SUMMARY
Excess serum free fatty acids (FFAs) are fundamental to the pathogenesis of insulin resistance. With high fat feeding, FFAs activate NF-κB in target tissues, initiating negative cross-talk with insulin signaling. However, the mechanisms underlying FFA-dependent NF-κB activation remain unclear. Here we demonstrate that the saturated FA, palmitate, requires Bcl10 for NF-κB activation in hepatocytes. Uptake of palmitate, metabolism to diacylglycerol, and subsequent activation of PKC appear to mechanistically link palmitate with Bcl10, known as a central component of a signaling complex that, along with CARMA3 and MALT1, activates NF-κB downstream of selected cell surface receptors. Consequently, Bcl10-deficient mice are protected from hepatic NF-κB activation and insulin resistance following brief high fat diet, suggesting that Bcl10 plays a major role in the metabolic consequences of acute overnutrition. Surprisingly, while CARMA3 also participates in the palmitate response, MALT1 is completely dispensable, thereby revealing an apparent non-classical role for Bcl10 in NF-κB signaling.
INTRODUCTION
The metabolic syndrome is a cluster of conditions that greatly increases the risk for type 2 diabetes mellitus (T2DM) and cardiovascular disease. A key component to the syndrome is chronic overnutrition and development of obesity (Unger and Scherer, 2010). Changes in adipose tissue that occur in central/visceral obesity contribute to a systemic, low-grade inflammatory state now known as “metabolic inflammation” which impairs insulin signaling in key tissues including liver, skeletal muscle, and brain, thereby producing systemic insulin resistance (Gregor and Hotamisligil, 2010).
While the insulin resistance associated with obesity unfolds over a long period of time and is associated with changes in the cellular makeup and function of adipose tissue, there is also a well-recognized short-term consequence of overnutrition that is likely due to the direct effects of nutrient excess (Kleemann et al., 2010; Schenk et al., 2008). This is particularly notable with the fat overnutrition that characterizes the Western diet. Under conditions of moderate fat intake, lipoprotein lipase expressed in the microvasculature releases fatty acids from triacylglycerols contained in circulating chylomicrons as a means for transferring dietary lipids to adipocytes for subsequent storage (Miles and Nelson, 2007). In this way, fatty acids obtained directly from the diet do not circulate freely, outside the confines of chylomicrons or related lipoproteins. In contrast, even a single high fat meal results in an increase in serum free fatty acids (FFAs), a consequence of the “spillover” effect whereby the lipoprotein lipase-mediated release of fatty acids exceeds the capacity of fatty acid uptake and storage in adipocytes (Miles and Nelson, 2007). This postprandial elevation in serum FFAs is only compounded by visceral obesity because spillover rates are particularly high in the splanchnic vascular bed, which expands to support the excess visceral fat (Nelson et al., 2007; Nielsen et al., 2004). In this scenario, FFAs become a pathologic nutrient, capable of disrupting metabolic homeostasis (Boden, 2011).
Hepatocytes are thought to be a major target for the deleterious effects of elevated FFAs, in part because FFAs released in the splanchnic bed are delivered through the portal circulation directly to the liver. Saturated FAs in particular activate the canonical NF-κB pathway in liver, which then mediates inhibitory cross-talk at various steps in insulin signaling pathways and causes hepatic insulin resistance (Baker et al., 2011). Despite the implications for the pathogenesis of T2DM, the mechanisms by which FFAs activate NF-κB are still unclear. There are likely multiple mechanisms at play, with increasing evidence suggesting that cell type- and tissue-specific differences may exist with regard to FFA-dependent signaling. Considerable data indicate that some FFAs act as ligands for toll-like receptor 4 (TLR4), expressed abundantly on macrophages, and therefore signal to NF-κB in a receptor-dependent manner (Lee et al., 2001; Saberi et al., 2009; Shi et al., 2006). Other data suggest that FFAs are taken up by target cells and undergo metabolism to produce signaling intermediates, such as diacylglycerols (DAGs) and ceramides, which can then signal to NF-κB through PKC intermediates (Erion and Shulman, 2010; Holland and Summers, 2008; Turban and Hajduch, 2011). Finally, FFA metabolites may also induce endoplasmic reticulum (ER) stress culminating in activation of kinases, such as the double-stranded RNA-dependent protein kinase (PKR), that interface with the NF-κB pathway (Nakamura et al., 2010; Ozcan et al., 2004). These mechanisms are not mutually exclusive and it is likely that different cells utilize different mechanisms depending upon the cellular repertoire of receptors, metabolic enzymes, and signaling intermediates.
In lymphocytes and some related immune cells, a critically important signaling event mediating adaptive immunity involves activation of a signaling complex (CBM signalosome) composed of a scaffolding protein, CARMA1, an adaptor protein, Bcl10, and an effector protein, MALT1, that communicates with the NF-κB machinery. In lymphocytes, assembly of the CBM signalosome first requires T or B cell receptor-dependent PKC activation and phosphorylation of CARMA1 (Wegener and Krappmann, 2007). Recently, we found that the CBM signaling components are present in non-immune cells including hepatocytes, with the highly homologous CARMA3 protein substituting for CARMA1 (McAllister-Lucas et al., 2007). Further, activation of the receptor for angiotensin II (AGTR1) on hepatocytes could also engage PKC-dependent CBM signaling and NF-κB activation (McAllister-Lucas et al., 2007). As a result, we wondered if FFA-dependent activation of PKC, through generation of intermediates such as DAG or ceramide, might harness the CBM signalosome as a means for activating NF-κB; this would represent the first physiologic example of receptor-independent NF-κB activation through the CBM signalosome. Results show that palmitate indeed activates NF-κB in hepatocytes in a Bcl10-dependent manner. Intriguingly, while CARMA3 also participates in palmitate-dependent NF-κB activation, MALT1 is completely dispensable. Further, Bcl10−/− mice are protected from hepatic NF-κB activation following high-fat feeding, and are also protected from systemic insulin resistance. These findings reveal a novel molecular mechanism through which FFAs can communicate with the canonical NF-κB machinery.
RESULTS
Palmitate induces hepatocellular NF-κB with delayed kinetics
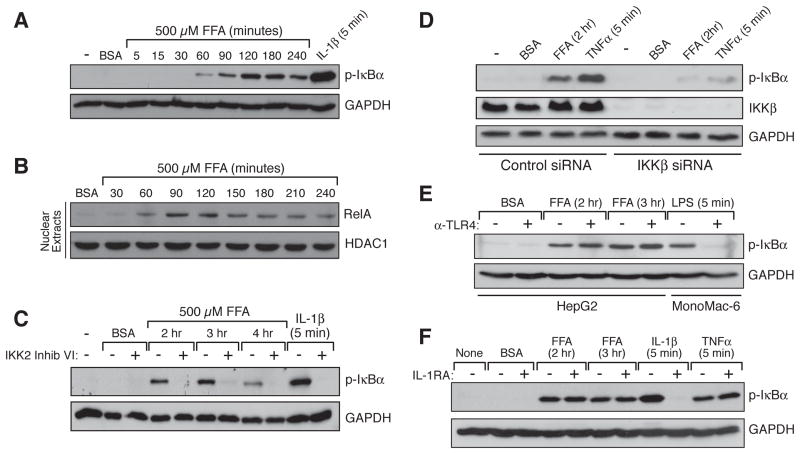
High fat feeding and obesity are associated with increased flux of FFAs into the circulation, thereby leading to enhanced uptake of the fatty acids into multiple tissues, including the liver. In order to mimic the influx of excess FFAs into hepatocytes, we treated human HepG2 cells with palmitate, a saturated FA found abundantly in the Western diet. We observed a time- and concentration-dependent induction of IκBα phosphorylation following palmitate treatment, indicating activation of the canonical NF-κB pathway (Figures 1A and S1A). In this pathway, stimulation of the multisubunit IκB Kinase (IKK) complex triggers phosphorylation-dependent proteasomal degradation of IκBα thus releasing transcriptionally active p50/RelA NF-κB dimers for translocation into the nucleus (Baker et al., 2011). As such, we also observed palmitate-dependent nuclear accumulation of RelA (Figure 1B). In addition, we demonstrated that IKK2 inhibitor VI, a selective inhibitor of the canonical pathway-specific IKKβ subunit, blocks palmitate-induced IκBα phosphorylation, as does siRNA-mediated knockdown of IKKβ protein (Figures 1C and 1D).
Figure 1. Palmitate-dependent NF-κB Activation in Hepatocytes.
(A and B) Time course for canonical NF-κB pathway activation in HepG2 cells following palmitate (FFA) treatment.
(C) Effect of IKK2 inhibitor VI (10 μM), an inhibitor of the canonical pathway kinase, IKKβ, on palmitate (FFA)-dependent NF-κB activation.
(D) Effect of IKKβ knockdown on palmitate (FFA) -dependent NF-κB activation.
(E and F) Palmitate (FFA) -dependent NF-κB activation in HepG2 cells in the absence or presence of either 10 μg/ml TLR4 blocking antibody (E) or 50 ng/ml IL-1R antagonist (F).
See also Figure S1.
Interestingly, NF-κB activation occurred much more slowly in response to palmitate as compared to interleukin-1β (IL-1β) (Figure 1A). IL-1β, a pro-inflammatory cytokine whose level is elevated during obesity, induces canonical NF-κB activation in hepatocytes via stimulation of its receptor, IL-1R (Schroder et al., 2010). IL-1β treatment of hepatocytes induced robust phosphorylation of IκBα within 5 minutes, typical for a receptor-mediated response. In contrast, no significant phosphorylation of IκBα or RelA nuclear translocation was observed until after 60 minutes of palmitate treatment, with peak activation occurring between 90 and 120 minutes. This difference in the time course suggests that palmitate stimulates canonical NF-κB signaling via a mechanism that is distinctly different from that used by IL-1β, and may indicate that palmitate-dependent activation of NF-κB does not occur via traditional receptor-mediated intracellular signaling.
Neither TLR4, nor an IL-1β/IL-1R autocrine loop, mediate palmitate-dependent NF-κB activation in hepatocytes
We then investigated whether TLR4, a pattern recognition receptor implicated in FFA-dependent signaling in macrophages, myocytes, and adipocytes (Fessler et al., 2009), is required for palmitate-induced canonical NF-κB activation in hepatocytes. Previous gene expression profiling studies had shown that TLR4 expression is quite low in the liver, as compared to several other tissues (Lattin et al., 2008; Wu et al., 2009). In particular, expression in liver cells is several orders of magnitude lower than in macrophages, myoblasts, and adipocytes (Figure S1B). Furthermore, high throughput analysis of gene transcriptional responses has revealed that treating primary rat hepatocytes with the classic TLR4 agonist, lipopolysaccharide (LPS), does not induce a notable NF-κB gene expression signature (Deng et al., 2010). With this in mind, we first evaluated the response of HepG2 cells to LPS; these cells represent a good model for testing hepatocellular sensitivity to bona fide TLR4 agonists because, albeit low, levels of TLR4 and its downstream signaling intermediates such as Myd88 are comparable in HepG2 cells and in primary hepatocytes (Liguori et al., 2008). We found that, even at high doses, LPS does not stimulate canonical NF-κB signaling in HepG2 cells (Figure S1C), consistent with what has been seen with a range of other hepatocyte cell lines (Preiss et al., 2008). Second, we demonstrated that α-TLR4, a receptor-blocking antibody that prevents LPS-mediated NF-κB activation in MonoMac-6 macrophages, has no effect on palmitate-induced IκBα phosphorylation in HepG2 cells (Figure 1E). Together, our results and the gene expression profiling studies suggest that the TLR4 signaling machinery is not robustly active in hepatocytes and that palmitate does not utilize TLR4 to stimulate canonical NF-κB activation in these cells.
Recent reports demonstrate that palmitate can induce activation of the NLRP3 inflammasome in macrophages and in liver (Csak et al., 2011; Vandanmagsar et al., 2011; Wen et al., 2011). Inflammasomes are large multi-protein complexes that, when activated by exogenous or endogenous “danger signals” such as cholesterol crystals, uric acid crystals and amyloid-β protein, stimulate the cleavage of pro-IL-1β, thereby promoting maturation and secretion of active IL-1β (Davis et al., 2011). This could potentially establish a feed-forward, inflammatory loop due to autocrine IL-1β-dependent NF-κB activation. We therefore investigated whether the palmitate-dependent NF-κB activation we observed in hepatocytes occurs via an IL-1β autocrine loop, a finding that might account for the time delay required for NF-κB activation. However, we found that while an IL-1R antagonist peptide (IL-1RA) completely prevents NF-κB activation in hepatocytes treated with IL-1β, the peptide has no effect on palmitate-dependent NF-κB activation (Figure 1F).
Taken together, these results indicate that the striking time delay required for palmitate-dependent NF-κB activation is not explained by an IL-1β autocrine loop, nor is it consistent with a direct, receptor-mediated response to palmitate, as might occur if TLR4 was present on hepatocytes and capable of recognizing palmitate as an agonist. Instead, we reasoned that the delay might reflect the need for internalization and metabolism of palmitate into signaling intermediates capable of stimulating the NF-κB machinery.
Palmitate-dependent canonical NF-κB activation in hepatocytes requires DAG-dependent stimulation of PKC, but not ceramide production
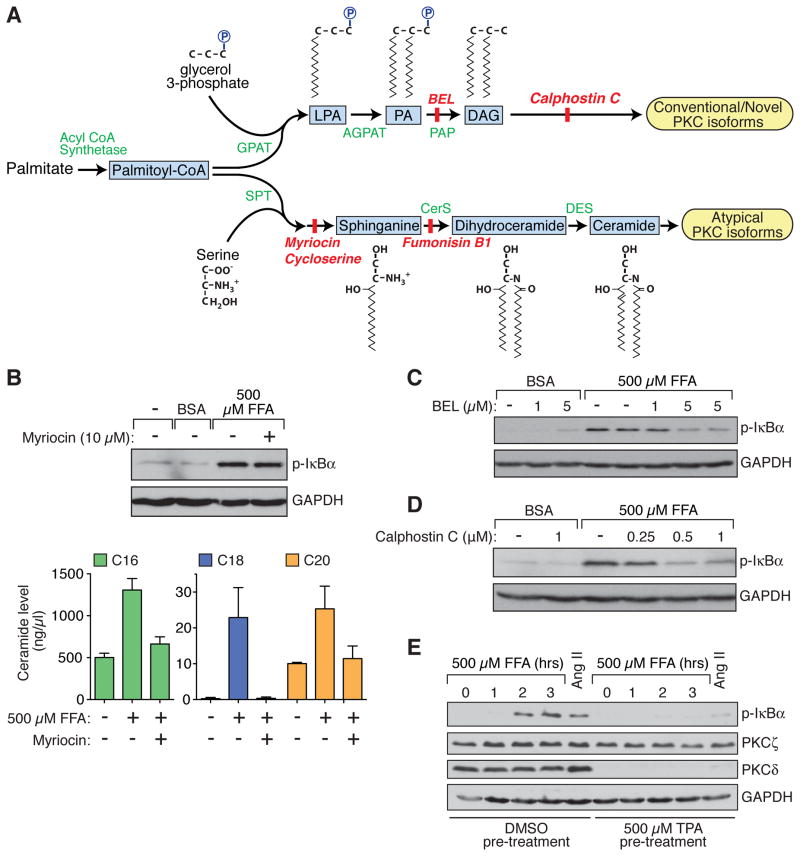
Upon entering the cell, palmitate can be incorporated into either of two bioactive metabolites that have been implicated in NF-κB stimulation and insulin resistance, DAG or ceramide (Figure 2A) (Deevska and Nikolova-Karakashian, 2011). In order to determine if ceramide production contributes to the observed palmitate-induced NF-κB activation in hepatocytes, we evaluated the effect of myriocin, an inhibitor of the rate-limiting enzyme in ceramide synthesis, serine palmitoyltransferase (SPT) (Summers, 2010). While myriocin completely prevented palmitate-dependent increases in intracellular ceramide species, it had no effect on palmitate-dependent IκBα phosphorylation (Figure 2B). Similarly, cycloserine and fumonisin B1, two other inhibitors of enzymes catalyzing committed steps in ceramide synthesis, had no effect on palmitate-dependent IκBα phosphorylation (Figure S2). In contrast, bromoenol lactone (BEL), which inhibits phosphatidic acid phosphohydrolase (PAP) activity and prevents the incorporation of palmitate into DAG, and Calphostin C, a potent inhibitor of DAG-sensitive PKC isoforms, both blocked palmitate-dependent IκBα phosphorylation (Figures 2C and 2D).
Figure 2. Hepatocellular Palmitate Metabolism and the Impact on NF-κB Activation.
(A) Schematic of palmitate metabolism.
(B) Lack of an effect of myriocin (10 μM), a ceramide synthesis inhibitor, on palmitate (FFA) -dependent NF-κB activation in HepG2 cells (upper panel). Cellular levels of three ceramide species (C16, C18, and C20) were induced by palmitate treatment, but induction of all three was blocked by myriocin (lower panel).
(C and D) Effect of DAG synthesis inhibition by BEL (C) or of blocking DAG-sensitive PKC isoforms with Calphostin C (D) on the palmitate (FFA) response.
(E) Overnight pre-treatment of HepG2 cells with TPA caused downregulation of DAG-sensitive PKCs (eg, PKCδ), but not atypical PKCs (eg, PKCζ), and prevented palmitate (FFA) -dependent NF-κB activation. As expected, TPA pre-treatment also prevented NF-κB activation in response to Ang II.
See also Figure S2.
We next attempted to use an siRNA approach to identify a specific PKC isoform that might be responsible for the palmitate effect. However, initial studies showed that downregulation of individual isoforms was largely ineffective, suggesting redundancy in the function of multiple PKCs. We therefore used an alternate approach to test whether the palmitate response depends on the group of DAG-responsive, conventional/novel PKC isoforms, or on the group of ceramide-responsive, atypical PKCs. This approach made use of the well-known ability of 12-O-tetradecanoylphorbol-13--acetate (TPA) to selectively downregulate DAG-responsive PKC isoforms after prolonged exposure. As expected, upon treating cells overnight with TPA, we found that levels of PKCδ, an example of a DAG-responsive isoform, were almost undetectable, while levels of PKCζ, an example of an atypical PKC, were completely unaltered (Figure 2E). Importantly, this TPA pre-treatment completely abolished palmitate-dependent IκBα phosphorylation (Figure 2E). Taken together, the results from the use of enzymatic inhibitors and TPA-mediated PKC downregulation suggest that DAG generation, but not ceramide generation, and subsequent activation of classic/novel PKC isoforms are critical for palmitate’s ability to stimulate canonical NF-κB signaling in hepatocytes.
Bcl10 is required for palmitate-dependent NF-κB stimulation in hepatocytes
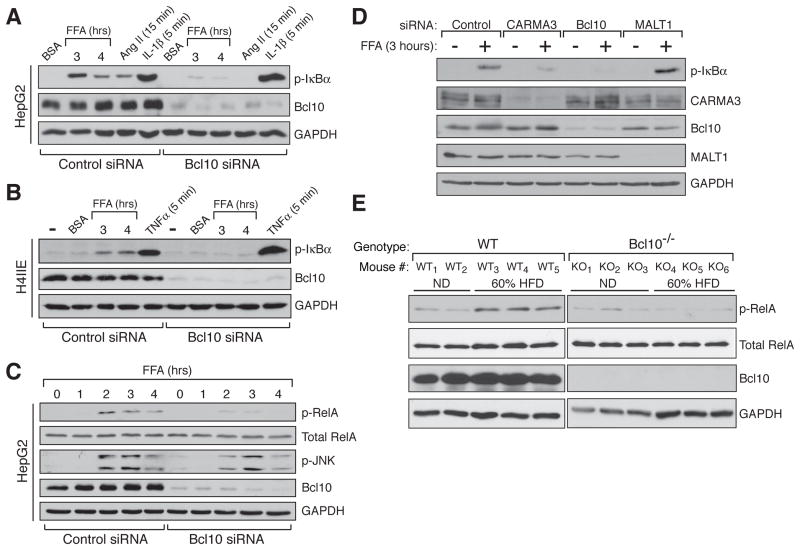
The CBM signalosome, composed of the proteins CARMA1/3, Bcl10 and MALT1, is assembled in response to cell-surface receptor dependent PKC activation, and mediates downstream canonical NF-κB activation. Thus, the signalosome functions as a bridge linking PKC to the NF-κB machinery, but to date this has only been demonstrated physiologically in the context of stimulating a select group of receptors that include the lymphocyte antigen receptors and a subset of G protein-coupled receptors (GPCRs) (Blonska and Lin, 2011; Wegener and Krappmann, 2007). Since our data indicated that PKC activation is required for palmitate to stimulate IκBαpalmitate-dependent PKC activation to stimulation of NF-κB, but in a unique, receptor-independent fashion. First, we demonstrated that siRNA-mediated depletion of Bcl10, a central linker protein required for assembly of the signalosome, prevents palmitate-dependent stimulation of IκBα phosphorylation in HepG2 cells (Figures 3A and S3A). This effect of Bcl10 depletion was also observed in rat H4IIE hepatoma cells, demonstrating that the requirement of Bcl10 is not a peculiarity of the HepG2 cells and is not a species-specific phenomenon (Figure 3B). Bcl10 knockdown also blocked palmitate-dependent phosphorylation of the RelA NF-κB subunit on Ser536 (Figure 3C). This phosphorylation event occurs as a consequence of IKKβ activation and contributes to optimal activity of the canonical NF-κB transcription factor (O’Mahony et al., 2004; Sakurai et al., 1999). Importantly, we found that role of Bcl10 in mediating a response to palmitate is relatively specific for the NF-κB pathway, since Bcl10 knockdown had only a slight effect on JNK1/2 phosphorylation (Figures 3C and S3B).
Figure 3. Bcl10 is a Critical Mediator of FFA-dependent NF-κB Activation in liver.
(A and B) Bcl10 knockdown by siRNA in either human HepG2 (A) or rat 4IIE (B) hepatocytes blocks palmitate (FFA) -dependent NF-κB activation. Activation by Ang II (1 μM) was included as a positive control, while activation by IL-1β (10 ng/ml) and TNFα (1 ng/ml) were used as negative controls to demonstrate the selective impact of Bcl10 knockdown on only certain pathways of IKK activation.
(C) Bcl10 knockdown blocked RelA phosphorylation, another marker of NF-κB activation, but did not significantly impact JNK activation.
(D) Effect of siRNA-mediated knockdown, in HepG2 cells, of each component of the CBM signalosome. CARMA3 knockdown, but not MALT knockdown, impaired the palmitate (FFA) response.
(E) NF-κB activation in livers taken from WT or Bcl10−/− mice following 3 days on either a normal diet (ND) or a 60% HFD.
See also Figure S3.
Next, we evaluated the contribution of the other principal components of the CBM signalosome, using a knockdown strategy. CARMA3 depletion was effective at blocking palmitate-dependent IκBα phosphorylation, but we were surprised to find that MALT1 depletion was inconsequential (Figure 3D). In fact, in many experiments the level of IκBα phosphorylation was somewhat higher in the absence of MALT1.
We then investigated the role of Bcl10 in FFA-dependent NF-κB activation in hepatocytes in vivo. First, we found that wild-type (WT) mice on a high saturated fat diet (HFD) responded with a peak of NF-κB activation in liver, as measured by p-RelA induction, after about three days (Figure S3C). Strikingly, HFD did not elicit this response in Bcl10-deficient (Bcl10−/−) mice (Figure 3E). JNK activation in livers was variable during the course of the brief HFD, without obvious differences between WT and Bcl10−/− mice (Figure S3D). However, given that Bcl10 knockdown in cultured hepatocytes had a slight impact on palmitate-dependent JNK1/2 phosphorylation (Figures 3C and S3B), we cannot completely rule out the possibility that differences in JNK responsiveness may exist between WT and Bcl10−/− mice at some point during HFD and ultimately influence insulin sensitivity in liver.
Bcl10 deficiency protects against high-fat diet induced insulin resistance
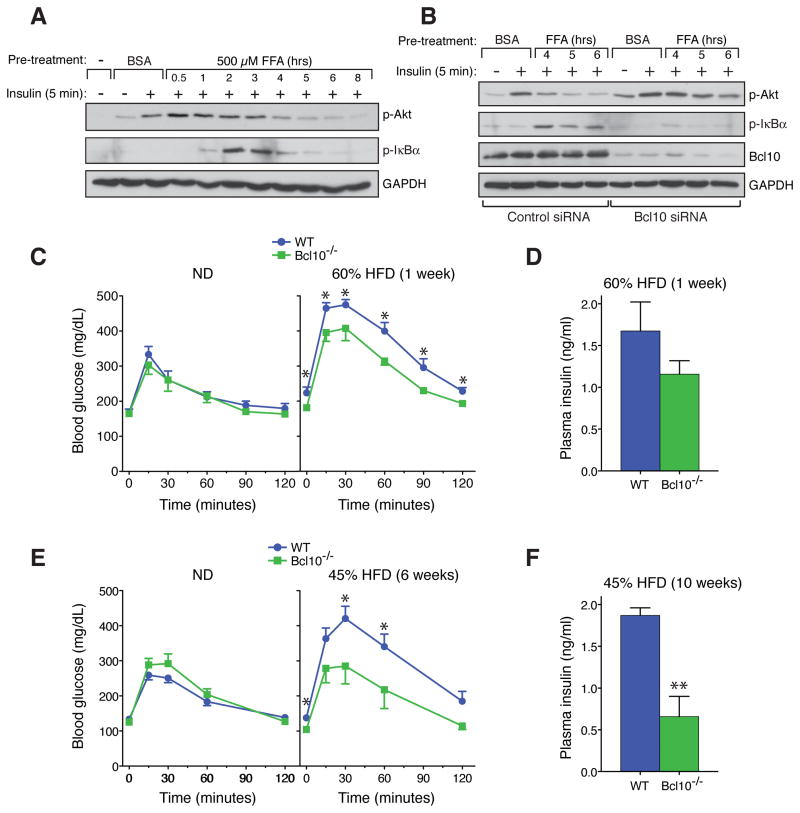
In the hepatocyte, insulin receptor stimulation leads to phosphorylation of the receptor substrate proteins, IRS1 and IRS2, which then coordinate phosphatidylinositol 3-kinase (PI3-kinase)-dependent activation of Akt (Taniguchi et al., 2006). One major function of activated Akt is to then direct the downregulation of genes required for gluconeogenesis. Several recent studies suggest that FFA-induced NF-κB activation contributes to hepatic insulin resistance via inhibitory cross-talk within the hepatocyte, resulting in blockade of specific steps in downstream insulin signaling pathways (Arkan et al., 2005; Cai et al., 2005; Kim et al., 2001; Yuan et al., 2001). Consistent with this notion, we found that palmitate pre-treatment of HepG2 cells caused a concentration- and time-dependent inhibition of insulin-responsive Akt phosphorylation, an effect that temporally followed NF-κB activation (Figures 4A and S4A). However, this effect of palmitate was not reversed by myriocin, cycloserine, or fumonisin B1, inhibitors of ceramide synthesis (Figure S4B). In contrast, siRNA-mediated depletion of Bcl10 did reverse the palmitate-dependent inhibition, restoring insulin sensitivity to the cells as measured by Akt phosphorylation (Figure 4B). These results indicate that Bcl10 is required for palmitate to inhibit insulin signaling, and implicate Bcl10 as a novel factor in promoting hepatocellular insulin resistance.
Figure 4. Bcl10 Deficiency Protects from HFD-induced Insulin Resistance.
(A) Palmitate (FFA) -dependent inhibition of insulin signaling in HepG2 cells.
(B) Bcl10 knockdown by siRNA in HepG2 cells restores insulin sensitivity in the face of palmitate (FFA) pre-treatment.
(C and E) GTTs following 1 week (C) on normal diet (ND) vs 60% HFD (n= 3–7 per group), or following 6 weeks (E) on ND vs 45% HFD (n= 4–10 per group). Data are mean ± SEM. *p<0.05. (D and F) Plasma insulin levels during GTTs. Data are mean ± SEM. **p<0.01.
See also Figure S4.
We next evaluated the contribution of Bcl10 to insulin resistance in vivo. We performed glucose tolerance tests (GTTs) with WT or Bcl10−/− mice that had been fed either a 60% HFD for 1 week or a 45% HFD for 6 weeks. While weight gain was not significantly different between the WT and Bcl10−/− groups (Figure S4C), this analysis revealed that under both diets, Bcl10−/− mice maintained better glucose control throughout the GTTs compared to WT mice (Figures 4C and 4E). Importantly, Bcl10−/− mice achieved better glucose control with less insulin production, indicating that the effect of Bcl10 deficiency is to protect the mice from HFD-induced insulin resistance (Figures 4D and 4F). These analyses demonstrate that Bcl10 plays a central role in the development of HFD-induced insulin resistance, in vivo.
DISCUSSION
Excess FFAs are key to the pathogenesis of the metabolic syndrome and likely play a role in both the acute insulin resistance seen after short-term high fat feeding, and the chronic insulin resistance associated with obesity. FFAs activate various signaling pathways that inhibit the intracellular actions of insulin; chief among these is the NF-κB pathway. Nevertheless, the mechanisms by which FFAs activate NF-κB are incompletely understood and probably vary depending on the target cell type.
We have identified a novel mechanism by which palmitate activates NF-κB in hepatocytes. In this case, activation depends upon intracellular accumulation of palmitate, metabolism to DAG, and activation of conventional/novel PKCs. Our data also demonstrate a critical downstream role for Bcl10 and CARMA3, supporting a model whereby activated PKC then stimulates the CARMA-Bcl10-MALT1 (CBM) signalosome, a signaling complex that until now has only been associated with cell surface receptor-dependent signaling to NF-κB (Wegener and Krappmann, 2007). One critical difference, however, is highlighted by the fact that MALT1 is completely dispensable for the palmitate response. This suggests that palmitate signaling to NF-κB is not completely analogous to the now classically understood mechanism whereby selected cell surface receptors (eg, TCR, AGTR1) signal to NF-κB. In the later case, signaling proceeds through the CBM signalosome with MALT1 acting as an obligatory effector protein, coordinating the downstream activation of the NF-κB machinery. In the case of palmitate, signaling proceeds to NF-κB through CARMA3 and Bcl10, but in a way that bypasses MALT1. This observation suggests that Bcl10 may have unexplored functions related to canonical NF-κB activation that, with certain stimuli, do not involve MALT1. Evidence for this concept can also be seen from work on Malt1−/− mice which do not completely phenocopy Bcl10−/− mice with respect to BCR-dependent NF-κB signaling (Ruland et al., 2003). Similarly, retinoic acid inducible gene I (RIG-1)-responsive cytokine production appears to depend on Bcl10 and subsequent NF-κB activation, but not on MALT1 (Poeck et al., 2010). Deciphering the MALT1-independent functions of Bcl10 will represent a major new frontier for the field of NF-κB signaling research.
Work presented here represents a paradigm shift in our understanding of how at least some components of the CBM signalosome can be engaged. Specifically, the findings suggest that coupling of CBM components at cell-surface receptor microdomains does not necessarily need to occur for downstream NF-κB activation. Additional work will be needed to fully determine how CARMA3, which is known to connect the signalosome with receptors and is the predominant CARMA family member expressed in non-immune cells, can be stimulated by palmitate metabolism and in the absence of receptor engagement. Since this signaling cascade likely occurs in the cytoplasm, and not at the cell surface, it is intriguing to speculate that the related CARD9 protein is also required; this scaffolding protein is structurally similar to CARMA3 and can be regulated by PKC, but lacks domains that are needed for membrane localization (Hara and Saito, 2009; Strasser et al., 2012). Although it has mostly been studied in myeloid cells, perhaps CARD9 could work in concert with CARMA3 to mediate the palmitate response in hepatocytes.
Our studies highlight the acute effects of FFAs on hepatocellular function and reveal a new mechanism by which short-term high fat feeding impacts systemic insulin sensitivity. These studies also underscore the notion that liver is one of the earliest sites affected by nutrient imbalance, in the form of excess dietary fat. We propose a model for hepatic insulin resistance during high fat feeding and subsequent obesity that takes into account both 1) acute effects of excess fat as a pathologic dietary nutrient and 2) chronic effects that involve the participation of inflammatory cells and the recently identified contribution of the inflammasome (Figure S4D). This model suggests that FFAs act through varied mechanisms that depend on both the cell type affected and the temporal stage in the development of insulin resistance. A more complete understanding of the pathogenesis of insulin resistance will require an understanding of how these varied mechanisms work in an integrated manner over time.
EXPERIMENTAL PROCEDURES
Mice
Bcl10−/− mice have been described (Ruland et al., 2001). Mice were backcrossed onto the C57BL/6J background for more than 10 generations and were used between 2–3 months of age for all experiments involving HFD.
Fatty Acid Preparation and Treatment
FFAs were prepared as described previously (Cousin et al., 2001). Briefly, a 100mM stock of palmitate (Sigma; Cat. No. P5585) was prepared in 0.1 M NaOH by heating to 70°C. Palmitate was then complexed with BSA to make a 5mM working stock via dropwise addition to 10% endotoxin/FA-free Fraction V BSA (MP Biomedicals; Cat. No. 194772), while vortexing. The palmitate/BSA mixture was sterile filtered (0.2μM pore size) before use.
Cell Culture and Western Analysis
Human hepatocellular carcinoma (HepG2) cells and H4IIE rat hepatocytes were obtained from ATCC and maintained in DMEM with 10% FBS. MonoMac-6 cells were cultured in RPMI with 10% FBS. Cells were treated and analyzed as detailed in the Supplemental Information.
RNA Interference
Cells were reverse transfected with siRNAs using Lipofectamine RNAiMAX (Invitrogen). Specific siRNA reagents are listed in the Supplemental Information.
Ceramide Quantification
Cells were extracted with a mixture of methanol and chloroform, including internal standards (D31-C16, C17, and C25 ceramide). Extractions were then dried under nitrogen gas and reconstituted in 100 μl of mobile phase B (60:40 acetonitrile:isopropanol). Analyses were carried out using liquid chromatography-triple quadrupole mass spectrometry (LC-QQQ) in the University of Michigan Lipidomics Core Lab, and data analyzed using the Agilent masshunter suite of programs, version B.04.00.
High Fat Diet Studies
Male WT or Bcl10−/− mice were fed high fat chow from Research Diets, Inc. containing either 60% calories from fat (D12492) for 1 week, or 45% calories from fat (D12451) for 6 weeks. GTTs were performed following a 6 hour fast by administering IP glucose (1.5g/kg). Blood glucose levels were measured using the OneTouch Ultra 2 glucose meter (Lifescan). Serum insulin was measured by ELISA (Crystal Chem, Inc., Cat No. 90080), 15 minutes into the GTT.
Statistics
Data are expressed as mean ± SEM. Differences between groups were compared using unpaired Student’s t tests. P values of < 0.05 were considered significant.
Supplementary Material
HIGHLIGHTS.
Palmitate activates hepatocellular NF-κB through CARMA3 and Bcl10, but not MALT1
Palmitate-dependent DAG/PKC activation triggers the Bcl10-containing signalosome
Bcl10 knockdown restores insulin sensitivity in hepatocytes exposed to palmitate
Bcl10 deficient mice are protected from insulin resistance during high fat diet
Acknowledgments
This work was supported by NIH grants R01-HL082914 and R01-DK079973 (P.C.L.), NIAID Training Grant AI007413 (M.V.B.), the Shirley K. Schlafer Foundation (L.M.M-L.) and a Pilot/Feasibility grant from the Michigan Diabetes Research and Training Center (P.C.L.). This work utilized core services supported by grant DK089503 to the University of Michigan. We thank Naohiro Inohara for advice on bioinformatics and Stephanie Daignault-Newton for help with statistics.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arkan MC, Hevener AL, Greten FR, Maeda S, Li ZW, Long JM, Wynshaw-Boris A, Poli G, Olefsky J, Karin M. IKK-beta links inflammation to obesity-induced insulin resistance. Nat Med. 2005;11:191–198. doi: 10.1038/nm1185. [DOI] [PubMed] [Google Scholar]
- Baker RG, Hayden MS, Ghosh S. NF-kappaB, inflammation, and metabolic disease. Cell Metab. 2011;13:11–22. doi: 10.1016/j.cmet.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blonska M, Lin X. NF-kappaB signaling pathways regulated by CARMA family of scaffold proteins. Cell Res. 2011;21:55–70. doi: 10.1038/cr.2010.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boden G. Obesity, insulin resistance and free fatty acids. Curr Opin Endocrinol Diabetes Obes. 2011;18:139–143. doi: 10.1097/MED.0b013e3283444b09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J, Shoelson SE. Local and systemic insulin resistance resulting from hepatic activation of IKK-β and NF-κB. Nat Med. 2005;11:183–190. doi: 10.1038/nm1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousin SP, Hugl SR, Wrede CE, Kajio H, Myers MG, Jr, Rhodes CJ. Free fatty acid-induced inhibition of glucose and insulin-like growth factor I-induced deoxyribonucleic acid synthesis in the pancreatic beta-cell line INS-1. Endocrinology. 2001;142:229–240. doi: 10.1210/endo.142.1.7863. [DOI] [PubMed] [Google Scholar]
- Csak T, Ganz M, Pespisa J, Kodys K, Dolganiuc A, Szabo G. Fatty acids and endotoxin activate inflammasome in hepatocytes which release danger signals to activate immune cells in steatohepatitis. Hepatology. 2011;54:133–144. doi: 10.1002/hep.24341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis BK, Wen H, Ting JP. The inflammasome NLRs in immunity, inflammation, and associated diseases. Annu Rev Immunol. 2011;29:707–735. doi: 10.1146/annurev-immunol-031210-101405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deevska GM, Nikolova-Karakashian MN. The twists and turns of sphingolipid pathway in glucose regulation. Biochimie. 2011;93:32–38. doi: 10.1016/j.biochi.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Y, Johnson DR, Guan X, Ang CY, Ai J, Perkins EJ. In vitro gene regulatory networks predict in vivo function of liver. BMC Syst Biol. 2010;4:153. doi: 10.1186/1752-0509-4-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erion DM, Shulman GI. Diacylglycerol-mediated insulin resistance. Nat Med. 2010;16:400–402. doi: 10.1038/nm0410-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fessler MB, Rudel LL, Brown JM. Toll-like receptor signaling links dietary fatty acids to the metabolic syndrome. Curr Opin Lipidol. 2009;20:379–385. doi: 10.1097/MOL.0b013e32832fa5c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregor MF, Hotamisligil GS. Inflammatory Mechanisms in Obesity. Annu Rev Immunol. 2010;29:415–445. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- Hara H, Saito T. CARD9 versus CARMA1 in innate and adaptive immunity. Trends Immunol. 2009;30:234–242. doi: 10.1016/j.it.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Holland WL, Summers SA. Sphingolipids, insulin resistance, and metabolic disease: new insights from in vivo manipulation of sphingolipid metabolism. Endocr Rev. 2008;29:381–402. doi: 10.1210/er.2007-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JK, et al. Prevention of fat-induced insulin resistance by salicylate. J Clin Invest. 2001;108:437–446. doi: 10.1172/JCI11559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleemann R, et al. Time-resolved and tissue-specific systems analysis of the pathogenesis of insulin resistance. PLoS One. 2010;5:e8817. doi: 10.1371/journal.pone.0008817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lattin JE, et al. Expression analysis of G Protein-Coupled Receptors in mouse macrophages. Immunome Res. 2008;4:5. doi: 10.1186/1745-7580-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JY, Sohn KH, Rhee SH, Hwang D. Saturated fatty acids, but not unsaturated fatty acids, induce the expression of cyclooxygenase-2 mediated through Toll-like receptor 4. J Biol Chem. 2001;276:16683–16689. doi: 10.1074/jbc.M011695200. [DOI] [PubMed] [Google Scholar]
- Liguori MJ, Blomme EA, Waring JF. Trovafloxacin-induced gene expression changes in liver-derived in vitro systems: comparison of primary human hepatocytes to HepG2 cells. Drug Metab Dispos. 2008;36:223–233. doi: 10.1124/dmd.107.017608. [DOI] [PubMed] [Google Scholar]
- McAllister-Lucas LM, et al. CARMA3/Bcl10/MALT1-dependent NF-kappaB activation mediates angiotensin II-responsive inflammatory signaling in nonimmune cells. Proc Natl Acad Sci U S A. 2007;104:139–144. doi: 10.1073/pnas.0601947103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles JM, Nelson RH. Contribution of triglyceride-rich lipoproteins to plasma free fatty acids. Horm Metab Res. 2007;39:726–729. doi: 10.1055/s-2007-990273. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Furuhashi M, Li P, Cao H, Tuncman G, Sonenberg N, Gorgun CZ, Hotamisligil GS. Double-stranded RNA-dependent protein kinase links pathogen sensing with stress and metabolic homeostasis. Cell. 2010;140:338–348. doi: 10.1016/j.cell.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson RH, Basu R, Johnson CM, Rizza RA, Miles JM. Splanchnic spillover of extracellular lipase-generated fatty acids in overweight and obese humans. Diabetes. 2007;56:2878–2884. doi: 10.2337/db07-0812. [DOI] [PubMed] [Google Scholar]
- Nielsen S, Guo Z, Johnson CM, Hensrud DD, Jensen MD. Splanchnic lipolysis in human obesity. J Clin Invest. 2004;113:1582–1588. doi: 10.1172/JCI21047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Mahony AM, Montano M, Van Beneden K, Chen LF, Greene WC. Human T-cell lymphotropic virus type 1 tax induction of biologically Active NF-κB requires IκB kinase-1-mediated phosphorylation of RelA/p65. J Biol Chem. 2004;279:18137–18145. doi: 10.1074/jbc.M401397200. [DOI] [PubMed] [Google Scholar]
- Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, Tuncman G, Gorgun C, Glimcher LH, Hotamisligil GS. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- Poeck H, et al. Recognition of RNA virus by RIG-I results in activation of CARD9 and inflammasome signaling for interleukin-1β production. Nat Immunol. 2010;11:63–69. doi: 10.1038/ni.1824. [DOI] [PubMed] [Google Scholar]
- Preiss S, Thompson A, Chen X, Rodgers S, Markovska V, Desmond P, Visvanathan K, Li K, Locarnini S, Revill P. Characterization of the innate immune signalling pathways in hepatocyte cell lines. J Viral Hepat. 2008;15:888–900. doi: 10.1111/j.1365-2893.2008.01001.x. [DOI] [PubMed] [Google Scholar]
- Ruland J, et al. Bcl10 is a positive regulator of antigen receptor-induced activation of NF-κB and neural tube closure. Cell. 2001;104:33–42. doi: 10.1016/s0092-8674(01)00189-1. [DOI] [PubMed] [Google Scholar]
- Ruland J, Duncan GS, Wakeham A, Mak TW. Differential requirement for Malt1 in T and B cell antigen receptor signaling. Immunity. 2003;19:749–758. doi: 10.1016/s1074-7613(03)00293-0. [DOI] [PubMed] [Google Scholar]
- Saberi M, Woods NB, de Luca C, Schenk S, Lu JC, Bandyopadhyay G, Verma IM, Olefsky JM. Hematopoietic cell-specific deletion of toll-like receptor 4 ameliorates hepatic and adipose tissue insulin resistance in high-fat-fed mice. Cell Metab. 2009;10:419–429. doi: 10.1016/j.cmet.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai H, Chiba H, Miyoshi H, Sugita T, Toriumi W. IκB kinases phosphorylate NF-κB p65 subunit on serine 536 in the transactivation domain. J Biol Chem. 1999;274:30353–30356. doi: 10.1074/jbc.274.43.30353. [DOI] [PubMed] [Google Scholar]
- Schenk S, Saberi M, Olefsky JM. Insulin sensitivity: modulation by nutrients and inflammation. J Clin Invest. 2008;118:2992–3002. doi: 10.1172/JCI34260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder K, Zhou R, Tschopp J. The NLRP3 inflammasome: a sensor for metabolic danger? Science. 2010;327:296–300. doi: 10.1126/science.1184003. [DOI] [PubMed] [Google Scholar]
- Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser D, et al. Syk kinase-coupled C-type lectin receptors engage protein kinase C-sigma to elicit Card9 adaptor-mediated innate immunity. Immunity. 2012;36:32–42. doi: 10.1016/j.immuni.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers SA. Sphingolipids and insulin resistance: the five Ws. Curr Opin Lipidol. 2010;21:128–135. doi: 10.1097/MOL.0b013e3283373b66. [DOI] [PubMed] [Google Scholar]
- Taniguchi CM, Emanuelli B, Kahn CR. Critical nodes in signalling pathways: insights into insulin action. Nat Rev Mol Cell Biol. 2006;7:85–96. doi: 10.1038/nrm1837. [DOI] [PubMed] [Google Scholar]
- Turban S, Hajduch E. Protein kinase C isoforms: mediators of reactive lipid metabolites in the development of insulin resistance. FEBS Lett. 2011;585:269–274. doi: 10.1016/j.febslet.2010.12.022. [DOI] [PubMed] [Google Scholar]
- Unger RH, Scherer PE. Gluttony, sloth and the metabolic syndrome: a roadmap to lipotoxicity. Trends Endocrinol Metab. 2010;21:345–352. doi: 10.1016/j.tem.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandanmagsar B, Youm YH, Ravussin A, Galgani JE, Stadler K, Mynatt RL, Ravussin E, Stephens JM, Dixit VD. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 2011;17:179–188. doi: 10.1038/nm.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegener E, Krappmann D. CARD-Bcl10-Malt1 signalosomes: missing link to NF-κB. Sci STKE. 2007;2007:pe21. doi: 10.1126/stke.3842007pe21. [DOI] [PubMed] [Google Scholar]
- Wen H, Gris D, Lei Y, Jha S, Zhang L, Huang MT, Brickey WJ, Ting JP. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat Immunol. 2011;12:408–415. doi: 10.1038/ni.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C, et al. BioGPS: an extensible and customizable portal for querying and organizing gene annotation resources. Genome Biol. 2009;10:R130. doi: 10.1186/gb-2009-10-11-r130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan M, Konstantopoulos N, Lee J, Hansen L, Li ZW, Karin M, Shoelson SE. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkbeta. Science. 2001;293:1673–1677. doi: 10.1126/science.1061620. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.