Abstract
Chemoprevention of cancer via herbal and dietary supplements is a logical approach to combat cancer and presently it is an attractive area of research investigations. Over the years, the use of isothiocyanates, such as sulforaphane (SFN) found in cruciferous vegetables, has been advocated as chemopreventive agents and their efficacy has been demonstrated in cell lines and animal models. In-vivo studies with SFN suggest that besides protecting normal healthy cells from environmental carcinogens it also exhibits cytotoxicity and apoptotic effects against various cancer cell types. Among several mechanisms for the chemopreventive activity of SFN against chemical carcinogenesis, its effect on drug metabolizing enzymes that causes activation/ neutralization of carcinogenic metabolites is well established. Recent studies suggest that SFN exerts its selective cytotoxicity to cancer cells via reactive oxygen species (ROS)-mediated generation of lipid peroxidation (LPO) products particularly 4-hydroxynonenal (HNE). Against the background of the known biochemical effects of SFN on normal and cancer cells, in this article we have reviewed the underlying molecular mechanisms responsible for the overall chemopreventive effects of SFN focusing on the role of HNE in these mechanisms that may also contribute to its selective cytotoxicity to cancer cells.
Introduction
Cancer is one of the major causes of morbidity and mortality throughout the world. Carcinogenesis is a multistep molecular process induced by genetic and epigenetic changes that disrupt pathways controlling cell proliferation, apoptosis, differentiation, and senescence [1–4]. A major approach to fight against cancer is based on prevention of the disease through use of non-toxic dietary supplements, micronutrients, and natural products. This approach is generally referred to as chemoprevention that is defined as the use of natural or synthetic agents to inhibit, reverse, or prevent the development of cancer. The major goal of chemoprevention is to delay the onset of cancer as well as to decrease its incidence. Therefore, effective chemoprevention requires the use of non-toxic agents that inhibit specific molecular steps in the carcinogenic pathway. It has been advocated that vegetarian diet may be an important source of cancer-inhibiting bioactive phytochemicals. Although these phytochemicals are generally viewed as non-essential for normal body functioning, an increasing number of these agents have been shown to possess biological activities that are not only relevant for their ability to fight various diseases but also in the prevention of cancer [5–7]. In last couple of decades the efficacies of isothiocyanates, particularly those of sulforaphane isolated from the cruciferous vegetables, in cancer chemoprevention have been recognized and continue to be extensively studied for their pharmacological effects.
Occurrence and chemistry of isothiocyanates
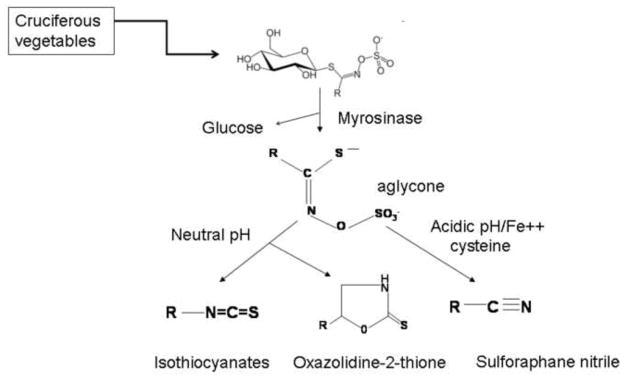
There is epidemiological evidence suggesting that dietary intake of cruciferous vegetables may reduce the risk of different types of malignancies, including the prostate cancer [8–11]. The anticarcinogenic effect of these cruciferous vegetables including broccoli has been attributed to the abundance of isothiocyanates (ITCs) in these plants. ITCs occur naturally as the thioglucoside conjugates (glucosinolates) in a variety of edible plants including watercress, broccoli and cabbage etc. Various ITCs can be released from the hydrolysis of their respective glucosinolates through catalytic action of myrosinase [Fig. 1]. For example, the principal glucosinolate present in broccoli is glucoraphanin, which is hydrolyzed by myrosinase to yield SFN. It has been shown [12] that the hydrolysis of glucosinolates by myrosinase is influenced by the pH. While at neutral pH, ITC is the dominant product, acidic pH lead to an enhanced formation of nitrile derivatives [Fig. 1]. Additionally, presence of ferrous ions (Fe2+) and epithiospecifier (ESP) protein also promotes the formation of nitrile during hydrolysis of glucosinolates and glucoaphanin respectively [13]. Most of the naturally occurring ITCs, including SFN, phenethyl-ITC (PEITC), and benzyl-ITC (BITC), have been shown to offer significant protection against cancer in animal models induced by a variety of chemicals including tobacco smoke-derived carcinogens [14,15]. It has been suggested that chemopreventive effects of different cruciferous plants may be influenced by the predominance of ITCs having characteristic side chains [14] such as methylsulfinyl-, benzyl-, 2-phenethyl-, methylthiopropyl and allyl groups [Table 1].
Figure 1.
Chemistry of naturally occurring isothiocyanates.
Table 1.
Isothiocyantes present in cruciferous vegetables
| Chemical Name | Structure | Vegetables |
|---|---|---|
| 4-methylsulfinylbutyl isothiocyanate (SFN) | CH3-S-CH2-CH2-CH2-CH2 N=C=S | Broccoli, Cauliflower |
| Benzyl isothiocyanate (BITC) | C6H5-CH2-N=C=S | Lepidium Cress |
| 2-Phenethyl isothiocyanate (PIETC) | C6H5-CH2-CH2-N=C=S | Water cress, Radishes, turnips |
| 3-Methylthiopropyl isothiocyanate | CH3-S-CH2-CH2-CH2-N=C=S | Cabbage |
| 4-Methylthiobutyl isothiocyanate | CH3-S-CH2-CH2-CH2-CH2-N=C=S | Arugula |
| 2-Propenyl isothiocyanate (AITC) | CH2=CH-CH2-N=C=S | Mustard, Cabbage, Cauliflower |
| 4-methylsulfinylbutyl isothiocyanate (SFN) | CH3-S-CH2-CH2-CH2-CH2 N=C=S | Broccoli, Cauliflower |
| Benzyl isothiocyanate (BITC) | C6H5-CH2-N=C=S | Lepidium Cress |
| 2-Phenethyl isothiocyanate (PIETC) | C6H5-CH2-CH2-N=C=S | Water cress, Radishes, turnips |
| 3-Methylthiopropyl isothiocyanate | CH3-S-CH2-CH2-CH2-N=C=S | Cabbage |
| 4-Methylthiobutyl isothiocyanate | CH3-S-CH2-CH2-CH2-CH2-N=C=S | Arugula |
| 2-Propenyl isothiocyanate (AITC) | CH2=CH-CH2-N=C=S | Mustard, Cabbage, Cauliflower |
Chemopreventive effects of Sulforaphane (SFN)
In vivo studies
Among the ITCs listed in Table 1, SFN has been studied more widely and is shown to provide significant protection against chemical carcinogenesis in rodent models [16–20]. For example, incidence, progression, and severity of dimethylbenz(a)anthracene (DMBA)-induced mammary tumors has been shown to be significantly reduced in mice pretreated with the extracts of broccoli sprouts [11, 21]. SFN isolated from broccoli also inhibited DMBA-induced preneoplastic lesions in mouse mammary glands, rat mammary tumors, benzo[a]pyrene (BaP)-induced fore stomach tumors in mice, and inhibited proliferation of human breast cancer cells by down regulating the expression of estrogen receptor α [22–25]. SFN effectively reduced the formation of colonic aberrant crypt foci in azoxymethane (AOM) treated rats and suppressed the growth of intestinal polyps in mice [26]. SFN has also been shown to inhibit skin tumor genesis by acting prior to its initiation stage in mice and also retard the growth of PC-3 human prostate cancer xenografts in nude mice [27]. Furthermore, it has been shown that SFN-mediated oxidative stress can activate pro-apoptotic signaling in cancer cells that may inhibit cancer progression [27, 28].
In vitro studies
In vitro studies suggest that SFN may selectively inhibit proliferation of cancer cells by targeting the factors that provide advantage to growth and motility of cancer cells. For example, hypoxia-inducible factor-1α (HIF-1α) protein often constitutively expressed in cancer cells and is believed to provide advantages to their growth and motility [29, 30], is targeted by SFN. Studies on the effect of SFN on oral carcinoma cell lines in vitro have shown that HIF-1α is down-regulated by SFN treatment in the human tongue squamous carcinoma cell line, Tca8113 [31]. It has been reported that SFN causes the enhancement of apoptosis through the inhibition of cyclooxygenase-2 expression and NFκB-DNA binding in the human bladder T24 cell line [32]. SFN was first thought to play only a blocking role in the prevention of carcinogenesis by inducing enzymes that are critical to the removal of carcinogens. Now there is overwhelming evidence that SFN suppresses tumor progression in all stages, including metastasis [32–40]. Studies in animal models as well as in vitro systems have provided evidence of the anti-metastatic activities of SFN for different types of carcinomas [33–36]. The mechanisms of these effects of SFN have not been investigated thoroughly. More recent studies demonstrate that SFN is capable of inhibiting angiogenesis, metastasis, neovascularization, pro-angiogenic signaling, basement membrane integrity and endothelial cell migration, and tube formation [37, 38]. These effects were associated with transcriptional down-regulation of vascular endothelial growth factor (VEGF), HIF-1α, c-Myc, and matrix metalloproteinase-2 (MMP-2). SFN also inhibited the proliferation and tubular formation of human umbilical vein endothelial cells on matrigel in vitro, and was responsible for suppression of MMP-9 activity and invasiveness of human MDA-MB-231 breast cancer cells [35–40]. SFN specifically targets cancer cells and prevent their proliferation. While these studies are consistent with the known chemopreventive effects of SFN, these findings also provide insight into the underlying mechanisms responsible for the selective cytotoxicity of SFN to cancer cells. It is possible that while SFN-mediated signaling for the induction of defense mechanisms such as those associated with Nrf2, HSF1, NFkB may contribute to the protective effect of SFN to normal cells from carcinogenic insult, SFN may also activate pro-apoptotic signaling specifically in cancer cells. Possible mechanisms that may be involved in chemopreventive functions of SFN are briefly discussed in the following sections.
Mechanisms of chemoprevention
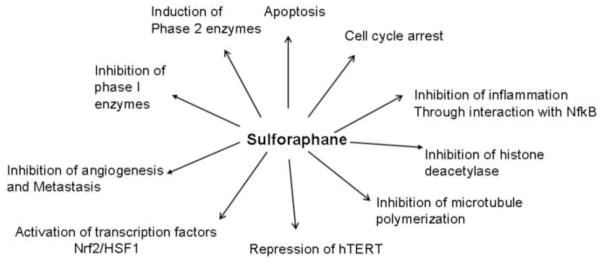
It was earlier believed that chemo preventive agents primarily inhibited chemical carcinogenesis by affecting the biotransformation enzymes of Phase I and Phase II and limiting the concentration of the ultimate carcinogens generated from procarcinogens. For example, the concentration of carcinogenic diol-epoxides generated from B(a)P can be minimized by inhibiting Phase I enzymes or by inducing Phase II enzymes [11,41]. There is ample evidence suggesting that SFN exerts at least some of its chemoprotective effects through the modulation of biotransformation enzymes. In addition, it can inhibit the development and proliferation of cancer cells by targeting various signaling pathways and the factors that provide advantage for the initiation, promotion, progression, and metastasis of cancer cells. The known biological activities of SFN are illustrated in Fig. 2.
Figure 2.
Biological activities of Sulforaphane.
Effect of SFN on biotransformation enzymes
Inhibition of Phase I cytochrome P450
It has been shown that SFN can modulate Phase I metabolism of xenobiotics through direct interactions with cytochrome P450 (CYP) enzymes or by regulating their transcript levels in cells. A dose dependent inhibition of CYP1A1 and CYP2B1/2 by SFN has been seen in rat hepatocytes. SFN has been shown to decrease the activity of CYP3A4 by suppressing its mRNA levels in human hepatocytes and there is additional indirect evidence indicating that SFN modulates the activity of various CYP enzymes [42–46]. Thus, SFN may at least partly exert its chempreventitive effect by protecting the normal cells from chemical carcinogenesis by inhibiting the activation of procarcinogens by cytochrome P450 enzymes.
Activation of Phase II enzymes
Induction of Phase II detoxification enzymes is perhaps one of the major mechanisms through which many chemopreventive agents including SFN inhibit carcinogenesis [40, 47]. Over the past decade SFN has received much attention in cancer chemoprevention as it has been shown to be among the most potent naturally occurring inducers of Phase II enzymes, where a strong inverse relationship exists between tissue levels of these enzymes and susceptibility to chemical carcinogenesis. Phase II enzymes in general catalyze the conjugation of various metabolites generated in Phase I biotransformation to endogenous ligands such as GSH and glucuronic acid for their elimination [40, 47, 48]. However, this classification of Phase II enzymes is further expanded to include enzymes that catalyze a wide variety of reactions to provide protection against the toxicity of various electrophiles and reactive oxygen species (ROS) [41, 49–51]. ITCs form conjugates with GSH (GS-ITCs) that are subsequently metabolized to mercapturic acids (N-acetyl cysteine conjugates of corresponding ITC) to be excreted in the urine. Similar to other xenobiotic substrates of GSTs, various ITCs also induce GSTs to varying degrees and the accelerated detoxification of electrophilic carcinogens has been suggested to be one of the major mechanisms for their protective effect against chemical carcinogens [49–53].
Besides, glutathione S-transferases (GSTs), and UDP- glucuronosyl transferases (UGTs), SFN is also a very potent inducer of quinone reductase (NAD[P]H): quinone oxidoreductase (NQO) [52]. The induction of Phase II gene expression and enzyme activity by SFN has been shown in a number of model cell lines of different origin, the most commonly utilized being those derived from liver hepatoma, human HepG2, and mouse Hepa1c1c7 [53–56]. SFN and its GSH-conjugates have been shown to promote a significant increase in both UGT1A1 and GSTA1 mRNA levels in HepG2 and HT29 cells. Up to three fold induction of NQO1 has been reported in Hepa1c1c7 cells exposed to increasing levels of SFN for 24h [57]. The enzymatic activities of GST, NQO1, aldo-keto reductase (AKR), and glutathione reductase (GR) in various cancer cell lines (HepG2, MCF7, MDA-MB-231, LNCaP, HeLa and HT-29) are increased by 11 to 17 fold by non-toxic doses of SFN [58–61]. In another study SFN has been shown to significantly induce the activity and expression of Phase II enzymes in the human prostate cell lines LNCaP, MDA PCa 2a, MDA PCa 2b, PC-3 and TSU-Pr1[49]. SFN caused a robust and sustained transcriptional induction of NQO1 gene expression in these cells that was accompanied by an increase in corresponding enzymatic activity. More recently, induction of GSTs and NQO1 has also been reported in cultured bladder cancer cells [52]. Induction of NQO1 may be particularly important to the mechanisms through which SFN provides protection to neighboring normal cells from oxidative stress.
Activation of Phase II enzymes by SFN is not only in cancer cells but also in their normal counterpart and non-transformed cell lines. For example, highly induced levels of NQO1 protein have been detected in the non-transformed rat RL34 epithelial cell line. SFN also induces expression of GST A1-1, A2-2 isoforms and NQO1 in primary rat hepatocytes in a dose and time-dependent manner, although prolonged treatment is required to obtain GST induction levels comparable to those obtained in hepatoma cell lines [43]. Similar results have been reported in primary cultures of freshly isolated human hepatocytes where NQO1 gene expression was induced by SFN without any significant effect on GSTA1 transcription. It has also been reported that SFN induces UDPG1A1 and GSTA1 mRNA expression in human hepatocytes, although UGT1A1 induction was found to be a matter of inter-individual variation [43, 53]. Similar to other ITCs, SFN also causes the induction of Phase II enzymes in vivo. Increased Phase II enzyme activities have been observed in the liver, lung, mammary gland, pancreas, stomach, small intestine and colon of rats and mice treated with SFN [22–28, 40, 42–52]. These studies suggest that SFN-mediated induction of Phase II enzymes not only attenuates the levels of activated carcinogens but also provides protection against oxidative stress and these effects collectively contribute to its chemopreventive activity. It must be realized that induction of phase II enzymes should also offer protection against electrophilic stress to cancer cells that would be a deterrent to chemotherapy due to the accelerated detoxification of electrophilic chemotherapeutic agents. In fact many drug resistant cancer cell line over express GST isozymes, particularly GSTP1-1. Thus the selective cytotoxicity of SFN to various cancer cells reported in recent studies appears to be independent of its effect on biotransformation enzymes. As mentioned above and elaborated later in article, SFN seems to exert its selective toxicity to cancer cells by targeting genes/proteins that provide growth advantage to cancer cells and it is likely that the oxidative stress induced by SFN via the generation of ROS plays a major role in these mechanisms.
Role of Reactive Oxygen Species (ROS) in chemopreventive activity of SFN
Oxidative stress is a cellular imbalance between production and elimination of ROS and accumulation of these species has been implicated in several mammalian patho-physiologies [62, 63]. It is well established that exogenous or endogenous electrophilic compounds induce oxidative stress in aerobic organisms because of the generation of ROS and reactive nitrogen species. It has been shown that the treatment of cells with purified SFN results in the generation of ROS and induction of ROS-mediated signaling that may contribute to at least some of its chemopreventive properties [63–65]. Recent studies have shown that the SFN-induced generation of ROS in U937 cells was evident as early as 2h after treatment, and that it caused the loss of mitochondrial membrane potential (MMP) suggesting that ROS-transduced signaling for apoptosis may be responsible for SFN-induced cell death [66]. These findings were further validated by quenching of ROS generation with N-acetylcysteine, which not only prevented ROS generation but also conferred near-complete protection against SFN-induced MMP disruption, and apoptosis [66]. Recent investigations suggest that damaged mitochondria stimulate increase in ROS, with subsequent activation of signaling pathways that control cancer cell growth. SFN treatment also leads to an increased ratio of Bax/Bcl2, release of cytochrome C, and subsequent activation of caspase3 in these cells further suggesting that ROS-mediated loss of MMP contributes to the activation of apoptotic signaling in cancer cell types [27, 66, 67]. These studies strongly suggest a role of ROS in SFN-induced signaling that may be relevant to its chemopreventive properties and its selective toxicity to cancer cells. For example, it has been shown that SFN induces cell cycle arrest, and apoptosis in cancer cells (LnCap, PC3) but not in normal cells (Pr-Ec). Likewise, SFN is reported to inhibit growth, activate apoptosis, up regulate HDAC activity, and suppress expression of key proteins involved in breast cancer progression. Thus ROS may play a dual role in chemoprotective activity of SFN by protecting normal cells from electrophilic stress through induction of defense mechanisms and also specifically inhibiting the growth and proliferation of cancer cells. As discussed later in this review, our recent studies demonstrate that many of the apoptotic signaling effects of SFN described above can be abrogated by inhibiting SFN-induced LPO and accumulation of HNE in cells indicating a major role of HNE in the mechanisms of the biological activity of SFN [68].
Role of Nrf2 in the SFN-induced chemoprevention
The transcription of ARE-driven genes is regulated, at least in part, by the nuclear factor (erythroid derived 2)-like 2 (Nrf2) which, under normal conditions, is sequestered in cytoplasm by Kelch-like ECH associated protein 1 (Keap1) [69–72]. Upon exposure of cells to inducers of oxidative stress and certain chemopreventive agents such as SFN, Nrf2 dissociates from Keap1, translocates to the nucleus, binds to antioxidant response element(s) (AREs), and transactivates Phase II detoxifying and antioxidant genes[70]. Consistent with the known induction of Phase II enzymes by SFN, many of the Nrf2-dependent genes were found to be SFN inducible as indicated by the results of comparative transcriptional profiles of the small intestine of Nrf2 (+/ +) and Nrf2 (−/−) female mice treated with SFN. Analysis of these gene expression profiles identified as many as 26 genes whose expression was Nrf2-dependent. These SFN-inducible genes included not only xenobiotic-metabolizing enzymes such as GSTs, but also GSH biosynthesizing and NADPH-generating enzymes [70, 72] that are crucial for defense against oxidative stress. This may suggest that many of the reported beneficial effects of SFN particularly in normal cells may be due to the induction of defense mechanisms against oxidative stress. Recently, several clusters of genes dependent on Nrf2 for their expression have been identified in the liver of SFN-fed wild-type and Nrf2-deficient mice by using gene chip microarrays [73]. The products of genes induced by SFN through an Nrf2-dependent pathway were classified as xenobiotic-metabolizing enzymes, antioxidants, ubiquitin/proteasome systems, stress response proteins, kinases and phosphatases, proteins related to immune response, cell adhesion, cell cycle and cell growth, metabolism, transport proteins and transcription factors [70, 73]. These findings suggest a much wider role of Nrf2 in the mechanisms of SFN activities besides the transcriptional activation of Phase II drug metabolizing and antioxidant enzymes. The effect of SFN on these signaling pathways associated with Nrf2 [70, 73] and its significance to the biological activities of SFN must be further investigated. In the context of oxidative stress, the activity of SFN clearly seems to be dichotomous. That is, while it causes ROS generation and oxidative stress in cells leading to the activation of pro-apoptotic signaling [27, 65], it simultaneously activates defense mechanisms e.g. Nrf2, and HSF1 for protection against oxidative stress and its own toxicity. The possibility of these dichotomous effects of SFN being responsible for its differential effects on normal and cancer cells should be explored. HNE generated during ROS-induced oxidative stress is also known to have such dichotomous effects [74–79]. HNE induces apoptosis in all cancer cell lines studied so far in our laboratory [79]. In addition HNE can also simultaneously activate the defense mechanisms against oxidative stress including Nrf2 and HSF1 in cell lines as well as in mice in vivo [74–76]. These studies together with our recent findings [68] that many of the signaling effects of SFN including apoptosis are blocked in cells transfected with GSTA1-1 or GSTA4-4, where formation and accumulation of HNE is inhibited, strongly suggest a key role of HNE in the mechanisms of SFN- induced signaling and chemoprevention [Fig. 3]. This is further elaborated in the following sections.
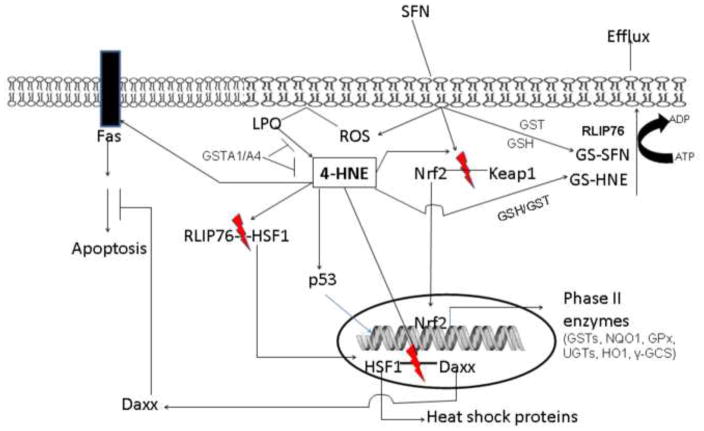
Figure 3.
ROS generated on exposure of SFN induces LPO [68] that leads to HNE formation. In turn, HNE causes activation and nuclear translocation of Nrf2, HSF1 and p53 [73–75] leading to enhanced transcription of genes associated with these transcription factors. Many of these genes contribute to the pro-survival pathways by inducing defense mechanisms against oxidative stress and enhanced detoxification of activated carcinogens/toxicants. Simultaneously, HNE can induce apoptosis in cancer cells through multiple pathways that may be self-regulated by HNE-mediated translocation of the transcription repressor Daxx which inhibits Fas-mediated apoptosis to protect neighboring cells from “run away” apoptosis. Thus SFN seems to provide protection against cancer by interrupting the initiation of cancer mediated by accelerating the detoxification of potential carcinogens as well as by killing cancer cells to prevent its progression.
HNE mediated signaling and its relevance to cancer
SFN mediated generation of ROS induces membrane LPO and generation of HNE [68] that is an inevitable consequence of ROS-induced stress [78–87]. Also as discussed above, there is ample evidence that the electrophilic products of LPO including lipid hydroperoxides and α, β-unsaturated carbonyls particularly, HNE play a crucial role in stress-induced apoptotic signaling [74–79, 82–87]. In recent years, HNE has emerged as an important second messenger molecule involved in signaling for cell proliferation, cell cycle arrest, differentiation, apoptosis, and the regulation of the expression of a multitude of genes in cells of diverse origin [82–87]. HNE has also been shown to modulate survival and death signaling pathways in a concentration dependent manner by interacting with several signaling proteins [74–79, 82–85]. Our studies have shown that HNE induces Fas-mediated extrinsic, as well as p53-mediated intrinsic pathways of apoptosis in different cell types [74–76]. The relevance of HNE-mediated signaling to cancer is evident from numerous studies showing that HNE induces apoptosis in various cell types studied so far and that at low levels, it could also limit its own toxicity by activating mechanisms for cell survival [74–76]. This appears to be true for SFN as well [Fig. 3].
Many of the effects of HNE on cell signaling have been determined by regulating its intracellular levels by GSTs that play a major role in regulating the levels of HNE in cells [78,79,82–87]. Apart from catalyzing the conjugation of carcinogenic electrophiles to GSH, the alpha class GSTs, GSTA1-1 and GSTA2-2 attenuate LPO by catalyzing the GSH-dependent reduction of phospholipid hydroperoxides (PL-OOH) and fatty acid hydroperoxides (FA-OOH) through its Se-independent glutathione peroxidase (GPx) activity thereby terminating the autocatalytic chain of LPO reactions resulting in decreased formation of HNE [86,87]. In addition, the isozyme GSTA4-4 can limit HNE levels in cells by catalyzing its conjugation of GSH in a highly efficient manner. A multitude of studies have demonstrated that HNE concentration in mammalian cells is regulated by a coordinated action of GSTA4-4 that conjugates HNE to GSH and RLIP76 that transports the conjugate, GS-HNE, out of cells [82–90]. It has been shown that elevated HNE levels in cells cause apoptosis and cancer cells can evade apoptosis by the up regulation of GSTs and RLIP76 and consequent lowering of HNE levels. The inhibition of RLIP76-mediated ATP-dependent transport of GS-HNE results in apoptosis in all cancer cell types studied so far in our laboratory [91–94]. More importantly, complete and sustained remission of the xenografts of melanoma, colon, lung, kidney and pancreas xenografts in nude mice has been demonstrated by blocking the transport of GS-HNE that leads to an increase in HNE concentrations in cells [91–94]. These rather remarkable findings further underscore the significance of HNE to cancer. HNE can promote survival pathways at low concentrations (sub-physiologic) and at higher (supra-physiologic) concentrations it promotes apoptosis via multiple pathways. Available evidence from a multitude of studies suggests that a basal constitutive level of HNE may be required for the normal cellular processes [79, 84]. It has been suggested that under the conditions of oxidative stress when concentration of HNE rise above this basal constitutive window it induces the pro-apoptotic signaling, while depletion of HNE to levels below this physiological window promotes proliferation and transformation [84].
Overlap in SFN and HNE-induced signaling
Similar to SFN, HNE has also been shown to induce stress-responsive pro- survival factors, such as Nrf2, heat shock factor 1 (HSF1) and its client heat shock proteins, and EGFR, and the transcription repressor Daxx, that can inhibit Fas mediated apoptosis [74–76, 82–86]. Some of the biological activities of SFN that are shared by HNE are listed in Table 2. Generation of HNE upon SFN exposure could therefore contribute to its protective effects against cancer through two mechanisms. First, it can activate the survival mechanisms to protect normal cells and second, it can promote apoptosis in cancer cells to inhibit their proliferation. In this model initially generated low levels of HNE may induce survival mechanisms beneficial for the normal cells but sustained oxidative stress caused by SFN and increase in HNE levels may selectively kill cancer cells and prevent their proliferation by targeting the factors that provide advantage to cancer cells in their proliferation. Our recent studies with human erythroleukemic cells are in line with this idea and show that indeed some of the biological activities associated with the chemoprotective properties of SFN are mediated through HNE generated during SFN-induced oxidative stress, and that these activities of SFN could be inhibited by the over expression of alpha class GST isozymes that attenuate HNE levels in cells [77]. For example, SFN induced cytotoxicity, cell cycle arrest, and apoptosis in HL60 and K562 cells is inhibited by forced over-expression of GSTA1-1 in these cells due to the attenuation of LPO and suppression of intracellular HNE. The idea of the biological activity of SFN being mediated via HNE finds support in many studies showing that HNE is a common denominator in the mechanisms of ROS-mediated signaling [68, 70]. HNE per se is known to cause cell cycle arrest, apoptosis, and cytotoxicity to cells via necrosis [78, 79, 95], the effects that are common with SFN. The observed effects of GSTA1-1 over expression on inhibition of the biological activities of SFN associated with the suppression of HNE levels [68] strongly implicate HNE in the mechanisms of chemopreventive effects of SFN. While it is possible that the increased SFN conjugating activity of GSTA1-1-overexpressing cells may lower the actual concentration of SFN by its accelerated conjugation with GSH, no significant alteration in the GSH levels of SFN-treated empty vector and hGSTA1-transfected cells was observed in these studies [68] suggesting that the protective effect of GSTA1-1 against SFN toxicity was preferentially imparted through the inhibition of SFN-induced LPO and consequent lowering of HNE levels, rather than GST-GSH-mediated detoxification of SFN.
Table 2.
Some of the common biological effects of HNE and SFN.
| Biological Effects | References for HNE
|
References for SFN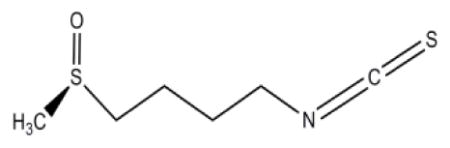
|
|
|---|---|---|---|
|
| |||
| 1 | Apoptosis | 74–77,82–87, 113. | 51, 63–66, 68, 97, 110–112, 119. |
| 2 | Induction of Bax, p21, Caspase3 | 75,79, 136. | 66, 135, 137. |
| 3 | Loss of Membrane Potential. | 138. | 65, 66, 137. |
| 4 | Cell Cycle Arrest | 113,117, 130 | 51, 65, 97, 105–107, 110, 112. |
| 5 | Interaction with NfkB | 123–126,129. | 51, 116, 120. |
| 6 | Inhibition of HDAC | 117, unpublished studies in our laboratory. | 109, 110, 118. |
| 7 | Activation of Nrf2 | 121,126. | 51, 73, 119, 120. |
| 8 | Activation of HSF1 | 122, 127. | 68,128. |
| 9 | Activation of p53 | 74–76. | 134. |
| 10 | Induction of HSP70 | 74, 122. | 128. |
| 11 | Repression of hTERT | 114, 131 | 115, 132. |
| 12 | Induction of oxidative stress | 78,82–87, 130. | 64–66,68 |
| 13 | Activation of MAPK | 125. | 133. |
| 14 | Angiogenesis | 83. | 36–40. |
The effects of SFN similar to those in HL60 and K562 cells have also been previously reported with colon and prostate cancer cells [65, 66, 96]. These effects include the induction of cell cycle arrest and apoptosis. It has been shown that SFN can arrest cell cycle at different stages of its progression, a mechanism by which it can inhibit growth of cancer cells. Arrest of cells in G0/G1, G2/M and S phases upon treatment with SFN have been reported in breast, bladder, colon and prostate cancer [27, 97–102]. A number of mechanisms have been proposed for the SFN-induced cell cycle arrest in different cell types. Cyclins and cyclin-dependent kinase complexes play an important role in the mechanisms of cell cycle progression [103,104]. By binding to Cdk1/2, cyclin B1 can activate Cdk1/2 (cdc2) to facilitate its nuclear accumulation for mitotic initiation in the late G2 phase of mammalian cells. It has been suggested that while SFN-induced cell cycle arrest in the G2/M phase appears to be regulated by cell cycle-related proteins cyclin B1 and Cdk1, the arrest in G1 phase is mediated by the inhibition of cyclin D1 and DNA synthesis [105–107]. Another suggested mechanism through which SFN induces cell cycle arrest is via the up regulation of CDKI such as p21 and p27 [65, 66, and 68]. Additionally, the SFN –induced cell cycle arrest has also been attributed to the disruption of normal mitotic microtubule polymerization and histone acetylation [108,109]. As summarized in Table 2, HNE shares many of these biological activities of SFN. Interestingly, many of these effects of SFN on cell cycle progression can be attenuated by over expression of GSTA1-1 or GSTA4-4 in cells [68]. HNE may be a causative factor for SFN-induced apoptosis via mitochondrial apoptotic pathways because in GSTA1-1-overexpressing cells SFN fails to induce apoptosis and unlike the wild type cells, HNE levels do not increase in these cells upon SFN treatment. In GSTA1-1-overexpressing cells, SFN-induced translocation of Bax to mitochondria, a pro apoptotic signal, is inhibited and anti-apoptotic signaling is activated as indicated by activation of Bcl-xL [68]. Furthermore, in GSTA1-1-overexpressing cells, the release of SFN-induced cytochrome c to the cytosol and nuclear accumulation of AIF is also inhibited. These studies suggest that caspase3 independent apoptosis by SFN is also HNE dependent that would further indicate a role of HNE in the biological activities of SFN [68]. Thus, at least some of the chemopreventive properties of SFN appear to be associated with generation of ROS and the accumulation of HNE in cells.
Both, SFN and HNE promote nuclear translocation of HSF1 and induction of the expression of Hsp70. SFN-induced up regulation of heat shock proteins [68] most likely results from the reverse nuclear-cytoplasmic trafficking of the transcription factor HSF1, its repressor protein Daxx. It has been shown that HNE also induces the translocation of Daxx from nucleus to cytoplasm and that of HSF1 from cytoplasm to nucleus [74]. Likewise both, HNE and SFN induce nuclear translocation and activation of Nrf2. SFN induced nuclear translocation of Nrf2 is more pronounced in GSTA1-1 over expressing HL60 and K562 cells as compared to empty vector transfected cells [68]. If HNE is the causative factor for such translocation, one may expect lesser nuclear translocation of Nrf2 and HSF1 in SFN-treated GSTA1-1 over expressing cells. This apparent anomaly could perhaps be due to the concentration dependent opposite effects of HNE on survival signaling discussed above. It is possible that initial low levels of HNE generated during SFN exposure act as a sensor to induce translocation of Nrf2 and HSF1 in both the vector and GSTA1-1 over expressing cells as a survival mechanism. But whereas in GSTA1-1 over expressing cells, low levels of HNE that are required for the translocation are maintained, in vector transfected cells sustained higher accumulation of HNE leads to apoptosis and apparently a lesser nuclear accumulation of Nrf2 and HSF1. This postulate however remains to be confirmed through further studies and the constitutive levels of HNE in cells that would promote either proliferation or cell death need to be clearly established. Thus available evidence strongly suggests that perhaps HNE plays a crucial role in the mechanisms of the biological activities of SFN including its chemoprotective properties i.e., protection of normal cells against oxidative/electrophilic stress by up regulating defense mechanisms, and specific killing of cancer cells by targeting signaling of pathways that provide selective growth advantage to cancer cells. Further studies to validate this conjecture may help in developing novel approaches for the search of effective chemoprotective agents.
Highlights.
Sulforaphane present in cruciferous vegetables is a potent anti cancer agent.
SFN can protect from chemical carcinogenesis and can selectively kill cancer cells.
Toxicity of SFN is due to Reactive oxygen species mediated lipid peroxidation.
4 Hydroxynonenal (HNE) plays a major role in anti cancer activity of SFN.
Acknowledgments
Supported in part by the NIH grants ES012171, EY004396 (YCA) and CA77495 (SA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Anand P, Kunnumakkara AB, Kunnumakara AB, et al. Cancer is a Preventable Disease that Requires Major Lifestyle Changes. Pharm Res. 2008;25(9):2097–116. doi: 10.1007/s11095-008-9661-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Irigaray P, Newby JA, Clapp R, et al. Lifestyle-related factors and environmental agents causing cancer: an overview. Biomed Pharmacother. 2007;61(10):640–58. doi: 10.1016/j.biopha.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 3.Jaffe LF. Epigenetic theories of cancer initiation. Advances in cancer research. 2003;90:209–30. doi: 10.1016/s0065-230x(03)90007-8. [DOI] [PubMed] [Google Scholar]
- 4.López-Lázaro M. A new view of carcinogenesis and an alternative approach to cancer therapy. Molecular medicine. 2010;16(3–4):144–153. doi: 10.2119/molmed.2009.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soria JC, Kim ES, Fayette J, et al. Chemoprevention of lung cancer. Lancet Oncol. 2003;4:659–669. doi: 10.1016/s1470-2045(03)01244-0. [DOI] [PubMed] [Google Scholar]
- 6.Ikegami T, Matsuzaki Y, Shoda J, et al. The chemopreventive role of ursodeoxycholic acid in azoxymethane-treated rats: suppressive effects on enhanced group II phospholipase A2 expression in colonic tissue. Cancer Lett. 1998;134:129–139. doi: 10.1016/s0304-3835(98)00248-1. [DOI] [PubMed] [Google Scholar]
- 7.Huber MH, Lee JS, Hong WK. Chemoprevention of lung cancer. Semin Oncol. 1993;20:128–141. [PubMed] [Google Scholar]
- 8.Wang LI, Giovannucci EL, Hunter D, Neuberg D, Su L, Christiani DC. Dietary intake of cruciferous vegetables, glutathione S-transferase (GST) polymorphisms and lung cancer risk in a Caucasian population. Cancer Causes Control. 2004;15:977–985. doi: 10.1007/s10552-004-1093-1. [DOI] [PubMed] [Google Scholar]
- 9.Brennan P, Hsu CC, Moullan N, Szeszenia-Dabrowska N, Lissowska J, Zaridze D, Rudnai P, Fabianova E, Mates D, Bencko V, Foretova L, Janout V, Gemignani F, Chabrier A, Hall J, Hung RJ, Boffetta P, Canzian F. Effect of cruciferous vegetables on lung cancer in patients stratified by genetic status: a mendelian randomisation approach. Lancet. 2005;366:1558–1560. doi: 10.1016/S0140-6736(05)67628-3. [DOI] [PubMed] [Google Scholar]
- 10.Fowke JH, Chung FL, Jin F, Qi D, Cai Q, Conaway C, Cheng JR, Shu XO, Gao YT, Zheng W. Urinary isothiocyanate levels, brassica, and human breast cancer. Cancer Res. 2003;63:3980–3986. [PubMed] [Google Scholar]
- 11.Fahey JW, Zhang Y, Talalay P. Broccoli sprouts: an exceptionally rich source of inducers of enzymes that protect against chemical carcinogens. Proc Natl Acad Sci USA. 1997;94:10367–10372. doi: 10.1073/pnas.94.19.10367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen S, Andreason E. Update on glucosinolate metabolism. Plant Physiol Biochem. 2001;39:743–758. [Google Scholar]
- 13.Fimognari C, Hrelia P. Sulforaphane as a promising molecule for fighting cancer. Mutat Res. 2007;635:90–104. doi: 10.1016/j.mrrev.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y, Talalay P. Anticarcinogenic activities of organic isothiocyanates: chemistry and mechanisms. Cancer Res. 1994;54:1976s–1981s. [PubMed] [Google Scholar]
- 15.Zhang Y, Talalay P, Cho CG, Posner GH. A major inducer of anticarcinogenic protective enzymes from broccoli: isolation and elucidation of structure. Proc Natl Acad Sci USA. 1992;89:2399–2403. doi: 10.1073/pnas.89.6.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu C, Huang MT, Shen G, Yuan X, Lin W, Khor TO, Conney AH, Tony Kong AN. Inhibition of 7,12- dimethylbenz(a)anthracene-induced skin tumorigenesis in C57BL/6 mice by sulforaphane is mediated by nuclear factor E2-related factor 2. Cancer Res. 2006;66:8293–8296. doi: 10.1158/0008-5472.CAN-06-0300. [DOI] [PubMed] [Google Scholar]
- 17.Singletary K, MacDonald C. Inhibition of benzo[a]pyrene- and 1,6-dinitropyrene-DNA adduct formation in human mammary epithelial cells by dibenzoylmethane and sulforaphane. Cancer Lett. 2000;155:47–54. doi: 10.1016/s0304-3835(00)00412-2. [DOI] [PubMed] [Google Scholar]
- 18.Prochaska HJ, Santamaria AB, Talalay P. Rapid detection of inducers of enzymes that protect against carcinogens. Proc Natl Acad Sci USA. 1992;89:2394–2398. doi: 10.1073/pnas.89.6.2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Y. Molecular mechanism of rapid cellular accumulation of anticarcinogenic isothiocyanates. Carcinogenesis. 2001;22:425–431. doi: 10.1093/carcin/22.3.425. [DOI] [PubMed] [Google Scholar]
- 20.Singh SV, Warin R, Xiao D, Powolny AA, Stan SD, Arlotti JA, Zeng Y, Hahm ER, Marynowski SW, Bommareddy A, Desai D, Amin S, Parise RA, Beumer JH, Chambers WH. Sulforaphane inhibits prostate carcinogenesis and pulmonary metastasis in TRAMP mice in association with increased cytotoxicity of natural killer cells. Cancer Res. 2009;69:2117–2125. doi: 10.1158/0008-5472.CAN-08-3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Conaway CC, Yang YM, Chung FL. Isothiocyanates as cancer chemopreventive agents: their biological activities and metabolism in rodents and humans. Curr Drug Metab. 2002;3:233–255. doi: 10.2174/1389200023337496. [DOI] [PubMed] [Google Scholar]
- 22.Fahey JW, Haristoy X, Dolan PM, Kensler TW, Scholtus I, Stephenson KK, Talalay P, Lozniewski A. Sulforaphane inhibits extracellular, intracellular, and antibiotic-resistant strains of Helicobacter pylori and prevents benzo[a]pyrene-induced stomach tumors. Proc Natl Acad Sci U S A. 2002;99:7610–7615. doi: 10.1073/pnas.112203099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramirez MC, Singletary K. Regulation of estrogen receptor alpha expression in human breast cancer cells by sulforaphane. J Nutr Biochem. 2009;20:195–201. doi: 10.1016/j.jnutbio.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 24.Cornblatt BS, Ye L, Dinkova-Kostova AT, et al. Preclinical and clinical evaluation of sulforaphane for chemoprevention in the breast. Carcinogenesis. 2007;28:1485–90. doi: 10.1093/carcin/bgm049. [DOI] [PubMed] [Google Scholar]
- 25.Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nature Rev Cancer. 2003;3:768–780. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- 26.Chung FL, Conaway CC, Rao CV, Reddy BS. Chemoprevention of colonic aberrant crypt foci in Fischer rats by sulforaphane and phenethyl isothiocyanate. Carcinogenesis. 2000;21:2287–2291. doi: 10.1093/carcin/21.12.2287. [DOI] [PubMed] [Google Scholar]
- 27.Singh AV, Xiao D, Lew KL, Dhir R, Singh SV. Sulforaphane induces caspase-mediated apoptosis in cultured PC-3 human prostate cancer cells and retards growth of PC-3 xenografts in vivo. Carcinogenesis. 2004;25:83–90. doi: 10.1093/carcin/bgg178. [DOI] [PubMed] [Google Scholar]
- 28.Chiao JW, Chung FL, Kancherla R, Ahmed T, Mittelman A, Conaway CC. Chemoprevention by sulforaphane its metabolite mediate growth arrest and apoptosis in human prostate cancer cells. Int J Oncol. 2002;20:631–636. doi: 10.3892/ijo.20.3.631. [DOI] [PubMed] [Google Scholar]
- 29.Huang LE, Willmore WG, Gu J, Goldberg MA, Bunn HF. Inhibition of hypoxia-inducible factor 1 activation by carbon monoxide, nitric oxide, Implications for oxygen sensing and signaling. J Biol Chem. 1999;274:9038–44. doi: 10.1074/jbc.274.13.9038. [DOI] [PubMed] [Google Scholar]
- 30.Chandel NS, Maltepe E, Goldwasser E, Mathieu CE, Simon MC, Schumacker PT. Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc Natl Acad Sci USA. 1998;95:11715–20. doi: 10.1073/pnas.95.20.11715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yao H, Wang H, Zhang Z, Jiang BH, Luo J, Shi X. Sulforaphane inhibited expression of hypoxia-inducible factor-1alpha in human tongue squamous cancer cells and prostate cancer cells. Int J Cancer. 2008;15:123(6):1255–61. doi: 10.1002/ijc.23647. [DOI] [PubMed] [Google Scholar]
- 32.Shan Y, Wu K, Wang W, Wang S, Lin N, Zhao R, Cassidy A, Bao Y. Sulforaphane down-regulates COX-2 expression by activating p38 and inhibiting NF-kappaB-DNA-binding activity in human bladder T24 cells. Int J Oncol. 2009;34(4):1129–34. doi: 10.3892/ijo_00000240. [DOI] [PubMed] [Google Scholar]
- 33.Xu K, Thornalley PJ. Studies on the mechanism of the inhibition of human leukaemia cell growth by dietary isothiocyanates and their cysteine adducts in vitro. Biochem Pharmacol. 2000;60:221–231. doi: 10.1016/s0006-2952(00)00319-1. [DOI] [PubMed] [Google Scholar]
- 34.Tang L, Zhang Y. Dietary isothiocyanates inhibit the growth of human bladder carcinoma cells. J Nutr. 2004;134:2004–2010. doi: 10.1093/jn/134.8.2004. [DOI] [PubMed] [Google Scholar]
- 35.Conaway CC, Wang CX, Pittman B, Yang YM, Schwartz JE, Tian D, McIntee EJ, Hecht SS, Chung FL. Phenethyl isothiocyanate and sulforaphane and their N-acetylcysteine conjugates inhibit malignant progression of lung adenomas induced by tobacco carcinogens in A/J mice. Cancer Res. 2005;65:8548–8557. doi: 10.1158/0008-5472.CAN-05-0237. [DOI] [PubMed] [Google Scholar]
- 36.Thejass P, Kuttan G. Antimetastatic activity of sulforaphane. Life Sci. 2006;78:3043–3050. doi: 10.1016/j.lfs.2005.12.038. [DOI] [PubMed] [Google Scholar]
- 37.Asakage M, Tsuno NH, Kitayama J, Tsuchiya T, Yoneyama S, Yamada J, Okaji Y, Kaisaki S, Osada T, Takahashi K, Nagawa H. Sulforaphane induces inhibition of human umbilical vein endothelial cells proliferation by apoptosis. Angiogenesis. 2006;9:83–91. doi: 10.1007/s10456-006-9034-0. [DOI] [PubMed] [Google Scholar]
- 38.Rose P, Huang Q, Ong CN, Whiteman M. Broccoli and watercress suppress matrix metalloproteinase-9 activity and invasiveness of human MDA-MB-231 breast cancer cells. Toxicol Appl Pharmacol. 2005;209:105–13. doi: 10.1016/j.taap.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 39.Bertl E, Bartsch H, Gerhauser C. Inhibition of angiogenesis and endothelial cell functions are novel sulforaphane-mediated mechanisms in chemoprevention. Mol Cancer Ther. 2006;5:575–85. doi: 10.1158/1535-7163.MCT-05-0324. [DOI] [PubMed] [Google Scholar]
- 40.Talalay P. Chemoprotection against cancer by induction of phase 2 enzymes. Biofactors. 2000;12:5–11. doi: 10.1002/biof.5520120102. [DOI] [PubMed] [Google Scholar]
- 41.Caldwell JA. Xenobiotic metabolism: mammalian aspectsACS Symp Ser. Am Chem Soc. 1986;299:2–28. [Google Scholar]
- 42.Barcelo S, Gardiner JM, Gescher A, Chipman JK. CYP2E1-mediated mechanism of anti-genotoxicity of the broccoli constituent sulforaphane. Carcinogenesis. 1996;17:277–282. doi: 10.1093/carcin/17.2.277. [DOI] [PubMed] [Google Scholar]
- 43.Maheo K, Morel F, Langouet S, Kramer H, Le Ferrec E, Ketterer B, Guillouzo A. Inhibition of cytochromes P-450 and induction of glutathione S-transferases by sulforaphane in primary human and rat hepatocytes. Cancer Res. 1997;57:3649–3652. [PubMed] [Google Scholar]
- 44.Barcelo S, Mace K, Pfeifer AM, Chipman JK. Production of DNA strand breaks by N-nitrosodimethylamine and 2-amino-3-methylimidazo[4,5-f]quinoline in THLE cells expressing human CYP isoenzymes and inhibition by sulforaphane. Mutat Res. 1998;402:111–120. doi: 10.1016/s0027-5107(97)00288-1. [DOI] [PubMed] [Google Scholar]
- 45.Yoxall V, Kentish P, Coldham N, Kuhnert N, Sauer MJ, Ioannides C. Modulation of hepatic cytochromes P450 and phase II enzymes by dietary doses of sulforaphane in rats: implications for its chemopreventive activity. Int J Cancer. 2005;117:356–362. doi: 10.1002/ijc.21191. [DOI] [PubMed] [Google Scholar]
- 46.Skupinska K, Misiewicz-Krzeminska I, Stypulkowski R, Lubelska K, Kasprzycka-Guttman T. Sulforaphane and its analogues inhibit CYP1A1 and CYP1A2 activity induced by benzo[a]pyrene. J Biochem Mol Toxicol. 2009b;23:18–28. doi: 10.1002/jbt.20259. [DOI] [PubMed] [Google Scholar]
- 47.Munday R, Munday CM. Induction of phase II detoxification enzymes in rats by plant-derived isothiocyanates: comparison of allyl isothiocyanate with sulforaphane and related compounds. J Agric Food Chem. 2004;52:1867–1871. doi: 10.1021/jf030549s. [DOI] [PubMed] [Google Scholar]
- 48.Petri N, Tannergren C, Holst B, Mellon FA, Bao Y, Plumb GW, Bacon J, O’Leary KA, Kroon PA, Knutson L, Forsell P, Eriksson T, Lennernas H, Williamson G. Absorption/metabolism of sulforaphane and quercetin, and regulation of phase II enzymes, in human jejunum in vivo. Drug Metab Dispos. 2003;31:805–813. doi: 10.1124/dmd.31.6.805. [DOI] [PubMed] [Google Scholar]
- 49.Brooks JD, Paton VG, Vidanes G. Potent induction of phase 2 enzymes in human prostate cells by sulforaphane. Cancer Epidemiol Biomarkers Prev. 2001;10:949–954. [PubMed] [Google Scholar]
- 50.Zhang Y, Marshall JR, Ambrosone CB. Cruciferous vegetables, genetic polymorphisms in glutathione S-transferases M1 and T1, and prostate cancer risk. Nutr Cancer. 2004;50:206–213. doi: 10.1207/s15327914nc5002_11. [DOI] [PubMed] [Google Scholar]
- 51.Juge N, Mithen RF, Traka M. Molecular basis for chemoprevention by sulforaphane: a comprehensive review. Cell Mol Life Sci. 2007;64:1105–1127. doi: 10.1007/s00018-007-6484-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang Y, Munday R, Jobson HE, Munday CM, Lister C, Wilson P, Fahey JW, Mhawech-Fauceglia P. Induction of GST and NQO1 in cultured bladder cells and in the urinary bladders of rats by an extract of broccoli (Brassica oleracea italica) sprouts. J Agric Food Chem. 2006;54:9370–9376. doi: 10.1021/jf062109h. [DOI] [PubMed] [Google Scholar]
- 53.Basten GP, Bao Y, Williamson G. Sulforaphane and its glutathione conjugate but not sulforaphane nitrile induce UDP-glucuronosyl transferase (UGT1A1) and glutathione transferase (GSTA1) in cultured cells. Carcinogenesis. 2002;23:1399–1404. doi: 10.1093/carcin/23.8.1399. [DOI] [PubMed] [Google Scholar]
- 54.Scharf G, Prustomersky S, Knasmuller S, Schulte-Hermann R, Huber WW. Enhancement of glutathione and gamma-glutamylcysteine synthetase, the rate limiting enzyme of glutathione synthesis, by chemoprotective plant derived food and beverage components in the human hepatoma cell line HepG2. Nutr Cancer. 2003;45:74–83. doi: 10.1207/S15327914NC4501_9. [DOI] [PubMed] [Google Scholar]
- 55.Zhang Y. Role of glutathione in the accumulation of anticarcinogenic isothiocyanates and their glutathione conjugates by murine hepatoma cells. Carcinogenesis. 2000;21:1175–1182. [PubMed] [Google Scholar]
- 56.Dinkova-Kostova AT, Holtzclaw WD, Cole RN, Itoh K, Wakabayashi N, Katoh Y, Yamamoto M, Talalay P. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc Natl Acad Sci USA. 2002;99:11908–11913. doi: 10.1073/pnas.172398899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McWalter GK, Higgins LG, McLellan LI, Henderson CJ, Song L, Thornalley PJ, Itoh K, Yamamoto M, Hayes JD. Transcription factor Nrf2 is essential for induction of NAD(P)H:quinone oxidoreductase 1, glutathione S-transferases, and glutamate cysteine ligase by broccoli seeds and isothiocyanates. J Nutr. 2004;134:3499S–3506S. doi: 10.1093/jn/134.12.3499S. [DOI] [PubMed] [Google Scholar]
- 58.Jiang ZQ, Chen C, Yang B, Hebbar V, Kong AN. Differential responses from seven mammalian cell lines to the treatments of detoxifying enzyme inducers. Life Sci. 2003;72:2243–2253. doi: 10.1016/s0024-3205(03)00101-2. [DOI] [PubMed] [Google Scholar]
- 59.Keck AS, Finley JW. Aqueous extracts of selenium-fertilized broccoli increase selenoprotein activity and inhibit DNA single-strand breaks, but decrease the activity of quinone reductase in Hepa 1c1c7 cells. Food Chem Toxicol. 2006;44:695–703. doi: 10.1016/j.fct.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 60.Brooks JD, Paton VG, Vidanes G. Potent induction of phase 2 enzymes in human prostate cells by sulforaphane. Cancer Epidemiol Biomarkers Prev. 2001;10:949–954. [PubMed] [Google Scholar]
- 61.Zhang Y, Munday R, Jobson HE, Munday CM, Lister C, Wilson P, Fahey JW, Mhawech-Fauceglia P. Induction of GST and NQO1 in cultured bladder cells and in the urinary bladders of rats by an extract of broccoli (Brassica oleracea italica) sprouts. J Agric Food Chem. 2006;54:9370–9376. doi: 10.1021/jf062109h. [DOI] [PubMed] [Google Scholar]
- 62.Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 63.Simon HU, Haj-Yehia A, Levi-Schaffer F. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis. 2000;5:415–418. doi: 10.1023/a:1009616228304. [DOI] [PubMed] [Google Scholar]
- 64.Pham NA, Jacobberger JW, Schimmer AD, Cao P, Gronda M, Hedley DW. The dietary isothiocyanate sulforaphane targets pathways of apoptosis, cell cycle arrest, and oxidative stress in human pancreatic cancer cells and inhibits tumor growth in severe combined immunodeficient mice. Mol Cancer Ther. 2004;3:1239–1248. [PubMed] [Google Scholar]
- 65.Singh SV, Srivastava SK, Choi S, Lew KL, Antosiewicz J, Xiao D, Zeng Y, Watkins SC, Johnson CS, Trump DL, Lee YJ, Xiao H, Herman-Antosiewicz A. Sulforaphane-induced cell death in human prostate cancer cells is initiated by reactive oxygen species. J Biol Chem. 2005;280:19911–19924. doi: 10.1074/jbc.M412443200. [DOI] [PubMed] [Google Scholar]
- 66.Choi WY, Choi BT, Lee WH, Choi YH. Sulforaphane generates reactive oxygen species leading to mitochondrial perturbation for apoptosis in human leukemia U937 cells. Biomed Pharmacother. 2008;62(9):637–44. doi: 10.1016/j.biopha.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 67.Choi S, Singh SV. Bax and Bak are required for apoptosis induction by sulforaphane, a cruciferous vegetable-derived cancer chemopreventive agent. Cancer Res. 2005;65:2035–43. doi: 10.1158/0008-5472.CAN-04-3616. [DOI] [PubMed] [Google Scholar]
- 68.Sharma R, Sharma A, Chaudhary P, Vatsyayan R, Pearce, Virginia, Singh SV, Awasthi S, Awasthi YC. Role of Lipid Peroxidation in Cellular Responses to D,L-Sulforaphane, A Promising Cancer Chemopreventive Agent. Biochemistry. 2010;49:3191–3202. doi: 10.1021/bi100104e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McMahon M, Itoh K, Yamamoto M, Hayes JD. Keap1-dependent proteasomal degradation of transcription factor Nrf2 contributes to the negative regulation of antioxidant response element-driven gene expression. J Biol Chem. 2003;278:21592–21600. doi: 10.1074/jbc.M300931200. [DOI] [PubMed] [Google Scholar]
- 70.Jeong WS, Jun M, Kong AN. Nrf2: a potential molecular target for cancer chemoprevention by natural compounds. Antioxid Redox Signal. 2006;8:99–106. doi: 10.1089/ars.2006.8.99. [DOI] [PubMed] [Google Scholar]
- 71.Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, Yamamoto M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999;13:76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hu R, Xu C, Shen G, Jain MR, Khor TO, Gopalkrishnan A, Lin W, Reddy B, Chan JY, Kong AN. Gene expression profiles induced by cancer chemopreventive isothiocyanate sulforaphane in the liver of C57BL/6J mice and C57BL/6J/Nrf2 (−/−) mice. Cancer Lett. 2006;243:170–192. doi: 10.1016/j.canlet.2005.11.050. [DOI] [PubMed] [Google Scholar]
- 73.Thimmulappa RK, Mai KH, Srisuma S, Kensler TW, Yamamoto M, Biswal S. Identification of Nrf2- regulated genes induced by the chemopreventive agent sulforaphane by oligonucleotide microarray. Cancer Res. 2002;62:5196–5203. [PubMed] [Google Scholar]
- 74.Sharma R, Sharma A, Dwivedi S, Zimniak P, Awasthi S, Awasthi YC. 4-hydroxynonenal self limits Fas-mediated DISC independent apoptosis by promoting export of Daxx from nucleus to cytosol and its binding to Fas. Biochemistry. 2008;47:143–156. doi: 10.1021/bi701559f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sharma A, Sharma R, Chaudhary P, Vatsyayan R, Pearce V, Jeyabal PV, Zimniak P, Awasthi S, Awasthi YC. 4-Hydroxynonenal induces p53-mediated apoptosis in retinal pigment epithelial cells. Arch Biochem Biophysics. 2008;480:85–94. doi: 10.1016/j.abb.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chaudhary P, Sharma R, Sharma A, Vatasyayan R, Yadav S, Singhal SS, Awasthi S, Awasthi YC. Mechanisms of 4-hydroxy-2-nonenal induced pro and anti apoptotic signaling. Biochemistry. 2010;49:6263–6275. doi: 10.1021/bi100517x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sharma R. Bryan Ellis and Abha Sharma, Role of alpha class glutathione transferases in chemoprevention: Human leukemia (HL60) cells overexpressin GSTA1and GSTA4 resist sulphorphane and curcumin induced cytotoxicity. Phytotherapy Res. 2011;25(4):563–568. doi: 10.1002/ptr.3297. [DOI] [PubMed] [Google Scholar]
- 78.Awasthi YC, Sharma R, Cheng JZ, Yang Y, Sharma A, Singhal SS, Awasthi S. Role of 4-hydroxynonenal in stress-mediated apoptosis signaling. Mol Aspects Med. 2003;24:219–230. doi: 10.1016/s0098-2997(03)00017-7. [DOI] [PubMed] [Google Scholar]
- 79.Awasthi YC, Sharma R, Sharma A, Yadav S, Singhal SS, Chaudhary P, Awasthi S. Self-regulatory role of 4-hydroxynonenal in signaling for stress-induced programmed cell death. Free Radic Biol Med. 2008;45:111–118. doi: 10.1016/j.freeradbiomed.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Leonarduzzi G, Arkan MC, Basaga H, Chiarpotto E, Sevanian A, Poli G. Lipid oxidation products in cell signaling. Free Radic Biol Med. 2000;28:1370–8. doi: 10.1016/s0891-5849(00)00216-1. [DOI] [PubMed] [Google Scholar]
- 81.Ames BN, Shigenaga MK, Hagen TM. Oxidants, antioxidants, and the degenerative diseases of aging. Proc Natl Acad Sci U S A. 1993;90:7915–22. doi: 10.1073/pnas.90.17.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sharma R, Brown D, Awasthi S, Yang Y, Sharma A, Patrick B, Saini MK, Singh SP, Zimniak P, Singh SV, Awasthi YC. Transfection with 4-hydroxynonenal-metabolizing glutathione S-transferase isozymes leads to phenotypic transformation and immortalization of adherent cells. Eur J Biochem. 2004;271:1690–1701. doi: 10.1111/j.1432-1033.2004.04067.x. [DOI] [PubMed] [Google Scholar]
- 83.Vatsyayan R, Chaudhary P, Sharma A, Sharma R, Rao Lelsani PC, Awasthi S, Awasthi YC. Role of 4-hydroxynonenal in epidermal growth factor receptor-mediated signaling in retinal pigment epithelial cells. Exp Eye Res. 2011;92(2):147–54. doi: 10.1016/j.exer.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Awasthi YC, Ansari GA, Awasthi S. Regulation of 4-hydroxynonenal mediated signaling by glutathione S-transferases. Methods Enzymol. 2005;401:379–407. doi: 10.1016/S0076-6879(05)01024-4. [DOI] [PubMed] [Google Scholar]
- 85.Cheng JZ, Sharma R, Yang Y, Singhal SS, Sharma A, Saini MK, Singh SV, Zimniak P, Awasthi S, Awasthi YC. Accelerated metabolism and exclusion of 4-hydroxynonenal through induction of RLIP76 and hGST5.8 is an early adaptive response of cells to heat and oxidative stress. J Biol Chem. 2001;276:41213–41223. doi: 10.1074/jbc.M106838200. [DOI] [PubMed] [Google Scholar]
- 86.Yang Y, Cheng JZ, Singhal SS, Saini M, Pandya U, Awasthi S, Awasthi YC. Role of glutathione S-transferases in protection against lipid peroxidation. Overexpression of hGSTA2-2 in K562 cells protects against hydrogen peroxide-induced apoptosis and inhibits JNK and caspase 3 activation. J Biol Chem. 2001;276(22):19220–30. doi: 10.1074/jbc.M100551200. [DOI] [PubMed] [Google Scholar]
- 87.Yang Y, Sharma R, Zimniak P, Awasthi YC. Role of alpha class glutathione S-transferases as antioxidant enzymes in rodent tissues. Toxicol Appl Pharmacol. 2002;182:105–115. doi: 10.1006/taap.2002.9450. [DOI] [PubMed] [Google Scholar]
- 88.Awasthi S, Singhal SS, Sharma R, Zimniak P, Awasthi YC. Transport of glutathione –conjugates and chemotherapeutic drugs by RLIP76 (RalBP1): a novel link between G-protein and tyrosine kinase signaling and drug resistance. Int J Cancer. 2003;106:635–646. doi: 10.1002/ijc.11260. [DOI] [PubMed] [Google Scholar]
- 89.Sharma R, Singhal SS, Cheng J, Yang Y, Sharma A, Zimniak P, Awasthi S, Awasthi YC. RLIP76 is the major ATP-dependent transporter of glutathione-conjugates and doxorubicin in human erythrocytes. Arch Biochem Biophys. 2001;391(2):171–9. doi: 10.1006/abbi.2001.2395. [DOI] [PubMed] [Google Scholar]
- 90.Awasthi S, Singhal SS, Yadav S, Singhal J, Drake K, Nadkar A, Zajac E, Wickramarachchi D, Rowe N, Yacoub A, Boor P, Dwivedi S, Dent P, Jarman WE, John B, Awasthi YC. RLIP76 is a major determinant of radiation sensitivity. Cancer Res. 2005;65(14):6022–28. doi: 10.1158/0008-5472.CAN-05-0968. [DOI] [PubMed] [Google Scholar]
- 91.Singhal SS, Awasthi YC, Awasthi S. Regression of melanoma in a murine model by RLIP76 depletion. Cancer Res. 2006;66(4):2354–60. doi: 10.1158/0008-5472.CAN-05-3534. [DOI] [PubMed] [Google Scholar]
- 92.Singhal SS, Singhal J, Yadav S, Dwivedi S, Boor PJ, Awasthi YC, Awasthi S. Regression of lung and colon cancer xenografts by depleting or inhibiting RLIP76 (Ral-binding protein 1) Cancer Res. 2007;67(9):4382–9. doi: 10.1158/0008-5472.CAN-06-4124. [DOI] [PubMed] [Google Scholar]
- 93.Awasthi S, Singhal SS, Awasthi YC, Martin B, Woo JH, Cunningham CC, Frankel AE. RLIP76 and Cancer. Clin Cancer Res. 2008;14(14):4372–7. doi: 10.1158/1078-0432.CCR-08-0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Singhal SS, Singhal J, Yadav S, Sahu M, Awasthi YC, Awasthi S. RLIP76: a target for kidney cancer therapy. Cancer Res. 2009;69(10):4244–51. doi: 10.1158/0008-5472.CAN-08-3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang H, Forman HJ. Signaling pathways involved in Phase II gene induction by alpha beta unsaturated aldehydes. Toxicol Ind Health. 2009;4–5:269–78. doi: 10.1177/0748233709102209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gamet-Payrastre L, Lumeau S, Gasc N, Cassar G, Rollin P, Tulliez J. Selective cytostatic and cytotoxic effects of glucosinolates hydrolysis products on human colon cancer cells in vitro. Anticancer Drugs. 1998;9:141–148. doi: 10.1097/00001813-199802000-00005. [DOI] [PubMed] [Google Scholar]
- 97.Gamet-Payrastre L, Li P, Lumeau S, Cassar G, Dupont MA, Chevolleau S, Gasc N, Tulliez J, Terce F. Sulforaphane, a naturally occurring isothiocyanate, induces cell cycle arrest and apoptosis in HT29 human colon cancer cells. Cancer Res. 2000;60:1426–1433. [PubMed] [Google Scholar]
- 98.Fowke JH, Chung FL, Jin F, Qi D, Cai Q, Conaway C, Cheng JR, Shu XO, Gao YT, Zheng W. Urinary isothiocyanate levels, brassica, and human breast cancer. Cancer Res. 2003;63:3980–3986. [PubMed] [Google Scholar]
- 99.Xu K, Thornalley PJ. Studies on the mechanism of the inhibition of human leukaemia cell growth by dietary isothiocyanates and their cysteine adducts in vitro. Biochem Pharmacol. 2000;60:221–231. doi: 10.1016/s0006-2952(00)00319-1. [DOI] [PubMed] [Google Scholar]
- 100.Fimognari C, Nusse M, Cesari R, Iori R, Cantelli-Forti G, Hrelia P. Growth inhibition, cell-cycle arrest and apoptosis in human T-cell leukemia by the isothiocyanate sulforaphane. Carcinogenesis. 2002;23:581–586. doi: 10.1093/carcin/23.4.581. [DOI] [PubMed] [Google Scholar]
- 101.Tang L, Zhang Y. Dietary isothiocyanates inhibit the growth of human bladder carcinoma cells. J Nutr. 2004;134:2004–2010. doi: 10.1093/jn/134.8.2004. [DOI] [PubMed] [Google Scholar]
- 102.Herman-Antosiewicz A, Johnson DE, Singh SV. Sulforaphane causes autophagy to inhibit release of cytochrome C and apoptosis in human prostate cancer cells. Cancer Res. 2006;66:5828–5835. doi: 10.1158/0008-5472.CAN-06-0139. [DOI] [PubMed] [Google Scholar]
- 103.Sherr CJ. Cancer cell cycles. Science. 1996;274:1672–1677. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- 104.Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 105.Shen G, Xu C, Chen C, Hebbar V, Kong AN. p53-independent G1 cell cycle arrest of human colon carci- noma cells HT-29 by sulforaphane is associated with induction of p21CIP1 and inhibition of expression of cyclin D1. Cancer Chemother Pharmacol. 2006;57:317–327. doi: 10.1007/s00280-005-0050-3. [DOI] [PubMed] [Google Scholar]
- 106.Singh SV, Herman-Antosiewicz A, Singh AV, Lew KL, Srivastava SK, Kamath R, Brown KD, Zhang L, Baskaran R. Sulforaphane-induced G2/M phase cell cycle arrest involves checkpoint kinase 2-mediated phosphorylation of cell division cycle 25C. J Biol Chem. 2004;279:25813–25822. doi: 10.1074/jbc.M313538200. [DOI] [PubMed] [Google Scholar]
- 107.Shan Y, Sun C, Zhao X, Wu K, Cassidy A, Bao Y. Effect of sulforaphane on cell growth, G0/G1 phase cell progression and apoptosis in human bladder cancer T24 cells. Int J Oncol. 2006;29:883–888. [PubMed] [Google Scholar]
- 108.Jackson SJ, Singletary KW, Venema RC. Sulforaphane suppresses angiogenesis and disrupts endothelial mitotic progression and microtubule polymerization. Vascul Pharmacol. 2006;46:77–84. doi: 10.1016/j.vph.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 109.Myzak MC, Karplus PA, Chung FL, Dashwood RH. A novel mechanism of chemoprotection by sulforaphane: inhibition of histone deacetylase. Cancer Res. 2004;64:5767–5774. doi: 10.1158/0008-5472.CAN-04-1326. [DOI] [PubMed] [Google Scholar]
- 110.Clarke J, Hsu A, Yu Z, Dashwood R, Ho E. Differential effects of sulforaphane on histone deacetylases, cell cycle arrest and apoptosis in normal prostate cells versus hyperplastic and cancerous prostate cells. Mol Nutr Foods Res. 2011;55:999–1009. doi: 10.1002/mnfr.201000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kallifatidis G, Labsch S, Rausch V, Mattern J, Gladkich J, Moldenhauer G, Büchler M, Salnikov A, Herr I. Sulforaphane increases drug-mediated cytotoxicity toward cancer stem-like cells of pancreas and prostate. Mol Ther. 2011;19:188–195. doi: 10.1038/mt.2010.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pledgie-Tracy A, Sobolewski M, Davidson N. Sulforaphane induces cell type-specific apoptosis in human breast cancer cell lines. Mol Cancer Ther. 2007;6:1013–1021. doi: 10.1158/1535-7163.MCT-06-0494. [DOI] [PubMed] [Google Scholar]
- 113.Ji C, Amarnath V, Pietenpol J, Marnett L. 4-hydroxynonenal induces apoptosis via caspase-3 activation and cytochrome c release. Chem Res Toxicol. 2001;14:1090–1096. doi: 10.1021/tx000186f. [DOI] [PubMed] [Google Scholar]
- 114.Pizzimenti S, Briatore F, Laurora S, Toaldo C, Maggio M, De Grandi M, Meaglia L, Menegatti E, Giglioni B, Dianzani M, Barrera G. 4-Hydroxynonenal inhibits telomerase activity and hTERT expression in human leukemic cell lines. Free Radic Biol Med. 2006;40:1578–1591. doi: 10.1016/j.freeradbiomed.2005.12.024. [DOI] [PubMed] [Google Scholar]
- 115.Meeran S, Patel S, Tollefsbol T. Sulforaphane causes epigenetic repression of hTERT expression in human breast cancer cell lines. PLoS One. 2010;5:e11457. doi: 10.1371/journal.pone.0011457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kallifatidis G, Rausch V, Baumann B, Apel A, Beckermann B, Groth A, Mattern J, Li Z, Kolb A, Moldenhauer G, Altevogt P, Wirth T, Werner J, Schemmer P, Büchler M, Salnikov A, Herr I. Sulforaphane targets pancreatic tumour-initiating cells by NF-kappaB-induced antiapoptotic signalling. Gut. 2009;58:949–963. doi: 10.1136/gut.2008.149039. [DOI] [PubMed] [Google Scholar]
- 117.Pettazzoni P, Pizzimenti S, Toaldo C, Sotomayor p, Tagliavacca L, Liu S, Liu D, Minelli R, Ellis L, Atadja P, Ciamporcero E, Dianzani M, Barrera G, Pili R. Induction of cell cycle arrest and DNA damage by the HDAC inhibitor panobinostat (LBH589) and the lipid peroxidation end product 4-hydroxynonenal in prostate cancer cells. Free Radic Biol Med. 2011;50:313–322. doi: 10.1016/j.freeradbiomed.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 118.Ho E, Clarke J, Dashwood R. Dietary sulforaphane, a histone deacetylase inhibitor for cancer prevention. J Nutr. 2009;139:2393–2396. doi: 10.3945/jn.109.113332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fawzy E, Nehad E. Potential health benefits of sulforaphane: A review of the experimental, clinical and epidemiological evidences and underlying mechanisms. J Med Plants Res. 2011;5:473–484. [Google Scholar]
- 120.Wagner A, Ernst I, Iori R, Desel C, Rimbach G. Sulforaphane but not ascorbigen, indole-3-carbinole and ascorbic acid activates the transcription factor Nrf2 and induces phase-2 and antioxidant enzymes in human keratinocytes in culture. Exp Dermatol. 2010;19:137–144. doi: 10.1111/j.1600-0625.2009.00928.x. [DOI] [PubMed] [Google Scholar]
- 121.Chen Z, Saito Y, Yoshida Y, Sekine A, Noguchi N, Niki E. 4-Hydroxynonenal induces adaptive response and enhances PC12 cell tolerance primarily through induction of thioredoxin reductase 1 via activation of Nrf2. J Biol Chem. 2005;280:41921–41927. doi: 10.1074/jbc.M508556200. [DOI] [PubMed] [Google Scholar]
- 122.Jacobs A, Marnett L. Heat shock factor 1 attenuates 4-Hydroxynonenal-mediated apoptosis: critical role for heat shock protein 70 induction and stabilization of Bcl-XL. J Biol Chem. 2007;282:33412–33420. doi: 10.1074/jbc.M706799200. [DOI] [PubMed] [Google Scholar]
- 123.Wang S, Kotamraju S, Konorev E, Kalivendi S, Joseph J, Kalyanaraman B. Activation of nuclear factor-κB during doxorubicin-induced apoptosis in endothelial cells and myocytes is proapoptotic: the role of hydrogen peroxide. Biochem J. 2002;367:729–740. doi: 10.1042/BJ20020752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ji C, Kozak K, Marnett L. IκB Kinase, a Molecular Target for Inhibition by 4-Hydroxy-2-nonenal. J Biol Chem. 2001;276:18223–18228. doi: 10.1074/jbc.M101266200. [DOI] [PubMed] [Google Scholar]
- 125.Lee S, Kim C, Seo K, Kim C. HNE-induced 5-LO expression is regulated by NF-κB/ERK and Sp1/p38 MAPK pathways via EGF receptor in murine macrophages. Cardiovasc Res. 2010;88:352–359. doi: 10.1093/cvr/cvq194. [DOI] [PubMed] [Google Scholar]
- 126.Malone P, Hernandez M. 4-Hydroxynonenal, a product of oxidative stress, leads to an antioxidant response in optic nerve head astrocytes. Exp Eye Res. 2007;84:444–454. doi: 10.1016/j.exer.2006.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jacobs A, Marnett L. HSF1-mediated BAG3 expression attenuates apoptosis in 4-hydroxynonenal-treated colon cancer cells via stabilization of anti-apoptotic Bcl-2 proteins. J Biol Chem. 2009;284:9176–9183. doi: 10.1074/jbc.M808656200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gan N, Wu Y, Brunet C, Chung F, Dai C, Mi L. Sulforaphane activates heat shock response and enhances proteasome activity through up-regulation of Hsp27. J Biol Chem. 2010;285:35528–35536. doi: 10.1074/jbc.M110.152686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Moodie F, Marwick J, Anderson C, Szulakowski P, Biswas S, Bauter M, Kilty I, Rahman I. Oxidative stress and cigarette smoke alter chromatin remodeling but differentially regulate NF-κB activation and proinflammatory cytokine release in alveolar epithelial cells. FASEB J. 2004;18:1897–1899. doi: 10.1096/fj.04-1506fje. [DOI] [PubMed] [Google Scholar]
- 130.Chen Z. Niki E4-hydroxynonenal (4-HNE) has been widely accepted as an inducer of oxidative stress, Is this the whole truth about it or can 4-HNE also exert protective effects? IUBMB Life. 2006;58:372–373. doi: 10.1080/15216540600686896. [DOI] [PubMed] [Google Scholar]
- 131.Pizzimenti S, Menegatti E, Berardi D, Toaldo C, Pettazzoni P, Minelli R, Giglioni B, Cerbone A, Dianzani M, Ferretti C, Barrera G. 4-hydroxynonenal, a lipid peroxidation product of dietary polyunsaturated fatty acids, has anticarcinogenic properties in colon carcinoma cell lines through the inhibition of telomerase activity. J Nutr Biochem. 2010;21:818–826. doi: 10.1016/j.jnutbio.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 132.Moon D, Kang S, Kim K, Kim M, Choi Y, Kim G. Sulforaphane decreases viability and telomerase activity in hepatocellular carcinoma Hep3B cells through the reactive oxygen species-dependent pathway. Cancer Lett. 2010;295:260–266. doi: 10.1016/j.canlet.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 133.Kong A, Yu R, Hebbar V, Chen C, Owuor E, Hu R, Ee R, Mandlekar S. Signal transduction events elicited by cancer prevention compounds. Mutat Res. 2001;480–481:231–241. doi: 10.1016/s0027-5107(01)00182-8. [DOI] [PubMed] [Google Scholar]
- 134.Gamet-Payrastre L. Signaling pathways and intracellular targets of sulforaphane mediating cell cycle arrest and apoptosis. Curr Cancer Drug Targets. 2006;6:135–145. doi: 10.2174/156800906776056509. [DOI] [PubMed] [Google Scholar]
- 135.Myzak Dashwood W, Orner G, Ho E, Dashwood R. Sulforaphane inhibits histone deacetylase in vivo and suppresses tumorigenesis in Apcmin mice. FASEB J. 2006;20:506–508. doi: 10.1096/fj.05-4785fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Laurora S, Tamagno E, Briatore F, Bardini P, Pizzimenti S, Toaldo C, Reffo P, Costelli P, Dianzani M, Danni O, Barrera G. 4-Hydroxynonenal modulation of p53 family gene expression in the SK-N-BE neuroblastoma cell line. Free Radic Biol Med. 2005;38:215–225. doi: 10.1016/j.freeradbiomed.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 137.Kaminski B, Weigert A, Brüne B, Schumacher M, Wenzel U, Steinhilber D, Stein J, Ulrich S. Sulforaphane potentiates oxaliplatin-induced cell growth inhibition in colorectal cancer cells via induction of different modes of cell death. Cancer Chemother Pharmacol. 2011;67:1167–1178. doi: 10.1007/s00280-010-1413-y. [DOI] [PubMed] [Google Scholar]
- 138.Anuradha C, Kanno S, Hirano S. Oxidative damage to mitochondria is a preliminary step to caspase-3 activation in fluoride-induced apoptosis in HL-60 cells. Free Radic Biol Med. 2001;31:367–373. doi: 10.1016/s0891-5849(01)00591-3. [DOI] [PubMed] [Google Scholar]
- 139.Fahey JW, Zalcmann AT, Talalay P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry. 2001;56:5–51. doi: 10.1016/s0031-9422(00)00316-2. [DOI] [PubMed] [Google Scholar]