Abstract
Chlorophyll b is synthesized by the oxidation of a methyl group on the B ring of a tetrapyrrole molecule to a formyl group by chlorophyllide a oxygenase (CAO). The full-length CAO from Arabidopsis (Arabidopsis thaliana) was overexpressed in tobacco (Nicotiana tabacum) that grows well at light intensities much higher than those tolerated by Arabidopsis. This resulted in an increased synthesis of glutamate semialdehyde, 5-aminolevulinic acid, magnesium-porphyrins, and chlorophylls. Overexpression of CAO resulted in increased chlorophyll b synthesis and a decreased chlorophyll a/b ratio in low light-grown as well as high light-grown tobacco plants; this effect, however, was more pronounced in high light. The increased potential of the protochlorophyllide oxidoreductase activity and chlorophyll biosynthesis compensated for the usual loss of chlorophylls in high light. Increased chlorophyll b synthesis in CAO-overexpressed plants was accompanied not only by an increased abundance of light-harvesting chlorophyll proteins but also of other proteins of the electron transport chain, which led to an increase in the capture of light as well as enhanced (40%–80%) electron transport rates of photosystems I and II at both limiting and saturating light intensities. Although the quantum yield of carbon dioxide fixation remained unchanged, the light-saturated photosynthetic carbon assimilation, starch content, and dry matter accumulation increased in CAO-overexpressed plants grown in both low- and high-light regimes. These results demonstrate that controlled up-regulation of chlorophyll b biosynthesis comodulates the expression of several thylakoid membrane proteins that increase both the antenna size and the electron transport rates and enhance carbon dioxide assimilation, starch content, and dry matter accumulation.
Light intensity is a major determinant of photosynthesis and plant growth. Under a low-light (LL) regime, both photosynthetic rate and crop productivity are low. Thus, it is essential to increase the rate of photosynthesis at LL intensity by efficiently harvesting the low amount of available solar energy. Solar energy is mostly captured by a light-harvesting chlorophyll protein complex (LHC) of the photosynthetic apparatus. In higher plants, both chlorophyll a (Chl a) and chlorophyll b (Chl b) are bound to the LHCs. The availability of Chl b is essential for the assembly and functioning of most LHC proteins (Bellemare et al., 1982; Peter and Thornber, 1991). Binding of Chl b to the LHC proteins stabilizes the latter in the thylakoid membranes (Paulsen et al., 1993; Lindahl et al., 1995). Therefore, in the absence of Chl b, the LHC proteins decrease (Thornber and Highkin, 1974; Murray and Kohorn, 1991; Murchie and Horton, 1997), partly due to the degradation of unbound LHC apoproteins by proteases (Hoober and Eggink, 2001). Under high light (HL) intensity, plants have a low amount of Chl b and a small LHC antenna, whereas plants grown under LL intensity accumulate more Chl b and have a bigger LHC antenna (Björkman et al., 1972; Leong and Anderson, 1984). This indicates the parallel regulation of Chl b biosynthesis and LHC proteins.
Chl b is synthesized from Chl a by oxidation of a methyl group on the B ring, of the latter molecule, to a formyl group at that position (Porra et al., 1993). The main function of Chl b is to gather light energy and transfer it to Chl a (Duysens, 1952). The gene encoding chlorophyllide a oxygenase (CAO), responsible for Chl b synthesis, has been isolated (Tanaka et al., 1998; Espineda et al., 1999; Tomitani et al., 1999; Nagata et al., 2004; Lee et al., 2005), and the recombinant CAO protein catalyzes the oxidation of chlorophyllide (Chlide) a to Chlide b (Oster et al., 2000). The mRNA and protein expression of CAO is highly regulated by light intensity, and the expression of CAO changes in parallel to that of lhcb (Masuda et al., 2003; Harper et al., 2004; Pattanayak et al., 2005; Tanaka and Tanaka, 2005). It is apparent that the regulation of CAO expression under different light intensities contributes significantly to control Chl b synthesis and consequently the chlorophyll a/chlorophyll b (Chl a/b) ratio in plants. Overexpression of CAO resulted in increased Chl b and LHCII levels, suggesting that enhanced CAO mRNA affects the size of LHCII (Satoh et al., 2001; Tanaka et al., 2001; Pattanayak et al., 2005; Tanaka and Tanaka, 2005). Further study on CAO overexpression in the cyanobacterium Synechocystis also revealed Chl b synthesis (Satoh et al., 2001), and simultaneous overexpression of both CAO and LHCP II in Synechocystis led to increased Chl b content that disturbed tetrapyrrole biosynthesis (Xu et al., 2001, 2002).
The CAO sequence has been classified into four parts: (1) the N-terminal transit peptide; (2) the regulatory A domain; (3) the B domain; and (4) the C domain that is sufficient for its catalytic activity (Nagata et al., 2004). The Clp protease and the A domain are involved in the regulation of Chl b biosynthesis through the destabilization of CAO by sensing the presence of overaccumulated Chl b (Yamasato et al., 2005; Nakagawara et al., 2007; Sakuraba et al., 2009). As the A domain of CAO regulates the level of CAO and thus prevents the excess accumulation of Chl b, overexpression of A domain-deleted CAO in Arabidopsis (Arabidopsis thaliana) results in the overaccumulation of Chl b. Consequently, the plants become vulnerable to photodamage, specifically when etiolated transgenic plants are exposed to either LL or HL immediately after etiolation (Yamasato et al., 2008). Similarly, overexpression of Prochlorothrix CAO, which lacks the regulatory A domain, in Arabidopsis led to the overaccumulation of Chl b, and the transgenic plants were photodamaged under HL intensity (Hirashima et al., 2006). From these studies, it is clear that unregulated excess accumulation of Chl b is deleterious for plants; therefore, overexpression of the A domain-deleted CAO protein in plants would not be useful in increasing the photosynthetic efficiency of the plants.
We have previously reported that overexpression of Arabidopsis full-length CAO (AtCAO) results in increased Chl b synthesis and decreased Chl a/b ratio in LL- and HL-grown tobacco (Nicotiana tabacum) plants (Pattanayak et al., 2005). In this study, we show that the overexpression of AtCAO modulates the flux of the chlorophyll biosynthesis pathway, leading to increased Chl b and total chlorophyll synthesis both in LL- and HL-grown transgenic tobacco plants. We further show that increased Chl b biosynthesis in AtCAO-overexpressing (CAOx) plants results in increased amounts of light-harvesting antenna proteins, efficient capture of solar energy, and increased electron transport at limiting as well as saturating light intensities. Furthermore, CAOx plants have increased carbon dioxide (CO2) fixation, starch content, and dry matter accumulation. These results suggest that engineering plants for larger antennae may have the potential for increasing photosynthesis in plants.
RESULTS
CAOx Plants Were Phenotypically Different from Wild-Type Plants
Substantial morphological differences were observed between wild-type and S2 lines of CAOx plants when grown in a greenhouse in a natural photoperiod for 6 weeks under light intensity of 400 to 450 μmol photons m−2 s−1 at 25°C ± 2°C. We tested 14 transgenic lines, and the S2 line was selected as it had the lowest Chl a/b ratio. CAOx plants had increased vegetative growth (Fig. 1A). Furthermore, the time taken for 50% flowering (day of anthesis) substantially increased; consequently, plant foliage and height continued to increase (Fig. 1B). Plants grown under the above-mentioned conditions were transferred to LL (70–80 μmol photons m−2 s−1) or HL (700–800 μmol photons m−2 s−1) for an additional 18 to 20 d in the greenhouse, and their morphological features were monitored. At the time of flowering, CAOx-LL and CAOx-HL plants were taller than wild-type plants by 15% and 30%, respectively (Table I). Among a similar age group of plants, as compared with the wild type, the number of leaves per plant also increased in both CAOx-LL and CAOx-HL plants (Table I).
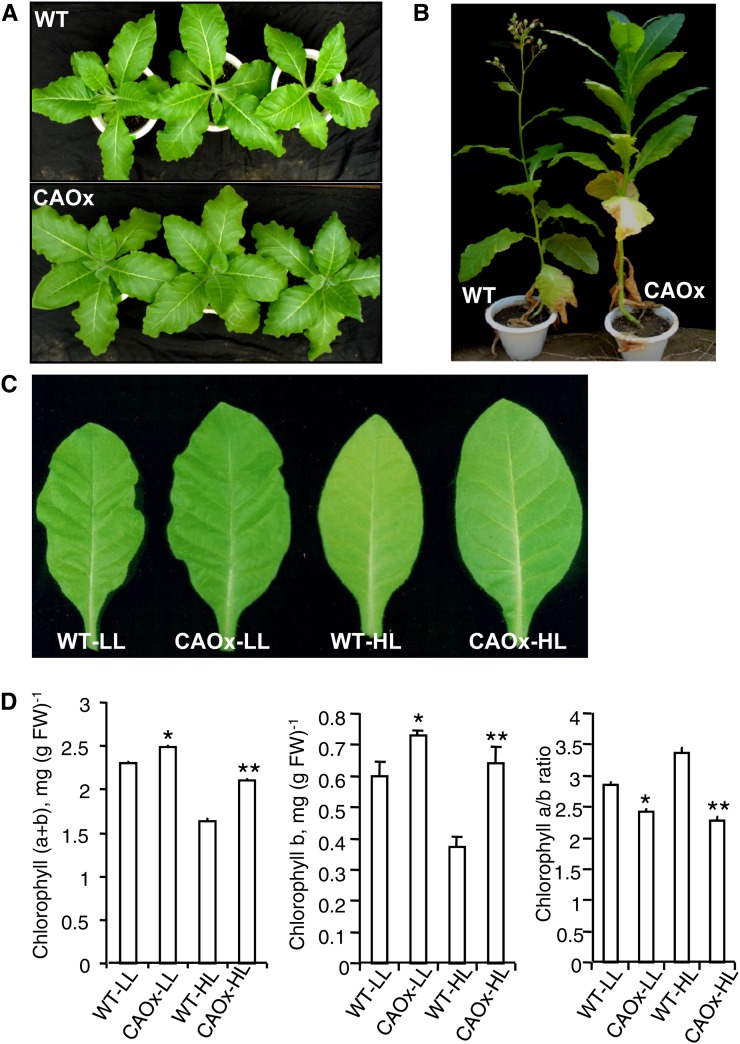
Figure 1.
Plant and leaf phenotypes and chlorophyll content of wild-type (WT) and CAOx tobacco plants grown under LL and HL. A, Phenotypes of 7-week-old wild-type and CAOx tobacco plants grown under normal growth light (400–450 μmol photons m−2 s−1). B, Fifteen-week-old wild-type and CAOx plants grown under normal growth light (400–450 μmol photons m−2 s−1). Note that flowering comes earlier in the wild-type plant than in the CAOx plant. C, Plants grown for up to 25 to 30 d under light intensity of 400 to 450 μmol m−2 s−1 were transferred to LL (70–80 μmol photons m−2 s−1) and HL (700–800 μmol photons m−2 s−1) for an additional 18 to 20 d in the greenhouse, and representative leaves (second leaf) of wild-type and CAOx tobacco plants grown in LL or HL are shown. Note that the leaves of CAOx plants are greener than those of wild-type plants. D, Total chlorophyll content, Chl b, and Chl a/b ratio of wild-type and CAOx plants grown in LL and HL. Each data point is the average of five replicates, and error bars are represented by se. Statistical analysis was performed using Statistica 5.0, and differences were analyzed with one-way ANOVA followed by Tukey’s multiple comparison test. Significance values are expressed as follows: * P < 0.05, ** P < 0.001. FW, Fresh weight. [See online article for color version of this figure.]
Table I. Morphological data of wild-type and CAOx plants grown in LL and HL intensities.
Wild-type and CAOx plants were grown in a greenhouse under LL and HL regimes in a natural photoperiod as described in “Materials and Methods.” Plant height, number of leaves, and days for anthesis (50% flowering) were measured from 15-week-old plants, and those parameters were significantly different in CAOx plants as compared with wild-type plants grown under LL and HL intensities (P < 0.001). As flowering was delayed in CAOx plants, the wild-type and CAOx plants belong to different age groups. The experiment was done three times with similar results. Each data point is the average of 15 replicates, and sd values are given.
CAOx Plants Grown in LL or HL Regimes Had Altered Chl a/b Ratio
The LL- or HL-grown CAOx plants had greener leaves that accumulated higher amounts of chlorophyll as compared with LL- and HL-grown wild-type (WT-LL and WT-HL) plants (28% and 10%, respectively; Fig. 1, C and D). Due to increased synthesis of Chl b in the CAOx-HL and CAOx-LL plants (71% and 22%, respectively), their Chl a/b ratio had declined (33% and 15%, respectively, as compared with WT-HL and WT-LL plants). The Chl a/b ratio and chlorophyll content of wild-type and CAOx plants showed some minor variations in different growth seasons.
There was no significant increase in the specific leaf weight (g dry weight m−2 leaf area) in CAOx-plants as compared with that in wild-type plants. However, as compared with that of LL-grown wild-type and CAOx plants, both WT-HL and CAOx-HL plants had increased (30%) specific leaf weight.
Reduced Chl a/b ratio usually results in increased grana stacking that is usually associated with the modulation of protein content. The chlorophyll content relative to protein of grana lamellae is known to be higher (e.g. 40% in some cases) than that of stroma lamellae from the same preparation (Allen et al., 1972). The chlorophyll-protein ratio of thylakoid membranes isolated from CAOx-LL and CAOx-HL plants had increased by 16% and 23%, respectively, as compared with LL- and HL-grown wild-type plants.
Overexpression of CAO Altered the Metabolic Flux of the Chlorophyll Biosynthesis Pathway
To understand the reasons for increased Chl a + Chl b and Chl b biosynthesis in CAOx plants, selected metabolites of the chlorophyll biosynthesis pathway were measured. As compared with wild-type plants, the steady-state accumulation of chlorophyll biosynthetic intermediates, such as protoporphyrin IX (Proto IX), Mg-protoporphyrin IX, its monoester (MPE), and protochlorophyllide (Pchlide) was higher in CAOx-LL and CAOx-HL plants (Fig. 2A).
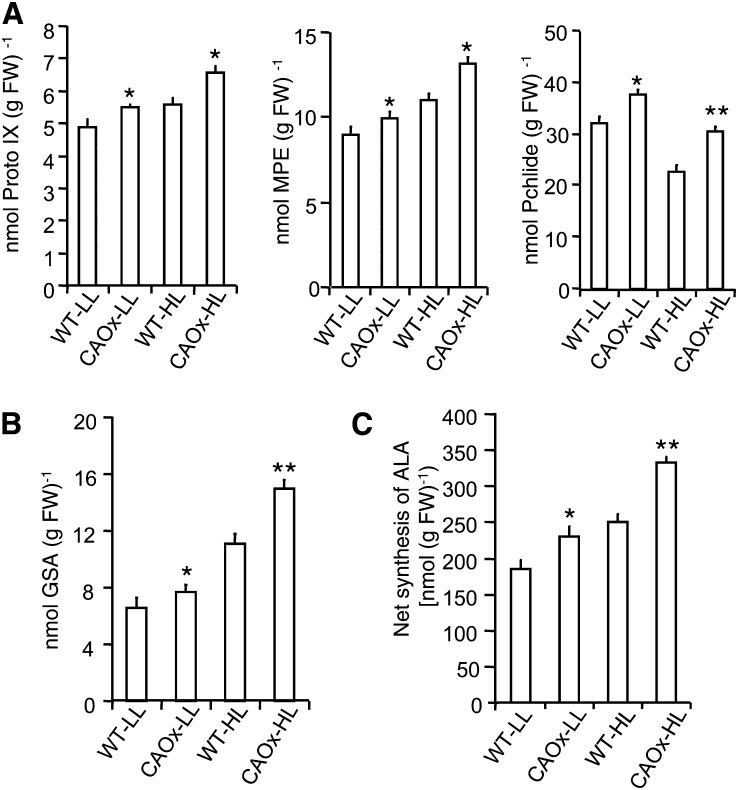
Figure 2.
Metabolites of the chlorophyll biosynthesis pathway of wild-type and CAOx plants grown under LL or HL. A, Steady-state tetrapyrrole content of wild-type and CAOx plants. Leaves were harvested from different plants (WT-LL, CAOx-LL, WT-HL, and CAOx-HL), and their steady-state tetrapyrrole intermediates (i.e. Proto IX, MPE, and Pchlide) were measured. B and C, GSA content (B) and net accumulation of ALA (C) from endogenous substrates of leaves harvested from different plants grown under LL and HL. These experiments were done three times with similar results, and error bars represent se (n = 6). Asterisks indicate significant differences determined by ANOVA followed by Tukey’s test (* P < 0.05, ** P < 0.001). FW, Fresh weight.
In order to understand the cause of the increased porphyrin content in CAOx plants, the content of porphyrin precursors, such as glutamate semialdehyde (GSA) and 5-aminolevulinic acid (ALA; for review, see Beale and Castelfranco, 1974; Tanaka and Tanaka, 2007), was measured. As compared with that in the wild type, GSA accumulation increased in CAOx-LL and CAOx-HL plants by 16% and 37%, respectively (Fig. 2B). Due to the increased availability of the substrate GSA, ALA synthesis also increased by 18% and 40% in CAOx-LL and CAOx-HL plants in comparison with wild-type plants (Fig. 2C).
Modulation of Enzymes Involved in Chlorophyll Biosynthesis in Wild-Type and CAOx Plants Grown in LL or HL Regimes
To understand the reason for the increased synthesis of tetrapyrroles in CAOx plants grown in LL and HL regimes, various important enzymes, such as 5-aminolevulinic acid dehydratase (ALAD), porphobilinogen deaminase (PBGD), protoporphyrinogen oxidase (Protox), Mg-chelatase, MPE cyclase, and protochlorophyllide oxidoreductase (POR), involved in chlorophyll biosynthesis were analyzed.
ALAD
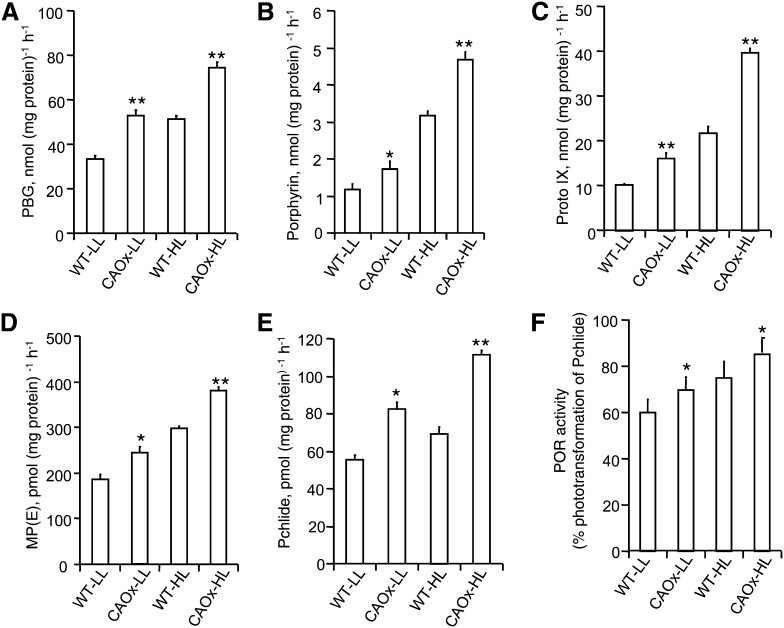
Two molecules of ALA are condensed together to yield one molecule of the pyrrole moiety porphobilinogen (PBG) by ALAD. In comparison with the wild type, the ALAD activity and, thus, PBG formation increased by 36% and 50% in CAOx-LL and CAOx-HL plants, respectively (Fig. 3A).
Figure 3.
Enzymatic activities of the chlorophyll biosynthetic pathway of wild-type and CAOx plants grown under LL or HL. A and B, ALAD (A) and PBGD (B) enzyme activities. Total leaf extracts were taken for both ALAD and PBGD activity test (see “Materials and Methods”). C to E, Protox (C), Mg-chelatase (D), and MPE cyclase (E) activities. Plastids were isolated from different plants, and the respective enzymatic assays were performed using a spectrofluorometer and expressed per mg of protein per h. F, POR activity as expressed as a percentage of the phototransformation of Pchlide to Chlide. LL- and HL-grown plants were incubated in the dark for 8 h, and their Pchlide contents were determined in the dark. After dark incubation, plants were transferred to light (100 μmol photons m−2 s−1) for 15 min, their Pchlide content was monitored, and the phototransformation of Pchlide to Chlide was determined. All the above experiments were done three times with similar results, and error bars represent se (n = 3). Asterisks indicate significant differences determined by ANOVA followed by Tukey’s test (* P < 0.05, ** P < 0.001).
PBGD
PBGD in concert with uroporphyrinogen III synthase converts four molecules of PBG to uroporphyrinogen III. LL- and HL-grown CAOx plants had higher activity of PBGD than identically grown wild-type plants (Fig. 3B).
Protox
Protox converts protoporphyrinogen IX to Proto IX. In comparison with the wild type, CAOx plants had elevated Protox activity and, thus, Proto IX formation in both the LL (57% more than the wild type) and the HL (81% more than the wild type) growth regime (Fig. 3C).
Mg-Chelatase
For chlorophyll formation, Mg-chelatase inserts Mg2+ into Proto IX by a complex ATP-dependent reaction to form Mg-protoporphyrin IX. Its activity increased in response to HL both in wild-type and CAOx plants. However, in CAOx plants, the Mg-chelatase activity was substantially higher (27%) in the HL growth regime than that in the wild type (Fig. 3D).
MPE Cyclase
MPE cyclase converts MPE to Pchlide. The MPE cyclase activity increased, as did Pchlide, in CAOx plants grown in LL and HL regimes by 35% and 70%, respectively (Fig. 3E).
POR
The phototransformation of Pchlide to Chlide was taken as a measure of the enzymatic function of POR. Its activity increased in response to HL both in wild-type and CAOx plants. The POR activity, measured as percentage phototransformation of Pchlide, increased in CAOx plants, especially in HL (Fig. 3F).
CAO Overexpression Modulates the Protein Abundance and Gene Expression of Certain Chlorophyll Biosynthetic Enzymes in LL and HL Intensities
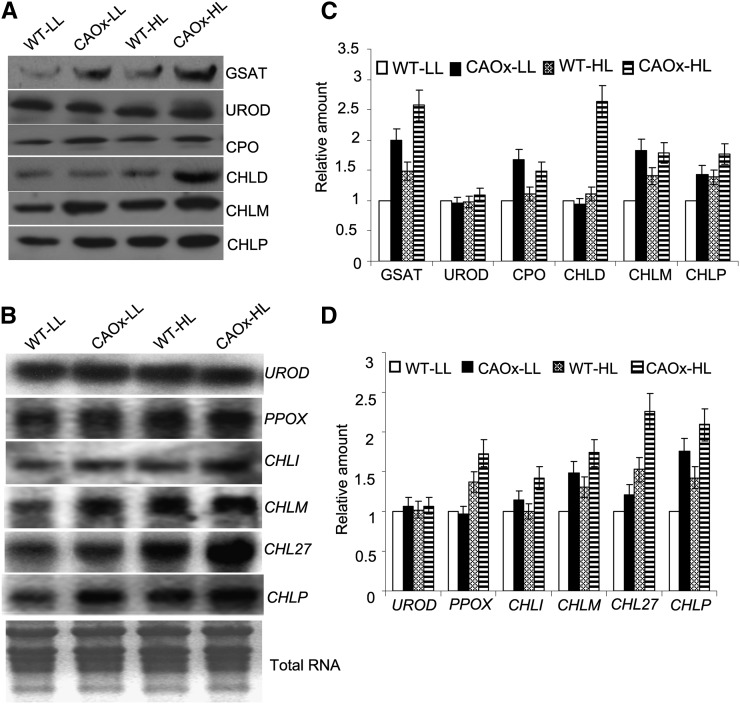
To ascertain if the increased activities of chlorophyll biosynthetic enzymes in LL- and HL-grown CAOx plants are due to increases in their protein abundance, western-blot analysis of selected enzymes was performed. Consistent with the increase in ALA biosynthetic activity, the protein abundance of Glu 1-semialdehyde aminotransferase increased in both LL-grown and HL-grown CAOx plants (Fig. 4A). The expression of uroporphyrinogen decarboxylase (UROD) was not altered in CAOx plants in LL or HL regimes. However, the protein abundance of coproporphyrinogen oxidase, CHLD, a subunit of Mg-chelatase, Mg-protoporphyrin IX:S-adenosyl-Met methyl transferase (CHLM), and geranyl geranyl reductase (CHLP), was increased in HL-grown CAOx plants.
Figure 4.
Western- and northern-blot analysis of chlorophyll biosynthetic pathway enzymes. A, Thirty micrograms of chloroplast proteins isolated from LL- and HL-grown wild-type and CAOx plants was loaded in each lane of an SDS-PAGE gel, and western-blot analysis was done as described in “Materials and Methods.” Each blot was repeated at least three times. The antibody dilutions used were as follows: for CPO, 1:500; for GSA aminotransferase (GSAT), UROD, CHLM, and CHLP, 1:1,000; for CHLD, 1:200. B, Twenty micrograms of total RNA isolated from the leaves of LL- and HL-grown wild-type and CAOx plants was processed (see “Materials and Methods”) for the gene expression study of the chlorophyll biosynthetic pathway enzymes. The cDNA sequences used for transcript quantification encode for UROD, PPOX, CHLI, CHLM, and CHLP. Reactions were repeated at least three times using independently treated samples. As a control for equal loading, ethidium bromide staining of total RNA is shown at the bottom. C and D, Quantification of band intensities (α Ease FC software) on the blots presented in A and B shown as bar diagrams. Signal intensities for each protein and mRNA were expressed relative to LL-grown wild-type plants. Each data point is the average of three replicates, and error bars represents sd.
To correlate the protein abundance with the regulation of gene expression of enzymes involved in chlorophyll biosynthesis in CAOx plants, northern-blot analysis of certain genes was performed. In agreement with protein abundance, the gene expression of UROD was not altered in CAOx plants (Fig. 4B). The transcript abundance of protoporphyrinogen IX oxidase (PPOX), CHLI, a subunit of Mg-chelatase, CHLM, CHL27 (a component of MPE cyclase), and CHLP was significantly increased in HL-grown CAOx plants (Fig. 4B).
Photosynthetic Responses of CAOx Plants Grown in LL and HL Regimes
To ascertain if increased Chl b content had the expected effect on the photosynthetic apparatus, the Chl a fluorescence of leaves of both wild-type and CAOx plants grown in LL or HL was measured.
Chl a fluorescence is used as a nondestructive and noninvasive signature of photosynthesis, particularly of PSII (for review, see Krause and Weis, 1991; Govindjee, 1995, 2004; Baker, 2008). As compared with that in the wild type, the minimal fluorescence (Fo) in dark-adapted leaves increased in LL- and HL-grown CAOx plants by 5% and 16%, respectively (Table II), whereas the maximum primary photochemical efficiency of PSII, measured as Fv/Fm (where Fv = Fm – Fo; see “Materials and Methods”), remained unchanged in LL- and HL-grown CAOx plants.
Table II. Chl a fluorescence parameter of LL- and HL-grown plants.
Leaves taken from LL- and HL-exposed wild-type and CAOx plants were dark adapted for 20 min before measurement of their minimal fluorescence (Fo), maximum fluorescence (Fm), and maximum primary photochemical efficiency (Fv/Fm) by a PAM 2100 fluorometer. The Fo, but not Fm or Fv/Fm, was significantly different (P < 0.001) in CAOx plants as compared with wild-type plants grown under LL and HL intensities. The experiment was repeated three times, and the values are means ± sd (n = 10).
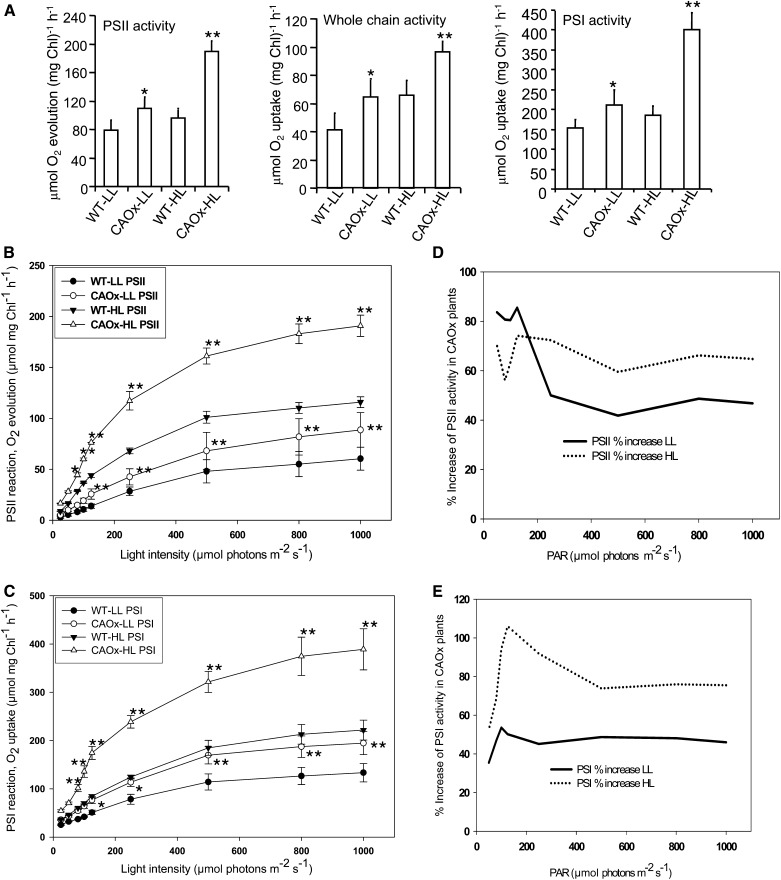
Whole-Chain, PSII, and PSI Reactions of Wild-Type and CAOx Plants Grown in the LL or HL Regime
The light-saturated whole-chain electron transport (water → methyl viologen [MV]), the partial reaction of PSII (water → phenylenediamine [PD]), and that of PSI (ascorbate/dichlorophenolindophenol → MV) were monitored polarographically in the thylakoid membranes of LL- and HL-grown wild-type and CAOx plants (Tripathy et al., 2007).
As expected from a typical sun plant, the electron transfer rates of PSI, PSII, and whole chain in HL-grown wild-type plants were higher than in WT-LL plants. This result is consistent with the previous work done on Pisum sativum and Arabidopsis (Leong and Anderson, 1984; Walters et al., 1999). In saturating light intensities, the CAOx plants had higher PSI (40%–120%) and PSII (40%–100%) reaction rates, on a chlorophyll basis, than the wild-type plants grown in LL or HL (Fig. 5A). In contrast, the increase in whole-chain electron transport rate, measured in saturating light intensity, was somewhat lower (20%–50%; Fig. 5A).
Figure 5.
Electron transport reactions of chloroplasts isolated from wild-type and CAOx plants. A, Electron transport through PSII (oxygen evolution; water to PD), whole chain (water to MV; oxygen uptake), and PSI (ascorbate to MV; oxygen uptake) was measured polarographically as described in “Materials and Methods.” B, Light intensity dependence of the PSII reaction. C, Light intensity dependence of the PSI reaction. D and E, Percentage increase of PSII and PSI supported electron transport activity in LL- and HL-grown CAOx plants, respectively. All the above experiments were done three times with similar results. Asterisks indicate significant differences determined by ANOVA followed by Tukey’s test (* P < 0.05, ** P < 0.001). Each data point is the average of three replicates, and error bars represent sd.
Light Saturation Curve of PSII
To further ascertain if the increase in PSII reaction in CAOx plants was due to increased light absorption, enhanced light-saturated electron transport rate, or both, the rates of PSII reactions in limiting and saturating light intensities were measured in thylakoid membranes isolated from LL- or HL-grown wild-type and CAOx plants. As compared with the wild type, the initial slopes of PSII reaction at limiting light intensities as well as the electron transport rates at saturating light intensities were much higher in CAOx plants grown in the LL or HL regime (Fig. 5B). Since CAOx plants had higher Chl b (Fig. 1) and more of it may be in LHCII, for the same amount of incident light more would go to PSII, and that could be the reason for increased PSII activity as compared with the wild type. As compared with the wild type, in CAOx plants, the percentage increase of PSII-supported electron transport was higher in limiting light intensities than in saturating light intensities (Fig. 5D). This suggests that an increase in the antenna size of PSII gives higher rates at LL intensities, whereas HL intensities added rate-limiting steps in the electron transport chain. Percentage increases in PSII activity at saturating light intensity in CAOx, as compared with wild-type, thylakoids were higher in HL-grown (approximately 70%) than in LL-grown (approximately 40%) samples (Fig. 5, B and D). The PSII reaction measured in CAOx plants grown in LL saturated at approximately 500 μmol photons m−2 s−1, whereas plants grown in HL attained the maximum at approximately 800 μmol photons m−2 s−1.
Light Saturation Curve of PSI
As compared with wild-type thylakoids, both the initial slope at limiting light intensities and light-saturated electron transport rates supported by PSI were higher in CAOx thylakoids isolated from LL- and HL-grown plants (Fig. 5C). Just as for the PSII reaction, CAOx plants, as compared with the wild type, had a higher percentage increase of PSI-mediated electron transport in limiting light intensities than in saturating light intensities in thylakoids isolated from HL samples. A higher amount of Chl b may be incorporated in LHCI, and for the same amount of incident light more would go to PSI, and that could be the reason for increased PSI activity as compared with the wild type. Thylakoids from LL samples did not show significant differences between limiting and saturating light intensities. Percentage increases in PSI activity at saturating light intensity in CAOx, as compared with wild-type, thylakoids were higher in HL-grown (70%) than in LL-grown (40%) samples (Fig. 5, C and E). However, the extent of increase of PSI activity was higher than that of PSII in both LL- and HL-grown CAOx plants when measured at LL intensities. However, when measured at HL intensities, the extent of increase was almost similar in both LL-grown and HL-grown CAOx, as compared with wild-type, plants (Fig. 5, C and E). The PSI reaction measured in both LL-grown and HL-grown wild-type and CAOx plants was almost fully saturated at around 800 μmol photons m−2 s−1. Just as in PSII, the PSI reaction measured in CAOx plants grown in LL saturated at approximately 500 μmol photons m−2 s−1, whereas those grown in HL attained the maximum at approximately 800 μmol photons m−2 s−1.
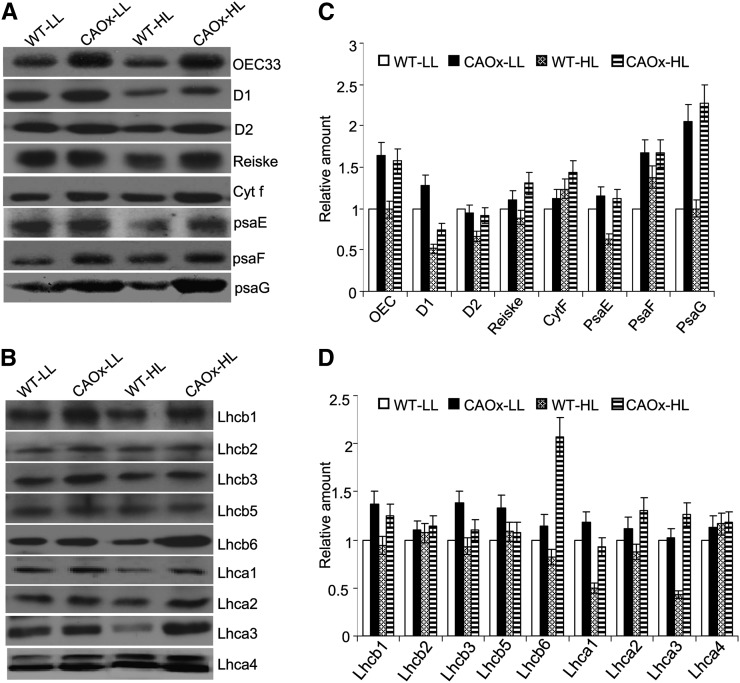
Western-Blot Analysis of Photosynthetic Proteins
To ascertain the reason why PSI- and PSII-dependent electron transfer rates increased at limiting as well as saturating light intensities in CAOx plants, certain components of the light-harvesting complex and electron transport chain were analyzed by western blot (Fig. 6). As compared with wild-type plants, the abundance of light-harvesting chlorophyll-binding proteins associated with PSII, Lhcb1, Lhcb3, and Lhcb6 increased in CAOx plants grown in LL and HL regimes (Fig. 6B). Similarly, the electron transport chain components of PSII (i.e. the reaction center proteins D1 and D2 and the oxygen-evolving complex proteins [e.g. OEC 33]) increased in both LL- and HL-grown CAOx plants (Fig. 6A).
Figure 6.
Immunoblot analysis of thylakoid membrane proteins in wild-type and CAOx plants. A, Thylakoid membranes were isolated from wild-type and CAOx plants grown under two different light intensities as described in the legend of Figure 1. Thirty micrograms of thylakoid membrane proteins was loaded in each lane of an SDS-PAGE gel, and immunoblotting was done as described in “Materials and Methods.” Three replicate membranes were immunolabeled with antibodies as indicated. B, Content of light-harvesting Chl a/b proteins of PSI and PSII. Thylakoid proteins were separated as described above, and the blots were incubated with antibodies as indicated. C and D, Quantification of band intensities (α Ease FC software) on the blots presented in A and B shown as bar diagrams. Signal intensities for each protein were expressed relative to LL-grown wild-type plants. Each data point is the average of three replicates, and error bar represents sd.
The different subunits of the cytochrome b/f complex substantially increased in CAOx plants, especially when grown under HL conditions. The abundance of both cytochrome f and Rieske proteins increased in HL-grown CAOx plants (Fig. 6A).
Among the light-harvesting antenna complex (LHCP1) proteins of PSI, the abundance of Lhca1, Lhca2, and Lhca3 proteins increased in LL- and HL-grown CAOx plants (Fig. 6B). Among other PSI proteins analyzed, the expression of psaE, psaF, and psaG proteins increased in HL-grown CAOx plants (Fig. 6A).
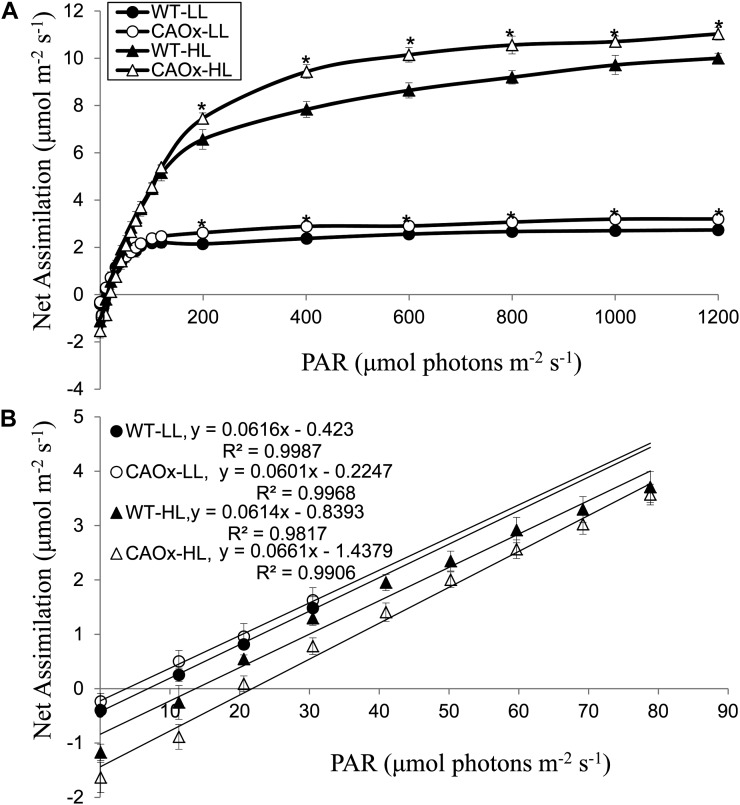
Light-Response Curve of Net CO2 Assimilation
To understand if increased electron transport resulted in increased CO2 assimilation and the efficiency of light utilization, the photosynthetic light response of attached leaves of wild-type and CAOx plants was monitored by an infrared gas analyzer in ambient CO2 concentration. As shown in Figure 7A, HL-grown wild-type plants had an approximately four times higher photosynthesis rate than LL-grown plants. The CO2 assimilation of LL-grown wild-type plants saturated at 150 μmol photons m−2 s−1, whereas saturation was achieved at approximately 600 μmol photons m−2 s−1 in HL-grown wild-type plants (Fig. 7A). The light compensation point of wild-type plants grown in HL was 13 μmol photons m−2 s−1, whereas that of LL-grown wild-type plants was 3 μmol photons m−2 s−1. The reduction of the light compensation point in LL-grown wild-type plants was due to a reduced rate of dark respiration. The relative quantum yield of CO2 fixation in wild-type plants grown in LL or HL was almost similar (i.e. 0.06).
Figure 7.
Photosynthesis (net CO2 assimilation rate) light-response curves and quantum yield of leaves from attached wild-type and CAOx plants grown in LL and HL intensities. A, Net CO2 assimilation rates of attached leaves of wild-type and CAOx plants were monitored with an infrared gas analyzer (Licor 6400-XT portable photosynthetic system) in ambient CO2 at different light intensities. Light-response curves were measured up to 1,800 μmol photons m−2 s−1 at 28°C. B, Relative quantum yield of CO2 fixation by leaves from wild-type and CAOx plants grown in LL or HL conditions. Quantum yield was measured from the above photosynthetic rate after the chamber reached a steady state. Light intensity curves were measured at LL intensities up to 80 μmol photons m−2 s−1; the slopes of these curves provide relative quantum yield of CO2 fixation by leaves. Leaves were preexposed for 15 min at 700 and 200 μmol photons m−2 s−1 for LL- and HL-grown plants, respectively, prior to CO2 assimilation measurement. These experiments were done three times with similar results. Each data point is the average of five replicates, and error bar represents se. Asterisks indicate significant differences determined by ANOVA followed by Tukey’s test (* P < 0.05). PAR, Photosynthetically active radiation.
In comparison with wild-type plants, CAOx plants grown in HL had a higher rate of dark respiration (Fig. 7B). One hour after sunset, the dark respiration rate was 1.7 μmol CO2 evolved m−2 s−1 in CAOx plants, whereas in the wild type, it was 1.2 μmol CO2 evolved m−2 s−1. Therefore, their light compensation point increased from 13 to 21 μmol photons m−2 s−1. Indeed, we found that the CO2 assimilation rates at several light intensities were 10% to 20% higher in CAOx plants than in wild-type plants. In LL-grown CAOx plants, the rate of dark respiration (0.49 μmol CO2 evolved m−2 s−1), measured 1 h after sunset, was higher than that in the wild type (0.33 μmol CO2 evolved m−2 s−1); consequently, their light compensation point increased from 3 to 5 μmol photons m−2 s−1. The net photosynthesis rate of LL-grown CAOx plants at saturating light intensity was higher than that of LL-grown wild-type plants. However, the (relative) quantum yields of photosynthetic CO2 assimilation of LL-grown or HL-grown CAOx plants, measured at limiting light intensities, were essentially the same (0.061–0.066) and were almost similar to that of the wild type (Fig. 7B).
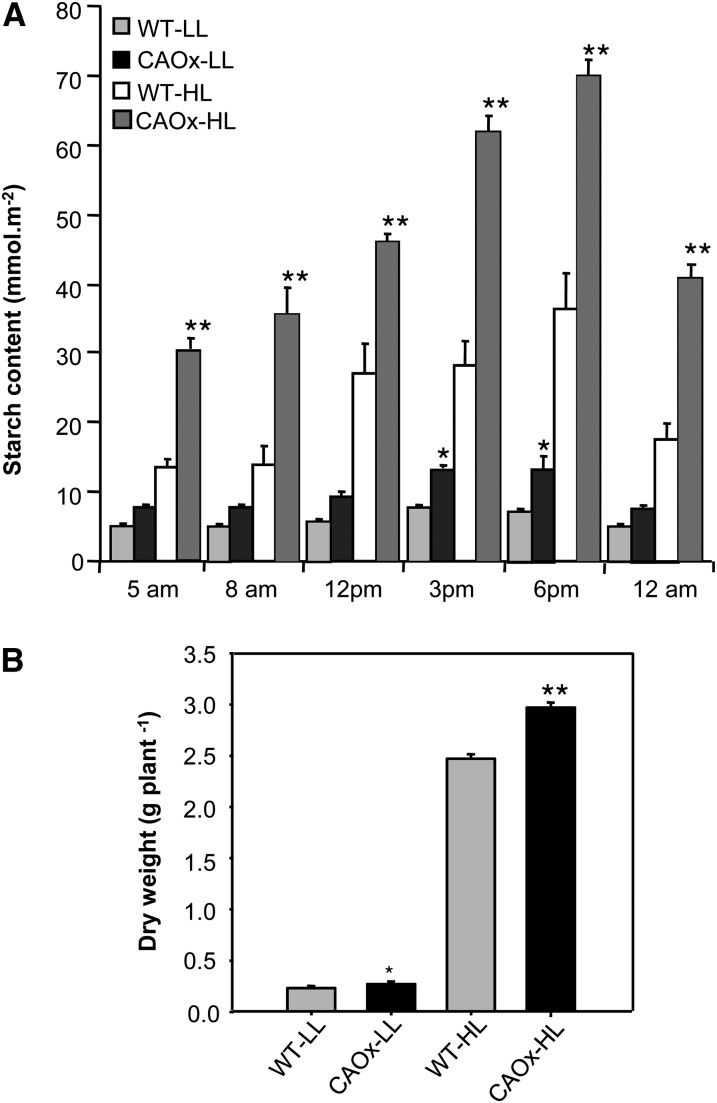
Starch Content
To ascertain if the increased rate of CO2 assimilation in CAOx plants resulted in increased starch production, the diurnal variation in starch accumulation was measured in 6-week-old plants at their vegetative stage (Fig. 8A).
Figure 8.
Diurnal starch content and dry weight measurement in wild-type and CAOx plants. A, Starch content was measured from mature leaves of wild-type and CAOx plants grown under LL and HL at various times over a diurnal cycle as described in “Materials and Methods.” Note that the diurnal starch accumulation was maximum between 3 and 6 pm and that CAOx-HL plants showed maximum starch accumulation. B, Dry weight of wild-type and CAOx plants was measured after aerial parts of the plant were dried at 70°C for 5 d. HL-grown wild-type and CAOx plants showed significant increases in dry matter accumulation in comparison with WT-LL and CAOx-LL plants. Asterisks indicate significant differences determined by ANOVA followed by Tukey’s test (* P < 0.05, ** P < 0.001). These experiments were done three times with similar results. Each data point is the average of four replicates (A) and 15 replicates (B), and error bars represent sd.
The starch content was quite low in LL-grown wild-type and LL-grown CAOx plants, although CAOx plants had a little higher starch content than wild-type plants at 3 or 6 pm. In HL-grown wild-type and CAOx plants, the starch content was higher than in LL-grown plants. In both light regimes, CAOx plants always had higher starch content than the wild-type plants. Diurnal variation in starch accumulation revealed that starch accumulation was lowest at dawn at 5 am and was maximum between 3 and 6 pm (Fig. 8A). After sunset, the starch content declined to a substantially lower value due to the respiratory consumption of stored starch. In HL-grown CAOx plants, the accumulation of photosynthate in the form of starch was always significantly higher at all time points as compared with that of wild-type plants.
Dry Matter Accumulation
As expected, the accumulation of plant dry matter was low in LL-grown plants and almost eight to 10 times higher in HL-grown plants (Fig. 8B). After 6 weeks of growth in HL, the dry weight per plant increased by 19% in CAOx plants. Under LL conditions, the CAOx plants accumulated around 8% higher dry mass than the wild-type plants after an identical period of growth. Beyond 9 to 10 weeks of growth, HL-grown CAOx plants continued to grow much taller, had higher leaf number, and accumulated much higher dry matter than the wild type. However, this was not taken into account for dry matter production, as they were in different developmental stages (i.e. wild-type plants entered the flowering phase and had arrested growth, whereas CAOx plants were in the vegetative phase and continued to grow vertically; Fig. 8B).
DISCUSSION
Overexpression of AtCAO increases the Chl b content and consequently decreases the Chl a/b ratio both in LL- and HL-grown tobacco or Arabidopsis plants (Pattanayak et al., 2005; Tanaka and Tanaka, 2005). In this study, we used the same S2 overexpression line that was used previously in our laboratory (Pattanayak et al., 2005). Instead of using T2 generation plants, we used T4 generation plants for all our experiments and, as expected, we observed increased Chl b content and reduced Chl a/b ratio in the T4 generation CAOx plants (Fig. 1). This demonstrates that the level of CAO overexpression is sustainable and that this trait is repeatedly transferred from one generation to the next. In our experiments, CAOx plants showed, as compared with wild-type tobacco plants, (1) 28% (HL) and 8% (LL) increases in total chlorophyll (this was partly reflected in the increase in the Fo level of fluorescence); (2) 71% (HL) and 22% (LL) increases in Chl b; (3) 33% (HL) and 15% (LL) increases in Chl a/b ratio (Table III).
Table III. Percentage changes [increase (+) and decrease (−)] in different parameters of CAOx plants grown in LL and HL intensities.
| Parameter | Plant Lines |
Percentage Change | Plant Lines |
Percentage Change | ||
|---|---|---|---|---|---|---|
| WT-HL | CAOx-HL | WT-LL | CAOx-LL | |||
| Fo | 0.19 | 0.22 | 16 (+) | 0.20 | 0.21 | 5 (+) |
| Fv/Fm | 0.80 | 0.80 | No change | 0.79 | 0.79 | No change |
| Chlorophyll-protein ratio of thylakoid membrane | 2.40 | 2.96 | 23 (+) | 2.70 | 3.13 | 16 (+) |
| Starch (3 pm; mmol m−2) | 28.27 | 61.0 | 116 (+) | 8.29 | 12.93 | 56 (+) |
| Starch (6 pm; mmol m−2) | 36.51 | 69.87 | 91 (+) | 7.96 | 13.6 | 71 (+) |
| Biomass (g plant−1) | 2.47 | 2.94 | 19 (+) | 0.25 | 0.27 | 8 (+) |
| Net CO2 assimilation (μmol m−2 s−1) | 9.49 | 11.03 | 16 (+) | 2.74 | 3.2 | 16 (+) |
| PSII (µmol oxygen evolution [at 1,500 µmol photons m−2 s−1] mg−1 chlorophyll h−1) | 96.52 | 189.12 | 96 (+) | 78.30 | 109.41 | 40 (+) |
| Whole chain (µmol oxygen uptake [at 1,500 µmol photons m−2 s−1] mg−1 chlorophyll h−1) | 65.9 | 96.9 | 47 (+) | 41.50 | 64.35 | 55 (+) |
| PSI (µmol oxygen uptake [at 1,500 µmol photons m−2 s−1] mg−1 chlorophyll h−1) | 167.75 | 401.25 | 139 (+) | 153.61 | 212.18 | 38 (+) |
| PSII (µmol oxygen evolution [at limiting light, 80 µmol photons m−2 s−1] mg−1 chlorophyll h−1) | 28.5 | 44.48 | 56 (+) | 8.1 | 14.63 | 81 (+) |
| PSI (µmol oxygen uptake [at limiting light, 80 µmol photons m−2 s−1] mg−1 chlorophyll h−1) | 60.16 | 101.48 | 69 (+) | 36.96 | 54.33 | 47 (+) |
| PSII (µmol oxygen evolution [at saturating light, 800 µmol photons m−2 s−1] mg−1 chlorophyll h−1) | 110.1 | 182.96 | 66 (+) | 55.0 | 81.76 | 47 (+) |
| PSI (µmol oxygen uptake [at saturating light, 800 µmol photons m−2 s−1] mg−1 chlorophyll h−1) | 212.82 | 374.48 | 76 (+) | 126.46 | 187.33 | 48 (+) |
| Total chlorophyll (mg g−1 fresh weight) | 1.64 | 2.10 | 28 (+) | 2.31 | 2.49 | 8 (+) |
| Chl b (mg g−1 fresh weight) | 0.375 | 0.641 | 71 (+) | 0.597 | 0.729 | 22 (+) |
| Chl a/b ratio | 3.37 | 2.27 | 33 (−) | 2.86 | 2.42 | 15 (−) |
The above changes were accompanied by increases in the antenna size in CAOx plants; there was (1) a 23% (HL) and a 16% (LL) increase in chlorophyll per thylakoid membrane protein; (2) a 47% (HL) and a 55% (LL) increase in whole-chain electron transport from water to MV at saturating light on a chlorophyll basis; (3) a 50% (HL) and a 100% (LL) increase in the quantum yield of PSII electron flow reaction (water to PD) at limiting light; and (4) a smaller (20% for HL and 30% for LL) increase in the quantum yield of PSI electron flow reaction (reduced dichlorophenolindophenol to MV; see “Materials and Methods”). These data suggest that CAOx plants not only have better quantum efficiency of electron flow, particularly of PSII, but have improved in other ways to have higher overall electron transport rate, due, perhaps, to an improved efficiency of (dark) enzymatic reactions. However, since the Fv/Fm, a measure of the yield of primary photochemistry (Govindjee, 2004), was identical in all cases, the effect must be in the secondary electron transport and not in the maximum yield of primary photochemistry. As compared with wild-type plants, the rates of individual PSII and PSI reactions were also higher in CAOx plants than in wild-type plants. In this context, we note that many thylakoid proteins (e.g. D1, D2, and several PSI proteins) increased in CAOx plants. Interestingly, these increases exceeded those in whole-chain electron transport, mentioned above (Table III). We explain this observation by suggesting that the whole-chain electron transport is limited by the bottleneck reaction of diffusion and oxidation of plastoquinol between the two photosystems (Haehnel, 1984).
We note that there was a significant (10%–20%) increase in CO2 fixation in CAOx over wild-type plants at saturating light intensities but not at limiting light intensities. Thus, the advantage of increased whole-chain electron flow did not translate into increased CO2 fixation at LL intensities; perhaps these electrons went into alternate electron sinks.
Another interesting observation is that there was a large increase (almost 100%) in starch formation. Although large increases in light-saturated electron transport rates (see above) and starch content were observed in HL-grown CAOx plants, their light-saturated carbon assimilation rate was rather small (16%). This was probably due to a down-regulation of carbon assimilation either by starch or intermediary sugars (Goldschmidt and Huber, 1992; Sharkey et al., 2004).
Increased Pigment Content and Associated Intermediates
In this study, we found that overexpression of AtCAO in tobacco plants affected the chlorophyll biosynthetic flux by modulating the gene and protein expression of several other chlorophyll biosynthetic pathway enzymes. A similar observation was also reported previously: that overexpression of other chlorophyll biosynthesis pathway genes (i.e. CHLM, PORC, and CHLG) comodulates the gene expression of several other chlorophyll biosynthetic genes (Alawady and Grimm, 2005; Shalygo et al., 2009; Pattanayak and Tripathy, 2011; for singlet oxygen-induced stress effects, see Tripathy and Pattanayak, 2010). In addition, Sakuraba et al. (2012) have demonstrated that increased Chl b synthesis delays senescence; thus, it retains the gene expression of several chlorophyll biosynthetic enzymes. These findings demonstrate the existence of a regulatory network among genes coding for enzymes involved in the greening process. Increased gene and protein expression of ALA biosynthetic enzymes led to augmented synthesis of GSA and ALA and increased porphyrin content in CAOx plants (Fig. 2). Increased ALA synthesis could lead to an overaccumulation of porphyrins (tetrapyrroles) that could, in principle, cause singlet oxygen-induced oxidative damage to plants (Tripathy and Chakraborty, 1991; Chakraborty and Tripathy, 1992; Pattanayak and Tripathy, 2011). However, the enhanced ALA synthesis did not result in the overaccumulation of Mg-porphyrin in CAOx plants because of the increased enzymatic activities of several other chlorophyll biosynthetic pathway enzymes, leading to higher mobilization of the substrate ALA to the final product chlorophyll (Fig. 3). We have also observed increases in the gene/protein expression of chlorophyll biosynthesis enzymes in HL-grown plants (Fig. 3). This result is in agreement with previously published results, where gene/protein expression of chlorophyll biosynthesis enzymes increased in response to HL (Tanaka et al., 1999; Oosawa et al., 2000; Masuda et al., 2003; Alawady and Grimm, 2005). This is probably because in HL, chlorophyll degrades faster than in LL, effectively reducing the half-life of chlorophyll (Vavilin et al., 2007). Therefore, it seems that in order to compensate for the loss of chlorophyll in HL, chlorophyll biosynthetic capacity increased; in other words, the turnover rate of chlorophyll biosynthesis increased at higher light intensities. Due to the increased turnover, the greater availability of the substrate Chlide for overexpressed CAO in HL-grown plants might be responsible for the increased Chl b synthesis and a consequent decrease in Chl a/b ratio. Unlike Arabidopsis, which usually grows best at 100 to 150 μmol photons m−2 s−1, a higher light intensity (800 μmol photons m−2 s−1) seemed optimal for tobacco. This could be one of the possible reasons why CAOx plants of tobacco had more Chl b than that in CAO-overexpressing Arabidopsis plants (Tanaka and Tanaka, 2005) at their optimal growth light intensity.
Photosynthetic organisms acclimatize to various light intensities by adjusting the size of the chlorophyll antenna complex of the two photosystems (Chow et al., 1990). The light-harvesting antenna system of higher plants consists of a core and a peripheral antenna complex (Green and Durnford, 1996; Barber et al., 1999). Sun and shade plants behave differently to LL and HL intensities; for example, shade plants have reduced Chl a/b ratio, higher levels of LHCP II, and hence larger antenna size of PSII (Anderson et al., 1988; Melis 1991), whereas HL-grown plants have increased Chl a/b ratio, increased PSII, ATP synthase, and Rubisco, but reduced chlorophyll and LHC contents (Björkman et al., 1972; Leong and Anderson, 1984; Bailey et al., 2001, Harper et al., 2004; Tanaka and Tanaka, 2005). Similarly, we also found that lhcb1, lhcb3, and lhcb6 as well as lcha1, lhca2, and lhca3, components of LHCII and LHCI, decreased in wild-type tobacco plants grown under HL intensity (700–800 μmol photons m−2 s−1). However, overexpression of AtCAO in tobacco (CAOx) resulted in an increase of the protein abundance of several components of LHCPI (i.e. lhca1, lcha2, and lhca3) and LHCPII (i.e. lhcb1 and lhcb6) in HL-grown plants and to a smaller extent in the LL growth regime (Fig. 6B). We note that individual PSI and PSII proteins were measured on an equal total thylakoid protein basis. However, electron transfer rates were measured on an equal chlorophyll basis. The chlorophyll-protein ratio of isolated thylakoid membranes was higher by 15% to 23% in CAOx than in wild-type plants. The reduced Chl a/b ratio usually results in increased grana stacking, which is usually associated with a modulation of protein content; the chlorophyll content relative to the protein of grana lamellae is about 40% higher than that in the stroma lamellae from the same preparation (Allen et al., 1972). Since western-blot analysis was done on an equal protein basis, more chlorophyll was loaded on each lane for the CAOx plants both in LL- and HL-grown samples. Since electron transport was plotted on a chlorophyll basis, higher rates in CAOx mean still higher rates if we would plot it per total protein. Thus, we explain this result as being due to higher specific increases in chlorophyll-enriched LHCs and key chlorophyll-deficient proteins of PSII and PSI and the cytochrome b/f complex or to their activities in the dark reactions of electron flow at saturating light intensities. Our observations explain the larger antenna size to be the reason behind higher rates and higher quantum yields at limiting light conditions and the increased specific PSI and PSII proteins to be the reason for higher electron transfer rates at saturating light intensities.
Electron Transport Rates
Our results further demonstrate that CAO overexpression not only increased the Chl b content and the antenna size but also enhanced the efficiency of the energy capture and its utilization at limiting as well as saturating light intensities when we measured PSI and PSII reactions separately. As compared with wild-type plants, both LL- and HL-grown CAOx plants had larger light-harvesting antenna size; consequently, the electron transfer rate of PSI and PSII in CAOx plants was higher than that in the wild type at limiting light intensities. At higher light intensities, due to the increased abundance of PSII reaction center and oxygen-evolving complex proteins (Fig. 6), the HL-grown CAOx plants had a higher electron transport rate than HL-grown wild-type plants. In isolated thylakoid membranes, the whole-chain electron transport using both photosystems and the partial reactions of PSI and PSII, expressed on an equal chlorophyll basis, also increased in CAOx plants in LL-grown plants and, more prominently, in HL-grown plants. The increase in the whole-chain electron transport rate at saturating light intensity was somewhat lower (50% in LL-grown plants and 20% in HL-grown plants) than the increases in partial reactions of PSI or PSII (Fig. 5), as noted above. As revealed from light intensity curves (Fig. 5), the PSI and PSII activities, measured separately, increased dramatically (by 40%–80%) in CAOx plants at saturating as well as limiting light intensities. However, in wild-type and CAOx thylakoids, the PSI and PSII reactions saturated at relatively lower light intensities in LL-grown plants than in HL-grown-plants. Due to the increased abundance of several components of LHCPI and LHCPII, the light-harvesting antenna size increased. The increased electron transfer rate at low light intensities in CAOx plants was mostly due to efficient energy capture by the larger antenna size. The increased electron transfer rate observed in CAOx plants at saturating light intensities is due to the increased protein abundance of electron transport chain components of PSII (i.e. D1, D2, and OEC33), PSI components (i.e. psaE, psaF, and psaG), and intersystem electron transport components (i.e. Rieske Fe-S center and cytochrome f in the cytochrome b/f complex).
The increased efficiency of photosynthetic (both PSII and PSI) electron transport in CAOx thylakoids at LL intensities was not accompanied by any increase in the relative quantum yield of CO2 assimilation. Their quantum yield of CO2 assimilation almost remained the same as that of the wild type, both in LL- or HL-grown wild-type and CAOx plants. The relative quantum yield of 0.061 to 0.066 for CO2 assimilation is similar to that observed in several C3 plant species (Ehleringer and Pearcy, 1983). As compared with wild-type plants, the electron transfer rate increased in CAOx plants at HL intensity. Because of the reduced metabolism of LL-grown plants, their respiration rate was minimal and, therefore, light compensation intensity was lower. The reduced rate of respiration, decreased light compensation point, and saturation of photosynthetic carbon reduction at LL intensity have been observed previously in shade plants (Nobel, 1976; Powles and Critchley, 1980). Our results clearly demonstrate that tobacco, a sun plant, could efficiently adapt itself to LL intensity and behave like a shade plant. The turnover of proteins is quite low in LL. Therefore, CAOx plants grown in LL do not have an increased rate of respiration.
Overexpression of Prochlorothrix hollandica CAO, which lacks the regulatory A domain responsible for the regulation of Chl b synthesis (Nagata et al., 2004), resulted in an uncontrolled synthesis of Chl b, leading to a highly reduced Chl a/b ratio (0.92), photodamage at HL intensities, and reduced Fv/Fm ratio of 0.19 (Hirashima et al., 2006). Similarly, transgenic Arabidopsis plants overexpressing the A domain-deleted AtCAO accumulated excess amounts of Chl b and were damaged by light (Yamasato et al., 2005). In the presence of extremely high amounts of Chl b and decreased quantities of Chl a, energy absorbed by Chl b might not be efficiently transferred to Chl a (Sakuraba et al., 2010), leading to the generation of reactive oxygen species and photodamage at HL intensities. Therefore, severe alteration of the Chl a/b ratio may be detrimental rather than beneficial to plants. However, controlled up-regulation of endogenous Chl b biosynthesis, by genetic manipulation of full-length CAO, partially increases protein expression and the function of LHCPs and several other components of photosynthetic apparatus and light absorption capacity and even increases electron transport rates.
Carbon Fixation, Starch Formation, and Biomass
Our data have shown clearly that, in response to CAO overexpression, the light-saturated photosynthetic carbon assimilation on a leaf area basis increased significantly in LL- and HL-grown CAOx plants. This corresponds well to our polarographic measurement of PSI and PSII activity in isolated thylakoid membranes, where we have also shown that CAOx plants had significantly higher photosynthetic capacity than wild-type plants. We have expressed the carbon assimilation rate on a leaf area basis and not on a chlorophyll basis because photosynthetic CO2 assimilation is not proportional to chlorophyll content (Yoshida, 1972). Moreover, it would not have been possible to determine the quantum yield and light compensation point. These data show that whole-plant photosynthesis can be increased by CAO overexpression as a genetic tool in tobacco.
An analysis of diurnal changes in starch accumulation revealed that the level of starch considerably increased between 3 and 6 pm toward the end of the day in wild-type plants. This is in agreement with end-of-the-day accumulation of starch in several plants (Zeeman et al., 2002; Lefebvre et al., 2005). In CAOx plants grown in a greenhouse, especially in the HL condition, starch content substantially increased as compared with that in wild-type plants throughout the diurnal cycle. In the leaves of both wild-type and CAOx plants, only low amounts of starch remained in the morning at 5 am. These results showed that the additional starch produced in the CAOx plants was being used in the dark period, possibly for plant growth. This is clearly indicated from the increase in dark respiration rate in CAOx plants. Our data, along with those of Lefebvre et al. (2005), demonstrate a clear correlation between increased photosynthesis and starch accumulation. Indeed, the total shoot biomass of greenhouse-grown CAOx plants increased by up to 8% to 19%, as compared with wild-type plants, under LL and HL growth regimes. This finding is in agreement with data from a previous report in plants overexpressing sedoheptulose-bisphosphatase enzyme, where PSII photosynthetic efficiency, carbon assimilation, starch content, and dry matter accumulation consistently increased (Lefebvre et al., 2005). In the same vein, transgenic tobacco plants expressing a bifunctional cyanobacterial Fru-1,6/sedoheptulose-1,7-bisphosphatase targeted to chloroplasts show enhanced photosynthetic efficiency, growth characteristics, and dry matter production under ambient atmospheric conditions (Miyagawa et al., 2001). Although the rate of photosynthesis per unit of leaf area usually does not increase in high-yielding crop cultivars (Loomis and Amthor, 1999), our results demonstrate that photosynthetic capacity per unit of leaf area and plant dry matter could be increased by overexpressing a single chloroplastic enzyme, CAO, involved in Chl b biosynthesis. In the same vein, previous work of Miyagawa et al. (2001) and Lefebvre et al. (2005) demonstrated that carbon reduction cycle enzyme(s) could be modulated to enhance plant productivity. Although we have overxpressed 35S-AtCAO only in tobacco, we believe that it can be successfully transformed to other high-light-tolerant crop plants, although this needs further experimental verification. The reliability of other native promoters (i.e. those of rbcS or Lhcb1) for the expression of AtCAO needs to be ascertained.
In conclusion, a multiple approach of modulating the photosynthetic apparatus involved in light harvesting, electron transport, and CO2 fixation may lead to increased photosynthesis and crop productivity.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Wild-type and CAOx tobacco (Nicotiana tabacum ‘Petit Havana’) plants (Pattanayak et al., 2005) were grown in a greenhouse in a natural photoperiod for 25 to 30 d under a light intensity of 200 μmol photons m−2 s−1 at 25°C ± 2°C. For the two different light treatments, these plants were transferred either to LL (70–80 μmol photons m−2 s−1) or to HL (700–800 μmol photons m−2 s−1) for an additional 18 to 20 d in a greenhouse. Plant material harvested for analysis was either immediately analyzed or stored at −80°C. In all the experiments, the second leaf was harvested from the different plant types for analysis. Leaf numbers were always counted from the top of the shoot.
Pigment and Protein Estimation
Chlorophyll content was estimated in 80% acetone, as described by Porra et al. (1989). The contents of chlorophyll biosynthesis intermediates (i.e. Proto IX, MPE, and Pchlide) were calculated from fluorescence emission spectra (E400, E420, and E440), as described elsewhere (Hukmani and Tripathy, 1992; Tewari and Tripathy, 1998). The protein content of the leaves and thylakoid membranes was measured according to the Bradford (1976) assay.
Estimation of ALA and GSA Contents
Leaves (200 mg) were incubated in 50 mm levulinic acid either in dark or light (30 μmol photons m−2 s−1) for 6 h, hand homogenized under green safelight in 5 mL of ice-cold 4% trichloroacetic acid, and centrifuged at 10,000 rpm for 10 min at 4°C. The supernatant was used for ALA estimation (Tewari and Tripathy, 1998; Sood et al., 2005). The results were expressed as net ALA synthesis (ALA synthesis in the dark subtracted from ALA synthesis in the light).
For the estimation of GSA, leaves (500 mg) were incubated in the presence of a GSA aminotransferase inhibitor, 500 μm gabaculine, for 6 h under light, hand homogenized in 5.0 mL of cold HCl (0.1 n), and centrifuged at 10,000 rpm for 10 min at 4°C. GSA was estimated in the supernatant, as described elsewhere (Tewari and Tripathy, 1998; Sood et al., 2005).
Isolation of Intact Chloroplasts and Thylakoid Membranes
Thylakoid membranes and intact chloroplasts were isolated from leaves of LL- and HL-grown wild-type and CAOx plants, as described before (Tripathy and Mohanty, 1980; Dutta et al., 2009).
Western-Blot Analysis
Thirty micrograms of chloroplastic proteins was resolved by 12.5% SDS-PAGE (Laemmli, 1970) and electrophoretically transferred to a nitrocellulose membrane as described (Pattanayak et al., 2005). After primary and secondary antibody treatment with appropriate dilution, blots were immunodetected using the ECL system (Amersham-Pharmacia).
Enzymatic Assays
For ALAD and PBGD assay, 250 mg of leaves was collected from LL- and HL-grown wild-type and CAOx plants and hand homogenized in 5 mL of 0.1 m Tris (pH 7.6) and 0.01 m β-mercaptoethanol solution at 4°C. The homogenate was centrifuged at 10,000 rpm for 10 min at 4°C. The supernatant was taken for the enzymatic assay. The ALAD and PBGD enzyme activities were determined by measuring the amount of PBG formed and the amount of porphyrin synthesized in 1.0 mL of reaction mixture, respectively, as described by Sood et al. (2005). PBG formed was calculated using the absorption coefficient 6.2 × 104 m−1 cm−1 (Hukmani and Tripathy, 1994).
Chloroplasts were isolated from leaves taken from wild-type and CAOx plants grown under different light intensities as described (Tewari and Tripathy, 1998) and were then subdivided for assays of Protox, Mg-chelatase, and MPE cyclase.
For Protox assay, the plastids were lysed in a buffer containing 10 mm Tris-HCl and 2.5 mm Na2EDTA (pH 7.7). Lysed plastids were centrifuged at 5,000 rpm for 3 min at 4°C, and the supernatant was used for the enzyme assay as described by Tewari and Tripathy (1998). Proto IX was estimated by spectrofluorometry as described elsewhere (Hukmani and Tripathy, 1992; Tewari and Tripathy, 1998). Heat-killed enzyme was obtained by keeping supernatant in a boiling-water bath for 10 min, which was taken as blank. For Mg-chelatase, chloroplasts were isolated at 4°C and assayed as described earlier (Tewari and Tripathy, 1998). For MPE cyclase, plastids were suspended in a suspension buffer containing 0.5 m Suc, 0.2 m Tris-HCl (pH 7.7), 20 mm MgCl2, 2.5 mm Na2EDTA, and 20 mm ATP. The reaction mixture consisted of 100 μL of chloroplast suspension, 100 μL of suspension buffer, and 1.5 μm MPE (Tewari and Tripathy, 1998). The incubation was carried out at room temperature for 1 h in the dark, and 1.7 mL of ice-cold 90% ammonical acetone was added to stop the reaction. The hexane-extracted acetone residue was prepared from the acetone extract, and the synthesis of Pchlide was estimated by spectrofluorometry (Hukmani and Tripathy, 1992).
Northern-Blot Analysis
Total RNA was isolated (Chomczynski and Sacchi, 1987), and 30 µg of total RNA was fractionated on a 1.2% agarose formaldehyde gel and blotted on a nylon membrane (Hybond NX) as described before (Pattanayak et al., 2005). The membranes were hybridized with [α-32P]dCTP-labeled (nick-translated) gel-purified DNA probes according to a standard procedure (Sambrook et al., 1989). Equal loading was checked by staining the gels with ethidium bromide.
Probes for hybridization were PCR amplified from Arabidopsis total cDNA using appropriate primers except for CHLP, which was the cDNA from tobacco. The primers used for the amplification of the different probes were as follows: for UROD, 5′-ATGAGCTTATCATCGCCAAC-3′ and 5′-GACAACCAATTCAGGTTCAG-3′; for PPOX, 5′-ATGGAGTTATCTCTTCTCCG-3′ and 5′-CTTGTAAGCGTACCGTGACA-3′; for CHLI, 5′-ATGGCGTCTCTTCTTGGAAC-3′ and 5′-GCTGAAAATCTCGGCGAACT-3′; for CHLM, 5′-ATGCCGTTTGCTCCTTCCTT-3′ and 5′-CATTGGAACAGCTTCGATGA-3′; and for CHL27, 5′-ATGGCGGCTGAAATGGCGTT-3′ and 5′-ATAGACAAGATTAGGCTCAAACT-3′.
Electron Transport Assay
Assays of electron transport activity of whole chain, PSII, and PSI were carried out using a glass cuvette fitted within a Clark-type oxygen electrode (Hansatech) as described before (Tripathy and Mohanty, 1980). The reaction was maintained at 25°C by using a temperature-controlled water bath and was illuminated for 20 s using a tungsten light source at a photon flux rate of 1,500 µmol photons m−2 s−1. The whole-chain electron transport from water to MV (1 mm) was monitored as oxygen uptake. The assay mixture (3 mL) consisted of 50 mm HEPES (pH 7.5), 10 mm NaCl, 1 mm NH4Cl, 3 mm MgCl2, 1.0 mm NaN3, and 0.5 mm MV. Chloroplast was added to the above reaction mixture to a total concentration of 50 µg. PSII activity was monitored as oxygen evolution from water to PD. The 3-mL reaction mixture for PD-supported oxygen evolution assay consisted of 50 mm HEPES (pH 7.5) buffer, 3 mm MgCl2, 10 mm NaCl, and freshly prepared PD (0.5 mm). The partial electron transport chain through PSI was measured as oxygen consumption. An ascorbate (1 mm)/dichlorophenolindophenol (0.1 mm) couple was used as electron donor to PSI, and MV (1 mm) was used as electron acceptor. Electron flow from PSII was blocked by 3-(3,4-dichlorophenyl)-1,1-dimethyl urea (20 μm).
Light Saturation Curve
The light saturation curve of PSI- and PSII-dependent oxygen uptake/evolution was measured at various light intensities. Maximum white light intensity (100% saturation) was 1,500 µmol photons m−2 s−1, obtained from a tungsten light source. Various lower light intensities were obtained using neutral density filters (Balzers).
Chl a Fluorescence Measurements
Chl a fluorescence from the upper surface of the leaves was measured with a PAM-2001 chlorophyll fluorometer (Walz) at room temperature, as described by Dutta et al. (2009). Before each measurement, the sample leaf was dark adapted for 20 min (Demmig et al., 1987). The initial fluorescence, Fo, and the maximal fluorescence, Fm, were recorded by turning on the weak measuring light, and the Fm after the saturation flash (of approximately 3,000 μmol photons m−2 s−1) was given. Optimum quantum efficiency of PSII was calculated as Fv/Fm = (Fm − Fo)/Fm (Schreiber and Armond, 1978).
Photosynthesis Light-Response Curve
Photosynthetic light-response curves of wild-type and CAOx plants grown in LL and HL were measured using an infrared gas analyzer (Licor 6400-XT portable photosynthetic system). Photosynthesis rate measurements were taken in the greenhouse. Sample-chamber CO2 concentration was maintained at 380 μL L−1. Chamber air temperature was maintained at 25°C. Leaves were preexposed for 15 min at 700 μmol photons m−2 s−1 for HL-grown plants and 200 μmol photons m−2 s−1 for LL-grown plants before CO2 assimilation was monitored.
Starch Estimation
Starch estimation was carried out by the acid digestion method (Rose et al., 1991) with a slight modification. Leaf discs of equal diameter from second leaves of plants were homogenized in liquid nitrogen. These homogenized samples were washed several times with acetone and hot 80% ethanol to remove interfering substances until the extract became colorless. The starch was extracted and solubilized by 35% perchloric acid. This starch solution is used for the colorimetric assay. Anthrone reagent (500 mL of concentrated sulfuric acid, 200 mL of water, and 1.146 g of anthrone powder) was used as a colorimetric reagent. Samples were boiled in a boiling-water bath for 12 min and placed in ice immediately. Absorbance was recorded at 625 nm on a spectrophotometer (Rose et al., 1991).
Measurement of Plant Dry Weight and Other Parameters
Aboveground plant parts (i.e. entire shoots) were harvested and dried at 70°C for 5 d, and their final dry weights were determined in 6-week-old plants. Several replicates (n = 15) were taken for dry matter measurements.
The plant height, number of leaves, and days for anthesis (50% flowering) were measured from 15-week-old plants.
Statistical Analysis
Statistical analysis was performed either by using GraphPad Prism 4.0 or Statistica 5.0. Differences were analyzed with one-way ANOVA followed by Tukey’s multiple comparison test. Significance was accepted at the level of P < 0.05.
Acknowledgments
We thank P.V.K. Gutla for assistance in some of the experiments. We are grateful to S. Jansson, N.P. Huner, B. Grimm, and R. Oelmüller for their gift of several reagents used in this research. Govindjee thanks L. Boise and M. Plummer for secretarial help.
Glossary
- LL
low-light/low light
- LHC
light-harvesting chlorophyll protein complex
- Chl a
chlorophyll a
- Chl b
chlorophyll b
- Chl a/b
Chl a/Chl b ratio
- HL
high light/high-light
- Chlide
chlorophyllide
- Proto IX
protoporphyrin IX
- MPE
Mg-protoporphyrin IX monoester
- Pchlide
protochlorophyllide
- GSA
glutamate semialdehyde
- ALA
5-aminolevulinic acid
- ALAD
5-aminolevulinic acid dehydratase
- PBGD
porphobilinogen deaminase
- Protox
protoporphyrinogen oxidase
- POR
protochlorophyllide oxidoreductase
- PBG
porphobilinogen
- MV
methyl viologen
- PD
phenylenediamine
- CAO
chlorophyllide a oxygenase
- CAOx
AtCAO-overexpressing
- CO2
carbon dioxide
References
- Alawady AE, Grimm B. (2005) Tobacco Mg protoporphyrin IX methyltransferase is involved in inverse activation of Mg porphyrin and protoheme synthesis. Plant J 41: 282–290 [DOI] [PubMed] [Google Scholar]
- Allen CF, Good P, Trosper T, Park RB. (1972) Chlorophyll, glycerolipid and protein ratios in spinach chloroplast grana and stroma lamellae. Biochem Biophys Res Commun 48: 907–913 [DOI] [PubMed] [Google Scholar]
- Anderson J, Chow W, Goodchild D. (1988) Thylakoid membrane organization in sun/shade acclimation. Aust J Plant Physiol 15: 11–26 [Google Scholar]
- Bailey S, Walters RG, Jansson S, Horton P. (2001) Acclimation of Arabidopsis thaliana to the light environment: the existence of separate low light and high light responses. Planta 213: 794–801 [DOI] [PubMed] [Google Scholar]
- Baker NR. (2008) Chlorophyll fluorescence: a probe of photosynthesis in vivo. Annu Rev Plant Biol 59: 89–113 [DOI] [PubMed] [Google Scholar]
- Barber J, Nield J, Morris EP, Hankamer B. (1999) Subunit positioning in photosystem II revisited. Trends Biochem Sci 24: 43–45 [DOI] [PubMed] [Google Scholar]
- Beale SI, Castelfranco PA. (1974) The biosynthesis of Δ-aminolevulinic acids in higher plants. I. Accumulation of Δ-aminolevulinic acid in greening plant tissues. Plant Physiol 53: 291–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellemare G, Bartlett SG, Chua NH. (1982) Biosynthesis of chlorophyll a/b-binding polypeptides in wild type and the chlorina f2 mutant of barley. J Biol Chem 257: 7762–7767 [PubMed] [Google Scholar]
- Björkman O, Pearcy RW, Harrison AT, Mooney H. (1972) Photosynthetic adaptation to high temperatures: a field study in Death Valley, California. Science 175: 786–789 [DOI] [PubMed] [Google Scholar]
- Bradford MM. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254 [DOI] [PubMed] [Google Scholar]
- Chakraborty N, Tripathy BC. (1992) Involvement of singlet oxygen in 5-aminolevulinic acid-induced photodynamic damage of cucumber (Cucumis sativus L.) chloroplasts. Plant Physiol 98: 7–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162: 156–159 [DOI] [PubMed] [Google Scholar]
- Chow WS, Melis A, Anderson JM. (1990) Adjustments of photosystem stoichiometry in chloroplasts improve the quantum efficiency of photosynthesis. Proc Natl Acad Sci USA 87: 7502–7506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demmig B, Winter K, Krüger A, Czygan FC. (1987) Photoinhibition and zeaxanthin formation in intact leaves: a possible role of the xanthophyll cycle in the dissipation of excess light energy. Plant Physiol 84: 218–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta S, Mohanty S, Tripathy BC. (2009) Role of temperature stress on chloroplast biogenesis and protein import in pea. Plant Physiol 150: 1050–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duysens LNM (1952) Transfer of excitation energy in photosynthesis. PhD thesis. State University, Utrecht, The Netherlands
- Ehleringer J, Pearcy RW. (1983) Variation in quantum yield for CO2 uptake among C3 and C4 plants. Plant Physiol 73: 555–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espineda CE, Linford AS, Devine D, Brusslan JA. (1999) The AtCAO gene, encoding chlorophyll a oxygenase, is required for chlorophyll b synthesis in Arabidopsis thaliana. Proc Natl Acad Sci USA 96: 10507–10511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidt EE, Huber SC. (1992) Regulation of photosynthesis by end-product accumulation in leaves of plants storing starch, sucrose, and hexose sugars. Plant Physiol 99: 1443–1448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindjee (1995) Sixty-three years since Kautsky: chlorophyll a fluorescence. Aust J Plant Physiol 22: 131–160 [Google Scholar]
- Govindjee (2004) Chlorophyll a fluorescence: a bit of basics and history. In GC Papageorgiou, Govindjee, eds, Chlorophyll Fluorescence: A Signature of Photosynthesis. Springer, Dordrecht, The Netherlands, pp 1–42
- Green BR, Durnford DG. (1996) The chlorophyll-carotenoid proteins of oxygenic photosynthesis. Annu Rev Plant Physiol Plant Mol Biol 47: 685–714 [DOI] [PubMed] [Google Scholar]
- Haehnel W. (1984) Photosynthetic electron transport in higher plants. Annu Rev Plant Physiol 35: 659–693 [Google Scholar]
- Harper AL, von Gesjen SE, Linford AS, Peterson MP, Faircloth RS, Thissen MM, Brusslan JA. (2004) Chlorophyllide a oxygenase mRNA and protein levels correlate with the chlorophyll a/b ratio in Arabidopsis thaliana. Photosynth Res 79: 149–159 [DOI] [PubMed] [Google Scholar]
- Hirashima M, Satoh S, Tanaka R, Tanaka A. (2006) Pigment shuffling in antenna systems achieved by expressing prokaryotic chlorophyllide a oxygenase in Arabidopsis. J Biol Chem 281: 15385–15393 [DOI] [PubMed] [Google Scholar]
- Hoober JK, Eggink LL. (2001) A potential role of chlorophylls b and c in assembly of light-harvesting complexes. FEBS Lett 489: 1–3 [DOI] [PubMed] [Google Scholar]
- Hukmani P, Tripathy BC. (1992) Spectrofluorometric estimation of intermediates of chlorophyll biosynthesis: protoporphyrin IX, Mg-protoporphyrin, and protochlorophyllide. Anal Biochem 206: 125–130 [DOI] [PubMed] [Google Scholar]
- Hukmani P, Tripathy BC. (1994) Chlorophyll biosynthetic reactions during senescence of excised barley (Hordeum vulgare L. cv. IB 65) leaves. Plant Physiol 105: 1295–1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause GH, Weis E. (1991) Chlorophyll fluorescence and photosynthesis: the basics. Annu Rev Plant Physiol Plant Mol Biol 42: 313–349 [Google Scholar]
- Laemmli UK. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685 [DOI] [PubMed] [Google Scholar]
- Lee S, Kim JH, Yoo ES, Lee CH, Hirochika H, An G. (2005) Differential regulation of chlorophyll a oxygenase genes in rice. Plant Mol Biol 57: 805–818 [DOI] [PubMed] [Google Scholar]
- Lefebvre S, Lawson T, Zakhleniuk OV, Lloyd JC, Raines CA, Fryer M. (2005) Increased sedoheptulose-1,7-bisphosphatase activity in transgenic tobacco plants stimulates photosynthesis and growth from an early stage in development. Plant Physiol 138: 451–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong T-Y, Anderson JM. (1984) Adaptation of the thylakoid membranes of pea chloroplasts to light intensities. I. Study on the distribution of chlorophyll–protein complexes. Photosynth Res 5: 105–115 [DOI] [PubMed] [Google Scholar]
- Lindahl M, Yang DH, Andersson B. (1995) Regulatory proteolysis of the major light-harvesting chlorophyll a/b protein of photosystem II by a light-induced membrane-associated enzymic system. Eur J Biochem 231: 503–509 [DOI] [PubMed] [Google Scholar]
- Loomis RS, Amthor JS. (1999) Yield potential, plant assimilatory capacity, and metabolic efficiencies. Crop Sci 39: 1584–1596 [Google Scholar]
- Masuda T, Tanaka A, Melis A. (2003) Chlorophyll antenna size adjustments by irradiance in Dunaliella salina involve coordinate regulation of chlorophyll a oxygenase (CAO) and Lhcb gene expression. Plant Mol Biol 51: 757–771 [DOI] [PubMed] [Google Scholar]
- Melis A. (1991) Dynamics of photosynthetic membrane composition and function. Biochim Biophys Acta 1058: 87–106 [Google Scholar]
- Miyagawa Y, Tamoi M, Shigeoka S. (2001) Overexpression of a cyanobacterial fructose-1,6-/sedoheptulose-1,7-bisphosphatase in tobacco enhances photosynthesis and growth. Nat Biotechnol 19: 965–969 [DOI] [PubMed] [Google Scholar]
- Murchie EH, Horton P. (1997) Acclimation of photosynthesis to irradiance and spectral quality in British plant species: chlorophyll content, photosynthetic capacity and habitat preference. Plant Cell Environ 20: 438–448 [Google Scholar]
- Murray DL, Kohorn BD. (1991) Chloroplasts of Arabidopsis thaliana homozygous for the ch-1 locus lack chlorophyll b, lack stable LHCPII and have stacked thylakoids. Plant Mol Biol 16: 71–79 [DOI] [PubMed] [Google Scholar]
- Nagata N, Satoh S, Tanaka R, Tanaka A. (2004) Domain structures of chlorophyllide a oxygenase of green plants and Prochlorothrix hollandica in relation to catalytic functions. Planta 218: 1019–1025 [DOI] [PubMed] [Google Scholar]
- Nakagawara E, Sakuraba Y, Yamasato A, Tanaka R, Tanaka A. (2007) Clp protease controls chlorophyll b synthesis by regulating the level of chlorophyllide a oxygenase. Plant J 49: 800–809 [DOI] [PubMed] [Google Scholar]
- Nobel PS. (1976) Photosynthetic rates of sun versus shade leaves of Hyptis emoryi Torr. Plant Physiol 58: 218–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oosawa N, Masuda T, Awai K, Fusada N, Shimada H, Ohta H, Takamiya K. (2000) Identification and light-induced expression of a novel gene of NADPH-protochlorophyllide oxidoreductase isoform in Arabidopsis thaliana. FEBS Lett 474: 133–136 [DOI] [PubMed] [Google Scholar]
- Oster U, Tanaka R, Tanaka A, Rüdiger W. (2000) Cloning and functional expression of the gene encoding the key enzyme for chlorophyll b biosynthesis (CAO) from Arabidopsis thaliana. Plant J 21: 305–310 [DOI] [PubMed] [Google Scholar]
- Pattanayak GK, Biswal AK, Reddy VS, Tripathy BC. (2005) Light-dependent regulation of chlorophyll b biosynthesis in chlorophyllide a oxygenase overexpressing tobacco plants. Biochem Biophys Res Commun 326: 466–471 [DOI] [PubMed] [Google Scholar]
- Pattanayak GK, Tripathy BC. (2011) Overexpression of protochlorophyllide oxidoreductase C regulates oxidative stress in Arabidopsis. PLoS ONE 6: e26532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen H, Finkenzeller B, Kühlein N. (1993) Pigments induce folding of light-harvesting chlorophyll a/b-binding protein. Eur J Biochem 215: 809–816 [DOI] [PubMed] [Google Scholar]
- Peter GF, Thornber JP. (1991) Biochemical composition and organization of higher plant photosystem II light-harvesting pigment-proteins. J Biol Chem 266: 16745–16754 [PubMed] [Google Scholar]
- Porra RJ, Thompson WA, Kriedmann PA. (1989) Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim Biophys Acta 975: 384–394 [Google Scholar]
- Porra RJW, Schäfer W, Cmiel E, Katheder I, Scheer H. (1993) Derivation of the formyl-group oxygen of chlorophyll b from molecular oxygen in greening leaves of a higher plant (Zea mays). FEBS Lett 323: 31–34 [DOI] [PubMed] [Google Scholar]
- Powles SB, Critchley C. (1980) Effect of light intensity during growth on photoinhibition of intact attached bean leaflets. Plant Physiol 65: 1181–1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose R, Rose CL, Omi SK, Forry KR, Durall DM, Big WL. (1991) Starch determination by perchloric acid vs enzymes: evaluating the accuracy of six colorimetrics methods. J Agric Food Chem 39: 2–11 [Google Scholar]
- Sakuraba Y, Balazadeh S, Tanaka R, Muller-Roeber B, Tanaka A. (2012) Overproduction of chlorophyll b retards senescence through transcriptional re-programming in Arabidopsis. Plant Cell Physiol 53: 505–517 [DOI] [PubMed] [Google Scholar]
- Sakuraba Y, Tanaka R, Yamasato A, Tanaka A. (2009) Determination of a chloroplast degron in the regulatory domain of chlorophyllide a oxygenase. J Biol Chem 284: 36689–36699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuraba Y, Yokono M, Akimoto S, Tanaka R, Tanaka A. (2010) Deregulated chlorophyll b synthesis reduces the energy transfer rate between photosynthetic pigments and induces photodamage in Arabidopsis thaliana. Plant Cell Physiol 51: 1055–1065 [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T (1989) Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
- Satoh S, Ikeuchi M, Mimuro M, Tanaka A. (2001) Chlorophyll b expressed in cyanobacteria functions as a light-harvesting antenna in photosystem I through flexibility of the proteins. J Biol Chem 276: 4293–4297 [DOI] [PubMed] [Google Scholar]
- Schreiber U, Armond PA. (1978) Heat-induced changes of chlorophyll fluorescence in isolated chloroplasts and related heat-damage at the pigment level. Biochim Biophys Acta 502: 138–151 [DOI] [PubMed] [Google Scholar]
- Shalygo N, Czarnecki O, Peter E, Grimm B. (2009) Expression of chlorophyll synthase is also involved in feedback-control of chlorophyll biosynthesis. Plant Mol Biol 71: 425–436 [DOI] [PubMed] [Google Scholar]
- Sharkey TD, Laporte M, Lu Y, Weise S, Weber APM. (2004) Engineering plants for elevated CO2: a relationship between starch degradation and sugar sensing. Plant Biol (Stuttg) 6: 280–288 [DOI] [PubMed] [Google Scholar]
- Sood S, Gupta V, Tripathy BC. (2005) Photoregulation of the greening process of wheat seedlings grown in red light. Plant Mol Biol 59: 269–287 [DOI] [PubMed] [Google Scholar]
- Tanaka A, Ito H, Tanaka R, Tanaka NK, Yoshida K, Okada K. (1998) Chlorophyll a oxygenase (CAO) is involved in chlorophyll b formation from chlorophyll a. Proc Natl Acad Sci USA 95: 12719–12723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka R, Koshino Y, Sawa S, Ishiguro S, Okada K, Tanaka A. (2001) Overexpression of chlorophyllide a oxygenase (CAO) enlarges the antenna size of photosystem II in Arabidopsis thaliana. Plant J 26: 365–373 [DOI] [PubMed] [Google Scholar]
- Tanaka R, Oster U, Kruse E, Rudiger W, Grimm B. (1999) Reduced activity of geranylgeranyl reductase leads to loss of chlorophyll and tocopherol and to partially geranylgeranylated chlorophyll in transgenic tobacco plants expressing antisense RNA for geranylgeranyl reductase. Plant Physiol 120: 695–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka R, Tanaka A. (2005) Effects of chlorophyllide a oxygenase overexpression on light acclimation in Arabidopsis thaliana. Photosynth Res 85: 327–340 [DOI] [PubMed] [Google Scholar]
- Tanaka R, Tanaka A. (2007) Tetrapyrrole biosynthesis in higher plants. Annu Rev Plant Biol 58: 321–346 [DOI] [PubMed] [Google Scholar]
- Tewari AK, Tripathy BC. (1998) Temperature-stress-induced impairment of chlorophyll biosynthetic reactions in cucumber and wheat. Plant Physiol 117: 851–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornber JP, Highkin HR. (1974) Composition of the photosynthetic apparatus of normal barley leaves and a mutant lacking chlorophyll b. Eur J Biochem 41: 109–116 [DOI] [PubMed] [Google Scholar]
- Tomitani A, Okada K, Miyashita H, Matthijs HC, Ohno T, Tanaka A. (1999) Chlorophyll b and phycobilins in the common ancestor of cyanobacteria and chloroplasts. Nature 400: 159–162 [DOI] [PubMed] [Google Scholar]
- Tripathy BC, Chakraborty N. (1991) 5-Aminolevulinic acid induced photodynamic damage of the photosynthetic electron transport chain of cucumber (Cucumis sativus L.) cotyledons. Plant Physiol 96: 761–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathy BC, Mohanty P. (1980) Zinc-inhibited electron transport of photosynthesis in isolated barley chloroplasts. Plant Physiol 66: 1174–1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathy BC, Mohapatra A, Gupta I. (2007) Impairment of the photosynthetic apparatus by oxidative stress induced by photosensitization reaction of protoporphyrin IX. Biochim Biophys Acta 1767: 860–868 [DOI] [PubMed] [Google Scholar]
- Tripathy BC, Pattanayak GK (2010) Singlet oxygen-induced stress in plants. In CA Rebeiz et al., eds, The Chloroplast: Basics and Applications. Springer, Dordrecht, The Netherlands, pp 397–412
- Vavilin D, Yao D, Vermaas W. (2007) Small Cab-like proteins retard degradation of photosystem II-associated chlorophyll in Synechocystis sp. PCC 6803: kinetic analysis of pigment labeling with 15N and 13C. J Biol Chem 282: 37660–37668 [DOI] [PubMed] [Google Scholar]
- Walters RG, Rogers JJ, Shephard F, Horton P. (1999) Acclimation of Arabidopsis thaliana to the light environment: the role of photoreceptors. Planta 209: 517–527 [DOI] [PubMed] [Google Scholar]
- Xu H, Vavilin D, Vermaas W. (2001) Chlorophyll b can serve as the major pigment in functional photosystem II complexes of cyanobacteria. Proc Natl Acad Sci USA 98: 14168–14173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Vavilin D, Vermaas W. (2002) The presence of chlorophyll b in Synechocystis sp. PCC 6803 disturbs tetrapyrrole biosynthesis and enhances chlorophyll degradation. J Biol Chem 277: 42726–42732 [DOI] [PubMed] [Google Scholar]
- Yamasato A, Nagata N, Tanaka R, Tanaka A. (2005) The N-terminal domain of chlorophyllide a oxygenase confers protein instability in response to chlorophyll B accumulation in Arabidopsis. Plant Cell 17: 1585–1597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasato A, Tanaka R, Tanaka A. (2008) Loss of the N-terminal domain of chlorophyllide a oxygenase induces photodamage during greening of Arabidopsis seedlings. BMC Plant Biol 8: 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S. (1972) Physiological aspects of grain yield. Annu Rev Plant Physiol 23: 437–464 [Google Scholar]
- Zeeman SC, Tiessen A, Pilling E, Kato KL, Donald AM, Smith AM. (2002) Starch synthesis in Arabidopsis: granule synthesis, composition, and structure. Plant Physiol 129: 516–529 [DOI] [PMC free article] [PubMed] [Google Scholar]