Abstract
We previously observed blunted phase-shift responses to morning bright light in women with Premenstrual Dysphoric Disorder (PMDD). The aim of this study was to determine if we could replicate these findings using a higher intensity, shorter duration light pulse and to compare these results with the effects of an evening bright light pulse.
In 17 PMDD patients and 14 normal control (NC) subjects, we measured plasma melatonin at 30 minute intervals from 18:00–10:00 h in dim (< 30 lux) or dark conditions the night before (night 1) and after (night 3) a bright light pulse (administered on night 2) in both follicular and luteal menstrual cycle phases. The bright light (either 3,000 lux for 6 h or 6,000 lux for 3 h) was given either in the AM, 7 h after the Dim Light Melatonin Onset (DLMO) measured the previous month, or in the PM, 3 h after the DLMO.
In the luteal, but not in the follicular, phase, AM light advanced melatonin offset between night 1 and night 3 significantly less in PMDD than in NC subjects. The effects of PM light were not significant, nor were there significant effects of the light pulse on melatonin measures of onset, duration, peak or area under the curve.
These findings replicated our previous finding of a blunted phase-shift response to morning bright light in the luteal, but not the follicular, menstrual cycle phase in PMDD compared with NC women, using a brighter (6,000 vs. 3,000 lux) light pulse for a shorter duration (3 vs. 6 h). As the effect of PM bright light on melatonin phase-shift responses did not differ between groups or significantly alter other melatonin measures, these results suggest that in PMDD there is a luteal phase subsensitivity or an increased resistance to morning bright light cues which are critical in synchronizing human biological rhythms. The resulting circadian rhythm malsynchonization may contribute to the occurrence of luteal phase depressive symptoms in women with PMDD.
Keywords: light, phase-shift, melatonin, women, PMDD, menstrual cycle
INTRODUCTION
We previously observed blunted phase-shift responses to morning bright light in the luteal, but not in the follicular, menstrual cycle phase in women with Premenstrual Dysphoric Disorder (PMDD) compared with normal control (NC) subjects (Parry et al., 1997b). This decreased phase-shift response occurred despite increased sensitivity to the suppressive effects of 200 lux light in the follicular, but not in the luteal, menstrual cycle phase in PMDD vs. NC women (Parry et al., 2010). Aging rodents (Benloucif et al., 1997; Kolker et al., 2003; Zhang et al., 1996) and humans (Klerman et al., 2001) also demonstrate blunted phase-shift responses to light. Benloucif et al. (2006) and Kripke et al. (2007), however, demonstrated that older adults are able to phase-delay melatonin circadian rhythms to the same extent as younger subjects. Thus PMDD subjects’ blunted phase-shift responses to light could reflect an overall subsensitivity to the phase-shifting effects of light, or an altered sensitivity at different times of the day. The abnormal phase-shift response could contribute to decreased entrainment with the environment and result in malsynchronized internal circadian rhythms. As a result, vulnerable women with PMDD might develop mood disturbances in the luteal menstrual cycle phase. Earlier work in rodents suggested that deficits in the serotonergic control of circadian function in aging contribute to the decrease in light-induced phase-advances occurring then (Penev et al., 1997; Penev et al., 1995). In PMDD, documented deficits in serotonergic function also might contribute to a decrease in phase-advance responses (Parry, 2001). The aims of the present study were to determine whether we could replicate our previous findings of a blunted phase-advance response to morning bright light using a more precise light stimulus (higher intensity, shorter duration) and to test the effects of evening bright light on the magnitude of phase-delay responses of plasma melatonin, contrasting PMDD vs. NC women during follicular and luteal menstrual cycle phases.
SUBJECTS AND METHODS
Subjects
We recruited potential patients with PMDD and NC subjects primarily by advertisements for participants in mood, sleep and light studies during the menstrual cycle. For the study of the phase-shifting effects of light in PMDD vs. NC subjects, we solicited physician referrals and posted advertisements in local papers, clinic flyers and on the internet. In response to these notices, we received 2,360 calls from interested subjects; screened 497 appropriate subjects by telephone or mailed screening packets, and scheduled 93 women for weekly visits over a 2-month diagnostic evaluation period. The primary reason for excluding subjects was that they did not meet diagnostic criteria, as is the case in most other PMDD studies nationwide. These rates of subject accrual match those of most PMDD studies based on a multi-site study (Hurt et al., 1992). We initially enrolled a total of 41 subjects (21 PMDD, 20 NC). Four women dropped from the initial Phase-Marker study (when the dim light melatonin onset –DLMO– was assessed) and one woman dropped from the subsequent Phase-Shift study (when the effects of late night or early morning bright light exposures were assessed). Reasons for not completing the study included difficulty with the blood draws, illness, family and work requirements. Five women who received placebo light intensity in the initial pilot study are not included in this report.
Screening procedures consisted of a structured menstrual assessment questionnaire (adapted by Parry and Mostofi from the Menstrual Assessment Form as described in Roy-Byrne et al. (1986)), the Structured Clinical Interview for DSM-IV (SCID) (First et al., 1995), a psychiatric interview, a physical examination, and laboratory tests, including chemistry panel, complete blood count, urinalysis, and measurements of thyroid indices. If the subject did not have other major medical, gynecologic, or psychiatric illness, had regular (26–32 day) menstrual cycles, reported recurrent premenstrual affective symptoms severe enough to disrupt social or occupational functioning (PMDD only), and was willing to endure the rigors of a research study over several months, she was admitted for a 2 to 3 month prospective evaluation for diagnostic assessment. A past, but not recent (within the last year) history of a depressive disorder was permitted for PMDD, but not NC, subjects. NC subjects had to be without a lifetime history of psychiatric illness (including alcohol dependence) and to have no active medical illnesses.
During the 2-month evaluation, both potential PMDD and NC subjects completed twice-daily (morning and evening) mood ratings (100-mm visual analogue scales of depression, anxiety, irritability, fatigue, withdrawal, physical symptoms, and appetite) (Aitken, 1969) and visited the clinic weekly for interview-based depression ratings (21-item Hamilton Rating Scale for Depression) (HRSD) (Hamilton, 1967) and self-report measures (Beck Depression Inventory) (BDI) (Beck et al., 1961). In addition, an addendum to the HRSD to assess atypical items of depression (Structured Interview Guide for the HRSD-Seasonal Affective Disorder (SIGH-SAD) Version (Williams et al., 1994)) and a hypomania rating scale to determine whether light interventions induced manic or hypomanic symptoms were used in evaluation (Rosenthal and Heffernan, 1986). On the basis of this examination, to be selected for the study, PMDD subjects had to meet DSM-IV criteria for Premenstrual Dysphoric Disorder (APA, 1994). To meet impairment criteria, PMDD subjects had to have a mean score of 14 or more on the HRSD, 10 or more on the BDI and a 30% increase in daily ratings in the late luteal phase (1 week before the onset of menses), and demonstrate a reduction in mean scores to 7 or less on the HRSD, 5 or less on the BDI and less than 50 mm on daily ratings by the week after the cessation of menses. All the PMDD subjects had debilitating affective symptoms that occurred during the late luteal phase of each menstrual cycle throughout the year (i.e., they did not have seasonal premenstrual symptoms). To be included in this study, NC subjects had to have mean HRSD scores less than 7 and BDI ratings less than 5 at all menstrual cycle phases and their daily ratings needed to show < 30% clinical variation in association with the menstrual cycle. PMDD versus NC classifications were not determined until the end of the 2-month evaluation.
Subjects were free of psychoactive medication for at least 2 months before study entry (during the diagnostic evaluation) and for the duration of the study. The use of natural remedies and herbs were excluded on the basis of interview and questionnaires. Substance abuse and recent prescription medication use was ruled out by obtaining urine toxicology screens prior to admissions. Subjects needed to be off oral contraceptives, and to have not been smoking for 3 months prior to entering the evaluation phase of the study, but their use before this time was not exclusionary.
We asked subjects to maintain their habitual sleep and wake times for at least a week before entering the study, as documented by sleep logs. In the screening forms and in the entrance interview, we asked subjects not to increase or decrease caffeine intake by more than one to two beverages per day (so as not to induce caffeine excess or withdrawal), and not to take NSAIDs (acetaminophen was allowed) as per the recommendations of the Associated Professional Sleep Societies workshop on measuring melatonin in humans (Benloucif et al., 2008).
The protocol was approved by the Human Subjects Committee of the University of California, San Diego (UCSD) and meets ethical standards (Portaluppi et al., 2010). All subjects gave written informed consent after the procedures had been explained fully.
Methods
Subjects who met criteria described above were studied during mid-follicular (MF) and late luteal (LL) menstrual cycle phases as determined by urine luteinizing hormone (LH) and serum estradiol and progesterone levels (see “Assays" described below). Admissions to the General Clinical Research Center (GCRC) were scheduled 8 ± 2 days after the onset of menses for the MF phase (when both PMDD and NC subjects were asymptomatic) and 2 to 4 days before the next predicted onset of menses (determined by the mid-cycle LH surge) for the LL phase (when PMDD, but not NC, subjects were symptomatic). We chose to study subjects in both the MF and LL menstrual cycle phases to determine whether abnormal phase-shift responses of melatonin to light, if replicated in PMDD, were trait or state markers for the illness.
Subjects were admitted to the GCRC at 16:00 h and placed in dim (<30 lux) light at bed rest. Lavatory facilities were provided in the room. Sleep technicians obtained polysomnography (PSG) recordings. Subjects were allowed to sleep at their usual bedtimes, which had been maintained for the week prior to admission. To allow time for adaptation, nurses inserted an intravenous catheter at 17:00 h, and threaded the catheter through a porthole in the wall to an adjoining room to allow for blood sample collection while subjects slept. Overnight blood samples were collected every 30 min, from 18:00-10:00h. Lights were turned out at 22:30 h.
We studied subjects during follicular and luteal phases of separate menstrual cycles (so that light administration in the follicular phase of one month would not alter melatonin responses in the luteal phase of the same month). During each of these 2 months, subjects were admitted for 3 consecutive nights of study: On Night 1, GCRC nurses placed an intravenous catheter and drew blood samples for baseline levels of plasma melatonin every 30 minutes from 18:00 to 10:00 h and serum reproductive hormones (estradiol & progesterone) at 18:00 and 06:00 h while the subjects reclined in dim light (<30 lux) while awake or dark while sleeping. Subjects were allowed to return to their work, school, or child care responsibilities at home during the day but were asked to wear goggles that block 99.9% of UVA and UVB rays (up to 400 nm) and 88% ambient light (Uvex Safety, Smithfield, RI), and return to the laboratory for studies by 17:00 h the same day. On Night 2, after the women’s usual sleep onset times, sleep technicians awakened subjects and exposed them to either 1) 3,000 lux of light (as documented by a photometer) for 6 h [7 h after the DLMO as determined the previous month in follicular and luteal menstrual cycle phases (Parry et al., 2010), generally between 03:00-09:00 h], or 2) 6,000 lux of light for 3 h starting either 3 h after the DLMO (generally between 23:00-02:00 h) for PM light or 8 h after the DLMO for AM light (generally between 04:00-07:00 h). Blood samples were not obtained on Night 2 because of the acute suppressive effects of light on melatonin secretion. On Night 3, subjects returned to the GCRC to repeat the blood sampling as on Night 1 from 18:00 to 10:00 h. Twenty-three subjects (13 PMDD, 10 NC) received light in the morning (AM light) and eight (4 PMDD, 4 NC) received light in the evening (PM light). The initial 18 subjects (10 PMDD, 8 NC) received 3000 lux light for 6 h; the remaining 13 subjects (7 PMDD, 6 NC) received 6000 lux light for 3 h.
Sleep and wake times were measured by PSG. Sleep technicians and GCRC nurses helped to ensure subjects' wakefulness during the times of light exposure. Subjects were asked to gaze at the lights for the duration of 1 min every 3 min. The light boxes (Apollo Brite Lite III (24 x 13 x 4-inch –4,100 Kelvin, 10,000 lux at 15”, with median irradiance of 3.82 x 10−3 w/cm2) (Apollo Light, Orem, UT)) were placed at eye level directly in front of the subjects 32 inches away for the 3000 lux light studies and 22 inches away for the 6000 lux light studies. They were portable illumination boxes comprised of full spectrum cool white fluorescent light bulbs with intensity adjusted and ultraviolet light blocked by a neutral density filter. Light boxes were calibrated and exposure intensity was documented by a photometer (United Detector, Orlando, FL) set at 3000 & 6000 lux. In addition, each subject was provided with an Actillume, a wrist monitor that measures the amount of activity and illumination, and was told to wear it during specific phases of the study. The Actillumes were worn 4 days prior to admission to the laboratory to assess baseline levels of activity and illumination as well as levels during the 3 days and nights of the study to document sleep and appropriate light intensities.
Melatonin Assays
Blood samples for melatonin were placed in ethylenediaminetetracetic acid-containing plastic tubes and centrifuged. The plasma was extracted and frozen immediately, and stored at −70° C until assayed. All samples from the same subject were run in duplicate in the same assay. Initial assays are described previously (Anderson et al., 1976; Brzezinski et al., 1988). With the exception of two subjects, we assayed plasma melatonin concentrations by radioimmunoassay (RIA) with kits manufactured by IBL Immuno-Biological Laboratories, Hamburg, Germany. As the manufacturer changed this kit, plasma samples for the last two NC subjects were assayed with Direct Melatonin RIA kits manufactured by Bühlmann Laboratories (ALPCO Diagnostics, Windham NH). This widely used RIA kit uses calibrators ranging from 1 – 81 pg/ml, with an analytical sensitivity of 0.8 pg/ml, and reports intra- and inter-assay CVs of 6.7 % and 10.4 %, respectively. For melatonin statistical analyses, assay type (IBL vs. Bühlmann) was included as a covariate to correct for differences between assays; no significant effect of the different assay methods was found.
Estimation of dim light melatonin parameters
As noted previously (Benloucif et al., 2008), there is no universally accepted method for estimating melatonin timing parameters. Based on our experience with various scoring methods (including two mathematically-derived curve-fitting procedures, multiple “threshold” methods which invoke plasma melatonin values of 3 or 10 pg/ml, or a multiple of the standard deviation of baseline values prior to the presumed melatonin onset) we chose to use a visual inspection method (Parry et al., 2008b) which, in our experience, has generated highly reliable estimates of melatonin timing parameters. Briefly, we defined the dim light melatonin onset (DLMO) as the time of the first elevated point when the slope (dy/dt) of the log-transformed melatonin concentration curve became steeply positive for at least three consecutive time points relative to the slope of the points immediately preceding it; the offset as the first time when the slope of the descending log-transformed melatonin curve approached zero for at least three consecutive time points; and synthesis offset (based on (Lewy et al., 1999), as the first time after the melatonin peak when the slope of the descending log-transformed melatonin curve became steeply negative for three consecutive time points. For all estimates we used the median of three visual inspection values derived from independent raters, blinded to each subject’s group. Cross correlation analyses showed visual inspection yielded inter-rater reliabilities ranging from r = .913 to .999 for melatonin onset, from r = .801 to .925 for melatonin offset and from r = .816 to .930 for synthesis offset. We also defined the melatonin duration as the difference in hours between onset and offset, while synthesis duration was defined as the difference in hours between onset and synthesis offset. We also defined the peak concentration as the single highest concentration during the nocturnal secretory episode, and area under the curve (AUC) as the integrated melatonin concentration (pmol/L/h) from onset to offset.
Reproductive Hormonal Assays
The timing of the mid-cycle LH surge as determined by a colorimetric urinary immunoassay (Unipath Limited, Bedford, UK) was used to document ovulation. Menstrual cycle phase was documented by serum estradiol and progesterone levels. Estradiol and progesterone assays are described previously (Anderson et al., 1976).
Statistical Analyses
We assessed pre-treatment (pre-light intervention) baseline differences in melatonin timing and quantity using a “mixed” (between/within) Diagnosis (NC vs. PMDD) x Phase (Follicular vs. Luteal) MANOVA, followed by univariate ANOVA on significant omnibus MANOVA effects. The effects of different light intensities (3,000 vs. 6,000 lux) were analyzed by comparing delta/change scores [baseline (night1) – post-light exposure (night 3)] in the follicular and luteal phases, separately, with one-factor, between subjects ANOVA. Subsequently, light intensity (3,000 vs. 6,000 lux) was included as a covariate where relevant in all data analyses. Similarly, we analyzed melatonin rhythms obtained before (night 1) and after (night 3) the bright (3,000 or 6,000 lux) light pulse administered on night 2 in the follicular and luteal phase for onset and standard and synthesis offset times, peak, duration, and area under the curve (AUC), using MANOVA, as described previously (Parry et al., 2008b). Onset and offset times were calculated as individualized threshold values, independent of the AUC; a positive value for the timing delta scores (night 1 - night 3) indicated a phase-advance (by convention), while for the quantitative measures (peak, duration, AUC), a positive value represented an increase (night 3 - night 1).
RESULTS
The mean age of NC women was 37.50 ± 5.4 years and for PMDD subjects, 34.71 ± 7.6 years. Parity range in PMDD and NC was 0–4 children. The PMDD women on average had 1.0 ± 1.1 while the NC women had 0.55 ± 1.0 children. In the NC women mean BMI was 24.71 ± 4.50 and in the PMDD 25.32 ± 3.86. There were no statistically significant differences between groups in age, parity, or body mass index. As determined by SCID interviews, PMDD subjects were significantly more likely to have had a previous history of a major depressive disorder (MDD) than NC subjects (70.6% vs. 0.0%, χ 2= 16.1, p = .001). Seven women reported Hispanic (23%) ethnic background and 24 (77%) reported non-Hispanic Caucasian ancestry. Eight women were studied in the spring (26%), six women in summer (20%), nine in the fall (29%) and eight women in winter (26%). At the time of the study, fourteen women were married (45%), 5 divorced (10%) and 14 single (45%). The difference between NC vs. DP was non-significant for ethnicity (χ2= 0.02, p = .889), season when tested (χ2= 0.49, p = .921), and marital status (χ2= 0.62, p = .733).
Baseline (Pre-Light Intervention) Differences in Melatonin (See Figure 1)
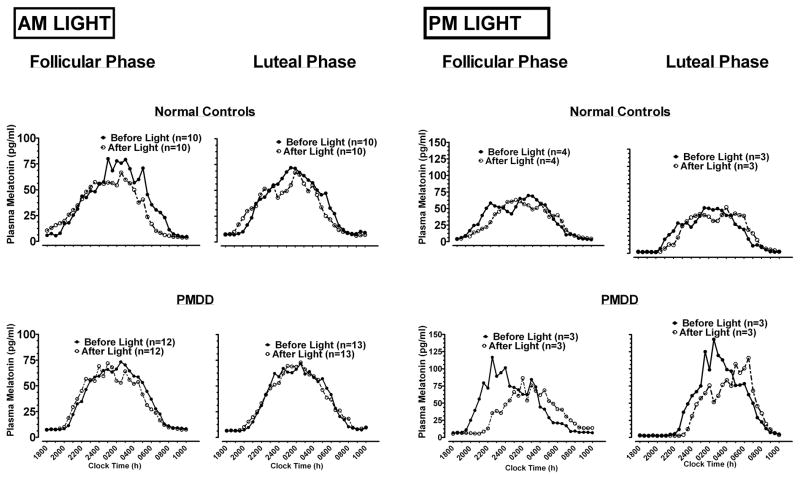
Figure 1.
Effects of single day/night exposure of AM and PM light on melatonin profiles from 18:00 to 10:00 h, in follicular and luteal phases, in normal controls and PMDD patients.
Analyses of pre-treatment baselines showed the omnibus MANOVA was significant for the main effect of Menstrual Phase (p = .041). Follow-up univariate ANOVA indicated melatonin duration was shorter across diagnoses in the luteal than in the follicular phase (11.3±0.4 vs. 11.8±0.4 h, p = .026), as was synthesis duration (7.9±0.3 vs. 8.5±0.3 h, p = .028); AUC was smaller in the luteal than in the follicular phase (1132±135 vs. 1320±138 pg/ml/h, p = .010). The Diagnosis x Phase interaction, however, was significant for AUC and Peak (ANOVA, both p < .05). Analyses of delta scores (luteal night 1 – follicular night 1) showed AUC decreased more in PMDD than NC as a consequence of the transition from the follicular to the luteal phase (−326±99 vs. −48±93 pg/ml/h, p = .050), as did Peak (−25.4±12 vs. 8.6±11 pg/ml, p = .043).
Phase-Shifting Effects of Light on Melatonin
In the follicular phase, 3 hours (6,000 lux) of AM light advanced melatonin onset to a greater extent than 6 hours (3,000 lux) of dimmer light did (1.5 vs. 0.4 h, p = .009), but melatonin offset (p = .371) and synthesis offset (p = .341) were not differentially affected by 3 vs. 6 h of light. In the luteal phase, differences between 3 and 6 h of light exposure were not significant for melatonin onset, offset or synthesis offset (all p > .05).
Analyses of Melatonin Variables Pre- and Post- Light Pulse
Analyses showed AM and PM light produced essentially opposite changes in melatonin timing (omnibus MANOVA p = .0001; see Figure 2 and 3).
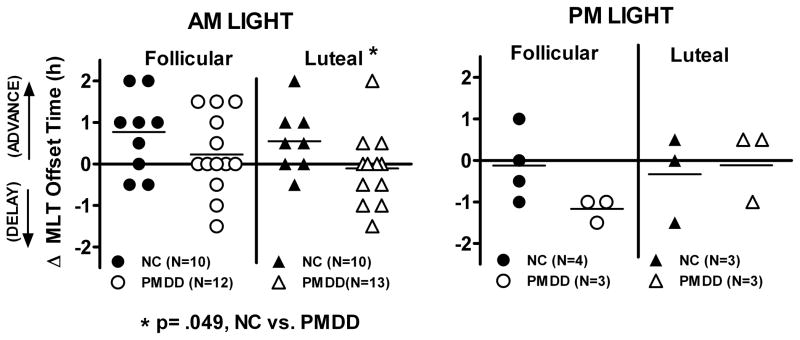
Figure 2.
Effects of AM and PM light on melatonin offset times in follicular and luteal phases. Data points represent change (delta) scores of individual subjects; horizontal lines denote means of the respective NC and PMDD groups.
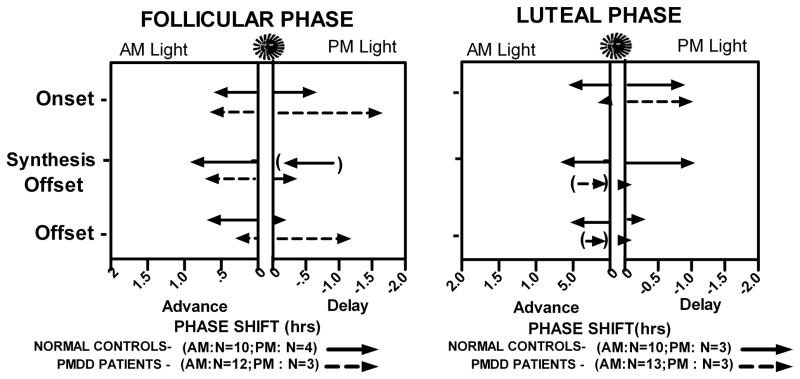
Figure 3.
Phase-shift responses to AM and PM light, during follicular and luteal menstrual phases, in normal control women and PMDD patients. Arrows in parentheses depict an advance with PM Light in Follicular Phase Synthesis Offset in NC, and delays with AM Light in Luteal Phase Synthesis Offset and Offset in PMDD.
Univariate ANOVA showed the phase advance in melatonin offset after AM light was significantly smaller in PMDD (.08±0.53 h) than in NC (0.65±0.53 h), F(1,20) = 6.29, p = 0.021); the main effect of menstrual phase (p = 0.388) and the diagnosis x phase interaction (p = 0.827) were both non-significant. However, based on our earlier finding of a blunted phase shift response in PMDD vs. NC (Parry et al., 1997b), we performed simple effects analyses which showed that in the luteal phase, AM Light advanced melatonin Offset (between night 1 and night 3) to a lesser extent in PMDD than in NC (−0.12±0.22 h vs. 0.56±0.24 h, p = .049; see Figs. 2, 3); in the follicular phase, phase advances in melatonin offset in PMDD vs. NC 0(0.25±0.99 vs. 0.75±0.89 h) did not differ significantly (p = .231). Changes in melatonin onset, duration, synthesis offset, synthesis duration, peak and AUC did not differ significantly between NC and PMDD in either the follicular or luteal phases (all p > .05). All changes due to PM light were not significant for phase (follicular vs. luteal), diagnosis, or the phase by diagnosis interaction (all p > .05).
PSG Data
Analyses of luteal phase change scores (night 1 – night 3) indicated AM and PM light produced essentially opposite changes in PSG parameters; e.g., AM light increased stage 4, delta sleep, REM and total sleep time minutes, while shortening sleep latency and REM onset time (see Table 1). AM light also delayed sleep end time to a greater degree in NC than PMDD (0.73±0.24 h vs. 0.0±0.20 h, p = .033).
Table 1.
Effects of AM vs. PM Light on Changes in PSG Variables [Min (±SD)]
| Total Sleep Time | Sleep Latency | Stage 4 | Delta Sleep | REM Onset Time | REM | |
|---|---|---|---|---|---|---|
| AM Light | 39.3 (3.0) | −4.5 (2.7) | 6.7 (2.9) | 6.0 (3.1) | −0.04 (1.3) | 21.5 (6.0) |
| PM Light | −33.8 (24.9) | 8.7 (5.2) | −7.2 (5.5) | −13.5 (6.0) | 13.6 (2.4) | −1.8 (11.5) |
| P | .016 | .035 | .036 | .009 | .001 | .047 |
Spectral Analyses of Sleep Data
Separate Fast-Fourier Transform (FFT) analyses were carried out on luteal and follicular phase data. There were no significant main effects or interactions on delta power change scores (night3– night1) for either AM or PM light, in either the luteal or follicular phases.
Phase-Angle Differences
Following methods of Lewy et al. (2006), we calculated phase angle difference (PAD) values representing the differences between mid-sleep time ((sleep offset time – sleep onset time)/2) and (a) melatonin onset time (PADon) and (b) melatonin offset time (PADoff) (represented schematically in Fig. 4).
Figure 4.
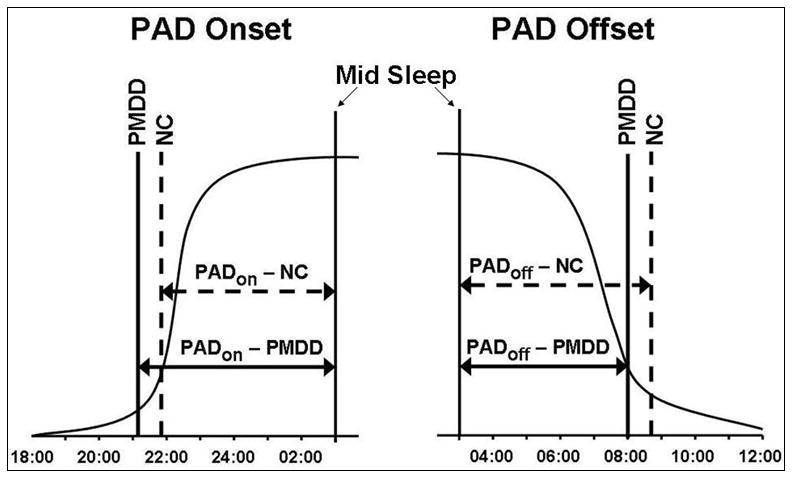
Schematic representation of differences between midsleep time ((sleep offset time sleep onset time –)/2) and melatonin onset time (PAD Onset), and melatonin offset time (PAD Offset).
AM Light Effects on PAD
In the follicular phase, no significant differences (p > .05) between NC and PMDD in baseline PAD scores or changes in PAD scores after exposure to AM light were found (data not shown). In the luteal phase, NC and PMDD groups did not differ significantly in PAD measures at baseline, prior to light exposure; see Table 2. AM light, however, reduced the PADon to a significantly smaller degree in PMDD than in NC (p = .049); thus, AM light advanced luteal melatonin onset, relative to midsleep, less in PMDD than in NC. Similar, but larger effects were found in PADoff, where AM light reduced the luteal midsleep-melatonin offset phase angle (PADoff) less in PMDD than in NC (p = .005; see Table 2). These group differences may be due, largely, to the fact that AM light delayed sleep end time more in NC than in PMDD subjects (0.73 vs. −0.01 h, respectively; p = .033).
Table 2.
Melatonin-Sleep Phase Angle Difference (PAD) [(midsleep – melatonin onset time), (midsleep – melatonin offset time)] (h±SD) before (Night 1) and after (Night 3) exposure to luteal AM light, in NC and PMDD subjects. Negative values indicate that midsleep follows melatonin onset; positive values indicate that midsleep precedes melatonin offset. The dPAD values represent differences between Night 3 and Night 1. P-values indicate significance of differences between NC and PMDD.
| Light Condition | Group |
PAD Onset Night1 |
PAD Onset Night3 |
dPAD Onset |
PAD Offset Night1 |
PAD Offset Night3 |
dPAD 0ffset |
|---|---|---|---|---|---|---|---|
| AM LIGHT | NC | -5.28(2.22) | −6.17 (2.60) | −0.89 (0.90) | 4.99 (0.94) | 4.00 (1.14) | −0.99 (0.70) |
| PMDD | −5.55 (1.61) | −5.63 (1.56) | −0.07(0.85) | 4.85 (2.02) | 5.18 (1.55) | 0.33 (1.17) | |
| P | 0.766 | 0.562 | 0.049 | 0.835 | 0.084 | 0.005 |
PM Light Effects on PAD
In the follicular phase, PM light decreased the phase-angle difference between melatonin onset and midsleep (dPADon) more in PMDD than in NC (2.26 vs. −0.38 h, p = .016), while increasing the difference between midsleep and melatonin offset time (dPADoff) more in PMDD than NC (1.76 vs. −0.38 h, p = .013). Luteal phase differences were not statistically significant (data not shown).
Reproductive Hormones
Estradiol and progesterone levels did not differ between PMDD and NC groups. As expected, progesterone levels increased in the luteal phase (p = 0.006), documenting an ovulation cycle. LH and FSH were not differentially affected by diagnosis or testing night, nor were there any significant interactions between night, diagnosis, or phase (all p > .05). Neither AM nor PM light modified baseline levels of reproductive hormones (all p > .05).
Actillume Data
The mesor, amplitude and acrophase for light exposure and average activity data were determined by cosinor analyses using the Action3 software (Ambulatory Monitoring, Inc.). A repeated measures ANOVA on the effects of diagnosis and phase (night 1 follicular vs. night 1 luteal) showed the omnibus MANOVA was not significant for Diagnosis (p = .297), Phase (p = .100) or the Diagnosis x Phase interaction (p = .272), as well as for all univariate ANOVAs (all p > .05).
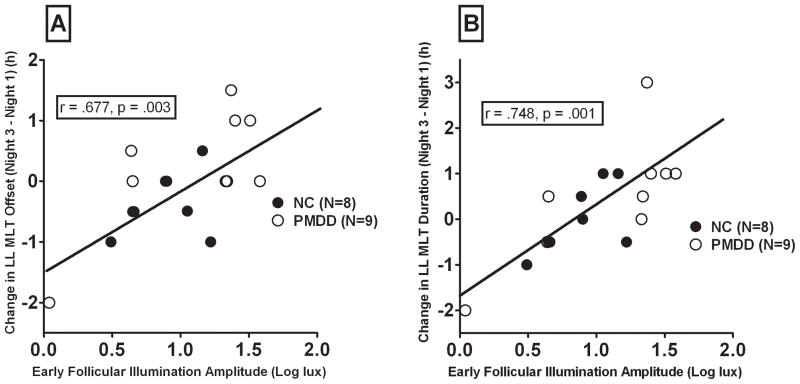
Consistent with our earlier findings, baseline follicular amplitude of illumination (cosine function) was positively correlated with magnitude of AM light-induced phase shifts in melatonin offset (r = .677, p = .003) and melatonin duration (r = .749, p = .001; see Fig. 5). Similarly, baseline follicular illumination mesor was positively correlated with magnitude of AM light-induced phase shifts in melatonin duration (r = .594, p = .012) and marginally with melatonin offset (r = .466, p = .060). Thus, the greater the mean daily illumination levels at baseline (prior to light interventions), the greater the magnitude of light-induced phase-shift responses (Parry et al., 1997b).
Figure 5.
Magnitude of change in late luteal melatonin (LL MLT) offset (A) and duration(B) after morning lightinrelation to follicular illumination amplitude (measured by Actillume) at baseline.
Mood Data
Analyses of baseline HRSD scores plus atypical and mania items, and BDI scores confirmed mood was significantly more depressed (p < .05, at least) in PMDD than in NC subjects during the late luteal phase; as expected, NC and PMDD women did not differ significantly during the follicular phase (p > .05). A comparison of HRSD and BDI measures, before and after luteal one-day AM or PM light exposure showed no significant effects of the exposure to light on mood; i.e., light exposure did not change mood measures, relative to baseline, in subjects receiving either AM or PM light (all p > .05). Pearson correlations relating mood measures (HRSD, Atypical, BDI) and melatonin timing and quantitative measures were non-significant (all p > .05).
DISCUSSION
The primary finding of this study is that, compared with NC subjects, women with PMDD showed a blunted phase-advance response of particularly melatonin offset time to bright AM light in the luteal, but not in the follicular, menstrual cycle phase, a result that replicated our earlier work (Parry et al., 1997b).
This lack of responsiveness to the phase-shifting effects of morning light compromises the ability of PMDD patients to entrain their internal circadian rhythms to the external environment and to synchronize other internal circadian rhythms with each other. As a result, these dysregulated circadian rhythms may contribute to the development of mood disturbances occurring in the luteal phase (Wirz-Justice, 2006).
For adaptive phase-shift responses to occur, adequate serotonin, melatonin and gonadal steroids may be required. Previous work in rodents showed that deficits in serotonergic control of circadian function in aging contributed to the decrease in light-induced phase-advances occurring then (Penev et al., 1997; Penev et al., 1995). Perhaps the serotonergic deficits in PMDD (Parry, 2001) likewise may contribute to the decrease in light-induced phase advances in the luteal phase in these symptomatic women. Most of the studies indicating serotonergic deficits in PMDD, however, find that the deficiency is present in both the asymptomatic follicular, as well as the symptomatic luteal, phase, thus indicating that serotonin abnormalities are more of a trait, rather than state, maker for the illness (Parry, 2001). As estrogen and progesterone modulate circadian rhythms (Leibenluft, 1993), their decline in the late luteal phase may diminish the serotonergic influence on phase-shift responses. Melatonin, as a regulator of internal circadian rhythms, is important in mediating phase-shift responses. We previously reported (Parry et al., 1990) and replicated (Parry et al., 1997a) low melatonin circadian rhythms in PMDD. The declining levels of gonadal steroids in the late luteal phase also may blunt the effects of melatonin in mediating phase-shift responses.
The multitude of menstrual cycle phase and circadian phase-response abnormalities in PMDD may be associated with an overall sub-sensitivity to the phase-shifting effects of light or confined to a particular time of day. Although our data on the phase-shifting effects of bright PM light are more limited than our data on the effects of AM bright light, the preliminary findings to date suggest that the blunted phase-shift responses occur more after AM, rather than PM light. In diurnal species such as humans, AM bright light has a more crucial role in synchronizing circadian rhythms. The circadian abnormalities may reflect a genuine alteration of the phase-response curve (PRC) in luteal-phase PMDD patients, indicating an abnormality of the underlying circadian pacemaker with extensive consequences on physiology and behavior.
There are several possible PRC-based explanations for the abnormal mixture of responses to AM light observed in PMDD patients. The primary finding of either a reduced or absent phase-advance response or an abnormally delayed phase-shift, particularly in melatonin offset time following a bright morning light stimulus, supports our hypothesis that PMDD subjects have an abnormal phase-shift response or an increased resistance to light-induced clock resetting, but the specific form of this difference is unknown. One possibility is that the PMDD PRC is sufficiently phase-delayed that the morning light pulse was administered at a time near the inflection point located between delay and advance portions of the PRC or even primarily in the delay region. Another, perhaps unlikely, possibility is that the PRC was so advanced that the light pulse fell in the “dead region” of the PRC. Alternatively, or additionally, PMDD subjects may have a PRC with an attenuated or abbreviated phase-advance region, or the entire PRC may have a low amplitude and/or irregular shape. The fact that offset of melatonin secretion, more than onset, showed a blunted phase-shift response to light further illustrates that the morning and evening oscillators are regulated independently (Illnerova and Vanecek, 1982).
The findings of decreased duration and AUC in the luteal compared with the follicular menstrual cycle phase replicate our previous findings in PMDD (Parry et al., 1997a). If melatonin is needed for a sufficient phase-shift response and to regulate other internal circadian rhythms, that AUC decreased more in PMDD than in NC as a consequence of the transition from the follicular to the luteal phase suggests that this decreased luteal phase melatonin may represent a vulnerability factor in PMDD.
The results from the PAD studies indicate that PMDD patients show smaller phase-shift responses in melatonin in relation to sleep after AM light than NC subjects and that phase-angle changes induced by AM light exposure are greater relative to melatonin offset than to onset. These findings also are consistent with our previous work in PMDD after 7 days of light treatment (Parry et al., 1997a; Parry et al., 1997b). Our collaborators found similar results with PAD measures using urinary melatonin (Kripke et al., 2007). Although the findings from the PM light studies included a relatively small sample size, the results based on the PAD analyses suggest that PMDD patients more readily delay their onset and offset times to PM light in relation to mid-sleep times, affecting the phase-angle differences. This tendency to phase-delay in PMDD patients (even when asymptomatic in the follicular phase) characterizes other depressed populations (Meliska et al., 2009).
The findings from the sleep studies suggest a more potent effect of bright AM vs. PM light on improving measures of sleep quality, and the more extensive effect of delaying sleep offset time in NC vs. PMDD patients. The shorter, more intense (3 h of 6,000 lux vs. 6 h of 3,000 lux) light pulse had greater effects in advancing melatonin offset, although these differences were confined to the follicular phase. In contrast, the blunted phase-advance responses to morning bright light in PMDD patients in the symptomatic luteal, but not in the asymptomatic follicular menstrual cycle phase, the primary finding in this study, suggests that this abnormality is a state, rather than trait, marker for the illness. Taken together with the findings of decreased luteal phase melatonin duration and AUC, the results suggest that PMDD patients may exhibit premature forms of aging in their decreased quantity of melatonin and their compromised ability to respond to important morning bright light cues. These vulnerabilities become manifest in the luteal phase, impairing their ability to entrain their internal circadian rhythms with the environment and regulate them internally with each other. Consequently, mood disturbances may result.
The clinical and treatment implications of these findings are that women with PMDD may require stronger zeitgebers or stimuli to entrain the circadian system in the luteal phase. These effects could be accomplished by administering chronobiological treatments such as more prolonged (for more than one day) bright morning light or one-night of late, but not early, wake therapy (therapeutic critically-timed partial sleep deprivation) (Parry et al., 2008a) that phase-advance melatonin offset time.
The limitations of this study include the relatively small sample size, particularly in the PM light conditions, the fact that we were not able to test phase-shift responses at other times of the day, and our not using constant routine conditions, which would be impractical in this population of women with family, work, or educational responsibilities (and the sleep deprivation components of which would confound results in patients with a depressive disorder). The relatively high homogeneity of the samples we studied also may limit the generalizability of the findings. Further work is needed to investigate whether mood response is related to the phase-shift or to the phase-angle difference (PAD) change between different circadian rhythms, as only one night of a light pulse used in this study may not be sufficient to alter mood in depressed patients.
Acknowledgments
This work was supported by NIH grant RO1 MH063462 and NIH Clinical Research Center (CRC) grant M01 RR00827. We thank Alan Turken, B.S., for his excellent work in performing the melatonin assays.
References
- Aitken RC. Measurement of feelings using visual analogue scales. Proc R Soc Med. 1969;62:989–93. doi: 10.1177/003591576906201005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DC, Hopper BR, Lasley BL, Yen SS. A simple method for the assay of eight steroids in small volumes of plasma. Steroids. 1976;28:179–96. doi: 10.1016/0039-128x(76)90108-2. [DOI] [PubMed] [Google Scholar]
- APA. Diagnostic and Statistical Manual of Mental Disorders. 4. American Psychiatric Association; Washington, D. C: 1994. [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Benloucif S, Burgess HJ, Klerman EB, Lewy AJ, Middleton B, Murphy PJ, Parry BL, Revell VL. Measuring melatonin in humans. J Clin Sleep Med. 2008;4:66–9. [PMC free article] [PubMed] [Google Scholar]
- Benloucif S, Green K, L'Hermite-Baleriaux M, Weintraub S, Wolfe LF, Zee PC. Responsiveness of the aging circadian clock to light. Neurobiol Aging. 2006;27:1870–9. doi: 10.1016/j.neurobiolaging.2005.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benloucif S, Masana MI, Dubocovich ML. Light-induced phase shifts of circadian activity rhythms and immediate early gene expression in the suprachiasmatic nucleus are attenuated in old C3H/HeN mice. Brain Res. 1997;747:34–42. doi: 10.1016/s0006-8993(96)01182-1. [DOI] [PubMed] [Google Scholar]
- Brzezinski A, Lynch HJ, Seibel MM, Deng MH, Nader TM, Wurtman RJ. The circadian rhythm of plasma melatonin during the normal menstrual cycle and in amenorrheic women. J Clin Endocrinol Metab. 1988;66:891–5. doi: 10.1210/jcem-66-5-891. [DOI] [PubMed] [Google Scholar]
- First MB, Gibbon M, Spitzer RL, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders- research version Biometerics Research Dept. New York State Psychiatric Institute; New York: 1995. [Google Scholar]
- Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967;6:278–96. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- Hurt SW, Schnurr PP, Severino SK, Freeman EW, Gise LH, Rivera-Tovar A, Steege JF. Late luteal phase dysphoric disorder in 670 women evaluated for premenstrual complaints. Am J Psychiatry. 1992;149:525–30. doi: 10.1176/ajp.149.4.525. [DOI] [PubMed] [Google Scholar]
- Illnerova H, Vanecek J. Two-oscillator structure of the pacemaker controlling the circadian rhythm of N-acetyltransferase in the rat pineal gland. J Comp Physiol. 1982;145:539–548. [Google Scholar]
- Klerman EB, Duffy JF, Dijk DJ, Czeisler CA. Circadian phase resetting in older people by ocular bright light exposure. J Investig Med. 2001;49:30–40. doi: 10.2310/6650.2001.34088. [DOI] [PubMed] [Google Scholar]
- Kolker DE, Fukuyama H, Huang DS, Takahashi JS, Horton TH, Turek FW. Aging alters circadian and light-induced expression of clock genes in golden hamsters. J Biol Rhythms. 2003;18:159–69. doi: 10.1177/0748730403251802. [DOI] [PubMed] [Google Scholar]
- Kripke DF, Elliott JA, Youngstedt SD, Rex KM. Circadian phase response curves to light in older and young women and men. J Circadian Rhythms. 2007;5:4. doi: 10.1186/1740-3391-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibenluft E. Do gonadal steroids regulate circadian rhythms in humans? J Affect Disord. 1993;29:175–81. doi: 10.1016/0165-0327(93)90031-e. [DOI] [PubMed] [Google Scholar]
- Lewy AJ, Cutler NL, Sack RL. The endogenous melatonin profile as a marker for circadian phase position. J Biol Rhythms. 1999;14:227–36. doi: 10.1177/074873099129000641. [DOI] [PubMed] [Google Scholar]
- Lewy AJ, Lefler BJ, Emens JS, Bauer VK. The circadian basis of winter depression. Proc Natl Acad Sci U S A. 2006;103:7414–9. doi: 10.1073/pnas.0602425103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meliska CJ, Parry BL, Lopez A, Martinez LF, Sorenson D, Nowakowski S. Horne-Ostberg Morningness-Eveningness Questionnaire (MEQ) predicts melatonin onset time, sleep end time and body mass index in peri- and post-menopausal depressed and normal control women (Abstract) Menopause. 2009;16:1250. [Google Scholar]
- Parry BL. The role of central serotonergic dysfunction in the aetiology of premenstrual dysphoric disorder: therapeutic implications. CNS Drugs. 2001;15:277–85. doi: 10.2165/00023210-200115040-00003. [DOI] [PubMed] [Google Scholar]
- Parry BL, Berga SL, Kripke DF, Klauber MR, Laughlin GA, Yen SS, Gillin JC. Altered waveform of plasma nocturnal melatonin secretion in premenstrual depression. Arch Gen Psychiatry. 1990;47:1139–46. doi: 10.1001/archpsyc.1990.01810240059010. [DOI] [PubMed] [Google Scholar]
- Parry BL, Berga SL, Mostofi N, Klauber MR, Resnick A. Plasma melatonin circadian rhythms during the menstrual cycle and after light therapy in premenstrual dysphoric disorder and normal control subjects. J Biol Rhythms. 1997a;12:47–64. doi: 10.1177/074873049701200107. [DOI] [PubMed] [Google Scholar]
- Parry BL, Meliska CJ, Martinez LF, Lopez A, Sorenson D, Hauger R, Elliott JA. Late, but not early, wake therapy reduces morning plasma melatonin: Relationship to mood in Premenstrual Dysphoric Disorder. Psychiatry Res. 2008a;161:76–86. doi: 10.1016/j.psychres.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry BL, Meliska CJ, Sorenson DL, Lopez A, Martinez LF, Hauger RL, Elliott JA. Increased sensitivity to light-induced melatonin suppression in premenstrual dysphoric disorder. Chronobiol Int. 2010;27:1438–53. doi: 10.3109/07420528.2010.503331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry BL, Meliska CJ, Sorenson DL, Lopez AM, Martinez LF, Nowakowski S, Hauger RL, Elliott JA. Increased melatonin and delayed offset in menopausal depression: role of years past menopause, follicle-stimulating hormone, sleep end time, and body mass index. J Clin Endocrinol Metab. 2008b;93:54–60. doi: 10.1210/jc.2006-2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry BL, Udell C, Elliott JA, Berga SL, Klauber MR, Mostofi N, LeVeau B, Gillin JC. Blunted phase-shift responses to morning bright light in premenstrual dysphoric disorder. J Biol Rhythms. 1997b;12:443–56. doi: 10.1177/074873049701200506. [DOI] [PubMed] [Google Scholar]
- Penev PD, Zee PC, Turek FW. Serotonin in the spotlight. Nature. 1997;385:123. doi: 10.1038/385123a0. [DOI] [PubMed] [Google Scholar]
- Penev PD, Zee PC, Wallen EP, Turek FW. Aging alters the phase-resetting properties of a serotonin agonist on hamster circadian rhythmicity. Am J Physiol. 1995;268:R293–8. doi: 10.1152/ajpregu.1995.268.1.R293. [DOI] [PubMed] [Google Scholar]
- Portaluppi F, Smolensky MH, Touitou Y. Ethics and methods for biological rhythm research on animals and human beings. Chronobiol Int. 2010;27:1911–29. doi: 10.3109/07420528.2010.516381. [DOI] [PubMed] [Google Scholar]
- Rosenthal NE, Heffernan MM. Bulimia, carbohydrate craving and depression: a central connection? In: Wurtman RJ, Wurtman JJ, editors. Nutrition and the Brain. New York: Raven Press; 1986. [Google Scholar]
- Roy-Byrne PP, Rubinow DR, Hoban MC, Parry BL, Rosenthal NE, Nurnberger JI, Byrnes S. Premenstrual changes: a comparison of five populations. Psychiatry Res. 1986;17:77–85. doi: 10.1016/0165-1781(86)90062-4. [DOI] [PubMed] [Google Scholar]
- Williams JB, Link MJ, Rosenthal NE, Amira L, Terman M. Structured Interview Guide for the Hamilton Depression Rating Scale, Seasonal Affective Disorders Version (SIGH-SAD), revised edition. New York Psychiatric Institute; New York: 1994. [Google Scholar]
- Wirz-Justice A. Biological rhythm disturbances in mood disorders. Int Clin Psychopharmacol. 2006;21(Suppl 1):S11–5. doi: 10.1097/01.yic.0000195660.37267.cf. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Kornhauser JM, Zee PC, Mayo KE, Takahashi JS, Turek FW. Effects of aging on light-induced phase-shifting of circadian behavioral rhythms, fos expression and CREB phosphorylation in the hamster suprachiasmatic nucleus. Neuroscience. 1996;70:951–61. doi: 10.1016/0306-4522(95)00408-4. [DOI] [PubMed] [Google Scholar]