Abstract
Progesterone may have actions independent of intracellular progestin receptors (PRs) to influence depressive behavior. To investigate this, we examined effects of progesterone (P; 10 mg/kg, SC) on the depressive behavior of mice in the forced swim test (FST). In Experiment 1, subjects were 4 to 6 months old, intact or ovariectomized (OVX) female and intact or gonadectomized (GDX) male, C57/BL6 mice. Progesterone reduced depressive behavior of young diestrous and OVX mice but male mice were impervious to effects of P. In Experiment 2, subjects were intact aged (20–28 months old) C57/BL6 female and male mice. Progesterone reduced depressive behavior of aged female and male C57/BL6 mice, albeit effects were greater among males. In Experiment 3, effects of P were examined in 4 to 6 months old, gonadally-intact, female and male mice that were wildtype or PR knockouts (PRKOs). Progesterone decreased depressive behavior of young adult, wildtype and PRKO mice, which showed greater immobility than did their wildtype counterparts. In Experiment 4, subjects were 18–24 months old wildtype or PRKO mice (Exp 4). Progesterone decreased immobility among wildtype and PRKO mice (which were not different in terms of their baseline depressive behavior). Together these data demonstrate that P decreases depressive behavior of young and older adult C57/BL6, wildtype and PRKO mice, which suggest that acute anti-depressant effects of P may occur independent of actions at “classic” PRs.
Keywords: Depression, Aging, Senescent, Progesterone
1. Introduction
Major depression affects 5 to 10% of the population, is a leading cause of disability, and has significant personal and therapeutic costs. The current conventional antidepressants therapeutics, such as selective serotonin reuptake inhibitors, have limited therapeutic efficacy, latencies of 2–6 weeks for effects, and side-effects which limit compliance (Shelton, 2009). The mechanisms underlying the etiology, symptomatology, and/or treatment of depression need to be better understood.
The prevalence of major depression is about twice as high among women than men, and is most evident during the reproductive years (Steiner et al., 2003). Beginning in adolescence, females experience depressive disorders at higher rates than do males (Steiner et al., 2003). Clinical studies show the prevalence of depression are increased with the hormonal transitions during the perimenstruum, post-partum or perimenopausal periods (Freeman et al., 2002; Paoletti et al., 2001; Pisani et al., 1998; Rasgon et al., 2005; Robinson, 2001). Among some women, depletion of ovarian hormones, due to surgical or natural menopause, is associated with increases in depression (Gilbert Evans et al., 2005; Girdler et al., 2001; Halbreich and Kahn, 2001; MacDonald et al., 1991; Osterlund et al., 2005; Rapkin et al., 2002; Stahl, 1997; Zonana and Gorman, 2005). These findings imply a role of gonadal hormones.
Progesterone (P) might be involved in the mechanisms underlying depression. Premenstrual syndrome, premenstrual dysphoric disorder, and post-partum depression are defined by their association with reproductive events, which are characterized by low endogenous levels of P. Some pharmacotherapies which are used to treat depression have some of their effects in association with increasing levels of progestogens (Griffin and Mellon, 1999; Uzunov et al., 1996, 1998). Given these clinical findings, the role and neurobiological mechanisms of P relevant for depression need to be better understood.
In animal models, P can reduce depressive behavior. First, less depressive behavior is seen when there are natural elevations in progestogen levels. For example, rats in behavioral estrus show less depressive behavior than do rodents in other phases of the estrous cycle (Becker and Cha, 1989; Bitran et al., 1991; Frye et al., 2000a; Gulinello et al., 2003; Walf et al., 2006). Pregnant rats also show less depressive behavior than do post-parturient rats, which have lower endogenous progestogen levels (Frye and Walf, 2002, 2004). Second, removal of the primary endogenous source of progestins, the ovaries (ovariectomy; OVX) increases depressive behaviors of rodents (Frye and Walf, 2002; Frye and Wawrzycki, 2003; Walf et al., 2006). Third, administration of P, compared to vehicle, to OVX rodents decreases depressive behavior (Frye et al., 2004; Frye and Walf, 2004; Walf et al., 2006). Thus, P can have anti-depressant effects in animal models.
Progesterone controls several biological processes through interactions with its classical or nuclear receptor (PR); however, some effects of P require its conversion by 5α-reductase enzymes to dihydroprogesterone, which is then metabolized to 5α-pregnan-3α-ol-20-one (3α,5α-THP) by 3α-hydroxysteriod dehydrogenase enzymes. In animal models, administration of P or 3α,5α-THP produce similar anti-depressant behavior (Frye and Walf, 2002; Hirani et al., 2002; Walf and Frye, 2007). The effects of P to reduce depressive behavior are attenuated when drugs that block conversion of P to 3α,5α-THP are co-administered or when P is administered to 5α-reductase knockout mice that cannot convert P to 3α,5α-THP (Khisti et al., 2000; Khisti and Chopde, 2000; Frye et al., 2004; Frye and Walf, 2004; Hirani et al., 2002; Walf et al., 2006). Moreover, the selective-serotonin reuptake inhibitor, fluoxetine, has anti-depressant effects and concomitantly increases formation of 3α,5α-THP (Hirani et al., 2002). Finally, an animal model of depression involves socially-isolating mice, which decreases 3α,5α-THP levels, but can be reversed by fluoxetine, P or 3α,5α-THP administration (Hirani et al., 2002; Matsumoto et al., 1999; Pinna et al., 2009). In physiological concentrations, 3α,5α-THP is devoid of affinity for PRs (Rupprecht et al., 1996). Some effects of P require its conversion into 3α,5α-THP, which interacts with GABAA/benzodiazepine receptors (GBRs) at low concentrations to increase the frequency and/or duration of openings of GABA-gated chloride channels (Lambert et al., 1995; Majewska et al., 1986; Paul and Purdy, 1992). As such, we examined effects of P on depressive behavior in the forced swim test (FST) of young and aged adult, C57/BL6 or wildtype and PR knockout (PRKO) mice. We hypothesized that if P has effects on depressive behavior that involve formation of 3α,5α-THP, and actions independent of PRs, then administration of P to PRKOs and wildtype mice should similarly reduce depressive behaviors.
2. Methods
2.1. Animal and housing
All procedures were pre-approved by The Institutional Animal Care and Use Committee at The University at Albany-SUNY. Mice were group-housed with free access to Purina Rat Chow and water in their home cages. Mice were housed in a room that had a reversed 12/12 h light/dark cycle (lights on 0800 h).
In Experiment 1, subjects were young (between 4 and 6 month of age) adult C57/BL6 mice that were bred in the Life Science Laboratory Animal Care Facility at SUNY-Albany. Mice (N=48) were female (intact or OVX n=6 in each condition) or male (intact or GDX n=6 in each condition). Intact females were tested in their diestrous phase to control for low hormonal levels that are typical of this phase in the estrous cycle (Frye and Walf, 2002, 2004; Walf et al., 2006).
In the Experiment 2, subjects were aged C57/BL6 mice (average age 24 months, range 20–28 months; N=16; n=8 in each sex) mice that were bred in the Life Science Laboratory Animal Care Facility at SUNY-Albany.
In Experiment 3, subjects were young adult (between 4 and 6 months of age) wildtype and PRKO mice that were bred in the Life Science Laboratory Animal Care Facility at SUNY-Albany. Mice (N=52) were intact female wildtype (n=12) or PRKO (n=12) mice and intact male wildtype (n=16) or PRKO mice (n=12).
In Experiment 4, subjects were wildtype or PRKO mice (average age 20 months, range 18–24 months; N=13; n=6 in each genotype) that were bred in the Life Science Laboratory Animal Care Facility at SUNY-Albany. Mice were intact female wildtype (n=3) or PRKO (n=3) mice and intact male wildtype (n=3) or PRKO mice (n=4).
2.2. Genotype
PRKO and wildtype mice are indistinguishable based upon their phenotype. As such, mice were genotyped by genomic DNA isolated from tails and subsequent analyses by polymerase chain reaction (PCR). PCR was performed by denaturing the DNA at 95 °C for 5 min, followed by 30 cycles of amplification: 94 °C for 1 min, 60 °C for 1 min, 72 °C for 1 min and a final primer extension step at 72 °C for 10 min. The following PR specific primers were used: P1 (5′-TAGACAGTGTCTTAGACTCGTTGTTG-3′), P2 (5′-GATGGGCACATGGATGAAATC-3′), and a neo gene-specific primer, N2 (5′-GCATGCTCCAGACTGCCTTGGGAAA-3′). Bands of approximately 565 and 500 bp were amplified for wildtype and PRKO mice, respectively.
2.3. Surgery
All mice in Experiment 1, around 4 months of age, were administered sodium pentobarbital anesthesia (70 mg/kg; intraperitoneally). Male mice were either gonadectomized (n=12) or sham surgerized (n=12). Female mice were either ovariectomized (n=12) or sham surgerized (n=12). Four to six weeks following surgery, which is the requisite time frame to enable androgen levels to reach nadir, males were randomly-assigned to receive P or vehicle and were tested, as described below, 1 h later. One to two weeks after surgery, which is the requisite time frame to enable estrogen and progestin levels to clear following ovariectomy, females were randomly-assigned to receive P or vehicle and were tested, as described below, 1 h later.
All mice in Experiments 2, 3 and 4 were used intact. Intact, aged mice can have significantly lower levels of hormones than do their younger counterparts (Nelson et al., 1992; Perrot-Sinal et al., 1998). Mice entering middle-age (typically 10–14 months of age) can have a period of constant estrous, as in rats, where they show relatively high and sustained levels of estrogens. This is followed by a persistent diestrous phase characterized by low levels of estrogens. As such, older mice were utilized in this study to minimize the potential that sex differences and/or age effects for forced swim test behavior were due to estrous cycle phase. Although older mice were not cycled daily to determine their endogenous hormone condition, we have previously reported that mice of this same age have significantly lower levels of estrogens and progestogens compared to younger female mice (Frye et al., 2006b). Moreover, surgery in aged mice would be expected to produce attrition. Mice in Experiments 2 and 4 were not surgerized to minimize attrition. Mice in Experiment 3 were not surgerized, so that they could serve as younger, comparison animals for those subjects in Experiment 4. Gonadally-intact female mice were tested when in the diestrous phase of the estrous cycle.
2.4. Hormonal milieu
Progesterone was obtained from Sigma Chemical Co. (St. Louis, MO) and dissolved in propylene glycol to a concentration of 10 mg/ml. Mice were randomly-assigned to receive subcutaneous injection of P (10 mg/kg) or vehicle (propylene glycol) 1 h prior to behavioral testing. We chose this regimen as it produces proestrous-like, physiologically-relevant levels of P and 3α,5α-THP, when administered to young or older adult mice (Frye and Vongher, 2001; Frye et al., 2006a,b).
2.5. Behavioral test: Porsolt forced swim task
FST remains one of the most commonly-used tools to investigate depressive behavior of mice. The FST is a good screening tool for anti-depressants as it has strong reliability and predictive validity among mice (Barros and Ferigolo, 1998; Petit-Demouliere et al., 2005). In the FST, immobility time is an indicator of depressive behavior. Immobility is defined as the absence of active behaviors (i.e. swimming, jumping, or diving) and after a brief period of vigorous activity in the water, mice adopt a characteristic immobile posture which is readily identifiable and quantifiable (Porsolt et al., 1977). The general activity test is commonly used as a complement of the FST to address non-specific actions of antidepressant treatments (Borsini and Meli, 1988; Porsolt et al., 1978). Compounds that increase the active behaviors in the FST, irrespective of effects on locomotor activity, are considered to possess antidepressant-like actions (Borsini and Meli, 1988; Porsolt et al., 1978). Although high concentrations of progestogens can decrease motor behaviors (Vivian et al., 1997), we have previously demonstrated that this P regimen to mice of these ages does not produce ataxia or disruptive effects on motor behavior (Frye et al., 2006a).
The protocol in our lab for the FST was modified from that previously described (Frye et al., 2004). Briefly, mice were placed in a glass cylinder, which was 20.5 cm in diameter and 21.5 cm in depth, and was filled with 18 cm of 25 °C water (Frye et al., 2004). Following the test, mice were removed from the pool, carefully dried with paper towels and placed in a single cage until they were dry, and then they were returned to their home cages.
The duration of immobility was recorded for 10 min in Experiments 1, 3, and 4. In Experiment 2, these older mice were only tested for 5 min because of concerns regarding how mice this old would fare. Mice in Experiment 4 were on average of about 4 months younger than those in Experiment 2. They were tested for the full 10 min of the test, without incident, so that their results could be assessed in comparison to the results in their younger counterparts in Experiment 3. However, direct comparisons could not be completed between Experiments 1 and 2.
2.6. Data analyses
For Experiment 1, three-way analyses of variance (ANOVAs) were used to evaluate effects of sex (female, male), gonadal status (intact, OVX/GDX), and hormone condition (vehicle, P). For Experiment 2, two-way ANOVAs were used to evaluate effects of sex (female, male) and hormone condition (vehicle, P). For Experiment 3, three-way ANOVAs were used to evaluate effects of sex (female, male), hormone condition (vehicle, P), and genotype (wildtype, PRKO). For Experiment 4, two-way ANOVAs were used to evaluate effects of genotype (wildtype, PRKO) and hormone condition (vehicle, P). There were too few observations to assess sex differences; however, data are depicted by sex to enable comparison with the results of Experiment 3. The α level for statistical significance was P<0.05 and ANOVAs were followed by Fisher’s post hoc tests, as appropriate, to determine group differences.
3. Results
3.1. Progesterone administration decreased depressive behavior of young adult, intact female>OVX, or male mice
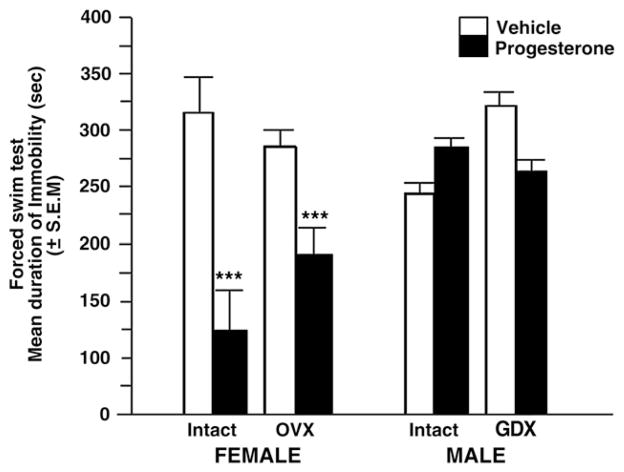
Sex (F (1, 40)=6.54, P=0.014), gonadal status (F (1, 40)=9.32, P=0.004), and P administration (F (1, 40)=36.82, P<0.001) produced significant main effects. Males spent more time immobile than did females. Removal of the gonads increased immobility compared to that observed in gonadally-intact mice. Progesterone reduced time spent immobile compared to vehicle-administration.
There were interactions between sex, gonadal status and P administration (F (1, 40)=18.81, P<0.001) to influence time spent immobile. Progesterone had the most salient effects to decrease the duration of immobility (depressive behavior) of gonadally-intact, diestrous female mice. Progesterone also significantly decreased immobility of OVX mice. However, young male mice seemed to be completely insensitive to the antidepressant-like effect of P. See Fig. 1.
Fig. 1.
Mean duration of immobility (s) in the forced swim task of young, adult (4–6 months of age) intact or ovariectomized female (far and middle left panels respectively) and male young, adult intact or gonadectomized (far and middle right panels respectively) C57/BL6 mice (n=6 in each conditions) administered vehicle control (white bar) or progesterone (black bar). Females showed significantly more immobility than did males. Mice that had their gonads removed spent significantly more time immobile. Progesterone significantly decreased immobility. *** denotes a significant interaction between sex, gonadal status, and progesterone condition, that was due to progesterone reducing depressive behavior most among intact, diestrous and ovariectomized mice (p<0.05).
3.2. Progesterone decreased depressive behavior of aged adult, female or male mice
There were main effects of P to decrease depressive behavior (F (1, 12)=15.75, P<0.002), but no main effects of sex (F (1, 12)=0.71, P=0.42), to influence time spent immobile. Progesterone reduced immobility compared to vehicle administration. However, there was a significant interaction between sex and P administration (F (1, 12)= 5.23, P=0.04), such that P decreased immobility more so among aged male, compared to aged female, mice (Fig. 2).
Fig. 2.
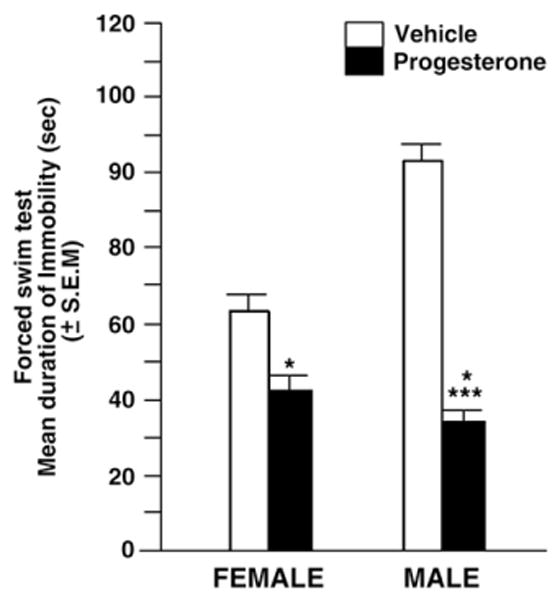
Mean duration of immobility (s) in the forced swim task of aged, adult (20–28 months of age) intact female (left panel) and male (right panel) C57/BL6 mice (administered vehicle control (white bar)) or P (black bar) tasks (n=4 in each conditions). Progesterone significantly decreased immobility. * denotes a main effect of progesterone to decrease immobility compared to vehicle. *** denotes a significant interaction between sex and progesterone condition, that was due to progesterone reducing depressive behavior most among male mice (p<0.05).
3.3. Progesterone decreases depressive behavior of young adult, wildtype and PRKO mice: PRKOs show greater immobility than do wildtype mice
Progesterone (F (1, 44)=4.85, P<0.032) and genotype (F (1, 44)= 0.71, P=0.42), but not sex (F (1, 44)=0.14, P=0.70), produced main effects on time spent immobile. Progesterone decreased the duration of time spent immobile compared to vehicle administration. Young adult, wildtype mice spent less time immobile than did young adult, PRKO mice (Fig. 3). There were no significant interactions between variables.
Fig. 3.
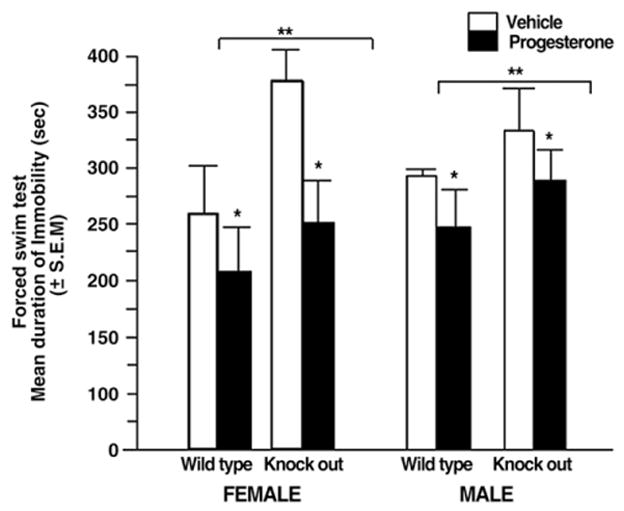
Mean duration of immobility (s) in the forced swim task of young adult (4–6 months of age) intact female wildtype and progestin receptor knockout mice (n=6 in each condition) (left panel) and male wildtype (n=8 in each condition) and progestin receptor knock mice (n=6 in each condition) (right panel) administered vehicle control (white bar) or P (black bar). * denotes a main effect of progesterone to decrease immobility compared to vehicle. ** denotes significantly more time spent immobile among progestin receptor knockout compared to wildtype mice (p<0.05).
3.4. Progesterone decreases depressive behavior of older adult, wildtype and PRKO mice
Progesterone (F (1, 8)=14.30, P<0.005), but not genotype (F (1, 8)= 0.17, P=0.69), influenced time spent immobile in the FST of older wildtype and PRKO mice. Progesterone similarly decreased immobility of older adult wildtype and PRKO mice (Fig. 4). [Although there were no sex differences, data are shown by sex for comparing age-related changes between Experiments 3 and 4.]
Fig. 4.
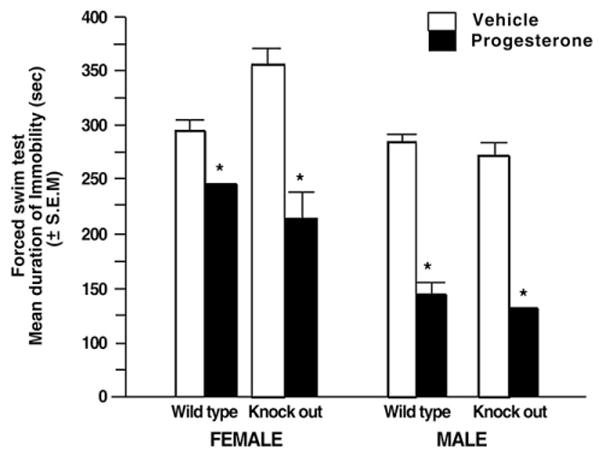
Mean duration of immobility (s) in the forced swim task of aged, adult (18–24 months of age) intact wildtype (left panel) and progestin receptor knockout (right panel) mice administered vehicle control (white bar) or P (black bar) (n=3–4 in each conditions). * denotes an effect of progesterone to decrease immobility compared to vehicle controls (p<0.05).
4. Discussion
These findings supported the hypothesis that P can reduce depressive behavior in the FST of younger and older, female and male, C57/BL6, wildtype and/or PRKO mice. In Experiment 1, P reduced depressive behavior among young adult intact females, more so than their OVX counterparts and these effects among females were greater than those seen among intact or GDX male mice whereas the young males seemed insensitive to progesterone. In Experiment 2, among older adult, gonadally-intact C57/BL6 mice, P reduced depressive behavior of female and male mice, albeit the effects were greater in male mice. In Experiment 3, P decreased immobility among young adult wildtype and PRKO mice, albeit PRKO mice showed more depressive behavior than did their wildtype counterparts. In Experiment 4, among older adult wildtype and PRKO mice, P reduced depressive behavior compared to that of vehicle-administration. Together, these data suggest P can exert acute activational effects to decrease depressive behavior among younger and older adult female and male C57/BL6, wildtype and/or PRKO mice.
There have been contradictory findings with respect to sex differences in the FST. Previous reports have indicated that females are more active than male rats (Dalla et al., 2008; Yang et al., 2007), male rats show more immobility than do females (Ferigolo et al., 1998; Paré and Redei, 1993), or there are no sex differences (Andrade et al., 2007; David et al., 2001). When comparisons are made between females in low P phases of the estrous cycle, such as diestrous, they typically demonstrate greater immobility than do males (Frye and Wawrzycki, 2003). Indeed, here we see that in Experiment 1, young diestrous or OVX mice show more depression-like behavior than did male mice. However, these sex differences in depressive behavior were not evident among older mice, in Experiments 2 and 4. The greater immobility of diestrous and OVX rodents suggests that young adult female mice with low levels of steroids, due to cycle phase or ovariectomy, may be predisposed to more depressive-like behavior.
Over reproductive cycles of female rodents, more depressive behavior typically occurs when hormone levels are low. For example, proestrous or pregnant rats that have higher levels of steroid hormones show less depressive behavior in the FST than do diestrous, post-partum or male rats that have lower level of hormones (Frye and Walf, 2002, 2004; Walf et al., 2006). Further, in the differential reinforcement model of depression, depressive behavior is increased among rats 3 days postpartum, which should have lower and transitioning levels of steroid hormones (Molina-Hernández et al., 2000). Although these findings imply that depressive behavior is greater when hormone levels are low, there are variations in both estrogens and progestogens, which make it difficult to attribute variations in behavior to hormonal status.
Experimental manipulations that involve removing the primary source of steroid hormones demonstrate that more depressive behavior occurs with extirpation of the ovaries. In general, results from most animal models, show that OVX, which decreases steroid levels, increases depressive behavior compared to that of intact controls. Between 7 and 18 days after OVX, increases in depression-like behavior, such as immobility in the FST, of young adult mice and rats are observed (Bekku et al., 2006; Frye and Walf, 2002, 2004; Galea et al., 2001; Stoffel and Craft, 2004). Notably, these OVX-induced increases in immobility occur as long as mice are not treated with hormones (Bekku and Yoshimura, 2005). While these results clearly demonstrate that the absence of steroids increase depressive behavior, OVX reduces both estrogens and progestogens, which make it difficult to attribute depressive behavior to changes in either hormone. Estrogen can mitigate depressive behavior, but some of these effects may also be related to estrogen’s capacity to increase formation of progestogens by enhancing activity of P metabolism enzymes (Cheng and Karavolas, 1975; Vongher and Frye, 1999). Another consideration to make is the role of gonadectomy. The antidepressant-like action of fluoxetine in the forced swim test is reduced by orchidectomy (Estrada-Camarena et al., 2004; Martinez-Mota and Fernandez-Guasti, 2004). Thus, removing endogenous steroids can increase depressive behavior in rodent models.
The results of the current studies confirm and extend prior reports that indicate that administration of P can reduce depressive behavior in rodents. Acute administration of P reverses the depressant effects of OVX on behavior of rats in the FST (Frye and Walf, 2002). As well, administration of P four times daily to mice reversed the depressant effect of OVX on immobility behavior in the tail suspension test (Bernardi et al., 1989). Interestingly, abrupt withdrawal from P administration can also increase depressive behavior. In support, OVX rats that were administered P daily for 5 days showed less depressive behavior when tested on the sixth day in the FST if they had received tapered P injections rather than having P abruptly discontinued (Saavedra et al., 2006). Here we see that P administration to young adult female mice with low P levels decreased their immobility, although young adult males were impervious to antidepressant effects of P (Experiment 1). Whereas, among older adult C57/BL6 mice, P decreased immobility among male and female mice (Experiment 2). Progesterone also reduced depressive behavior when administered to young or older adult wildtype or PRKO mice, irrespective of sex (Experiments 3 and 4). Thus, these results largely show that actions at “classic” PRs do not seem to mediate the acute antidepressant-like effect of P in young adult or aged female or male mice; however, the lack of effects of P in young adult males suggests a potential difference or age-related change in P’s mechanism of action.
The results of the current studies also provide insight into sex differences and age-related changes in depressive behavior. In Experiment 2, there was a difference in the control levels of immobility between aged male (~95 s) and female (~65 s) mice. Progesterone reduced depressive behavior to ~40 s for both sexes. Although P decreased immobility in each of our studies, these sex differences observed in Experiments 1 and 2 were not confirmed in Experiments 3 and 4. One explanation for seeing sex differences in Experiments 1 and 2 but not in 3 and 4, is that these are different strains of mice. Other factors may have something to do with the shorter duration of the test period for subjects in Experiment 2. This was among some of our first studies examining effects on FST in mice up to 28 months of age. We were concerned that these aged mice would not perform well, and so we chose to test them in abbreviated session. This was unfortunate as we were unable to compare whether older mice of the same strain would show more immobility compared to their younger counterparts. Indeed, there is evidence that older mice may spend more time immobile than do their younger counterparts (Chaves et al., 2009). However, comparing across Experiments 3 and 4, we see some indication for the duration of immobility to be longer among the older female, but not male, wildtype and PRKO mice. As well, it is important to consider possible strain differences as there are clear differences among baseline levels of immobility among females in Experiments 1 and 3.
An intriguing possibility is that sex and/or age difference in the antidepressant-like effects of P may occur through its conversion to 3α,5α-THP. Progesterone can have actions through intracellular and membrane-bound PRs (mPRs), but 3α,5α-THP is a positive allosteric modulator of GBRs (Belelli et al., 1990; Majewska, 1992; Morrow et al., 1987). Formation of 3α,5α-THP may be essential for some anti-depressant effects of steroid hormones. First, acute administration of 3α,5α-THP decreases immobility time of mice or rats in the FST (Frye and Walf, 2004; Hirani et al., 2002; Rodríguez-Landa et al., 2007). Second, when formation of 3α,5α-THP is attenuated pharmacologically, or due to a genetic deficiency in metabolism enzymes, P does not produce anti-depressive behavior in the FST of mice or rats (Frye et al., 2004; Frye and Walf, 2004; Hirani et al., 2002). Third, several antidepressant medications enhance the production or synaptic accumulation of 3α,5α-THP (Frye and Seliga, 2003; Griffin and Mellon, 1999; Marx et al., 2003; Pinna et al., 2006; Schüle et al., 2006). Moreover, when these anti-depressant are administered to mice they enhance the anti-depressant effects of 3α,5α-THP in the FST (Hirani et al., 2002; Urani et al., 2001). Fourth, results of our experiment suggest that mice that have higher endogenous 3α,5α-THP levels may be more responsive to P. In support, P administration produced the greatest anti-depressant behavior among young female intact or OVX mice as compared to males. Female mice have higher endogenous 3α,5α-THP levels than do male mice (Finn et al., 2004a). As well, aged mice in the present study showed less anti-depressive behavior compared to their younger counterparts, and there is age related decline in the capacity to metabolize P to 3α,5α-THP (Genazzani et al., 2004). Notably, aged males in the present study had a better response to P and the capacity to form 3α,5α-THP declines less dramatically at reproductive senescence among males compared to females. Anti-depressant effects of 3α,5α-THP to decrease FST immobility of rats or mice is blocked by co-administration of GBR antagonists (Hirani et al., 2002; Rodríguez-Landa et al., 2007). Although 3α,5α-THP has agonist-like actions at GBRs (Majewska et al., 1986), it is devoid of affinity for PRs (Iswari et al., 1986; Smith et al., 1974). Results of the present experiments, that P has acute, anti-depressant effects when administered to wildtype and PRKO mice, suggest that actions at PRs may not be required for its anti-depressant effects. However, this does not rule out acute actions of progestogens at mPRs or other neurotransmitter targets. Moreover, it is possible that PRs may play an organizing action relevant for depressive behavior, as suggested by the longer duration of immobility among older PRKO, compared to wildtype, mice.
Anti-depressant like effects of 3α,5α-THP may involve neurogenesis in brain regions, such as the hippocampus. In people, there is an inverse relationship between 3α,5α-THP concentrations and severity of depression and therapeutic effects of antidepressants coincide with normalization in 3α,5α-THP levels (Nappi et al., 2001; reviewed in Pinna, 2010; Uzunova et al., 1998). Impaired neurogenesis may be an important factor in vulnerability to depression and stimulation of neurogenesis may be essential for effective antidepressant treatment (Kempermann and Kronenberg, 2003; Malberg and Blendy, 2005). Whether 3α,5α-THP’s effects relevant for depression involve neurogenesis in the hippocampus is a topic of ongoing investigation.
The nature of progestogens effects need to be better understood. Indeed, a role of P or 3α,5α-THP has been implicated in pain perception (Bradshaw and Berkley, 2003; Bradshaw et al., 1999; Choi et al., 2006; Frye et al., 2004), anxiety (Frye et al., 2004; Frye and Walf, 2002, 2004; Löfgren et al., 2006; Lonstein, 2007; Smith et al., 2004, 2006), learning and memory (Djebaili et al., 2004; Walf et al., 2006), seizure susceptibility (Belelli et al., 1990; Finn and Gee, 1994; Frye et al., 2000a,b, 2002; Frye and Muscatiello, 2001; Hsu and Smith, 2003; Kokate et al., 1994, 1996), depression (Frye et al., 2004, 2006a,b; Frye and Walf, 2004; Rasmusson et al., 2006; Walf et al., 2006), stress-related disorders (Dong et al., 2001; Matsumoto et al., 1999, 2005, 2007; Pinna et al., 2003; Pinna, 2010; Serra et al., 2000, 2007) and drug effects (Cagetti et al., 2004; Devaud et al., 1996; Finn et al., 2004a,b, 2006). How, these effects relate to the anti-depressant effects seen here require further investigation. Moreover, there is also evidence that P or 3α,5α-THP can have paradoxical effects on many of these parameters (Shen et al., 2007). As such, further research is required to fully understand the role of progestogens in the etiological, therapeutic treatment, and/or prognosis of affective processes.
Acknowledgments
This research was supported by grants from the National Science Foundation (03–16083; IOS-0957148) and the National Institute of Health (MH0676980; RMH067698B).
References
- Andrade S, Silveira SL, Gomez R, Barros HM, Ribeiro MF. Gender differences of acute and chronic administration of dehydroepiandrosterone in rats submitted to the forced swimming test. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:613–21. doi: 10.1016/j.pnpbp.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Barros HM, Ferigolo M. Ethopharmacology of imipramine in the forced-swimming test: gender differences. Neurosci Biobehav Rev. 1998;23:279–86. doi: 10.1016/s0149-7634(98)00029-3. [DOI] [PubMed] [Google Scholar]
- Becker JB, Cha JH. Estrous cycle-dependent variation in amphetamine-induced behaviors and striatal dopamine release assessed with microdialysis. Behav Brain Res. 1989;35:117–25. doi: 10.1016/s0166-4328(89)80112-3. [DOI] [PubMed] [Google Scholar]
- Bekku N, Yoshimura H. Animal model of menopausal depressive-like state in female mice: prolongation of immobility time in the forced swimming test following ovariectomy. Psychopharmacology (Berl) 2005;183:300–7. doi: 10.1007/s00213-005-0179-0. [DOI] [PubMed] [Google Scholar]
- Bekku N, Yoshimura H, Araki H. Factors producing a menopausal depressive-like in mice following ovariectomy. Psychopharmacology (Berl) 2006;187:170–80. doi: 10.1007/s00213-006-0395-2. [DOI] [PubMed] [Google Scholar]
- Belelli D, Lan NC, Gee KW. Anticonvulsant steroids and the GABA/benzodiazepine receptor chloride ionophore complex. Neurosci Biobehav Rev. 1990;14:315–22. doi: 10.1016/s0149-7634(05)80041-7. [DOI] [PubMed] [Google Scholar]
- Bernardi M, Vergoni AV, Sandrini M, Tagliavini S, Bertolini A. Influence of ovariectomy, estradiol and progesterone on the behavior of mice in an experimental model of depression. Physiol Behav. 1989;45:1067–8. doi: 10.1016/0031-9384(89)90238-2. [DOI] [PubMed] [Google Scholar]
- Bitran D, Hilvers RJ, Kellogg CK. Anxiolytic effects of 3α-hydroxy-5α[β]-pregnan-20-one: endogenous metabolites of progesterone that are active at the GABAA receptor. Brain Res. 1991;561:157–61. doi: 10.1016/0006-8993(91)90761-j. [DOI] [PubMed] [Google Scholar]
- Borsini F, Meli A. Is the forced swimming test a suitable model for revealing antidepressant activity? Psychopharmacology (Berl) 1988;94:147–60. doi: 10.1007/BF00176837. [DOI] [PubMed] [Google Scholar]
- Bradshaw HB, Berkley KJ. The influence of ovariectomy with or without estrogen replacement on responses of rat gracile nucleus neurons to stimulation of hindquarter skin and pelvic viscera. Brain Res. 2003;986:82–90. doi: 10.1016/s0006-8993(03)03175-5. [DOI] [PubMed] [Google Scholar]
- Bradshaw HB, Temple JL, Wood E, Berkley KJ. Estrous variations in behavioral responses to vaginal and uterine distention in the rat. Pain. 1999;82:187. doi: 10.1016/S0304-3959(99)00049-4. [DOI] [PubMed] [Google Scholar]
- Cagetti E, Pinna G, Guidotti A, Baicy K, Olsen RW. Chronic intermittent ethanol (CIE) administration in rats decreases levels of neurosteroids in hippocampus, accompanied by altered behavioral responses to neurosteroids and memory function. Neuropharmacology. 2004;46:570–9. doi: 10.1016/j.neuropharm.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Chaves G, Moretti M, Castro AA, Dagostin W, da Silva GG, Boeck CR, et al. Effects of long-term ovariectomy on anxiety and behavioral despair in rats. Physiol Behav. 2009;97:420–5. doi: 10.1016/j.physbeh.2009.03.016. [DOI] [PubMed] [Google Scholar]
- Cheng YJ, Karavolas HJ. Properties and subcellular distribution of delta4-steroid (progesterone) 5α-reductase in rat anterior pituitary. Steroids. 1975;26:57–71. doi: 10.1016/0039-128x(75)90006-9. [DOI] [PubMed] [Google Scholar]
- Choi JC, Park SK, Kim YH, Shin YW, Kwon JS, Kim JS. Different brain activation patterns to pain and pain-related unpleasantness during the menstrual cycle. Anesthesiology. 2006;105:120–7. doi: 10.1097/00000542-200607000-00021. [DOI] [PubMed] [Google Scholar]
- Dalla C, Antoniou K, Kokras N, Dossopoulou G, Papathanasiou G, Bekris S, et al. Sex differences in the effects of two stress paradigms on dopaminergic neurotransmission. Physiol Behav. 2008;93:595–605. doi: 10.1016/j.physbeh.2007.10.020. [DOI] [PubMed] [Google Scholar]
- David DJ, Nic Dhonnchadha BA, Jolliet P, Hascoët M, Bourin M. Are there gender differences in the temperature profile of mice after acute antidepressant administration and exposure to two animal models of depression? Behav Brain Res. 2001;119:203–11. doi: 10.1016/s0166-4328(00)00351-x. [DOI] [PubMed] [Google Scholar]
- Devaud LL, Purdy RH, Finn DA, Morrow AL. Sensitization of γ-aminobutyric acidA receptors to neuroactive steroids in rats during ethanol withdrawal. J Pharmacol Exp. 1996;278:510–7. [PubMed] [Google Scholar]
- Djebaili M, Hoffman SW, Stein DG. Allopregnanolone and progesterone decrease cell death and cognitive deficits after a contusion of the rat pre-frontal cortex. Neuroscience. 2004;123:349–59. doi: 10.1016/j.neuroscience.2003.09.023. [DOI] [PubMed] [Google Scholar]
- Dong E, Matsumoto K, Uzunova V, Sugaya I, Takahata H, Nomura H, et al. Brain 5α-dihydroprogesterone and allopregnanolone synthesis in a mouse model of protracted social isolation. Proc Natl Acad Sci U S A. 2001;98:2849–54. doi: 10.1073/pnas.051628598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada-Camarena E, Fernandez-Guasti A, Lopez-Rubalcava C. Interaction between estrogens and antidepressants in the forced swimming test in rats. Psychopharmacology (Berl) 2004;173:139–45. doi: 10.1007/s00213-003-1707-4. [DOI] [PubMed] [Google Scholar]
- Ferigolo M, Barros HM, Marquardt AR, Tannhauser M. Comparison of behavioral effects of moclobemide and deprenyl during forced swimming. Pharmacol Biochem Behav. 1998;60:431–7. doi: 10.1016/s0091-3057(98)00011-2. [DOI] [PubMed] [Google Scholar]
- Finn DA, Gee KW. The estrus cycle, sensitivity to convulsants and the anticonvulsant effect of a neuroactive steroid. J Pharmacol Exp. 1994;271:164–70. [PubMed] [Google Scholar]
- Finn DA, Ford MM, Wiren KM, Roselli CE, Crabbe JC. The role of pregnane neurosteroids in ethanol withdrawal: behavioral genetic approaches. Pharmacol Ther. 2004a;102:91–112. doi: 10.1016/j.pharmthera.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Finn DA, Long SL, Tanchuck MA, Crabbe JC. Interaction of chronic ethanol exposure and finasteride: sex and strain differences. Pharmacol Biochem Behav. 2004b;78:435–43. doi: 10.1016/j.pbb.2004.04.016. [DOI] [PubMed] [Google Scholar]
- Finn DA, Douglass AD, Beadles-Bohling AS, Tanchuck MA, Long SL, Crabbe JC. Selected line difference in sensitivity to a GABAergic neurosteroid during ethanol withdrawal. Genes Brain Behav. 2006;5:53–63. doi: 10.1111/j.1601-183X.2005.00137.x. [DOI] [PubMed] [Google Scholar]
- Freeman EW, Frye CA, Rickels K, Martin PA, Smith SS. Allopregnanolone levels and symptom improvement in severe premenstrual syndrome. J Clin Psychopharmacol. 2002;22:516–20. doi: 10.1097/00004714-200210000-00013. [DOI] [PubMed] [Google Scholar]
- Frye CA, Muscatiello NA. 3α,5α-THP in the raphe magnus attenuates PTZ-induced myoclonic seizures. Brain Res. 2001;911:146–51. doi: 10.1016/s0006-8993(01)02560-4. [DOI] [PubMed] [Google Scholar]
- Frye CA, Seliga AM. Olanzapine’s effects to reduce fear and anxiety and enhance social interactions coincide with increased progestin concentrations of ovariectomized rats. Psychoneuroendocrinology. 2003;28:657–73. doi: 10.1016/s0306-4530(02)00049-5. [DOI] [PubMed] [Google Scholar]
- Frye CA, Vongher JM. Progesterone and 3α,5α-THP enhance sexual receptivity in mice. Behav Neurosci. 2001;115:1118–28. [PubMed] [Google Scholar]
- Frye CA, Walf AA. Changes in progesterone metabolites in the hippocampus can modulate open field and forced swim test behavior of proestrous rats. Horm Behav. 2002;41:306–15. doi: 10.1006/hbeh.2002.1763. [DOI] [PubMed] [Google Scholar]
- Frye CA, Walf AA. Hippocampal 3α,5α-THP may alter depressive behavior of pregnant and lactating rats. Pharmacol Biochem Behav. 2004;78:531–40. doi: 10.1016/j.pbb.2004.03.024. [DOI] [PubMed] [Google Scholar]
- Frye CA, Wawrzycki J. Effect of prenatal stress and gonadal hormone condition on depressive behaviors of female and male rats. Horm Behav. 2003;44:319–26. doi: 10.1016/s0018-506x(03)00159-4. [DOI] [PubMed] [Google Scholar]
- Frye CA, Manjarrez J, Camacho-Arroyo I. Infusion of 3α,5α-THP to the pontine reticular formation attenuates PTZ-induced seizures. Brain Res. 2000a;881:98–102. doi: 10.1016/s0006-8993(00)02897-3. [DOI] [PubMed] [Google Scholar]
- Frye CA, Petralia SM, Rhodes ME. Estrous cycle and sex differences in performance on anxiety tasks coincide with increases in hippocampal progesterone and 3α,5α-THP. Pharmacol Biochem Behav. 2000b;67:587–96. doi: 10.1016/s0091-3057(00)00392-0. [DOI] [PubMed] [Google Scholar]
- Frye CA, Rhodes ME, Walf AA, Harney J. Progesterone reduces pentylenetetrazol-induced ictal activity of wild-type mice but not those deficient in type I 5α-reductase. Epilepsia. 2002;5:14–7. doi: 10.1046/j.1528-1157.43.s.5.19.x. [DOI] [PubMed] [Google Scholar]
- Frye CA, Walf AA, Rhodes ME, Harney JP. Progesterone enhances motor, anxiolytic, analgesic, and antidepressive behavior of wild-type mice, but not those deficient in type 1 5 α-reductase. Brain Res. 2004;1004:16–24. doi: 10.1016/j.brainres.2004.01.020. [DOI] [PubMed] [Google Scholar]
- Frye CA, Sumida K, Dudek BC, Harney JP, Lydon JP, O’Malley BW, et al. Progesterone’s effects to reduce anxiety behavior of aged mice do not require actions via intracellular progestin receptors. Psychopharmacology (Berl) 2006a;186:312–22. doi: 10.1007/s00213-006-0309-3. [DOI] [PubMed] [Google Scholar]
- Frye CA, Sumida K, Lydon JP, O’Malley BW, Pfaff DW. Mid-aged and aged wild-type and progestin receptor knockout (PRKO) mice demonstrate rapid progesterone and 3α,5α-THP-facilitated lordosis. Psychopharmacology. 2006b;185:423–32. doi: 10.1007/s00213-005-0300-4. [DOI] [PubMed] [Google Scholar]
- Galea LA, Wide JK, Barr AM. Estradiol alleviates depressive-like symptoms in a novel animal model of post-partum depression. Behav Brain Res. 2001;122:1–9. doi: 10.1016/s0166-4328(01)00170-x. [DOI] [PubMed] [Google Scholar]
- Genazzani AR, Stomati M, Bernardi F, Luisi S, Casarosa E, Puccetti S, et al. Conjugated equine estrogens reverse the effects of aging on central and peripheral allopregnanolone and beta-endorphin levels in female rats. Fertil Steril. 2004;81:757–66. doi: 10.1016/j.fertnstert.2003.08.022. [DOI] [PubMed] [Google Scholar]
- Gilbert Evans SE, Ross LE, Sellers EM, Purdy RH, Romach MK. 3α-reduced neuroactive steroids and their precursors during pregnancy and the postpartum period. Gynecol Endocrinol. 2005;21:268–79. doi: 10.1080/09513590500361747. [DOI] [PubMed] [Google Scholar]
- Girdler SS, Straneva PA, Light KC, Pedersen CA, Morrow AL. Allopregnanolone levels and reactivity to mental stress in premenstrual dysphoric disorder. Biol Psychiatry. 2001;49:788–97. doi: 10.1016/s0006-3223(00)01044-1. [DOI] [PubMed] [Google Scholar]
- Griffin LD, Mellon SH. Selective serotonin reuptake inhibitors directly alter activity of neurosteroidogenic enzymes. Proc Natl Acad Sci. 1999;96:13512–7. doi: 10.1073/pnas.96.23.13512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulinello M, Gong QH, Smith SS. Progesterone withdrawal increases the anxiolytic actions of gaboxadol: role of alpha4betadelta GABAA receptors. Neuroreport. 2003;14:43–6. doi: 10.1097/01.wnr.0000050303.92401.9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbreich U, Kahn LS. Role of estrogen in the aetiology and treatment of mood disorders. CNS Drugs. 2001;15:797–817. doi: 10.2165/00023210-200115100-00005. [DOI] [PubMed] [Google Scholar]
- Hirani K, Khisti RT, Chopde CT. Behavioral action of ethanol in Porsolt’s forced swim test: modulation by 3α-hydroxy-5α-pregnan-20-one. Neuropharmacology. 2002;43:1339–50. doi: 10.1016/s0028-3908(02)00330-1. [DOI] [PubMed] [Google Scholar]
- Hsu FC, Smith SS. Progesterone withdrawal produces paired-pulse inhibition in rat hippocampus: dependence on GABAA receptor α4 subunit upregulation. J Neurophysiol. 2003;89:186–98. doi: 10.1152/jn.00195.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iswari S, Colas AE, Karavolas HJ. Binding of 5α-dihydroprogesterone and other progestins to female rat anterior pituitary nuclear extracts. Steroids. 1986;47:189–203. doi: 10.1016/0039-128x(86)90088-7. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kronenberg G. Depressed new neurons? –Adult hippocampal neurogenesis and a cellular plasticity hypothesis of major depression. Biol Psychiatry. 2003;54:499–503. doi: 10.1016/s0006-3223(03)00319-6. [DOI] [PubMed] [Google Scholar]
- Khisti RT, Chopde CT. Serotonergic agents modulate antidepressant-like effect of the neurosteroid 3α-hydroxy-5α-pregnan-20-one in mice. Brain Res. 2000;86:291–300. doi: 10.1016/s0006-8993(00)02373-8. [DOI] [PubMed] [Google Scholar]
- Khisti RT, Chopde CT, Jain SP. Antidepressant-like effect of the neurosteroid 3α-hydroxy-5α-pregnan-20-one in mice forced swim test. Pharmacol Biochem Behav. 2000;67:137–43. doi: 10.1016/s0091-3057(00)00300-2. [DOI] [PubMed] [Google Scholar]
- Kokate TG, Svensson BE, Rogawski MA. Anticonvulsant activity of neurosteroids: correlation with γ-aminobutyric acid-evoked chloride current potentiation. J Pharmacol Exp Ther. 1994;270:1223–9. [PubMed] [Google Scholar]
- Kokate TG, Cohen AL, Karp E, Rogawski MA. Neuroactive steroids protect against pilocarpineand kainic acid-induced limbic seizures and status epilepticus in mice. Neuropharmacology. 1996;35:1049–56. doi: 10.1016/s0028-3908(96)00021-4. [DOI] [PubMed] [Google Scholar]
- Lambert JJ, Belelli D, Hill-Venning C, Peters JA. Neurosteroids and GABAA receptor function. Trends Pharmacol Sci. 1995;16:295–303. doi: 10.1016/s0165-6147(00)89058-6. [DOI] [PubMed] [Google Scholar]
- Löfgren M, Johansson IM, Meyerson B, Lundgren P, Bäckström T. Progesterone withdrawal effects in the open field test can be predicted by elevated plus maze performance. Horm Behav. 2006;50:208–15. doi: 10.1016/j.yhbeh.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Lonstein JS. Regulation of anxiety during the postpartum period. Front Neuroendocrinol. 2007;28:115–41. doi: 10.1016/j.yfrne.2007.05.002. [DOI] [PubMed] [Google Scholar]
- MacDonald PC, Dombroski RA, Casey ML. Recurrent secretion of progesterone in large amounts: an endocrine/metabolic disorder unique to young women? Endocr Rev. 1991;12:372–401. doi: 10.1210/edrv-12-4-372. [DOI] [PubMed] [Google Scholar]
- Majewska MD. Neurosteroids: endogenous bimodal modulators of the GABAA receptor. Mechanism of action and physiological significance. Prog Neurobiol. 1992;38:379–95. doi: 10.1016/0301-0082(92)90025-a. [DOI] [PubMed] [Google Scholar]
- Majewska MD, Harrison NL, Schwartz RD, Barker JL, Paul SM. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science. 1986;232:1004–7. doi: 10.1126/science.2422758. [DOI] [PubMed] [Google Scholar]
- Malberg JE, Blendy JA. Antidepressant action: to the nucleus and beyond. Trends Pharmacol Sci. 2005;26:631–8. doi: 10.1016/j.tips.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Martinez-Mota L, Fernandez-Guasti A. Testosterone-dependent antidepressant-like effect of noradrenergic but not of serotonergic drugs. Pharmacol Biochem Behav. 2004;78:711–8. doi: 10.1016/j.pbb.2004.05.016. [DOI] [PubMed] [Google Scholar]
- Marx CE, VanDoren MJ, Duncan GE, Lieberman JA, Morrow AL. Olanzapine and clozapine increase the GABAergic neuroactive steroid allopregnanolone in rodents. Neuropsychopharmacology. 2003;28:1–13. doi: 10.1038/sj.npp.1300015. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Uzunova V, Pinna G, Taki K, Uzunov DP, Watanabe H, et al. Permissive role of brain allopregnanolone content in the regulation of pentobarbital-induced righting reflex loss. Neuropharmacology. 1999;38:955–63. doi: 10.1016/s0028-3908(99)00018-0. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Pinna G, Puia G, Guidotti A, Costa E. Social isolation stress-induced aggression in mice: a model to study the pharmacology of neurosteroidogenesis. Stress: Int J Biol Stress. 2005;8:85–93. doi: 10.1080/10253890500159022. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Puia G, Dong E, Pinna G. GABA(A) receptor neurotransmission dysfunction in a mouse model of social isolation-induced stress: possible insights into a non-serotonergic mechanism of action of SSRIs in mood and anxiety disorders. Stress. 2007;10:3–12. doi: 10.1080/10253890701200997. [DOI] [PubMed] [Google Scholar]
- Molina-Hernández M, Contreras CM, Téllez-Alcántar P. Antidepressant-like effects of pregnancy and progesterone in Wistar rats as measured in the differential reinforcement of the low-rate 72 s task. Psychopharmacology (Berl) 2000;151:306–11. doi: 10.1007/s002130000496. [DOI] [PubMed] [Google Scholar]
- Morrow AL, Suzdak PD, Paul SM. Steroid hormone metabolites potentiate GABA receptor mediated chloride ion flux with nanomolar potency. Eur J Pharmacol. 1987;142:483–5. doi: 10.1016/0014-2999(87)90094-x. [DOI] [PubMed] [Google Scholar]
- Nappi RE, et al. Serum allopregnanolone in women with postpartum “blues”. Obstet Gynecol. 2001;97:77–80. doi: 10.1016/s0029-7844(00)01112-1. [DOI] [PubMed] [Google Scholar]
- Nelson JF, Felicio LS, Osterburg HH, Finch CE. Differential contributions of ovarian and extraovarian factors to age-related reductions in plasma estradiol and progesterone during the estrous cycle of C57BL/6J mice. Endocrinology. 1992;130:805–10. doi: 10.1210/endo.130.2.1733727. [DOI] [PubMed] [Google Scholar]
- Osterlund MK, Witt MR, Gustafsson JA. Estrogen action in mood and neurodegenerative disorders: estrogenic compounds with selective properties—the next generation of therapeutics. Endocrine. 2005;28:235–42. doi: 10.1385/ENDO:28:3:235. [DOI] [PubMed] [Google Scholar]
- Paoletti AM, Floris S, Mannias M, Orru M, Crippa D, Orlandi R, et al. Evidence that cyproterone acetate improves psychological 26 symptoms and enhances the activity of the dopaminergic system in postmenopause. J Clin Endocrinol Metab. 2001;86:608–12. doi: 10.1210/jcem.86.2.7179. [DOI] [PubMed] [Google Scholar]
- Paré WP, Redei E. Sex differences and stress response of WKY rats. Physiol Behav. 1993;54:1179–85. doi: 10.1016/0031-9384(93)90345-g. [DOI] [PubMed] [Google Scholar]
- Paul SM, Purdy RH. Neuroactive steroids. FASEB J. 1992;6:2311–22. [PubMed] [Google Scholar]
- Perrot-Sinal TS, Kavaliers M, Ossenkopp KP. Spatial learning and hippocampal volume in male deer mice: relations to age, testosterone and adrenal gland weight. Neuroscience. 1998;86:1089–99. doi: 10.1016/s0306-4522(98)00131-6. [DOI] [PubMed] [Google Scholar]
- Petit-Demouliere B, Chenu F, Bourin M. Forced swimming test in mice: a review of antidepressant activity. Psychopharmacology (Berl) 2005;177:245–55. doi: 10.1007/s00213-004-2048-7. [DOI] [PubMed] [Google Scholar]
- Pinna G. In a mouse model relevant for post-traumatic stress disorder, selective brain steroidogenic stimulants (SBSS) improve behavioral deficits by normalizing allopregnanolone biosynthesis. Behav Pharmacol. 2010;21:438–50. doi: 10.1097/FBP.0b013e32833d8ba0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinna G, Dong E, Matsumoto K, Costa E, Guidotti A. In socially isolated mice, the reversal of brain allopregnanolone down-regulation mediates the anti-aggressive action of fluoxetine. Proc Natl Acad Sci U S A. 2003;18(100):2035–40. doi: 10.1073/pnas.0337642100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinna G, Costa E, Guidotti A. Fluoxetine and norfluoxetine stereospecifically and selectively increase brain neurosteroid content at doses that are inactive on 5-HT reuptake. Psychopharmacology (Berl) 2006;186:362–72. doi: 10.1007/s00213-005-0213-2. [DOI] [PubMed] [Google Scholar]
- Pinna G, Costa E, Guidotti A. SSRIs act as selective brain steroidogenic stimulants (SBSSs) at low doses that are inactive on 5-HT reuptake. Curr Opin Pharmacol. 2009;9:24–30. doi: 10.1016/j.coph.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisani G, Facioni F, Fiorani G, Pisani G. Psychosexual problems in menopause. Minerva Ginecol. 1998;50:77–81. [PubMed] [Google Scholar]
- Porsolt RD, Bertin A, Jalfre M. Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther. 1977;229:327–36. [PubMed] [Google Scholar]
- Porsolt RD, Anton G, Blavet N, Jalfre M. Behavioural despair in rats: a new model sensitive to antidepressant treatments. Eur J Pharmacol. 1978;47:379–91. doi: 10.1016/0014-2999(78)90118-8. [DOI] [PubMed] [Google Scholar]
- Rapkin AJ, Mikacich JA, Moatakef-Imani B, Rasgon N. The clinical nature and formal diagnosis of premenstrual, postpartum, and perimenopausal affective disorders. Curr Psychiatry Rep. 2002;4:419–28. doi: 10.1007/s11920-002-0069-7. [DOI] [PubMed] [Google Scholar]
- Rasgon N, Shelton S, Halbreich U. Perimenopausal mental disorders: epidemiology and phenomenology. CNS Spectr. 2005;10:471–8. doi: 10.1017/s1092852900023166. [DOI] [PubMed] [Google Scholar]
- Rasmusson AM, Pinna G, Paliwal P, Weisman D, Gottschalk C, Charney D. Decreased cerebrospinal fluid allopregnanolone levels in women with posttraumatic stress disorder. Biol Psychiatry. 2006;60:704–13. doi: 10.1016/j.biopsych.2006.03.026. [DOI] [PubMed] [Google Scholar]
- Robinson GE. Psychotic and mood disorders associated with the perimenopausal period: epidemiology, aetiology and management. CNS Drugs. 2001;15:175–84. doi: 10.2165/00023210-200115030-00002. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Landa JF, Contreras CM, Bernal-Morales B, Gutiérrez-García AG, Saavedra M. Allopregnanolone reduces immobility in the forced swimming test and increases the firing rate of lateral septal neurons through actions on the GABAA receptor in the rat. J Psychopharmacol. 2007;21:76–84. doi: 10.1177/0269881106064203. [DOI] [PubMed] [Google Scholar]
- Rupprecht R, Hauser CA, Trapp T, Holsboer F. Neurosteroids: molecular mechanisms of action and psychopharmacological significance. J Steroid Biochem Mol Biol. 1996;56:163–8. doi: 10.1016/0960-0760(95)00233-2. [DOI] [PubMed] [Google Scholar]
- Saavedra M, Contreras CM, Azamar-Arizmendi G, Hernández-Lozano M. Differential progesterone effects on defensive burying and forced swimming tests depending upon a gradual decrease or an abrupt suppression schedules. Pharmacol Biochem Behav. 2006;83:130–5. doi: 10.1016/j.pbb.2005.12.016. [DOI] [PubMed] [Google Scholar]
- Schüle C, Zill P, Baghai TC, Eser D, Zwanzger P, Wenig N, et al. Brain-derived neurotrophic factor Val66Met polymorphism and dexamethasone/CRH test results in depressed patients. Psychoneuroendocrinology. 2006;31:1019–25. doi: 10.1016/j.psyneuen.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Serra M, Pisu MG, Littera M, Papi G, Sanna E, Tuveri F, et al. Social isolation-induced decreases in both the abundance of neuroactive steroids and GABAA receptor function in rat brain. J Neurochem. 2000;75:732–40. doi: 10.1046/j.1471-4159.2000.0750732.x. [DOI] [PubMed] [Google Scholar]
- Serra M, Sanna E, Mostallino MC, Biggio G. Social isolation stress and neuroactive steroids. Eur Neuropsychopharmacol. 2007;17:1–11. doi: 10.1016/j.euroneuro.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Shelton C. Factors impacting the selection of antidepressant treatment in patients with major depressive disorder at risk for nonadherence. CNS Spectr. 2009;14(12 Suppl 12):15–9. doi: 10.1017/s1092852900026365. [DOI] [PubMed] [Google Scholar]
- Shen H, Gong QH, Aoki C, Yuan M, Ruderman Y, Dattilo M, et al. Reversal of neurosteroid effects at alpha4beta2delta GABA(A) receptors triggers anxiety at puberty. Nat Neurosci. 2007;10:469–77. doi: 10.1038/nn1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith HE, Smith RG, Toft DO, Neergaard JR, Burrows EP, O’Malley BW. Binding of steroids to progesterone receptor proteins in chick oviduct and human uterus. J Biol Chem. 1974;249:5924–32. [PubMed] [Google Scholar]
- Smith SS, Ruderman Y, Gong QH, Gulinello M. Effects of a low dose of ethanol in an animal model of premenstrual anxiety. Alcohol. 2004;33:41–9. doi: 10.1016/j.alcohol.2004.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SS, Ruderman Y, Frye CA, Homanics G, Yuan M. Steroid withdrawal in the mouse results in anxiogenic effects of 3α,5β-THP: a possible model of premenstrual dysphoric disorder. Psychopharmacology (Berl) 2006;186:323–33. doi: 10.1007/s00213-005-0168-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl SM. Sex therapy in psychiatric treatment has a new partner: reproductive hormones. J Clin Psychiatry. 1997;58:468–9. doi: 10.4088/jcp.v58n1101. [DOI] [PubMed] [Google Scholar]
- Steiner M, Dunn E, Born L. Hormones and mood: from menarche to menopause and beyond. J Affect Disord. 2003;74:67–83. doi: 10.1016/s0165-0327(02)00432-9. [DOI] [PubMed] [Google Scholar]
- Stoffel EC, Craft RM. Ovarian hormone withdrawal-induced “depression” in female rats. Physiol Behav. 2004;83:505–13. doi: 10.1016/j.physbeh.2004.08.033. [DOI] [PubMed] [Google Scholar]
- Urani A, Roman FJ, Phan VL, Su TP, Maurice T. The antidepressant-like effect induced by sigma(1)-receptor agonists and neuroactive steroids in mice submitted to the forced swimming test. J Pharmacol Exp Ther. 2001;298:1269–79. [PubMed] [Google Scholar]
- Uzunov DP, Cooper TB, Costa E, Guidotti A. Fluoxetine-elicited changes in brain neurosteroid content measured by negative ion mass fragmentography. Proc Natl Acad Sci U S A. 1996;93:12599–604. doi: 10.1073/pnas.93.22.12599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzunova V, Sheline Y, Davis JM, Rasmusson A, Uzunov DP, Costa E, et al. Increase in the cerebrospinal fluid content of neurosteroids in patients with unipolar major depression who are receiving fluoxetine or fluvoxamine. Proc Natl Acad Sci U S A. 1998;95:3239–44. doi: 10.1073/pnas.95.6.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivian JA, Barros HM, Manitiu A, Miczek KA. Ultrasonic vocalizations in rat pups: modulation at the gamma-aminobutyric acid A receptor complex and the neurosteroid recognition site. J Pharmacol Exp Ther. 1997;282:318–25. [PubMed] [Google Scholar]
- Vongher JM, Frye CA. Progesterone in conjunction with estradiol has neuroprotective effects in an animal model of neurodegeneration. Pharmacol Biochem Behav. 1999;64:777–85. doi: 10.1016/s0091-3057(99)00140-9. [DOI] [PubMed] [Google Scholar]
- Walf AA, Frye CA. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc. 2007;2:322–8. doi: 10.1038/nprot.2007.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Sumida K, Frye CA. Inhibiting 5α-reductase in the amygdala attenuates antianxiety and antidepressive behavior of naturally receptive and hormone-primed ovariectomized rats. Psychopharmacology. 2006;186:302–11. doi: 10.1007/s00213-005-0100-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Li W, Zhu B, Liu Y, Yang B, Wang H, et al. Sex differences in antidepressant-like effect of chronic repetitive transcranial magnetic stimulation in rats. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:735–40. doi: 10.1016/j.pnpbp.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Zonana J, Gorman JM. The neurobiology of postpartum depression. CNS Spectr. 2005;10:792–9. doi: 10.1017/s1092852900010312. [DOI] [PubMed] [Google Scholar]