Abstract
Luminescent bacteria in the family Vibrionaceae (Bacteria: γ-Proteobacteria) are commonly found in complex, bilobed light organs of sepiolid and loliginid squids. Although morphology of these organs in both families of squid is similar, the species of bacteria that inhabit each host has yet to be verified. We utilized sequences of 16S ribosomal RNA, luciferase α-subunit (luxA) and the glyceraldehyde-3-phosphate dehydrogenase (gapA) genes to determine phylogenetic relationships between 63 strains of Vibrio bacteria, which included representatives from different environments as well as unidentified luminescent isolates from loliginid and sepiolid squid from Thailand. A combined phylogenetic analysis was used including biochemical data such as carbon use, growth and luminescence. Results demonstrated that certain symbiotic Thai isolates found in the same geographic area were included in a clade containing bacterial species phenotypically suitable to colonize light organs. Moreover, multiple strains isolated from a single squid host were identified as more than one bacteria species in our phylogeny. This research presents evidence of species of luminescent bacteria that have not been previously described as symbiotic strains colonizing light organs of Indo-West Pacific loliginid and sepiolid squids, and supports the hypothesis of a non-species-specific association between certain sepiolid and loliginid squids and marine luminescent bacteria.
Members of the families Loliginidae and Sepiolidae (Mollusca: Cephalopoda) have been previously shown to contain luminous bacteria that reside in specialized light organ complexes. These light organs vary in structure and complexity, from simple, spherically shaped structures [Rondeletiola and Sepiolina; (Naef, 1912a; Nesis, 1982)], to complex, bilobed organs (Naef, 1912b; McFall-Ngai and Montgomery, 1990; Foster et al., 2002; Nishiguchi et al., 2004).
Squid use the light produced from the bacteria for a behavior known as counterillumination (Young and Roper, 1977; Young et al., 1980; Jones and Nishiguchi, 2004). Luminescence emitted from the light organ reduces the squid’s silhouette to match the intensity and wavelength of down-welling light (Young and Roper, 1977). This provides squid with a mechanism that allows them to evade predators by camouflage. All bacteria housed in the light organs are able to produce light via the lux operon both inside the light organs and in their free-living state, although intensity of light and differences between strains of bacteria have never been thoroughly investigated. Likewise, regulation of the lux operon inside the light organ of squid has only been extensively studied in the Euprymna scolopes–Vibrio fischeri symbiosis, where reduction in the amount of light produced affects symbiotic competence (Visick et al., 2000).
Previous investigations have demonstrated that two genera, Vibrio and Photobacterium, are prevalent in both sepiolid and loliginid squid (Fidopiastis et al., 1998; Nishiguchi et al., 2004). Sepiolid squid primarily contain two species of Vibrio, V. fischeri and V. logei (Fidopiastis et al., 1998; Nishiguchi, 2002), although the genera Rondeletiola and Sepiolina have been shown to contain Photobacterium leiognathi in their light organs (Nishiguchi and Nair, 2003; Nishiguchi et al., 2004). Photobacteria are more commonly found in the genera Uroteuthis and Loliolus (Nishiguchi, 2002), with no cases of Vibrio species detected in bacteriogenic light organ of loliginids. Because there is geographic overlap between several species of Uroteuthis, Loliolus and Euprymna in the Indo-West Pacific, we were interested in isolating and identifying bacteria from these squid, determining whether these strains were phylogenetically related to one another, and whether there was any biogeographical or host-specific patterns evident between the symbionts. We used the complete 16S rRNA sequence from each isolate in a combined analysis with the luciferase α-subunit gene (luxA) and the glyceraldehyde phosphate dehydrogenase gene (gapA) sequences to determine phylogenetic relationships among closely related species. In addition, we measured both growth and luminescence to determine whether substantial differences occurred between these isolates. Previously isolated squid symbionts from host species collected in close or distant geographic locations were also compared in order to evaluate whether luminous bacteria that have been environmentally transmitted are promiscuous across hosts, or alternatively, whether symbionts exhibit squid family specificity. These comparisons would then allow us to determine if geographic location of the species investigated was a determining factor in predicting the associations between light organ symbionts and loliginid squid.
Materials and methods
Collection of squid and isolation of bacteria from light organs
Squid were collected from either fishermen in the local Thai markets, or by trawl net and brought into the lab for identification. Squid were collected from two geographically separated locations: Rayong, Thailand, which is located on the Gulf of Thailand, and the island of Phuket, in southern Thailand, which is located in the Andaman Sea (Table 1, in bold). Animals collected in Rayong were brought back to the Rayong Coastal Aquaculture and Fisheries Research Center for confirmation of identity. Specimens collected in Phuket were identified at the Phuket Marine Biological Station. Host specimens were used for isolation of symbiotic bacteria according to previous protocols (Nishiguchi, 2002; Nishiguchi and Nair, 2003). Mantle cavities were opened and the entire light organ complex was removed and placed in sterile seawater (700 µL). Adult light organs were homogenized, serially diluted to 10−5 and plated on seawater tryptone (SWT) agar media (Ruby and Asato, 1993). Cultures were incubated overnight at room temperature, and kept at 5 °C upon return to New Mexico State University. Approximately 20–30 light-producing colonies from each plate were subsequently grown overnight in SWT medium at 20–28 °C, and cultures were either frozen in a glycerol stock for further biological assays or bacterial DNA was extracted for isolate identification and phylogenetic analyses.
Table 1.
Bacterial strain, location, host, 16S ribosomal DNA, gapA and luxA accession number. Strains in bold were isolated for this study
| Accession numbers | |||||
|---|---|---|---|---|---|
| Bacterial strains | Source | Location | 16S rRNA | gapA | luxA |
| Vibrio fischeri CG101 | Cleidopus gloriamaris | Australia | AY292939 | AY292958 | DQ026810 |
| Vibrio fischeri ES 114 | Euprymna scolopes | Hawaii | AY292919 | AF034845 | DQ026811 |
| Vibrio fischeri ES915 | Euprymna scolopes | Hawaii | AY292920 | AY292953 | DQ026812 |
| Vibrio fischeri ET101 | Euprymna tasmanica | Australia | AY292923 | AF034847 | DQ026813 |
| Vibrio fischeri MJ101 | Monocentrus japonicus | Japan | AY292946 | DQ026814 | |
| Vibrio fischeri SL518 | Sepiola ligulata | France | AY292950 | AY292962 | DQ026815 |
| Vibrio fischeri SR5 | Sepiola robusta | France | AY292926 | AF034851 | DQ026816 |
| Vibrio fischeri WH1 | Free-living | USA | AY292930 | AY292955 | DQ026817 |
| Photobacterium leiognathi LN101 | Uroteuthis noctiluca | Australia | AY292944 | AF034849 | DQ026808 |
| Photobacterium leiognathi RM1 | Rondeletiola minor | France | AY292947 | DQ026809 | |
| EHR1 | Euprymna hyllebergi | Thailand | AY332401 | ||
| LAR1 | Loliolus affinis | Thailand | AY332398 | ||
| EHP8 | Euprymna hyllebergi | Thailand | AY332400 | ||
| UCP6 | Uroteuthis chinensis | Thailand | AY332404 | ||
| UDP1 | Uroteuthis duvauceli | Thailand | AY332407 | ||
| Photobacterium phosphoreum ATCC 11040 | Chlorophthalmid fishes | AY341437 | AY341063 | ||
| Photobacterium leiognathi ATCC 25521 | Leiognathus splendens | Thailand | D25309 | M63594 | |
| Vibrio fischeri ET401 | Euprymna tasmanica | Australia | AY292943 | AY292960 | |
| Vibrio fischeri SA1G | Sepiola affinis | France | AY292924 | AF034848 | |
| Vibrio fischeri SI1D | Sepiola intermedia | France | AY292948 | AY292961 | |
| Vibrio logei ATCC15382 | Gadus macrocephalus | USA | AY292932 | AY292956 | |
| Vibrio logei SR181 | Sepiola robusta | France | AY292934 | AY292957 | |
| Vibrio anguillarum ATCC 19264 | Gadus morhua | Denmark | X16895 | ||
| Vibrio alginolyticus ATCC 17749 | Trachurus trachurus | Japan | X56576 | ||
| Vibrio campbellii ATCC 25920 | Seawater | X56575 | |||
| Vibrio harveyi EH701 | Euprymna hyllebergi | Thailand | AY292941 | ||
| Vibrio cholerae N16961 | Cholera patient | Bangladesh | AE004096 | AE004274 | |
| Vibrio fischeri EB12 | Euprymna berryi | Japan | AY292921 | AY292954 | |
| Vibrio fischeri EM17 | Euprymna morsei | Japan | AY292922 | AF034846 | |
| Vibrio fischeri ET301 | Euprymna tasmanica | Australia | AY292942 | AY292959 | |
| Vibrio vulnificus YJ016 | Clinical isolate | Taiwan | BA000037 | BA000037 | |
| Vibrio parahaemolyticus RIMD 2210633 | BA000031 | BA000031 | |||
| Vibrio salmonicida NCMB 2262 | Fanned Salmo salar | Norway | X70643 | AF452135 | |
| Vibrio pelagius ATCC 25916T | Succinate enriched seawater | X74722 | |||
| Salinivibrio costicola ATCC 33508T | Bacon curing brine | X74699 | |||
| Photobacterium damselae ATCC 33539T | Chromis punctipinnis | USA | X74700 | ||
| Escherichia coli W3110 | USA | AC_000091 | AP009048 | ||
| Photobacterium damselae damselae ATCC 33539 | Chromis punctipinnis | USA | AB032015 | ||
| Vibrio splendidus ATCC 33125 | Marine fish | X74724 | |||
| Vibrio lentus 40M4T CECT 5110 | Oysters | Spain | AJ278881 | ||
| Vibrio orientalis ATCC 33934 | Seawater | China | X74719 | ||
| Vibrio tasmaniensis LMG 20012 | Salmo salar | Australia | AJ316192 | ||
| Vibrio rumoiensis S 1FERM P-14531 | Fish product processing plant | Japan | AB013297 | ||
| Vibrio fischeri ES 191 | Euprymna scolopes | Hawaii | DQ026825 | DQ026819 | |
| Vibrio fischeri PP3 | Free-living | Hawaii | DQ026824 | DQ026818 | |
| Vibrio fischeri PP42 | Free-living | Hawaii | DQ026826 | DQ026820 | |
| Vibrio fischeri VLS2 | Free-living | Hawaii | DQ026827 | DQ026821 | |
| EHP1 | Euprymna hyllebergi | Thailand | DQ530284 | DQ530298 | |
| EHP2 | Euprymna hyllebergi | Thailand | DQ530285 | DQ520299 | |
| EHP3 | Euprymna hyllebergi | Thailand | DQ530286 | DQ520300 | |
| EHP4 | Euprymna hyllebergi | Thailand | DQ530287 | DQ520301 | |
| EHP5 | Euprymna hyllebergi | Thailand | DQ530288 | DQ520302 | |
| EHR2 | Euprymna hyllebergi | Thailand | DQ530289 | DQ520303 | |
| EHR3 | Euprymna hyllebergi | Thailand | DQ530290 | DQ520304 | |
| EHR4 | Euprymna hyllebergi | Thailand | AY332402 | DQ520305 | |
| LAR2 | Loliolus affinis | Thailand | DQ530291 | DQ520306 | |
| LAR3 | Loliolus affinis | Thailand | DQ530292 | DQ520307 | |
| LARS | Loliolus affinis | Thailand | DQ530293 | DQ520308 | |
| UCP4 | Uroteuthis chinensis | Thailand | DQ530294 | DQ520309 | |
| UCR4 | Uroteuthis chinensis | Thailand | DQ530295 | DQ520310 | |
| UCR6 | Uroteuthis chinensis | Thailand | DQ530296 | DQ520311 | |
| UCR7 | Uroteuthis chinensis | Thailand | DQ530297 | DQ520312 | |
| Vibrio harveyi ATCC 14126 | Amphipod Talorchestia sp. | USA | X74706 | AF147084 | |
ATCC: American type culture collection (USA)
LMG: Belgian Coordinated Collections of Microorganisms (BCCM). Division of Bacteria: BCCM/LMG (Belgium) CECT: Coleccion espanola de cultivos tipo (Spanish type culture collection) (Spain)
FERM: Patent and Bio-Resource Center (Japan)
NCMB: National Collections of Industrial and Marine Bacteria Ltd (United Kingdom)
RIMD: Research Institute for Microbial Diseases (Japan)
Determination of bacterial growth and light production
Samples of symbiotic strains were recovered from each stock by streaking onto SWT agar plates and growing for 16 h at 28 °C. One CFU from this culture was used to inoculate 5 mL of seawater tryptone (SWT) broth and grown for 16 h on a shaking incubator (250 r.p.m.) at the same temperature. Ten microliters of this culture was then re-inoculated in 5 mL of SWT broth and incubated for 3 h on a shaking incubator (250 r.p.m.) at 28 °C. The starting aliquot for inoculation of the growth flask containing 50 mL of SWT was taken from this culture. Optical density was measured at 600 nm to guarantee that all strains tested would have the same density at time T0 by adjusting the volume of each culture. Flasks were maintained in a shaking incubator (250 r.p.m.) at 28 °C for the entire 8- or 16-h period.
Measurements for growth and light production were obtained simultaneously every 30 min for both 8- and 16-h incubation assays. For growth curves, OD600 was determined using 1 mL of the initial culture transferred to a 1000 µL cuvette. Optical density was measured using an Uvikon XL spectrophotometer (Bio-tek Instruments, San Diego, CA, USA) and transferred to a PC using the Laboratory Power Junior 2.06 software. Sterile SWT broth was used as the blank.
For measurements of light emitted by cell suspensions(bioluminescence), 100 µL of the liquid culture was transferred to a 20 mL disposable scintillation vial and placed in a TD-20/20 luminometer (Turner Designs, Sunnyvale, CA, USA). Light production was measured in light units per milliliter and also indicated as luminescence per cell (luminescence per OD600). The volume of culture was reduced whenever light production exceeded the maximum reading allowed by the luminometer (9999 light units). Light production values were adjusted using dilution factors corresponding to each volume reduction.
Carbon source utilization for phylogenetic analysis
Strains from frozen stock were plated on SWT agar plates and grown overnight at 28 °C. Single colony forming units (CFU) of each strain were recovered after incubation and grown in 10 mL of SWT medium to an OD600 of 0.5 (Nishiguchi and Nair, 2003). Bacterial cells were harvested by centrifugation at 5000 r.p.m. (2655 g)for 10 min after being washed three times using 5 mL of PBS buffer (pH 7.3). The pellet was finally resuspended in 10 mL of PBS. A 100 µL aliquot of the cell suspension was added to each of the 96 wells of a GN2 Micro Plate™ (Biolog, Hayward, CA, USA) plate and incubated overnight at 28 °C. Carbon use was determined using an ELx800 absorbance microplate reader (Bio-Tek instruments, Winooski, VT, USA). Each strain was analyzed in duplicate plates. Biolog data were converted into binary information giving a value of 1 for positive results (i.e., use of carbon source) and 0 to the negative ones (i.e., carbon source not utilized). These phenotypic values were then converted into Hennig 86/Nona input data to be used in a combined analysis with the nucleotide data.
DNA extraction, amplification and sequencing of bacterial genes
To extract DNA from isolates, bacteria were grown overnight at 28 °C at 250 r.p.m. in 5 mL of SWT. 2 mL of each culture was centrifuged for one minute, and media removed. Bacterial DNA was isolated using the DNA easy kit (Qiagen Inc., Valencia, CA, USA) according to manufacturer’s instructions. One to 10 ng of template DNA was used for polymerase chain reaction (PCR) amplifications. For the 16S rRNA locus, four sets of primers (each amplifying approximately 420 bp) were used to determine the entire 16S rRNA sequence (~1600 bp). Primer sequences were obtained from Nishiguchi and Nair (2003) and are shown in Table 2. A single PCR reaction (50 µL) contained 2.5 mm of MgCl2, 0.5 mm dNTPs (25 µm each; Promega, Madison, WI, USA), 0.2 µm of forward and reverse primer, 10 × reaction buffer (10 mm Tris–HCl, pH 9.0, 50 mm KCl, and 0.1% Triton X-100) and 0.05 U/µL of Taq DNA Polymerase (Promega; Madison, WI), Amplitaq Gold® or AmpliTaq® DNA polymerase (Applied Biosystems, Foster City, CA, USA).
Table 2.
Oligonucleotide primers and PCR conditions used in this study
| Cycle | |||||||
|---|---|---|---|---|---|---|---|
| Primer name and amplified region |
Primer sequence |
||||||
| Cycles | Hot start | Denaturation | Annealing | Extension | Final extension | ||
| 16S rRNA** | IF 5′-AGA GTT TGA TCM TGG CTC AG-3′ | 94 °C | 94 °C | 49 °C | 72 °C | 72 °C | |
| 4R 5′-AGG CCT TCT TCA TAC ACG CG-3′ | 25 | 2 min | 15 s | 15 s | 15 s | 7 min | |
| 16S rRNA** | 2F 5′-GCA AGC CTG ATG CAG CCA TG-3′ | 94 °C | 94 °C | 49 °C | 72 °C | 72 °C | |
| 3R 5′-ATC GTT TAC GGC GTG GAC TA-3′ | 25 | 2 min | 15 s | 15 s | 15 s | 7 min | |
| 16S rRNA** | 3F 5′-AAA CAG GAT TAG ATA CCC TG-3′ | 94 °C | 94 °C | 49 °C | 72 °C | 72 °C | |
| 2R 5′-CTG GTC GTA AGG GCC ATG AT-3′ | 25 | 2 min | 15 s | 15 s | 15 s | 7 min | |
| 16S rRNA** | 4F 5′-AGG TGG GGA TGA CGT CAA GT-3′ | 94 °C | 94 °C | 49 °C | 72 °C | 72 °C | |
| 1R 5′-AAG GAG GTG WTC CAR CC-3′ | 25 | 2 min | 15 s | 15 s | 15 s | 7 min | |
| luxA-specific* | F 5′-GTTCTTAGTTGGATTATTGG-3’ | 94 °C | 94 °C | 44 °C | 72 °C | 72 °C | |
| Positions 568–995 | R 5′-TCAGTTCCCATTAGCTTCAAATCC-3’ | 29–33 | 2 min | 15 s | 30 s | 30 s | 7 min |
| 1st luxA† | F 5′-CAGAATCACCAAAAAGGAATAGGT-3’ | 30 | 94 °C | 94 °C | 55 °C | 72 °C | 72 °C |
| Positions 1–590 | R 5′-CAATTTCATTATAGAGTTCCATCTGTG-3’ | 2 min | 15 s | 30 s | 30 s | 7 min | |
| 2nd luxA† | F 5′-GTTCTTAGTTGGATTATTGGTA-3’ | 26–30 | 94 °C | 94 °C | 50 °C | 72 °C | 72 °C |
| Positions 450–1068 | R 5′-AACTTGTTCCTTATTATCTCTAGTAT-3’ | 2 min | 15 s | 30 s | 30 s | 7 min | |
| luxA 1–569† | F 5′-GCGAAGATTAAGAAATAAAA-3’ | 25–30 | 94 °C | 94 °C | 47 °C | 72 °C | 72 °C |
| Positions 1–569 | R 5′-TTCTTTAGAGTACTTTGTTGG-3’ | 2 min | 15 s | 30 s | 30 s | 7 min | |
| luxA 400–1168† | F 5′-ATTTAATTTTGGTGTTGTTA-3’ | 25–30 | 94 °C | 94 °C | 43 °C | 72 °C | 72 °C |
| Positions (400–1168) | R 5′-TTATTTTTGAGGTTCTTTTA-3’ | 2 min | 15 s | 30 s | 30 s | 7 min | |
| luxA 401–11686 | F 5′-TTTAATTTTGGTGTTGTT-3’ | 25–30 | 94 °C | 94 °C | 43 °C | 72 °C | 72 °C |
| Positions 401–1168 | R 5′-TTATTTTTGAGGTTCTTTT-3’ | 2 min | 15 s | 30 s | 30 s | 7 min | |
| luxA ext† | F 5′-AATTTATTTAGGTTCTTTTAAG-3’ | 28 | 94 °C | 94 °C | 42.5 °C | 72 °C | 72 °C |
| R 5′-AAYATTTRTTTTTCGTATCA-3’ | 2 min | 15 sec | 30 s | 30 s | 7 min | ||
| luxA intl† | F 5′-CTATAATCAACACGTCGATTS-3’ | 28 | 94 °C | 94 °C | 48.4 °C | 72 °C | 72 °C |
| R 5′-CAATGGTWCTTAGTTGGATTAT-3’ | 2 min | 15 s | 30 s | 30 s | 7 min | ||
| luxA int2† | F 5′-CCATYTGTGYTTTTTTYTCATT-3’ | 28 | 94 °C | 94 °C | 48.5 °C | 72 °C | 72 °C |
| R 5′-KTTTGATACATATTGGACSTT-3’ | 2 min | 15 s | 30 sec | 30 s | 7 min | ||
| luxA int3† | F 5′-TGATAATCRTAACCWCGAGT-3’ | 28 | 94 °C | 94 °C | 47.5 °C | 72 °C | 72 °C |
| R 5′-AAGGTCGWTTTAATTTTGG-3’ | 2 min | 15 s | 30 s | 30 s | 7 min | ||
| Pleiogleiog A‡ | F 5′-GTTTAAGATCAACTGTCTAAAGGRCG-3’ | 25–30 | 94 °C | 94 °C | 56 °C | 72 °C | 72 °C |
| R 5′-TCAGAACCATTCGCTTCAAATCCAAC-3’ | 2 min | 15 s | 30 s | 30 s | 7 min | ||
| Pleiogleiog AB‡ | F 5′-CGTCGCCGACTTGATTACAGTAACG-3’ | 25–30 | 94 °C | 94 °C | 60 °C | 72 °C | 72 °C |
| R 5′-ATCCTTTTCTTGCTGCCCATTCAACC-3’ | 2 min | 15 s | 30 s | 30 s | 7 min | ||
| Pphosp A‡ | F 5′-CTTTTAGATCCAATGTCAAAAGGCCG-3’ | 25–30 | 94 °C | 94 °C | 58 °C | 72 °C | 72 °C |
| R 5′-TCAGAGCCATTTGCTTCGAAACCAAG-3’ | 2 min | 15 s | 30 s | 30 s | 7 min | ||
| Pphosp AB‡ | F 5′-AAACGTCGTGTTGATTATAGCAACG-3’ | 25–30 | 94 °C | 94 °C | 55.4 °C | 72 °C | 72 °C |
| R 5′-TCCAACGATATGTTAGTGGAAGC-3’ | 2 min | 15 s | 30 s | 30 s | 7 min | ||
| luxAl27§ | F 5′-GANCANCANTTNACNGAGTTTGG-3’ | 30 | 94 °C | 94 °C | 58 °C | 72 °C | 72 °C |
| R 5′-ATTTCNTCTTCAGNNCCATTNGCTTCAAANCC-3’ | 5 min | 30 s | 30 s | 90 s | 7 min | ||
| luxAf Wimpee¶ | F 5′-CTACTGGATCAAATGTCAAAAGGA-3’ | 30 | 94 °C | 94 °C | 56 °C | 72 °C | 72 °C |
| R 5′-TCAGAACCGTTTGCTTCAAAACC-3’ | 5 min | 1.5 min | 30s | 2 min | 7 min | ||
| gapA** | F 5′-GTG TAC TTC GAG CGT TAT AC-3′ | 29 | 95 °C | 95 °C | 40 °C | 70 °C | 70 °C |
| 5′-GCC CAT TAC TCA CCC TTG TT-3′ | 5 min | 15 s | 15 s | 1 min | 7 min | ||
Oligonucleotide primers designed in this study.
Modified from Budsberg et al., 2003.
The luxA gene was used as a second molecular marker for additional phylogenetic analyses. A group of primers was used for the amplification of this locus that codes for the α-subunit of luciferase. PCR protocol and conditions for each of these sets of primers are shown in Table 2. PCR products were cleaned with Gene Clean II DNA purification kit (Q-biogene, Irvine, CA, USA).
gapA is a gene that codes for the glyceraldehyde phosphate dehydrogenase enzyme, and was used as the third phylogenetic marker in the analysis. For its amplification, primers gapA F and gapA R were used. Primer sequences and conditions for amplification are shown in Table 2.
PCR products were sequenced using Applied Biosystems Big Dye© (v.3.1). Excess fluorescently labeled dNTPs were removed by spin column or plate (Edge Biosystems, Gaithersburg, MD, USA) according to manufacturer instructions. Samples were sequenced in an Applied Biosystems 3100 automated capillary sequencer. Independent forward and reverse sequences obtained were combined and edited using Sequencher v.4.6. (Gene Codes™, Ann Arbor, MI, USA). Edited sequences were imported to BBEdit v.7.0.3 and converted into UNIX format to be revised, prealigned, and compared through the Genetic Data Environment v.2.2. (Smith et al., 1994).
Phylogenetic analysis
Sequences were analyzed using the direct optimization method described by Wheeler (Wheeler, 1996, 1998) and implemented in the computer program POY (Wheeler, 1995; Wheeler et al., 2002). The method assesses the number of transformations in DNA sequences required by a phylogenetic topology without using pair-wise optimization through multiple sequence alignment. POY treats insertions and deletions as processes, which place it apart from multiple sequence alignment (Wheeler, 1998).
Several analyses were implemented with character transformations weighted differently to determine how different phylogenetic hypotheses were affected (sensitivity analysis sensu Wheeler, 1995). Final analysis was performed with characters equally weighted, using a molecular matrix in which a value of 1 was given to each transition, transversion, insertion, deletion and gap. Combined phylogenetic analysis produced a total of 54 875 156 trees examined in TBR after the initial construction of a total of 3948 trees. In total, there were 54 879 104 trees examined in all branch swapping strategies. This process took a total of 102.8 h. A total of 241 838 974 alignments were performed by direct optimization during this time.
Tree search was implemented by TBR (tree bisection and reconnection) branch-swapping on the best of 100 random addition replicates, holding 1000 trees per round and performing one round of tree-fusing (Goloboff, 1999). At the same time, the command “exact” was used to guarantee a more accurate calculation of tree costs for direct optimization on the downpass; it also performs a complete Sankoff optimization (Sankoff and Rousseau, 1975). Binary trees were plotted in TreeView 0.4.1 and the consensus tree was calculated using PAUP*4.0.10 (Swofford, 2002). Analysis was executed in the NetBSD biology computer system at New Mexico State University.
All newly sequenced 16S rRNA, gapA and luxA loci have been deposited in GenBank with the accession numbers shown in Table 1 (strains isolated from this study are in bold type in Table 1). Previously determined gene sequences were obtained from GenBank.
Results and discussion
Quantification of bacterial numbers of loliginid and sepiolid light organ symbionts from Thailand
Colonies isolated from light organ homogenates of either Uroteuthis, Loliolus or Euprymna hosts were luminescent on SWT plates. In average, 10 light organs were extracted from this location in Thailand and used for strain isolation. A total of 40 isolates from each host specimen were extracted, with one to five isolates randomly selected for analyses (Table 1). Colony forming units (CFUs) from each light organ homogenate were enumerated to calculate the total number of CFUs present in each squid light organ. Adult light organs from both U. chinensis and U. duvauceli ranged from 1011 to 1012 CFU/light organ, whereas E. hyllebergi light organs contained approximately 109–1010 CFU/light organ.
Bacterial growth and light production
Results of the luminescence assays are shown in Table 3. Growth and light production clearly demonstrated that peak luminescence (the point of time in which the amount of light units per milliliter reaches its maximum), coincides in most cases with a period at which bacteria are in stationary phase. Strains in Table 3 showed a unique peak of maximum luminescence occurring in culture. From this group of strains, symbionts from Euprymna (ES114 and ET101) exhibited the lowest light production, registering between 0.045 and 0.6 light units per milliliter. Strains PP3 and PP42, both free-living V. fischeri strains from Hawaii, emitted their peak luminescence simultaneously at 330 min, but their intensities varied slightly, with PP3 higher (~2 light units per milliliter). It is noticeable that strains with the lowest light production grouped together in the combined phylogenetic tree (Fig. 1), in a clade containing other sepiolid squid symbionts, such as V. fischeri ES191, ES915 and EM17.
Table 3.
Light emission of bacterial isolates
| Bacterial isolate |
Maximum luminescence (light units/ mL) |
Bacterial isolate luminescence (luminescence/ cell) |
Time (min) of maximum luminescence |
|---|---|---|---|
| Vibrio fischeri ES114 | 0.045 | 0.023 | 600 |
| Vibrio fischeri ET101 | 0.59 | 0.52 | 420 |
| Vibrio harveyi EH701 | 3490 | 7759.6 | 420 |
| Vibrio fischeri WH1 | 159.1 | 158.9 | 480 |
| Vibrio fischeri CG101 | 4.25 | 3.24 | 540 |
| Vibrio fischeri VLS2 | 136.9 | 91.6 | 450 |
| Photobacterium leiognathi RM1 | 123.4 | 227.1 | 300 |
| Vibrio fischeri PP3 | 2.05 | 1.39 | 330 |
| Vibrio fischeri PP42 | 0.27 | 0.17 | 330 |
| UCP1 | 119.8 | 68.25 | 420 |
| UCR1 | 134.9 | 91.74 | 300 |
| UCR4 | 195.2 | 107.4 | 240 |
| UCR6 | 3011 | 1347.6 | 420 |
| UCR7 | 9998 | 4489.3 | 270 |
| EHR1 | 44.14 | 19.48 | 300 |
| EHR2 | 16.13 | 7.61 | 270 |
| EHR3 | 29.1 | 13.06 | 330 |
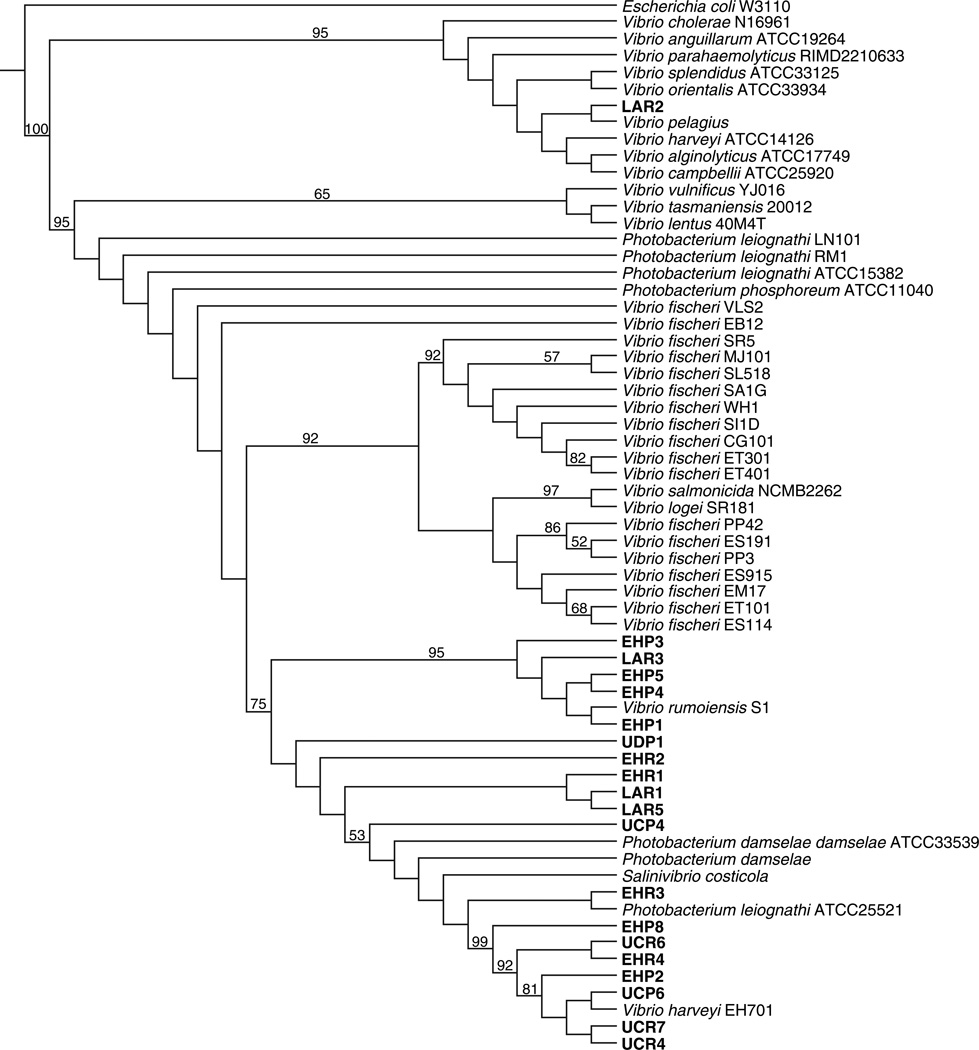
Fig. 1.
Phylogenetic tree of Vibrionaceae species and isolates, based on DNA sequences of three loci and 95 biochemical characters. The tree was obtained with insertions, deletions, transitions and transversions equally weighed. Jackknife percentages of more than 50% are shown as numbers on nodes. Strains isolated from this study are in bold.
The remaining free-living strains VLS2 and WH1 exhibited similar values of light production (Table 3). Both strains produced peak luminescence after 450 min and production of light was similar: 140 and 160 light units per milliliter for VLS2 and WH1, respectively.
Even though UCP1 and UCR1 displayed relatively similar luminescence, their peak luminescence occurred at different times during growth. UCP1 isolated from Uroteuthis chinensis from Phuket, emitted its peak luminescence at 420 min, whereas UCR1, also from the same squid species but collected in a different location (Rayong), emitted higher luminescence at 300 min. UCR4, an isolate from U. chinensis (Rayong), displayed 195.2 light units/mL (specific luminescence of 107.4). This value is close in range to ones emitted by other isolates from the same host, but peaked earlier in the assay. Also, light production values from Thailand strains were comparatively higher than levels exhibited by symbiotic V. fischeri strains ES114, ET101, CG101 and free-living V. fischeri strains PP3 and PP42. High values of light produced by VLS2 are due to the very luminous phenotype of this V. fischeri strain isolated from Hawaii (Lee and Ruby, 1995).
Thailand strains showing high levels of bioluminescence are found forming a group that also includes Vibrio harveyi EH701. Previous research has shown that luminescence in V. harveyi strain B392 reaches a higher intensity than V. fischeri strain ATCC 7744 (Meighen, 1999). These results indicate that Thailand strains are comparable with V. harveyi in that their luminescence is greater than V. fischeri. Measurements of light intensity from this study may indicate that Thailand symbiotic strains are more closely related to V. harveyi than to V. fischeri, which is confirmed by the phylogenetic data (see next section) in Fig. 1.
Combined phylogenetic analysis of 16S rRNA, gapA, luxA sequences and biological data
Using several loci and combining molecular information with biochemical attributes has been described as an appropriate approach to understanding relationships among members of a group of taxa (Giribet and Wheeler, 2002; Nishiguchi and Nair, 2003). By exploring our data set using a sensitivity analysis it was possible to generate hypotheses of relationships and at the same time evaluate their stability.
Direct optimization produces more parsimonious and congruent explanations of sequence variation than multiple sequence alignment (Giribet et al., 2001). Without the use of multiple sequence alignment, the method assesses the number of transformations in DNA sequence required by a phylogenetic topology (Giribet and Wheeler, 2002). Therefore, it is clear that the most parsimonious hypothesis is represented by the phylogeny reported here.
The most parsimonious tree (Fig. 1) produced from the analyses using all parameter sets that minimized overall incongruence among the 16S rRNA, gapA, luxA genes and phenotypic data, demonstrates that the clade containing symbiotic Vibrio fischeri is not monophyletic. A number of internal clades contain symbiotic isolates, irrespective of host or geographic location, although support for some internal nodes is below the 50% threshold. This group also includes free-living bacteria, as well as both fish and squid symbionts, indicating that symbiosis in these V. fischeri strains has arisen multiple times. Vibrio salmonicida and V. logei SR181 group in a single clade for both 16S rRNA analysis (data not shown) and the combined phylogenetic analysis (Fig. 1). Previous molecular and biochemical data (Fidopiastis et al., 1998; Nishiguchi and Nair, 2003) has demonstrated that V. salmonicida is a close relative (and possible sibling species) to V. logei, which provides additional evidence that these species of bacteria may share a common ancestor that was psychrophilic in nature.
Our analysis also resolved the placement of V. fischeri fish isolates CG101 and MJ101 within a more uniform group with strains from France (SR5 and SL518) and Australia (ET401 and ET301) as well as the free-living strain WH1. Interestingly, symbiotic strain LAR2, isolated from Loliolus affinis (Loliginidae), did not group with clades containing the remaining squid strains. This clade contains only Vibrio species, including recently described species found either associated with or causing disease to marine organisms (Banatvala et al., 1997; Thompson et al., 2001, 2003). Additionally the node was well supported, with a jackknife value of 95% (Fig. 1). Species V. vulnificus, V. tasmaniensis and V. lentus also grouped in one clade with 65% support. Thompson et al. (2004) reported a close relationship between V. lentus and V. tasmaniensis, which coincides with the phylogenetic hypotheses presented here that these species are sister taxa.
All Thailand strains isolated from either Uroteuthis, Loliola or Euprymna squid hosts (with the aforementioned exception of isolate LAR2) grouped in one separate clade with Vibrio harveyi EH701 (previously described by Nishiguchi and Nair, 2003), Vibrio rumoiensis, some Photobacterium species and Salinivibrio costicola (Fig. 1). This result suggests that Thailand molecular variants, found colonizing light organs in loliginid and sepiolid squid are neither Vibrio fischeri nor Vibrio logei, which are the bacterial species commonly found as light organ symbionts. This is a relationship not previously reported in squid.
Our combined analysis produced a phylogeny that was able to resolve Thailand isolates with a relatively high bootstrap value (75%). Within this clade, there is one node (95% jackknife support), containing isolates EHP3, LAR3, EHP5, EHP4 and EHP1, which includes the recently described species Vibrio rumoiensis (Yumoto et al., 1999). This facultative psychrophilic bacterium is known to exhibit high catalase activity, which may explain why Thailand squid symbionts exhibit a closer relationship to identified strains of V. rumoiensis. Previous studies with E. scolopes light organs have demonstrated high levels of halide peroxidase present in this organ (Small and McFall-Ngai, 1998). This enzyme produces potent microbicides in response to pathogens by generating hypohalous acids from hydrogen peroxide. In bacteria, catalase is used to detoxify any host generated hydrogen peroxide (Ruby and McFall-Ngai, 1999) therefore giving an enormous ecological advantage to those bacteria exhibiting high catalase activity in the light organ. Production of this enzyme would allow bacteria to combat any immunological response, and successfully colonize light organ tissue. Any host-generated hydrogen peroxide would therefore be neutralized before it has the chance to enter the bacterium. Ecological variants related to V. rumoiensis and possibly sharing this beneficial defensive phenotype will easily colonize squid light organs.
With respect to isolates UDP1, EHR2, EHR1, LAR1, LAR5 and UCP4, there is no clear resolution in the phylogenetic tree. However, they are clearly part of the clade containing the Thailand isolates, which separate them from the V. fischeri group.
In addition, Vibrio harveyi EH701 is also included in the Thailand clade, indicating a possible relationship between luminescent symbionts and V. harveyi. V. harveyi has been found to produce much higher luminescence in culture than V. fischeri (Meighen, 1999), which may increase its ecological advantage over potential but low light producing symbionts. V. harveyi has been recognized as a cause of disease in marine invertebrates, particularly crustaceans used for aquacultural farms. Similarly, a V. harveyi-like organism, classified as Vibrio carchariae has been isolated from sharks (Austin and Zhang, 2006). Thus, V. harveyi is classified as a pathogen of invertebrates and vertebrates. Furthermore, Pizzutto and Hirst (1995) reported the high diversity within the species by genetic fingerprinting and protein profiling in an attempt to differentiate virulent from avirulent strains of V. harveyi. The authors concluded that virulence and phylogenetic history are not associated, which led them to consider that virulence was acquired by association with mobile genetic elements, such as plasmids or transposons. It is then possible that the Vibrionaceae strains found colonizing light organs in Thai squid correspond to a non-virulent form of V. harveyi with the conserved ecological advantage of being highly luminescent.
Within this clade other species such as Salinivibrio costicola, Photobacterium damselae, and Photobacterium leioghathi are also found, supporting previous tested hypotheses proposed by Kita-Tsukamoto et al. (1993), Alsina and Blanch (1994) and Ruimy et al. (1994).
Of importance to this study is the fact that isolates obtained from a common host found in the same geographic area are embedded within a large clade that contains a variety of bacterial species with characteristics that make them excellent candidates as bacterial symbionts. However, strains isolated from a particular squid host are not found to be phylogenetically related to a unique bacterial species. This result indicates a possible non-specific association between Thai squid with marine luminescent bacteria. The results presented here clearly suggest that symbioses between Vibrionaceae bacteria and their squid hosts are family specific and not strain specific. This is in contrast to earlier studies of sepiolid squid symbionts (Nishiguchi et al., 1998), where parallel cladogenesis occurred between native strains and their host squid. More recently, better taxon sampling among strains within and between populations of host squid has provided a more complex picture of Vibrio-sepiolid squid dynamics than previously thought (Jones et al., 2006). Introgression of shared haplotypes among strains isolated from different host species seems to be more common than previously thought, with genetic breaks observed at locations where abiotic factors (i.e., temperature) seem to be a major influence. As sepiolid and loliginid squid in Thailand are colonized by more than one species from the Vibrionaceae, this suggests that specificity for genera of this family still exists, but is influenced by additional factors besides host species. Future studies to determine whether homologous genes are co-opted for similar functions between these bacteria will help determine the level of specificity that is required for symbiosis within the Vibrionaceae, and whether abiotic or biotic factors have an effect for determining species level colonization in this environmentally transmitted association.
Acknowledgments
The authors would like to thank Dr Jaruwat Nabhitabhata, Dr Renu Yashiro, Pitiporn Nilaphat, and Pichitra Promboon from the Rayong Coastal Aquaculture Station for help collecting in Rayong, Thailand, and Dr Praulai Nootmorn and her students from the Andaman Sea Fisheries Development Center for help collecting in Phuket, Thailand. We would also like to thank G. Giribet for his advice with the phylogenetic analyses. William Soto, Serena Rogers, Latoya Dale and Clayton Gorman contributed with sequencing bacterial loci. Special thanks to Co Thai Ngo for her assistance with the NMSU cluster. This work was supported in part by NSF DEB-01316516, DBI-0079820 and SBE-123690, and NIH S06-GM08136-26 to M.K.N.
References
- Alsina M, Blanch AR. Improvement and update of a set of keys for biochemical identification of Vibrio species. J. Appl. Bacteriol. 1994;77:719–721. doi: 10.1111/j.1365-2672.1994.tb04419.x. [DOI] [PubMed] [Google Scholar]
- Ast JC, Dunlap PV. Phylogenetic analysis of the lux operon distinguishes two evolutionarily distinct clades of Photobacterium leiognathi. Arch. Microbiol. 2004;181:352–361. doi: 10.1007/s00203-004-0663-7. [DOI] [PubMed] [Google Scholar]
- Austin B, Zhang XH. Vibrio harveyi: a significant pathogen of marine vertebrates and invertebrates. Lett. Appl. Microbiol. 2006;43:119–124. doi: 10.1111/j.1472-765X.2006.01989.x. [DOI] [PubMed] [Google Scholar]
- Banatvala N, Hlady WG, Ray BJ, McFarland LM, Thompson S, Tauxe RV. Vibrio vulnificus infection reporting on death certificates: the invisible impact of an often fatal infection. Epidemiol. Infect. 1997;118:221–225. doi: 10.1017/s0950268897007425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budsberg KJ, Wimpee CF, Braddock JF. Isolation and identification of Photobacterium phosphoreum from an unexpected niche: migrating salmon. Appl. Environ. Microbiol. 2003;69:6938–6942. doi: 10.1128/AEM.69.11.6938-6942.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidopiastis PM, Boletzky SV, Ruby EG. A new niche for Vibrio logei, the predominant light organ symbiont of squids in the genus Sepiola. J. Bacteriol. 1998;180:59–64. doi: 10.1128/jb.180.1.59-64.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster JS, Boletzky SV, McFall-Ngai MJ. A comparison of the light organ development of Sepiola robusta Naef and Euprymna scolopes Berry (Cephalopoda: Sepiolidae) Bull. Mar. Sci. 2002;70:141–153. [Google Scholar]
- Giribet G, Wheeler WC. On bivalve phylogeny: a high-level analysis of the Bivalvia (Mollusca) based on combined morphology and DNA sequence data. Invert. Zool. 2002;121:271–324. [Google Scholar]
- Giribet G, Edgecombe GD, Wheeler WC. Arthropod phylogeny based on eight molecular loci and morphology. Nature. 2001;413:157–161. doi: 10.1038/35093097. [DOI] [PubMed] [Google Scholar]
- Goloboff PA. Analyzing large data sets in reasonable times: solutions for composite optima. Cladistics. 1999;15:415–428. doi: 10.1111/j.1096-0031.1999.tb00278.x. [DOI] [PubMed] [Google Scholar]
- Jones BW, Nishiguchi MK. Counterillumination in the bobtail squid, Euprymna scolopes. Mar. Biol. 2004;144:1151–1155. [Google Scholar]
- Jones BW, Lopez JE, Huttenburg J, Nishiguchi MK. Population structure between environmentally transmitted vibrios and bobtail squids using nested clade analysis. Mol. Ecol. 2006;15:4317–4329. doi: 10.1111/j.1365-294X.2006.03073.x. [DOI] [PubMed] [Google Scholar]
- Kita-Tsukamoto K, Oyaizu H, Nanba K, Simidu U. Phylogenetic relationships of marine bacteria, mainly members of the family Vibrionaceae, determined on the basis of 16S rRNA sequences. Int. J. Syst. Bacteriol. 1993;43:8–19. doi: 10.1099/00207713-43-1-8. [DOI] [PubMed] [Google Scholar]
- Lee K-H, Ruby EG. Symbiotic role of the viable but nonculturable state of Vibrio fischeri in Hawaiian coastal seawater. Appl. Environ. Microbiol. 1995;61:278–283. doi: 10.1128/aem.61.1.278-283.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFall-Ngai MJ, Montgomery MK. The anatomy and morphology of the adult bacterial light organ of Euprymna scolopes Berry (Cephalopoda: Sepiolidae) Biol. Bull. 1990;179:332–339. doi: 10.2307/1542325. [DOI] [PubMed] [Google Scholar]
- Meighen EA. Autoinduction of light emission in different species of bioluminescent bacteria. Luminescence. 1999;14:3–9. doi: 10.1002/(SICI)1522-7243(199901/02)14:1<3::AID-BIO507>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Naef A. Teuthologische Notizen. 2. Gattungen Sepioliden. Zool. Anzeiger. 1912a;39:244–248. [Google Scholar]
- Naef A. Teuthologische notizen. 3. Die Arten der Gattungen Sepiola und Sepietta. Zool. Anzeiger. 1912b;39:262–271. [Google Scholar]
- Nesis KN. Cephalopods of the World. Neptune City, NJ: T.F.H. Publications; 1982. [Google Scholar]
- Nishiguchi MK. Host recognition is responsible for symbiont composition in environmentally transmitted symbiosis. Microbiol. Ecol. 2002;44:10–18. doi: 10.1007/BF03036870. [DOI] [PubMed] [Google Scholar]
- Nishiguchi MK, Nair VS. Evolution of symbioses in the Vibrionaceae: a combined approach using molecules and physiology. Int. J. Syst. Evol. Microbiol. 2003;53:2019–2026. doi: 10.1099/ijs.0.02792-0. [DOI] [PubMed] [Google Scholar]
- Nishiguchi MK, Lopez JE, Boletzky SV. Enlightenment of old ideas from new investigations: The evolution of cephalopod bacteriogenic light organs. Evol. Dev. 2004;6:41–49. doi: 10.1111/j.1525-142x.2004.04009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiguchi MK, Ruby EG, McFall-Ngai MJ. Competitive dominance among strains of luminous bacteria provides an unusual form of evidence for parallel evolution in sepiolid squid-Vibrio symbiases. Appl. Environ. Microbiol. 1998;64:3209–3213. doi: 10.1128/aem.64.9.3209-3213.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzutto M, Hirst RG. Classification of isolates of Vibrio harveyi virulent to Penaeus monodon larvae by protein profile analysis and M13 DNA fingerprinting. Dis. Aquat. Organ. 1995;21:61–68. [Google Scholar]
- Ruby EG, Asato LM. Growth and flagellation of Vibrio fischeri during initiation of the sepiolid squid light organ symbiosis. Arch. Microbiol. 1993;159:160–167. doi: 10.1007/BF00250277. [DOI] [PubMed] [Google Scholar]
- Ruby EG, McFall-Ngai MJ. Oxygen-utilizing reactions and symbiotic colonization of the squid light organ by Vibrio fischeri. Trends Microbiol. 1999;7:414–420. doi: 10.1016/s0966-842x(99)01588-7. [DOI] [PubMed] [Google Scholar]
- Ruimy R, Breittmayer V, Elbaze P, Lafay B, Boussemart O, Gauthier M, Christen R. Phylogenetic analysis and assessment of the genera Vibrio, Photobacterium, Aeromonas, and Plesiomonas deduced from small-subunit rRNA sequences. Int. J. Syst. Bacteriol. 1994;44:416–426. doi: 10.1099/00207713-44-3-416. [DOI] [PubMed] [Google Scholar]
- Sankoff D, Rousseau P. Locating the vertices of a Steiner tree in arbitrary space. Math. Program. 1975;9:240–246. [Google Scholar]
- Small AL, McFall-Ngai MJ. A halide peroxidase in tissues interacting with bacteria in the squids Euprymna scolopes. J. Cell. Biochem. 1998;72:445–457. [PubMed] [Google Scholar]
- Smith SW, Overbeek R, Woese CR, Gilbert W, Gillevet PM. The genetic data environment an expandable GUI for multiple sequence analysis. Comput. Appl. Biosci. 1994;10:671–675. doi: 10.1093/bioinformatics/10.6.671. [DOI] [PubMed] [Google Scholar]
- Swofford DL. PAUP* Phylogenetic Analysis Using Parsimony (*and other methods), Version 4.08b8. Sunderland, MA: Sinauer Associates; 2002. [Google Scholar]
- Thompson CC, Thompson FL, Vandemeulebroecke K, Hoste B, Dawyndt P, Swings J. Use of recA as an alternative phylogenetic marker in the family Vibrionaceae. Int. J. Syst. Evol Microbiol. 2004;54:919–924. doi: 10.1099/ijs.0.02963-0. [DOI] [PubMed] [Google Scholar]
- Thompson FL, Hoste B, Vandemeulebroecke K, Swings J. Genomic diversity amongst Vibrio isolates from different sources determined by fluorescent amplified fragment length polymorphism. Syst. Appl. Microbiol. 2001;24:520–538. doi: 10.1078/0723-2020-00067. [DOI] [PubMed] [Google Scholar]
- Thompson FL, Thompson CC, Vicente AC, Theophilo GN, Hofer E, Swings J. Genomic diversity of clinical and environmental Vibrio cholerae strains isolated in Brazil between 1991 and 2001 as revealed by fluorescent amplified fragment length polymorphism analysis. J. Clin. Microbiol. 2003;41:1946–1950. doi: 10.1128/JCM.41.5.1946-1950.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visick KL, Foster JS, Doino JA, McFall-Ngai MJ, Ruby EG. Vibrio fischeri lux genes play an important role in colonization and development of the host light organ. J. Bacteriol. 2000;182:4578–4586. doi: 10.1128/jb.182.16.4578-4586.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler WC. Sequence alignment, parameter sensitivity, and the phylogenetic analysis of molecular data. Syst. Biol. 1995;44:321–331. [Google Scholar]
- Wheeler WC. Optimization alignment: the end of multiple sequence alignment in phylogenetics? Cladistics. 1996;12:1–9. [Google Scholar]
- Wheeler WC. Alignment characters, dynamic programming and heuristic solutions. In: DeSalle R, Schierwater B, editors. Alignment Characters, Dynamic Programming and Heuristic Solutions. Basel: Birkhauser-Verlag; 1998. pp. 243–251. [Google Scholar]
- Wheeler WC, Gladstein D, DeLaet J. POY v. 3.0. New York: American Museum of Natural History; 2002. [Google Scholar]
- Wimpee CF, Nadeau TL, Nealson KH. Development of species-specific hybridization probes for marine luminous bacteria by using in vitro DNA amplification. Appl. Environ. Microbiol. 1991;57:1319–1324. doi: 10.1128/aem.57.5.1319-1324.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RE, Kampa EM, Maynard SD, Mencher FM, Roper CFE. Counterillumination and the upper depth limits of midwater animals. Deep Sea Res. 1980;27A:671–691. [Google Scholar]
- Young RE, Roper CFE. Intensity regulation of bioluminescence during countershading in living midwater animals. Fish. Bull. 1977;75:239–252. [Google Scholar]
- Yumoto II, Iwata H, Sawabe T, Ueno K, Ichise N, Matsuyama H, Okuyama H, Kawasaki K. Characterization of a facultatively psychrophilic bacterium, Vibrio rumoiensis sp. nov., that exhibits high catalase activity. Appl. Environ. Microbiol. 1999;65:67–72. doi: 10.1128/aem.65.1.67-72.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]