Highlights
► We classified four different populations among human spiral ganglion cells. ► Spatial organization of human spiral ganglion cells and nuclei along the cochlea was determined. ► Size of spiral ganglion perikarya influences action potential initiation site. ► Simulation of single nerve fiber response showed different stimulation threshold. ► Temporal parameters of the AP are affected by perikaryal size.
Abbreviations: AP, action potential; cpcc, cophenetic correlation coefficient; E1, electrode position 1; E2, electrode position 2; E3, electrode position 3; E4, electrode position 4; IS, initiation site; LAV, lower adjacent value; LSD, Fisher’s least significant difference test; SGCs, spiral ganglion cells; UAV, upper adjacent value
Key words: spiral ganglion cells, spatial organization, morphometric classification, hierarchical clustering, computer simulation, microstimulation
Abstract
The unique, unmyelinated perikarya of spiral ganglion cells (SGCs) in the human cochlea are often arranged in functional units covered by common satellite glial cells. This micro anatomical peculiarity presents a crucial barrier for an action potential (AP) travelling from the sensory receptors to the brain. Confocal microscopy was used to acquire systematically volumetric data on perikarya and corresponding nuclei in their full dimension along the cochlea of two individuals.
Four populations of SGCs within the human inner ear of two different specimens were identified using agglomerative hierarchical clustering, contrary to the present distinction of two groups of SGCs. Furthermore, we found evidence of a spatial arrangement of perikarya and their accordant nuclei along the cochlea spiral. In this arrangement, the most uniform sizes of cell bodies are located in the middle turn, which represents the majority of phonational frequencies.
Since single-cell recordings from other mammalians may not be representative to humans and human SGCs are not accessible for physiological measurements, computer simulation has been used to quantify the effect of varying soma size on single neuron response to electrical micro stimulation. Results show that temporal parameters of the spiking pattern are affected by the size of the cell body. Cathodic stimulation was found to induce stronger variations of spikes while also leading to the lowest thresholds and longest latencies. Therefore, anodic stimulation leads to a more uniform excitation profile among SGCs with different cell body size.
Introduction
Within the mammalian cochlea the existence of two different types of spiral ganglion cells (SGCs) based on morphological criteria is well described. A vast majority of 90–95% belongs to ‘large’ type I cells while only 5–10% are assigned to ‘small’ type II SGCs (Ota and Kimura, 1980; Spoendlin, 1981). One significant feature of the human spiral ganglion cells is the lack of myelin layers around the majority of their perikarya. Only 5% of the neurons are surrounded by several loose myelin layers (Ota and Kimura, 1980; Arnold, 1987; Spoendlin and Schrott, 1989) often arranged in functional units covered by common satellite glial cells (Liu et al., 2011).
Prior research has led to speculation that the unique human morphology of SGC somata (Tylstedt and Rask-Andersen, 2001; Glueckert et al., 2005) affects excitability and spiking pattern (Rattay et al., 2001; Rusznak and Szucs, 2009) since these unmyelinated neuronal parts are the first energy barrier for an action potential travelling from the sensory epithelium to the auditory cortex (Rattay, 1995; Rattay et al., 2001). Nevertheless, ever since Hodgkin and Huxley (1952) developed their model of a nonmyelinated squid axon, functional electrical stimulation has focused on myelinated nerve structures and nodes of Ranvier. Thus, in order to simulate the response of single SGCs to micro stimulation, some modelers have considered the soma to be myelinated (Frijns et al., 1994) or passive (Woo et al., 2009b), while others have completely neglected the presence of a soma (Mino et al., 2004). Since detailed data of this specific human feature are currently available, theoretical analysis concerning single neuron response shall incorporate fundamental anatomical data. These peculiarities of human SGCs are not considered so far while a growing amount of studies about cochlear implant strategies, electrode positioning within the cochlea, psychological measurements and recorded audiograms emerge. Nonetheless, the physiology of hearing is not yet fully understood, e.g., complex coding strategies as speech recognition and perception in noisy environment (Drennan et al., 2007), attenuation effects (Smit et al., 2009) and the temporal fine structure of the neural pattern.
In order to gain further knowledge of the shape, distribution and size of this unique human neuronal part, we acquired 3D morphometrics confocal stacks of normal human SGCs systematically and analyzed them using an agglomerative hierarchical cluster algorithm. Determination of neuronal classes based on micro-anatomic features of cochlea perikarya provides crucial geometric information from human spiral ganglion neurons.
Since vital human SGCs are not experimentally accessible, we used computer simulation to study possible changes in excitability with respect to varying soma sizes. To do this, we adapted the model of Rattay et al. (2001), because it is the only cochlear nerve model which accounts for the poorly myelinated soma region in man. Based on our analysis of morphometric data, this paper sheds light on the difference in thresholds and action potential (AP) properties of single neurons stimulated by microelectrodes with mono- and biphasic pulses at different positions.
Experimental procedures
Specimens
The present study evaluated two temporal bones from individuals aged 74 (specimen 1, male) and 56 (specimen 2, male), respectively, without any known inner ear disease or hearing loss (audiograms not available). The temporal bones were obtained during routine autopsy at the Institute of Pathology, Innsbruck Medical University. Cochleae were fixed with ice cold 4% paraformaldehyde buffered with 0.1 M phosphate-buffered saline (PBS, pH 7.4) within <5 h post mortem via the round- and oval window and were immersed in the same fixative for 24 h at 4 °C. Small-sized diamond drills were used to mill off cochleae surrounding the bone. The cochleae were then dissected using a stereo microscope and forceps and subsequently decalcified using 20% ethylenediaminetetraacetic acid at pH 7.4 for 6 weeks, after which they were rinsed thoroughly in PBS. Inner ears were prepared for cryoembedding according to Coleman et al. (2009). Specimens were serially sectioned with a cryomicrotome perpendicularly and radially respectively, to the modiolus at 35 μm thicknesses, thereby providing bipolar spiral ganglion somata in their full dimensions (Tylstedt et al., 1997; Tylstedt and Rask-Andersen, 2001) on each cryosection.
Immunohistochemistry
In order to delineate perikarya from their surrounding satellite glial cells, antibodies against β-III-tubuline and the intermediate filament S-100 were used. After washing the sections in PBS (pH 7.4; 3 × 10 min), non-unspecific sites were blocked with a dilution containing 0.1 M PBS, 30% normal donkey-serum and 0.3% Triton X-100 for 2 h at room temperature. Cryosections were incubated overnight in a humid chamber at 4 °C with a mixed solution of mouse-monoclonal anti-β-III-tubuline antibodies (1:500, Chemicon, Billerica, MA, USA) and rabbit-polyclonal anti-S-100 antibodies (raised against whole S-100 protein purified from bovine brain, 1:200, Sigma–Aldrich, St. Louis, MO, USA), followed by 75 min at 37 °C.
After being rinsed with PBS (3 × 10 min), sections were incubated with secondary antibodies conjugated to Alexa Fluor 488 (donkey anti-mouse, 1:1000, Invitrogen, Carlsbad, CA, USA) and Alexa Fluor 633 (donkey anti-rabbit, 1:400, Invitrogen, Carlsbad, CA, USA), diluted in PBS for 2 h at RT and mounted using Vectashield mounting medium with DAPI (Vector Laboratories, Burlingame, CA, USA) for additional nuclei-counterstaining. Fig. 1 presents an example of immunolabeling of TuJ1 (green), S-100 (red) and DAPI (blue) staining of the human spiral ganglion. White arrows indicate cell-body candidates for volumetric reconstruction. Only perikarya completely surrounded by satellite glial cells without showing any shrinkage were chosen for three dimensional (3D) reconstructions.
Fig. 1.
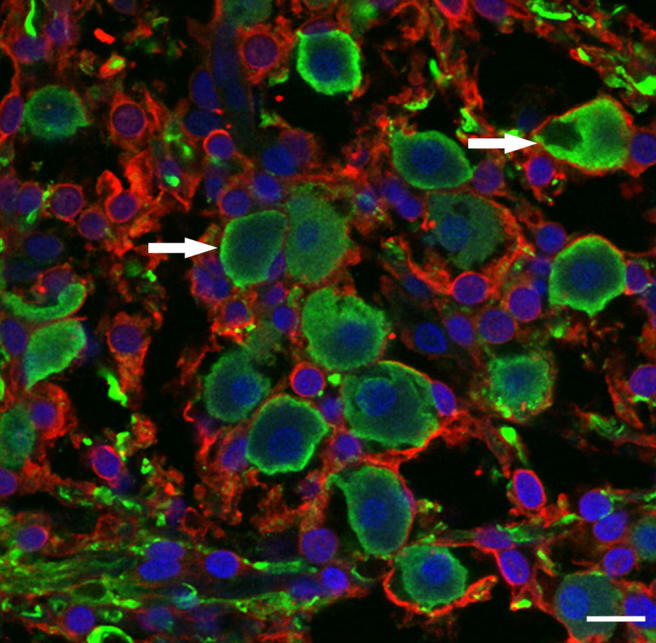
Immunolabeling for TuJ1 and S-100 in human spiral ganglion. SGCs are stained positively for TuJ1 (green). The satellite glial cells surrounding SGCs are strongly stained for S-100 (red). Nuclei of neurons and satellite cells are illustrated blue. White arrows indicate cell-body-candidates for volumetric reconstruction. Scale bar represents 20 μm.
Acquisition of confocal data
Sections were analyzed on a Zeiss LSM 510 Meta or a Leica SP5 confocal laser-scanning microscope equipped with a 63×/1.4 NA oil immersion lens. Simultaneous detection of perikarya and satellite glial cells involved excitation with the 488 nm line of an argon ion laser and a 633 nm HeNe laser. Cell nuclei were visualized using a 405 nm diode laser.
Pixel sizes selected according to the Nyquist criterion were used for the acquisition of 3D-stacks, resulting in the following resolution: x = 38 nm, y = 38 nm, z = 122 nm.
Image processing
Confocal image stacks of the SGCs were processed with a high-performance workstation (Z800, Hewlett–Packard, Palo Alto, CA, USA, 32 GB RAM, a six-core Intel® Xeon® processor and a Quadro FX4600 graphical board) using Amira 5.3.3 (Mercury Computer Systems Inc., San Diego, CA, USA). Each stack was separated subsequently into its component channels to allow for image restoration via individual deconvolution. As a result, a theoretical point spread function was computed for each channel. The point spread functions were then used to deconvolute each channel using a non-blind maximum-likelihood image restoration algorithm (Holmes and Liu, 1989) over 40 iterations. To improve signal-to-noise-ratio a median filter was applied to the data prior to structure segmentation. Perikarya and nuclei were manually segmented using the Amira’s ‘Segmentation Editor’. Segmentation data were saved in a label field which was used to determine the volume of each structure using the ‘MaterialStatistics’ module from Amira. For visualization, data were smoothed by producing a surface from a resampled label field.
Statistical analysis
Statistical significance was calculated using one-way analysis of variance followed by Fisher’s least significant difference (LSD) test and the Bonferroni correction. Normality of data was carried out using quantile–quantile plots. Hierarchical cluster analysis was performed on volumes of segmented SGC and corresponding nuclei in order to identify clusters with comparable similarities. In the absence of an expectation as to the number of clusters present, we used an agglomerative (bottom-up) approach that starts with as many clusters as objects. Clusters are gradually merged in the following way until each object belongs to a cluster. All members of a cluster feature comparable similarities. For determining similarities between each pair of objects the distance matrix was calculated using the Chebyshev distance (maximum coordinate difference). This distance information was applied to ascertain the proximity of cell volumes to each other computed by the unweighted average distance algorithm.
To choose the best fitting distance metric and algorithm examining the present structures in the pairwise distance matrix of data, the cophenetic correlation coefficient was calculated systematically (data not shown). Both above-mentioned algorithms reached the highest cophenetic correlation coefficient (0.84 ± 0.02).
To gain more information about the distribution of the volumes, the normalized eigenvalues of the correlation matrix were calculated and visualized in a scree plot (Cattell, 1966). These values indicate the percentage of variance explained. A linear fit (Nelson, 2005) calculated with the first 14 normalized eigenvalues was used to overcome reported problems of finding the distinctive sharp break (Costello and Osborne, 2005).
The ‘sharp break’ was defined at the number of populations where R2 of fitting was ⩾0.8. Statistics were performed using MatLab® 2011a (MathWorks, Natick, MA, USA).
Computational model
In order to analyze the effect of the collected variations in soma size of human cochlear neurons, a detailed compartment model of one specific neuron was used. The two processes were split up into cylindrical compartments consisting of passive internodes (six of varying size for the peripheral process with 40 layers of myelin, five central internodes with 80 layers of myelin) and active nodes of Ranvier. The spherical soma as well as the postsomatic and also the preceding presomatic region, consisting of three compartments, are assumed to be unmyelinated, i.e., only surrounded by three membrane layers (one SGC membrane, two satellite glial membranes) and modeled as active compartments with Hodgkin Huxley ion channel kinetics. Note that the original standard value of 30 μm for the diameter of the soma is certainly higher than the observed values, as discussed below. For details about geometric and electrical parameters see Rattay et al. (2001).
For the simulations in this study all geometric and electrical model parameters are regarded to be constant, except the value of the soma diameter that was calculated from collected volumetric data of different human SGCs. By varying the diameter of the soma the influence on action potential (AP) properties and thresholds to micro stimulation was examined. Parameters like the AP height and AP width are explained in detail in Bean (2007). For the stimulation by a point electrode, thresholds for different electrode positions and four pulse shapes have been calculated (i.e., 0.1 ms long monophasic pulses for cathodic amplitudes and anodic pulses as well as biphasic pulses of equal amplitudes with 0.05 ms/phase for leading cathodic phase or pulses with anodic phase first).
Four active electrode positions that correspond to cochlear implant situations in the basal turn have been examined and are illustrated in Fig. 2. Two electrode positions (E1 and E2) simulate electrodes placed at the transition between bony columns housing nerve fiber bundles ad osseous spiral lamina (E1) and at the distal end of the osseous spiral lamina (E2). E1 was previously introduced by Rattay et al. (2001).
Fig. 2.
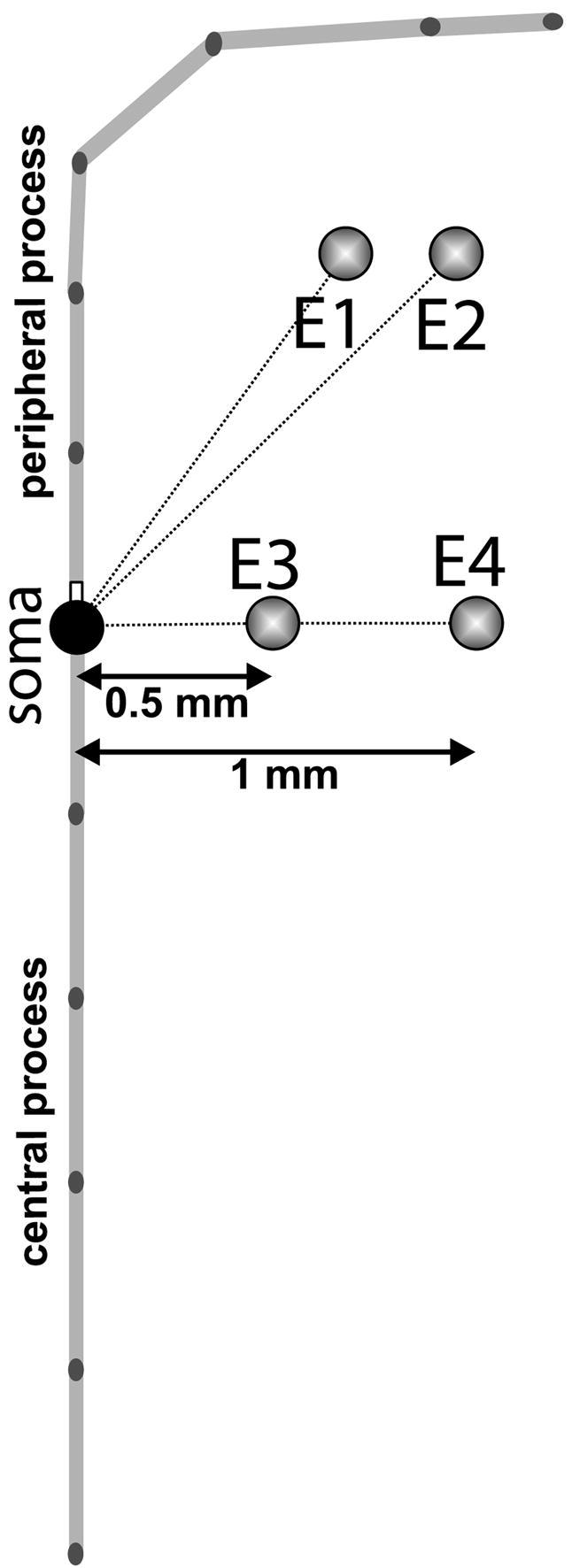
Electrode positions and spatial arrangement of the model neuron compartments. Peripheral and central processes are segmented in light gray internodes and black nodes of Ranvier. E1 and E2 simulate electrodes placed at the transition between bony columns and at the osseous spiral lamina. E3 and E4 are placed normal to the soma at to two different distances.
E3 and E4 correspond to positions of cochlear implant electrodes placed at the level of Rosenthal’s canal that contain SGC somata orthogonal to the neural axis (Fig. 2) at a distance of 0.5 mm (E3) and 1 mm (E4). Therefore E1 and E2 represent typical electrode positions with straight electrode arrays, whereas E3 and E4 illustrate perimodiolar electrode placement (Fig. 2).
Additionally, the following parameters were acquired during simulation: the initiation site (IS), the corresponding time of the peak as well as the arrival time of the spike at the soma and the time at which the AP occurs at the end of the neuron as defined by the last compartment.
We assumed a spherical electrode in an infinite homogeneous extracellular medium with a resistivity of pe = 300 Ω cm. Under quasi-static conditions the extracellular potentials Ve were calculated by
where r is the distance from a compartment to the electrode and IEL is the amplitude of the stimulating current pulse. The current to the center of the nth compartment of the model neuron consists of the following components: capacitive current, ion currents across the membrane and ohmic currents to the left and right neighbors.
The following system of differential equations is deduced by introducing the transmembrane voltage
to compute the time course of Vn in every compartment. Applying Kirchhoff’s law for compartment n results in
where Vi, R and Cm denote the intracellular potential, axial resistance and membrane capacity, respectively. In order to compute these parameters for each compartment, an intracellular specific resistivity of 150 Ω cm and a specific membrane capacity of 1 μF/cm2 (Rattay et al., 2001) were used. Note that the resistance and capacitance of the somatic compartment will also change with varying diameter since the surface is also altered.
Results
Descriptive statistics
To provide an overview of quantified volumetric data we quantitatively described the main features of spiral ganglion cell somata and their corresponding nuclei. For this purpose we discuss the median (M) in this section as it best describes the central tendency of the reconstructed perikarya.
Perikarya
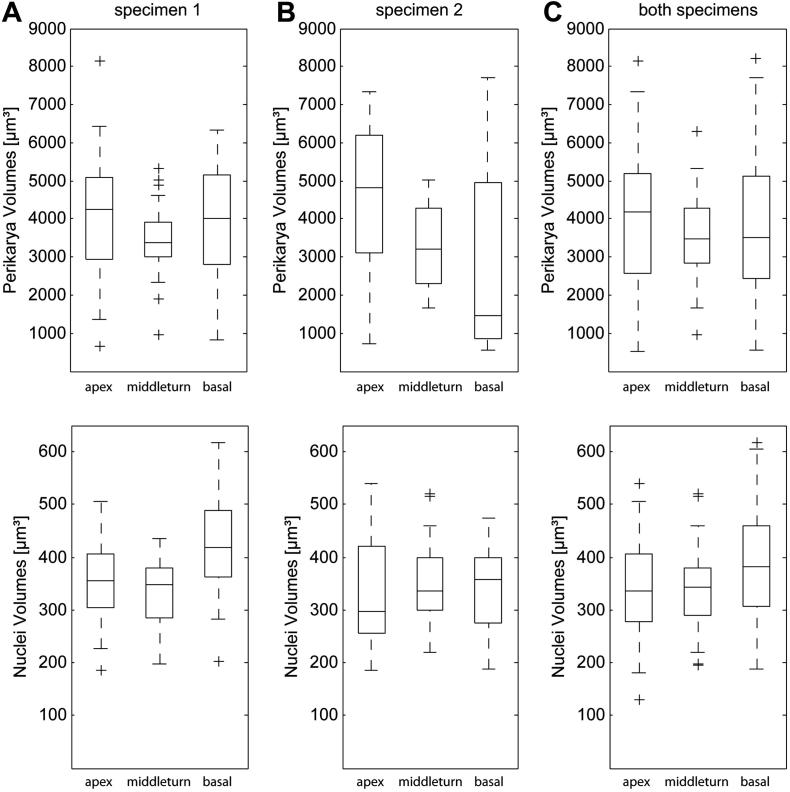
A closer look at specimen 1 shows that the perikaryal size of the apical- and basal turn are comparable (Mapical = 4235 μm3, Mbasal′ = 4011 μm3), varying only by 5.2%, with the largest cells located in the apex. The smallest SGCs from this inner ear are identified within the middle turn (M = 3379 μm3) representing a volumetric difference of 20.2% compared to the median apical value. There is no significant difference regarding the perikarya volumes determined in specimen 1 (Fig. 3A).
Fig. 3.
Box–Whisker-plot depicts distributions of volumes from reconstructed perikarya and their nuclei. Data illustrates all acquired volumes of specimen 1 (column A, n = 83), specimen 2 (column B, n = 55) and both specimens (column C, n = 138) according to their location within the human cochlea. The upper horizontal row illustrates perikaryal volumes; lower horizontal row represents the corresponding nuclei.
The SGCs in the apical turn of specimen 2 (Fig. 3B) likewise reach the highest evaluated volumes (M = 4818 μm3). In addition, the smallest SGCs (M = 1474 μm3) in this specimen are also identified in the basal turn, reaching a volume 69.4% smaller than the perikarya in the low frequency region. The SGCs in the middle turn (M = 3208 μm3) show a 33.4% lower volume compared to cell bodies of the apical region. Significant difference in SGC volume is found in this specimen between the apical- and the basal turn (p < 0.1, LSD).
The biggest SGCs in both specimens (M = 4197 μm3) are found in the apical turn (Fig. 3A, Table 1). These SGCs show 17.3% higher volume compared to the perikarya measured in the middle turns (M = 3473 μm3). Reconstructed SGCs from the basal turn of both specimens reach an M of 3510 μm3, which is only 1.1% larger than the cells from the middle turn. No significant difference is found between perikaryal volumes of apical-, middle- and basal turn; however, the largest cells are observed in the low-frequency region (Fig. 3B and C).
Table 1.
Summary of the determined perikarya- and nuclei volumes and their diameters. Presented data were calculated from manually segmented data. The first part presents the evaluated perikarya volumes of both analyzed cochleae (n = 138), specimen 1 (n = 83) and specimen 2 (n = 55) according to their location within the inner ear. Volumetric data of corresponding nuclei is shown in the lower part of the table in the same manner by the median (M [μm3]), the lower quartile (QL [μm3]), upper quartile (QU [μm3]) as well as the lower adjacent value (LAV [μm3]) and the upper adjacent value (UAV [μm3]); d is shown as μm
| Perikarya | Region | Median | d | QL | Qu | UAV | LAV |
|---|---|---|---|---|---|---|---|
| Specimen 1 | apical | 4235 | 20.07 | 2948 | 5103 | 6436 | 1345 |
| middle turn | 3379 | 18.62 | 2996 | 3928 | 4621 | 2321 | |
| basal | 4011 | 19.71 | 2789 | 5165 | 6325 | 825.9 | |
| Specimen 2 | apical | 4818 | 20.96 | 3118 | 6190 | 7345 | 732 |
| middle turn | 3208 | 18.30 | 2291 | 4298 | 5024 | 1659 | |
| basal | 1474 | 14.12 | 861.8 | 4962 | 7713 | 570.2 | |
| Both specimens | apical | 4197 | 20.01 | 2572 | 5184 | 7345 | 531.6 |
| middle turn | 3473 | 18.79 | 2821 | 4298 | 5337 | 1659 | |
| basal | 3510 | 18.86 | 2439 | 5121 | 7713 | 570.2 | |
| Nuclei | |||||||
| Specimen 1 | apical | 355.1 | 8.79 | 303.3 | 407.2 | 506.5 | 226.3 |
| middle turn | 346.9 | 8.72 | 284.8 | 380.3 | 434.9 | 196.5 | |
| basal | 419.4 | 9.29 | 361.7 | 489.8 | 617.8 | 282.8 | |
| Specimen 2 | apical | 298.2 | 8.29 | 256.4 | 421.2 | 540 | 186.2 |
| middle turn | 334.9 | 8.62 | 300.4 | 398.3 | 458.9 | 218.4 | |
| basal | 357.9 | 8.81 | 276.1 | 399.3 | 473 | 187 | |
| Both specimens | apical | 335.9 | 8.62 | 277 | 406.3 | 506.1 | 180.5 |
| middle turn | 344 | 8.70 | 289.5 | 379.5 | 458.9 | 218.4 | |
| basal | 381.7 | 9.00 | 306.5 | 459.8 | 606.2 | 187 | |
An individual difference in SGC volume between specimen 1 and 2 is found in the basal region (p < 0.1, LSD). Perikarya of the basal region from specimen 1 are 63.3% bigger than neurons of the corresponding region in specimen 2.
Moreover, the difference in volume between the apical regions of the cochleae is 13.8%. Surprisingly, SGC volumes in the middle turn are rather uniform; the percentage difference between SGC volumes from the middle turns of both cochleae is found to be only 5.1%. Note the upper adjacent value (UAV) and lower adjacent value (LAV) of the middle turns presented on Table 1.
Remarkably, the absolute differences between these two values are 2300 μm3 (n = 25) for specimen 1 and 3365 μm3 (n = 21) for specimen 2, respectively. This absolute difference is only about 50% compared with other regions of specimen 1 (apical turn = 5091 μm3, basal turn = 5499 μm3) and specimen 2 (apical turn = 6613 μm3, basal turn = 7143 μm3).
SGC volumes of the middle turn in specimen 1 and the apical turn of specimen 2 were calculated to be significantly different (p < 0.1, LSD).
Nuclei
Specimen 1 shows slight differences between apical- (M = 355.1 μm3) and middle turn (M = 346.9 μm3) nuclei (−2.3%), whereas basal nuclei are 18.1% bigger (M = 419.4 μm3) compared to the apical turn (Fig. 3A). Significant differences in the volume of nuclei are determined when comparing the apical- and middle turn with the basal region in specimen 1 (p < 0.05, Bonferroni).
SGC nuclei in specimen 2 are 12.3% bigger in the apical (M = 298.2 μm3) compared to the middle turn (M = 334.9 μm3) and 20% bigger compared to the basal (M = 357.9 μm3) SGC nuclei, respectively (Fig. 3B). However, nuclei volumes are not found to be significantly different along the cochlea spiral in specimen 2.
Analyzing data from both specimens (Fig. 3C), we found the smallest nuclei of SGCs (Table 1) in the apical region of the cochleae (M = 335.9 μm3), slightly larger (+2.4%) nuclei in the middle turn reached a median of 344 μm3 and in the basal turn 381.9 μm3. This increase in volume (basal turn nuclei) corresponds to 13.7% bigger nuclei compared to the nuclei in the apical turn. A significant difference is found when comparing volumes of the apical- and middle turn with the nuclei of the basal turn (p < 0.05, Bonferroni).
Furthermore, between the basal turn of specimen 1 and the apical-, middle- and basal turns of specimen 2 the volumetric difference is calculated to be significant (p < 0.05, Bonferroni).
Hierarchical clustering
Agglomerative hierarchical cluster analysis of volumetric data was used to identify similarities among reconstructed SGCs. The volume distribution was examined with scree plots of the percentage of variance explained which is described as a function of the amount of clusters.
Specimen 1
The applied algorithm of the cluster analysis identified four distinct populations of SGCs in specimen 1 (n = 83). Fig. 4A presents the results of this hierarchical clustering. The distinct groupings of the determined SGC-populations are color-coded in the scatterplot. These populations differ considerably in perikaryal size as well as in their incidence. The smallest identified population 1 of this cochlea is formed by 12% of reconstructed perikarya (Fig. 4A, violet colored) with a mean volume of 1288 ± 496 μm3. Interestingly, their corresponding nuclei are also found to be the smallest within the four populations (228.2 ± 60.4 μm3). The majority of measured cell bodies belonging to specimen 1 are classified as population 2 (80.7%) and have a mean volume of 3878 ± 932.6 μm3 (green colored). Their nuclei are identified to be slightly larger (372.8 ± 80 μm3) compared to the nuclei from population 1. Cell population 3 is represented by 5.8% (6297 ± 140.7 μm3, red) of reconstructed perikarya (red colored). Their associated nuclei have a mean volume of 436.1 ± 97.5 μm3 and represent the third-largest reconstructed group. The largest population 4 is represented by a single cell body with a mean volume of 8148 μm3 (cyan color). The volume of this perikaryon is 632.7% bigger compared to the mean volume calculated for population 1. Similarly, its nucleus measures 506.5 μm3, the largest reconstructed from this specimen. The volumetric difference compared with the corresponding value of the smallest population is 222%.
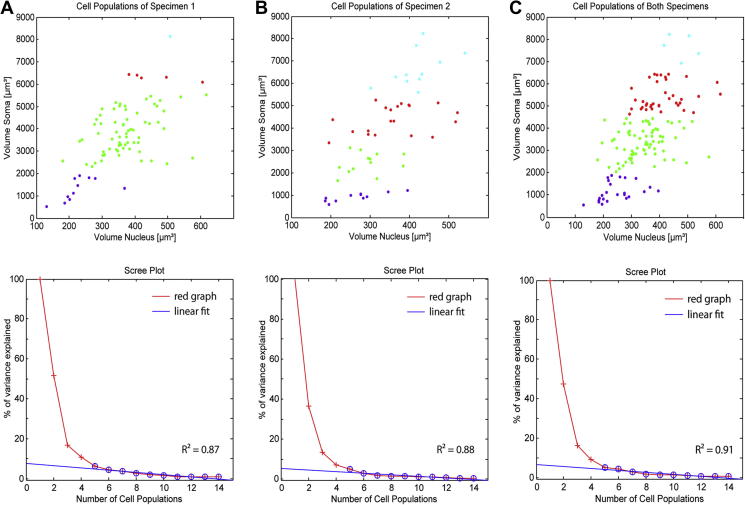
Fig. 4.
Result of the cluster analysis of reconstructed perikarya from specimen 1 (A, n = 83), specimen 2(B, n = 55) and both specimens (C, n = 138). The scatterplot illustrates arrangement of clusters in a color-coded manner, identified by the agglomerative hierarchical cluster algorithm applied on volumetric data. Purple dots represent the smallest cell population 1 while green and red colored dots illustrate population 2 and population 3 respectively. The biggest cell population 4 is visualized by cyan-colored dots. The screeplot strongly support the determined four cluster solution for each of the three data sets. The red graph presents the calculated percentage of variance explained. For determining the ‘sharp break’ in the scree plot a linear fit (blue line) of the percentage of variance explained was calculated. The “sharp break” was defined at the number of populations where R2 of fitting was ⩾0.8.
The existence of four different populations among the analyzed SGCs of specimen 1 is strongly supported by the scree plot shown in Fig. 4A, which shows a linear fit (R2 = 0.87) of the percentage of variance explained. Furthermore, the volumes of the four identified perikaryal SGC populations and their corresponding nuclei are calculated to be significantly different from each other (p < 0.001 for perikarya and p < 0.01 for nuclei respectively, Bonferroni).
The determined classes of SGCs are also identified among cell bodies from the apical-, middle- and basal turn of the cochlea and show very large differences in the incidence of cells within the populations of distinct turns. Detailed data are given in Table 2.
Table 2.
Summary of all determined perikarya- and nuclei volumes. Calculated mean ± SD volumes (μm3), diameter (d) (μm), number of cells (n) and frequency (%) of reconstructed perikarya and their corresponding nuclei of human cochleae are presented for pooled data of both cochleae as well as specimen-separated. Calculated volumes are additionally shown for apical-, middle- and basal turn of investigated specimen. Furthermore, evaluated values are presented for each population classified by the hierarchical cluster algorithm. The fist horizontal row (whole cochlea(e)) presents data calculated from all reconstructed cell bodies and their nuclei from specimen 1, specimen 2 and pooled data of both specimens respectively. The following horizontal rows (apical-, middle- and basal turn) show calculated data of analyzed SGCs according to their origin along the cochlea spiral
 | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (μm3) | SD (μm3) | Mean (μm3) | SD (μm3) | n= | Frequ. (%) | Mean (μm3) | SD (μm3) | Mean (μm3) | SD (μm3) | n= | Frequ. (%) | Mean (μm3) | d (μm) | SD (μm3) | Mean (μm3) | SD (μm3) | n= | Frequ. (%) | ||
| Whole cochlea(e) | Population 1 | 1288 | 496 | 228.2 | 60.4 | 10 | 12.0 | 919.3 | 192 | 264 | 67 | 11 | 20 | 1154 | 13.01 | 429.7 | 247.9 | 64 | 24 | 17.4 |
| Population 2 | 3878 | 932.6 | 372.8 | 80 | 67 | 80.7 | 2441 | 461.3 | 290.2 | 53.4 | 13 | 23.6 | 3352 | 18.57 | 661.3 | 343.4 | 77.7 | 77 | 52.2 | |
| Population 3 | 6297 | 140.7 | 436.1 | 97.5 | 1 | 5.8 | 4393 | 620.5 | 359.9 | 89.9 | 20 | 36.4 | 5407 | 21.78 | 592.5 | 409.6 | 74.7 | 37 | 26.8 | |
| Population 4 | 8148 | 0 | 506.5 | 0 | 1 | 1.1 | 6636 | 826 | 418 | 60.6 | 11 | 20 | 7675 | 24.47 | 543.3 | 475.1 | 50.6 | 5 | 3.6 | |
| Apical turn | Population 1 | 1860 | 829.1 | 254.3 | 69.8 | 12 | 37.5 | 874.4 | 201.6 | 231.4 | 63.9 | 2 | 14.3 | 1162 | 13.04 | 472.6 | 236.3 | 69.1 | 5 | 19.5 |
| Population 2 | 4416 | 558.3 | 373.7 | 58.7 | 17 | 53 1 | 3616 | 551.2 | 256.5 | 34.2 | 5 | 35.7 | 3599 | 19.01 | 642.6 | 323.5 | 78.3 | 20 | 43.5 | |
| Population 3 | 6418 | 25 | 394.5 | 16.7 | 2 | 6.3 | 5844 | 453.8 | 370.7 | 59 | 5 | 35.7 | 5723 | 22.19 | 803.3 | 391 | 64.4 | 16 | 34.8 | |
| Population 4 | 8184 | 0 | 506.5 | 0 | 1 | 3.1 | 7142 | 286.8 | 508.4 | 44 | 2 | 14.3 | 8148 | 24.97 | 0 | 506.5 | 0 | 1 | 2.2 | |
| Middle turn | Population 1 | 970.9 | 0 | 196.5 | 0 | 1 | 4.2 | 2294 | 359.7 | 281 | 55.8 | 8 | 38.1 | 2049 | 15.76 | 450.7 | 258.3 | 53.1 | 10 | 21.7 |
| Population 2 | 2211 | 280.6 | 251.8 | 19.8 | 3 | 12.5 | 3621 | 222.6 | 315.4 | 108.6 | 4 | 19 | 3376 | 18.61 | 385.1 | 337.5 | 64.5 | 22 | 47.8 | |
| Population 3 | 3513 | 480.8 | 343.4 | 54 | 17 | 70.8 | 4683 | 338.1 | 402.5 | 74.1 | 8 | 38.1 | 4739 | 20.84 | 352.6 | 383 | 68.9 | 13 | 28 3 | |
| Population 4 | 5078 | 234.4 | 375 | 52.6 | 3 | 12.5 | 6293 | 0 | 364.7 | 0 | 1 | 4.8 | 6293 | 22.91 | 0 | 364.7 | 0 | 1 | 2.2 | |
| Basal turn | Population 1 | 852.9 | 0 | 201.9 | 0 | 1 | 3.7 | 1012 | 322.5 | 275.9 | 66.8 | 10 | 50 | 1025 | 12.51 | 298.7 | 274.8 | 64.7 | 11 | 23.9 |
| Population 2 | 2942 | 438.6 | 402.4 | 81.8 | 13 | 48.2 | 3036 | 529.9 | 361.5 | 58.3 | 3 | 15 | 3035 | 17.96 | 446.5 | 388.9 | 71.2 | 16 | 34.8 | |
| Population 3 | 4822 | 535.8 | 456 | 94.6 | 10 | 37 | 5519 | 688.9 | 413.5 | 39.9 | 5 | 25 | 5250 | 21.56 | 750.4 | 452.9 | 84.5 | 17 | 37 | |
| Population 4 | 6227 | 132.7 | 507.9 | 93.2 | 3 | 11.1 | 7970 | 363.9 | 425.7 | 12.5 | 2 | 10 | 7970 | 24.78 | 363.9 | 425.7 | 12.5 | 2 | 4.3 | |
Specimen 2
Fig. 4B (same color-coded manner as used for specimen 1) presents the result of hierarchical cluster analysis of reconstructed perikarya of specimen 2. The evaluated SGCs of the four identified populations are much more homogenously distributed, as seen in Table 2. One-fifth of the cell bodies scanned in specimen 2 are classified to form population 1 (919 ± 192 μm3, violet colored). The appropriate nuclei of this smallest population are again the smallest reconstructed from specimen 2 (264 ± 67 μm3). The second population, illustrated in green contains 23.6% of all analyzed cell bodies within this specimen and has a mean volume of (2441 ± 461.3 μm3). Compared to population 1, their nuclei are found to be slightly larger (290.2 ± 53.4 μm3). Population 3 is composed of 36.4% of reconstructed perikarya with a mean volume of 4393 ± 620.5 μm3 (red). Their corresponding nuclei have a mean volume of 359.9 ± 89.9 μm3. Another fifth of all analyzed SGCs from specimen 2 are classified as the largest population 4 with a mean volume of 6636 ± 826 μm3 (cyan). The reconstructed nuclei from the cells of this population represent the largest from this specimen with a mean volume of 418 ± 60.6 μm3.
The scree plot (R2 = 0.88) strongly supports the existence of the identified four population solution in specimen 2. Moreover, the reconstructed volumes of the four populations and their corresponding nuclei are shown to be significantly different from each other (p < 0.001 for perikarya and p < 0.1 for nuclei respectively, Bonferroni).
After dividing scanned cell bodies according to their origin along the cochlea spiral, the described four population solution is also determined for the apical-, middle- and basal region of this cochlea and is strongly supported by the scree plot (for details see Table 2).
Individual differences
Comparing both analyzed specimens with respect to the distribution of SGC populations and incidence of cells within the populations in distinct turns offers striking individual variations (Table 2). As already stated, it is found that the majority of measured cell bodies belonging to specimen 1 are classified as population 2 (80.7%, n = 83), whereas in specimen 2 some shifts of relative distribution in SGC populations are apparent. The corresponding group identified in specimen 2 contains 23.6% (n = 55) with a mean volume that is 37.1% smaller compared to specimen 1.
Another example of individual differences is found within the middle turn. Only 4.2% (n = 24) of SGCs are assigned to the smallest group in specimen 1. However, no comparable ‘small’ population of cells is found in specimen 2.
The second biggest perikaryon is found in the apical turn of specimen 1, representing population 4 (3.1%, n = 32). In contrast, the mean volume of the corresponding population of specimen 2 is 18.6% smaller while containing 20% (n = 55) of the reconstructed somata.
Both the largest and smallest perikarya were found in specimen 2. 80% of the identified ‘giant’ neurons (n = 5) were localized in specimen 2 within the apical- as well as the basal turn. In the basal turn, representing the high frequency region in cochleae, 50% (n = 20) are classified as the smallest group (population 1), whereas only 3.7% (n = 27) are classified as this population in specimen 1.
Pooled data of both specimens
Hierarchical cluster analysis of the pooled volumetric data set demonstrates the distribution of reconstructed perikarya of both cochleae (n = 138).
Fig. 4C presents the results of this hierarchical clustering where the distinct grouping of the determined SGC-populations is shown in the same color-coded manner as was previously introduced. The existence of four classes of cell somata with respect to micro-anatomical features is found once again in pooled data of these two individual human cochleae.
17.4% of neurons could be assigned to the group with smallest cell bodies (population 1, violet) with a mean volume of 1154 ± 429.7 μm3. Similarly, their corresponding nuclei are found to be the smallest within the identified populations with a mean volume of 247.9 ± 64 μm3. Population 2 (green) contains the majority (52.2%) of all reconstructed SGCs with a mean soma volume of 3352 ± 661.3 μm3. Interestingly, their corresponding nuclei range in volume from 180.4 μm3 (smallest) to 576 μm3 (largest), a volumetric difference of 314% within this determined population. This increase of size represents the largest variation of nuclei volume within all detected populations. The third identified population (population 3, red) of SGC covers 26.8% of all analyzed cells with a mean volume of 5407 ± 592.5 μm3. Their corresponding nuclei have a mean volume of 409.6 ± 74.7 μm3. The largest SGCs form population 4 (cyan). Only 3.6% of all cells comprise this giant population of SGCs with a mean volume of 7675 ± 543.3 μm3. The mean volume of these cells is 6.65 times larger than that of population 1. The volume of their nuclei is quantified as 475.1 ± 50.6 μm3.
The existence of four different populations among the analyzed SGCs of both cochleae is strongly supported by the scree plot (Fig. 4C) with a linear fit (R2 = 0.91) of the normalized eigenvalues. Furthermore, the volumes of the four identified perikaryal SGC populations and their corresponding nuclei are found to be significantly different from each other (p < 0.001 for perikarya and p < 0.01 for nuclei respectively, Bonferroni).
Likewise, the four classes of SGCs were individually examined in the apical-, middle- and basal turns of the cochleae presented in Table 2.
Within the apical- (population 4), middle- (population 1) and basal (population 1) turn of specimen 1, as well as in the middle turn region of specimen 2, only one cell body from each region is found to be an outlier. After removal of these four values, which represent 2.9% of all reconstructed SGCs, our hierarchical cluster algorithm was again applied to the data in order to determine the difference in cluster composition and the incidence of cells within the newly classified populations.
The specified four population solution supported by the scree plot is identified in both specimen 1 and 2. Furthermore, the four classes of neurons are determined among cell bodies separated according to their origin along the cochlea spiral (data not shown). It is observed that the newly determined mean volumes of the cell classes are ‘shifted’, resulting in more similar populations among the two cochleae. These shifts of populations are accompanied by a decrease in the standard deviation calculated from the reconstructed volumes among these subgroups. However, the incidence of cells within the four determined different populations remains unchanged representing huge individual variations.
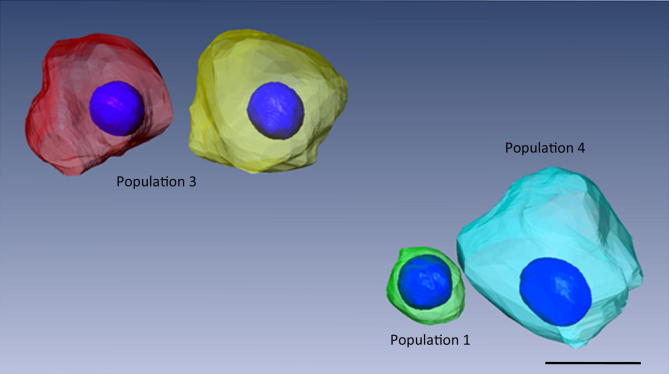
The volumetric differences of the cell bodies and their corresponding nuclei are visualized in Fig. 5 using a 3D rendering of a microscopic view comprising three distinct populations of SGCs. A small soma (population 1, green) of the apical turn of specimen 2 is shown alongside a ‘giant’ soma (population 4, cyan) and medium-sized SGCs of population 3 (red and yellow) which compose approximately one fourth of all scanned neurons.
Fig. 5.
Surface rendering of manually segmented perikarya with their corresponding nuclei. The green-colored perikaryon represents a SGC from the apical turn of specimen 2. It was one of the smallest scanned cell bodies and classified as population 1 by the cluster algorithm. On the other hand, the cyan-colored SGC represents one of the largest reconstructed perikaryon belonging to Population 4. Identified population 3 is shown by the red- and yellow-colored soma. Scale bar represents 15 μm.
Computer simulation
To quantify the effect of different soma volumes the model neuron has been analyzed during two different stimulation modes. The natural spiking behavior was examined with the injection of a current impulse into the first compartment (i.e., the distal end of the peripheral axon). Additionally, the stimulation by an extracellular point electrode simulates micro-stimulation in the case of cochlear implants.
Intracellular stimulation
Changes in soma diameter of the model neuron have insignificant influence on conduction velocity of an AP initiated in the first dendritic compartment. When the soma diameter is varied from minimum values of 10.05 μm to maximum values of 25.05 μm, the threshold amplitude for a 0.1 ms pulse is 60–70 pA. The traveling time of the spike for threshold current injection increases only slightly (i.e., 0.58 ms for the smallest cell to 0.65 ms for the largest one). The AP height at the soma shows a spread of only 2.3 mV. The AP width at the soma is always about 0.13 ms and its small variations do not correlate with soma diameter.
The unmyelinated soma region which is typical for human SGCs impedes spike traveling. Rattay et al. (2001) report sensitive model parameters for the signal transmission of a spike, e.g., the number of membrane layers at the soma, the length of the presomatic region or the diameter of the peripheral process. Even a small deviation from standard values can reduce the safety factor for successful spike conduction. The experiments performed (data not shown) demonstrate that this impediment to successful AP propagation is more pronounced for neurons with larger cell bodies.
Extracellular stimulation with electrodes E1 and E2
For both electrodes and all four stimulus configurations, the thresholds as well as initiation site (IS) did not change much within the range of all collected soma diameters. Temporal parameters of the spike were collected for threshold stimulation and additionally for pulses of doubled amplitude. We recorded the peak time of the AP at three different sites, namely the IS, the soma and compartment 27 which is the model neurons’ end.
These temporal parameters are displayed in Fig. 6 which demonstrates different spiking behavior when a selected model cell with d = 19.08 μm (which is the mean value of all evaluated diameters) is stimulated by electrode E1 with the four different pulse forms. The time courses of membrane potential of this cell are plotted for monophasic and biphasic threshold stimulations.
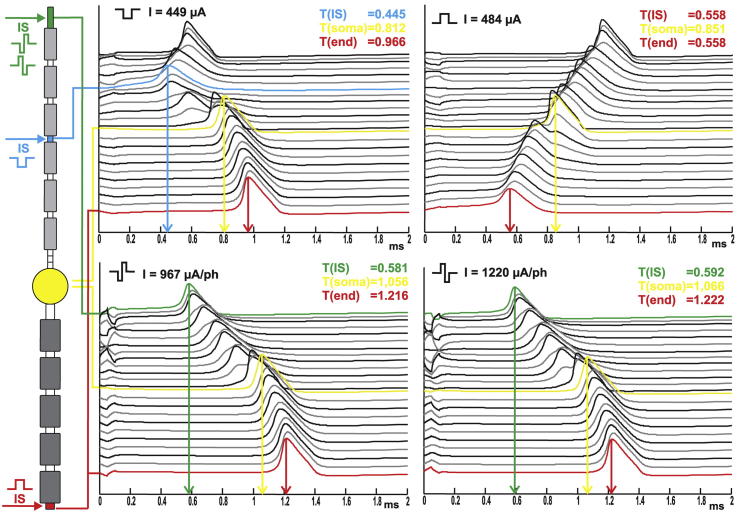
Fig. 6.
Spike initiation sites depend on stimulus pulse type. The left side illustrates the compartment model of a target neuron with spike origin (horizontal arrows) at threshold for four different pulses. Stimulating electrode E1. Traveling APs are plotted at the right. Each line represents the temporal change in transmembrane potential. The top line is the neurons peripheral end, the soma is always plotted in yellow. The values for the time at which the spike occurs at the IS, the soma and the end of the model neuron are given in ms and they are marked by vertical arrows.
When stimulated with a 0.1 ms long cathodic pulse, the threshold value for E1 is about −449 μA and −186 μA for E2 regardless of the cell size. Note the difference in magnitude of the two values, although the electrodes are quite close (compare Fig. 2). APs were generated in compartment 7 for E1 and in compartment 9 for E2, both pertaining to the peripheral axon. The IS for twice the threshold is changed to compartment 9 for E1 and remains the same for E2. Since for all cases the AP is initiated peripherally, the spike travels orthodromically over the soma to the central end of the neuron. This can be observed in the top left panel of Fig. 6, where a threshold impulse initiates a spike after 0.445 ms at compartment 7, indicated by the blue line. The spike appears later at the yellow soma after 0.812 ms, and then reaches the axonal end 0.966 ms after stimulus onset, plotted as a red line.
Anodic thresholds slightly decrease with increasing soma size for E1 from 496 μA to 482 μA and cause the spike to start at the central end of the neuron at compartment 27. For E2 the threshold remains constant at 289 μA initiating the spike at the very peripheral end of the neuron in compartment 1. The IS stays the same for both electrodes and all cell sizes when stimulated with twice the threshold value. The spike travels orthodromically as it can be seen at the top right panel of Fig. 6, where the AP at the soma (indicated by yellow line) appears later than the graph of the last compartment, indicated by the red line which represents both, the IS and the end of the neuron. Interestingly, the onset of the spike is delayed compared to the cathodic stimulation with a peak time value of 0.558 ms. Still, since it is also the end time of the spike, the delay of information processing is certainly reduced.
The thresholds for biphasic pulses from E1 remained constant at −967 μA for cathodic first and 1220 μA for anodic first pulses, both initiated the spike at compartment 1 for all cell sizes, indicated by the green lines at the bottom panels of Fig. 6. Since the peak time values for both leading phases are quite similar, almost the same value of about 1.2 ms can be observed for the delay of spike transmission, which is certainly longer than for monophasic pulses.
No matter which cell size is observed, when the pulse amplitudes are doubled, the ISs are shifted to compartment 9 for biphasic cathodic first pulses and to multiple sites including compartment 27 and 3 for biphasic anodic first pulses. The corresponding threshold values for E2 are −483 μA and 661 μA, although it should be stated that the largest cell of the basal turn requires about 15 μA more threshold current amplitude compared to the constant values of all other possible cell diameters. Also, the IS is not constant for all cells, but rather switched between compartment 7 and 9 for both biphasic pulse forms of E2. When stimulated with twice the threshold value, the IS was constant for all cell sizes (i.e., compartment 9 for cathodic first and compartment 1 for anodic first biphasic pulses) This behavior demonstrates the overall trend that temporal fluctuations decrease for increased stimulus amplitudes.
Regardless of the electrode position and pulse form, and despite some fluctuations, it was observed that the peak time values of the spike increased with cell size. No matter which pulse form is chosen, changing the soma diameter alters the temporal spiking behavior and larger soma size delays information processing.
Extracellular stimulation with electrodes E3 and E4
These electrode positions were tested for all evaluated soma diameters. Both electrodes are placed normal to the soma at different distances, where E3 denotes the short distance case of 0.5 mm and E4 the longer, doubled distance of 1 mm (Fig. 2). Initially the individual monophasic and biphasic threshold currents have been determined for varying soma size. While analyzing all soma diameters, the spike is initiated either peripherally at compartment 13, which is the last node of Ranvier before the soma, or at the central end of the model neuron.
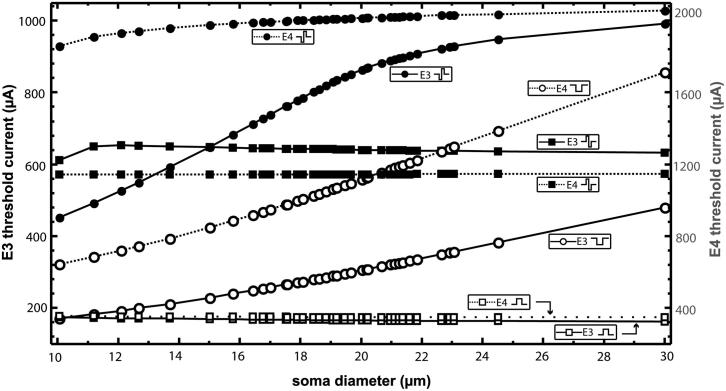
Due to the phase duration ratio of 100 μs to 50 μs, the monophasic threshold currents for both electrode positions are approximately half the values of their biphasic counterparts, as can be seen in Fig. 7.
Fig. 7.
Threshold currents of E3 and E4 as function of soma diameter for all detected cells. The black E3 axis on the left is half of the grey E4 axis on the right. Corresponding threshold curves have the same color. For better visualization, the markers display a selection of evaluations.
When the soma-electrode distance is 0.5 mm, the cathodic threshold increases with soma diameter monotonically from −171 μA to −393 μA, always initiating the AP at compartment 13. Contrarily, the anodic threshold decreases monotonically from 174 μA to 162 μA with a constant IS at compartment 27. For the biphasic pulses of E3, the thresholds as well as the IS changes with varying soma diameter. When stimulated with biphasic cathodic first pulses, the thresholds again increase with cell size. The values range from −451 μA to −954 μA, although the slope of the curve decreases for diameters larger than 19.07 μm correlating with the change of IS from compartment 13 to 27 (compare top panel of Fig. 8). For biphasic anodic first pulses, this change appears already for diameters above 11.64 μm. As a result, the threshold values ascend steeply at first from 611 μA to 654 μA, but then again decrease to 635 μA (compare bottom panel of Fig. 8).
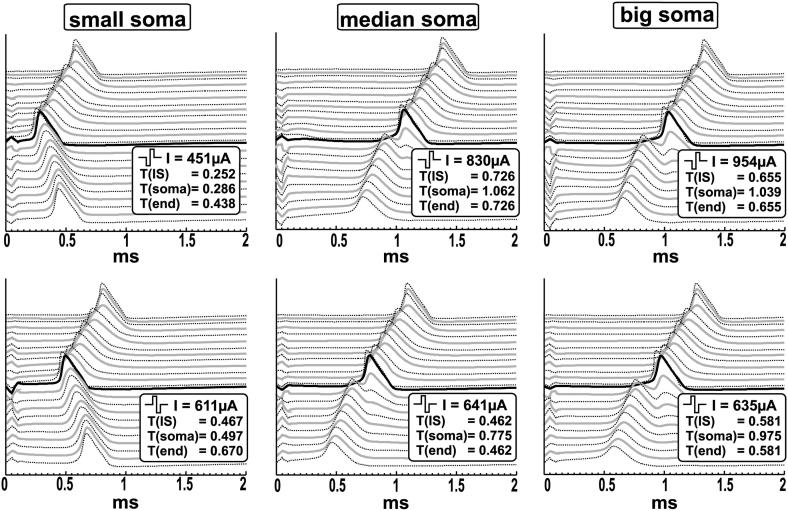
Fig. 8.
Biphasic threshold stimulation by E3 of three neurons with different soma size. The traveling APs (lines from top to bottom correspond to the transmembrane potential of every compartment from the peripheral to the central end) are simulated for the smallest (d = 10.05 μm), mean value (d = 19.08 μm) and biggest detected cell (d = 25.05 μm).
When the electrode is moved further away from the soma to a distance of 1 mm, the application of a cathodic pulse initiates the AP at compartment 13 for each soma diameter. For all other pulse forms and variations in diameter, the IS is the central end of the model neuron (i.e., compartment 27). The threshold values for both monophasic and biphasic cathodic pulses increase with cell size, although with a variation between −643 μA and −1420 μA, the monophasic values show a steeper slope than those of biphasic pulses with values ranging from −1858 μA to −2040 μA. For anodic stimulation, the threshold values remain virtually the same (i.e., for monophasic pulses the values decrease only slightly from 353 μA to 348 μA, while the threshold values for biphasic stimulation fluctuate between 1142 μA and 1146 μA, Fig. 7).
While recording E3 and E4 threshold currents and ISs, we also collected temporal parameters of the spike, namely values of the AP peak time at the IS, the soma and the last model compartment. Corresponding values vary for different soma sizes when stimulated with the individual thresholds. For monophasic stimulation, the values for the spike onset, the AP peak time at the IS, seem to increase with broad distribution when the soma diameter is raised from its minimum of 10.05 μm to its maximum of 25.05 μm.
The influence of the soma diameter for biphasic stimulation by E3 is illustrated in Fig. 8. The shift in IS occurring for altering soma diameter is observed for the biphasic cathodic first pulses radiated by E3. The values jump from a lower level, around 0.25 ms, to a peak time around 0.7 ms at the IS for diameters larger than 19.07 μm (compare top row of Fig. 8). For anodic first pulses (bottom row) the latencies, the time at which the AP occurs at the end, are longest for small cells.
Table 3 summarizes the results of the whole set of soma diameters and compares different pulse forms for two different electrode-soma distances. Monophasic cathodic pulses lead to smaller E3 values of the AP peak time at the IS compared to their counterparts of E4 with doubled distance. Corresponding anodic values are higher, but the effect of increasing the electrode-soma distance is less pronounced. For biphasic pulses the values of E3 include spikes initiated at two different sites, compartment 13 and 27. Still, the mean value of E3 cathodic first pulses of 0.46 ms is significantly higher than 0.583 ms for E4. By contrast, anodic first pulses by E4 cause a slightly earlier onset. The fastest onset among all pulse forms of the two electrode positions appears for E3 stimulation with monophasic cathodic pulses.
Table 3.
Summary of temporal spike parameter values induced by E3 (left) and E4 (right). Values (in ms) for each pulse form are calculated from the whole set of soma diameters
 |
 |
 |
 |
 |
 |
 |
 |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Peak timeE3 | min max mean |
0.332 0.360 0.346 |
0.464 0.486 0.475 |
0.252 0.668 0.460 |
0.458 0.467 0.462 |
Peak timeE4 | min max mean |
0.400 0.445 0.422 |
0.494 0.551 0.522 |
0.572 0.594 0.583 |
0.367 0.508 0.438 |
| difference var svd |
0.027 0.000 0.019 |
0.022 0.000 0.016 |
0.416 0.087 0.294 |
0.009 0.000 0.007 |
difference var svd |
0.045 0.001 0.032 |
0.057 0.002 0.040 |
0.022 0.000 0.015 |
0.142 0.010 0.100 |
||
| Peak time somaE3 | min max mean |
0.363 0.454 0.408 |
0.675 0.937 0.806 |
0.286 1.128 0.707 |
0.497 0.948 0.723 |
Peak time somaE4 | min max mean |
0.476 0.496 0.486 |
0.747 0.920 0.834 |
0.816 1.007 0.912 |
0.572 0.946 0.759 |
| difference var svd |
0.091 0.004 0.065 |
0.262 0.034 0.185 |
0.843 0.355 0.596 |
0.452 0.102 0.319 |
difference varsvd | 0.020 0.000 0.014 |
0.174 0.015 0.123 |
0.190 0.018 0.135 |
0.374 0.070 0.264 |
||
| End timeE3 | min max mean |
0.526 0.605 0.566 |
0.464 0.486 0.475 |
0.438 0.668 0.553 |
0.458 0.670 0.564 |
End timeE4 | min max mean |
0.638 0.649 0.643 |
0.494 0.551 0.522 |
0.572 0.594 0.583 |
0.367 0.508 0.438 |
| difference var svd |
0.079 0.003 0.056 |
0.022 0.000 0.016 |
0.230 0.026 0.162 |
0.213 0.023 0.150 |
difference var svd |
0.011 0.000 0.008 |
0.057 0.002 0.040 |
0.022 0.000 0.015 |
0.142 0.010 0.100 |
||
Although changing the pulse shape or electrode position has the same consequences for the peak time at the soma, the variations among corresponding values are now higher. The influence of the soma size is most obvious for this temporal parameter, as the largest values of variance, standard deviation and overall range, appear in the middle part of Table 3. Moreover, the results predict that irrespective of the electrode-soma distance, the peak time values at the soma elevate with increasing diameter for each of the four pulse forms (data not shown).
The bottom part of Table 3 represents the peak time at the end of the model neuron. Since in most cases the spike is initiated at compartment 27, the values of the peak time at the IS and at the end are equal. Spikes which are generated peripherally, as for monophasic cathodic stimulation, need to pass the delaying soma region and therefore have longer latencies than the other pulse forms. The shortest latency appears for E4 stimulation with biphasic anodic first stimulation with a minimum of 0.367 ms.
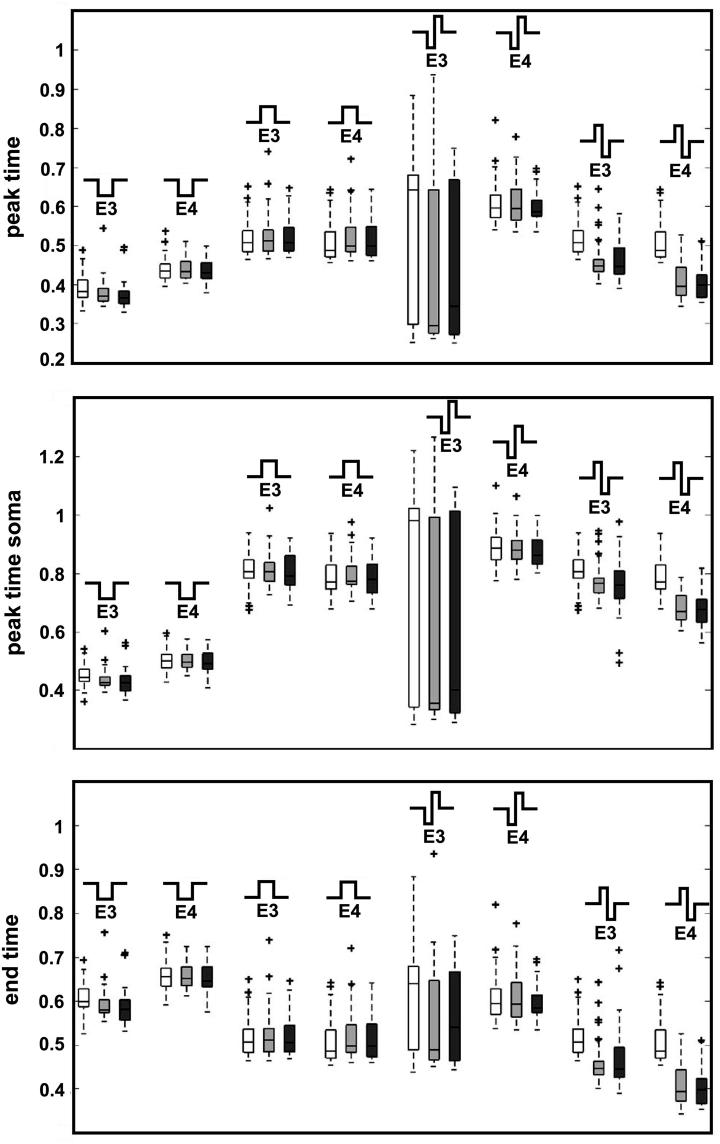
We not only acquired the volumetric data of a certain amount of SGCs, but also assigned cells to their site within the modiolus. We used this additional information to analyze the distribution of temporal spike parameters along the cochlear turns. Fig. 9 shows Boxplots of the peak time values of SGCs from the base, the apex and the middle turn which were collected for threshold stimulation of E3 and E4.
Fig. 9.
Comparison of temporal parameters in the three cochlear regions; Boxplot of temporal parameters of the spike induced by E3 and E4. The peak time at the IS (top) the peak time at the soma (middle) and the end time of the spike (bottom) are compared for apical (white boxes), middle turn (light gray boxes) and basal neurons (dark gray boxes).
Biphasic cathodic first stimulation radiated by E3 leads to the widest variation in values of the peak time. Note that this electrode configuration also produces the lowest values of the peak time, although the mean value of apical neurons is certainly higher than those of middle turn and basal neurons. This is also visible for cathodic pulses of E3 and biphasic anodic first pulses of both electrodes that confirm the trend of increasing values for increasing cell body diameter.
The results for the peak time at the soma are very similar. Since for cathodic pulses the AP is initiated peripherally, and therefore propagates orthodromically, the values of the peak time at the soma are higher than the peak time at the IS. The low outliers for biphasic anodic first pulses of E3 correspond to APs initiated in compartment 13.
Although neurons will respond earlier to cathodic pulses, the delay of information processing to the next cell is lowest for biphasic anodic first pulses (Fig. 9, bottom panel), since the spike is initiated at the central end instead of propagating from peripheral sites over the soma. For monophasic and biphasic pulses, longer latencies appear for cathodic pulses. Surprisingly, E3 leads to smaller values than E4 when radiating cathodic pulses, although for biphasic anodic first pulses E4 shows shorter latencies.
Discussion
The unmyelinated perikarya of human cochlea neurons are the first energy dissipater for an AP on the way from the hair cells to the auditory cortex. To overcome this barrier the AP must load the membrane capacitance of the cell body, which is directly proportional to its surface (Rattay, 1995; Rattay et al., 2001).
Since experimental investigations and electrical recordings of cat neurons do not account for this human characteristic, computer simulation which takes into account the volumetric nature of unmyelinated human SGCs is an important tool for enhancing the understanding of AP initiation and propagation triggered by micro stimulation. The mathematical models used for computer simulation are based on geometric parameters specified by the micro-anatomical characteristics of the simulated structures. Therefore, we systematically collected 3D-data of human SGCs and classified them according to these anatomical properties in order to acquire information about this critical parameter for cochlea neuron simulations.
Present knowledge concerning size of human SGC is based mainly on transmission electron microscopy studies taking morphological criteria into account. The main characteristics extracted from such studies include the maximum cross sectional diameter of perikarya and nuclei (Kiang et al., 1984) as well as their maximum circumference (Nadol et al., 1990; Rosbe et al., 1996). Analyzing these independent data, the authors suggest that there may be subgroups among large and small SGCs. Spoendlin (1984) analyzed cat cochleae at light- and electron microscope resolutions and distinguished three different types of spiral ganglion neurons. Another group (Jagger and Housley, 2003) studied 400 μm thick rat-cochlea slices but could not discern between type I and II cells based on morphological criteria. The diverging shapes of human SGC were also presented by Glueckert et al. (2005). This group analyzed cochleae from normal-hearing subjects using scanning electron microscopy. The shapes of the SGCs range from spherical, elongated cylinder-like somata to (partly) squeezed lemon-like perikarya, which were also illustrated by Rusznak and Szucs (2009). We have seen in our study that this squeezing mainly results from perikarya-circumjacent, space-claiming satellite glial cells (especially their nuclei) and from myelinated nerve fibers surrounding the perikarya.
Hence, the use of 2D-parameters such as the cross sectional area is not sufficient for obtaining information about non-uniform 3D-structures and reduces the possibility of drawing appropriate conclusions concerning their shape, and therefore, their actual size.
Sections used in ultrastructural studies have an average thickness of 90 nm. Assuming a SGC with a diameter of 20 μm, this section represents only about 0.45% from the whole available 3D-data of the structure. By scanning the evaluated perikarya and corresponding nuclei in their full dimension, our study overcomes the above-mentioned morphological limitations and accounts for irregular shapes. In taking the whole available 3D-information of these two independent parameters into account, more precise data are available for statistical analyses.
For this analysis, we used an agglomerative hierarchical cluster algorithm to determine possible similarities. Using this approach we identified four different types of SGCs among all reconstructed SGCs. A closer look at scanned SGC from the apical-, middle- and basal turn representing different frequency-specific regions within this sensory organ shows the uniform existence of these four populations of cells in both investigated individuals.
The findings of small volumetric variation of SGCs in the middle turn may provide new insights into the adaption of speech. According to the Greenwood function (Greenwood, 1990; Loizou, 1999), this section of the cochlea is of prime importance for speech recognition (Hollien et al., 1971). The greatest density of SGCs (Nadol, 1988; Spoendlin and Schrott, 1988) as well as the maximum number of myelinated fibers and outer hair cells (Spoendlin and Schrott, 1989) per millimeter ensures that this region is at most sensitive within the human cochlea. In this region we identified, surprisingly, the smallest SGCs, which are boosting the chance that an arriving AP from the peripherals can load the membrane capacitance of the perikarya. These electric charging of the cell bodies ensure further signal transmission. Consequently, smaller cell bodies in this region should reduce the risk of information loss caused by ‘stucked’ AP within these important frequencies.
In contrast, we depict huge individual volumetric variations of SGCs in the apical and particularly in the basal turn of the analyzed cochleae. Such morphological differences and variations of SGCs along the cochlea spiral are also described by Nadol et al. (1990) and Thiers et al. (2000). A major problem imposed by the use of human material of heterogeneous genetic background and incomplete documentation of pre-mortem pathologic influence is that the observed morphology may reflect unknown pathology (Rosbe et al., 1996). Considerable variations in size as well as number of spiral ganglion neurons in the high frequency region are frequently reported and coincide with our own observations on human temporal bones (not published). In a recent large human temporal bone study by Makary et al. (2011), an SGC decline at a mean rate of 100 cells per year of life was found, with pronounced decline of neurons in the basal turn. This degeneration was evaluated from 100 normal hearing subjects and represents a ‘normal’ type of degeneration in our society. A pronounced loss of neurons in the basal turn results in an increased amount of slowly degenerating SGCs. Cochlear neuron deterioration often involves loss of peripheral processes and cell shrinkage that may explain the high variation of SGC size in the base of the cochlea. Middle and apical regions of the cochlea showed fewer declines of neurons in this study, reflecting a more stable population of nerve cells.
Furthermore we have identified a spatial organization of SGCs (Liberman and Oliver, 1984; Glueckert et al., 2005) and their corresponding nuclei. Larger nuclei may be associated with higher metabolism.
The identified volumetric variations of SGCs were incorporated into our model neuron to systematically test each collected diameter. Computer simulation of micro stimulation by electrodes E1–E4 demonstrates that changing the soma size affects the excitability of neurons in noticeably different degrees depending on electrode positions and stimulus shapes.
Although Miller et al. (2001) state interspecies difference in spiking behavior, the morphologically distinct soma region of human SGCs often remains unaccounted for. Goldwyn et al. (2010) suggest that the varying soma diameter may contribute to channel-to-channel variability of implants. With the acquired volumetric data of SGCs from different cochlea regions, these speculations can be tested systematically. Therefore, forthcoming studies should include varying soma sizes according to detected data, since thresholds and temporal parameters change significantly under certain circumstances (cathodic pulses for E3). Still, our threshold investigations for the four electrode positions support common findings of previous studies. They consider different electrode configurations concerning their position (Mino et al., 2004) and pulse forms, including monophasic (Miller et al., 1999), biphasic (Miller et al., 2001; van Wieringen et al., 2006) and triphasic pulses (Bierer, 2007; Goldwyn et al., 2010) as well as pulse trains (Miller et al., 1997; Woo et al., 2009a).
Greater latencies appear for cathodic stimulation (Miller et al., 1999; Shepherd and Javel, 1999) which also leads to lower thresholds compared to anodic pulses (Miller et al., 1999) and also to their biphasic counterparts (Shepherd and Javel, 1999; Miller et al., 2001). In accordance with our detected ISs, the results of Miller et al. (1999) also support the fact that neurons are mainly stimulated at central processes and that only a minority of fibers close to the electrode is excitable at the respective peripheral axons. This is of special interest for retrograde degeneration of peripheral processes following loss of sensory cells reported in humans (Spoendlin, 1984; Glueckert et al., 2005). Van Wieringen et al. (2006) also state that the trend of lower monophasic thresholds is also valuable for pulse trains and it will occur for different CI devices, different electrode configurations, and generally, for different phase duration.
Although recordings in cats may not be valid for humans, the neural response induced by micro stimulation can alternatively be studied with recordings of electrically evoked compound APs. Briaire and Frijns (2005) compared one model including a myelinated cell body with the unmyelinated cell body case. They also reported a delay caused by the lack of myelin layers and examined the difference in excitation by biphasic pulses of both leading phases. With the inclusion of our new morphological analysis, more precise results might be obtained. Macherey et al. (2008) not only studied the evoked compound AP, they also report that the human auditory system is more sensitive to anodic stimulation, yielding larger response at an equal stimulus level compared to cathodic pulses. Our results, which predict a quite uniform anodic threshold value for all soma diameters, are in accordance with this finding. Therefore, one anodic impulse might excite a larger number of SGCs compared to cathodic pulses with highly changing threshold current amplitude for varying soma size.
Many more parameters which affect the neurons excitability vary along the length of the cochlea. For pending studies about electrode position and pulse configuration, three-dimensional data from a greater amount of traced cochlear neurons is crucial. Although the obtained results clearly show divergent single fiber response for different soma sizes, the model would certainly benefit from further adjustments.
Conclusion
We acquired volumetric data, reconstructed as well as analyzed soma size of human SGCs and identified four different populations of SGCs within the cochlea of humans. Moreover, we found evidence of a spatial arrangement of perikarya and their accordant nuclei from high- to low-frequency regions where the most uniform sizes of cell bodies are located in the middle turn representing the majority of phonational frequencies. This more accurate volume information of the unmyelinated human perikarya was used to analyze stimulation thresholds, initial sites and travel latencies of AP triggered by micro stimulation. Our results show that temporal parameters of the AP are affected by the size of the cell body with stronger variations for spikes induced by cathodic stimulation. Therefore, a more uniform excitation profile of the whole amount of cochlear neurons can be expected for anodic stimulation. Together with our morphometric findings, our results deliver new insight into the unique micro anatomical features of the human spiral ganglion and their impact on AP onset and propagation initiated by micro stimulation.
Acknowledgements
This research project was supported by the Austrian Science Fund (FWF) project 21848-N13. We want to thank Matthew D. DiFranco, Ph.D., for proofing and editing this manuscript.
References
- Arnold W. Myelination of the human spiral ganglion. Acta Otolaryngol Suppl. 1987;436:76–84. doi: 10.3109/00016488709124979. [DOI] [PubMed] [Google Scholar]
- Bean B.P. The action potential in mammalian central neurons. Nat Rev Neurosci. 2007;8:451–465. doi: 10.1038/nrn2148. [DOI] [PubMed] [Google Scholar]
- Bierer J.A. Threshold and channel interaction in cochlear implant users: evaluation of the tripolar electrode configuration. J Acoust Soc Am. 2007;121:1642–1653. doi: 10.1121/1.2436712. [DOI] [PubMed] [Google Scholar]
- Briaire J.J., Frijns J.H. Unraveling the electrically evoked compound action potential. Hear Res. 2005;205:143–156. doi: 10.1016/j.heares.2005.03.020. [DOI] [PubMed] [Google Scholar]
- Cattell R.B. The scree test for the number of factors. Multivariate Behav Res. 1966;1:245–276. doi: 10.1207/s15327906mbr0102_10. [DOI] [PubMed] [Google Scholar]
- Coleman B., Rickard N.A., de Silva M.G., Shepherd R.K. A protocol for cryoembedding the adult guinea pig cochlea for fluorescence immunohistology. J Neurosci Methods. 2009;176:144–151. doi: 10.1016/j.jneumeth.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello A.B., Osborne J.W. Best practices in exploratory factor analysis: four recommendations for getting the most from your analysis. Pract Assess Res Eval. 2005;10:1–9. [Google Scholar]
- Drennan W.R., Won J.H., Dasika V.K., Rubinstein J.T. Effects of temporal fine structure on the lateralization of speech and on speech understanding in noise. J Assoc Res Otolaryngol. 2007;8:373–383. doi: 10.1007/s10162-007-0074-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frijns J.H., Mooij J., ten Kate J.H. A quantitative approach to modeling mammalian myelinated nerve fibers for electrical prosthesis design. IEEE Trans Biomed Eng. 1994;41:556–566. doi: 10.1109/10.293243. [DOI] [PubMed] [Google Scholar]
- Glueckert R., Pfaller K., Kinnefors A., Rask-Andersen H., Schrott-Fischer A. The human spiral ganglion: new insights into ultrastructure, survival rate and implications for cochlear implants. Audiol Neurootol. 2005;10:258–273. doi: 10.1159/000086000. [DOI] [PubMed] [Google Scholar]
- Goldwyn J.H., Bierer S.M., Bierer J.A. Modeling the electrode–neuron interface of cochlear implants: effects of neural survival, electrode placement, and the partial tripolar configuration. Hear Res. 2010;268:93–104. doi: 10.1016/j.heares.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood D.D. A cochlear frequency-position function for several species – 29 years later. J Acoust Soc Am. 1990;87:2592–2605. doi: 10.1121/1.399052. [DOI] [PubMed] [Google Scholar]
- Hodgkin A.L., Huxley A.F. A quantitative description of membrane current and its application conduction and excitation in nerve. J Physiol. 1952;117:500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollien H., Dew D., Philips P. Phonational frequency ranges of adults. J Speech Hear Res. 1971;14:755–760. doi: 10.1044/jshr.1404.755. [DOI] [PubMed] [Google Scholar]
- Holmes T.J., Liu Y.H. Richardson–Lucy/maximum likelihood image restoration algorithm for fluorescence microscopy: further testing. Appl Opt. 1989;28:4930–4938. doi: 10.1364/AO.28.004930. [DOI] [PubMed] [Google Scholar]
- Jagger D.J., Housley G.D. Membrane properties of type II spiral ganglion neurones identified in a neonatal rat cochlear slice. J Physiol. 2003;552:525–533. doi: 10.1113/jphysiol.2003.052589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiang N.Y., Liberman M.C., Gage J.S., Northrup C.C., Dodds L.W., Oliver M.E. Afferent innervation of the mammalian cochlea. In: Bolis L., Keynes R.D., Maddrell H.P., editors. Comparative Physiology of Sensory Systems. Cambridge UP; Cambridge: 1984. pp. 143–161. [Google Scholar]
- Liberman M.C., Oliver M.E. Morphometry of intracellularly labeled neurons of the auditory nerve: correlations with functional properties. J Comp Neurol. 1984;223:163–176. doi: 10.1002/cne.902230203. [DOI] [PubMed] [Google Scholar]
- Liu W., Bostrom M., Kinnefors A., Linthicum F., Rask-Andersen H. Expression of myelin basic protein in the human auditory nerve – an immunohistochemical and comparative study. Auris Nasus Larynx. 2011 doi: 10.1016/j.anl.2011.04.007. [DOI] [PubMed] [Google Scholar]
- Loizou P.C. Introduction to cochlear implants. IEEE Eng Med Biol Mag. 1999;18:32–42. doi: 10.1109/51.740962. [DOI] [PubMed] [Google Scholar]
- Macherey O., Carlyon RP., van WA., Deeks JM., Wouters J. Higher sensitivity of human auditory nerve fibers to positive electrical currents. J Assoc Res Otolaryngol. 2008;9:241–251. doi: 10.1007/s10162-008-0112-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makary C.A., Shin J., Kujawa S.G., Liberman M.C., Merchant S.N. Age-related primary cochlear neuronal degeneration in human temporal bones. J Assoc Res Otolaryngol. 2011;12:711–717. doi: 10.1007/s10162-011-0283-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A.L., Morris D.J., Pfingst B.E. Interactions between pulse separation and pulse polarity order in cochlear implants. Hear Res. 1997;109:21–33. doi: 10.1016/s0378-5955(97)00037-3. [DOI] [PubMed] [Google Scholar]
- Miller C.A., Abbas P.J., Robinson B.K., Rubinstein J.T., Matsuoka A.J. Electrically evoked single-fiber action potentials from cat: responses to monopolar, monophasic stimulation. Hear Res. 1999;130:197–218. doi: 10.1016/s0378-5955(99)00012-x. [DOI] [PubMed] [Google Scholar]
- Miller C.A., Robinson B.K., Rubinstein J.T., Abbas P.J., Runge-Samuelson C.L. Auditory nerve responses to monophasic and biphasic electric stimuli. Hear Res. 2001;151:79–94. doi: 10.1016/s0300-2977(00)00082-6. [DOI] [PubMed] [Google Scholar]
- Mino H., Rubinstein J.T., Miller C.A., Abbas P.J. Effects of electrode-to-fiber distance on temporal neural response with electrical stimulation. IEEE Trans Biomed Eng. 2004;51:13–20. doi: 10.1109/TBME.2003.820383. [DOI] [PubMed] [Google Scholar]
- Nadol J.B., Jr. Quantification of human spiral ganglion cells by serial section reconstruction and segmental density estimates. Am J Otolaryngol. 1988;9:47–51. doi: 10.1016/s0196-0709(88)80007-3. [DOI] [PubMed] [Google Scholar]
- Nadol J.B., Jr., Burgess B.J., Reisser C. Morphometric analysis of normal human spiral ganglion cells. Ann Otol Rhinol Laryngol. 1990;99:340–348. doi: 10.1177/000348949009900505. [DOI] [PubMed] [Google Scholar]
- Nelson L.R. Some observations on the scree test, and on coefficient alpha. Thai J Educ Res Meas. 2005;3:1–17. [Google Scholar]
- Ota C.Y., Kimura R.S. Ultrastructural study of the human spiral ganglion. Acta Otolaryngol. 1980;89:53–62. doi: 10.3109/00016488009127108. [DOI] [PubMed] [Google Scholar]
- Rattay F. Propagation and distribution of neural signals: a modeling study of axonal transport. Phys Alive. 1995;3:60–66. [Google Scholar]
- Rattay F., Lutter P., Felix H. A model of the electrically excited human cochlear neuron. I. Contribution of neural substructures to the generation and propagation of spikes. Hear Res. 2001;153:43–63. doi: 10.1016/s0378-5955(00)00256-2. [DOI] [PubMed] [Google Scholar]
- Rosbe K.W., Burgess B.J., Glynn R.J., Nadol J.B., Jr. Morphologic evidence for three cell types in the human spiral ganglion. Hear Res. 1996;93:120–127. doi: 10.1016/0378-5955(95)00208-1. [DOI] [PubMed] [Google Scholar]
- Rusznak Z., Szucs G. Spiral ganglion neurones: an overview of morphology, firing behaviour, ionic channels and function. Pflugers Arch. 2009;457:1303–1325. doi: 10.1007/s00424-008-0586-2. [DOI] [PubMed] [Google Scholar]
- Shepherd R.K., Javel E. Electrical stimulation of the auditory nerve: II. Effect of stimulus waveshape on single fibre response properties. Hear Res. 1999;130:171–188. doi: 10.1016/s0378-5955(99)00011-8. [DOI] [PubMed] [Google Scholar]
- Smit J.E., Hanekom T., Hanekom J.J. Estimation of stimulus attenuation in cochlear implants. J Neurosci Methods. 2009;180:363–373. doi: 10.1016/j.jneumeth.2009.03.024. [DOI] [PubMed] [Google Scholar]
- Spoendlin H. Differentiation of cochlear afferent neurons. Acta Otolaryngol. 1981;91:451–456. doi: 10.3109/00016488109138527. [DOI] [PubMed] [Google Scholar]
- Spoendlin H. Factors inducing retrograde degeneration of the cochlear nerve. Ann Otol Rhinol Laryngol Suppl. 1984;112:76–82. doi: 10.1177/00034894840930s415. [DOI] [PubMed] [Google Scholar]
- Spoendlin H., Schrott A. The spiral ganglion and the innervation of the human organ of Corti. Acta Otolaryngol. 1988;105:403–410. doi: 10.3109/00016488809119493. [DOI] [PubMed] [Google Scholar]
- Spoendlin H., Schrott A. Analysis of the human auditory nerve. Hear Res. 1989;43:25–38. doi: 10.1016/0378-5955(89)90056-7. [DOI] [PubMed] [Google Scholar]
- Thiers F.A., Burgess B.J., Nadol J.B., Jr. Prevalence and ultrastructural morphology of axosomatic synapses on spiral ganglion cells in humans of different ages. Hear Res. 2000;150:119–131. doi: 10.1016/s0378-5955(00)00193-3. [DOI] [PubMed] [Google Scholar]
- Tylstedt S., Kinnefors A., Rask-Andersen H. Neural interaction in the human spiral ganglion: a TEM study. Acta Otolaryngol. 1997;117:505–512. doi: 10.3109/00016489709113429. [DOI] [PubMed] [Google Scholar]
- Tylstedt S., Rask-Andersen H. A 3-D model of membrane specializations between human auditory spiral ganglion cells. J Neurocytol. 2001;30:465–473. doi: 10.1023/a:1015628831641. [DOI] [PubMed] [Google Scholar]
- van Wieringen A., Carlyon R.P., Macherey O., Wouters J. Effects of pulse rate on thresholds and loudness of biphasic and alternating monophasic pulse trains in electrical hearing. Hear Res. 2006;220:49–60. doi: 10.1016/j.heares.2006.06.015. [DOI] [PubMed] [Google Scholar]
- Woo J., Miller C.A., Abbas P.J. Biophysical model of an auditory nerve fiber with a novel adaptation component. IEEE Trans Biomed Eng. 2009;56:2177–2180. doi: 10.1109/TBME.2009.2023978. [DOI] [PubMed] [Google Scholar]
- Woo J., Miller C.A., Abbas P.J. Simulation of the electrically stimulated cochlear neuron: modeling adaptation to trains of electric pulses. IEEE Trans Biomed Eng. 2009;56:1348–1359. doi: 10.1109/TBME.2008.2005782. [DOI] [PubMed] [Google Scholar]