Abstract
Studies of metabolic adaptation during environmental stress have broad applications to human disease. Adenosine signaling has been implicated in cardiac adaptation to limited oxygen availability. Serendipitously, a wide search for adenosine receptor A2b (Adora2b)-elicited cardio-adaptive responses identified the circadian rhythm protein period2 (Per2). Subsequent pharmacologic and genetic studies confirmed Adora2b-dependent stabilization of Per2 during myocardial ischemia. Functional studies of myocardial ischemia in Per2−/− mice revealed larger infarct sizes and abolished cardio-protection by ischemic preconditioning. Metabolic studies during myocardial ischemia uncovered a limited ability of Per2−/− mice to utilize carbohydrates via oxygen-efficient glycolysis. These metabolic alterations were associated with a failure in Per2−/− mice to stabilize hypoxia-inducible-factor Hif1a. Moreover, cardiac stabilization of Per2 via light-exposure transcriptionally enhanced glycolysis, and provided period-specific cardio-protection from ischemia. Together, these studies identify Per2 as key regulator of ischemia tolerance through reprogramming of cardiac metabolism and implicate Per2 as novel therapeutic modality during acute myocardial ischemia.
INTRODUCTION
Metabolic adaptation during environmental stress is currently an area of intense investigation, as metabolic alterations have broad applications to human disease1,2. For instance, myocardial ischemia leads to the activation of pathways directed towards enhancing myocardial oxygen efficiency3. In fact, a metabolic switch from more “energy-efficient” utilization of fatty acids to more “oxygen-efficient” utilization of glucose as the main source for energy generation is pivotal to allow the myocardium to function under ischemic conditions.4,5
Extracellular adenosine is a signaling molecule implicated in cellular adaptation to hypoxia2. In the extracellular compartment, adenosine stems from phosphohydrolysis of AMP via the ecto-5’-nucleotidase (NT5E)6 and signals through four adenosine receptors (ARs,ADORA1, ADORA2A, ADORA2B, ADORA3)7. During conditions of hypoxia, adenosine generation is significantly enhanced, and activation of ARs plays a critical role in counterbalancing deleterious effects of hypoxia1,8. Particularly during conditions of myocardial ischemia, adenosine signaling events have been implicated in cardio-protection from ischemia. Similarly, cardio-protective responses elicited by ischemic preconditioning (IP) are abolished following pharmacological inhibition or genetic ablation of extracellular adenosine production or signaling9. In the present studies we identified the circadian rhythm protein Period 2 (Per2) as an important mediator in Adora2b elicited cardio-protection by enhancing the glycolytic capacity of the ischemic heart.
RESULTS
Adenosine signaling events mediate cardiac adaptation to ischemia
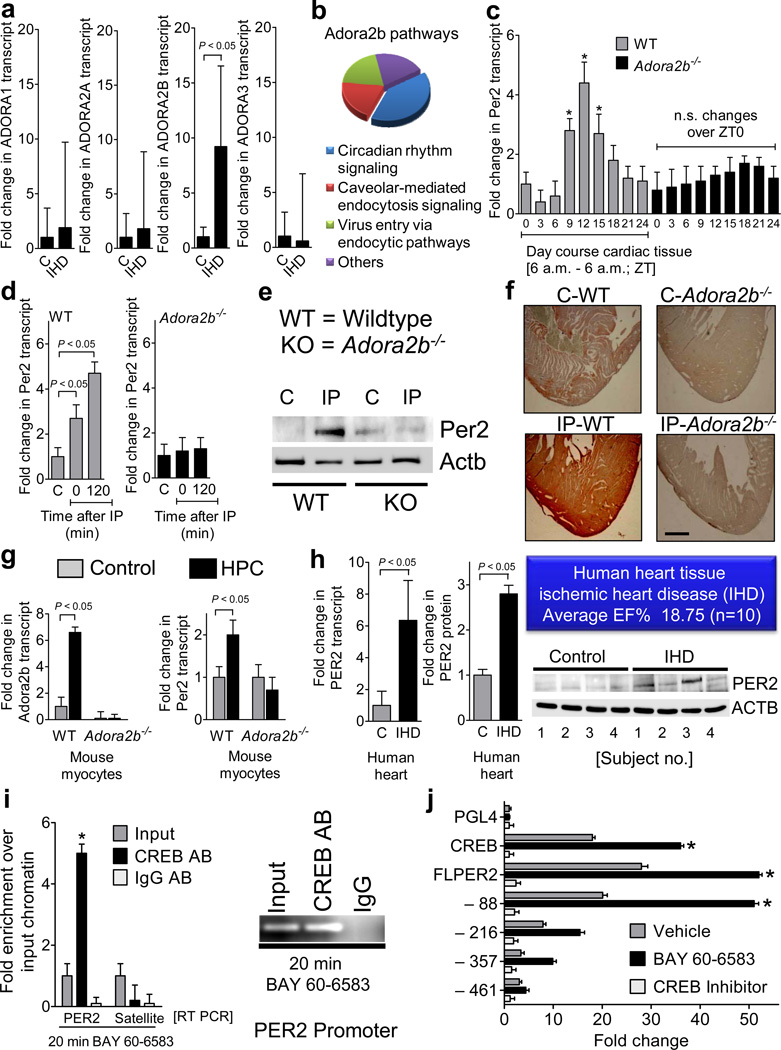
Previous studies have implicated adenosine receptor signaling in myocardial adaptation to ischemia or hypoxia in mice10. Here, we studied these pathways in cardiac tissues obtained from patients suffering from ischemic heart disease (Supplementary Table S1). In comparison to cardiac tissues derived from healthy hearts, we found a selective induction of the ADORA2B (Fig. 1a). Together with previous studies in gene-targeted mice10, these findings in human patients implicate extracellular adenosine signaling via the ADORA2B in cardio-protection from ischemia.
Figure 1. Consequences of adenosine signaling on Period 2 induction.
(a) Transcript levels of individual adenosine receptors (ADORA1 ADORA2A, ADORA2B, or ADORA3) in cardiac tissue from patients with severe ischemic heart disease (IHD) or controls (C; mean±SD, n=10 patients per condition). (b) Canonical pathway analysis. Wild-type mice or gene-targeted mice for the Adora2b (Adora2b−/−) were exposed to ischemic preconditioning (4 cycles consistent of 5min ischemia followed by 5 min of reperfusion). Following two hours of reperfusion, cardiac tissues from preconditioned myocardium were compared to control cardiac tissues without preconditioning. (c–f) Hearts from Adora2b−/− or littermate control mice matched in age, gender and weight were analyzed for diurnal variations of Per2 (c) or subjected to in situ preconditioning with 4 cycles of IP (5 minutes of ischemia, 5 minutes of reperfusion), followed by indicated time periods of reperfusion (d–f). (c, d) Per2 transcript levels (mean±SD, n=6). (e) Per2 protein levels determined by Western blot following IP-treatment without reperfusion (IP0). One representative blot of three is displayed. (f) Comparison of immunoreactivity for Per2 on pre-conditioned (IP) cardiac tissue or sham operated controls (magnification × 20, one of three representative images is displayed, scale bar represents 100 µm). (g) Adora2b or Per2 transcript level in isolated adult murine cardiomyocytes from wild-type or Adora2b−/− mice following in vitro exposure to hypoxic preconditioning (HPC; see also Supplementary Fig. S8, mean±SD, n=6). (h) PER2 transcript (left) or protein (middle, right) levels in cardiac tissues from human patients with severe ischemic heart (IHD) disease or controls (C) (see also Supplementary Fig. S4 and Supplementary Table S1; mean±SD, n=10 patients per condition). (i) Chromatin immunopreciptitation using human endothelia (HMEC-1). Following synchronization by serum starvation, human endothelia (HMEC-1) were treated with the ADORA2B agonist BAY 60-6583 for 20 minutes. Real-time RT PCR for human PER2 promoter or Satellite DNA (negative control) was performed (left). Products obtained by PER2 promoter PCR were also analyzed by using an 1% agarose gel (right; mean±SD, n=3, *p<0.05). (k) Full length PER2 promoter constructs and indicated truncations were sub-cloned into the pGL4 luciferase reporter vector. To measure promoter activity, cells were treated with BAY 60-6583 for 6 h. To control for circadian activity, cells were co-transfected with the CREB dominant negative vector from Clontech (CREB inhibitor; *significant increase of luciferase activity over baseline activity, p<0.05, n=6). Schematic of plasmids expressing sequence corresponding to full length PER2 promoter (FLPER2) or indicated truncations: −88, −216, −357,−461 with putative CREB binding sites are shown in Supplementary Fig. S11.
Identification of the circadian rhythm protein Per2 as an Adora2b target-gene
Given the prominent role of adenosine receptor signaling in IP, we next pursued microarray studies comparing transcriptional responses elicited by IP treatment (Supplementary Fig. S1) in wild-type or Adora2b−/− mice (Fig. 1b, Supplementary Fig. S2–4, Supplementary Table S2, http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE19875). The gene with highest differential readout was the circadian rhythm protein Per2. Per2 is a member of the Period family of genes expressed in a circadian rhythm pattern in the suprachiasmatic nucleus11. In addition to Per2, the microarray studies also demonstrated a similar regulatory pattern for Per1, while other members of the circadian rhythm family were not Adora2b-dependently induced (Supplementary Fig. S3–5, Supplementary Table S2). However, studies in Per1−/− mice failed to identify a functional role for Per1 in myocardial ischemia (Supplementary Fig. S6 and Fig.S7). We therefore focused on Adora2b-elicited alterations of Per2. Indeed, the circadian expression pattern of cardiac Per2 mRNA over a 24h zeitgeber period was abolished in Adora2b−/− mice, similar to the induction of Per2 transcript and protein levels following ischemic preconditioning of the heart (Fig. 1 c, d, e, f, Supplementary Fig. S1). Studies in isolated cardiac myocytes exposed to in vitro hypoxic preconditioning (HPC, Supplementary Fig. S8) demonstrated enhanced Adora2b and Per2 transcript in wild-type, but not in Adora2b−/− myocytes (Fig. 1g). Moreover, we found elevated PER2 transcript and protein levels in cardiac tissues of patients with ischemic heart disease (n=10 per group; Fig. 1h, Supplementary Fig. S9, Supplementary Table S1). Studies in a cultured endothelial cell line (HMEC-1, Supplementary Fig. S10) revealed binding of cAMP response element binding protein (CREB) to the PER2 promoter upon ADORA2B agonist treatment (BAY 60-658310, Fig. 1i). Studies with truncated PER2 promoter constructs identified a CREB binding site responsible for ADORA2B-inducibility of the PER2 promoter that is conserved between mice and human (Fig. 1j, Supplementary Fig. S11). Together, these studies indicate that Adora2b/ADORA2B signaling transcriptionally induces Per2/PER2 transcript and protein levels.
ADORA2B signaling events attenuate proteasomal degradation of PER2 via CULLIN-deneddylation
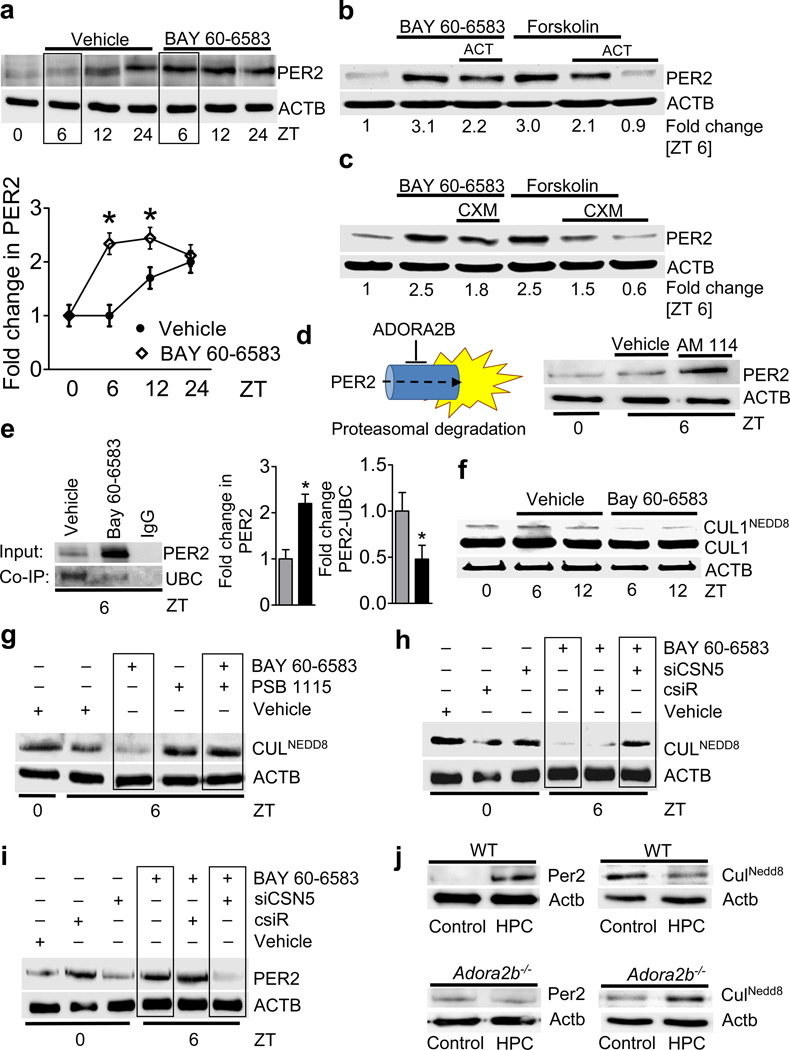
The rapid kinetics of PER2 stabilization following adenosine receptor activation in conjunction with previous reports indicating posttranslational mechanisms in regulating PER2 protein levels12 prompted us to investigate additional post-translational mechanisms of ADORA2B-dependent regulation for PER2. As differences between PER2 expression in controls or ADORA2B agonist treated HMEC-1 were maximal after 6h (Fig. 2a), we examined the influence of inhibition of transcription by actinomycin or inhibition of translation with cycloheximide at 6h (Fig. 2 b and c, Supplementary Figure 12a). These studies demonstrated a combination of transcriptional and post-translational mechanisms in ADORA2B-dependent PER2 stabilization.
Figure 2. Influence of transcriptional, translational or post-translational mechanisms on Period 2 protein levels.
(a) Synchronized HMEC-1 were treated with vehicle, or ADORA2B agonist BAY 60-6583 and blotted after indicated time periods; one of three representative experiments is displayed, and quantified below (*p<0.05, n=3). (b,c) Synchronized HMEC-1 treated with ADORA2B agonist (10mM) or forskolin (30mM) with and without actinomycin (ACT, 2µM, b) or cycloheximide (CXM, 40 µg/ml, c). Effectiveness of ACT or CXM are shown in Supplementary Fig S12 a,b. (d, left) Proposed model of adenosine-dependent alteration in post-translational PER2 protein stability; ADORA2B: A2B adenosine receptor. (d, right) PER2 protein levels following inhibition of proteasomal degradation (AM114 (10 µM; one of three representative blots is displayed). (e) Synchronized HMEC-1 were treated with vehicle, or ADORA2B agonist BAY 60-6583 and protein lysates were isolated for native protein complexes using a PER2 antibody covalently coupled (immobilized) onto an amine-reactive resin. Immunoprecipitated protein was analyzed using immunoblot against ubiquitin. One representative blot of three is displayed. (e, middle, right) Changes in protein shown by densitometry (n=3). (f) Synchronized HMEC-1 treated with vehicle, or ADORA2B agonist BAY 60-6583 and blotted for total CUL1 or neddylated CUL1 using a specific CUL1 antibody on a gradient gel. (g) HMEC-1 at 6h following synchronization treated with ADORA2B agonist BAY 60-6583 alone (10 µM), or following additional pre-treatment with ADORA2B antagonist PSB 1115 (1 µM) and blotted for neddylated CULLIN using a NEDD8 antibody (one of three representative experiments is displayed). (h, i) HMEC-1 following siRNA repression of CSN5 (siCSN5) or treatment with non-specific control siRNA (csiR) were synchronized by serum starvation, lysed and blotted for neddylated CULLIN (h) or PER2 (i) at indicated time points (one of three representative experiments is displayed). (j) Cardiac myocytes were isolated from wild-type (WT) or Adora2b−/− mice, exposed to hypoxic preconditioning (HPC) or control conditions and blotted for Per2 or neddylated Cullin (one representative blot of three independent experiments is displayed, one animal per experiment).
Next, we pursued the ADORA2B as inhibitor of proteasomal PER2 degradation (Fig. 2d, left). As first step, we pretreated HMEC-1 with the proteasomal inhibitor AM114, which resulted in prominent PER2 stabilization (Fig. 2d, right). Previous studies have indicated that post-translational degradation of Per2 involves the SCF E3 ubiquitin ligase complex resulting in polyubiquitination and subsequent degradation by the 26S proteasome13. This SCF complex is active only when CUL1 is covalently modified by the ubiquitin-like protein NEDD814. Indeed, immunoprecipitation of PER2 and immunoblotting for ubiquitin demonstrates attenuated PER2 ubiquitination following ADORA2B agonist treatment (Fig. 2e). Given that ADORA2B signaling has been shown to deneddylate CUL115, we pursued ADORA2B-dependent alterations of the neddylation status of CUL1. Here, we confirmed that deneddylation of CUL1 was enhanced following ADORA2B agonist treatment (Fig. 2f, Supplementary Fig. S12c,d). Furthermore, pretreatment with an ADORA2B antagonist (PSB1115) blocked ADORA2B-agonist-dependent deneddylation of all CULLINs (Fig. 2g). Deneddylation of CULLINs is accomplished through interaction with subunits of the COP9 signalosome (e.g., subunit 5 or CSN5/JAB1)16. Here, we examined whether repression of CSN5 expression (and thereby inhibition of CULLIN-deneddylation) might influence ADORA2B-dependent stabilization of PER2. Following siRNA-mediated CSN5 repression (Supplementary Fig. S12 e,f), BAY 60-6583-induced CULLIN-deneddylation was attenuated compared with controls (Fig. 2h). Moreover, cells treated with siRNA targeting CSN5 lost their ability to stabilize PER2 in response to ADORA2B agonist (Fig. 2i). Finally, we utilized isolated adult cardiac myocytes from wild-type or Adora2b−/− mice. Exposure to HPC was associated with Per2 stabilization in wild-type, but not in Adora2b−/− myocytes. Similarly, HPC-induced Cullin-deneddylation in wild-type mice was abolished in Adora2b−/− myocytes (Fig. 2j). Taken together, these studies indicate that Adora2b/ADORA2B-dependent stabilization of Per2/PER2 levels involves Adora2b/ADORA2B-induced increases in Per2/PER2 transcription together with attenuation of its posttranslational breakdown.
Impaired myocardial adaptation to ischemia in Per2−/− mice
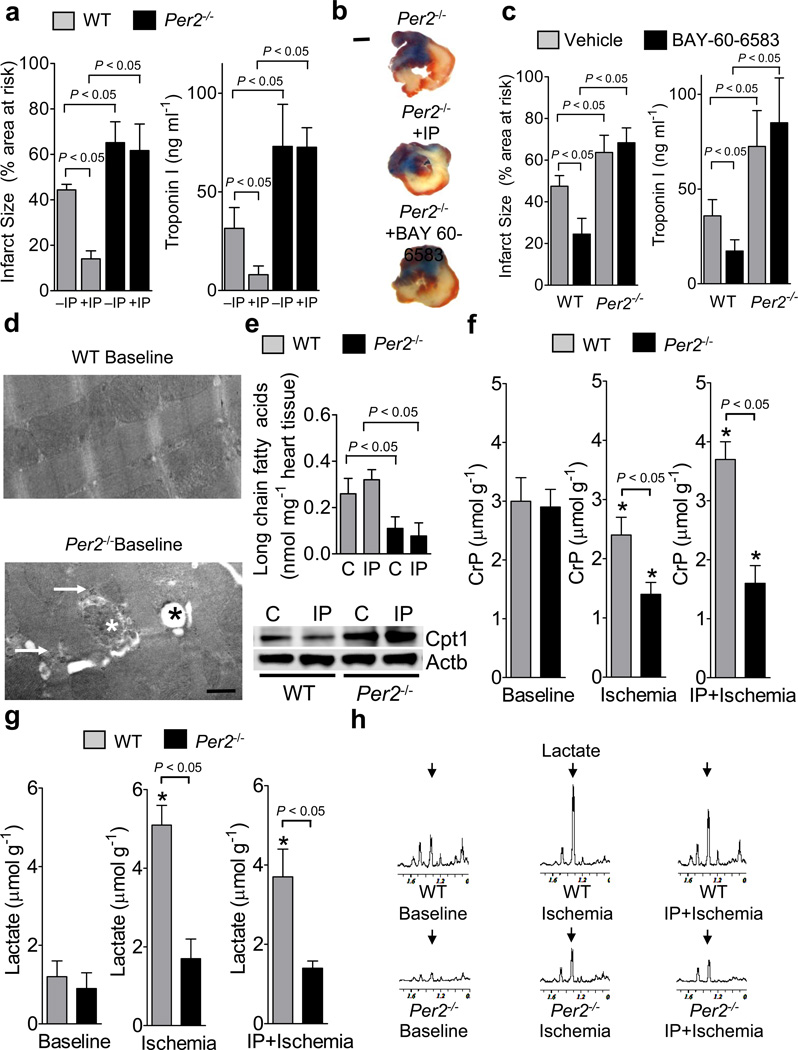
We next studied previously characterized mice gene-targeted for Per2 (Supplementary Fig. S13a)11. Studies of myocardial ischemia in Per2−/− mice revealed enhanced tissue injury with myocardial ischemia, and abolished cardio-protection by IP (Fig. 3a, b). Moreover, treatment with the Adora2b agonist BAY 60-658310 led to a significant reduction of infarcted tissue or Troponin I levels in wild-type, but no cardio-protection in Per2−/− mice, indicating that Adora2b-dependent cardio-protection is abolished in Per2−/− mice (Fig. 3 b,c). Baseline electron microscopic imaging of the cardiac ultrastructure in Per2−/− mice demonstrated isolated mitochondrial swelling and glycogen accumulation, with no major structural alterations of the myofibrillar apparatus (Fig. 3d). Baseline cardiac glycogen levels were elevated (Supplementary Fig. 13b), while long chain fatty acids were decreased (Supplementary Fig. 13c). Consistent with these findings, Per2−/− mice exhibited elevated protein levels for glycogen synthase 1 (Supplementary Fig. 13d) and carnitine-palmitoyltransferase 1 (Supplementary Fig. 13e). However, baseline cardiac function assessed by echocardiography was unaltered (Supplementary Fig. 13f). Consistent with recent studies on the role of Per2 in fatty acid metabolism,17 we observed decreased long chain fatty acids with enhanced Cpt1 protein levels following IP treatment (Fig. 3e). Since these studies indicate enhanced fatty acid metabolism at baseline and following myocardial ischemia of Per2−/− mice, we next utilized magnetic-resonance (NMR) studies to characterize the metabolic role of Per2. We exposed Per2−/− mice or controls to ischemia alone (60min), or IP-treatment prior to ischemia and analyzed cardiac tissues. While baseline creatine phosphate levels were similar, ischemia-associated creatine phosphate depletion was significantly enhanced in Per2−/− mice and conservation of creatine phosphate levels by IP-treatment was abolished (Fig. 3f). Parallel measurements of lactate levels demonstrated that ischemia-induced increases of cardiac lactate were impaired in Per2−/− mice (Fig. 3g, h), indicating a role for Per2 in glycolytic utilization of carbohydrates during myocardial ischemia.
Figure 3. Functional role of Period 2 during myocardial ischemia and ischemic preconditioning.
(a–c) Per2−/− mice or littermate controls matched in age, weight and gender were exposed to 60 min of in situ myocardial ischemia followed by 2h or reperfusion, or received IP pretreatment or Adora2b agonist (BAY 60-6583) treatment prior to myocardial ischemia. IP consisted of 4 cycles of 5 minutes of myocardial ischemia and 5 minutes of reperfusion. Infarct sizes are expressed as the percent of the area at risk that was exposed to myocardial ischemia. In parallel, measurements of the myocardial injury marker troponin I were performed (mean±SD; n=6). (b) Representative infarct staining from Per2−/− mice exposed to 60 min of ischemia, and 2h reperfusion alone (−IP), or additional IP or Adora2b agonist treatment (+IP/BAY 60-6583,c) are displayed (blue indicates retrograde Evan’s blue staining; red and white: area at risk; white: infarcted tissue; scale bar represents 50 µm). (d) Electron microscopy from wildtype and Per2−/− heart tissue. Baseline wildtype and Per2−/− mice showed normal sarcolemal structures, however, in some areas Per2−/− mice exhibited enhanced glycogen content (white arrow), swollen mitochondria (white star) and lipid accumulation within mitochondria (black star); scale bar represents 500 nm. (e) Per2−/− mice or littermate controls matched in age, weight and gender were subjected to in situ IP treatment consisting of 4 cycles of IP (5 minutes of ischemia, 5 minutes of reperfusion). Cardiac preconditioned tissue was shock-frozen and analyzed for long chain fatty acids using an enzymatic ELISA KIT from Biovision (mean±SD; n=3) and protein levels of carnitine-palmitoyltransferase 1 (Cpt1). One representative blot of three is displayed. (f–h) Per2−/− mice or littermate controls matched in age, weight and gender were exposed to 60 min of in situ myocardial ischemia with or without ischemic preconditioning (IP; 4 cycles of 5 min ischemia followed by 5 min of reperfusion) prior to myocardial ischemia. For nuclear magnetic resonance (NMR) analysis of cardiac metabolites, cardiac tissue was shock frozen immediately after ischemia. (f) Creatinephosphate (CrP) levels in wildtype and Per2−/− mice using NMR. Note: IP mediated conservation of CrP storage is abolished in Per2−/− mice. (g, h) Lactate levels and corresponding NMR spectra. (n=3, * significant changes compared to baseline conditions, p<0.05).
Impaired glycolysis during myocardial ischemia in Per2−/− mice
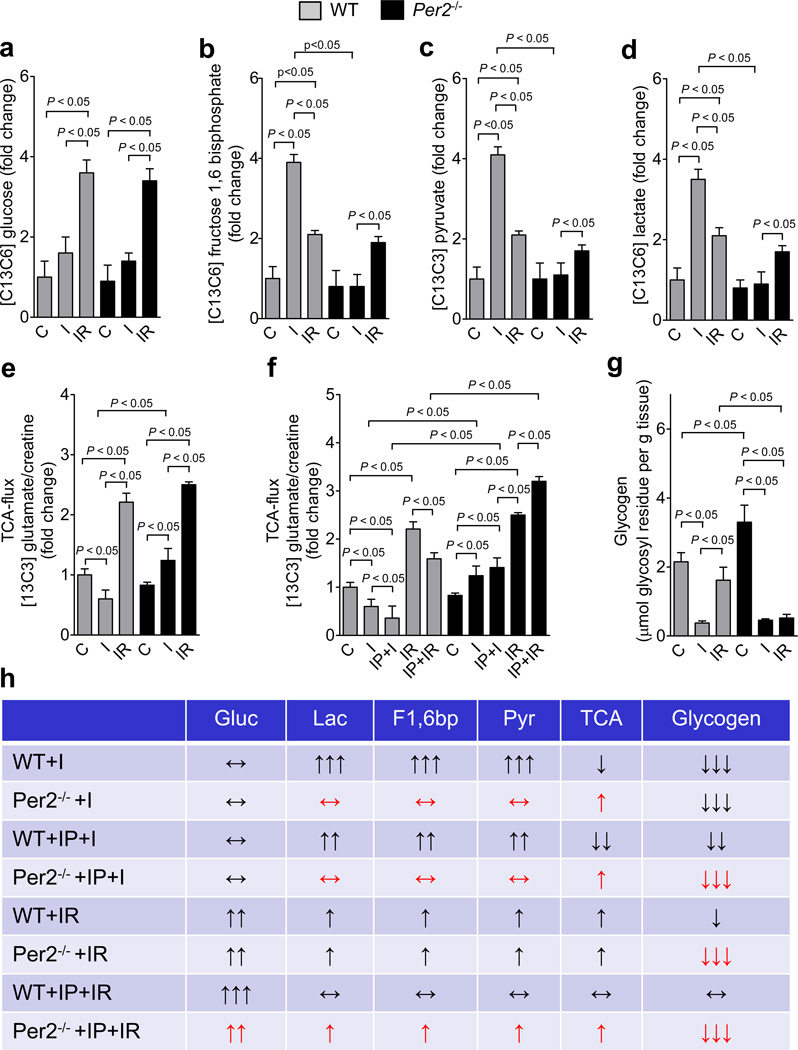
Analysis of glycolytic enzymes revealed IP-elicited induction of their transcript levels in wild-type mice which was completely abolished in Per2−/− mice (Supplementary Fig. S14). To further characterize metabolic alterations in Per2−/− mice, we next used liquid chromatography–tandem mass spectrometry studies following the infusion of 13C-glucose to assess glucose metabolism during ischemia (I) or reperfusion (IR). While we observed no difference in global 13C-glucose flux at baseline, during ischemia or at reperfusion (Fig. 4a, Supplementary Fig. 15a), detailed analysis of glycolytic flux revealed that ischemia-associated increases of 13C-fructose-1,6-bisphosphate levels were completely abolished in Per2−/− mice, indicating that hypoxia-elicited enhancement of glycolysis during ischemia involves Per2 (Fig. 4b, Supplementary Fig. 15b). Moreover, we observed that the ischemia-induced elevation of 13C-pyruvate or 13C-lactate was abolished in Per2−/− mice (Fig. 4c,d, Supplementary Fig. 15c). In addition, while ischemia in wild-type mice attenuated glucose oxidation, glucose TCA flux in Per2−/− was increased (Fig. 4 e). Finally, IP treatment of wild-type mice was associated with an additional reduction in TCA cycle flux, which was abolished in Per2−/− mice (Fig. 4f, Fig. Supplementary Fig. 15d).
Figure 4. Consequences of Period 2 deficiency on cardiac metabolism during myocardial ischemia and reperfusion.
(a–h) Per2−/− mice or littermate controls matched in age, weight and gender were exposed to 60 min of in situ myocardial ischemia with or without ischemic preconditioning (IP; 4 cycles of 5 min ischemia followed by 5 min of reperfusion) prior to myocardial ischemia. 13C glucose (Cambridge Isotopes) was administered intra- arterially in Per2−/− mice or littermate controls either 30 minutes before ischemia (ischemia group: I) or at the onset of reperfusion following 60 min of in situ myocardial ischemia (reperfusion group: R) with or without ischemic preconditioning (IP; 4 cycles of 5 min ischemia followed by 5 min of reperfusion). Determination of 13C glucose and 13C carbohydrates during ischemia or reperfusion was performed using liquid chromatography–tandem mass spectrometry (LC-MS). Glycogen was determined using an enzymatic ELISA KIT from Biovision (mean±SD; n=3). (a) 13C glucose. (b) 13C fructose 1,6 bisphosphate. (c) 13C pyruvate. (d) 13C lactate. (e,f) (TCA cycle) flux rates determined by the ratio of 13C glutamate and total creatine. (g) Glycogen; (mean±SD; n=3).
While glycolytic utilization of carbohydrates is an important adaptive mechanism during ischemia18, increased glycolysis during reperfusion is considered detrimental as it frequently indicates mitochondrial dysfunction.19 Indeed, tissue reperfusion attenuated glycolysis reflected as reduced production of 13C-fructose-1,6-biosphosphate, 13C-pyruvate or 13C-lactate in wild-type mice (Fig. 4b–d). Although ischemia alone failed to enhance glycolysis in Per2−/− mice, their glycolytic flux was increased during reperfusion (Fig. 4e,f). Studies on the effect of IP pretreatment on reperfusion metabolism in wild-type mice demonstrated a further reduction of glycolysis20 and restoration of a glucose metabolism comparable to baseline conditions (Fig. 4f, Supplementary Fig. 15b–d). In contrast thereof, Per2−/− mice maintained lactate production, indicating uncoupling glycolysis from glucose oxidation as a sign of mitochondrial dysfunction21 (Supplementary Fig. 15c). Since we did not observe differences in glucose uptake between wild-type and Per2−/− mice during ischemia or reperfusion, we next analyzed the effect of metabolic changes on cardiac glycogen. Here, ischemia significantly reduced glycogen storages in both, wild-type or Per2−/− mice, even though Per2−/− started from a higher glycogen baseline level (Fig. 4g, Supplementary Fig. 15f). While reperfusion led to the restoration of glycogen in wild-type mice, this was abolished in Per2−/− mice. Together these data indicate that Per2−/− are severely compromised in effectively utilizing carbohydrates during ischemia or reperfusion (Fig. 4h).
Hypoxia-inducible factor 1 alpha (Hif1a) links adenosine-mediated Per2 stabilization to cardiac metabolism during ischemia
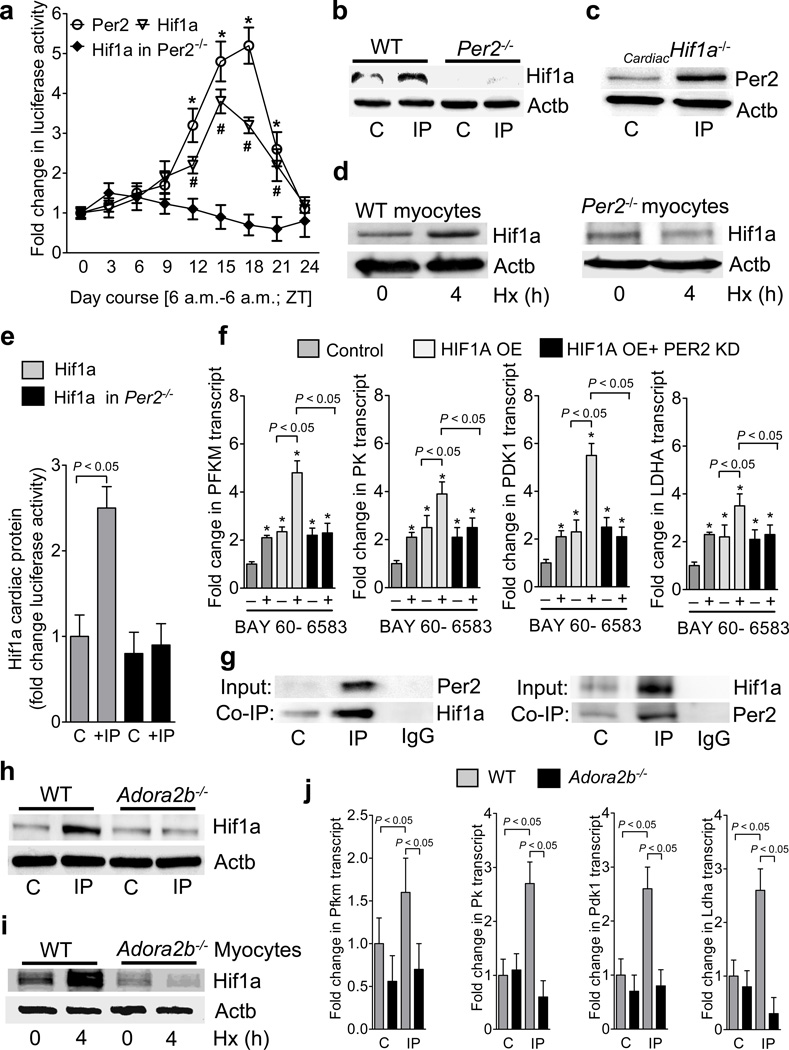
We next addressed transcriptional mechanisms controlling glycolysis during limited oxygen availability, as IP treatment of wild-type mice was associated with a robust induction of glycolytic enzymes, which was completely abolished in gene-targeted mice for Per2 (Supplementary Table S6). Based on the notion that Hif1a plays a key role in transcriptional control of the glycolytic pathway,22 we pursued the functional status of Hif1a in gene-targeted mice for Per2 in a Hif1a reporter mouse23. Surprisingly, we observed a diurnal kinetic for cardiac Hif1a protein levels (Fig. 5a), transcript levels of its isoforms Hif1.1, Hif1.2 as well as the glycolytic enzymes Pdk1 and Ldh (Supplementary Fig. S16) during a 24h time period. Moreover, genetic deletion of Per2 in Hif1a reporter mice abolished cycling of Hif1a (Fig. 5a). In addition, stabilization of Hif1a with IP treatment in wild-type was abolished in Per2−/− mice (Fig. 5b), while mice with induced deletion of Hif1a in cardiac myocytes (Fig. 5c, Supplementary Fig. S17) retained their ability to stabilize Per2 upon IP treatment (Fig. 5c). Similarly, hypoxic Hif1a stabilization was abolished in isolated myocytes from Per2−/− mice (Fig. 5d). Moreover, IP treatment of Hif1a reporter mice was associated with increased reporter activity, which was abolished following genetic deletion of Per2 (Fig. 5e). We next assessed transcriptional regulation of glycolytic enzymes in oxygen-stable HIF1A overexpressing HMEC-1 cells24 with or without siRNA mediated PER2 knockdown. Oxygen-stable HIF1A or treatment with the ADORA2B agonist BAY 60-6583 was associated with elevated transcript levels of glycolytic enzymes. While treatment with BAY 60-6583 further enhanced glycolytic transcripts in oxygen-stable HIF1A overexpressing cells, this was abolished after PER2 knockdown (Fig. 5f). Additional studies utilizing co-immunoprecipitation indicated a direct protein-protein interaction between Hif1a and Per2 in cardiac tissues following exposure to IP (Fig. 5g).
Figure 5. Hif1a as link between adenosine mediated period 2 signaling and metabolism.
(a) Hearts from Hif1a reporter, Per2 reporter or Per2−/−-Hif1a reporter double mutant mice were analyzed for Hif1a or Per2 protein during a 24 h zeitgeber period (*,# p<0.05 over baseline, n=3). (b,c) Per2−/− or cardiac specific Hif1a−/− mice were subjected to IP (IP; 4 cycles of 5 min ischemia followed by 5 min of reperfusion) and Western blot analysis for Hif1a or Per2 protein from the area at risk was performed, respectively. One representative blot of three is displayed. (d) Isolated adult cardiomyocytes from wild-type or Per2−/− mice were exposed to ambient hypoxia [1%, 4h] and analyzed for Hif1a protein. One representative blot of three is displayed. (e) Hif1a reporter or Per2−/−-Hif1a reporter double mutant mice were exposed to IP (IP; 4 cycles of 5 min ischemia followed by 5 min of reperfusion) and the area at risk was analyzed for luciferase activity indicating Hif1a protein (mean±SD; n=4). (f) Transcriptional regulation of glycolytic enzymes in oxygen-stable HIF1A overexpressing HMEC-1 cells with or without siRNA mediated PER2 knockdown. Cells were treated with vehicle or Adora2b agonist BAY 60-6583 and analyzed for transcript levels of phosphofructokinase-m (PFKM), pyruvate kinase (PK), pyruvate dehydrogenase kinase 1 (PDK1) and lactate dehydrogenase a (LDHA) (mean±SD; n=3). (g) Hearts from wild-type mice were subjected to IP (IP; 4 cycles of 5 min ischemia followed by 5 min of reperfusion) and protein lysates were isolated for native protein complexes using a Per2 antibody covalently coupled (immobilized) onto an amine-reactive resin. Co-immunoprecipitated protein was analyzed using immunoblot against Hif1a. One representative blot of three is displayed. (h,i) Hearts or isolated myocytes from wildtype or Adora2b−/− were exposed to IP (IP; 4 cycles of 5 min ischemia followed by 5 min of reperfusion) or ambient hypoxia [1%, 4h], respectively, and analyzed for Hif1a protein using immunoblot. One representative blot of three is displayed. (j) Transcript levels of phosphofructokinase-m (Pfkm), pyruvate kinase (Pk), pyruvate dehydrogenase kinase 1 (Pdk1) and lactate dehydrogenase a (Ldha) from wildtype or Adora2b−/− mice after IP (IP; 4 cycles of 5 min ischemia followed by 5 min of reperfusion) treatment (mean±SD; n=3).
As we had previously shown that Adora2b signaling plays a critical role in the transcriptional induction and protein stability for Per2, we next examined Hif1a in the hearts of gene-targeted mice for the Adora2b. Similar to the above findings in Per2−/− mice, we observed lower expression of the transcript levels for Hif1.1 and Hif1.2 isoforms in gene-targeted mice for the Adora2b, in conjunction with abolished circadian expression over a 24h period (Supplementary Fig. S18). Moreover, hypoxia induced stabilization of Hif1a was abolished in Adora2b−/− mice (Fig. 5h,i). Finally, we observed a similar defect for the transcriptional induction of the glycolytic enzymes with cardiac IP treatment of Adora2b−/− as previously seen in Per2−/− mice (Fig. 5j) Together, these data indicate that Adora2b-dependent control of Per2 plays an important role in the hypoxia-elicited induction of the glycolytic machinery during myocardial ischemia.
Stabilization of cardiac Per2 by light exposure mediates cardio-protection from ischemia
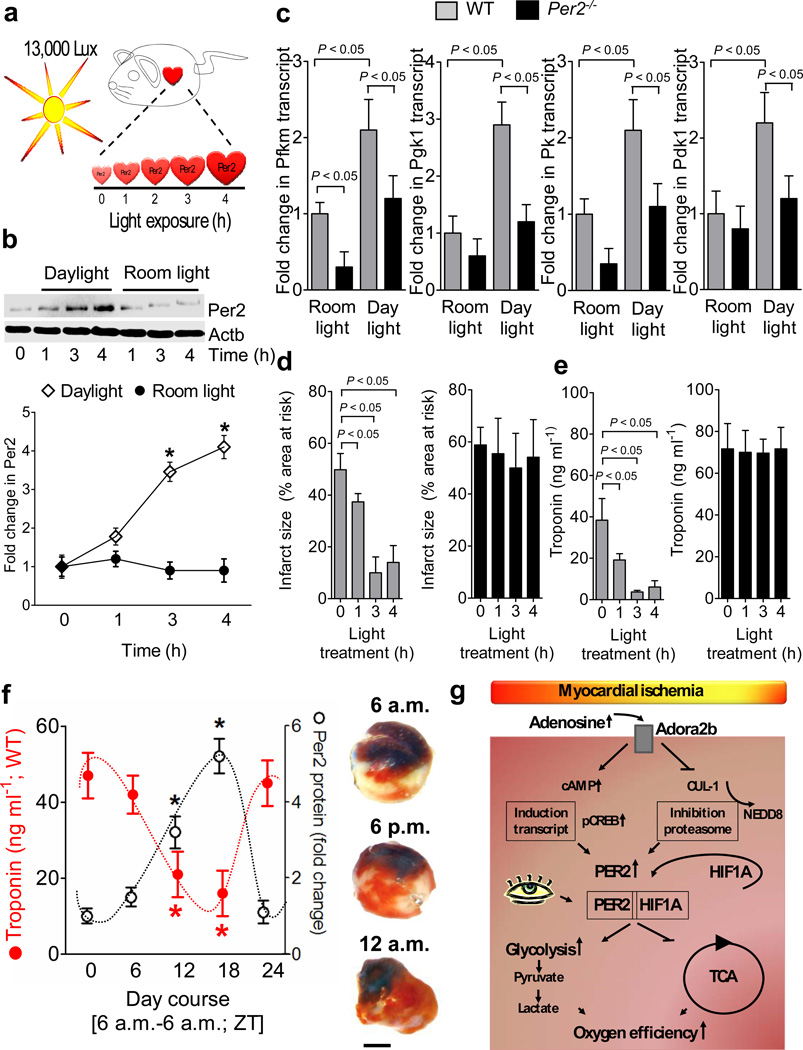
We next attempted to achieve enhanced cardiac Per2 stabilization via light exposure25,26. Therefore, we exposed mice over 0 to 4h to daylight (13,000 lux, Fig. 6a) and assessed cardiac levels of Per2 protein. We found time-dependent increases in cardiac Per2 protein levels with daylight exposure (Fig. 6b) compared to mice maintained at room light (200 lux, Fig. 6b, Supplementary Fig. 19a). Light exposure of wild-type mice over 4h was associated with induction of cardiac transcript levels for glycolytic enzymes (Fig. 6c). In contrast, light-dependent induction of glycolytic enzymes was abolished in Per2−/− mice.
Figure 6. Light-induced stabilization of cardiac Per2 provides potent protection from myocardial ischemia.
(a) Experimental model for studying light-induced stabilization of cardiac Per2 levels. (b) Following exposure to 12h of darkness, mice were exposed to indicated times of intense daylight (13,000 lux) and compared to controls that were maintained at room light. Cardiac Per2 levels were analyzed by Western blotting (n=4 mice per group). (c) Alterations of transcript levels due to daylight exposure. Cardiac transcript levels of glycolytic key enzymes in Per2−/− mice or matched littermate controls after 4 hrs of light exposure (n=4 animals per condition; Pfkm: 6-phosphofructokinase-m; Pgk1: phosphoglycerate kinase 1; Pk: pyruvate kinase; Pdk1: pyruvate dehydrogenase kinase, isozyme 1). (g,e) Per2−/− mice or littermate controls matched in age, gender and weight underwent light therapy as described above over indicated time periods, followed by exposure to in situ myocardial ischemia (60 min) followed by 2h of reperfusion. Myocardial injury was assessed by measurement of Troponin I plasma levels (n=6 mice per experimental group) or infarct staining (see also Supplementary Fig S19b, n=6 animals per group, mean±SD). (f) Wild-type mice were subjected to in situ myocardial ischemia (60 min) followed by 2h of reperfusion over a 24 h time period. Troponin or infarct sizes (scale bar represents 50 µm) were correlated to Per2 protein levels using Per2 reporter mice (*p<0.05, n=8, mean±SD, Fig. 5a, Supplementary Fig. S19c). (g) Schematic model of Adora2b-dependent Per2 stabilization and its role in regulating anaerobic glycolysis and cardiac metabolism during myocardial ischemia.
We next tested a potential association of light exposure-elicited stabilization of Per2 with cardio-protection (Fig. 6d,e). We observed a time-dependent attenuation of myocardial infarct sizes and plasma troponin I levels in wild-type mice pre-exposed to intense light. In contrast, reduction of myocardial infarct sizes (Fig. 6d, Supplementary Fig. 19b) or plasma troponin I levels (Fig. 6e) following intense light exposure was abolished in gene-targeted mice for Per2. Consistent with these findings, myocardial infarct sizes showed a diurnal variation, with smallest infarct sizes and lowest troponin I levels around midnight (Fig. 6f, Supplementary Fig.19c). Taken together, these findings indicate that exposure to intense light enhances cardiac Per2 levels, and is associated with Per2-dependent cardio-protection from ischemia (Fig. 6g).
DISCUSSION
Myocardial adaptation to conditions of limited oxygen availability involves a metabolic switch towards more oxygen efficient utilization of carbohydrates27. The present studies demonstrate that Adora2b/ADORA2B-dependent stabilization of Per2/PER2 plays an important role in this cardio-adaptive response. We observed that Per2−/− mice showed diminished levels of cellular energy stores during ischemia, while they failed to generate lactate, indicating a metabolic phenotype. In fact, Per2−/− mice lacked the capacity to enhance oxygen efficient glycolysis, ultimately resulting in depletion of energy-rich phosphate levels, and increased myocardial cell death during ischemia. Together, these studies indicate a previously unrecognized role for Per2 as a metabolic master-switch during cardio-adaption to ischemia, driving the utilization of oxygen-efficient carbohydrate-dependent metabolic pathways (Fig. 6g).
We observed that Adora2b signaling increased Per2 expression and protein stability. Consistent with our findings, Adora2b signaling has previously been implicated in regulating target genes. One study demonstrated that Adora2b signaling activates MAPK or p38 via cAMP/Creb pathways,28 both which have been implicated in Per2 regulation.29 Consistent with the notion that Adora2b signaling alters intracellular cAMP responses, recent studies identified CLOCK-independent regulation of Per2 via cAMP-dependent signaling30. Other studies have shown that Adora2b signaling regulates protein stability of adenosine target-genes. In fact, studies on the role of hypoxic preconditioning of the lungs demonstrated that Adora2b signaling has protective and anti-inflammatory effects by regulating the post-translational stability of NFκB15.
Our studies demonstrate that cardiac Per2 stabilization can be achieved by daylight exposure. Indeed, stabilization of Per2 within the suprachiasmatic nuclei of the hypothalamus involves light-induced Creb phosphorylation31. Moreover, a previous study examined whether profiles of Per1 or Per2 proteins in peripheral organs are affected by the photoperiod26. For this purpose, the authors maintained rats under different photoperiods and found that timing of light exposure significantly affected the circadian profile of Per1 or Per2 protein levels in lungs and hearts26,32. At present, the mechanisms linking central circadian rhythm regulation and peripheral Per2 stabilization are under investigation and could involve hormonal pathways33, or cyclic alterations in 5'-AMP or adenosine34. Indeed, a recent study identified 5'-AMP in the initiation of hypo-metabolism in mammals35. In addition, it is intriguing to think about light-dependent stabilization of Per2 and cardio-protection from ischemia in the context of well-documented variations in the frequency of the onset of acute myocardial infarction36. In fact, two recent studies found that patients have larger infarct sizes in the early morning hours37,38. For the first time, it was shown that myocardial infarct size and left ventricular function after acute myocardial infarction have a circadian dependence on the time of day onset of ischemia.38 However, a different study in humans on cardiac PER2 oscillation reports a 12h delay compared to murine Per239. As such, observations in patients indicating larger infarct sizes in the morning hours37 would be in contrast to the present findings. However, this study included a high proportion of patients with coronary heart disease and cardiomyopathy compared to healthy controls. As such it is not clear if these findings are a reflection of a circadian pattern in healthy patients or in patients with heart disease. Moreover, epidemiologic studies in human indicate that other factors than daytime (e.g. sun exposure, exercise, social factors) are critical for synchronization of circadian rhythms40. Therefore, the present findings from mice cannot simply be extrapolated to the circadian rhythmicity of myocardial ischemia in humans. In fact, additional studies will be necessary to define the expression levels and circadian rhythmicity of PER2 in the human heart, as well as its functional role in human heart disease.
The critical role of anaerobic glycolysis in providing ATP in severe ischemia has been well documented and observed in different gene targeted mice. As such GLUT4−/− or AMPK−/− mice41,42 exhibited reduced lactate production which is associated with increased tissue injury after low flow ischemia and diminished regeneration of high-energy phosphate compounds on reperfusion. These findings are in line with the present studies showing larger infarct sizes in Per2−/− who failed to utilize glycolysis during ischemia and to restore glycogen levels during reperfusion. Similar to GLUT4−/−, Per2−/− also showed higher glycogen levels at baseline but were unable to sufficiently utilize carbohydrates. While diminished uptake of exogenous glucose contributes to the phenotype in GLUT4−/−, we did not observe differences in 13C6 Glucose uptake between wild-type or Per2−/− mice.
The present studies indicate that Per2 enhances glycolytic capacity during ischemia and genetic data implicate Hif1a as molecular regulator for this adaptation. These findings are consistent with recent studies of myocardial ischemia in hypoxia-inducible factor prolyl 4-hydroxylase-2 hypomorphic mice43. These mice show increased protein levels of Hif1a and Hif2a, in conjunction with cardio-protection from ischemia. While the present studies in gene-targeted mice for Per2 demonstrate attenuated stabilization of Hif1a, larger infarct sizes and attenuated glycolytic flow, the above studies demonstrate that Hif1a overexpression by Phd hypomorphism is associated with increased lactate levels and glycolytic capacity.43 While Ldh regulation by Hif1a has been shown earlier44, studies on cardiac Ldh implicated the Clock:Bmal1 as regulating transcription factor45. Clock:Bmal1 has also been shown to regulate the circadian pattern of Per2. However, photic induction of Per2 in the SCN is suggested to be Creb dependent.46 Similarly, in the present study, enhanced Adora2b signaling during ischemia led to Creb induction and Per2 stabilization. Together, such findings support the concept that multiple pathways can function to regulate the circadian network.47
Consistent with our findings for Hif1a as circadian protein with highest protein levels in the late evening (ZT12-ZT18), we found a significant reduction in infarct sizes at ZT12 and ZT18 compared to ZT0. Surprisingly, a different study on diurnal variations in myocardial ischemia/reperfusion tolerance revealed increased infarct sizes at ZT12 compared to ZT0.48 The authors used a closed chest model for myocardial ischemia, and a potent opioid (buprenorphine) was used for anesthesia. The differences between both studies could potentially be explained by the fact that the open chest model used in the current study has been associated with increased inflammation which may have influenced experimental results.49 Moreover, a study of permanent cardiac occlusion in Per2−/− mice revealed attenuated infarct sizes following Per2 deletion, which could be due to differences in the model system or methods (e.g. identification of an area at risk)50. In line with our findings, other studies on the role of Per2 during ischemia and reperfusion reported impaired endothelial progenitor cell function and auto-amputation of the distal limb when Per2−/− mice were subjected to hind-limb ischemia.51,52 The present findings of Per2-dependent regulation of cardiac metabolism provide some level of specificity for Per2, as gene-targeted mice for Per1 did not show a phenotype in myocardial ischemia nor had alterations in their ability to stabilize Hif1a. This is consistent with previous studies showing non-redundant roles for Per1 and Per2 in the mammalian circadian clock53. Studies on clock, cryptochrome 1 or timeless following IP showed no induction of their transcript levels, while protein stabilization occurred in an Adora2b-independent fashion Supplementary Fig. S4). These results are consistent with previous findings indicating posttranslational mechanisms in the regulation of the mammalian circadian clock. 12
Taken together, the present results identify Adora2b-dependent stabilization of Per2 as an endogenous mechanism allowing the ischemic myocardium to adapt its metabolism towards oxygen-efficient utilization of carbohydrates. Future challenges will involve understanding the axis between light exposure and cardiac Per2 stabilization, as well as defining approaches to stabilize cardiac Per2 levels in a therapeutic setting. Moreover, the use of a germline Per2−/− mouse in the current studies does not allow conclusions regarding the contributions of different tissues to the observed phenotype. Therefore, additional challenges will include studies in mice with tissue-specific Per2 deletion.
Experimental Procedures
Human cardiac tissue
Patient heart samples were obtained from patients undergoing orthotopic cardiac transplantation. Clinical samples screened are given in Supplementary Table S1. Collection and use of patient samples were approved by the appropriate IRB of each Institution in addition to the study having Colorado Multiple Institutional Review Board (COMIRB) approval.
Mice
Experimental protocols were approved by the Institutional Review Board at the University of Colorado Denver, USA. They were in accordance with the Protection of Animals and the National Institutes of Health guidelines for use of live animals. Adora2b−/− mice were generated by Deltagen 10, Per2−/− mice 11, BL6C57, Hif1aloxp/loxp 54 and Myh6-cre/Esr155, mPer2Luc56 and ROSA26 ODD-Luc+23 mice were obtained from the Jackson Laboratories. To obtain cardiac specific Hif1a−/− mice, Hif1aloxp/loxp mice were crossed with Myh6-cre/Esr1 mice. Tissue specific knockout was achieved by a 5 day treatment of tamoxifen (1mg/day) i.p.
Murine Model for cardiac ischemia
Murine model for in situ ischemia and IP of the heart was performed using a hanging weight system57.
Microarray analysis
Array data have been deposited at http://www.ncbi.nlm.nih.gov/geo/ (accession number GSE19875).
Transcriptional analysis
Total RNA was isolated from human heart tissue or human endothelial cells (HMEC) and transcript levels were determined by real-time RT-PCR (iCycler; Bio-Rad Laboratories Inc.)58.
Immunoblotting experiments
All antibodies used were rabbit polyclonal: Per1 (Abcam, ab3443), Clock (Abcam, ab43106), Timeless (Abcam, ab84502), Cry1 (Abcam, ab104736), Prkaa1 (Abcam, ab32047), Per2 (Abcam, ab467), Ubiquitin (Cell Signal, ab3933), Nedd8 (Abcam, ab38634), JAB1/CSN5 (Abcam, ab12323), Cul1 (Abcam, ab2964), Gys1 (Abcam, ab2479), Scd1 (Abcam, ab19862), Cpt1 (Alpha Diagnostic, CPT1M11-A), Hif1a (Abcam, ab1).
Isolation of adult cardiomyocytes
Myocytes from C57BL/6N (The Jackson Laboratory), Adora2b−/−, Per2−/− or cardiacHIF1a−/− mice were isolated as described previously59.
In vitro preconditioning
Cellular preconditioning was performed on adult cardiomyocytes that were plated on either 6- or 24-well plates following a modified in vivo protocol optimized for cells 15.
Cell culture and treatments
Human microvascular endothelial cells (HMEC-1) or HMEC-1 expressing oxygen-stable HIF1a24 were cultured as described previously58.
Chromatin immunoprecipitation (ChIP) assay
ChIP assays were performed using the ChIP-IT™ Express Enzymatic Kit from Active Motif (Carlsbad, CA, USA). Phospho-CREB (Phospho-CREB [Ser133] [87G3] Rabbit mAb, #9198) and control (Normal Rabbit IgG #2729) antibodies were purchased from Cell Signal.
PER2 promoter studies
Full length PER2 promoter constructs and truncations were sub-cloned into pGL4 luciferase reporter vector. HMEC-1 cells were co-transfected with the pGL4 construct expressing firefly luciferase and with pRL-TK plasmid expressing Renilla luciferase. To measure promoter activity, the activity of firefly luciferase was corrected against Renilla luciferase activity. To control for circadian activity, cells were co-transected with the CREB dominant negative vector from Clontech.
COP9 or PER2 suppression with RNA interference
HMEC-1were either grown on inserts or in 60-mm Petri dishes. SMARTpool siRNA targeting CSN5 or PER2 was synthesized by Dharmacon (Lafayette, CO, USA). siRNA sequences are listed in Table S59,58.
Electron Microscopy
The samples were imaged with an FEI Tecnai G2 Spirit Biotwin TEM (Hillsboro, OR) at an operating voltage of 120 kV.
Glycogen and Long Chain Fatty Acid (LCFA) measurements
Glycogen and LCFA were determined using Glycogen Assay Kit and Free Fatty Acid Quantification Kit from Biovision.
Echocardiography
For echocardiography, mice were anesthetized with 2% isoflurane and cardiac function was assessed by 2D-transthoracic echocardiography using a Visual Sonics Vevo 770 high resolution ultrasound imager equipped with a 35-MHz transducer. The heart rates was maintained above 500 beats/min throughout.60
PK and LDH activity
Tissues were homogenized and enzyme activity was determined using a Pyruvate Kinase Assay Kit and and LDH Assay kit from Biovison.
NMR Analysis on Cell and Tissue Extracts
All 1H-NMR spectra were obtained at the Bruker 500 MHz DRX NMR spectrometer using an inverse Bruker 5-mm TXI probe.
Determination of 13C glucose and 13C metabolites using liquid chromatography–tandem mass spectrometry (UPLC-MS)
Isotopically labeled 13C-glucose was purchased from Cambridge Isotope Labs. All UPLC-MS data were acquired with a Waters Acquity UPLC system coupled to a Water Synapt HDMS quadrupole time-of-flight mass spectrometer. Experimental details are given in the “Supplementary Information”.
Co-Immunoprecipitation studies
Co-IP studies were performed using the Thermo Scientific Pierce Co-Immunoprecipitation (Co-IP) Kit.
Data analysis
Data were compared by two-factor ANOVA with Bonferroni's posttest, or by Student’s t test where appropriate. Values are expressed as mean ± SD from 3–6 animals per condition. For analysis of changes in transcript a one-way ANOVA was carried out and multiple comparisons between control and treatment groups were made using the Dunnett post test. Data are expressed as mean ± SD. P<0.05 was considered statistically significant. For metabolic analysis 3 repeats were performed. All numerical data are presented as mean ± SD from the replicate experiments. Unpaired T-test and/or one-way analysis of variance (ANOVA) test were used to determine differences between groups. The significance level was set at p<0.05 for all tests. For all statistical analysis GraphPad Prism 5.0 software for Windows XP was used. The authors had full access to and take full responsibility for the integrity of the data. All authors have read and agree to the manuscript as written.
Supplementary Material
Acknowledgement
The authors wish to acknowledge Shelley A. Eltzschig for artwork during manuscript preparation and Allen Medway, Katharina Hoffmann, Jadranka Macas, Cornelia Zachskorn and Megan Bonney for technical assistance and Dr. Cheng-Chi Lee for kindly providing the Per1−/− mice. The present research work is supported by National Heart, Lung, and Blood Institute Grant R01-HL0921, R01-DK083385 and R01-HL098294 to H. K. Eltzschig and R01-HL60569 to S.P. Colgan, and by 1K08HL102267-01 to TE, and Foundation for Anesthesia Education and Research Grants to T. E. and H. K. Eltzschig, American Heart Association Grant to T. E. and Crohn’s and Colitis Foundation of America.
Footnotes
Supplemental information
Supplemental Information includes extended experimental procedures, nineteen figures, and seven tables.
Author Contributions
T.E. designed and supervised the study, wrote the manuscript and did animal surgery, K.H. did western blots and siRNA knockdown studies, S.B. did western blots, co-immunoprecipitation and promoter studies, S.R. did western blots and animal experiments, M.M. did immunohistochemistry and electron microscopy, L.W. isolated mouse myocytes, B.L. provided human heart samples, J.H., C.B. and D.K. did metabolic analysis, B.P., S.C. and H.E. supervised the study and wrote the manuscript.
References
- 1.Eltzschig HK, Carmeliet P. Hypoxia and inflammation. N Engl J Med. 2011;364:656–665. doi: 10.1056/NEJMra0910283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sitkovsky MV, et al. Physiological control of immune response and inflammatory tissue damage by hypoxia-inducible factors and adenosine A2A receptors. Annual Review of Immunology. 2004;22:657–682. doi: 10.1146/annurev.immunol.22.012703.104731. [DOI] [PubMed] [Google Scholar]
- 3.Eltzschig HK, Eckle T. Ischemia and Reperfusion - From Mechanism to Translation. Nat Med. 2011 doi: 10.1038/nm.2507. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neubauer S. The failing heart--an engine out of fuel. N Engl J Med. 2007;356:1140–1151. doi: 10.1056/NEJMra063052. [DOI] [PubMed] [Google Scholar]
- 5.Aragones J, Fraisl P, Baes M, Carmeliet P. Oxygen Sensors at the Crossroad of Metabolism. Cell Metab. 2009;9:11–22. doi: 10.1016/j.cmet.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Thompson LF, et al. Crucial role for ecto-5'-nucleotidase (CD73) in vascular leakage during hypoxia. J. Exp. Med. 2004;200:1395–1405. doi: 10.1084/jem.20040915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fredholm BB. Adenosine, an endogenous distress signal, modulates tissue damage and repair. Cell Death Differ. 2007;14:1315–1323. doi: 10.1038/sj.cdd.4402132. [DOI] [PubMed] [Google Scholar]
- 8.Eckle T, et al. A2B adenosine receptor dampens hypoxia-induced vascular leak. Blood. 2008;111:2024–2035. doi: 10.1182/blood-2007-10-117044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eckle T, Kohler D, Lehmann R, El Kasmi K, Eltzschig HK. Hypoxia-inducible factor-1 is central to cardioprotection: a new paradigm for ischemic preconditioning. Circulation. 2008;118:166–175. doi: 10.1161/CIRCULATIONAHA.107.758516. [DOI] [PubMed] [Google Scholar]
- 10.Eckle T, et al. Cardioprotection by ecto-5'-nucleotidase (CD73) and A2B adenosine receptors. Circulation. 2007;115:1581–1590. doi: 10.1161/CIRCULATIONAHA.106.669697. [DOI] [PubMed] [Google Scholar]
- 11.Zheng B, et al. The mPer2 gene encodes a functional component of the mammalian circadian clock. Nature. 1999;400:169–173. doi: 10.1038/22118. [DOI] [PubMed] [Google Scholar]
- 12.Lee C, Etchegaray JP, Cagampang FR, Loudon AS, Reppert SM. Posttranslational mechanisms regulate the mammalian circadian clock. Cell. 2001;107:855–867. doi: 10.1016/s0092-8674(01)00610-9. [DOI] [PubMed] [Google Scholar]
- 13.Gallego M, Virshup DM. Post-translational modifications regulate the ticking of the circadian clock. Nat Rev Mol Cell Biol. 2007;8:139–148. doi: 10.1038/nrm2106. [DOI] [PubMed] [Google Scholar]
- 14.Wu JT, Lin HC, Hu YC, Chien CT. Neddylation and deneddylation regulate Cul1 and Cul3 protein accumulation. Nat Cell Biol. 2005;7:1014–1020. doi: 10.1038/ncb1301. [DOI] [PubMed] [Google Scholar]
- 15.Khoury J, Ibla JC, Neish AS, Colgan SP. Antiinflammatory adaptation to hypoxia through adenosine-mediated cullin-1 deneddylation. J Clin Invest. 2007;117:703–711. doi: 10.1172/JCI30049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mikus P, Zundel W. COPing with hypoxia. Semin Cell Dev Biol. 2005;16:462–473. doi: 10.1016/j.semcdb.2005.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grimaldi B, et al. PER2 controls lipid metabolism by direct regulation of PPARgamma. Cell Metab. 2010;12:509–520. doi: 10.1016/j.cmet.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jennings RB, Reimer KA. The cell biology of acute myocardial ischemia. Annu Rev Med. 1991;42:225–246. doi: 10.1146/annurev.me.42.020191.001301. [DOI] [PubMed] [Google Scholar]
- 19.Jaswal JS, Keung W, Wang W, Ussher JR, Lopaschuk GD. Targeting fatty acid and carbohydrate oxidation--a novel therapeutic intervention in the ischemic and failing heart. Biochim Biophys Acta. 2011;1813:1333–1350. doi: 10.1016/j.bbamcr.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 20.Finegan BA, Lopaschuk GD, Coulson CS, Clanachan AS. Adenosine alters glucose use during ischemia and reperfusion in isolated rat hearts. Circulation. 1993;87:900–908. doi: 10.1161/01.cir.87.3.900. [DOI] [PubMed] [Google Scholar]
- 21.Lopaschuk GD, Stanley WC. Glucose metabolism in the ischemic heart. Circulation. 1997;95:313–315. doi: 10.1161/01.cir.95.2.313. [DOI] [PubMed] [Google Scholar]
- 22.Semenza GL, Roth PH, Fang HM, Wang GL. Transcriptional regulation of genes encoding glycolytic enzymes by hypoxia-inducible factor 1. J Biol Chem. 1994;269:23757–23763. [PubMed] [Google Scholar]
- 23.Safran M, et al. Mouse model for noninvasive imaging of HIF prolyl hydroxylase activity: assessment of an oral agent that stimulates erythropoietin production. Proc Natl Acad Sci U S A. 2006;103:105–110. doi: 10.1073/pnas.0509459103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kong T, Westerman KA, Faigle M, Eltzschig HK, Colgan SP. HIF-dependent induction of adenosine A2B receptor in hypoxia. Faseb J. 2006;20:2242–2250. doi: 10.1096/fj.06-6419com. [DOI] [PubMed] [Google Scholar]
- 25.Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418:935–941. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- 26.Bendova Z, Sumova S. Photoperiodic regulation of PER1 and PER2 protein expression in rat peripheral tissues. Physiol Res. 2006;55:623–632. doi: 10.33549/physiolres.930849. [DOI] [PubMed] [Google Scholar]
- 27.Wolff AA, Rotmensch HH, Stanley WC, Ferrari R. Metabolic approaches to the treatment of ischemic heart disease: the clinicians' perspective. Heart Fail Rev. 2002;7:187–203. doi: 10.1023/a:1015384710373. [DOI] [PubMed] [Google Scholar]
- 28.Schulte G, Fredholm BB. The G(s)-coupled adenosine A(2B) receptor recruits divergent pathways to regulate ERK1/2 and p38. Exp Cell Res. 2003;290:168–176. doi: 10.1016/s0014-4827(03)00324-0. [DOI] [PubMed] [Google Scholar]
- 29.Petrzilka S, Taraborrelli C, Cavadini G, Fontana A, Birchler T. Clock gene modulation by TNF-alpha depends on calcium and p38 MAP kinase signaling. J Biol Rhythms. 2009;24:283–294. doi: 10.1177/0748730409336579. [DOI] [PubMed] [Google Scholar]
- 30.O'Neill JS, Maywood ES, Chesham JE, Takahashi JS, Hastings MH. cAMP-dependent signaling as a core component of the mammalian circadian pacemaker. Science. 2008;320:949–953. doi: 10.1126/science.1152506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ginty DD, et al. Regulation of CREB phosphorylation in the suprachiasmatic nucleus by light and a circadian clock. Science. 1993;260:238–241. doi: 10.1126/science.8097062. [DOI] [PubMed] [Google Scholar]
- 32.Dibner C, Schibler U, Albrecht U. The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu Rev Physiol. 2010;72:517–549. doi: 10.1146/annurev-physiol-021909-135821. [DOI] [PubMed] [Google Scholar]
- 33.Peliciari-Garcia RA, et al. Expression of circadian clock and melatonin receptors within cultured rat cardiomyocytes. Chronobiol Int. 2011;28:21–30. doi: 10.3109/07420528.2010.525675. [DOI] [PubMed] [Google Scholar]
- 34.Zhang J, Kaasik K, Blackburn MR, Lee CC. Constant darkness is a circadian metabolic signal in mammals. Nature. 2006;439:340–343. doi: 10.1038/nature04368. [DOI] [PubMed] [Google Scholar]
- 35.Daniels IS, et al. A role of erythrocytes in adenosine monophosphate initiation of hypometabolism in mammals. J Biol Chem. 2010;285:20716–20723. doi: 10.1074/jbc.M109.090845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muller JE, et al. Circadian variation in the frequency of onset of acute myocardial infarction. N Engl J Med. 1985;313:1315–1322. doi: 10.1056/NEJM198511213132103. [DOI] [PubMed] [Google Scholar]
- 37.Suarez-Barrientos A, et al. Circadian variations of infarct size in acute myocardial infarction. Heart. 2011 doi: 10.1136/hrt.2010.212621. [DOI] [PubMed] [Google Scholar]
- 38.Reiter R, Swingen C, Moore L, Henry TD, Traverse JH. Circadian Dependence of Infarct Size and Left Ventricular Function After ST Elevation Myocardial Infarction. Circ Res. 2012;110:105–110. doi: 10.1161/CIRCRESAHA.111.254284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leibetseder V, et al. Clock genes display rhythmic expression in human hearts. Chronobiol Int. 2009;26:621–636. doi: 10.1080/07420520902924939. [DOI] [PubMed] [Google Scholar]
- 40.Roenneberg T, Kumar CJ, Merrow M. The human circadian clock entrains to sun time. Curr Biol. 2007;17:R44–R45. doi: 10.1016/j.cub.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 41.Tian R, Abel ED. Responses of GLUT4-deficient hearts to ischemia underscore the importance of glycolysis. Circulation. 2001;103:2961–2966. doi: 10.1161/01.cir.103.24.2961. [DOI] [PubMed] [Google Scholar]
- 42.Russell RR, 3rd, et al. AMP-activated protein kinase mediates ischemic glucose uptake and prevents postischemic cardiac dysfunction, apoptosis, and injury. J Clin Invest. 2004;114:495–503. doi: 10.1172/JCI19297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hyvarinen J, et al. Hearts of hypoxia-inducible factor prolyl 4-hydroxylase-2 hypomorphic mice show protection against acute ischemia-reperfusion injury. J Biol Chem. 2010;285:13646–13657. doi: 10.1074/jbc.M109.084855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Denko NC. Hypoxia, HIF1 and glucose metabolism in the solid tumour. Nat Rev Cancer. 2008;8:705–713. doi: 10.1038/nrc2468. [DOI] [PubMed] [Google Scholar]
- 45.Durgan DJ, Young ME. The cardiomyocyte circadian clock: emerging roles in health and disease. Circ Res. 2010;106:647–658. doi: 10.1161/CIRCRESAHA.109.209957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rutter J, Reick M, McKnight SL. Metabolism and the control of circadian rhythms. Annu Rev Biochem. 2002;71:307–331. doi: 10.1146/annurev.biochem.71.090501.142857. [DOI] [PubMed] [Google Scholar]
- 47.Albrecht U. Invited review: regulation of mammalian circadian clock genes. J Appl Physiol. 2002;92:1348–1355. doi: 10.1152/japplphysiol.00759.2001. [DOI] [PubMed] [Google Scholar]
- 48.Durgan DJ, et al. Short communication: ischemia/reperfusion tolerance is time-of-day-dependent: mediation by the cardiomyocyte circadian clock. Circ Res. 2010;106:546–550. doi: 10.1161/CIRCRESAHA.109.209346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nossuli TO, et al. A chronic mouse model of myocardial ischemia-reperfusion: essential in cytokine studies. Am J Physiol Heart Circ Physiol. 2000;278:H1049–H1055. doi: 10.1152/ajpheart.2000.278.4.H1049. [DOI] [PubMed] [Google Scholar]
- 50.Virag JA, et al. Attenuation of myocardial injury in mice with functional deletion of the circadian rhythm gene mPer2. Am J Physiol Heart Circ Physiol. 2010;298:H1088–H1095. doi: 10.1152/ajpheart.01280.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang CY, et al. Increased vascular senescence and impaired endothelial progenitor cell function mediated by mutation of circadian gene Per2. Circulation. 2008;118:2166–2173. doi: 10.1161/CIRCULATIONAHA.108.790469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Viswambharan H, et al. Mutation of the circadian clock gene Per2 alters vascular endothelial function. Circulation. 2007;115:2188–2195. doi: 10.1161/CIRCULATIONAHA.106.653303. [DOI] [PubMed] [Google Scholar]
- 53.Zheng B, et al. Nonredundant roles of the mPer1 and mPer2 genes in the mammalian circadian clock. Cell. 2001;105:683–694. doi: 10.1016/s0092-8674(01)00380-4. [DOI] [PubMed] [Google Scholar]
- 54.Ryan HE, et al. Hypoxia-inducible factor-1alpha is a positive factor in solid tumor growth. Cancer Res. 2000;60:4010–4015. [PubMed] [Google Scholar]
- 55.Sohal DS, et al. Temporally regulated and tissue-specific gene manipulations in the adult and embryonic heart using a tamoxifen-inducible Cre protein. Circ Res. 2001;89:20–25. doi: 10.1161/hh1301.092687. [DOI] [PubMed] [Google Scholar]
- 56.Yoo SH, et al. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci U S A. 2004;101:5339–5346. doi: 10.1073/pnas.0308709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Eckle T, et al. Systematic evaluation of a novel model for cardiac ischemic preconditioning in mice. Am J Physiol Heart Circ Physiol. 2006;291:H2533–H2540. doi: 10.1152/ajpheart.00472.2006. [DOI] [PubMed] [Google Scholar]
- 58.Eltzschig HK, et al. Coordinated adenine nucleotide phosphohydrolysis and nucleoside signaling in posthypoxic endothelium: role of ectonucleotidases and adenosine A2B receptors. J Exp Med. 2003;198:783–796. doi: 10.1084/jem.20030891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wolska BM, Solaro RJ. Method for isolation of adult mouse cardiac myocytes for studies of contraction and microfluorimetry. Am J Physiol. 1996;271:H1250–H1255. doi: 10.1152/ajpheart.1996.271.3.H1250. [DOI] [PubMed] [Google Scholar]
- 60.Walker LA, Walker JS, Ambler SK, Buttrick PM. Stage-specific changes in myofilament protein phosphorylation following myocardial infarction in mice. J Mol Cell Cardiol. 2010;48:1180–1186. doi: 10.1016/j.yjmcc.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.