Abstract
Background
In animal models of cardiac arrest, the benefit afforded by hypothermia is closely linked to the rapidity in body temperature decrease after resuscitation. Since total liquid ventilation (TLV) with temperature controlled perfluorocarbons induces a very rapid and generalized cooling, we aimed to determine whether this could limit the post-cardiac arrest syndrome in a rabbit model. We especially focused on neurological, cardiac, pulmonary, liver and kidney dysfunctions.
Methods and Results
Anesthetized rabbits were submitted to either 5 or 10-min of untreated ventricular fibrillation. After cardiopulmonary resuscitation and resumption of a spontaneous circulation, the animals underwent either normothermic life support (control) or therapeutic hypothermia induced by TLV. The latter procedure decreased esophagal and tympanic temperatures to 32–33°C within only 10-min. After rewarming, the animals submitted to TLV exhibited an attenuated neurological dysfunction and decreased mortality 7 days later as compared to control. The neuroprotective effect of TLV was confirmed by a significant reduction in brain histological damages. We also observed limitation of myocardial necrosis, along with a decrease in troponin I release and a reduced myocardial caspase 3 activity with TLV. The beneficial effects of TLV were directly related to the rapidity at inducing hypothermia since neither conventional cooling (cold saline infusion + external cooling) nor normothermic TLV elicited a similar protection.
Conclusions
Ultra-fast cooling instituted by TLV exerts potent neurological and cardiac protections in an experimental model of cardiac arrest in rabbits. This could be a relevant approach to afford a global and protective hypothermia against the post-cardiac arrest syndrome.
Keywords: Animals; Cardiopulmonary Resuscitation; Disease Models, Animal; Fluorocarbons; Heart; physiology; Heart Arrest; mortality; physiopathology; therapy; Hypothermia, Induced; methods; Kidney; physiology; Liquid Ventilation; Liver; physiology; Lung; physiology; Nervous System Physiological Phenomena; Rabbits; Reperfusion Injury; mortality; physiopathology; prevention & control; Time Factors; Ventricular Fibrillation; mortality; physiopathology; therapy
Keywords: Cardiopulmonary resuscitation, Fibrillation, Heart arrest, Ischemia, Ventilation
Introduction
Institution of mild “therapeutic” hypothermia (32–34°C) during 24 to 36 hours after resuscitation is known to improve survival and neurological recovery in comatose survivors of cardiac arrest.1, 2 However, experimental studies in dogs,3, 4 pigs5, 6 and rodents7, 8 demonstrated that the neuroprotection afforded by hypothermia was related to the rapidity in body temperature decrease after resuscitation. When achieved rapidly, hypothermia could also be beneficial for other organs since it can be, for example, also potently cardioprotective during myocardial ischemia.9–12 Accordingly, many strategies were proposed to afford such a rapid hypothermia, including intravenous infusion of cold fluid,13 endovascular 14 or intranasal cooling.15, 16
Another strategy that can experimentally afford a very rapid and generalized cooling is liquid ventilation of the lungs with temperature-controlled perfluorocarbons.11, 17–22 These liquids can use the lungs as heat exchangers while maintaining normal gas exchanges. 18–20 In addition, this ventilation procedure also protects the lung integrity.20 Using a prototype of total liquid ventilator that alternatively instillates and removes a tidal volume of perfluorocarbon from the lung, we were able to decrease the left atrial temperature to 32°C within only 5 min in anesthetized rabbits.11, 17, 18 This was associated with a very potent protection against myocardial infarction and subsequent contractile dysfunction in animal models of coronary artery occlusion.11, 17, 18 In a swine model of ventricular fibrillation, liquid ventilation also induced a rapid convective cooling that further improves the chances for subsequent resumption of spontaneous circulation. 21, 22 However, the effect of hypothermic total liquid ventilation (TLV) has never been investigated to our knowledge in animal models of post-cardiac arrest dysfunction when instituted after resumption of spontaneous circulation.
Accordingly, the main purpose of the present study was to investigate the long term effect of ultrafast cooling induced by TLV in a rabbit model of post-cardiac arrest dysfunction following ventricular fibrillation and resuscitation. In order to determine whether hypothermic TLV properly protects through very fast cooling, we investigated two additional groups submitted to a conventional hypothermia (cold saline infusion + external cooling) or to normothermic TLV. The primary outcome was the survival during 7 days of follow-up. The secondary outcomes were clinical, biochemical, hemodynamic and histological parameters describing neurological, cardiac, pulmonary, liver and kidney potential dysfunctions. We also aimed to investigate whether ultra-fast cooling can protect the heart through an early inhibition of cardiac cell death. The latter point was also critical to further support the relevance of very fast cooling to limit the subsequent dysfunction following cardiac arrest.
Methods
The animal instrumentation and ensuing experiments were conducted in accordance with French official regulations (agreement A94-046-13) after approval by the local ethical committee. The investigation conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health.
Animal preparation
New Zealand rabbits (3.0–3.5 kg) were anesthetized using zolazepam, tiletamine and pentobarbital (all 20–30 mg/kg i.v.). They were intubated and mechanically ventilated. After administration of pancuronium bromide (200 μg/kg i.v.), two electrodes were implanted upon the chest and inserted into the esophagus for subsequent induction of ventricular fibrillation. Rectal, esophageal and tympanic temperatures were continuously monitored using thermal probes (Harvard Apparatus, Paris, France). Throughout the protocol, external electrocardiogram was recorded, as well as arterial blood pressure from a catheter implanted into the ear artery. Data were digitalized and analyzed using the data acquisition software HEM v3.5 (Notocord, Croissy-sur-Seine, France).
Cardiac arrest and cardiopulmonary resuscitation
After animal preparation and subsequent stabilisation, ventricular fibrillation was induced by passing an alternative current (10 V, 4 mA; 2 min) between the implanted electrodes. Mechanical ventilation was stopped at the onset of fibrillation and throughout the subsequent period of cardiac arrest. After either 5 or 10 min of untreated fibrillation, cardiopulmonary resuscitation was started using cardiac massage (~ 200 beats/min), electric attempts of defibrillation (5–10 J/kg) and intravenous administration of epinephrine (15 μg/kg i.v.). Resumption of spontaneous circulation (ROSC) was considered as an organized cardiac rhythm associated with a mean arterial pressure above 40 mmHg during at least 1 min. After ROSC, administration of epinephrine was further permitted during a maximum of 7 h at a dosage appropriately adjusted to maintain mean arterial pressure at ~80 mmHg. Mechanical ventilation was continued until weaning and awakening of the animals. Rabbits subsequently returned to their cage for a survival follow-up. They received antibiotics (enrofloxacine, 5 mg/kg i.m.) during 7 days and analgesics (buprenorphine, 30 μg/kg s.c.) during 3 days.
Experimental protocol
As shown in Figure 1, rabbits randomly underwent either 5 or 10 min of cardiac arrest with subsequent cardiopulmonary resuscitation. For each duration of cardiac arrest, rabbits were randomly allocated to resuscitation under normothermic conditions (Control5′ and Control10′ groups, respectively) or with hypothermia induced by TLV (H-TLV5′ and H-TLV10′ groups, respectively). In these last two groups, TLV was started at the 10th min following cardiopulmonary resuscitation (i.e., after ROSC) by filling the lung with 10 ml/kg of perfluorocarbon (Fluorinert, 3M, Cergy, France) and then connecting the endotracheal tube to our prototype of liquid ventilator (Supplemental Figure 1).11, 17, 18 This ventilator was set to a tidal volume of ~7–10 ml/kg of body weight with a respiratory rate of 6 breaths/min. For each breath, the ventilator pumped into and out of the lungs the tidal volume of liquids. The perfluorocarbon mixture was bubbled with 100% O2. The temperature of the heat exchanger was adjusted to maintain esophageal and tympanic temperatures at a target temperature of ~32°C. After 20 min of TLV and achie vement of the hypothermic target temperature, the perfluorocarbon was evacuated from the lungs by gravity and the endotracheal tube was again connected to a conventional mechanical ventilator. Hypothermia was further maintained at 32°C during 3 h, if necessary using cold blankets. Animals were subsequently rewarmed using infra-red lights and thermal pads until weaning from conventional ventilation and awakening. Animals were housed in a closed cage enriched in O2 during 2–3 days to avoid hypoxic episodes. In order to determine whether hypothermic TLV properly protects through very fast cooling, we investigated two randomly allocated additional groups submitted to 10 min of cardiac arrest. The first of these groups (Saline 10′) was submitted to 3 h of conventional hypothermia through the combination of cold saline administration (30 ml/kg i.v., NaCl 0.9% at 4°C) and external cooling. The second additional group was submitted to an episode of TLV with normothermic perfluorocarbons (N-TLV10′ group) to determine their proper effects.
Figure 1. Experimental protocol.
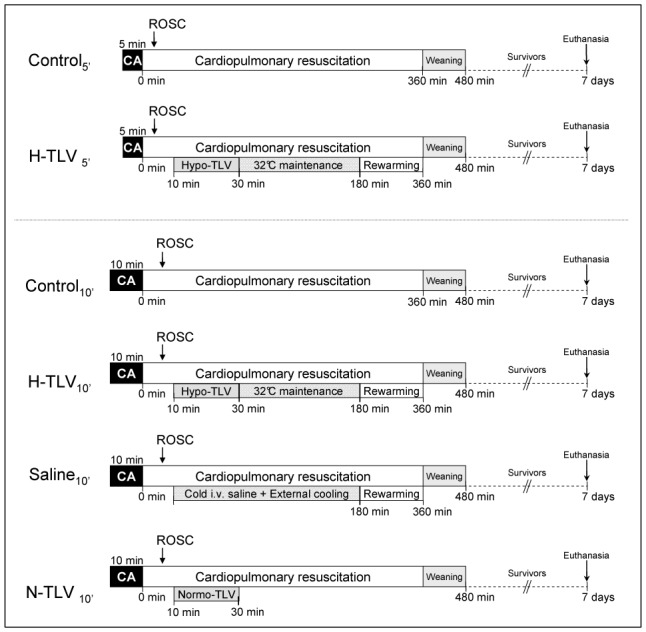
CA, cardiac arrest; TLV, total liquid ventilation initiated; H-TLV, hypothermic TLV; N-TLV, normothermic TLV; Saline, hypothermia induced by intravenous administration of cold saline combined to external cooling; ROSC, resumption of spontaneous circulation.
In order to further investigate the effects of hypothermic TLV, additional rabbits were included in the Control10′ and H-TLV10′ groups, respectively. These animals were euthanized one hour after the cardiac arrest episode for collection of myocardial and blood samples for caspase activity assays and measurement of circulating troponin I, respectively.
Neurological and cardiac dysfunction assessment
Neurological dysfunction was evaluated daily in surviving animals using a clinical score previously validated in rabbits,23 as shown in Supplemental Table 1 (0–10% = normal; 100% = brain death). After 7 days of follow-up, surviving rabbits were reanesthetized and a pressure catheter (SciSense, London, Ontario, Canada) was introduced into the left ventricle through the right carotid artery for measurement of end-diastolic pressures as well as positive and negative left ventricular rate of pressure development (dP/dtmax and dP/dtmin). These parameters were also measured in a group of Sham rabbits that were neither submitted to cardiac arrest nor hypothermia.
Blood chemistry and caspase activity assay
Blood pH, carbon dioxide and oxygen partial pressures (pCO2 and pO2, respectively) were assessed from arterial blood samples with an ABL 77 series analyser (Radio-meter SA, France). Blood lactate was determined on an Accutrend® Plus analyser (Roche Diagnostics, Mannheim, Germany). Liver and renal functions were evaluated by measuring the alanine aminotransferase (ALAT) and creatinine concentrations (Prestige 24i, Tokyo-Boehi, Japan). Troponin I and Creatinine Kinase were measured by an off-site laboratory (IDEXX Laboratories, Alfortville, France).
Caspase 3 activity was assayed from cardiac samples, as previously described.24 Briefly, tissues were homogenized in cold buffer (25 mM HEPES pH 7.5, 5 mM MgCl2, 2 mM EDTA, 0.1% Triton X-100, 2 mM dithiothreitol, 1 mM PMSF, 5 μl/ml protease cocktail inhibitor P8340; Sigma-Aldrich, St Louis, MO, USA). Homogenates were centrifuged and supernatants collected. Proteins (90 μg) were incubated in caspase assay buffer (50 mM HEPES pH 7.4, 100 mM NaCl, 1 mM EDTA, 10 mM dithiothreitol, Triton X-100 0.1%, glycerol 10%). Enzymatic reaction was started by addition of 0.2 mM of the fluorogenic substrates ac-DEVD-AFC (Biomol Research Laboratories, Hambourg, Germany). Fluorescent arbitrary units were converted into pmol/mg protein/h using a standard curve of free AFC (Biomol Research Laboratories, Hambourg, Germany).
Histological analyses
After 7 days of follow-up after cardiac arrest, the surviving rabbits were finally euthanized for pathological analyses of the heart, lung, kidney, liver and brain. These organs were also removed and analyzed in the animals died before the end of the follow-up. For lungs, a slice was sampled from each lobe (5 per lung). For the heart, we analyzed a mid heart transversal biventricular section. For kidneys, two slices were studied for each organ. We used a 0–3 score system to blindly quantify the severity of each organ alteration, as shown in Supplemental Tables 2 and 3 (0=normal; 3=very severe lesion). The overall brain score was the mean value obtained for cortex, hippocampus and cerebellum, as previously described.23 For lungs, we assessed two separate scores for cardiogenic lesions (serous edema and/or congestion) and infectious complication of bronchopneumonia, respectively. The first panels of Figures 2 and 3 illustrate typical normal and pathological aspects of the different organs.
Figure 2.
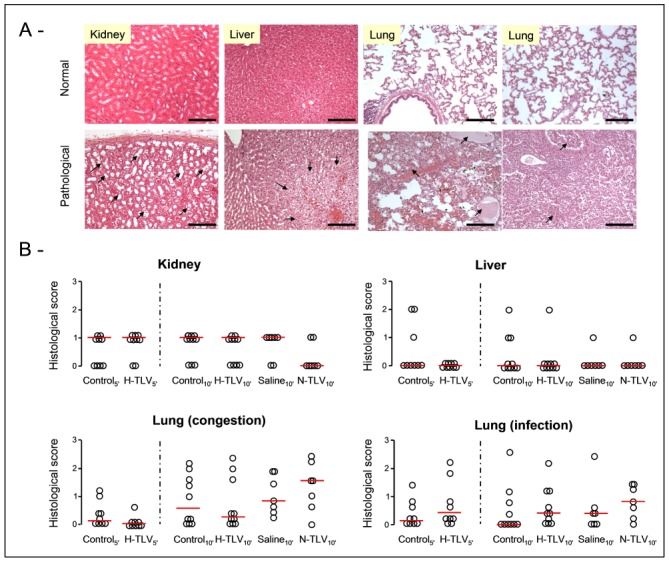
Panel A: Examples of normal or pathological histological appearances of the kidney, liver and lungs in the TLV and control groups, respectively. In kidney, lesions consisted in dilated regenerative proximal tubules (arrows, bar=120 μm). In liver, we observed systematized clarification of hepatocytes (arrows, bar=120 μm). In lungs, lesions were congestion and serous edema (arrows in the left lung panel, bar=120 μm) or foci of bronchopneumonia (arrows in the right lung panel, bar=120 μm).
Panel B: Histological scores of alteration in kidney, liver and lungs of rabbits from the different groups. For lungs, we assessed two separate scores for cardiogenic lesions and infection complications, respectively. Open circles represents individual scores and the thick line represents the median value of corresponding group.
* p<0.05 vs corresponding control; TLV, total liquid ventilation; H-TLV, hypothermic TLV; N-TLV, normothermic TLV; Saline, hypothermia induced by intravenous administration of cold saline combined to external cooling.
Figure 3.
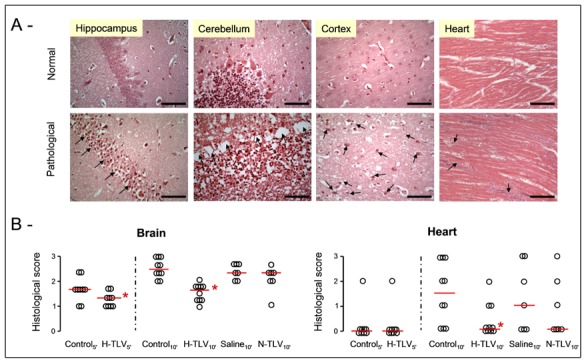
Panel A: Examples of normal or pathological histological appearances of the brain and the heart in the TLV and control groups, respectively. In brain, ischemic disorders consisted in ischemic pyramidal cells with pycnotic nucleus in the hippocampus (arrows, bar=30 μm), in laminar necrosis of Purkinje cells in the cerebellum (arrows, bar=30 μm) or in numerous ischemic neurons in the cortex (arrows, bar=30 μm), respectively. In the myocardium, we observed foci of cardiomyocytes necrosis (arrows, bar=120 μm).
Panel B: Histological scores of alteration in the brain and heart of rabbits from the different groups. Open circles represents individual scores and the thick line represents the median value of the corresponding group.
* p<0.05 vs corresponding control; TLV, total liquid ventilation; H-TLV, hypothermic TLV; N-TLV, normothermic TLV; Saline, hypothermia induced by intravenous administration of cold saline combined to external cooling.
Statistical analyses
Data were expressed as mean±SEM. Hemodynamic and biochemical parameters were compared between the different groups and corresponding controls using a two-way ANOVA for repeated measures. Post-hoc analyses were performed at each time-point as compared to controls using a Student t-test with Bonferroni correction. Values were not compared between the different time-points in order to avoid multiple comparisons. In each experimental group, neurological dysfunction and histological scores were compared to the corresponding control group using a Mann-Whitney non parametric test. Survival curves were obtained using a Kaplan-Meier analysis and were compared to the corresponding control group using a log-rank test. These last analyses took only into account the animals that achieved ROSC. Significant differences were determined at P≤0.05.
Results
Seventy rabbits were included in the present study and submitted to cardiac arrest (n=10, 10, 15, 15, 10 and 10 in the Control5′, H-TLV5′, Control10′, H-TLV10′, Saline10′ and N-TLV10′ groups, respectively).
As shown in Table 1, all rabbits subjected to 5 min of cardiac arrest were successfully resuscitated (Control5′ and H-TLV5′ groups) whereas only 10/15 were successfully resuscitated in the Control10′ and H-TLV10′ groups, respectively. This rate was 7/10 in Saline10′ and N-TLV10′ groups. Regardless the duration of the cardiac arrest, the time to resumption of spontaneous circulation was not significantly different among groups for each duration of cardiac arrest.
Table 1.
Groups characteristics during cardiopulmonary resuscitation, including the rate of successful resuscitation, the time to resumption of spontaneous circulation (ROSC) and the total amount of epinephrine administered throughout the protocol.
| n
|
Rate of successfull resuscitation
|
ROSC (min)
|
Epinephrine dose (μg/kg)
|
|
|---|---|---|---|---|
| Control 5′ | 10 | 10/10 | 2.4±0.3 | 207±58 |
| H-TLV 5′ | 10 | 10/10 | 2.3±0.3 | 174±81 |
| Control 10′ | 15 | 10/15 | 4.8±0.4 | 684±118 |
| H-TLV 10′ | 15 | 10/15 | 4.2±0.8 | 128±128 * |
| Saline 10′ | 10 | 7/10 | 3.7±0.7 | 430±126 |
| N-TLV 10′ | 10 | 7/10 | 3.7±0.4 | 509±64 |
p<0.05 vs corresponding control value; TLV, total liquid ventilation; H-TLV, hypothermic TLV; N-TLV, normothermic TLV; Saline, hypothermia induced by intravenous administration of cold saline combined to external cooling.
As illustrated in Figure 4, esophageal, tympanic and rectal temperatures were not significantly different among groups at baseline. A mild and passive decrease in body temperature was observed in the Control5′ and Control10′ groups after cardiac arrest but this remained within the normothermic range. In H-TLV groups, esophageal and tympanic temperatures decreased very rapidly after the institution of TLV. As example, tympanic temperature achieved 33.3±0.5 and 32.5±0.3 °C in H- TLV5′ and H-TLV10′ within 10 min after the onset of the cooling protocol, respectively. In the Saline 10′ group, such tympanic temperatures were achieved after ~30 min. Regarding esophageal and rectal temperatures, the time to achieve 32 to 33°C was less than 5 and 20 min in H-TLV10′ whereas this was more than 45 and 60 min in Saline10′, respectively. In the N-TLV10′ group, body temperatures did not significantly differ from the Control10′ values throughout the experimental protocol.
Figure 4. Esophageal, tympanic and rectal temperatures in the different experimental groups.
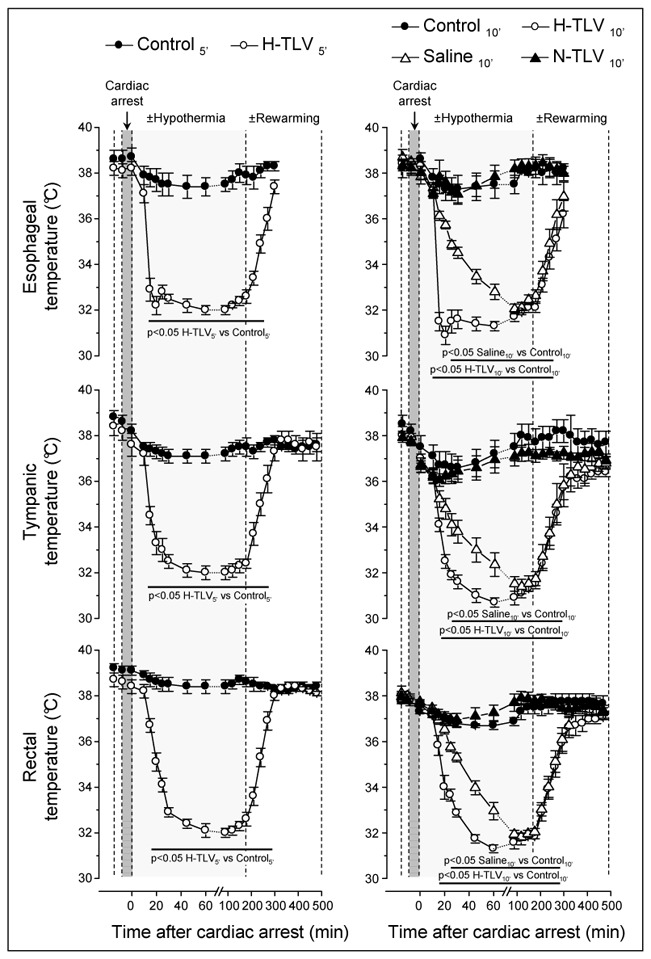
* p<0.05 vs corresponding control; n=10 in each experimental group; TLV, total liquid ventilation; H-TLV, hypothermic TLV; N-TLV, normothermic TLV; Saline, hypothermia induced by intravenous administration of cold saline combined to external cooling.
As shown in Table 2, heart rate significantly decreased during the hypothermic phase in hypothermic groups as compared to corresponding controls (e.g., −21%, −28% and −31% at 60 min after cardiac arrest in H-TLV5′, H-TLV10′ and Saline10′ vs corresponding controls, respectively). Mean arterial pressure was not significantly different between groups throughout the experimental protocol since epinephrine administration was permitted to maintain a ~80 mmHg value during 7 h after cardiac arrest. As shown in Table 1, the total amount of epinephrine administered throughout cardiopulmonary resuscitation was however significantly lower in H-TLV10′ vs Control10′ (128±128 vs 684±118 μg/kg, respectively), suggesting a favorable hemodynamic effect of hypothermic TLV. We did not observe such a significant difference in H-TLV5′ vs Control5′ but epinephrine dosages were much lower (174±81 vs 207±58 μg/kg, respectively). In Saline10′ and N-TLV10′ groups, epinephrine dosages were also not different from Control10′. After discontinuation of any pharmacological support (e.g., at 8 h after cardiac arrest), the lactate levels were significantly lower in H-TLV5′ vs Control5′ (1.2±0.2 vs 4.8±1.7 mmol/l) and in H-TLV10′ vs Control10′ (3.6±0.7 vs 7.0±1.7 mmol/l). Those levels were not significantly different among Saline10′ and N-TLV10′ groups as compared to Control10′ (5.9±0.7 and 7.6±0.6 vs 7.0±1.7 mmol/l).
Table 2.
Mean arterial pressure, heart rate, plasma creatinine and alanine aminotransferase concentrations (ALAT) throughout the experimental protocol in the different groups.
| n | Baseline | Cardiopulmonary resuscitation
|
Day 1 (n) | |||||
|---|---|---|---|---|---|---|---|---|
| 15min | 60min | 180min | 360min | 480min | ||||
| Epinephrine perfusion | No | Yes | Yes | Yes | Yes | No | ||
| Heart rate (beat/min) | ||||||||
| Control 5′ | 10 | 257±11 | 222±8 | 221±7 | 243±11 | 216±7 | 220±9 | 234±8 (10) |
| H-TLV 5′ | 10 | 259±10 | 202±12 | 174±6 * | 177±9 * | 245±9 | 234±8 | 244±10 (10) |
| Control 10′ | 10 | 263±10 | 219±6 | 220±10 | 198±8 | 221±11 | 231±13 | 256±17 (7) |
| H-TLV 10′ | 10 | 267±8 | 167±10 * | 158±8 * | 167±11 | 208±12 | 240±11 | 252±7 (8) |
| Saline 10′ | 7 | 266±7 | 200±10 | 153±7 * | 155±13 * | 219±10 | 218±10 | 226±16 (6) |
| N-TLV 10′ | 7 | 256±13 | 216±19 | 207±9 | 213±12 | 207±9 | 221±15 | 240±28 (2) |
| Mean arterial pressure (mmHg) | ||||||||
| Control 5′ | 10 | 81±3 | 83±4 | 82±3 | 83±1 | 83±4 | 80±4 | 83±4 (10) |
| H-TLV 5′ | 10 | 80±7 | 81±3 | 82±5 | 82±3 | 83±3 | 79±3 | 82±4 (10) |
| Control 10′ | 10 | 80±5 | 82±3 | 83±3 | 81±4 | 83±2 | 80±3 | 79±4 (7) |
| H-TLV 10′ | 10 | 83±4 | 81±4 | 82±3 | 81±3 | 81±4 | 80±4 | 79±6 (8) |
| Saline 10′ | 7 | 80±8 | 86±6 | 89±2 | 78±5 | 82±5 | 76±6 | 83±9 (6) |
| N-TLV 10′ | 7 | 78±7 | 78±5 | 78±1 | 78±4 | 78±5 | 75±7 | 88±4 (2) |
| Plasma creatinine concentrations (mg/l) | ||||||||
| Control 5′ | 10 | 10±1 | 11±1 | - | 10±1 | - | - | 10±1 (10) |
| H-TLV 5′ | 10 | 10±0 | 12±1 | - | 11±1 | - | - | 10±1 (10) |
| Control 10′ | 10 | 9±1 | 13±1 | - | 14±2 | - | - | 11±1 (7) |
| H-TLV 10′ | 10 | 10±0 | 13±1 | - | 12±1 | - | - | 11±1 (8) |
| Saline 10′ | 7 | 9±1 | 10±1 | - | 10±1 | - | - | 10±1 (6) |
| N-TLV 10′ | 7 | 9±1 | 11±1 | - | 12±1 | - | - | 13±6 (2) |
| Plasma ALAT concentrations (UI/l) | ||||||||
| Control 5′ | 10 | 29±5 | 31±4 | - | 33±4 | - | - | 35±9 (10) |
| H-TLV 5′ | 10 | 25±3 | 26±2 | - | 43±5 | - | - | 30±6 (10) |
| Control 10′ | 10 | 44±13 | 79±25 | - | 115±32 | - | - | 60±17 (7) |
| H-TLV 10′ | 10 | 48±3 | 65±5 | - | 111±27 | - | - | 83±14 (8) |
| Saline 10′ | 7 | 32±2 | 48±4 | - | 101±30 | - | - | 62±27 (6) |
| N-TLV 10′ | 7 | 31±5 | 66±10 | - | 96±13 | - | - | 94±37 (2) |
p<0.05 vs corresponding control value; TLV, total liquid ventilation; H-TLV, hypothermic TLV; N-TLV, normothermic TLV; Saline, hypothermia induced by intravenous administration of cold saline combined to external cooling.
As shown in Figure 5, we observed a severe acidosis with an increase in pCO2 and a decrease in pO2 in all groups following cardiac arrest. In H-TLV5′, pO2 was lower 15 min after cardiac arrest as compared to Control5′. This could be expected as control animals were ventilated with oxygen whereas TLV rabbits underwent liquid ventilation by that time. At 180 min, gas exchanges were conversely improved in H-TLV groups as compared to controls. As example, blood pH and pO2 increased whereas pCO2 decreased in H-TLV10′ vs Control10′, respectively. Importantly, all the animals were submitted to conventional ventilation at that time point, with standardized ventilation parameters. As illustrated in Figure 2, the safety of TLV for lungs was also documented by histology demonstrating cardiogenic lesions (serous edema and/or congestion) or infectious complications of bronchopneumonia to a similar extent in TLV groups vs corresponding controls.
Figure 5. Blood pH, pCO2 and pO2 in the different experimental groups.
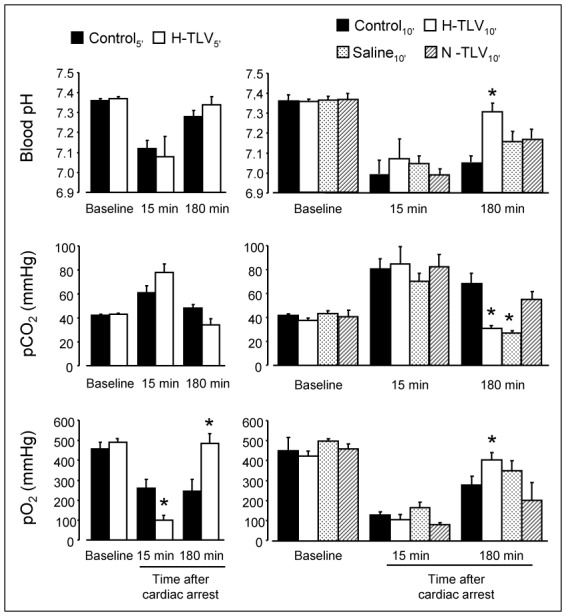
* p<0.05 vs corresponding control; n=10 in each experimental group; TLV, total liquid ventilation; H-TLV, hypothermic TLV; N-TLV, normothermic TLV; Saline, hypothermia induced by intravenous administration of cold saline combined to external cooling.
As shown in Table 2, renal function was not affected after cardiac arrest in all groups since plasma creatinine levels remained within usual values. Conversely, we observed an increase in the liver enzyme ALAT with no difference among TLV and corresponding controls. Kidney and liver lesions were mild with no difference among groups (Figure 2, panel B).
As illustrated in Figure 6, neurological dysfunction was significantly attenuated in H-TLV groups as compared to controls. This difference was significant as early as the 2nd day following cardiac arrest in H-TLV5′ vs Control5′ (panel A) whereas this was observed within 24h of follow-up in H-TLV10′ vs Control10′ (panel B). In Saline10′, a transient improvement in neurological recovery was observed at day 1 but this was no longer significant at day 2 after cardiac arrest. As illustrated in Figure 3 (panel B), the neuroprotective effect of hypothermic TLV was further demonstrated by a significant decrease in the severity of the ischemic disorders in the brain in H-TLV5′ and H-TLV10′ as compared to Control5′ and Control10′ groups, respectively. No any protection was conversely seen in Saline10′ and N-TLV10′ as compared to Control10′.
Figure 6.
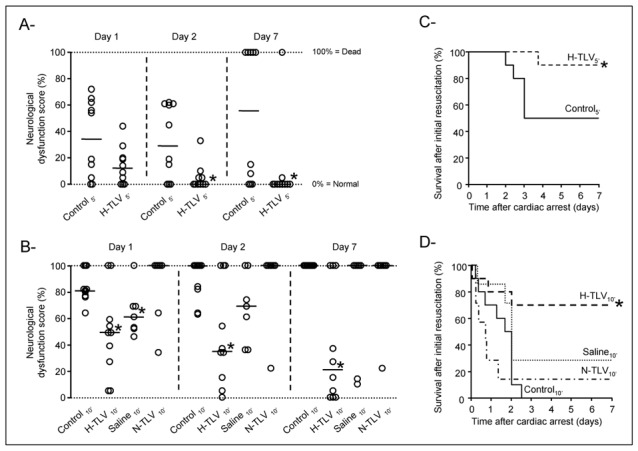
Panels A and B: Neurological dysfunction scores at days 1, 2 and 7 following resuscitation in the different experimental groups submitted to 5 min or 10 min of cardiac arrest, respectively. Open circles represent individual scores and the thick line represents the median value of the corresponding group. Only animals achieving resumption of spontaneous circulation were included.
Panels C and D: Kaplan-Meyer survival curves in the different experimental groups submitted to 5 min or 10 min of cardiac arrest, respectively. Only animals achieving resumption of spontaneous circulation were included.
* p<0.05 vs corresponding control; TLV, total liquid ventilation; H-TLV, hypothermic TLV; N-TLV, normothermic TLV; Saline, hypothermia induced by intravenous administration of cold saline combined to external cooling.
A significant difference in survivals was also evidenced between H-TLV groups and corresponding controls, as illustrated in Figure 6 (panels C and D). At the end of the follow-up, 9/10 and 7/10 rabbits survived in the H-TLV5′ and H-TLV10′ groups as compared to 5/10 and 0/10 in Control5′ and Control10′, respectively. Conversely, survival was not significantly improved in Saline10′ and N-TLV10′ as compared to Control10′.
As illustrated in Figure 3 (panel B), myocardial foci of necrosis were less frequent in H-TLV10′ vs Control10′, demonstrating a cardioprotective effect of hypothermic TLV. No difference was conversely seen between Saline10′ and N-TLV10′ as compared to Control10′. In surviving animals, the functional myocardial sequels of cardiac arrest were also evaluated after 7 days of follow-up. As shown in Supplemental Table 4, mean blood pressure and heart rate in the conscious state were not different among groups as compared to a Sham group. After anesthesia, end diastolic left ventricular pressure, dP/dtmax and dP/dtmin min were also not different between groups, suggesting that there was no major functional myocardial alterations in those surviving animals.
To further explore the cardioprotective effect of hypothermic TLV, eight additional rabbits were included in the Control10′ and H-TLV10′ groups for a surrogate study dedicated to the caspase activity assays and measurement of troponin I levels. As shown in supplemental Figure 2, troponin I measured 60 min after cardiac arrest significantly decreased in H-TLV10′ as compared to Control10′ (1.3±0.3 vs 70.7±30.4 ng/ml, respectively). The cardioprotective effect of hypothermic TLV was also supported by a decrease in caspase 3 activity as compared to control (6.2±1.2 vs 10.0±1.2 pmol/mg prot/h, respectively).
Discussion
The present study provides the proof of concept that ultra-fast whole body cooling with hypothermic TLV limits the post-cardiac arrest syndrome when instituted after ROSC in a rabbit model of ventricular fibrillation. Interestingly, we observed potent neuro- and cardioprotections with hypothermic TLV which remains a safe procedure for the lungs. As we used only 3 h of hypothermia, this also suggested that very early hypothermia after ROSC does not need to be prolonged to produce a strong clinical benefit. Importantly, this benefit was directly related to cooling rapidity with TLV since a conventional cooling with cold saline and external blankets was not significantly protective in similar conditions. Proper effects of the perfluorocarbon are unlikely as related to the lack of protection with normothermic TLV.
Our first finding is the rapidity of TLV-induced cooling since esophagal and brain temperatures achieved ~32–33°C within only 10 min. In comparison, a conventional hypothermic protocol (cold saline infusion + external cooling) requires ~30 and 45 min to similarly reduce these temperatures. The rapid cooling elicited by TLV was directly related to the tidal exchange of the liquid since simple repetitive pulmonary lavages with a 4°C perfluorocarbon requires more than 60 min to decrease the tympanic temperature to 32°C in the same species.20 In large animals, hypothermic TLV was also reported to afford a very fast cooling and to reduce the pulmonary artery temperature to 32°C within 9–10 min when instituted intra-arrest in a ventricular fibrillation model in swine.22
Importantly, the rapid hypothermia elicited by TLV was associated with a potent neurological protection and an increase in survival rate as compared to control conditions. In animal studies, it is admitted that the neuroprotective effect of hypothermia is time dependent and that a large part of the protection is lost when cooling is delayed.25 For example, in a canine model of cardiac arrest, the neurological protection was lost after only 15 min of delay before the onset of hypothermia after ROSC. 25 In the present study, we observed a very potent benefit of hypothermia when achieved rapidly after ROSC with TLV whereas a conventional hypothermia was not significantly protective. Recent experiments have also shown that hypothermia started before ROSC (e.g., intra-arrest hypothermia) can afford an additional benefit7, 8 but this might be difficult to translate this concept into human clinical practice. All these findings demonstrate that most of the possible benefits of hypothermia can be lost within minutes after ROSC, further supporting the need of devices eliciting ultra-fast cooling such as TLV in the present study.
Importantly, the benefit of hypothermic TLV observed in our conditions was produced by a short hypothermic episode (3 h), whereas the current recommendations in humans are maintenance of hypothermia during 24–36 h.1, 2 We choose this short duration since previous experiments have shown that when achieved very early, the duration of the hypothermia does not need to be prolonged to afford an effective neuroprotection, e.g., in a gerbil model of global ischemia. 26 Mice studies also noted that 1 h of cooling after ROSC was sufficient to generate a significant clinical benefit. 7, 8 When the severity of the ischemic insult increases or when the onset of cooling is delayed, it is conversely well established that prolonging hypothermia is critical for achieving a maximal neurological protection.27, 28 As example, a prolonged cooling allowed to provide enduring behavioral and histological protection in rats submitted to permanent middle cerebral artery occlusion, even when delayed after onset of ischemia.27
Another important beneficial effect of hypothermic TLV is the cardioprotection observed here like that previously shown in animal models of coronary artery occlusion.11, 17, 18 This was especially observed after 10 min of cardiac arrest since myocardial lesions were minor in the groups submitted to only 5 min of cardiac arrest. This was evidenced by a limitation in myocardial necrosis and a preservation of myocardial functional performance in surviving rabbits. Cardioprotection was also observed very early after cardiac arrest since troponin I release and caspase 3 activity were significantly decreased within 60 min after resuscitation in H-TLV10′ vs Control10′. In animal models of focal myocardial ischemia, the window of protection with hypothermia is virtually limited to the ischemic phase, whereas cooling at reperfusion is ineffective at reducing infarct-size in most experimental studies.12 In the present study, hypothermia was instituted after global reperfusion (ROSC) but it is reasonable to speculate that the myocardium remains momentarily and partially ischemic even after ROSC. This can explain that a very rapid cooling with TLV can still afford a beneficial effect even if instituted after ROSC and systemic reperfusion. Improved post-resuscitation myocardial function have interestingly also be observed with intra-arrest rapid head cooling.29 Generalized hypothermia could even potentially afford a protection of the liver and/or the kidney.30 As these organs were mildly altered in control conditions in the present study, we were not able to show any difference with hypothermic TLV.
Importantly, TLV was a safe procedure for the lungs. Even, we observed improved gas exchanges using standardized ventilatory parameters in TLV vs control groups at 3 h after cardiac arrest. After weaning from ventilation, animals were however maintained in a cage enriched in oxygen to avoid hypoxic episodes.11 In pigs, intra-arrest liquid ventilation was indeed demonstrated to alter lung function since activation of pulmonary macrophages might alter gas exchanges after resumption to conventional ventilation.21, 22 In our study, the tolerance of TLV was shown by histological examinations and this is supported by several reports from the literature demonstrating that liquid ventilation can even protect the lungs.19, 20 Several prototypes of liquid ventilator have been developed and the clinical translation of this concept might accordingly be feasible when those devices will be available for a clinical use. 31 To date, the current prototypes are mostly developed for a paediatric use31 and the translation of TLV-induced hypothermia would be accordingly first possible in newborns presenting global ischemia. Further developments might also ultimately permit a translation in adult patients.
Our study has several limitations. First, neurological dysfunctions were assessed on the basis of clinical and histological parameters. Other more functional tests or imaging would also be important. Second, histological analyses were performed in all animals, irrespectively of their survival time. This would have lead to an underestimation of the histological scores in some animals who died very early after the cardiac arrest. However, since the lower scores were observed in the group that lived for the longer time (H-TLV10′), the latter limitation should not actually impact our conclusions.
In conclusion, ultra-fast cooling instituted by hypothermic TLV limits the post-cardiac arrest dysfunction with associated neuro- and cardioprotective effects. Importantly, TLV was a safe procedure for the lungs in our experimental conditions. The beneficial effects of hypothermic TLV were probably directly related to the rapidity in temperature decrease since myocardial cell death inhibition was evidenced even very early following resuscitation.
Clinical perspective.
Mild therapeutic hypothermia is known to improve survival and neurological recovery in patients resuscitated from cardiac arrest. Previous experimental studies however demonstrated that the benefit afforded by hypothermia was closely linked to the rapidity in body temperature decrease after resuscitation. The present article investigates an original approach offering a very rapid and generalized cooling using total liquid ventilation (TLV) with temperature controlled perfluorocarbons. These liquids can use the lungs as heat exchangers while maintaining normal gas exchanges. We showed that this strategy potently limits the post-cardiac arrest syndrome when instituted after resumption of spontaneous circulation in a rabbit model of cardiac arrest. The protection was evidenced regarding survival as well as neurological and cardiac sequels. The benefit was directly related to cooling rapidity since a conventional cooling with infusion of cold saline and external blankets was not significantly protective in similar conditions. Proper effects of the perfluorocarbon are also unlikely as normothermic TLV was not protective. These results offer promising perspectives for the induction of a neuroprotective and cardioprotective rapid cooling using TLV in resuscitated patients. Several prototypes of liquid ventilator have been developed and the clinical translation of this concept will be feasible when they will be available for a clinical use. The current prototypes are mostly developed for a paediatric use and the translation of hypothermic TLV would be first possible in newborns presenting global ischemia. The current development of devices devoted to liquid ventilation in adult patients will further expand the possible applications of this original approach.
Supplementary Material
Acknowledgments
The authors are indebted to Dr J.M. Downey, Dr M.V Cohen and Dr J.C. Parker for their insightful comments and creative ideas at the beginning of these investigations. We are also greatly indebted to Pr J. Grassi (ITMO “Technologies pour la Santé”) and Dr C. Cans (INSERM-transfert) for their important support and advices. We thank Sandrine Bonizec for her excellent administrative support and the central laboratory of the National Veterinary School of Alfort that performed the biochemical analyses of the kidney and liver blood parameters.
Funding source
This study was supported by grant TLV-CARDAREST (R10028JS) from INSERM and ITMO “Technologies pour la Santé” and grant ET7-460 from the “Fondation de l’Avenir”. Mourad Chenoune was supported by a grant from the “Groupe de Reflexion sur la Recherche Cardiovasculaire” and by a “Poste d’accueil INSERM 2009”. Renaud Tissier was also a recipient of a “Contrat d’Interface INSERM-ENV” (2010) and of a grant from the “Société Française de Cardiologie” (“Edouard Corraboeuf” grant, 2010).
Footnotes
Disclosure
None.
The present study was partially presented during the Resuscitation Symposium of the American Heart Association in Chicago in 2010 (12–13 Nov).
References
- 1.Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, Smith K. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557–563. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- 2.The Hypothermia After Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346:549–556. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- 3.Nozari A, Safar P, Stezoski SW, Wu X, Henchir J, Radovsky A, Hanson K, Klein E, Kochanek PM, Tisherman SA. Mild hypothermia during prolonged cardiopulmonary cerebral resuscitation increases conscious survival in dogs. Crit Care Med. 2004;32:2110–2116. doi: 10.1097/01.ccm.0000142700.19377.ae. [DOI] [PubMed] [Google Scholar]
- 4.Nozari A, Safar P, Stezoski SW, Wu X, Kostelnik S, Radovsky A, Tisherman S, Kochanek PM. Critical time window for intra-arrest cooling with cold saline flush in a dog model of cardiopulmonary resuscitation. Circulation. 2006;113:2690–2696. doi: 10.1161/CIRCULATIONAHA.106.613349. [DOI] [PubMed] [Google Scholar]
- 5.Guan J, Barbut D, Wang H, Li Y, Tsai MS, Sun S, Inderbitzen B, Weil MH, Tang W. A comparison between head cooling begun during cardiopulmonary resuscitation and surface cooling after resuscitation in a pig model of cardiac arrest. Crit Care Med. 2008;36:S428–433. doi: 10.1097/ccm.0b013e31818a8876. [DOI] [PubMed] [Google Scholar]
- 6.Tsai MS, Barbut D, Tang W, Wang H, Guan J, Wang T, Sun S, Inderbitzen B, Weil MH. Rapid head cooling initiated coincident with cardiopulmonary resuscitation improves success of defibrillation and post-resuscitation myocardial function in a porcine model of prolonged cardiac arrest. J Am Coll Cardiol. 2008;51:1988–1990. doi: 10.1016/j.jacc.2007.12.057. [DOI] [PubMed] [Google Scholar]
- 7.Zhao D, Abella BS, Beiser DG, Alvarado JP, Wang H, Hamann KJ, Hoek TL, Becker LB. Intra-arrest cooling with delayed reperfusion yields higher survival than earlier normothermic resuscitation in a mouse model of cardiac arrest. Resuscitation. 2008;77:242–249. doi: 10.1016/j.resuscitation.2007.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abella BS, Zhao D, Alvarado J, Hamann K, Vanden Hoek TL, Becker LB. Intra-arrest cooling improves outcomes in a murine cardiac arrest model. Circulation. 2004;109:2786–2791. doi: 10.1161/01.CIR.0000131940.19833.85. [DOI] [PubMed] [Google Scholar]
- 9.Hale SL, Dae MW, Kloner RA. Hypothermia during reperfusion limits ‘no-reflow’ injury in a rabbit model of acute myocardial infarction. Cardiovasc Res. 2003;59:715–722. doi: 10.1016/s0008-6363(03)00456-5. [DOI] [PubMed] [Google Scholar]
- 10.Miki T, Swafford AN, Cohen MV, Downey JM. Second window of protection against infarction in conscious rabbits: real or artifactual. J Mol Cell Cardiol. 1999;31:809–816. doi: 10.1006/jmcc.1998.0917. [DOI] [PubMed] [Google Scholar]
- 11.Tissier R, Couvreur N, Ghaleh B, Bruneval P, Lidouren F, Morin D, Zini R, Bize A, Chenoune M, Belair MF, Mandet C, Douheret M, Dubois-Rande JL, Parker JC, Cohen MV, Downey JM, Berdeaux A. Rapid cooling preserves the ischaemic myocardium against mitochondrial damage and left ventricular dysfunction. Cardiovasc Res. 2009;83:345–353. doi: 10.1093/cvr/cvp046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tissier R, Chenoune M, Ghaleh B, Cohen MV, Downey JM, Berdeaux A. The small chill: mild hypothermia for cardioprotection? Cardiovasc Res. 2010;88:406–414. doi: 10.1093/cvr/cvq227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Larsson IM, Wallin E, Rubertsson S. Cold saline infusion and ice packs alone are effective in inducing and maintaining therapeutic hypothermia after cardiac arrest. Resuscitation. 2010;81:15–19. doi: 10.1016/j.resuscitation.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 14.Dixon SR, Whitbourn RJ, Dae MW, Grube E, Sherman W, Schaer GL, Jenkins JS, Baim DS, Gibbons RJ, Kuntz RE, Popma JJ, Nguyen TT, O’Neill WW. Induction of mild systemic hypothermia with endovascular cooling during primary percutaneous coronary intervention for acute myocardial infarction. J Am Coll Cardiol. 2002;40:1928–1934. doi: 10.1016/s0735-1097(02)02567-6. [DOI] [PubMed] [Google Scholar]
- 15.Yu T, Barbut D, Ristagno G, Cho JH, Sun S, Li Y, Weil MH, Tang W. Survival and neurological outcomes after nasopharyngeal cooling or peripheral vein cold saline infusion initiated during cardiopulmonary resuscitation in a porcine model of prolonged cardiac arrest. Crit Care Med. 2010;38:916–921. doi: 10.1097/CCM.0b013e3181cd1291. [DOI] [PubMed] [Google Scholar]
- 16.Boller M, Lampe JW, Katz JM, Barbut D, Becker LB. Feasibility of intra-arrest hypothermia induction: A novel nasopharyngeal approach achieves preferential brain cooling. Resuscitation. 2010;81:1025–1030. doi: 10.1016/j.resuscitation.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chenoune M, Lidouren F, Ghaleh B, Couvreur N, Dubois-Rande J-L, Berdeaux A, Tissier R. Rapid cooling of the heart with total liquid ventilation prevents transmural myocardial infarction following prolonged ischemia in rabbits. Resuscitation. 2010;81:359–362. doi: 10.1016/j.resuscitation.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 18.Tissier R, Hamanaka K, Kuno A, Parker JC, Cohen MV, Downey JM. Total liquid ventilation provides ultra-fast cardioprotective cooling. J Am Coll Cardiol. 2007;49:601–605. doi: 10.1016/j.jacc.2006.09.041. [DOI] [PubMed] [Google Scholar]
- 19.Wolfson MR, Shaffer TH. Pulmonary applications of perfluorochemical liquids: ventilation and beyond. Paediatr Respir Rev. 2005;6:117–127. doi: 10.1016/j.prrv.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 20.Yang SS, Jeng MJ, McShane R, Chen CY, Wolfson MR, Shaffer TH. Cold perfluorochemical-induced hypothermia protects lung integrity in normal rabbits. Biol Neonate. 2005;87:60–65. doi: 10.1159/000081245. [DOI] [PubMed] [Google Scholar]
- 21.Riter HG, Brooks LA, Pretorius AM, Ackermann LW, Kerber RE. Intra-arrest hypothermia: both cold liquid ventilation with perfluorocarbons and cold intravenous saline rapidly achieve hypothermia, but only cold liquid ventilation improves resumption of spontaneous circulation. Resuscitation. 2009;80:561–566. doi: 10.1016/j.resuscitation.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Staffey KS, Dendi R, Brooks LA, Pretorius AM, Ackermann LW, Zamba KD, Dickson E, Kerber RE. Liquid ventilation with perfluorocarbons facilitates resumption of spontaneous circulation in a swine cardiac arrest model. Resuscitation. 2008;78:77–84. doi: 10.1016/j.resuscitation.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baker AJ, Zornow MH, Grafe MR, Scheller MS, Skilling SR, Smullin DH, Larson AA. Hypothermia prevents ischemia-induced increases in hippocampal glycine concentrations in rabbits. Stroke. 1991;22:666–673. doi: 10.1161/01.str.22.5.666. [DOI] [PubMed] [Google Scholar]
- 24.Souktani R, Pons S, Guegan C, Bouhidel O, Bruneval P, Zini R, Mandet C, Onteniente B, Berdeaux A, Ghaleh B. Cardioprotection against myocardial infarction with PTD-BIR3/RING, a XIAP mimicking protein. J Mol Cell Cardiol. 2009;46:713–718. doi: 10.1016/j.yjmcc.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 25.Kuboyama K, Safar P, Radovsky A, Tisherman SA, Stezoski SW, Alexander H. Delay in cooling negates the beneficial effect of mild resuscitative cerebral hypothermia after cardiac arrest in dogs: a prospective, randomized study. Crit Care Med. 1993;21:1348–1358. doi: 10.1097/00003246-199309000-00019. [DOI] [PubMed] [Google Scholar]
- 26.Carroll M, Beek O. Protection against hippocampal CA1 cell loss by post-ischemic hypothermia is dependent on delay of initiation and duration. Metab Brain Dis. 1992;7:45–50. doi: 10.1007/BF01000440. [DOI] [PubMed] [Google Scholar]
- 27.Clark DL, Penner M, Wowk S, Orellana-Jordan I, Colbourne F. Treatments (12 and 48 h) with systemic and brain-selective hypothermia techniques after permanent focal cerebral ischemia in rat. Exp Neurol. 2009;220:391–399. doi: 10.1016/j.expneurol.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 28.Wu X, Drabek T, Kochanek PM, Henchir J, Stezoski SW, Stezoski J, Cochran K, Garman R, Tisherman SA. Induction of profound hypothermia for emergency preservation and resuscitation allows intact survival after cardiac arrest resulting from prolonged lethal hemorrhage and trauma in dogs. Circulation. 2006;113:1974–1982. doi: 10.1161/CIRCULATIONAHA.105.587204. [DOI] [PubMed] [Google Scholar]
- 29.Tsai MS, Barbut D, Wang H, Guan J, Sun S, Inderbitzen B, Weil MH, Tang W. Intra-arrest rapid head cooling improves postresuscitation myocardial function in comparison with delayed postresuscitation surface cooling. Crit Care Med. 2008;36:S434–439. doi: 10.1097/ccm.0b013e31818a88b6. [DOI] [PubMed] [Google Scholar]
- 30.Kang J, Albadawi H, Casey PJ, Abbruzzese TA, Patel VI, Yoo HJ, Cambria RP, Watkins MT. The effects of systemic hypothermia on a murine model of thoracic aortic ischemia reperfusion. J Vasc Surg. 2010;52:435–443. doi: 10.1016/j.jvs.2010.03.021. [DOI] [PubMed] [Google Scholar]
- 31.Robert R, Micheau P, Avoine O, Beaudry B, Beaulieu A, Walti H. A regulator for pressure-controlled total-liquid ventilation. IEEE Trans Biomed Eng. 2010;57:2267–2276. doi: 10.1109/TBME.2009.2031096. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


