Abstract
While the benefits of exercise for managing cancer-and treatment-related side effects has been shown among various populations of cancer survivors, a relative dearth of information exists among older cancer patients.
Objectives
To determine the prevalence of exercise participation during and after primary cancer treatment in older (≥65 years) and the oldest (≥80 years) cancer patients and to examine the relationships between exercise, symptoms, and self-rated health (SRH).
Materials and Methods
408 newly diagnosed older cancer patients (mean age=73, range=65-92) scheduled to receive chemotherapy and/or radiation therapy reported symptoms and SRH prior to, during, and 6 months after treatment, and exercise participation during and following treatment.
Results
Forty-six percent of older and 41% of the oldest patients reported exercising during treatment. Sixty percent of older and 68% of the oldest patients reported exercising in the 6 months thereafter. Older patients who exercised during treatment reported less shortness of breath and better SRH during treatment, and better SRH following treatment. The oldest patients who exercised during treatment reported less memory loss and better SRH during treatment and less fatigue and better SRH following treatment. The oldest patients who exercised following treatment reported less fatigue, skin problems, and total symptom burden following treatment.
Conclusion
These data suggest a willingness of older cancer patients to attempt exercise during and after treatment. Exercise during these times is associated with less severe symptoms; further clinical research examining the efficacy of formal exercise interventions to reduce symptoms and improve SRH in older cancer patients is needed.
Keywords: elderly, exercise, physical activity, neoplasms, side effects, symptoms, chemotherapy, radiation therapy
Introduction
Aging is associated with declines in physical (1-4) and psychological (3-4) function and cancer treatments can further exacerbate these functional decrements. Younger cancer patients often experience a transient decline in function, whereas older cancer patients may never fully recover (5). These decrements in physiological and psychological function can ultimately lead to reduced quality of life (6-7).
Older adults are at much greater risk of developing cancer than those who are younger. In the United States, approximately 60% of new cancer diagnoses occur in those over 65 years of age. By 2030, 70% of cancer patients will be elderly (8). Compared to older adults without a history of cancer, older cancer survivors suffer from a greater incidence of frailty and more limitations in activities of daily living (9), lower self-rated health (9), reduced quality of life (10), and a greater prevalence of geriatric syndromes such as dementia, depression, falls, incontinence, and osteoporosis (9). The severity of cancer-related fatigue, another problem facing many cancer patients during and following treatment, is related to the cancer and its treatment as well as age-related factors (11).
Regular physical activity reduces the risk of developing many chronic conditions, such as coronary artery disease, hypertension , osteoporosis , type II diabetes , obesity, and chronic obstructive pulmonary disease, while playing a role in the management of anxiety, depression, dementia, and pain (12). The American College of Sports Medicine (ACSM) recommends that older adults, even those with chronic medical conditions, participate in regular aerobic exercise training (150-300 minutes per week) and resistance exercise training (at least 2 days a week) (12). Evidence also supports the use of exercise for side-effect and symptom management in cancer survivors (13-15). Among elders, especially those who are frail, physical activity intervention programs have resulted in improved muscular mass and strength (16), improved aerobic capacity (17), fall reduction (18-20), improved perceived health status (21), and improved physical functioning (22).
Exercise is a promising behavioral intervention with the potential to mitigate multiple symptoms and improve physical functioning in older cancer patients (23-27). However, it is important to investigate the proportion of older cancer patients who participate in exercise during and following treatment and to investigate the possible associations between exercise and symptom severity in older cancer patients. The purpose of this paper is to describe the proportion of older cancer patients both during and following treatment who report using exercise and to describe the associations between exercise and symptom severity during these time frames.
Materials and Methods
Study sample
Participants were newly-diagnosed cancer patients who were scheduled to receive chemo- or radiation-therapy. Patients who participated in this investigation met the following criteria: a) had a diagnosis of breast, gynecological, lung, hematologic, gastrointestinal, genitourinary tract, or head and neck cancer, b) had not received chemotherapy or radiation therapy in the past, c) had an estimated life expectancy of 10 months or more, d) were 65 years of age or older, and e) were able to read and understand English.
Recruitment and Procedures
This is a secondary data analysis of a longitudinal study designed to assess the information needs of cancer patients during and following chemotherapy and radiation therapy with original results published by Hofman et al (28). Participants in this study were recruited from 17 Community Clinical Oncology Program (CCOP) affiliates located throughout the United States, all of which were members of the University of Rochester Cancer Center (URCC) CCOP Research Base.
Data Collection
Participants were given a semi-structured clinical interview and a series of questionnaires at three time points throughout the study. Data was collected in two parts, using a semi-structured interview and self-report questionnaires. The semi-structured interview and self-report questionnaires were used to obtain patient information and demographic information, self-rated health, and symptom presence and severity. The semi-structured interview was conducted by study coordinators on-site, used to develop a rapport with the participants and to explain the rest of the study. The questionnaires were self-administered. They were provided to the participants during the interview, completed at home, and mailed back in preaddressed, stamped envelopes. Clinical diagnosis, Karnofsky performance status, and date of birth were obtained from the patients’ medical records. Participants completed a symptom inventory and reported self-rated health prior to beginning chemo- or radiation-therapy, within the two weeks after completing these treatments, and six months thereafter. Participants reported if they “used exercise since diagnosis” at each of the two later time points.
Outcome Measures
Symptom presence and severity were assessed using the 12-item URCC Symptom Inventory, adapted from a measure created at the M.D. Anderson Cancer Center (29). Participants were asked to rate their symptom severity on an 11-point scale ranging from 0 (not present) to 10 (as bad as you can imagine). The 12 symptoms assessed included pain, fatigue, nausea, sleep problems, feelings of depression, shortness of breath, memory loss, weight loss, hair loss, difficulty concentrating, hot flashes, and skin problems. Total symptom burden was calculated by summing the severity of all 12 symptoms. Prior to treatment, patients reported their symptom severity during the previous five days. In the two weeks following treatment, patients reported their symptom severity at its worst during treatment. Six months after treatment, patients reported their symptom severity during the previous five days.
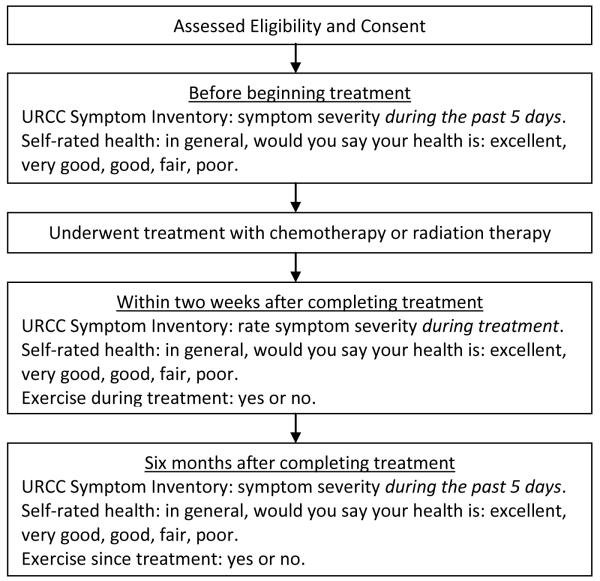
General self-rated health was assessed using a single question, “In general, would you say that your health is?” from which patients selected excellent, very good, good, fair or poor. Self-rated health is related to mortality with those reporting poor self-rated health having twice the mortality risk as those rating their health as excellent, even when adjusting for co-morbidities and functional status (30). The use of exercise was assessed with a single question, “Since your cancer diagnosis, have you used exercise” with possible answers being “I HAVE used this” or “Did NOT use”. See Figure 1 for the study timeline and flow.
Figure 1.
Study Timeline and Flow.
Plan of Analysis
Data analysis methods included descriptive statistics to characterize baseline characteristics of the sample including age, race, marital status, gender, education, Karnofsky performance score, treatment type, and cancer diagnosis. In those who did and did not use exercise during and following treatment, analysis of covariance (ANCOVAs) with baseline symptom severity scores or self-rated health as the covariates were used to test for differences between symptom severity and self-rated health, respectively. SPSS PASW version 18, JMP and R were used for all statistical analyses.
Institutional Review Board Approval
The study was approved by the University of Rochester Research Subjects Review Board and the Institutional Review Boards of the participating CCOPs. Written informed consent was obtained prior to enrollment in the study.
Results
Sample Characteristics
A total of 408 patients 65 to 92 years of age provided evaluable information. Of those, 361 were between the ages of 65 and 79 and 47 were 80 years of age or older. The mean age of patients in this study was 73 years. Males comprised just over half of the sample. A majority of patients were white and married, had a Karnofsky performance status of 90 or greater and had undergone surgery for their cancer prior to chemotherapy and/or radiation therapy. The largest proportion of cancer diagnoses were breast and genitourinary. Patient characteristics are presented in Table 1.
Table 1.
Patient characteristics
| Age |
|||
|---|---|---|---|
| Variable | ≥ 65 (n= 408) | 65-79 (n = 361) | ≥ 80 (n = 47) |
| Demographics | |||
| Age (years ± SEM) | 72.82 ± 0.26 | 71.59 ± 0.07 | 82.26 ± 0.40 |
| Race - white (N, %) | 384 (94) | 340 (94) | 44 (94) |
| Married (N, %) | 287 (70) | 261 (72) | 26 (55) |
| Gender (N, %) | |||
| Female | 199 (49) | 181 (50) | 18 (38) |
| Male | 209 (51) | 180 (50) | 29 (62) |
| > High school education | 183 (45) | 162 (45) | 21 (45) |
| Clinical Characteristics | |||
| Karnofsky performance status ≥ 90 (N, %) | 317 (78) | 280 (78) | 37 (79) |
| Treatment type (N, %) | |||
| Surgery | 247 (60) | 223 (62) | 24 (51) |
| Chemotherapy | 144 (35) | 129 (36) | 15 (32) |
| Radiation therapy | 195 (48) | 172 (48) | 23 (49) |
| Chemotherapy and radiation therapy | 69 (17) | 60 (17) | 9 (19) |
| Cancer Diagnosis (N, %) | |||
| Breast | 122 (30) | 114 (32) | 8 (17) |
| Genitourinary | 123 (30) | 109 (30) | 14 (30) |
| Lung | 77 (19) | 65 (18) | 12 (26) |
| Gastrointestinal | 36 (9) | 34 (9) | 2 (4) |
| Hematologic | 25 (6) | 19 (5) | 6 (13) |
| Gynecologic | 17 (4) | 14 (4) | 3 (6) |
| Head/neck | 8 (2) | 6 (2) | 2 (4) |
Exercise during Treatment
Patients reported their use of exercise during treatment at the follow-up time point which was within two weeks after primary treatment completion with chemotherapy and/or radiation therapy. Forty-six percent of patients 65 years of age and older reported using exercise during radiation therapy and/or chemotherapy treatments. Similar proportions of males (45%) and females (47%) reported exercising during treatment, and 50% of patients undergoing chemotherapy, 48% undergoing radiation therapy, and 35% undergoing chemotherapy and radiation therapy reported exercise use during treatment.
Forty percent of patients 80 years of age and older reported using exercise during radiation therapy and/or chemotherapy treatments. A greater proportion of males (48%) than females (29%) reported exercising during treatment and 30% of patients undergoing chemotherapy, 56% of patients undergoing radiation therapy, and 22% of patients undergoing chemotherapy and radiation therapy reported exercising during treatment. There was not a significant difference in the proportion of patients who exercised compared to those who did not based on the type of treatment they received.
Exercise followingTreatment
Patients reported their use of exercise following treatment at the follow-up time point which was 6 months after primary treatment completion. Patients could have been receiving adjuvant treatments including hormone therapies or aromatase inhibitors during that 6 month period following primary treatment. Of patients 65 years of age and older, 60% reported using exercise following treatment, with 66% of females and 55% of males reporting exercise following treatment. Fifteen percent of patients who did not exercise during treatment began to exercise following treatment. Sixty-five percent of patients who had received chemotherapy, 58% of patients who had received radiation therapy, and 59% of patients who had received both chemotherapy and radiation therapy exercised following treatment.
Of patients 80 and older, 68% reported using exercise following radiation therapy and/or chemotherapy. Only 7% of patients who did not exercise during treatment reported exercising following treatment. More females (75%) than males (65%) reported exercising following treatment, and 63% of patients who had completed chemotherapy, 71% of patients who had completed radiation therapy, and 67% of patients who had completed both radiation therapy and chemotherapy reported exercising after treatment completion. Of patients 65 years of age and over, 32% exercised both during and after treatment, whereas 42% did not exercise during either time frame. Thirty percent exercised both during and after treatment, whereas 43% did not exercise during that time frame.
Exercise and Symptom Severity during Treatment
ANCOVAs with baseline symptom severity scores or self-rated health, respectively, as the covariates revealed patients 65 years of age and older and patients between the ages of 65 and 79 who used exercise reported less severe shortness of breath (≥ 65: exercise = 1.96±0.21, no exercise = 2.84±0.24, p=0.04; 65-79: exercise = 1.92±0.21, no exercise = 2.73±0.26, p=0.05) and better self-rated health (≥ 65: exercise = 2.37±0.07, no exercise = 2.88±0.07, p<0.01; 65-79: exercise = 2.37±0.07, no exercise = 2.88±0.08, p<0.01). Patients between 65 and 79 years of age reported less severe weight loss (exercise = 1.33±0.19, no exercise = 1.94±0.22, p=0.03) and patients 65 and over showed a statistical trend for less severe weight loss (exercise = 1.43±0.19, no exercise = 2.01±0.21, p=0.07) throughout the course of treatment when compared to patients who did not exercise. Self-rated health was reported as very good or excellent by 60% of patients 65 years of age or older who exercised and by 33% of patients who did not. In patients 80 years of age and older, those who exercised reported less severe memory loss (exercise = 1.67±0.75, no exercise = 4.14±0.69, p=0.05), better self-rated health (exercise = 2.40±0.24, no exercise = 2.86±0.22, p=0.01), and a statistical trend towards having less difficulty concentrating (exercise = 1.20±0.62, no exercise = 3.05±0.57, p=0.10) throughout the course of treatment compared to patients who did not exercise. Self-rated health was reported as excellent or very good by 47% of patients 80 years of age or older who exercised and by 33% of patients who did not exercise. See Table 2 for symptom severity and self-rated health during treatment based on exercise participation during treatment.
Table 2.
Symptom severity and self-rated health during treatment reported by patients who used exercise and those who did not use during treatment.
| ≥ 65 years of age |
65-79 years of age |
≥ 80 years of age |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Symptom | Exercise (n = 141) |
No exercise (n = 163) |
p-value | Exercise (n = 126) |
No exercise (n = 142) |
p-value | Exercise (n = 15) |
No exercise (n = 21) |
p-value |
| Pain | 2.84 ± 0.26 | 3.13 ± 0.26 | 0.93 | 2.86 ± 0.27 | 3.18 ± 0.28 | 0.91 | 2.67 ± 0.84 | 2.81 ± 0.76 | 0.81 |
| Fatigue | 4.94 ± 0.23 | 5.41 ± 0.25 | 0.16 | 5.02 ± 0.25 | 5.37 ± 0.27 | 0.27 | 4.27 ± 0.70 | 5.67 ± 0.66 | 0.21 |
| Nausea | 1.95 ± 0.25 | 1.58 ± 0.20 | 0.28 | 1.94 ± 0.26 | 1.51 ± 0.21 | 0.31 | 2.00 ± 0.92 | 2.10 ± 0.67 | 0.99 |
| Sleep problems | 3.48 ± 0.25 | 3.28 ± 0.24 | 0.49 | 3.47 ± 0.26 | 3.24 ± 0.26 | 0.47 | 3.60 ± 0.93 | 3.57 ± 0.77 | 0.92 |
| Feelings of depression | 2.51 ± 0.24 | 2.86 ± 0.24 | 0.13 | 2.52 ± 0.25 | 2.78 ± 0.26 | 0.23 | 2.51 ± 0.24 | 2.86 ± 0.24 | 0.13 |
| Shortness of breath | 1.96 ± 0.21 | 2.84 ± 0.24 | 0.04* | 1.92 ± 0.21 | 2.73 ± 0.26 | 0.05* | 2.33 ± 0.89 | 3.62 ± 0.65 | 0.42 |
| Memory loss | 2.35 ± 0.23 | 2.29 ± 0.21 | 0.82 | 2.44 ± 0.25 | 2.02 ± 0.21 | 0.24 | 1.67 ± 0.75 | 4.14 ± 0.69 | 0.05* |
| Weight loss | 1.43 ± 0.19 | 2.01 ± 0.21 | 0.07^ | 1.33 ± 0.19 | 1.94 ± 0.22 | 0.03* | 2.27 ± 0.88 | 2.52 ± 0.69 | 0.79 |
| Hair loss | 2.43 ± 0.33 | 3.11 ± 0.32 | 0.17 | 2.57 ± 0.36 | 3.13 ± 0.34 | 0.23 | 1.20 ± 0.83 | 2.95 ± 0.93 | 0.35 |
| Difficulty concentrating | 2.06 ± 0.22 | 2.21 ± 0.21 | 0.75 | 2.17 ± 0.24 | 2.08 ± 0.22 | 0.74 | 1.20 ± 0.62 | 3.05 ± 0.57 | 0.10^ |
| Hot flashes | 2.22 ± 0.26 | 2.01 ± 0.23 | 0.80 | 2.25 ± 0.28 | 2.04 ± 0.24 | 0.91 | 1.93 ± 0.83 | 1.18 ± 0.67 | 0.28 |
| Skin problems | 2.43 ± 0.25 | 2.18 ± 0.23 | 0.41 | 2.48 ± 0.26 | 2.14 ± 0.25 | 0.47 | 1.93 ± 0.90 | 2.48 ± 0.65 | 0.78 |
| Total symptom burden | 30.60 ± 1.85 | 32.93 ± 1.77 | 0.40 | 30.97 ± 1.97 | 32.16 ± 1.88 | 0.63 | 27.47 ± 5.44 | 38.10 ± 5.33 | 0.32 |
| Self-rated health | 2.37 ± 0.07 | 2.88 ± 0.07 | < 0.01* | 2.37 ± 0.07 | 2.88 ± 0.08 | < 0.01* | 2.40 ± 0.24 | 2.86 ± 0.22 | 0.01* |
Note: =p ≤ 0.05,
=p≤ 0.10.
Exercise during Treatment and Symptom Severity following Treatment
Patients 65 years of age and older who used exercise during chemotherapy or radiation therapy reported less severe shortness of breath (exercise = 1.34±0.18, no exercise = 2.14±0.25, p=0.04), better self-rated health (exercise = 2.44±0.08, no exercise = 2.85±0.08, p<0.01), and a statistical trend toward less severe total symptom burden (exercise = 15.30±1.20, no exercise = 17.96±1.36, p=0.09) in the six months after treatment compared to patients who did not exercise. Patients between the ages of 65 and 79 who used exercise during treatment also reported better self-rated health (exercise = 2.44±0.08, no exercise = 2.88±0.09, p<0.01) six months after treatment completion with self-rated health reported as very good or excellent by 53% of patients who exercised and by 32% of patients who did not. In patients 80 years of age and older, those who used exercise reported less fatigue (exercise = 3.07±0.68, no exercise = 4.47±0.72, p=0.04), better self-rated health (exercise = 2.43±0.29, no exercise = 2.65±0.24, p=0.04), and statistical trends for less shortness of breath (exercise = 1.43±0.50, no exercise = 3.06±0.82, p=0.09) and less total symptom burden (exercise = 15.14±3.01, no exercise = 22.41±4.03, p=0.10) compared to those who did not exercise in the six months after treatment completion. See Table 3 for symptom severity and self-rated health following treatment based on exercise participation during treatment.
Table 3.
Symptom severity and self-rated health following treatment reported by patients who used exercise and those who did not use exercise during treatment.
| ≥ 65 years of age |
65-79 years of age |
≥ 80 years of age |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Symptom | Exercise (n = 148) |
No exercise (n = 96) |
p-value | Exercise (n =111) |
No exercise (n =108) |
p-value | Exercise (n = 14) |
No exercise (n = 17) |
p-value |
| Pain | 1.04 ± 0.17 | 1.25 ± 0.18 | 0.48 | 1.10 ± 0.19 | 1.24 ± 0.19 | 0.83 | 0.57 ± 0.36 | 1.29 ± 0.50 | 0.31 |
| Fatigue | 3.00 ± 0.24 | 3.50 ± 0.27 | 0.14 | 2.99 ± 0.26 | 3.35 ± 0.29 | 0.42 | 3.07 ± 0.68 | 4.47 ± 0.72 | 0.04* |
| Nausea | 0.30 ± 0.08 | 0.46 ± 0.13 | 0.20 | 0.33 ± 0.09 | 0.35 ± 0.11 | 0.72 | 0.07 ± 0.07 | 1.12 ± 0.60 | 0.15 |
| Sleep problems | 2.06 ± 0.22 | 2.49 ± 0.26 | 0.21 | 2.07 ± 0.24 | 2.39 ± 0.27 | 0.44 | 1.93 ± 0.71 | 3.12 ± 0.87 | 0.18 |
| Feelings of depression | 1.63 ± 0.21 | 2.00 ± 0.23 | 0.16 | 1.52 ± 0.22 | 1.94 ± 0.26 | 0.19 | 2.50 ± 0.72 | 2.41 ± 0.58 | 0.71 |
| Shortness of breath | 1.34 ± 0.18 | 2.14 ± 0.25 | 0.04* | 1.32 ± 0.19 | 2.00 ± 0.25 | 0.17 | 1.43 ± 0.50 | 3.06 ± 0.82 | 0.09^ |
| Memory loss | 1.86 ± 0.19 | 2.10 ± 0.22 | 0.20 | 1.87 ± 0.21 | 2.10 ± 0.24 | 0.25 | 1.79 ± 0.56 | 2.12 ± 0.55 | 0.57 |
| Weight loss | 0.62 ± 0.13 | 0.98 ± 0.20 | 0.14 | 0.59 ± 0.14 | 1.00 ± 0.22 | 0.13 | 0.86 ± 0.53 | 0.88 ± 0.33 | 0.94 |
| Hair loss | 0.70 ± 0.18 | 1.10 ± 0.24 | 0.24 | 0.74 ± 0.20 | 1.08 ± 0.26 | 0.28 | 0.36 ± 0.84 | 1.18 ± 0.57 | 0.43 |
| Difficulty concentrating | 1.47 ± 0.18 | 1.41 ± 0.19 | 0.80 | 1.50 ± 0.20 | 1.40 ± 0.20 | 0.64 | 1.29 ± 0.43 | 1.47 ± 0.47 | 0.68 |
| Hot flashes | 1.82 ± 0.26 | 1.18 ± 0.24 | 0.18 | 1.84 ± 0.27 | 1.19 ± 0.22 | 0.15 | 1.64 ± 0.84 | 1.12 ± 0.44 | 0.29 |
| Skin problems | 0.94 ± 0.17 | 0.76 ± 0.16 | 0.46 | 0.95 ± 0.18 | 0.62 ± 0.14 | 0.18 | 0.93 ± 0.40 | 1.65 ± 0.70 | 0.38 |
| Total symptom burden | 15.30 ± 1.20 | 17.96 ± 1.36 | 0.09^ | 15.32 ± 1.30 | 17.27 ± 1.43 | 0.32 | 15.14 ± 3.01 | 22.41 ± 4.03 | 0.10^ |
| Self-rated health | 2.44 ± 0.08 | 2.85 ± 0.08 | < 0.01* | 2.44 ± 0.08 | 2.88 ± 0.09 | < 0.01* | 2.43 ± 0.29 | 2.65 ± 0.24 | 0.04* |
Note: =p ≤ 0.05,
=p≤ 0.10.
Exercise and Side-Effect Severity following Treatment
Patients 80 years of age and older who reported using exercise following treatment reported less severe fatigue (exercise = 3.14±0.60, no exercise = 5.90±0.67, p<0.01), skin problems (exercise = 0.67±0.29, no exercise = 2.90±1.02, p=0.01), and total symptom burden(exercise = 15.14±2.99, no exercise = 30.10±4.87, p<0.01) six months after treatment, with a statistical trend towards less nausea (exercise = 0.19±0.15, no exercise = 1.60±0.97, p=0.07), fewer sleep problems (exercise = 2.00±0.62, no exercise = 4.20±1.17, p=0.09), fewer feelings of depression (exercise = 2.24±0.57, no exercise = 3.30±0.82, p=0.08), less shortness of breath (exercise = 1.90±0.56, no exercise = 3.70±1.27, p=0.07), and less memory loss (exercise = 1.67±0.40, no exercise = 2.90±0.80, p=0.08) compared to those who did not exercise. See Table 4 for symptom severity and self-rated health following treatment based on exercise participation following treatment.
Table 4.
Symptom severity and self-rated health six months after treatment reported by patients who used exercise and those who did not use exercise following treatment.
| ≥ 65 years of age |
65-79 years of age |
≥ 80 years of age |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Symptom | Exercise (n = 156) |
No exercise (n = 102) |
p-value | Exercise (n = 135) |
No exercise (n = 92) |
p-value | Exercise (n = 21) |
No exercise (n = 10) |
p-value |
| Pain | 1.15 ± 0.15 | 1.16 ± 0.20 | 0.90 | 1.20 ± 0.16 | 1.10 ± 0.21 | 0.51 | 0.81 ± 0.36 | 1.70 ± 068 | 0.17 |
| Fatigue | 3.06 ± 0.22 | 3.51 ± 0.30 | 0.55 | 3.04 ± 0.24 | 3.25 ± 0.31 | 0.75 | 3.14 ± 0.60 | 5.90 ± 0.67 | < 0.01* |
| Nausea | 0.33 ± 0.07 | 0.43 ± 0.15 | 0.37 | 0.36 ± 0.08 | 0.30 ± 0.12 | 0.91 | 0.19 ± 0.15 | 1.60 ± 0.97 | 0.07^ |
| Sleep problems | 2.28 ± 0.21 | 2.25 ± 0.29 | 0.99 | 2.32 ± 0.23 | 2.03 ± 0.28 | 0.51 | 2.00 ± 0.62 | 4.20 ± 1.17 | 0.09^ |
| Feelings of depression | 1.74 ± 0.19 | 1.88 ± 0.25 | 0.61 | 1.66 ± 0.20 | 1.73 ± 0.26 | 0.91 | 2.24 ± 0.57 | 3.30 ± 0.82 | 0.08^ |
| Shortness of breath | 1.69 ± 0.18 | 1.82 ± 0.27 | 0.95 | 1.66 ± 0.19 | 1.62 ± 0.25 | 0.40 | 1.90 ± 0.56 | 3.70 ± 1.27 | 0.07^ |
| Memory loss | 1.88 ± 0.18 | 2.16 ± 0.24 | 0.50 | 1.92 ± 0.20 | 2.08 ± 0.26 | 0.94 | 1.67 ± 0.40 | 2.90 ± 0.80 | 0.08^ |
| Weight loss | 0.69 ± 0.14 | 0.89 ± 0.21 | 0.25 | 0.68 ± 0.15 | 0.86 ± 0.22 | 0.35 | 0.71 ± 0.37 | 1.20 ± 0.51 | 0.39 |
| Hair loss | 0.96± 0.20 | 0.74 ± 0.20 | 0.43 | 1.01 ± 0.22 | 0.72 ± 0.22 | 0.12 | 0.57 ± 0.27 | 0.90 ± 0.46 | 0.59 |
| Difficulty concentrating | 1.49 ± 0.16 | 1.31 ± 0.20 | 0.42 | 1.53 ± 0.18 | 1.25 ± 0.21 | 0.18 | 1.19 ± 0.31 | 1.90 ± 0.72 | 0.30 |
| Hot flashes | 1.65 ± 0.22 | 1.25 ± 0.23 | 0.24 | 1.72 ± 0.24 | 1.20 ± 0.24 | 0.12 | 1.24 ± 0.53 | 1.80 ± 0.84 | 0.11 |
| Skin problems | 0.89 ± 0.13 | 0.80 ± 0.20 | 0.80 | 0.93 ± 0.15 | 0.58 ± 0.17 | 0.16 | 0.67 ± 0.29 | 2.90 ± 1.02 | 0.01* |
| Total symptom burden | 16.31 ± 1.10 | 16.89 ± 1.54 | 0.76 | 16.50 ± 1.19 | 15.46 ± 1.56 | 0.35 | 15.14 ± 2.99 | 30.10 ± 4.87 | < 0.01* |
| Self-rated health | 2.54 ± 0.07 | 2.76 ± 0.09 | 0.33 | 2.54 ± 0.07 | 2.75 ± 0.10 | 0.41 | 2.52 ± 0.27 | 2.80 ± 0.25 | 0.63 |
Note: =p ≤ 0.05,
=p≤ 0.10.
Discussion
The number of adults over 65 years of age is increasing more rapidly than any other age group, with those over 80 growing the fastest and expected to account for 20% of people over 65 by the year 2050 (31). Cancer is a disease that disproportionally affects those over the age of 65 (32). To our knowledge, this is one of the few studies to focus on symptom severity, self-rated health and exercise participation in older cancer patients undergoing chemotherapy and/or radiation therapy and one of the few that provides data on cancer patients 80 years of age and older. Overall, these data suggest that many older cancer patients are interested in and able to exercise during treatment for cancer, as nearly half of the cancer patients age 65 and older in this study reported using exercise during treatment. Following treatment, over half of patients over 65 years of age reported using exercise. In addition, nearly half of patients 80 and older reported exercising during treatment, and over half reported using exercise following treatment. In a sample of stage I-IIIa breast cancer patients of all ages, Irwin et al. reported a reduction in physical activity from pre to post-diagnosis of 14% and 9%, respectively (33). Pinto and colleagues also found that breast cancer patients who were younger and farther out from diagnosis were more likely to exercise (34).
In general, older patients who reported using exercise during and following treatment reported less severe symptoms during and following treatment. Specifically, certain subsets of older patients who used exercise reported less shortness of breath, weight loss, fatigue, and total symptom burden during and following treatment. However, previous research has shown that co-morbidities, physical declines with aging, and physical limitations and symptoms resulting from treatment serve as barriers to exercise participation (35), so it may be that those who have more severe symptoms are less likely to exercise. These data are not definitive for causality, but rather show an association between exercise and symptom severity. Other researchers have found exercise beneficial for the reduction of symptoms, but these studies, especially in older cancer patients, are limited in number.
Dyspnea at rest or during exertion is a symptom experienced by some cancer patients (36). Exercise interventions in lung cancer patients in particular have resulted in less breathlessness (37). Sarcopenia is a type of muscle loss characterized by muscle fiber atrophy that occurs with age(38) . Cancer cachexia is another troubling side effect of cancer and its treatment affecting an estimated 2 to 15% of cancer patients (39). Exercise is a promising intervention for the reduction of both sarcopenia and cancer cachexia (40-42). Cancer-related fatigue is a common symptom in cancer patients of all ages (43-44).Cancer-related fatigue can be reduced in cancer patients between ages 21 and 65 (45-46) but until recently, little was known about the relationship between physical activity and fatigue in cancer patients 65 years of age and older. Luctkar-Flude and colleagues have found that higher physical activity levels predict lower fatigue levels in older cancer survivors (47), however few studies have focused on older cancer patients. Patients who exercised also reported better self-rated health than those who did not use exercise during or following treatment. Self-rated health has been found to be a predictor of mortality, independent of health status (48).
Research to assess why some cancer patients exercise and others do not is necessary. Research is desperately needed to determine the safety and efficacy of exercise in older cancer patients and survivors. Feasibility studies to assess intensity and dose of exercise needed for these patients and survivors to benefit from a reduction in symptom burden are also warranted. Exercise interventions must be designed to meet the unique needs of older cancer survivors and should be easily disseminated to all patients, regardless of social economic status and geographic location. Interventions that can be performed at retirement and assisted living communities may be of the greatest value and using modes of delivery that provide motivation and feedback, such as exergames, are promising interventions for this population. Lastly, research into the relationship between exercise and overall survival in older cancer patients is needed.
This study had a number of strengths in that it was conducted prospectively and systematically in one of the largest samples of older cancer patients to date. Moreover, it was conducted in multiple CCOP sites, thus increasing the generalizability of the data. However, there were some limitations. For example, the question used to ascertain the use of exercise during treatment did not provide any information on exercise type or dose. Additionally, a number of factors could have affected whether cancer patients participated in exercise during or following chemotherapy, radiation therapy or both. For example, information on cancer stage was not obtained, which may have affected exercise participation and symptoms. Although we found no significant difference in Karnofsky performance status between those who exercised and those who did not, a randomized controlled trial is needed to further investigate these relationships. Too, it may be that symptom presence or absence influences participation in exercise rather than exercise participation influencing symptoms. These patients represent a select group who are willing and able to participate in a clinical trial, perhaps limiting the generalizability of this investigation.
As the population ages, physicians will increasingly be faced with the challenges of managing the symptoms and side effects of cancer treatment in elderly populations. Exercise may be a promising strategy to reduce multiple symptoms and side effects with a single intervention. However, there is little data on the prevalence of participation of exercise in community-dwelling older adults during cancer treatment. The objective of this study was to evaluate the prevalence of exercise participation in older patients and to examine associations between exercising and treatment symptoms and self-rated health.
In conclusion, this investigation suggests that many older cancer patients are willing and able to use exercise during treatment. Evidence supporting the benefits of exercise for reducing symptom severity will likely increase the proportion of older adults willing to exercise during treatment. However, we must develop evidence-based exercise interventions that are proven to be effective for side-effect management in older cancer survivors. Ascertaining the type/s and dose of exercise that are most beneficial for reducing specific symptoms while developing exercise interventions that patients enjoy must be a priority given the growing number of older cancer survivors in the United States.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Short KR, Bigelow ML, Kahl J, Singh R, Coenen-Schimke J, Raghavakaimal S, et al. Decline in skeletal muscle mitochondrial function with aging in humans. Proc Natl Acad Sci U S A. 2005 Apr 12;102(15):5618–23. doi: 10.1073/pnas.0501559102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ishizaki T, Kai I, Kobayashi Y, Matsuyama Y, Imanaka Y. The effect of aging on functional decline among older Japanese living in a community: a 5-year longitudinal data analysis. Aging Clin Exp Res. 2004 Jun;16(3):233–9. doi: 10.1007/BF03327389. [DOI] [PubMed] [Google Scholar]
- 3.Payette H, Gueye NR, Gaudreau P, Morais JA, Shatenstein B, Gray-Donald K. Trajectories of physical function decline and psychological functioning: the Quebec longitudinal study on nutrition and successful aging (NuAge) J Gerontol B Psychol Sci Soc Sci. 2011 Jul;66(Suppl 1):i82–90. doi: 10.1093/geronb/gbq085. [DOI] [PubMed] [Google Scholar]
- 4.Costarella M, Monteleone L, Steindler R, Zuccaro SM. Decline of physical and cognitive conditions in the elderly measured through the functional reach test and the mini-mental state examination. Arch Gerontol Geriatr. 2010 May-Jun;50(3):332–7. doi: 10.1016/j.archger.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 5.Satariano WA, Ragheb NE, Branch LG, Swanson GM. Difficulties in physical functioning reported by middle-aged and elderly women with breast cancer: a case-control comparison. J Gerontol. 1990;45(1):M3–11. doi: 10.1093/geronj/45.1.m3. [DOI] [PubMed] [Google Scholar]
- 6.Courneya KS, Friedenreich CM. Physical exercise and quality of life following cancer diagnosis: a literature review. Ann Behav Med. 1999 Spring;21(2):171–9. doi: 10.1007/BF02908298. [DOI] [PubMed] [Google Scholar]
- 7.Pedro LW. Quality of life for long-term survivors of cancer: influencing variables. Cancer Nurs. 2001 Feb;24(1):1–11. doi: 10.1097/00002820-200102000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Balducci L. Epidemiology of cancer and aging. J Oncol Manag. 2005 Spring;14(2):47–50. [PubMed] [Google Scholar]
- 9.Mohile SG, Xian Y, Dale W, Fisher SG, Rodin M, Morrow GR, et al. Association of a cancer diagnosis with vulnerability and frailty in older Medicare beneficiaries. Journal of the National Cancer Institute. 2009;101(17):1206–15. doi: 10.1093/jnci/djp239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baker F, Haffer SC, Denniston M. Health-related quality of life of cancer and noncancer patients in Medicare managed care. Cancer. 2003 Feb 1;97(3):674–81. doi: 10.1002/cncr.11085. [DOI] [PubMed] [Google Scholar]
- 11.Deimling GT, Bowman KF, Wagner LJ. The effects of cancer-related pain and fatigue on functioning of older adult, long-term cancer survivors. Cancer Nurs. 2007 Nov-Dec;30(6):421–33. doi: 10.1097/01.NCC.0000300168.88089.2b. [DOI] [PubMed] [Google Scholar]
- 12.Chodzko-Zajko WJ, Proctor DN, Fiatarone Singh MA, Minson CT, Nigg CR, Salem GJ, et al. American College of Sports Medicine position stand. Exercise and physical activity for older adults. Med Sci Sports Exerc. 2009 Jul;41(7):1510–30. doi: 10.1249/MSS.0b013e3181a0c95c. [DOI] [PubMed] [Google Scholar]
- 13.Galvao DA, Newton RU. Review of exercise intervention studies in cancer patients. J Clin Oncol. 2005 Feb 1;23(4):899–909. doi: 10.1200/JCO.2005.06.085. [DOI] [PubMed] [Google Scholar]
- 14.Knols R, Aaronson NK, Uebelhart D, Fransen J, Aufdemkampe G. Physical exercise in cancer patients during and after medical treatment: a systematic review of randomized and controlled clinical trials. J Clin Oncol. 2005 Jun 1;23(16):3830–42. doi: 10.1200/JCO.2005.02.148. [DOI] [PubMed] [Google Scholar]
- 15.Stevinson C, Lawlor DA, Fox KR. Exercise interventions for cancer patients: systematic review of controlled trials. Cancer Causes Control. 2004 Dec;15(10):1035–56. doi: 10.1007/s10552-004-1325-4. [DOI] [PubMed] [Google Scholar]
- 16.Rydwik E, Lammes E, Frandin K, Akner G. Effects of a physical and nutritional intervention program for frail elderly people over age 75. A randomized controlled pilot treatment trial. Aging Clin Exp Res. 2008 Apr;20(2):159–70. doi: 10.1007/BF03324763. [DOI] [PubMed] [Google Scholar]
- 17.Binder EF, Schechtman KB, Ehsani AA, Steger-May K, Brown M, Sinacore DR, et al. Effects of exercise training on frailty in community-dwelling older adults: results of a randomized, controlled trial. J Am Geriatr Soc. 2002 Dec;50(12):1921–8. doi: 10.1046/j.1532-5415.2002.50601.x. [DOI] [PubMed] [Google Scholar]
- 18.Faber MJ, Bosscher RJ, Chin APMJ, van Wieringen PC. Effects of exercise programs on falls and mobility in frail and pre-frail older adults: A multicenter randomized controlled trial. Arch Phys Med Rehabil. 2006 Jul;87(7):885–96. doi: 10.1016/j.apmr.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 19.Freiberger E, Menz HB, Abu-Omar K, Rutten A. Preventing falls in physically active community-dwelling older people: a comparison of two intervention techniques. Gerontology. 2007;53(5):298–305. doi: 10.1159/000103256. [DOI] [PubMed] [Google Scholar]
- 20.Sattin RW, Easley KA, Wolf SL, Chen Y, Kutner MH. Reduction in fear of falling through intense tai chi exercise training in older, transitionally frail adults. J Am Geriatr Soc. 2005 Jul;53(7):1168–78. doi: 10.1111/j.1532-5415.2005.53375.x. [DOI] [PubMed] [Google Scholar]
- 21.Greenspan AI, Wolf SL, Kelley ME, O’Grady M. Tai chi and perceived health status in older adults who are transitionally frail: a randomized controlled trial. Phys Ther. 2007 May;87(5):525–35. doi: 10.2522/ptj.20050378. [DOI] [PubMed] [Google Scholar]
- 22.Fahlman MM, McNevin N, Boardley D, Morgan A, Topp R. Effects of resistance training on functional ability in elderly individuals. Am J Health Promot. 2011 Mar-Apr;25(4):237–43. doi: 10.4278/ajhp.081125-QUAN-292. [DOI] [PubMed] [Google Scholar]
- 23.Demark-Wahnefried W, Clipp EC, Morey MC, Pieper CF, Sloane R, Snyder DC, et al. Lifestyle intervention development study to improve physical function in older adults with cancer: outcomes from Project LEAD. J Clin Oncol. 2006 Jul 20;24(21):3465–73. doi: 10.1200/JCO.2006.05.7224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.LaStayo PC, Marcus RL, Dibble LE, Smith SB, Beck SL. Eccentric exercise versus usual-care with older cancer survivors: the impact on muscle and mobility--an exploratory pilot study. BMC Geriatr. 2011;11:5. doi: 10.1186/1471-2318-11-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morey MC, Snyder DC, Sloane R, Cohen HJ, Peterson B, Hartman TJ, et al. Effects of home-based diet and exercise on functional outcomes among older, overweight long-term cancer survivors: RENEW: a randomized controlled trial. JAMA : the journal of the American Medical Association. 2009;301(18):1883–91. doi: 10.1001/jama.2009.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Courneya KS, Vallance JKH, McNeely ML, Karvinen KH, Peddle CJ, Mackey JR. Exercise issues in older cancer survivors. Crit Rev Oncol Hematol. 2004;51(3):249–61. doi: 10.1016/j.critrevonc.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 27.Rao AV, Cohen HJ. Fatigue in older cancer patients: etiology, assessment, and treatment. Seminars in oncology. 2008;35(6):633–42. doi: 10.1053/j.seminoncol.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 28.Hofman M, Morrow GR, Roscoe JA, Hickok JT, Mustian KM, Moore DF, et al. Cancer patients’ expectations of experiencing treatment-related side effects: a University of Rochester Cancer Center-- Community Clinical Oncology Program study of 938 patients from community practices. Cancer. 2004 Aug 15;101(4):851–7. doi: 10.1002/cncr.20423. [DOI] [PubMed] [Google Scholar]
- 29.Cleeland CS, Mendoza TR, Wang XS, Chou C, Harle MT, Morrissey M, et al. Assessing symptom distress in cancer patients: the M.D. Anderson Symptom Inventory. Cancer. 2000 Oct 1;89(7):1634–46. doi: 10.1002/1097-0142(20001001)89:7<1634::aid-cncr29>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 30.DeSalvo KB, Bloser N, Reynolds K, He J, Muntner P. Mortality prediction with a single general self-rated health question. A meta-analysis. J Gen Intern Med. 2006 Mar;21(3):267–75. doi: 10.1111/j.1525-1497.2005.00291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.World Population Prospects: The 2010 Revision. New York: 2010. [Google Scholar]
- 32.Balducci L. Epidemiology of cancer and aging. The Journal of oncology management : the official journal of the American College of Oncology Administrators. 2005;14(2):47–50. [PubMed] [Google Scholar]
- 33.Irwin ML, Crumley D, McTiernan A, Bernstein L, Baumgartner R, Gilliland FD, et al. Physical activity levels before and after a diagnosis of breast carcinoma: the Health, Eating, Activity, and Lifestyle (HEAL) study. Cancer. 2003;97(7):1746–57. doi: 10.1002/cncr.11227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pinto BM, Trunzo JJ, Reiss P, Shiu SY. Exercise participation after diagnosis of breast cancer: trends and effects on mood and quality of life. Psychooncology. 2002 Sep-Oct;11(5):389–400. doi: 10.1002/pon.594. [DOI] [PubMed] [Google Scholar]
- 35.Craike MJ, Livingston PM, Botti M. An exploratory study of the factors that influence physical activity for prostate cancer survivors. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2011;19(7):1019–28. doi: 10.1007/s00520-010-0929-3. [DOI] [PubMed] [Google Scholar]
- 36.Salminen E, Clemens KE, Syrjanen K, Salmenoja H. Needs of developing the skills of palliative care at the oncology ward: an audit of symptoms among 203 consecutive cancer patients in Finland. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2008;16(1):3–8. doi: 10.1007/s00520-007-0252-9. [DOI] [PubMed] [Google Scholar]
- 37.Adamsen L, Stage M, Laursen J, Rorth M, Quist M. Exercise and relaxation intervention for patients with advanced lung cancer: a qualitative feasibility study. Scand J Med Sci Sports. 2011 May 23; doi: 10.1111/j.1600-0838.2011.01323.x. [DOI] [PubMed] [Google Scholar]
- 38.Thomas DR. Loss of skeletal muscle mass in aging: examining the relationship of starvation, sarcopenia and cachexia. Clinical nutrition (Edinburgh, Scotland) 2007;26(4):389–99. doi: 10.1016/j.clnu.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 39.Fox KM, Brooks JM, Gandra SR, Markus R, Chiou C-F. Estimation of Cachexia among Cancer Patients Based on Four Definitions. J Oncol. 2009;2009:693458. doi: 10.1155/2009/693458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rolland Y, Dupuy C, Abellan van Kan G, Gillette S, Vellas B. Treatment strategies for sarcopenia and frailty. The Medical clinics of North America. 2011;95(3):427–38. ix. doi: 10.1016/j.mcna.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 41.Zinna EM, Yarasheski KE. Exercise treatment to counteract protein wasting of chronic diseases. Current opinion in clinical nutrition and metabolic care. 2003;6(1):87–93. doi: 10.1097/00075197-200301000-00013. [DOI] [PubMed] [Google Scholar]
- 42.Ardies CM. Exercise, cachexia, and cancer therapy: a molecular rationale. Nutrition and cancer. 2002;42(2):143–57. doi: 10.1207/S15327914NC422_1. [DOI] [PubMed] [Google Scholar]
- 43.Esbensen BA, Osterlind K, Hallberg IR. Quality of life of elderly persons with cancer: a 3-month follow-up. Cancer Nurs. 2006 May-Jun;29(3):214–24. doi: 10.1097/00002820-200605000-00008. quiz 25-6. [DOI] [PubMed] [Google Scholar]
- 44.Kenefick AL. Patterns of symptom distress in older women after surgical treatment for breast cancer. Oncol Nurs Forum. 2006 Mar;33(2):327–35. doi: 10.1188/06.ONF.327-335. [DOI] [PubMed] [Google Scholar]
- 45.Burnham TR, Wilcox A. Effects of exercise on physiological and psychological variables in cancer survivors. Medicine and science in sports and exercise. 2002;34(12):1863–7. doi: 10.1097/00005768-200212000-00001. [DOI] [PubMed] [Google Scholar]
- 46.Dimeo FC, Stieglitz RD, Novelli-Fischer U, Fetscher S, Keul J. Effects of physical activity on the fatigue and psychologic status of cancer patients during chemotherapy. Cancer. 1999;85(10):2273–7. [PubMed] [Google Scholar]
- 47.Luctkar-Flude MF, Groll DL, Tranmer JE, Woodend K. Fatigue and physical activity in older adults with cancer: a systematic review of the literature. Cancer Nurs. 2007;30(5):E35–45. doi: 10.1097/01.NCC.0000290815.99323.75. [DOI] [PubMed] [Google Scholar]
- 48.Idler EL, Benyamini Y. Self-rated health and mortality: a review of twenty-seven community studies. Journal of health and social behavior. 1997;38(1):21–37. [PubMed] [Google Scholar]