Abstract
Rationale
Addictive drugs are commonly delivered in the organism by means of intravenous (iv) injections. Since saline mimics the blood environment by basic ionic properties and pH, it is generally assumed that it should not have any physiological effects, serving as a control for the effects induced by drugs.
Objective
To examine central, behavioral and physiological effects of stress- and cue-free iv saline injection in freely moving rats.
Methods
We examined how typical, low-volume and slow-speed saline injections affects cortical EEG and neck EMG, locomotor activity as well as central and peripheral temperatures.
Results
Saline injection made during slow-wave synchronized activity induces rapid, transient EEG desynchronization, manifesting as a drop of EEG total power, decrease in alpha activity, and increases in beta and gamma activities. Saline injection did not affect locomotor activity as well as brain and body temperatures, but induced a transient increase in neck EMG activity and rapid, brief drop in skin temperature, suggesting peripheral vasoconstriction. These responses were virtually fully absent when saline injection was made during naturally occurring desynchronized EEG activity during behavioral activity.
Conclusions
Since iv injection is able to produce a peripheral sensory signal that is transmitted rapidly to the CNS and followed by a more prolonged effect of the injected drug on brain cells, with repeated drug administrations the injection itself could play a role of drug-related sensory cue, thus inducing conditioned physiological responses and altering the effects of injected drugs.
Keywords: EEG, EMG, visceral sensory stimulus, desynchronization, neural activation, vasoconstriction, brain temperature, repeated drug administration, learning
Introduction
Addictive drugs are commonly delivered in the organism by means of intravenous (iv) injections. Since saline mimics the blood environment by basic ionic properties and pH, there is a general assumption that iv saline injection should not have any physiological effects, serving as a control for the effects induced by drugs. However, in our previous thermorecording studies, we often observed that iv saline injections performed in awake, freely moving rats at quiet rest could induce transient effects, particularly weak decreases in skin temperature (Kiyatkin and Brown 2005).
In the present study, we examined this phenomenon in detail using such sensitive measures of neural activity as electroencephalograpy (EEG) and neck electromyography (EMG) in freely moving rats. It is known that EEG provides a reliable measure of a variable brain's activity state and EEG desynchronization is a universal manifestation of neural activation that is induced by somato-sensory stimuli, occurs spontaneously during awakening and behavioral activation, and induced by certain drugs (see Buzsaki 2006; Hobson 1999; Kiyatkin and Smirnov 2010; McClung et al. 1977; Sasaki et al. 1996). In contrast to highly variable locomotor activity, which is often monitored to measure behavioral activation, EMG reflects both tonic and phasic alterations in electrical activity of the muscle, being a centrally mediated physiological response to the afferent challenge. In addition to the cerebral cortex, the most common area of EEG recording, we also simultaneously monitored electrical activity from the ventral tegmental area of midbrain (VTA), a deep subcortical area heavily implicated in regulating motivational and reinforcement processes (Le Moal and Simon 1991). Therefore, high-speed analysis of cortical and VTA electrical activity was used in this study as a basic technique to examine the effects of a typical stress- or cue-free saline injection in freely moving rats. In a separate experiment, we also examined the effects of iv saline injection on locomotor activity measured by infrared beams breaks and temperatures recorded from the VTA, temporal muscle, and facial skin. Temperature monitoring from these recording locations allowed us to assess the effect of the procedure on such important physiological parameters as brain and body temperatures as well to evaluate alterations in brain metabolic activity and peripheral vascular tone (vasoconstriction/ vasodilatation) (see Kiyatkin 2010 for details).
Methods
Animals and Surgery
The data presented in this study were obtained from 15 male Long-Evans rats (400±50 g) supplied by Charles River Laboratories (Greensboro, NC). All animals were housed individually in a temperature-, humidity- and light-controlled room (12-hr light cycle beginning at 07:00) with free access to food and water. Protocols were performed in compliance with the Guide for the Care and Use of Laboratory Animals (NIH, Publication 865-23) and were approved by the NIDA-IRP Animal Care and Use Committee.
For Experiment I, each of six rats was surgically prepared for chronic EEG and EMG recording as previously described in detail (Kiyatkin and Smirnov 2010). Under general anesthesia (Equithesin 0.33 ml/100 g ip.), each rat was implanted with three stainless steel screws threaded into the skull (two active screws on the left side: A-L 4.0 mm-2.0 mm; P-L 4.0 mm-1.5 mm and a ground screw on the right side: A-L 2.0 mm-2.0 mm; according to Paxinos and Watson atlas, 1998) and two stainless steel EMG electrodes implanted bilaterally in deep neck muscle. In addition, an insulated stainless still electrode (with 0.5 mm open active area at the tip) was implanted in the VTA (P-L 5.5 mm-2.0 mm, depth 8.4 mm with 10° angle). Screws with extension wires and gold-pin connectors were purchased from Pinnacle Technology, Inc. (Lawrence, KS, USA) and EMG electrodes were custom-made from four insulated 50 μm wires, which were twisted together, insulated (except the last 0.5mm at the top), and soldered to a gold-pin connector, similar to that for the EEG electrodes. Following the implantation, all six electrode pins were inserted into a plastic socket and fixed with dental acrylic as a head assembly. During the same surgical session, each rat was implanted with a chronic iv jugular catheter, which was run subcutaneously to the head mount and secured with dental acrylic in the same head assembly. After a 3-4 day period of recovery and habituation to the experimental chamber, recording sessions were held once daily over the next 5-8 days.
Electrical signals were recorded differentially in two brain areas. For cortical recordings, we used two active screws threaded into the skull on the left side and a ground screw implanted on the right side. For VTA recordings, we used an electrode implanted in the VTA and the most frontal screw (A-L: 4 mm-2 mm). The same ground screw was also used as a reference electrode for differential EMG recording, with two active EMG electrodes implanted bilaterally in deep neck muscle. Electrical activity from EEG and EMG electrodes passed through the pre-amplifier (Pinnacle Technology) incorporated inside of an extension cord and electrical swivel to the main amplifiers (P15D and P55; Grass Electronics, USA), which were used for additional signal amplification and filtering. EEG and EMG signals were filtered from 1 to 100 Hz and 100 to 1000Hz, respectively. The filtered signals then entered a Micro 1401 MK2 interface (Cambridge Electronic Design, Cambridge, UK), allowing its acquisition, recording, and analysis using a Spike2 interface (Cambridge Electronic Design).
For Experiment II, each of nine rats under the same anesthesia was implanted with four copper-constantan thermocouple electrodes as described in details previously (Brown and Kiyatkin 2005). One electrode was implanted stereotaxically in the VTA (P-L: 6.0 mm-2.0 mm, 8.4 mm depth with 10% angle) according to coordinates of Paxinos and Watson, 1998. A second thermocouple probe was implanted subcutaneously along the nasal ridge with the tip approximately 15 mm anterior to bregma. A third thermocouple probe was implanted in the deep temporal muscle (musculus temporalis). The probes were secured with dental cement to the three stainless steel screws threaded into the skull. During the same surgery session, animals were also implanted with a jugular iv catheter. Rats were allowed three days recovery and two more days of habituation (6 h sessions) to the testing environment before the start of testing.
Temperatures were recorded continuously with a time resolution of 10 s using thermal recording hardware (Thermes 16, Physitemp, Clifton, NJ, USA). Locomotor activity was recorded as the number of infrared beam breaks per 1 min. Room temperature was maintained at 23-24°C and controlled by another thermocouple located in the recording chamber.
Experimental protocol
A similar protocol was used in both experiments. Recordings were conducted in an electrically insulated cage (38 × 47 × 47 cm) placed inside a sound- and light-attenuated box (60 × 56 × 70 cm), under continuous weak light illumination (20 W) in view of a small USB camera mounted above the cage. After placement in the cage, the rat was connected to the recording cable and a plastic catheter extension. This catheter extension was connected to a liquid swivel and an additional catheter extension, allowing stress-free drug delivery from outside of the cage and thus minimizing detection of the iv injection procedure by the animal. To eliminate possible sound cues associated with the injections, they were performed manually. Each rat was intensively habituated to the recording environment both before (2-3 daily sessions, 5-7 hours each) and after (2-3 sessions) surgery.
Each rat was exposed to several (n=2-4) iv saline injections (0.15 ml over 15 s) during the next two to four recording sessions; ~2-hour intervals were maintained between each injection. The injections were made when the rat was either in sleep/drowsiness state (no visual movements, stable high-magnitude synchronized EEG activity, no phasic changes in EMG activity) or in quiet wakefulness state (no gross movements, sitting or standing posture, low-magnitude desynchronized EEG activity, minor phasic or tonic changes in EMG). Cases in which we observed appearance of movements or sudden changes in EEG during 120-s pre-injection periods were removed from analysis. Quiet, sleep-like state with no overt movements within 10-min pre-injection was also the requirement for accepting temperature and locomotion data.
Data analysis
EEG and EMG signals were analyzed with 5-s bin resolution based on 7-min recording durations, with two min preceding and 5 min following the injection. The following parameters were determined and analyzed for each test: total power of EEG (filtered within 2-58 Hz), total power of EMG (58-1000 Hz), change in the power of delta (2-4 Hz), theta (4-8 Hz), alpha (8-15 Hz), beta (15-29 Hz), and gamma (29-58 Hz) frequencies. Since EEG and EMG signals in individual rats differ in their magnitude, absolute values of total power were transferred into relative changes, taking a basal value (mean for 60 s pre-injection) as 100%. Changes in each individual EEG frequency band were analyzed in percents with respect to the pre-event baseline. EEG and EMG total powers are integral indices of electrical activity, dependent upon both signal amplitude and frequency and the powers of individual EEG waves reflect respective proportions of the EEG total power calculated for specified signal frequencies; the sum of all five EEG wave powers equals total EEG power (100%). Since EMG signals typically showed robust and highly variable increases, especially during locomotion, changes in EMG activity were analyzed statistically as natural logarithmic derivatives.
Temperature and locomotion data were analyzed with 1-min time bins for 60 min post-injection and were presented as both absolute and relative changes with respect to baseline (0-10 min pre-injection). To evaluate rapid temperature fluctuations, the same data were also analyzed with 10-s quantification bins for 5 min post-injection.
Both a one-way ANOVA with repeated measures and post-hoc Fisher tests were used for statistical evaluation of changes in EEG/EMG, temperatures, and locomotion. Student's t-test was used for comparisons of between-site and between-condition differences in tested parameters. The use of the words ‘increase’ or ‘decrease’ as well as ‘significant’ refers to the presence of a statistically significant change in the parameter or in the differences between the compared groups or conditions (with at least p <0.05) revealed by either one-way ANOVA with repeated measurements or Student's t-test.
Results
Data reported in this study were obtained in freely moving rats intensively habituated to the recording cage and tested during several recording sessions. After the initial period of behavioral activation following transfer from the animal facility to the recording cage (1-2 hrs), rats became quiet, showing periods of inactivity intermixed with transient periods of locomotion, grooming, and rearing. All tests were conducted within these periods of quiet rest during light time recordings (11:00-16:00).
EEG/EMG recordings
Periodic fluctuations in EEG and EMG activity occurred consistently in rats during these periods of quiet rest. Periodically, the rats were in sleep-like inactivity with high magnitude EEG fluctuations (synchronization) and minimal EMG activity, but when they were awake and active, they showed low-magnitude desynchronized EEG activity coupled with increased EMG activity.
Sleep state, synchronized EEG activity
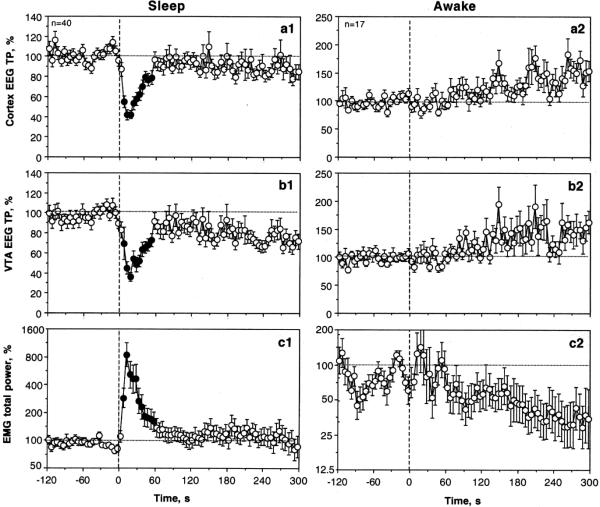
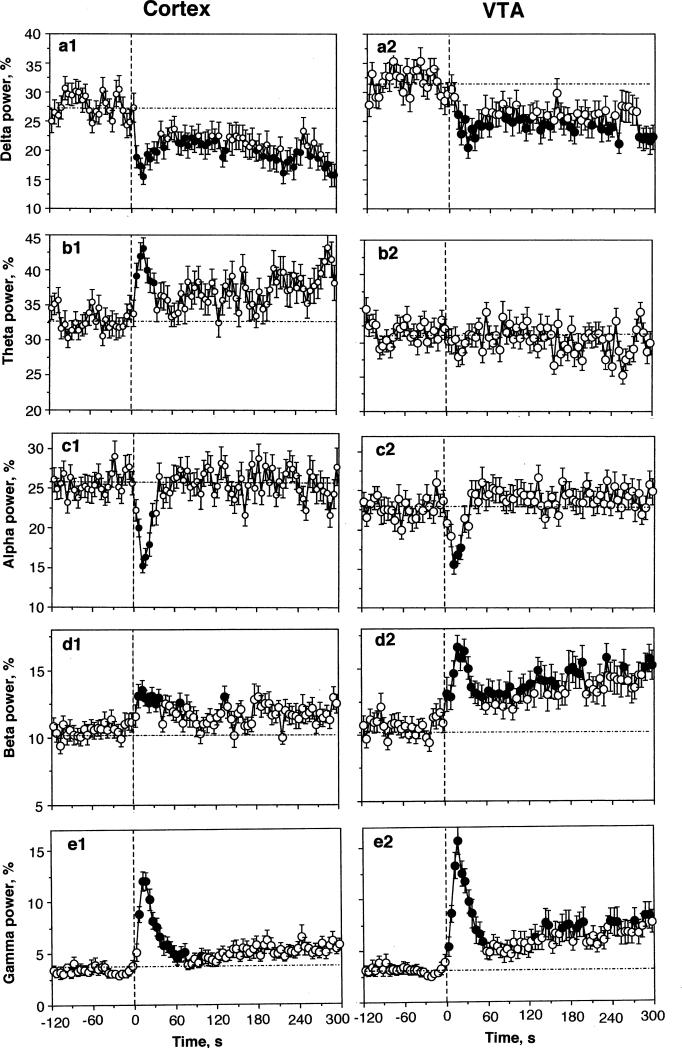
Most of our saline tests (n=40 in 6 rats during 16 sessions) were conducted during periods of motor inactivity when the EEG signals showed high-magnitude synchronized fluctuations. Under these conditions, slow saline administration (0.15 ml over 15 s) induced rapid, transient decreases in cortical and VTA EEG total power (Fig. 1) as well as significant changes in individual EEG waves (Fig. 2). Original examples of changes in EEG and EMG induced by saline during sleep state are shown in Online Resource 1 (Fig. S1). Despite some minor differences seen both in baseline and response characteristics, alterations in electrical activity were highly significant and similar in magnitude and time-course in both the cortex and VTA (F39,1140=8.59 and 5.57, respectively; both p<0.001). In each case, we saw a typical activation triad (phasic decreases in alpha [F39,1140=4.92 and 3.29 for cortex and VTA; p<0.001] and phasic increases in beta [cortex=2.05 and VTA 2.38; p<0.05] and gamma [cortex=3.41; VTA=10.89; p<0.001] activities) as well as a rapid but more prolonged drop in delta activity (2.01 and 2.05 for cortex and VTA, respectively; p<0.02).
Fig. 1.
The effects of iv saline injection on cortical EEG total power (A), VTA total power (B), and EMG total power (C) shown separately for sleep and active state. Vertical hatched line shows the onset of saline injection. Filled symbols mark values significantly different from pre-injection baseline (at least p<0.05).
Fig. 2.
Mean changes in individual EEG waves in the cortex and VTA following iv saline injections in slow-wave sleep state. With exception of one case (VTA, theta activity), saline has significant effects on each EEG frequency in both structures (see text). Vertical hatched line shows the onset of saline injection. Values significantly different from pre-injection baseline (at least p<0.05) are shown as filled symbols.
The EEG changes were tightly coupled with the injection, showing significant changes within the injection duration (Fig. 1 and 2). Both the EEG total power, an integral measure of EEG response, and powers of individual waves became significantly different from the pre-injection baselines at the second time bin (or 5-10 s from the start of 15-s injection). While alpha, beta, and gamma activities peaked rapidly and returned to baseline slowly, delta activity remained in decreased levels during the entire analyzed interval (5 min).
Despite a similar response pattern seen in the cortex and VTA, certain between-structure differences in frequency characteristics were found during statistical analyses (see Table). First, basal electrical activity in the VTA differed from that in the cortex by a large proportion of delta and lower proportion of alpha activities. Second, in the VTA, there were no saline-induced changes in theta activity, but this change was clear and strong in the cortex. Third, responses seen in both beta and gamma activities were stronger in the VTA than in the cortex, with long-term tails seen after the initial, injection-related peak.
Table.
Distribution of EEG waves in the cortex and VTA in sleep and awake states
| Sleep | Awake | |||
|---|---|---|---|---|
| Cortex | VTA | Cortex | VTA | |
| Delta power, % | 27.16±1.04 | 31.77±0.98*** | 17.93±0.96ooo | 22.35±1.30**,ooo |
| Theta power, % | 32.85±0.59 | 31.93±0.63 | 37.84±2.04o | 30.47±1.36*** |
| Alpha power, % | 25.95±0.83 | 22.97±0.79* | 19.88±1.00oo | 19.42±0.88oo |
| Beta power, % | 10.73±0.36 | 10.50±0.43 | 14.69±0.63ooo | 15.97±0.80ooo |
| Gamma power, % | 3.33±0.37 | 2.99±0.32 | 11.29±0.91ooo | 11.36±1.06ooo |
Each value of individual wave powers represents the mean±standard errors for 60-s period preceding saline injection (baseline). Two types of symbols (* and o) are used to show between-structure differences in the same state and between-state differences in the same structure Student's t-test.
p<0.05
p<0.01
p<0.01
p<0.05
p<0.01
p<0.001
EEG desynchronization was consistently accompanied by EMG activation (F39,1140=13.81, p<0.001), often without obvious motor effects (see Fig. 1). However, on average, the EMG response was evident at 5-10 s, peaked at the injection end (10-15 s), and returned slowly to the pre-injection baseline within the next 60 s.
Awake state, desynchronized EEG activity
We also examined the effects of the same saline injection made under conditions of quiet wakefulness during preceding EEG activation. During these periods, the rat was awake, sometimes in minor motor activity, and the patterns of basal EEG and EMG drastically differed from those in slow-wave sleep (see Table). In awake state, mean values of EEG total power in both structures were much lower, the delta and alpha waves were reduced drastically, and high-frequency beta and gamma waves were much more prominent than during slow-wave sleep. Similar to sleep state, there were minor between-structure differences in basal electrical activity; the VTA showed a significantly higher proportion of delta and lower proportion of theta waves than did the cortex.
As shown in Figs. 1 (and Fig. S2 in Online Resource 2), saline injected under these conditions has no significant effect on all analyzed parameters. However, EEG total power increased slightly but gradually within the analyzed interval, reflecting the appearance of sleep in some rats. Similarly, EMG activity showed a tendency to decrease. Since all recordings were started during the active state, these slow changes are not related to saline injection itself and reflect a higher probability of sleep episodes, when all recordings were started in active state. Although the effect of saline on individual EEG waves was insignificant in both structures for each individual wave, a weak, significant (p<0.05) increase in gamma activity was seen in the VTA but absent in the cortex (see Fig. S2).
State-dependence of saline effects
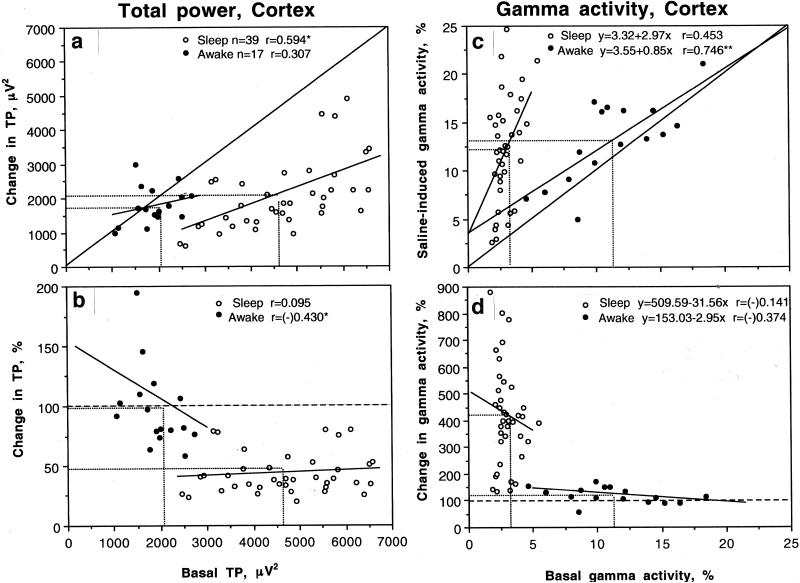
While these data suggest that the effects of saline differ drastically depending upon basal activity state, correlation and regression analyses were used to characterize this state-dependency quantitatively. These analyses are shown only for the cortex, but the results were similar in both structures.
Fig. 3A shows the relationships between absolute values of cortical total power and its changes induced by iv saline shown separately for ‘sleep’ and ‘activity’ states. All data points in the sleep state were located below the line of no effect (i.e., total power always decreased following saline injection), but in active state the data points were located on this line (i.e., total power did not change following saline injection). The values in the sleep state showed a positive correlation (r=0.594, p<0.05), i.e. the effect was stronger when total power was large (i.e., when basal activity was more synchronized), but correlation was absent in the awake state. The same relationships are evident when the effects of saline were shown as relative change (Fig. 3B). In this case, EEG total power dropped about 50% in the sleep state but did not change in the active state. In the latter case, a negative correlation emerged (r=-0.430, p<0.05) within the continuum of awake state. The EEG total power decreased when its basal values were higher, closer to sleep, and increased when they were lower, (i.e., in high activity state).
Fig. 3.
The relationships between basal cortical EEG activity and its changes following iv saline injection. A and B show the relationships between absolute values of EEG total power (TP, μV2) and its changes (A, absolute and B, relative) after saline injection during awake (filled symbols) and sleep states (open symbols). C and D show similar relationships calculated for basal gamma power (%) and its changes after saline injection. Basal values of EEG total power and gamma power were calculated for 60-s interval before saline injection. Saline data were calculated as a mean of 4 values (second to fifth data points in Fig. 1, i.e., 5-25 s). Each graph shows line of no effect, regression lines (where correlation is significant) and mean values in each group (hatched lines).
Similar to our previous data with other sensory stimuli (Kiyatkin and Smirnov, 2010), EEG gamma activity showed the largest relative changes following saline injection (see Fig. 2). As can be seen in Fig. 3C, the increase in cortical gamma activity was strongly dependent upon its basal levels. When basal gamma activity in individual tests was low (low activity state, sleep), it showed a large increase, but the effect was absent when basal gamma activity was high (high activity state, awake). While the degree of gamma change was independent of basal activity within each group, the effect was clearly evident for all tests as a whole (D).
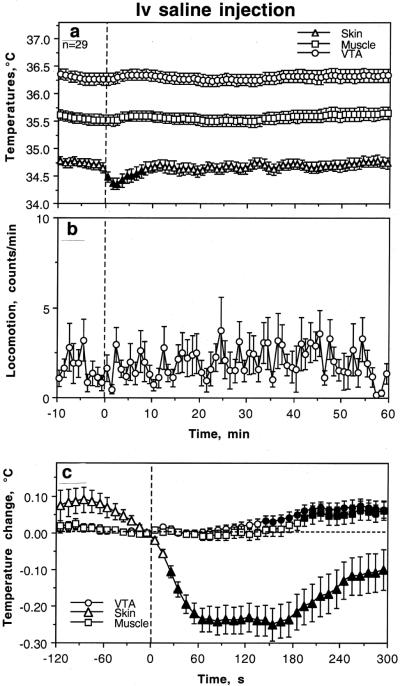
Temperature and locomotor recordings
Fig. 4 shows changes in temperatures and locomotion assessed before and 60 min after iv saline injection at minute time bins. Saline injection made under quiet rest did not affect locomotion and temperatures in the VTA and temporal muscle, but significantly decreased (-0.25°C) skin temperatures (F28,898=4.04, p<0.001). This effect was rapid but transient, disappearing at ~ 8 min post-injection. When the same data were reanalyzed with high temporal resolution (10-s bins) for the same time interval (300 s), the effect of saline injection was significant for each parameter (F28,898=7.14, 5.21 and 7.20 for VTA, muscle, and skin, respectively, each at least p<0.01). In this case, decrease in skin temperature was evident at the third data point (20-30 s after the injection start) and peaked at the second min post-injection. With this time resolution, we also observed weak and delayed effects on VTA and muscle temperature, with the first significant point at 140-150 s and 190-200 s post-injection, respectively. However, this effect was small in magnitude (~0.07°C) and could be detected as a significant change only in very large data samples.
Fig. 4.
Changes in brain (VTA), temporal muscle (Muscle) and facial skin (Skin) temperatures (°C) and locomotion (infrared beams breaks per min) following iv saline injection in freely moving rats in quiet rest. Top two graphs (A and B) show changes at slow time resolution (bin=1 min) for 60 min post-injection. The bottom graph (C) shows changes in VTA, Muscle and Skin temperatures assessed at high temporal resolution (bin=10 s) for the initial five min post-injection. n=number of tests. The values significantly different from pre-injection baseline are shown as filled symbols. The decrease in skin temperature (C) became significant at the third data point (20-30 s) but in the VTA and Muscle the effect was opposite but weak and delayed from the moment of injection (first significant value at 140-150 s and 190-200 s, respectively). Data were obtained in 9 rats (n=29 injections).
Discussion
Psychoactive drugs are often delivered into the organism by means of iv injection that provides rapid drug delivery to the brain and high brain drug levels. The present study revealed that a typical stress- and cue-free iv injection of pharmacologically neutral saline delivered to quietly resting freely moving rats induces rapid, transient and generalized EEG desynchronization, a neural effect that would precede direct effect of any injected drug on neural cells. Therefore, if the procedure of injection per se creates a rapid sensory signal from the periphery, thus affecting neural activity, its repeated pairing with the neural effects induced by drugs in the brain would result in Pavlovian conditioning, i.e., changes in the effects of drugs and the appearance of conditioned activating effects of the injection procedure. While various environmental and cognitive factors associated with drug intake could play prominent roles as drug-related cues, such roles should also be attributed to the interoceptive stimulation associated with the procedure of iv drug administration.
Peripheral sensory signal induced by iv saline administration
The immediate effects of saline injection were similar to those induced by natural somato-sensory stimuli. For example, a brief sound also induced a transient cortical EEG desynchronization, with decreases in alpha and increases in beta and gamma frequencies (Kiyatkin and Smirnov 2010). In contrast to the immediate response to sound, the change induced by saline injection had an onset latency of several seconds, reflecting the time necessary for the released saline to interact with vascular neural substrates and transmit the signal to the CNS. Saline injection also mimicked sound stimulus in its ability to induce neck EMG activation, but the effect of sound was immediate and larger in magnitude. Although tonic increase in EMG activity was prevalent in both cases, saline injection could induce a transient phasic EMG change, especially following initial presentations. Importantly, EMG activity in both cases had longer onset latencies and later peaks than did EEG activity, confirming its central mediation. However, when motor activity was evaluated by breaks of infrared beams in minute time scale, both saline injection and sound did not induce any significant effect. Saline injection performed in quiet resting conditions was also unable to change significantly brain and muscle temperatures, but rapidly and transiently decreased skin temperature, suggesting peripheral vasoconstriction—a sensitive, centrally mediated physiological response to various arousing stimuli that is aimed at heat retention inside of an organism (Altschule 1951; Baker et al. 1968; Solomon et al. 1964). Importantly, this rapid and brief effect was evident at high-temporal resolution analyses and virtually disappeared at the scale of minutes that is usually used to analyze temperatures.
Similar to that with simple sensory stimuli, the activating effect of saline injection was clearly evident during slow-wave synchronized EEG activity and virtually fully disappeared in active states, when the EEG was naturally desynchronized. This state-dependency is evident in our correlative analyses performed for two activity extremes: deep sleep and behavioral activation (see Fig 3). When saline was injected during low activity state (high-magnitude, low-frequency EEG signal), it induced EEG activation to the levels seen in the awake state. In contrast, in the awake, desynchronized state, saline injection lost its activating (arousing) potential with virtually no effects on each EEG wave. Only high-frequency gamma activity, the most sensitive index of activation, showed a minimal effect, which was evident only in the VTA.
In addition to the cortex, the traditional area of EEG recordings, electrical activity was also recorded from the VTA (in this case, the recorded signal is usually called ‘local field potentials’). Surprisingly, both structures had similar distributions of signal frequencies in baseline, mostly similar differences between awake and sleep states, and a similar pattern of activation response following saline injection. However, there were certain between-structure differences in slow-frequency waves (see Table). In the awake state, VTA activity showed a higher proportion of delta and lower proportion of theta waves than did the cortex. In the sleep state, VTA still showed a higher proportion of delta, but lower proportion of alpha waves than the cortex. Saline injection resulted in significant changes in all individual cortical EEG waves. In contrast, in the VTA theta activity was stable and changes in high-frequency beta and gamma activities were stronger and more prolonged than in the cortex. While it is difficult to speculate on neuronal mechanisms underlying differences in basal activity and responsiveness in the cortex and VTA, these data suggest that visceral sensory signal elicited by saline injection equally rapidly reaches cortical and subcortical brain structures.
Mechanisms that trigger a visceral sensory signal
While sensory stimulation is an evident cause of sound-induced EEG desynchronization, several physical factors could be responsible for triggering a similar neural effect following iv saline injection. Since saline was injected at room temperature (~22°C), which is ~15°C lower than that in the body core (~37°C), temperature impact of the injection appears to be the most obvious factor. Sensory nerves abundantly innervate peripheral vessels, including veins, and afferent endings of these nerves express a number of transient receptor potential (TRP) channels (see Chapham 2003 for review) that are sensitive to temperature and, being stimulated, could produce an ascending excitatory signal to the CNS. In contrast to other channels, which have Q10 within 3-5 (i.e., increase in activity with a 10°C temperature change), TRP channels have exceptionally high Q10 (TRPV3 >20; TRPV1 and 4, 10-20), making them suitable to detect even slight and transient temperature fluctuations. Baroreceptors, a subtype of mechanoreceptors that are sensitive to pressure, are also expressed abundantly within the cardio-vascular system and they could be another possible contributor. Thus, sudden changes in pressure that could accompany iv saline injection into the jugular vein in close proximity to the heart could be another source of ascending afferent signals to the CNS. In order to verify the role of temperature factor, it is important to inject saline at body core temperature but it is quite a difficult task in rats because of a long catheter extension, the additional volume of a liquid swivel, and a relatively small injection volume. Importantly, iv injections in most studies in animals are performed at room temperatures, often using high injection speeds. Therefore, based on our data it is reasonable to assume that stronger subjective (Abreu et al. 2000), physiological, and behavioral effects (Brown and Kiyatkin 2005; Panlilio et al. 1998; Samaha et al. 2002, 2004; Wakabayashi et al. 2010) of high-speed drug injections could be at least in part associated with stronger impacts of temperature, pressure, and ionic change—major physical factors associated with the injection procedure.
Functional implications
Independently of the precise nature of physical factors associated with iv saline injection and the nature of receptive neural substrates activated by these factors, this study demonstrates that the procedure of stress- and cue-free iv saline injection elicits weak, transient neural activation, manifesting in EEG desynchronization and EMG activation. Although this procedure does not affect gross locomotion and brain and body temperatures (evaluated at minute time scale), it induces transient peripheral vasoconstriction. It is likely that a similar phenomenon will be observed in humans if other conditions remain the same, but the volume of iv injection in humans is typically much less than in rats (rats: 0.15 ml/450g, human, 0.5-2 ml/70 kg), the injected liquid is often warmed, the injection speed is lower, and the injection site is typically in the peripheral vein far from the heart. However, this phenomenon is clearly relevant for animal research (especially in rats and mice); when drug solutions are not warmed to body temperature, the injection volumes are relatively large (0.1-0.4 ml), and the injection speed is often very high (4-10 s).
If the iv injection per se is able to produce a peripheral sensory signal that results in detectable CNS response that is followed by a more prolonged direct effect of the drug on brain cells, with repeated drug use, the procedure of iv injection becomes a conditioned stimulus (Pavlov 1927) and could play the role of a drug-related sensory cue. It is known that multiple factors that precede drug administration and accompany it act as conditioned drug-related cues that trigger drug craving in dependent individuals and alter the effects of drugs. This study suggests that this role could also be attributed to the procedure of iv drug administration – the critical event of drug experience. This role could explain well-known clinical observations that habitual cocaine users often perceive a similar immediate subjective effect as well as similar (but weaker) autonomic responses when neutral saline or other saline-like substances are substituted for cocaine (Cascella et al. 1989; Muntaner et al. 1989). Finally, our study demonstrates the importance of visceral sensory systems in providing the CNS with continuous information about the state of an organism's internal environment. While under physiological conditions this peripheral afferent inflow is critical for detecting any potentially dangerous homeostatic shifts and structural damages, it could play an important, although not yet appreciated, role in drug addiction. All major addictive drugs are self-administered in areas densely innervated by sensory nerves (i.e., lung alveoli, nasal and oral cavities, veins) and afferents of these nerves abundantly express numerous ionic channels (i.e., K+, Ca++, TRP, etc) and neuroreceptors (i.e., nicotinic cholinoreceptors) that could be directly affected by these drugs. Therefore, in addition to “non-specific” activation of visceral sensory afferents induced by any injection, drug-induced activation of these afferents could provide the CNS with rapid neural signal that always precedes more delayed and prolonged direct effects of drugs on neural cells. This peripherally driven pharmacological mechanism appears to play an important role in drug addiction (Kiyatkin and Smirnov 2010; Wise et al. 2008).
Supplementary Material
Acknowledgements
This research was supported by the Intramural Research Program of the NIH, NIDA. We wish to thank Jeremy Tang for participation in Exp. 2 of this study.
References
- Abreu ME, Bigelow GE, Fleisher L, Walsh SL. Effect of intravenous injection speed on responses to cocaine and hydromorphone in humans. Psychopharmacology. 2000;154:76–84. doi: 10.1007/s002130000624. [DOI] [PubMed] [Google Scholar]
- Altschule MD. Emotion and circulation. Circulation. 1951;3:444–454. doi: 10.1161/01.cir.3.3.444. [DOI] [PubMed] [Google Scholar]
- Baker M, Cronin M, Mountjoy D. Variability of skin temperature in the waking monkey. Am J Physiol. 1976;230:449–455. doi: 10.1152/ajplegacy.1976.230.2.449. [DOI] [PubMed] [Google Scholar]
- Berridge MS, Apana SM, Nagano KK, Berridge CE, Leisure GP, Boswell MV. Smoking produces rapid rise of [11C]nicotine in human brain. Psychopharmacology. 2010;209:383–394. doi: 10.1007/s00213-010-1809-8. [DOI] [PubMed] [Google Scholar]
- Brown PL, Kiyatkin EA. Brain temperature change and movement activation induced by intravenous cocaine delivered at various injection speeds in rats. Psychopharmacology. 2005;181:299–308. doi: 10.1007/s00213-005-2244-0. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. Rhythms of the brain. Oxford University Press; Oxford: 2006. [Google Scholar]
- Cascella NG, Nagoshi CT, Muntaner C, Walter D, Haertzen CA, Kumor KM. Impulsiveness and subjective effects of intravenous cocaine administration in the laboratory. J Subst Abuse. 1994;6:355–66. doi: 10.1016/s0899-3289(94)90278-x. [DOI] [PubMed] [Google Scholar]
- Chapham DE. TRP channels as cellular sensors. Nature. 2003;426:517–24. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- Hobson JA. Sleep and dreaming. In: Zigmond MJ, Bloom FE, Landis SC, Roberts JL, Squire LR, editors. Fundamental Neuroscience. Academic Press; San Diego: 1999. pp. 1207–1227. [Google Scholar]
- Kiyatkin EA. Brain temperature homeostasis: physiological fluctuations and pathological shifts. Front Biosci. 2010;15:73–92. doi: 10.2741/3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyatkin EA, Brown PL. Dopamine-dependent and dopamine-independent actions of cocaine as revealed by brain thermorecording in freely moving rats. Eur J Neurosci. 2005;22:930–938. doi: 10.1111/j.1460-9568.2005.04269.x. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA, Smirnov MS. Rapid EEG desynchronization and EMG activation induced by intravenous cocaine in freely moving rats: a peripheral, nondopamine neural triggering. Amer J Physiol. 2010;298:R285–R300. doi: 10.1152/ajpregu.00628.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Moal M, Simon H. Mesocorticolimbic dopaminergic network: functional and regulatory roles. Physiol Rev. 1991;71:155–234. doi: 10.1152/physrev.1991.71.1.155. 1991. [DOI] [PubMed] [Google Scholar]
- McClung R, Reilly E, Dafny N. Urethane modification of EEG-like activity and acoustically evoked field potentials recorded from deep nuclei. Appl Neurophysiol. 1976-1977;39:11–26. doi: 10.1159/000102471. [DOI] [PubMed] [Google Scholar]
- Muntaner C, Cascella NG, Kumor KM, Nagoshi C, Herning R, Jaffe J. Placebo responses to cocaine administration in humans: effects of prior administrations and verbal instructions. Psychopharmacology (Berl) 1989;99:282–286. doi: 10.1007/BF00442823. [DOI] [PubMed] [Google Scholar]
- Panlilio LV, Goldberg SR, Gilman JP, Jufer R, Cone EJ, Schindler CW. Effects of delivery rate and non-contingent infusion of cocaine on cocaine self-administration in rhesus monkeys. Psychopharmacology. 1998;137:253–258. doi: 10.1007/s002130050618. [DOI] [PubMed] [Google Scholar]
- Pavlov IP. Conditioned reflexes (Ed and trans. By GV Anrep) Dover, New York: 1927. [Google Scholar]
- Paxinos J, Watson C. The rat brain in stereotaxic coordinates. Academic Press; Sydney: 1996. [Google Scholar]
- Samaha AN, Li Y, Robinson TE. The rate of intravenous cocaine administration determines susceptibility to sensitization. J Neurosci. 2002;22:3244–3250. doi: 10.1523/JNEUROSCI.22-08-03244.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaha AN, Mallet N, Ferguson SM, Gonon F, Robinson TE. The rate of cocaine administration alters gene regulation and behavioral plasticity: implications for addiction. J Neurosci. 2004;24:6362–6370. doi: 10.1523/JNEUROSCI.1205-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki H, Coffey P, Villegas-Perez MP, Vidal-Sanz M, Young MJ, Lund RD, Fukuda Y. Light induced EEG desynchronization and behavioral arousal in rats with restored retinocollicular projection by peripheral nerve graft. Neurosci Lett. 1996;218:45–48. doi: 10.1016/0304-3940(96)13121-9. [DOI] [PubMed] [Google Scholar]
- Solomon GF, Moos RH, Stone GC, Fessel WJ. Peripheral vasoconstriction induced by emotional stress in rats. Angiology. 1964;15:362–365. doi: 10.1177/000331976401500806. [DOI] [PubMed] [Google Scholar]
- Wakabayahi KT, Weiss MJ, Picup KN, Robinson TE. Rats markedly escalate their intake and show a persistent susceptibility to reinstatement only when cocaine is injected rapidly. J Neurosci. 2010;30:11346–55. doi: 10.1523/JNEUROSCI.2524-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA, Wang B, You ZB. Cocaine serves as a peripheral interoceptive conditioned stimulus for central glutamate and dopamine release. PLoS One. 2008;6:e2846. doi: 10.1371/journal.pone.0002846. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.