Abstract
Cognitive and memory deficits can be caused or exacerbated by dietary folate deficiency, which has been combatted by the addition of folate to grains and dietary supplements. The recommended dose of the B9 vitamin folate is 400 μg/day for adolescents and non-pregnant adults, and consumption above the recommended daily allowance is not considered to be detrimental. However, the effects of excess folate have not been tested in adolescence when neuro and endocrine development suggest possible vulnerability to long-term cognitive effects. We administered folate-supplemented (8.0 mg folic acid/kg diet) or control lab chow (2.7 mg folic acid/kg diet) to rats ad libitum from 30 to 60 days of age, and subsequently tested their motivation and learning and memory in the Morris water maze. We found that folate-supplemented animals had deficits in motivation and spatial memory, but they showed no changes of the learning- and memory-related molecules growth-associated protein-43 or Gs-α subunit protein in the hippocampus. They had decreased levels of thyroxine (T4) and triiodothyronine (T3) in the periphery and decreased protein levels of thyroid receptor-α1 and -α2 (TRα1 and TRα2) in the hippocampus. The latter may have been due to an observed increase of cytosine–phosphate–guanosine island methylation within the putative thyroid hormone receptor-α promoter, which we have mapped for the first time in the rat. Overall, folate supplementation in adolescence led to motivational and spatial memory deficits that may have been mediated by suppressed thyroid hormone function in the periphery and hippocampus.
Keywords: DNA methylation, folate, hippocampus, memory, motivation, thyroid hormone
Methyl group availability is important for many cellular functions of neurons, including DNA methylation and gene expression control, nucleic acid synthesis and repair and protein metabolism. Dietary vitamins including vitamins B-6, B-9, B-12 and choline are critical in methyl group transfer and availability. As a precaution, folate (vitamin B-9) supplementation has been recommended for pregnant women, as this simple intervention greatly reduces the incidence of neural tube defects in the offspring (Blencowe et al. 2010). Some evidence suggests that when given to adults, folate also prevents neurodegeneration (Zhuo & Pratico 2010), improves cognition, and ameliorates depressive symptoms (Beydoun et al. 2010; Brocardo et al. 2009; Kronenberg et al. 2009; de Lau et al. 2007; Taylor & Geddes 2004). It is estimated that between 33% and 67% of the population in USA takes folate supplements regularly (Smith et al. 2008). However, conclusions regarding the effectiveness of folate supplementation are often drawn from correlational evidence. Experimental studies directly evaluating its effects are few (Balk et al. 2007; Bhat 2009), with some reporting that folate has no cognitive benefit in humans (Nelson et al. 2009).
The methylation status of cytosine–phosphate–guanosine (CpG) islands can be altered by diet, suggesting that excess intake of folate could cause detrimental epigenetic changes (Smith et al. 2008). A recent study of the fetal and neonatal growth-related gene IGF-2 found that maternal folic acid supplementation in pregnancy was correlated with 4.5% higher methylation in a regulatory region of the gene along with lower birthweight (Steegers-Theunissen et al. 2009). This study suggests that in addition to the known benefits of folate in pregnancy, other adverse effects could occur that affect fetal physiology. Although it is less well studied, adolescence is another developmental period that may be vulnerable to elevated folate levels. Neural circuitry underlying working memory is recruited differently in early vs. late adolescence, showing that there are rapid changes in hippocampal structures during the peripubertal period (Finn et al. 2010). In rodents, adolescent events can affect the hippocampus such that deficits in cognitive and learning behaviors are observed later in life (Lupien et al. 2009). We hypothesized that dietary intervention with excess folate, a known epigenetic modifier, during adolescence could affect future hippocampal-related behaviors.
Methods
Animals
All animal procedures were approved by the Northwestern University Animal Care and Use Committee. Female Sprague Dawley (S) and male Brown Norway (B) rats (Harlan, Indianapolis, IN, USA) were mated to obtain F1 ‘SB’ hybrid litters (N = 6). Male SB hybrid rats were previously shown to be vulnerable to prenatal alcohol exposure in several hippocampal-related behaviors (Sittig et al. 2011b). Therefore, we chose to use male SB rats to evaluate the effects of folate supplementation on motivation and memory performance. Male SB offspring remained with the mother until 21 days of age, and one or two males from each litter were assigned to each of the two treatment groups, folate-supplemented (FOL) or control (CTL) (N = 9/group). Animals were housed together by treatment group (two to three per cage) and kept in a temperature- and humidity-controlled vivarium with regular light/dark cycles (on at 0600 h, off at 1800 h).
Folate supplement
Folic acid (Sigma-Aldrich, St. Louis, MO, USA) was incorporated into Harlan laboratory chow (additional 5.3 mg/kg lab chow) by mixing with a small volume of dH2O and evenly coating food for a final folate content of 8.0 mg folate/kg diet in folate-supplemented chow. Folate-supplemented chow was given to juvenile rats ad libitum from 30 to 60 days of age and control rats received normal Harlan lab chow (containing 2.7 mg folate/kg chow) ad libitum. A folate dose five times higher than that used in the current study, 40 mg folate/kg diet given to weaning rats for 4 weeks, did not alter food intake, weight or various measures of the methionine cycle (Achon et al. 2007).
Behavioral testing
Open field test (OFT) and Morris water maze (MWM) were administered to test locomotor activity, and motivation, learning and memory, respectively. Testing began roughly 45 days after folate supplementation ended, and 2 weeks’ rest was given between tests. Behavioral tests were video recorded and conducted as previously described (Sittig et al. 2011b). For OFT (N = 9/group), rats were placed in a circular arena 82 cm in diameter for 10 min and allowed to freely explore while activity was tracked by the software (Nosek et al. 2008). The arena was lit with a brightness of 60 lux.
TSE VIDEOMOT 2 software (version 5.75; TSE Systems, Bad Homburg, Germany) was used to collect and analyze the data including total distance traveled and time spent in the outer and inner (50 cm diameter) regions. For MWM, rats (N = 9 control; N = 7 folate-supplemented as this was the upper limit of animals that could be tested together) were given four consecutive days of learning trials with four trials/day. The tank was 180 cm in diameter, and the water depth was 25 cm. The water temperature was 21–22 °C. A hidden platform (15 cm diameter) remained in a fixed position 1 cm below the water level during all trials. Rats were placed in the tank in a randomized quadrant order each day and were required to remain in the tank until they located the platform or until 1 min passed. If the platform was not found, the animal was guided to the platform and allowed to remain for 20 seconds before being removed. Three hours after the final trial on day 4, the probe trial was conducted in which animals were placed in the tank without the platform for 1 min. A visual platform task was given on day 5; all animals were able to navigate to a visible platform. TSE VIDEOMOT 2 software was used to collect and analyze the data including path tracing of each trial, latency to the platform and swim speed.
Tissue collection
Animals were sacrificed by decapitation after a 2-week rest period. Brains were immediately dissected and the right hippocampus of each animal was stored at −80°C for protein or genomic DNA extraction. Brain slices were first made in a rat brain matrix using Paxinos coordinates anterior/posterior −2.12 to −6.0 and the entire hippocampal structure was dissected out from this section (Chiu et al. 2007). Blood was collected on ice and plasma was obtained and stored as previously described (Sittig & Redei 2010).
Genomic DNA preparation and bisulfite conversion
Genomic DNA was prepared by standard phenol–chloroform extraction. Bisulfite modification of genomic DNA was performed with the EZ DNA Methylation Gold kit (Zymo Research, Orange, CA, USA) according to the manufacturer’s instructions. Briefly, 0.5 μg genomic DNA was bisulfite treated, eluted with 10 μl elution solution and stored at −20°C until ready for use.
PCR and pyrosequencing
Bisulfite pyrosequencing reactions were conducted essentially as described (Xie et al. 2009). Briefly, to amplify the target promoter regions, a 50 μl polymerase chain reaction (PCR) mixture was prepared with 2 μl (100 ng) bisulfite-treated DNA and 50 pmol each forward and reverse primers. After denaturation at 94°C for 15 min, PCR conditions were as follows: 45 cycles of 95°C, 30 seconds, 56°C, 1 min, 72°C 30 seconds; 72°C, 5 min using the HotStar Taq Plus Master Mix (Qiagen, Valencia, CA, USA). PCR products were stored at 4°C until ready for pyrosequencing. Primers for amplifying the bisulfite modified promoter region were F 5′-GGG GAG GGA GGA GTT AGA-3′ and R 5′-CTC CCT CCT CCT TAC CTC-3′ with either the forward or reverse primers biotinylated. Primers for pyrosequencing were 5′-GGG AGG GAG GAG TTA GA-3′ or 5′-TCC CTC CTC CTT ACC T-3′ for reverse or forward labeled products, respectively.
Pyrosequencing was performed (N = 3/group) using the PyroMark MD Pyrosequencing System (Biotage, Charlottesville, VA, USA). The final PCR product was purified using streptavidin-Sepahrose HP beads (GE Healthchare, Uppsala, Sweden) and processed to yield single-stranded DNA. The single-stranded DNA was prepared for pyrosequencing using the PyroMark Vacuum Prep Tool (Biotage). In brief, the PCR product was bound onto Sepharose beads. Beads containing the immobilized PCR product were washed, denatured using a 0.2 M NaOH solution, washed again and neutralized. Pyrosequencing primer at a concentration of 0.3 μM was annealed to the purified single-stranded PCR products at 28°C. Methylation quantification was performed using the manufacturer-provided software.
Radioimmunoassay
Total T4 and T3 as well as thyroid stimulating hormone (TSH) in plasma (N = 9/group) were measured by radioimmunoassay (RIA) as previously described (Sittig & Redei 2010). T4 and T3 assays were manufactured by MP Biomedicals (Irvine, CA, USA). Assay sensitivities and coefficients of variation were 0.8 μg/dl; 3.3% and 47 ng/dl; 3.1%, respectively. The rat TSH RIA (Alpco Diagnostics, Salem, NH, USA) was used according to manufacturer’s instructions with assay sensitivity of 2.8 ng/ml and intra-assay coefficient of variation of 6.5%.
Western blots
Hippocampi were homogenized in cold lysis buffer and 60 μg protein/sample (N = 4/group) was electrophoresed on 12% (w/v) sodium dodecyl sulphate polyacrylamide gel and transferred to polyvinylidene difluoride membranes as described previously (Shukla et al. 2010). Blots were incubated with primary antibody (below) and horseradish peroxidase-linked secondary antibodies. ECL Plus Western Blotting Detection System (GE Healthcare, Piscataway, NJ, USA) was used to detect signals. Optical density was normalized to β-actin signal for quantification using IMAGEJ v. 1.43u software. TRα1 and TRα2 were detected by a single antibody, rabbit antithyroid receptor-α (Santa Cruz Biotechnology, Santa Cruz, CA, USA), 1:1000, with sizes of 46 and 55 kDa, respectively (Katz et al. 1995). Other primary antibodies were rabbit anti-GAP-43 (Santa Cruz Biotechnology), 1:1000; rabbit anti-Gsα (Santa Cruz Biotechnology), 1:500; mouse anti-β-actin (Sigma-Aldrich), 1:10 000.
Statistics
Since only males were used, diet was the only factor in all analyses except in the MWM trial measures, where day of testing was added as a factor. In this case, a two-way repeated measures ANOVA was used to compare animals’ performance on subsequent testing days, with diet and day as factors. Bonferroni adjusted post hoc tests were used to identify days on which performance differed between the two groups. For probe trial performance, two-tailed Student’s t-tests were conducted to evaluate group differences. Methylation data obtained by pyrosequencing were analyzed across sites using a General Linear Model. Post hoc analysis was performed using a sliding window approach with Student’s t-test. Data are reported as mean ± SEM. Significance was set at P < 0.05.
Results
Motivation and spatial memory
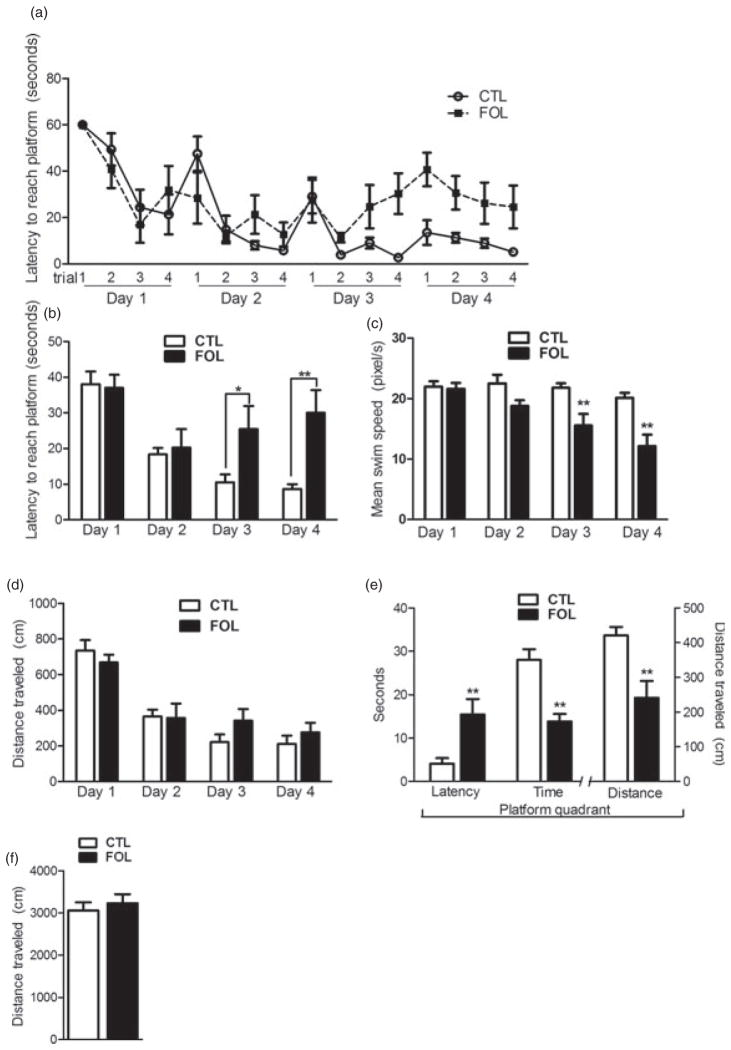
Behavior observed in the MWM test has several components. Performance during the daily trials measures motivation for the task as well as learning, while the probe trial is considered a measure of spatial memory itself (Lubbers et al. 2007). In the MWM training period (days 1–4), there was no difference between CTL and FOL rats in average latency to reach the platform on day 1 or day 2 (Fig. 1a,b). Animals from both diet groups took about half as long to locate the hidden platform on the second day of training compared to the first day. Control rats learned to locate the hidden platform as seen by their decreasing average latency to reach the platform on each successive training day (training day: F3,42 = 17.3, P < 0.001) (Fig. 1b). However, folate-supplemented rats had a progressively longer latency to reach the platform on day 3 and 4 (Fig. 1a,b) in contrast to controls, whose latency continued to decrease over all four training days (interaction of day and diet: F3,42 = 5.6, P < 0.01). Folate-supplemented rats took significantly longer than control rats to reach the platform on day 3, and the group difference was even greater on day 4 (Fig. 1b). Daily mean swim speed of folate-supplemented rats was inversely related to the pattern of increased latency on days 3 and 4 (Fig. 1c). Control animals maintained a similar swim speed across all days of learning trials, and folate animals did not differ on days 1 and 2. However, folate animals had decreased swim speed on days 3 and 4 (interaction of day and diet: F3,39 = 7.6, P < 0.001). Distance traveled during the trials did not differ between the groups on any of the trial days (Fig. 1d).
Figure 1. Behavioral effects of excess folate in the MWM (a–e) and OFT (f) tests.
(a) Latency in seconds to reach the hidden platform in each learning trial of the MWM for control (CTL) and folate-supplemented (FOL) adult male rats. (b) Daily average latency to the hidden platform. Values were generated by averaging daily individual animals’ latencies across trials and taking the mean of these values for each group. (c) Daily average swimming speed. Values were generated by averaging daily individual animals’ swim speeds across trials and taking the mean of these values for each group. Folate-supplemented animals showed no difference in swimming speed ability on the first 2 days of testing, while their average swimming speed was decreased compared to controls on days 3 and 4. (d) Daily mean distance traveled on days 1–4. Values were generated as in (b) and (c). The two groups showed no differences, suggesting folate-supplemented animals were not impaired in motor function. (e) MWM probe trial test with the platform missing. Folate-supplemented animals took longer to reach the platform quadrant and spent less time there. In agreement, they traveled less distance in the correct quadrant. (f) Activity in the OFT was indistinguishable between groups. Unless otherwise noted, values are reported as means ± SEM. *P < 0.05, **P < 0.01.
During the 1-min probe trial of memory, folate-supplemented rats took longer to arrive in the platform quadrant [t(11) = 3.6, P < 0.01], spent less time there [t(10) = 3.8, P < 0.01] and traveled less distance [t(11) = 3.7, P < 0.01] in the platform quadrant compared to controls (Fig. 1e).
Activity
The groups exhibited no difference in exploratory behavior (activity) in the OFT (Fig. 1f). There were no group differences in time spent or distance traveled in the inner region of the field (data not shown). All animals spent at least five times as much time in the outer ring of the OFT as they did in the inner rings combined (data not shown), indicating that the center of the field was aversive and could be considered anxiety provoking.
Peripheral thyroid hormones and hippocampal thyroid hormone receptors
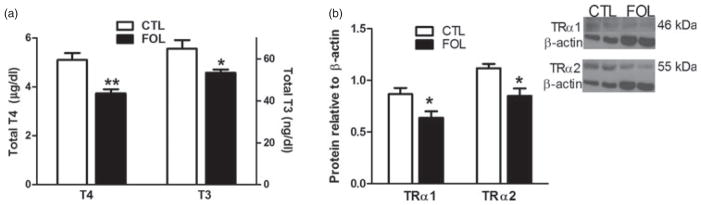
Because the memory and motivational deficits seen in folate-supplemented rats were similar to those associated with thyroid hormone deficiency in humans, we measured thyroid hormone levels in the periphery (Correia et al. 2009; Demartini et al. 2010; He et al. 2011). Folate-supplemented rats had significantly decreased levels of plasma thyroid hormones T4 [t(16) = 4.1, P < 0.001] and T3 [t(16) = 2.4, P < 0.05] (Fig. 2a) while TSH levels did not differ (control: 3.9 ± 0.33 ng/ml; folate: 4.0 ± 0.39 ng/ml). This pattern indicates that adolescent folate supplementation resulted in a peripheral hypothyroid milieu in adulthood.
Figure 2. Suppressive effect of excess folate on measures of thyroid function.
(a) Total plasma thyroxine (T4) and triiodothyrinine (T3) levels of CTL and FOL adult male rats. Hormone levels were measured by radioimmunoassay. (b) Thyroid hormone receptor-α1 and -α2 protein levels in hippocampal tissue, normalized to β-actin levels as determined by western blot. Insert shows representative western blots. Data are means ± SEM. *P < 0.05, **P < 0.01.
Thyroid hormone receptor levels in relevant brain regions could mediate the behavioral changes observed in folate-supplemented rats. We measured TRα because mice lacking TRα (TRαo/o) also exhibit spatial learning and memory deficits in the MWM (Wilcoxon et al. 2007). In addition, we measured TRα2 because it is abundant in brain (Katz et al. 1995), could share genomic regulatory mechanisms, and has dominant negative effect on TRα1 DNA binding. Protein levels were used rather than mRNA to assess isoform-specific TRα levels in this study due to the numerous transcript variants derived from the Thra gene, including TR-α1, TRΔ-α1, TR-α2, TRΔ-α2 and TR-α3. Our attempt to design TRα1-specific primers was unsuccessful due to sequence overlaps among these transcripts. In addition, the existence of several untranslated, processed TRα transcripts in the mouse (see Ensembl.org) suggests that protein determination is a more specific measure. Protein levels of TRα1 [t(5) = 2.6, P < 0.05] and TRα2 [t(6) = 3.3, P < 0.05] were decreased in the hippocampus of folate-supplemented rats (Fig. 2b). In contrast, protein levels of the learning and memory-related molecules GAP-43 (Wilcoxon et al. 2007) and Gs-α (Bourtchouladze et al. 2006) in the hippocampus were not altered by folate supplementation (data not shown).
Identification of putative rat Thra promoter
The promoter for rat thyroid hormone receptor-α has not been characterized. We utilized the human Thra promoter sequence (Ensembl: EBSR00000094047 human Chr 17:38218480–38220039), with NCBI’s BLAST to identify a 300 bp homologous region in the rat (rat Chr 10:87514294–87514616). We made the assumption that the 12 kb region between this and the 5′ end of Thra (10:87514294–87526260) contains the rat Thra promoter elements, including potential regulatory CpG dinucleotides. Using EMBOSS CpG plot, (http://www.ebi.ac.uk/Tools/emboss/cpgplot/) we analyzed the CpG frequency in this region, specifically identifying a 190 bp CpG-rich region using an observed/expected parameter of 0.7. This region (rat Chr 10:87514382–87514572) is 94% identical to the human Thra promoter and is likely a functional part of the Thra promoter in the rat. The 28 CpG dinucleotides contained within this region were analyzed by bisulfite pyrosequencing.
Methylation at CpG islands of the putative rat Thra promoter
We aimed to determine whether dietary folate impacted the DNA methylation of the Thra promoter as a whole or at specific target CpG sites. The Thra promoter was generally hypomethylated across the region studied, averaging 12% methylation across the locus. Of the 28 CpG sites analyzed, 25 yielded usable data. General Lineal Model analysis of methylation levels across these sites indicated that individual CpG islands differed in methylation level (F24,190 = 10.4, P < 0.01). Post hoc analysis using a sliding window approach showed that CpGs 10–15 as a group showed increased long-term methylation levels in animals who had consumed the folate diet as adolescents (Student’s t-test, P < 0.023) (Fig. 3). In the small sample size tested, this corresponded to a 56% increase in average methylation from 12.1% to 18.9% across these six CpG dinucleotides, with a 73% increase in average methylation from 12.7% to 21.9% across CpGs 12–15 alone (P < 0.05). Comparative sequence analysis shows that these CpGs are in a region with 94% identity to a portion of the known human Thra promoter (not shown).
Figure 3. CpG methylation across the Thra promoter region (see Results section) in CTL (C) and FOL (F) adult male rats as measured by pyrosequencing analysis.
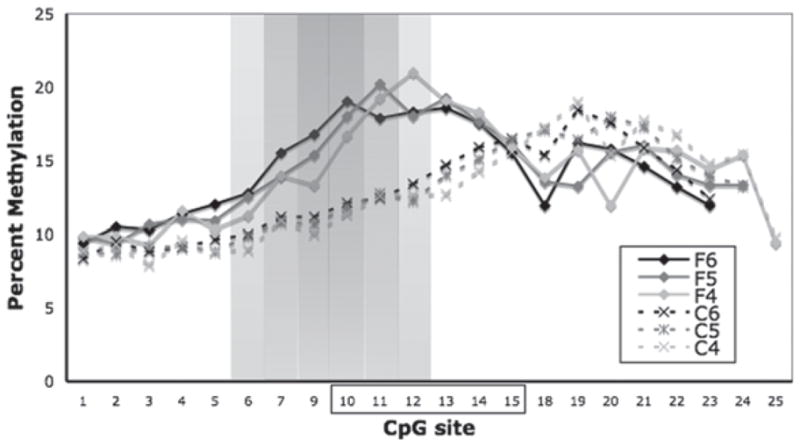
Regional enhancement of methylation (sites 10–15, boxed) was determined following post hoc analysis between groups using a sliding window approach with window sizes of four to six CpGs (e.g. C4 vs. F4) as shown. Percent methylation for each window is plotted at the first CpG of that window. Shading indicates significance (P < 0.05) for a single (light) to all three (dark) window sizes starting at a given CpG site.
Discussion
Our study is the first to test the prolonged effects of folate supplementation in adolescence. Adolescent rats were given lab chow with threefold the normal folic acid from days 30–60, which covers approximately 15 days before and after the onset of puberty. This treatment impaired motivation and spatial memory, both hippocampal-related behaviors, during adulthood. Concomitantly, we found reduced plasma thyroid hormone concentrations and lowered hippocampal levels of thyroid receptor-α1 and -α2 protein together with increased methylation within the CpG-rich region upstream of the Thra gene in folate-supplemented rats. Together, these findings suggest that folate supplementation affected peripheral thyroid function and local thyroid hormone-mediated signaling in the hippocampus.
Folate-supplemented rats exhibited a spatial memory deficit as evidenced by their impaired ability to navigate during the probe trial. They also showed a motivational deficit as evidenced by increased latency to the platform and decreased swimming speed on days 3 and 4 of the MWM trials. A very similar behavioral pattern in the same 4-day MWM paradigm has previously been ascribed to a motivational deficit (Lubbers et al. 2007). It is possible that repeated exposure to the same situation over several days led to or revealed a lack of motivation for the task. This is consistent with findings in TRαo/o mice, which show intact performance during the first trials of the MWM task but a deficit in subsequent trials (Wilcoxon et al. 2007). Thus, the motivational deficits seen in the folate-supplemented animals may have been partially due to altered thyroid receptor-mediated signaling resulting from decreased TRα levels. The fact that we detected no motor deficits in the OFT, no differences in days 1 and 2 of MWM and no differences in anxiety-like behavior in the OFT further suggest a motivational rather than motor or learning deficit.
It is possible that the folate-induced motivational and memory impairments were secondary to the lower thyroid hormone levels, although a causal relationship between the endocrine changes and behavioral phenotypes has yet to be showed. Clinical and subclinical hypothyroidisms are associated with deficits in these behaviors in humans and animals (Gunnarsson et al. 2001; Montero-Pedrazuela et al. 2006). The mechanism for how folate supplementation lowered T4 and T3 levels permanently (or at least well into adulthood) is unclear, but could relate to the methylation of TSH receptor in the thyroid gland since there was no effect of folate administration on TSH levels. We hypothesize that such a strong effect of folate supplementation on thyroid hormone levels was seen because it occurred during the developmentally important adolescent period. This postnatal critical period for thyroid function maturation is not well characterized, but our data and others’ in humans (Kaloumenou et al. 2010) indicate that thyroid function is changing during puberty. Lower free T4 concentrations are related to depressive symptoms and attention deficit behaviors in human adolescents (Dorn et al. 1996) and subclinical hypothyroidism has been associated with affective and behavioral problems in youth (Haviland et al. 2006; Holtmann et al. 2010). We have not confirmed that our animals were functionally hypothyroid during the supplementation period, and it would be interesting to determine when this state arose. Regardless, negative cognitive and depressive effects during a hypothyroid state are seen in human adults (Correia et al. 2009; Demartini et al. 2010; He et al. 2011). Following from such evidence, if the same effect of high dose folate can occur in women who take excessive amounts during pregnancy, decreases in maternal plasma thyroid hormones would have significant implications for the health of the fetus.
The decrease of peripheral thyroid hormones and the changes in hippocampal thyroid receptor levels are consistent with both motivational and memory impairments observed in folate-supplemented rats, but a related question is whether the lowered peripheral thyroid hormones led to decreased TRα levels in the hippocampus. The MWM is a hippocampus-mediated learning task and its motivational aspects are also hippocampal-mediated (Burton et al. 2009; Holscher et al. 2003; Kobayashi et al. 1997; Singer & Frank 2009). Lower thyroid hormone levels in the periphery have been shown to elicit higher TRα1 but lower TRα2 levels in hippocampus (Constantinou et al. 2005). This suggests that the regulation of TR by thyroid hormones in the hippocampus may be local rather than peripheral, as evidenced by findings showing that peripheral thyroid hormone levels may not reflect the thyroid hormone milieu of specific brain regions including the hippocampus (Shukla et al. 2010; Sittig et al. 2011a,b). Another potential mechanism for lowered hippocampal TRα is the methylation increase of ~50% in a group of six CpG islands within the putative rat Thra promoter. These CpG sites harbor predicted binding sites for Sp-1 and TTF-1 transcription factors. Sp-1 has been implicated in the control of neuronal differentiation and is increased in rat hippocampus by kainic acid treatment (Feng et al. 1999; Ravache et al. 2010). TTF-1 is also found in the hippocampus (Suzuki et al. 1998) and could mediate thyroid hormone-related processes, although its role there has not been identified. However, it is known to direct the development of multiple tissues and is thought to influence neuron differentiation and morphogenesis (Pohlenz et al. 2002; Provenzano et al. 2010). Thus, methylation-induced altered DNA binding of Sp-1 and/or TTF-1 to the promoter of Thra could affect hippocampal Thra gene expression and consequently expression of other genes involved in cognitive and motivational processes. Whether the change in methylation status that we observed is functional will require future studies, but others have found that <5% changes can be functional (Steegers-Theunissen et al. 2009).
In industrialized nations including the USA, fortified grain products are estimated to provide an additional 100 μg folate/day, one quarter of the 400 μg recommended daily allowance (Anderson et al. 2010). This intervention has been found to substantially increase plasma folate levels (Jacques et al. 1999). In addition, dietary supplements commonly provide 400 μg, another 100% of the recommended daily allowance for adolescents and adults. Thus, depending on their grain consumption, many people likely consume more than 200% of the 400 μg recommended allowance. In fact, up to 2000 μg folate/day is used in humans for clinical trials (Anderson et al. 2010). Just as folate deficiency can be harmful, especially to vulnerable populations such as the elderly (Selhub et al. 1993), our results suggest long-term harm can occur from excess folate consumed during the adolescent period. Other adverse effects of folate have been found, including its stimulation of Gs gamma proteins in brain membranes with its greatest effect occurring in the hippocampus and cerebellum (Hartley & Snodgrass 1990). Deleterious effects of folate may occur in other conditions as well; evidence indicates that in some cases folate promotes carcinogenesis or its progression (Collin et al. 2010; Protiva et al. 2011). Here, we found that thyroid hormone status in both the brain and periphery was permanently suppressed by excess adolescent folate consumption, providing an example of wide-ranging effects of this dietary intervention on endocrine and behavioral parameters. In the future, the mechanisms and downstream consequences of folate-induced thyroid hormone deficiency will be elucidated. It will be especially interesting to determine whether the global or brain region-specific imprinting status of neurobehaviorally important genes could be altered, either favorably or detrimentally, by folate treatment (Sittig et al. 2011b). Overall, the current work suggests that ‘less is more’ when it comes to dietary folate supplementation.
References
- Achon M, Alonso-Aperte E, Ubeda N, Varela-Moreiras G. Supranormal dietary folic acid supplementation: effects on methionine metabolism in weanling rats. Br J Nutr. 2007;98:490–496. doi: 10.1017/S0007114507721499. [DOI] [PubMed] [Google Scholar]
- Anderson CA, Jee SH, Charleston J, Narrett M, Appel LJ. Effects of folic acid supplementation on serum folate and plasma homocysteine concentrations in older adults: a dose-response trial. Am J Epidemiol. 2010;172:932–941. doi: 10.1093/aje/kwq197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balk EM, Raman G, Tatsioni A, Chung M, Lau J, Rosenberg IH. Vitamin B6, B12, and folic acid supplementation and cognitive function: a systematic review of randomized trials. Arch Intern Med. 2007;167:21–30. doi: 10.1001/archinte.167.1.21. [DOI] [PubMed] [Google Scholar]
- Beydoun MA, Shroff MR, Beydoun HA, Zonderman AB. Serum folate, vitamin B-12, and homocysteine and their association with depressive symptoms among U.S. adults. Psychosom Med. 2010;72:862–873. doi: 10.1097/PSY.0b013e3181f61863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat RS. You are what you eat: of fish, fat and folate in late-life psychiatric disorders. Curr Opin Psychiatry. 2009;22:541–545. doi: 10.1097/YCO.0b013e3283308e3a. [DOI] [PubMed] [Google Scholar]
- Blencowe H, Cousens S, Modell B, Lawn J. Folic acid to reduce neonatal mortality from neural tube disorders. Int J Epidemiol. 2010;39 (Suppl 1):i110–i121. doi: 10.1093/ije/dyq028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourtchouladze R, Patterson SL, Kelly MP, Kreibich A, Kandel ER, Abel T. Chronically increased Gsalpha signaling disrupts associative and spatial learning. Learn Mem. 2006;13:745–752. doi: 10.1101/lm.354106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocardo PS, Budni J, Lobato KR, Santos AR, Rodrigues AL. Evidence for the involvement of the opioid system in the antidepressant-like effect of folic acid in the mouse forced swimming test. Behav Brain Res. 2009;200:122–127. doi: 10.1016/j.bbr.2009.01.004. [DOI] [PubMed] [Google Scholar]
- Burton BG, Hok V, Save E, Poucet B. Lesion of the ventral and intermediate hippocampus abolishes anticipatory activity in the medial prefrontal cortex of the rat. Behav Brain Res. 2009;199:222–234. doi: 10.1016/j.bbr.2008.11.045. [DOI] [PubMed] [Google Scholar]
- Chiu K, Lau WM, Lau HT, So KF, Chang RC. Micro-dissection of rat brain for RNA or protein extraction from specific brain region. J Vis Exp. 2007:269. doi: 10.3791/269. Epub Aug 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin SM, Metcalfe C, Refsum H, et al. Circulating folate, vitamin B12, homocysteine, vitamin B12 transport proteins, and risk of prostate cancer: a case-control study, systematic review, and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2010;19:1632–1642. doi: 10.1158/1055-9965.EPI-10-0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinou C, Margarity M, Valcana T. Region-specific effects of hypothyroidism on the relative expression of thyroid hormone receptors in adult rat brain. Mol Cell Biochem. 2005;278:93–100. doi: 10.1007/s11010-005-6934-z. [DOI] [PubMed] [Google Scholar]
- Correia N, Mullally S, Cooke G, Tun TK, Phelan N, Feeney J, Fitzgibbon M, Boran G, O’Mara S, Gibney J. Evidence for a specific defect in hippocampal memory in overt and subclinical hypothyroidism. J Clin Endocrinol Metab. 2009;94:3789–3797. doi: 10.1210/jc.2008-2702. [DOI] [PubMed] [Google Scholar]
- Demartini B, Masu A, Scarone S, Pontiroli AE, Gambini O. Prevalence of depression in patients affected by subclinical hypothyroidism. Panminerva Med. 2010;52:277–282. [PubMed] [Google Scholar]
- Dorn LD, Burgess ES, Dichek HL, Putnam FW, Chrousos GP, Gold PW. Thyroid hormone concentrations in depressed and nondepressed adolescents: group differences and behavioral relations. J Am Acad Child Adolesc Psychiatry. 1996;35:299–306. doi: 10.1097/00004583-199603000-00010. [DOI] [PubMed] [Google Scholar]
- Feng Z, Chang RC, Bing G, Hudson P, Tiao N, Jin L, Hong JS. Long-term increase of Sp-1 transcription factors in the hippocampus after kainic acid treatment. Brain Res Mol Brain Res. 1999;69:144–148. doi: 10.1016/s0169-328x(99)00099-6. [DOI] [PubMed] [Google Scholar]
- Finn AS, Sheridan MA, Kam CL, Hinshaw S, D’Esposito M. Longitudinal evidence for functional specialization of the neural circuit supporting working memory in the human brain. J Neurosci. 2010;30:11062–11067. doi: 10.1523/JNEUROSCI.6266-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnarsson T, Sjoberg S, Eriksson M, Nordin C. Depressive symptoms in hypothyroid disorder with some observations on biochemical correlates. Neuropsychobiology. 2001;43:70–74. doi: 10.1159/000054869. [DOI] [PubMed] [Google Scholar]
- Hartley DM, Snodgrass SR. Folate interactions with cerebral G proteins. Neurochem Res. 1990;15:681–686. doi: 10.1007/BF00973648. [DOI] [PubMed] [Google Scholar]
- Haviland MG, Sonne JL, Anderson DL, Nelson JC, Sheridan-Matney C, Nichols JG, Carlton EI, Murdoch WG. Thyroid hormone levels and psychological symptoms in sexually abused adolescent girls. Child Abuse Negl. 2006;30:589–598. doi: 10.1016/j.chiabu.2005.11.011. [DOI] [PubMed] [Google Scholar]
- He XS, Ma N, Pan ZL, Wang ZX, Li N, Zhang XC, Zhou JN, Zhu DF, Zhang DR. Functional MRI assessment of altered brain function in hypothyroidism during working memory processing. Eur J Endocrinol. 2011;164:951–959. doi: 10.1530/EJE-11-0046. [DOI] [PubMed] [Google Scholar]
- Holscher C, Jacob W, Mallot HA. Reward modulates neuronal activity in the hippocampus of the rat. Behav Brain Res. 2003;142:181–191. doi: 10.1016/s0166-4328(02)00422-9. [DOI] [PubMed] [Google Scholar]
- Holtmann M, Duketis E, Goth K, Poustka L, Boelte S. Severe affective and behavioral dysregulation in youth is associated with increased serum TSH. J Affect Disord. 2010;121:184–188. doi: 10.1016/j.jad.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Jacques PF, Selhub J, Bostom AG, Wilson PW, Rosenberg IH. The effect of folic acid fortification on plasma folate and total homocysteine concentrations. N Engl J Med. 1999;340:1449–1454. doi: 10.1056/NEJM199905133401901. [DOI] [PubMed] [Google Scholar]
- Kaloumenou I, Duntas LH, Alevizaki M, Mantzou E, Chiotis D, Mengreli C, Papassotiriou I, Mastorakos G, Dacou-Voutetakis C. Gender, age, puberty, and BMI related changes of TSH and thyroid hormones in schoolchildren living in a long-standing iodine replete area. Horm Metab Res. 2010;42:285–289. doi: 10.1055/s-0029-1246184. [DOI] [PubMed] [Google Scholar]
- Katz D, Reginato MJ, Lazar MA. Functional regulation of thyroid hormone receptor variant TR alpha 2 by phosphorylation. Mol Cell Biol. 1995;15:2341–2348. doi: 10.1128/mcb.15.5.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, Nishijo H, Fukuda M, Bures J, Ono T. Task-dependent representations in rat hippocampal place neurons. J Neurophysiol. 1997;78:597–613. doi: 10.1152/jn.1997.78.2.597. [DOI] [PubMed] [Google Scholar]
- Kronenberg G, Colla M, Endres M. Folic acid, neurodegenerative and neuropsychiatric disease. Curr Mol Med. 2009;9:315–323. doi: 10.2174/156652409787847146. [DOI] [PubMed] [Google Scholar]
- de Lau LM, Refsum H, Smith AD, Johnston C, Breteler MM. Plasma folate concentration and cognitive performance: Rotterdam Scan Study. Am J Clin Nutr. 2007;86:728–734. doi: 10.1093/ajcn/86.3.728. [DOI] [PubMed] [Google Scholar]
- Lubbers ME, van den Bos R, Spruijt BM. Mu opioid receptor knockout mice in the Morris Water Maze: a learning or motivation deficit? Behav Brain Res. 2007;180:107–111. doi: 10.1016/j.bbr.2007.02.021. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Montero-Pedrazuela A, Venero C, Lavado-Autric R, Fernandez-Lamo I, Garcia-Verdugo JM, Bernal J, Guadano-Ferraz A. Modulation of adult hippocampal neurogenesis by thyroid hormones: implications in depressive-like behavior. Mol Psychiatry. 2006;11:361–371. doi: 10.1038/sj.mp.4001802. [DOI] [PubMed] [Google Scholar]
- Nelson C, Wengreen HJ, Munger RG, Corcoran CD. Dietary folate, vitamin B-12, vitamin B-6 and incident Alzheimer’s disease: the cache county memory, health and aging study. J Nutr Health Aging. 2009;13:899–905. doi: 10.1007/s12603-009-0249-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosek K, Dennis K, Andrus BM, Ahmadiyeh N, Baum AE, Woods LC, Redei EE. Context and strain-dependent behavioral response to stress. Behav Brain Funct. 2008;4:23. doi: 10.1186/1744-9081-4-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohlenz J, Dumitrescu A, Zundel D, Martine U, Schonberger W, Koo E, Weiss RE, Cohen RN, Kimura S, Refetoff S. Partial deficiency of thyroid transcription factor 1 produces predominantly neurological defects in humans and mice. J Clin Invest. 2002;109:469–473. doi: 10.1172/JCI14192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protiva P, Mason JB, Liu Z, Hopkins ME, Nelson C, Marshall JR, Lambrecht RW, Pendyala S, Kopelovich L, Kim M, Kleinstein SH, Laird PW, Lipkin M, Holt PR. Altered folate availability modifies the molecular environment of the human colorectum: implications for colorectal carcinogenesis. Cancer Prev Res (Phila) 2011;4:530–543. doi: 10.1158/1940-6207.CAPR-10-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provenzano C, Pascucci B, Lupari E, Civitareale D. Large scale analysis of transcription factor TTF-1/NKX2.1 target genes in GnRH secreting cell line GT1-7. Mol Cell Endocrinol. 2010;323:215–223. doi: 10.1016/j.mce.2010.02.038. [DOI] [PubMed] [Google Scholar]
- Ravache M, Weber C, Merienne K, Trottier Y. Transcriptional activation of REST by Sp1 in Huntington’s disease models. PLoS One. 2010;5:e14311. doi: 10.1371/journal.pone.0014311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selhub J, Jacques PF, Wilson PW, Rush D, Rosenberg IH. Vitamin status and intake as primary determinants of homocysteinemia in an elderly population. JAMA. 1993;270:2693–2698. doi: 10.1001/jama.1993.03510220049033. [DOI] [PubMed] [Google Scholar]
- Shukla PK, Sittig LJ, Andrus BM, Schaffer DJ, Batra KK, Redei EE. Prenatal thyroxine treatment disparately affects peripheral and amygdala thyroid hormone levels. Psychoneuroendocrinology. 2010;35:791–797. doi: 10.1016/j.psyneuen.2009.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer AC, Frank LM. Rewarded outcomes enhance reactivation of experience in the hippocampus. Neuron. 2009;64:910–921. doi: 10.1016/j.neuron.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sittig LJ, Redei EE. Paternal genetic contribution influences fetal vulnerability to maternal alcohol consumption in a rat model of fetal alcohol spectrum disorder. PLoS One. 2010;5:e10058. doi: 10.1371/journal.pone.0010058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sittig LJ, Herzing LB, Shukla PK, Redei EE. Parent-of-origin allelic contributions to deiodinase-3 expression elicit localized hyperthyroid milieu in the hippocampus. Mol Psychiatry. 2011a;16:786–787. doi: 10.1038/mp.2011.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sittig LJ, Shukla PK, Herzing LB, Redei EE. Strain-specific vulnerability to alcohol exposure in utero via hippocampal parent-of-origin expression of deiodinase-III. FASEB J. 2011b;25:2313–2324. doi: 10.1096/fj.10-179234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AD, Kim YI, Refsum H. Is folic acid good for everyone? Am J Clin Nutr. 2008;87:517–533. doi: 10.1093/ajcn/87.3.517. [DOI] [PubMed] [Google Scholar]
- Steegers-Theunissen RP, Obermann-Borst SA, Kremer D, Lindemans J, Siebel C, Steegers EA, Slagboom PE, Heijmans BT. Periconceptional maternal folic acid use of 400 microg per day is related to increased methylation of the IGF2 gene in the very young child. PLoS One. 2009;4:e7845. doi: 10.1371/journal.pone.0007845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Kobayashi Y, Katoh R, Kohn LD, Kawaoi A. Identification of thyroid transcription factor-1 in C cells and parathyroid cells. Endocrinology. 1998;139:3014–3017. doi: 10.1210/endo.139.6.6126. [DOI] [PubMed] [Google Scholar]
- Taylor MJ, Geddes J. Folic acid as ultimate in disease prevention: folate also improves mental health. BMJ. 2004;328:768–769. doi: 10.1136/bmj.328.7442.768-c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcoxon JS, Nadolski GJ, Samarut J, Chassande O, Redei EE. Behavioral inhibition and impaired spatial learning and memory in hypothyroid mice lacking thyroid hormone receptor alpha. Behav Brain Res. 2007;177:109–116. doi: 10.1016/j.bbr.2006.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie H, Wang M, Bonaldo Mde F, Smith C, Rajaram V, Goldman S, Tomita T, Soares MB. High-throughput sequence-based epigenomic analysis of Alu repeats in human cerebellum. Nucleic Acids Res. 2009;37:4331–4340. doi: 10.1093/nar/gkp393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuo JM, Pratico D. Acceleration of brain amyloidosis in an Alzheimer’s disease mouse model by a folate, vitamin B6 and B12-deficient diet. Exp Gerontol. 2010;45:195–201. doi: 10.1016/j.exger.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]