sepiolidae, which receives a jackknife support value of 86% and is stable to parameter variation.
Fig. 3.
Phylogeny of representative Sepiolidae species and selected outgroups (for reference). Sepiolid taxa in red have no light organs. After a combined analysis of four loci (mtCOI, mt12S, mt16S rRNA, and partial sequence of the nuclear 28S rRNA, total approximately 2.4 kb) using direct optimization as implemented in POY (Wheeler et al. 2002). We analyzed six parameter sets varying the relative weights of insertion/deletion events and of base transformations. The tree on the left represents the best hypothesis using the parameter set that minimizes incongruence among all gene partitions (for all transformation receiving equal weights). On the right, a more conservative hypothesis, the strict consensus of all parameter sets examined, is presented, with all the nodes in this tree indicating parameter independence. Numbers on branches (left tree) indicate jackknife proportions (branches with no value have jackknife values<50%).
The basic identical pattern underlying the anatomical complexity and the positional relation of the symbiotic light organ to the ink sac suggests a common phylogenetic origin (Fig. 1B). For the family Sepiolidae, inferring a common ancestor that had a symbiotic light organ raises no major problem, because the known genera lacking such an organ (Rossia and Sepietta, see taxa in red, Fig. 3) are likely derived from forms in which differentiation of the light organ was secondarily suppressed. Considering the morphogenetic onset of light organ formation (Fig. 1B), it is easy to imagine a modification entailing a blockade of crypt formation, so that the tissue complex associated with the hindgut and ink sac remains in a situation as that found at stage XIV (Fig. 1A). This is supported by our most parsimonious phylogeny where both Sepietta and Rossia are clearly grouped within clades of light organ containing sepiolids (Fig. 3), this result being supported under all analytical parameters explored here. The “Rondeletiola minor clade,” which contains all Sepietta species, Sepiola ligulata, and R. minor, provides phylogenetic evidence that the light organ was lost in extant Sepietta (Fig. 3, left); however, support for such clade is low. The node that contains Rossia and Semirossia (72% jackknife support and stable to parameter variation) is sister to part of the Heteroteuthinae, inferring a loss of light organs in the genus Rossia. Sepiola ligulata, one of the “true endemics” (Mangold and Boletzky 1988) of the Mediterranean Sepiolidae, did not cluster with the rest of the species of Sepiola but rather is an outlier within the entire Sepiola/Sepietta/Rondeletiola cluster, supporting the fact that this clade is not stable. The position of S. birostrata as sister to E. morsei is surprising from a morphological standpoint (100% jackknife support; parameter independent). Additional sampling of Sepiola species in the Pacific will help determine whether S. birostrata is found isolated from other Sepiola species and whether the genus Sepiola is indeed polyphyletic. As for other taxa that represent outgroups to the Sepiolidae, some resolution for these species was determined by our analyses from the consensus tree. Our analyses do show that for the most parsimonious tree, both loliginid species are sister to each other (86% jackknife support), and this clade is most closely related to Idiosepius (54% support). As to the Loliginidae, the presence of symbiotic light organs in species of Photololigo and possibly other genera (Pringgenies and Jorgensen 1994) also suggests the presence of such light organs in an ancestral loliginid and subsequent loss of the organs in derived forms (Loligo/P. noctiluca clade).
Discussion
Morphology and organogenesis of the light organ
Organogenesis of bacteriogenic light organs raises several questions regarding the acquisition of bacteria at the outset of the association, as well as the formation of an organ complex that has a specific function (such as light production). First, how do bacteria evolve specificity for particular groups of tissues, and second, how does the tissue(s) respond to the presence of these bacteria to produce a specialized milieu? Initially, a primitive ANG may have been the pacemaker for establishment of a bacterial association, from which two specialized complexes could then evolve, but the inverse (i.e., light organ function first, ANG second) cannot be excluded. Indeed, the embryological data (Fig. 1) would seem to suggest that the light organ came first, because it is formed long before a rudiment of an ANG (or ANG relic in males) is recognizable. But this sequence of morphogenetic events is no proof, because heterochronic shifts may have changed an originally different order in the course of evolution. For what we can be certain of, there is compelling evidence that such an association exists in the extant ANG in a wide variety of decabrachian species, especially (though not only) in females. The presence of bacteria in nidamental jellies (Biggs and Epel 1991; Barbieri et al. 2001) emphasizes the female-related function of the ANG, but there is no reason to entirely exclude an originally similar function in males that may have disappeared more or less completely. Examples of Rondeletiola (Naef 1923) and Semirossia (Boletzky 1971) seem to emphasize a developmental connection, but unfortunately we do not yet know the embryonic development of these two genera. Obtaining eggs to study the embryos of at least one representative is the next step to understanding the morphogenetic course of organ diversification.
Colonization of bacteria from the ANG
Whatever the answer to the above question, one has to address the following problem: How can colonization from a large pool of bacteria lead to a monotypic (i.e., one species or strain) association in such a developed organ complex? Could this also mean that less specific bacterial colonization of ANG crypts reflects a more permissive relationship? Because ANG crypts have over 20 isolates of bacteria present (Kaufman et al. 1998; Grigioni et al. 2000), there is little reason to suspect that this organ complex's function is dependent on the presence of only one of many species. Also, whether the entire population of ANG bacteria is transmitted vertically or environmentally may direct the evolution of specificity in these bacteria (Nishiguchi et al. 1998; Nishiguchi 2002a). In contrast, advanced light organ morphogenesis is dependent on only one strain of bacteria that is transmitted environmentally. Although this association is highly specific, somehow each symbiont has evolved a particular signature that distinguishes itself from all others (Nishiguchi 2001, 2002b). Evolution of the specificity is probably a consequence of an immunological host response that only allows the colonization of a few types of bacteria that have adapted to this particular habitat, namely the light organ. If bacterial adaptation has occurred in the light organ or similar tissues (environmental response), the change would be in either recognition of specific type(s) of receptor(s) (i.e., mannose or other glycosylated residues; McFall-Ngai et al. 1998) or use of adhesins or pili to attach to the surrounding tissues (Stabb and Ruby 2003). These evolutionary changes, once in place, would allow only certain types of bacteria to not only colonize but also out-compete other bacteria that have not undergone these specific adaptations. What is selecting for these types of characteristics that allow increased fitness of bacteria in the light organ or other bacteriogenic tissues has yet to be discovered.
Evolution of autogenic versus bacteriogenic light organs
How then did the tissues respond to the presence of bacteria to form this highly complex light-emitting organ? Ultimately, the question can be raised in relation to a given function rather than to a particular cell type. For example, light emission (no matter how achieved) and light reception could be coupled from the outset. We know that autogenic light organs are involved in ventral counter-illumination much the same way as the bacteriogenic light organs (Young 1977). A structural contrast between these two light emitting organs is the integumental in situ differentiation in autogenic light organs (Chun 1903) versus an integumental ex situ differentiation (by invagination of host tissue) in bacteriogenic light organs. The morphogenetic process involved in this ex situ differentiation is reminiscent of the formation of extraocular photoreceptors, some of which appear to be coadapted to visceral light organs (Young 1977). Perhaps the question of incipient photogenic function in an ancestral form can be addressed in such a context of functional coadaptation. In particular, the photoreceptors described by Young (1977) and nuchal organs (Sundermann 1990; Parry 2000) provide some hints. Ultimately, these results point to the epistellar bodies of octopods, which are undeniable photoreceptors; however, these are not formed by invagination and hence remain superficial inside the mantle cavity (Nishioka et al. 1962). Recruitment of proteins that comprise the cephalopod eye lens may also be closely involved in the development of the light organ lens (Tomarev et al. 1995; Tomarev and Piatigorsky 1996). How these proteins are expressed and then subsequently recruited in response to bacterial colonization remains yet to be discovered in bacteriogenic light organ cephalopods (Tomarev et al. 1997; Hartmann et al. 2003).
Bacterial induction of light organ tissues
Invagination of surface epithelia in prospective symbiosis-competent tissue always occurs in an axenic environment (perivitellin fluid). Given this, it seems difficult to envisage an ancestral condition in which an integumental contact with bacteria caused invagination to occur. It seems more likely that existing crypts were colonized by bacteria and subsequently became increasingly specialized to interact with them. If this occurred, plasticity in the tissues may have been maintained, as suggested by the observations of the microvilli in the crypt's epithelia (Lamarcq and McFall-Ngai 1998). Before colonization light organ epithelial tissue has very small microvilli, and those present are shorter and stouter. Once bacteria colonize the light organ, these microvilli increase in number and become more filiform. If bacteria are removed (using an antibiotic), the microvilli return to their initial morphology (Lamarcq and McFall-Ngai 1998). Similar phenomena are known from a number of mammalian hosts and their enterogastric symbionts (Lawrence et al. 1991) as well as leguminous plants and their root-nodule symbionts (van Rhijn and Vanderleyden 1995).
In addition, host tissues can respond to the presence of bacteria in an immunological reaction. It has been shown in previous studies that the presence of high concentrations of haloperoxidase in the light organ is correlated to high numbers of symbiotic bacteria. The amount of haloperox-idase is concentrated at the apical end of epithelial cells that line the crypts during the evening hours when the light organ is fully colonized. During the daytime after squids vent 90– 95% of their bacteria, haloperoxidase levels decrease and the enzyme is located at the basal end of the epithelial cells (Tomarev et al. 1993; Weis et al. 1996; Small and McFall-Ngai 1998). This spatial and temporal control of squid haloperoxidase has two consequences: (a) It is very similar to a large number of invertebrate immunological systems that use antimicrobial agents to prevent infection by undesirable bacteria and (b) it allows only certain types of bacteria to colonize the light organ (i.e., symbionts that produce high levels of catalase; Visick and Ruby 1998). In many cases, invertebrate immunological systems have been coadapted such that they not only prevent invasion and infection from a large variety of pathogens, but they have also been integrated into very specific associations with a particular partner/symbiont (Ruby 1999). More detailed analyses of the phylogenetic origin of bacteria isolated from symbiotic light organs have demonstrated the multiple radiation of a number of mutualistic and pathogenic strains of Vibrio (Nishiguchi and Nair 2003). Phylogenetic relationships between bacteria that are benign or cause disease-related infections may be important for investigating origins of specialized bacterial-containing organs such as the ANG and light organ complex that enable animals to house bacteria without having the onset of disease or tissue necrosis.
Phylogenetic implications and future studies
Our phylogenetic investigations imply a distant relationship between the Sepiolidae and Sepiadariidae (Fig. 3). By the same token, Chtenopteryx and the Loliginidae are removed from such a relationship. At this point, it is premature to speculate about the evolution of light organs in all cephalopods. Answering such questions will require a much denser taxon sampling outside of the Sepiolidae (Idiosepidae, Loliginidae), and addition of phylogenetic markers that evolve at slower rates to recover deeper divergences. Additionally, inclusion of other light organ containing genera (Family Loliginidae: Uroteuthis, Photololigo, and Loliolus) will perhaps give us a better perspective on the evolution of bacteriogenic light organs in other cephalopod families. Comparative morphological evidence from various states of squids with (Sepiola and Euprymna) or without (Rossia and Sepietta) light organs and those with intermediate light organs (Rondeletiola, Sepiolina, Semirossia, Heteroteuthis, and Stoloteuthis) will provide more support of a common origin of this interesting complex.
Acknowledgments
Funding was provided by NSF DBI-0079820, NSF SBE-0123690, and NIH SO6-GM08136-26 to M. K. N. and NIH-RISE GM61222-01 at NMSU for support of J. E. L. We especially thank G. Giribet at Harvard University for assistance with analysis of phylogenetic data. Many thanks to J. Browne-Silva, B. Jones, A. Lindgren, V. Nair, T. Powers, W. Soto, and two anonymous reviewers for help with reviewing the manuscript before submission. Histological preparations for the reconstructions from serial sections were made by M. V. v. Boletzky. Thanks to Angel Guerra, Hitoshi Honda, Jaruwat Nabhitabhata, Claude Nozeres, and the crew of the Neres at Laboratoire Arago with help collecting specimens.
References
- Barbieri E, et al. Phylogenetic characterization of epibiotic bacteria in the accessory nidamental gland and egg capsules of the squid Loligo pealei (Cephalopoda: Loliginidae) Environ Microbiol. 2001;3:151–167. doi: 10.1046/j.1462-2920.2001.00172.x. [DOI] [PubMed] [Google Scholar]
- Biggs J, Epel D. Egg capsule and sheath of Loligo opalescens Berry: structure and association with bacteria. J Exp Zool. 1991;259:263–267. [Google Scholar]
- Bloodgood RA. The squid accessory nidamental gland: ultra-structure and association with bacteria. Tissue Cell. 1977;9:197–208. doi: 10.1016/0040-8166(77)90016-7. [DOI] [PubMed] [Google Scholar]
- Boletzky Sv. Biological results of the University of Miami deep-sea expeditions, 54. On the presence of light organs in Semirossia Steenstrup, 1887 (Mollusca: Cephalopoda) Bull Mar Sci. 1970;20:374–388. [Google Scholar]
- Boletzky Sv. Neorossia n.g. pro Rossia (Allorossia) caroli Joubin, 1902, with remarks on the generic status of Semirossia Steenstrup, 1887 (Mollusca: Cephalopoda) Bull Mar Sci. 1971;21:964–969. [Google Scholar]
- Boletzky Sv. Cephalopod development and evolutionary concepts. In: Clarke MR, Trueman ER, editors. Paleontology and Neontology of Cephalopods, The Mollusca. Vol. 12. Academic Press; San Diego: 1988. pp. 185–202. [Google Scholar]
- Chun C. Ueber leuchtorgane und augen von tiefsee-Cephalopoden. Verh Dtsch Zool Ges. 1903;12:67–91. [Google Scholar]
- Fidopiastis PM, Boletzky Sv, Ruby EG. A new niche for Vibrio logei, the predominant light organ symbiont of squids in the genus. Sepiola J Bacteriol. 1998;180:59–64. doi: 10.1128/jb.180.1.59-64.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster J, Boletzky Sv, McFall-Ngai MJ. A comparison of the light organ development of Sepiola robusta Naef and Euprymna scolopes Berry (Cephalopoda: Speiolidae) Bull Mar Sci. 2002;70:141–153. [Google Scholar]
- Goloboff PA. Analyzing large data sets in reasonable times: solutions for composite optima. Cladistics. 1999;15:415–428. doi: 10.1111/j.1096-0031.1999.tb00278.x. [DOI] [PubMed] [Google Scholar]
- Grigioni S, Boucher-Rodini R, Demarta A, Tonolla M, Peduzzi R. Phylogenetic characterization of bacterial symbionts in the accessory nidamental glands of the sepioid Sepia officinalis (Cephalopoda: Decapoda) Mar Biol. 2000;136:217–222. [Google Scholar]
- Hartmann B, Lee PN, Kang YY, Tomarev S, de Couet HG, Callaerts P. Pax6 in the sepiolid squid Euprymna scolopes: evidence for a role in eye, sensory organ and brain development. Mech Dev. 2003;120:177–183. doi: 10.1016/s0925-4773(02)00456-2. [DOI] [PubMed] [Google Scholar]
- Herring PJ. Luminescence in Cephalopods and Fish. Sym Zool Soc Lond. 1977;38:127–159. [Google Scholar]
- Kaufman MR, Ikeda Y, Patton C, van Dykhuizen G, Epel D. Bacterial symbionts colonize the accessory nidamental gland of the squid Loligo opalescens via horizontal transmission. Biol Bull. 1998;194:36–43. doi: 10.2307/1542511. [DOI] [PubMed] [Google Scholar]
- Lamarcq LH, McFall-Ngai MJ. Induction of a gradual, reversible morphogenesis of its host's epithelial brush border by Vibrio fischeri. Infect Immun. 1998;66:777–785. doi: 10.1128/iai.66.2.777-785.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence JG, Ochman H, Hartl DL. Molecular and evolutionary relationships among enteric bacteria. J Gen Microbiol. 1991;137:1911–1921. doi: 10.1099/00221287-137-8-1911. [DOI] [PubMed] [Google Scholar]
- Lemaire J. Differenciation sexuelle de la gonade embryonnarie de Sepia officinalis. I- cultivee in vitro. C R Acad Sci Paris. 1972;275:475–478. [PubMed] [Google Scholar]
- Mangold K, Boletzky Sv. Mediterranean cephalopod fauna. In: Clarke MR, Trueman ER, editors. The Mollusca. Academic Press; San Diego: 1988. pp. 315–330. [Google Scholar]
- McFall-Ngai MJ, Brennan C, Weis V, Lamarcq LH. Mannose adhesin-glycan interactions in the Euprymna scolopes-Vibrio fischeri symbiosis. In: Le Gal Y, Halvorson HO, editors. New Developments in Marine Biotechnology. Plenum Press; New York: 1998. pp. 273–276. [Google Scholar]
- McFall-Ngai MJ, Ruby EG. Symbiont recognition and subsequent morphogenesis as early events in an animal-bacterial symbiosis. Science. 1991;254:1491–1494. doi: 10.1126/science.1962208. [DOI] [PubMed] [Google Scholar]
- Meyer WT. Ueber das Leuchtorgan der Sepiolini. Zool Anz. 1906;30:388–392. [Google Scholar]
- Montgomery MK, McFall-Ngai MJ. The muscle-derived lens of a squid bioluminescent organ is biochemically convergent with the ocular lens. J Biol Chem. 1992;267:20999–21003. [PubMed] [Google Scholar]
- Montgomery MK, McFall-Ngai MJ. Embryonic development of the light organ of the sepiolid squid Euprymna scolopes Berry. Biol Bull. 1993;184:296–308. doi: 10.2307/1542448. [DOI] [PubMed] [Google Scholar]
- Naef A. Die Cephalapoden (Systematik) Fauna Flora Golf Napoli (Monograph) 1923;35(I-2):1–863. [Google Scholar]
- Naef A. Die Cephalapoden (Embryologie) Fauna Flora Golf Napoli (Monograph) 1928;35(I-2):1–357. [Google Scholar]
- Nishiguchi MK. The use of physiological data to corroborate cospeciation events in symbiosis. In: DeSalle R, Wheeler W, Giribet G, editors. Molecular Systematics and Evolution: Theory and Practice. Birkhäuser, Basel; Germany: 2002a. pp. 237–246. [DOI] [PubMed] [Google Scholar]
- Nishiguchi MK. Host recognition is responsible for symbiont composition in environmentally transmitted symbiosis. Microbiol Ecol. 2002b;44:10–18. doi: 10.1007/BF03036870. [DOI] [PubMed] [Google Scholar]
- Nishiguchi MK. Co-evolution of symbionts and hosts: the sepiolid-Vibrio model. In: Seckbach J, editor. Symbiosis: Mechanisms and Model Systems. Cole-Kluwer Academic; Dordrecht, The Netherlands: 2001. pp. 757–774. [Google Scholar]
- Nishiguchi MK, Nair VS. Evolution of pathogenicity and symbiosis in Vibrionaceae: a combined approach using molecules and physiology. Int J Syst Evol Microbiol. 2003;53:2019–2026. doi: 10.1099/ijs.0.02792-0. [DOI] [PubMed] [Google Scholar]
- Nishiguchi MK, Ruby EG, McFall-Ngai MJ. Competitive dominance among strains of luminous bacteria provides an unusual form of evidence for parallel evolution in sepiolid squid-Vibrio symbioses. Appl Environ Microbiol. 1998;64:3209–3213. doi: 10.1128/aem.64.9.3209-3213.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishioka RS, Hagadorn IR, Bern HA. Ultrastructure of the epistellar body of the octopus. Zeitschr Zellforsch Mikrosk Anat. 1962;57:406–421. doi: 10.1007/BF00343327. [DOI] [PubMed] [Google Scholar]
- Parry M. A description of the nuchal organ, a possible photoreceptor, in Euprymna scolopes and other cephalopods. J Zool Lond. 2000;252:163–177. [Google Scholar]
- Pringgenies D, Jorgensen JM. Morphology of the luminous organ of the squid Loligo duvauceli d'Orbigny, 1839. Acta Zool. 1994;75:305–309. [Google Scholar]
- Ruby EG. Ecology of a benign “infection”: colonization of the squid luminous organ by Vibrio fischeri. In: Rosenberg E, editor. Microbiol Ecology and Infectious Disease. American Society of Microbiology; Washington, DC: 1999. pp. 217–231. [Google Scholar]
- Small AL, McFall-Ngai MJ. A halide peroxidase in tissues interacting with bacteria in the squids Euprymna scolopes. J Cell Biochem. 1998;72:445–457. [PubMed] [Google Scholar]
- Stabb EV, Ruby EG. Contribution of pilA to competitive colonization of the squid Euprymna scolopes by Vibrio fischeri. Appl Environ Microbiol. 2003;69:820–826. doi: 10.1128/AEM.69.2.820-826.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundermann G. Development and hatching state of ectodermal vesicle-organs in the head of Sepia officinalis, Loligo vulgaris and Loligo forbesi (Cephalopoda, Decabrachia) Zoomorph. 1990;109:343–352. [Google Scholar]
- Swofford DL. PAUP* Phylogenetic Analysis Using Parsimony (*and Other Methods) Sinauer Associates; Sunderland, MA: 2002. [Google Scholar]
- Tomarev SI, et al. Squid Pax6 and eye development. Proc Natl Acad Sci USA. 1997;94:2421–2426. doi: 10.1073/pnas.94.6.2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomarev SI, Chung S, Piatigorsky J. Glutathione S-transferase and S-crystallins of cephalopods: evolution from active enzyme to lens-refractive proteins. J Mol Evol. 1995;41:1048–1056. doi: 10.1007/BF00173186. [DOI] [PubMed] [Google Scholar]
- Tomarev SI, Piatigorsky J. Lens crystallins of invertebrates. Diversity and recruitment from detoxification enzymes and novel proteins. Eur J Biochem. 1996;235:449–465. doi: 10.1111/j.1432-1033.1996.00449.x. [DOI] [PubMed] [Google Scholar]
- Tomarev SI, Zinovieva RD, Weis VM, Chepelinsky AB, Piatigorsky J, McFall-Ngai MJ. Abundant mRNAs in the squid light organ encode proteins with a high similarity to mammalian peroxidases. Gene. 1993;132:219–226. doi: 10.1016/0378-1119(93)90199-d. [DOI] [PubMed] [Google Scholar]
- van Rhijn P, Vanderleyden J. The Rhizobium-plant symbiosis. Microbiol Rev. 1995;59:124–142. doi: 10.1128/mr.59.1.124-142.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visick KL, Ruby EG. The periplasmic, group III catalase of Vibrio fischeri is required for normal symbiotic competence and is induced both by oxidative stress and by approach to stationary phase. J Bacteriol. 1998;180:2087–2092. doi: 10.1128/jb.180.8.2087-2092.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis VM, Montgomery MK, McFall-Ngai MJ. Enhanced production of ALDH-like proteins in the bacterial light organ of the sepiolid squid Euprymna scolopes. Biol Bull. 1993;184:309–321. doi: 10.2307/1542449. [DOI] [PubMed] [Google Scholar]
- Weis VM, Small AL, McFall-Ngai MJ. A peroxidase related to the mammalian antimicrobial protein myeloperoxidase in the Euprymna-Vibrio mutualism. Proc Natl Acad Sci USA. 1996;93:13683–13688. doi: 10.1073/pnas.93.24.13683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler WC. Optimization alignment: the end of multiple sequence alignment in phylogenetics? Cladistics. 1996;12:1–9. [Google Scholar]
- Wheeler WC, Gladstein D, DeLaet J. POY. American Museum of Natural History; New York: 2002. [Google Scholar]
- Young JZ. Ctenopteryx the comb-fin squid is related to Loligo. Bull Mar Sci. 1991;49:148–161. [Google Scholar]
- Young RE. Ventral bioluminescent countershading in midwater cephalopods. Symp Zool Soc Lond. 1977;38:161–190. [Google Scholar]