Abstract
Previous studies demonstrate a role for β epithelial Na+ channel (βENaC) protein as a mediator of myogenic constriction in renal interlobar arteries. However, the importance of βENaC as a mediator of myogenic constriction in renal afferent arterioles, the primary site of development of renal vascular resistance, has not been determined. We colocalized βENaC with smooth muscle α-actin in vascular smooth muscle cells in renal arterioles using immunofluorescence. To determine the importance of βENaC in myogenic constriction in renal afferent arterioles, we used a mouse model of reduced βENaC (βENaC m/m) and examined pressure-induced constrictor responses in the isolated afferent arteriole-attached glomerulus preparation. We found that, in response to a step increase in perfusion pressure from 60 to 120 mmHg, the myogenic tone increased from 4.5 ± 3.7 to 27.3 ± 5.2% in +/+ mice. In contrast, myogenic tone failed to increase with the pressure step in m/m mice (3.9 ± 0.8 to 6.9 ± 1.4%). To determine the importance of βENaC in myogenic renal blood flow (RBF) regulation, we examined the rate of change in renal vascular resistance following a step increase in perfusion pressure in volume-expanded animals. We found that, following a step increase in pressure, the rate of myogenic correction of RBF is inhibited by 75% in βENaC m/m mice. These findings demonstrate that myogenic constriction in afferent arterioles is dependent on normal expression of βENaC.
Keywords: epithelial sodium ion channel, ion channel, degenerin, blood pressure
the myogenic mechanism is an inherent response of certain vessels, particularly in the mesenteric, cerebral, and renal circulations. It is characterized by reciprocal changes in diameter in response to perfusion pressure (5, 13). With increasing pressure, myogenically responsive vessels constrict; with decreasing pressure, they dilate. In the kidney, myogenic-dependent rapid adaptations of preglomerular vascular resistance are thought to prevent transmission of sudden fluctuations in perfusion pressure to the glomerular capillaries (24). The myogenic response is initiated by a pressure-induced change in vascular wall stretch, where stretch activates a mechanodependent signaling cascade leading to vasoconstriction (5, 13). The sensing mechanism has not been identified yet.
Our laboratory has considered epithelial Na+ channel (ENaC) proteins as candidates for the mechanosensor because they have a strong evolutionary link to mechanosensing. ENaC proteins are members of the same protein family as nematode Caenorhabditis elegans degenerin proteins and share amino acid homology and a common structure of intracellular NH2 and COOH termini, two membrane-spanning domains, and a large extracellular domain (1, 10, 19, 33). A substantial body of evidence demonstrates that nematode degenerin proteins form the ion-conducting pore of mechanosensors in neurons and muscle (10, 33). Therefore, the strong evolutionary link to nematode mechanosensing provides a reasonable basis that certain ENaC proteins may also function as a mechanosensor.
At least one specific ENaC protein, βENaC, is essential to transduction of myogenic constriction in renal interlobar arteries. βENaC is expressed in renal vascular smooth muscle cells (VSMCs) (14, 15). Transient gene silencing, using small-interfering RNA (siRNA) or dominant-negative constructs, demonstrates inhibition of βENaC alone is sufficient to nearly abolish myogenic constriction in mouse renal interlobar arteries (14). Although the interlobar artery is a small resistance artery (∼75–100 μm diameter), the role of βENaC in the myogenic response must be extended to vascular beds that generate most of the renal vascular resistance to be physiologically relevant. A few recent pharmacological studies have addressed the importance of ENaC proteins on the afferent arteriolar (primary site of renal vascular resistance) myogenic response using broad-spectrum ENaC channel inhibitors amiloride and benzamil with equivocal results (12, 37). Thus, the importance of ENaC protein-mediated afferent arteriolar myogenic constriction remains unresolved.
The goal of this study was to determine the importance of βENaC in myogenic constriction of the afferent arteriole by using a genetically modified mouse model with reduced levels of βENaC (βENaC m/m). The βENaC m/m model was generated using standard gene-targeting approaches in the course of generating a model of Liddle's syndrome (increased βENaC) by inserting a premature stop codon in the COOH-terminus coding region. However, the presence of the neomycin selection marker disrupts the βENaC gene locus, resulting in reduced βENaC expression. Thus, a mouse model that under-, rather than overexpresses, βENaC was generated (27). Mice homozygous for the mutation (m/m) express very low levels of βENaC transcripts and protein in the lung and kidney as well as reduced βENaC protein in cerebral VSMCs (11, 27, 38). We found that 1) βENaC is localized in small renal arterioles in wild-type animals, 2) myogenic constriction of the afferent arteriole using the intact afferent arteriole-glomerulus preparation is nearly abolished in the βENaC m/m, and 3) loss of βENaC-mediated myogenic constriction contributes to a loss of myogenic regulation of whole kidney blood flow immediately after a change in blood pressure. Results of this investigation demonstrate that βENaC mediates myogenic constriction in renal afferent arterioles and contributes to regulation of whole kidney blood flow.
METHODS
Studies were conducted in genetically modified mice. Heterozygote βENaC +/m mating pairs on a mixed genetic background were generously provided by E. Hummler and B. Rossier (University of Lausanne, Lausanne, Switzerland). Animals were provided standard rodent chow containing 0.4% Na+ (Teklad) and water ad libitum. Offspring of heterozygote mating pairs were genotyped at 3 wk of age using DNA isolated (DirectTail PCR; Viagen) from tail samples and reconfirmed following phenotypic analysis using liver samples as previously described (38). The University of Mississippi Medical Center's Institutional Animal Care and Use Committee approved all animal protocols. Animals were exposed to 12:12-h light (0600–1800)-dark (1800–0600) cycles.
Protocol 1: Localization of βENaC in small renal arterioles.
We used confocal microscopy to localize βENaC in small arterioles in paraformaldehyde-fixed, frozen renal sections. For these experiments, kidneys from adult βENaC +/+ and m/m littermates were harvested and postfixed in 4% paraformaldehyde overnight. The kidneys were rinsed in phosphate buffer salt solution (PBS), embedded in freezing medium, and sectioned at 40 μm. For staining, sections were thoroughly air-dried, then rinsed with PBS and blocked with 5% normal donkey serum (NDS) for 1 h at 37°C. Samples were incubated with affinity-purified rabbit anti-βENaC developed in our laboratory (1:100, 1 h, 37°C) and mouse smooth muscle α-actin (1:100; Sigma Chemicals) as a positive control for VSMCs (14, 38). Samples without primary antibody served as an additional negative control. Samples were rinsed three times with PBS and incubated with Alexa 488-conjugated donkey anti-mouse IgG (1:1,000; Invitrogen) and Cy3-conjugated donkey anti-rabbit Fab (1:100; Jackson Immunologicals) in 5% NDS for 1 h at 37°C. Samples from +/+ and m/m kidneys were rinsed in PBS, mounted with GelMount, covered with a cover slip, and examined under identical conditions using a Leica TSC-SP2 laser scanning confocal microscope. Images were prepared identically for presentation in Adobe Photoshop.
Protocol 2: Determination of myogenic constriction of mouse afferent arterioles.
To evaluate myogenic constrictor responses in the afferent arteriole of βENaC mice, we used methods described previously (22, 28). Briefly, male βENaC +/+ (8.2 ± 1.7 wk) and m/m (8.0 ± 0.6 wk) mice were anesthetized with isoflurane, and kidneys were removed and thinly sliced along the corticomedullary axis. Slices were placed in ice-cold minimum essential medium (MEM; GIBCO, Grand Island, NY) containing 5% bovine serum albumin (BSA), and single superficial glomeruli with an attached afferent arteriole were microdissected under a stereomicroscope (model SMZ 1500; Nikon). Afferent arteriole-glomerulus complexes were dissected within 90 min at 8°C. The isolated afferent arteriole and attached glomerulus were transferred to a temperature-regulated chamber mounted on an inverted microscope (Eclipse Ti; Nikon) cannulated with an array of glass pipettes and gradually warmed to 37°C. The afferent arterioles were perfused with MEM from the proximal end in an orthograde direction and gently pressurized to 60 mmHg. Following a 30-min equilibration period, images were collected to determine afferent arteriole internal diameter. The imaging system consisted of a microscope (Eclipse Ti; Nikon), digital CCD camera (CoolSnap; Photometrics), xenon light (LB-LS/30; Shutter Instruments), and optical filter changer (Lambda 10–3; Shutter Instruments). Images were displayed and analyzed with NIS-Elements imaging software (Nikon). To determine the active response to pressure, perfusion pressure was increased from 60 to 120 mmHg for 5 min, and another image was collected. To determine passive response, the perfusion pressure was lowered to 60 mmHg, and the perfusion and bathing solutions were exchanged with a Ca2+-free MEM and reequilibrated for another 30 min. The response to a 60-mmHg step increase in pressure was repeated under Ca2+-free conditions. At the end of the experiment, the arteriolar segments were reequilibrated with Ca2+ MEM at 60 mmHg, and the vasoconstrictor response to the α-adrenergic receptor agonist norepinephrine (NE, 10−5 M) was obtained. Myogenic tone was calculated as (diameter Ca2+ free - diameter Ca2+ plus)/diameter Ca2+ free. The sensitivity of the response was quantified as the slope of the pressure-myogenic tone relationship at 60 and 120 mmHg.
Previous studies in our laboratory have used the renal interlobar artery to study the renal myogenic response; however, concern that myogenic activity of the small artery may not behave similarly to the afferent arteriole has been raised. To determine if the small renal interlobar artery is similarly affected, we evaluated myogenic constriction as previously described (14, 15) in interlobar arteries from a small group of βENaC+/+ (n = 3; 14 ± 1 wk of age; female) and mice harboring one or two mutant alleles (m/m or +/m; n = 5, 14 ± 1 wk of age; female).
Protocol 3: Determination of myogenic renal blood flow regulation.
To gain further insight into the importance of βENaC-mediated myogenic control of renal blood flow (RBF), we evaluated RBF and renal vascular resistance (RVR) responses to a step increase in perfusion pressure under conditions where tubuloglomerular feedback (TGF), a slower mechanism involved in the control of RBF, was suppressed by volume expansion (9, 18, 26, 30, 35, 37).
Determination of RBF regulation was conducted as described previously (11) with a few significant modifications. Mice were maintained under isoflurane anesthesia on a heating pad to maintain body temperature at 37°C (rectal) for the duration of the study. The depth of anesthesia was monitored by the response to toe pinching. Mice were instrumented with a fluid-filled carotid artery catheter to measure mean arterial pressure (MAP) and a jugular vein catheter for fluid infusion. For determination of RBF, the left kidney was approached through a retroperitoneal flank incision. The renal artery, celiac artery, mesenteric artery, and lower abdominal aorta were freed from adjacent tissue. The celiac and superior mesenteric arteries were ligated, and a temporary ligature was placed around the lower abdominal aorta. A perivascular flow probe (0.5 PSB; Transonic, Ithaca, NY) was positioned on the left renal artery. RBF was measured using an ultrasound transit-time flowmeter (TS420, low-pass filter 30–40 Hz; Transonic) recorded, along with blood pressure, with a computerized chart recorder (LabChart 6.0, PowerLab; ADInstruments, Colorado Springs, CO). After 10 min of stabilization, a blood volume expansion (equivalent to 5% body weight) was initiated to suppress TGF contribution to RBF regulation, an approach used by others (9, 18, 26, 30, 35, 37). The volume expansion was accomplished by infusing a 5% BSA solution in lactated Ringer solution delivered at 100 μl/min (10- to 15-min infusion). Following this period, 20 min were allowed for equilibration. To achieve a 10- to 15-mmHg step increase in MAP, the ligature around the lower abdominal aorta was tightened. At the conclusion of the experiments, the animals were killed by an overdose of isoflurane followed by decapitation. MAP and RBF data were recorded at 1,000 Hz. RBF was normalized to left kidney weight and reported as milliliters per minute per gram kidney weight. RBF responses were obtained in 7 m/m (3 females, 4 males) and 6 +/+ (2 females, 4 males) littermate controls.
Data analysis for RBF regulation.
RVR was calculated as MAP/RBF and reported as resistance units (RU = mmHg·ml−1·min−1·g kidney wt−1). To quantify the RVR response to changes in perfusion pressure, we continuously recorded pressure and RBF for at least 10 s before and 30 s after a step increase in pressure. The contribution of myogenic regulation to steady-state RBF cannot be assessed from this investigation because the myogenic and TGF responses are suppressed, but not abolished, and residual activity from these mechanisms and an intact “third mechanism” will contribute to steady-state RBF regulation. Data were extracted and averaged over each 1-s interval in LabChart and exported to Microsoft Excel for further analysis. The first time point where MAP increased 5% or more above baseline was used to identify the zero time point. Responses were normalized to control baseline values. The sensitivity of the myogenic mechanism was assessed by the slope of the time-RVR relationship between 0 and 5 s [i.e., the rate of correction of RVR during the time frame where the myogenic mechanism is active as previously shown by Just and Arendshorst (16)].
Statistical analysis.
All data are presented as means ± SE. Data were analyzed using paired or unpaired t-test with one (predicted direction of response) or two tails where appropriate. We also used repeated-measures ANOVA followed by a Student-Neuman-Keuls post hoc test for differences among the means where appropriate. Statistical significance was set at P < 0.05. Specific P values are provided for certain data sets to demonstrate the high level of confidence in our statistical analyses.
RESULTS
βENaC localization in VSMC of small renal arterioles.
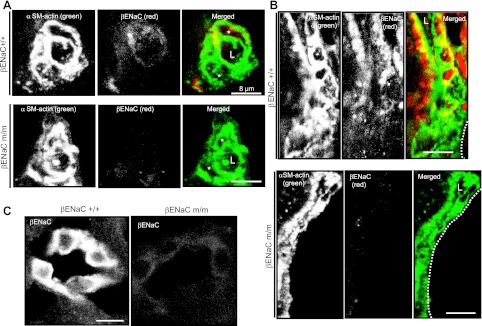
We used confocal microscopy to localize βENaC in small renal arterioles. In preliminary studies, we were unable to detect βENaC immunolabeling in renal vasculature in paraffin-embedded renal sections. Because others and we have previously demonstrated βENaC immunolabeling in enzymatically dissociated renal VSMCs, we considered the possibility that the lack of signal was due “antigen blockade.” To address this, we used proteinase K treatment. Following treatment with proteinase K, a small but consistent signal difference was observed in renal vascular tissue between +/+ and m/m mice; however, general background staining was also enhanced. We then turned to frozen renal sections. Using frozen sections, we could identify brightly stained portions of the distal cortical nephron (Fig. 1C) and less well-stained vascular tissue. Although vascular βENaC labeling was weak relative to the tubules, βENaC labeling in the vasculature was consistently greater in +/+ vs. m/m mice (Fig. 1, A and B). βENaC localization in afferent arterioles is shown in a cross section (Fig. 1A) and longitudinal section (Fig. 1B) adjacent to the glomerular border. The studies were repeated in two separate trials. Immunolabeling was absent in the no primary antibody negative controls.
Fig. 1.
β−Epithelial Na+ channel (βENaC) labeling is decreased in afferent arterioles and distal nephron segments of a mouse model of reduced βENaC (βENaC m/m). All images were obtained in the cortex. A: localization of smooth muscle (SM) α-actin (green) and βENaC (red) in a cross-sectional image of a renal arteriole from a βENaC +/+ (top) and m/m (bottom) mouse. In the merged image, the arteriolar lumen is identified by an “L,” and the vascular smooth muscle cell (VSMC) bodies are identified by an asterisk (*). B: localization of SM α-actin (green) and βENaC (red) in a longitudinal section of a renal arteriole from a βENaC +/+ (top) and m/m (bottom) mouse. Dashed lines in merged image represent the glomerular border. Colocalization of βENaC and SM α-actin is identified as yellow labeling in the merged images in A and B. C: βENaC labeling in portions of the distal nephron in βENaC +/+ and m/m mice. Images are representative of 2 trials. Scale bar is equivalent to 8 μm.
Afferent arteriole myogenic constriction is inhibited in βENaC m/m mice.
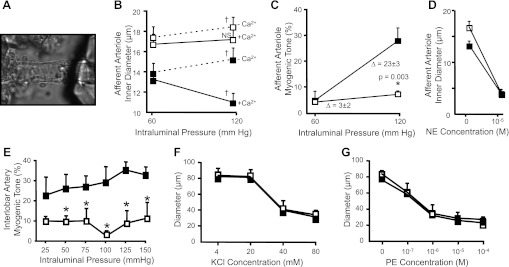
We examined myogenic constrictor responses in βENaC +/+ and m/m mice using the isolated afferent arteriole-attached glomerular preparation (Fig. 2A). The average inner diameter of renal afferent arterioles before and after a step increase in intraluminal pressure under Ca2+-containing and Ca2+-free conditions in +/+ and m/m mice is shown in Fig. 2B. When pressure is raised from 60 to 120 mmHg, afferent arterioles from βENaC +/+ mice constrict to below resting diameter (P = 0.003, paired t-test); however, the diameter remains steady in m/m mice (P = 0.099, paired t-test). Under Ca2+-free conditions, the afferent arterioles from +/+ and m/m mice passively dilate with the step increase in pressure (P = 0.013 for +/+ and m/m groups, paired t-test; Fig. 2B). The significance of the increased diameter of the βENaC m/m afferent arteriole, compared with +/+, is not clear. Under Ca2+ conditions, it may reflect a loss of basal myogenic tone. However, under Ca2+-free conditions, it may reflect vascular remodeling that has occurred over the life span of the animal. When calculated as myogenic tone, afferent arterioles from +/+ mice generate nearly 27 ± 5% (n = 6) myogenic tone at 120 mmHg; however, afferent arterioles from m/m mice generate 7 ± 1% (n = 6, P = 0.003) (Fig. 2C). Constrictor responses to 10−5 M NE were similar between +/+ and m/m groups, suggesting afferent arterioles from βENaC m/m do not have a generalized loss in their ability to vasoconstrict, but rather have a deficit in their ability to constrict in response to pressure (Fig. 2D).
Fig. 2.
Myogenic constriction in the afferent arteriole and interlobar artery is impaired in βENaC m/m mice. A–D: myogenic constriction in the afferent arteriole. A: image of the isolated afferent arteriole-glomerular preparation. B: inner diameter response to incubation at 60 and 120 mmHg under Ca2+-containing (solid lines) and Ca2+-free (broken lines) conditions in βENaC +/+ (■) and m/m (□) mice (n = 6). C: calculated myogenic tone is inhibited in βENaC m/m mice at 120 mmHg. D: constriction to the α-adrenergic agonist norepinephrine (NE) is similar between +/+ and m/m animals. E–G: myogenic constriction in the renal interlobar artery is impaired in βENaC mutant mice. For these experiments, m/m and m/+ mice were grouped together because responses were similar. E: calculated %myogenic tone is reduced in βENaC m/m or +/m (□, n = 5) compared with +/+ (■, n = 3) mice with stepwise increases in intraluminal pressure. F and G: vasoconstrictor responses to KCl (F) and phenylephrine (G) were identical. Data are presented as means ± SE. †Statistically different from 60 mmHg using paired, 2-tailed t-test, P < 0.05. NS, not significantly different. *Statistically different from +/+ using unpaired, 2-tailed t-test at P value indicated or 2-way repeated-measures ANOVA, with Student-Newman-Keuls post hoc test, P < 0.05.
To determine whether the myogenic response is also altered in small renal arteries, we examined myogenic constriction in renal interlobar artery segments (Fig. 2, E–G). For these experiments, data from mice harboring one or two βENaC mutant alleles were combined. While myogenic tone increases with increasing pressure in +/+ animals, tone remains flat in mice with the βENaC mutant gene(s) (Fig. 2E). Constriction to phenylephrine and KCl was not different (Fig. 2, F and G). These findings suggest that βENaC also plays an important role in myogenic tone in renal interlobar arteries.
Myogenic regulation of RBF is impaired in βENaC m/m mice.
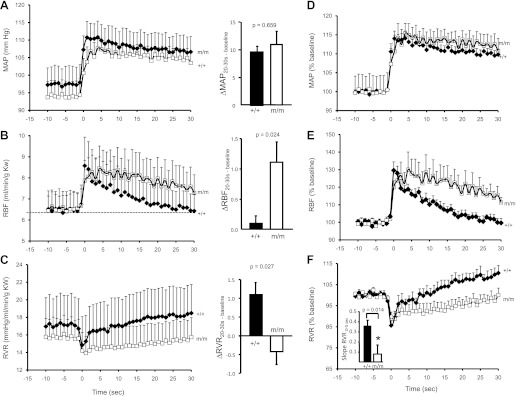
Baseline MAP, RBF, and RVF were not different between +/+ and m/m mice (Table 1 and Fig. 3, A–C). The temporal responses of MAP, RBF, and RVR following the volume expansion protocol are presented as absolute data (Fig. 3, A–C) and normalized as the percentage of baseline (Fig. 3, D–F). With the step increase in perfusion pressure, MAP increases ∼10 mmHg (15%) and remains elevated in +/+ and m/m groups (Fig. 3, A and D). The change in MAP between baseline and 20–30 s was not different between +/+ and m/m mice (Fig. 3A, right). RBF increases transiently with the increase in MAP in both +/+ and m/m mice (Fig. 3, B and E). Although RBF returns to baseline levels by 30 s in +/+, it remains elevated in m/m mice (Fig. 3, B and E). At 20–30 s, the change in RBF from baseline was significantly greater in m/m mice (Fig. 3B, right). RVR falls transiently with the step increase in MAP as expected due to passive compliance (Fig. 3, C and F). In +/+ animals, the decrease in RVR is followed by an initial rapid increase within the first 5s (the expected time frame for initiation of the myogenic response), followed by a slower secondary increase. In m/m animals, the rapid increase in RVR is less dramatic (Fig. 3, C and F). To investigate the speed of the myogenic mechanism, we calculated the slope of the linear regression lines for RVR between 0 and 5 s. The speed of the myogenic mechanism is reduced in βENaC m/m (RVR0–5 s, 0.08 ± 0.09 vs. 0.36 ± 0.06 RU/s, P = 0.014) (Fig. 3F, inset). At 20–30 s, the increase in RVR from baseline was significantly less in m/m mice (Fig. 3B, right). These data suggest the apparent speed of the myogenic response is reduced by more than fourfold in βENaC m/m mice. As shown in Table 2, 30 s following the pressure step, RBF (%baseline) is significantly greater and RVR significantly lower, in the βENaC m/m vs. +/+. These findings suggest the deficit in the myogenic mechanism delays the correction of RBF and RVR following a change in perfusion pressure.
Table 1.
Baseline MAP, RBF, and RVR in βENaC +/+ and m/m mice following volume expansion
| Genotype |
|||
|---|---|---|---|
| βENaC +/+ | βENaC m/m | P Value* | |
| MAP, mmHg | 97 ± 5 | 94 ± 4 | 0.68 |
| RBF, ml · min−1 · g kidney wt−1 | 6.5 ± 0.9 | 6.5 ± 0.7 | 0.98 |
| RVR, mmHg · ml−1 · min−1 · g kidney wt | 16.9 ± 3.4 | 15.7 ± 2.0 | 0.75 |
| Sample size, n | 6 | 7 | |
| Age, wk | 18.6 ± 1.9 | 15.7 ± 1.7 | 0.52 |
Values are means ± SE; n, no. of mice. MAP, mean arterial pressure; RBF, renal blood flow; RVR, renal vascular resistance; βENaC, β-epithelial Na+ channel; ENaC m/m, mouse model of reduced βENaC.
P value from 2-tailed t-test.
Fig. 3.
Myogenic regulation of renal blood flow (RBF) and renal vascular resistance (RVR) is altered in βENaC m/m mice. Time course of the regulatory response of mean arterial pressure (MAP; A and D), RBF (B and E), and RVR (C and F) in wild-type (+/+, filled symbols, n = 6) and mutant (m/m, open symbols, n = 7) mice 10 s before and 30 s after the step increase in pressure. Data are presented as absolute values in A–C and normalized changes to minimize variance in D–F. The change in MAP, RBF, and RVR from 20 to 30 s to baseline is shown on right in A–C. Baseline values of MAP (mmHg), RBF (ml·min−1·g kidney wt−1), and RVR were not different between +/+ and m/m mice (see Table 1). Following a similar increase in MAP, the transient increase in RBF and decrease in RVR are similar between +/+ and m/m mice. Immediately following the transient drop, RVR begins to increase in the +/+, but remains low in the m/m. The inset in F shows the rate of increase in RVR during the first 5 s following the drop in RVR is significantly greater in +/+ vs. m/m mice (P = 0.014). By 20–30 s following the step increase in MAP, RBF in the +/+ is completely corrected while RBF remains elevated in m/m animals (P = 0.024, B, right). By 20–30 s following the step increase in MAP, the change in RVR from baseline is significantly greater in the +/+ vs. m/m (P = 0.027, C, right). Data are means ± SE. *Significantly different from βENaC +/+ group at the P value indicated.
Table 2.
MAP, RBF, and RVR as a percent of baseline 30 s after the step increase in pressure in βENaC +/+ and m/m mice
| Genotype |
|||
|---|---|---|---|
| βENaC +/+ | βENaC m/m | P Value* | |
| MAP, %baseline | 110 ± 1 | 112 ± 4 | 0.59 |
| RBF, %baseline | 102 ± 2 | 117 ± 5 | 0.02 |
| RVR, %baseline | 108 ± 3 | 97 ± 3 | 0.02 |
| Sample size, n | 6 | 7 | |
Values are means ± SE; n, no. of mice.
P value from 2-tailed t-test.
DISCUSSION
Previous studies have suggested certain members of the degenerin protein family may contribute to the transduction of the myogenic response. Because degenerin proteins are closely related to proteins thought to form the pore of a large mechanosensor in the nematode, we suspect that they may also form the pore of a mechanosensor in renal VSMCs. βENaC is one degenerin expressed in renal VSMCs. Transient inhibition of βENaC inhibits myogenic constriction in renal interlobar arteries. However, the importance of βENaC in myogenic constriction of renal afferent arterioles, the primary site of vascular resistance development in the kidney, is not clear. In the current investigation, we determined the importance of βENaC in renal afferent arteriolar myogenic response in a mouse model of reduced βENaC (βENaC m/m). Here, we provide evidence that 1) βENaC is expressed in small renal arterioles, 2) myogenic constriction of afferent arterioles is inhibited in βENaC m/m, and 3) myogenic regulation of RBF is inhibited in βENaC m/m mice.
Role of βENaC as a mechanosensor.
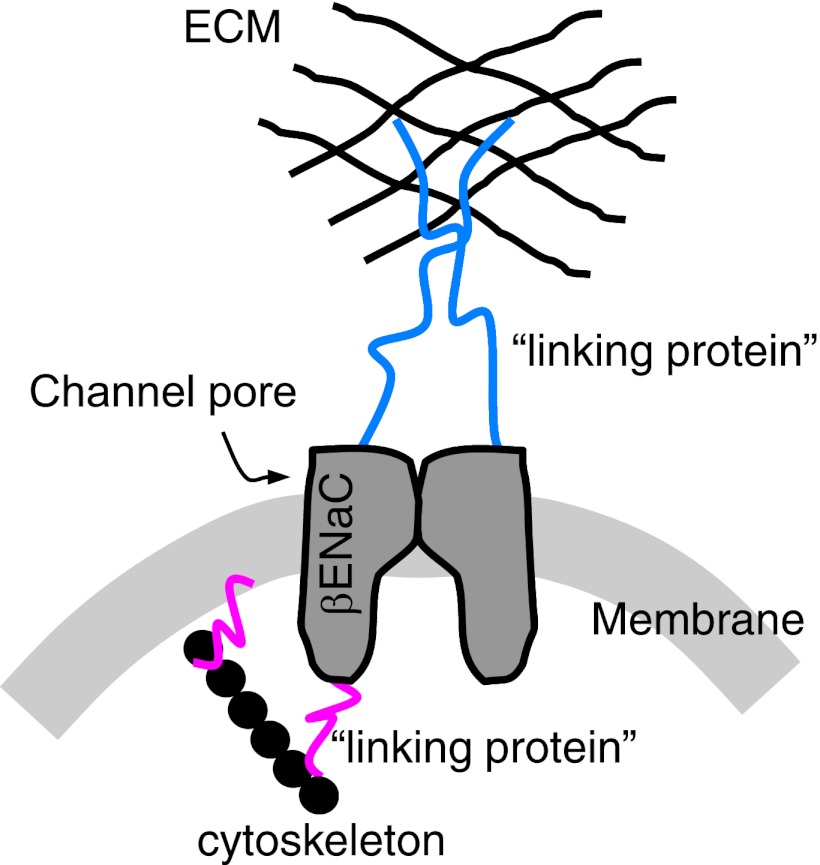
ENaC proteins are members of a larger, novel family of proteins termed degenerins. Degenerins were first identified in the nematode where chemically induced mutations caused neurons to swell and lyse, or degenerate, hence the name degenerin. Subsequent studies demonstrated that nematode degenerin proteins likely form the pore of large, heteromeric mechanosensors present in neurons and muscle (10, 32, 33). Because ENaC proteins are evolutionarily related to mechanosensory nematode mechanosensor proteins, they have been considered as potential mammalian mechanosensors (7, 8, 10, 32). In support of this possibility, mammalian degenerins have been identified in mechanosensitive tissues such as sensory neurons, bone cells, and smooth muscle cells (2, 21, 34, 36). Our laboratory has focused on the role of βENaC in VSMC mechanotransduction. A hypothetical model of a mammalian mechanosensor in VSMCs, based on the C. elegans model, is shown in Fig. 4. In this model, degenerin proteins form the ion-conducting pore. We hypothesize that βENaC contributes to the pore. The pore is tethered to the extracellular matrix and cytoskeleton, either directly or indirectly. Gating of the complex by mechanical forces allows influx of Na+, which then leads to membrane depolarization and initiates the signaling cascade leading to VSMC contraction.
Fig. 4.
Hypothetical model of a vascular mechanosensor containing βENaC. This model is based on the degenerin mechanosensor in the nematode Caenorhabditis elegans. In our model, we hypothesize that βENaC is a component of the ion-conducting pore. The pore is tethered to the extracellular matrix (ECM) and possibly the cytoskeleton, either directly or indirectly. Gating of the mechanosensor by mechanical forces is proposed to lead to Na+ influx, membrane depolarization, and activation of downstream signaling, leading to VSMC contraction.
Evidence suggests βENaC plays an important role in mediating the myogenic response in renal vessels. βENaC protein is localized at or near the cell surface of renal VSMCs. Inhibition of βENaC expression, using siRNA and dominant-negative isoforms, inhibits myogenic constriction in renal interlobar arteries. However, the importance of βENaC in myogenic constriction of renal afferent arterioles, the primary site of vascular resistance development in the kidney, is not clear. A few recent investigations have addressed the importance of ENaC protein family members in afferent arteriolar myogenic constriction using broad-spectrum inhibitors, such as amiloride and benzamil (12, 39); however, these studies have not provided a clear picture of the importance of ENaC proteins. To address the importance of βENaC, we turned to a genetically modified mouse model, since it offers the advantage of target specificity over pharmacological approaches. For the experiments described in this investigation, we used the βENaC m/m model with low expression of βENaC. We used this model rather than a traditional knockout model since complete elimination of βENaC by knockout is lethal shortly after birth (25).
Importance of βENaC in renal afferent arteriolar myogenic constriction.
A previous study showed that transient βENaC gene-silencing approaches in renal interlobar arteries suppressed myogenic constriction (11). The findings of our current study are consistent with this earlier study: myogenic constriction in interlobar artery segments is inhibited in βENaC m/m mice. Furthermore, findings from the current study indicate that myogenic constriction in the afferent arteriole, the primary site of RVR development, is also mediated by βENaC. Therefore, these findings suggest βENaC plays an important role in transduction of the myogenic response in afferent arterioles and small arteries of the renal vasculature.
Importance of myogenic constriction in RBF regulation.
In response to a change in renal perfusion pressure, RBF is maintained constant by the concerted action of at least two mechanisms (myogenic constriction and TGF). Myogenic constriction is fast acting, adjusting vascular resistance to a change in perfusion pressure within 5–10 s, while TGF is slower, adjusting vascular resistance within 6–25 s (3, 4, 16, 17). In a previous study, we demonstrated the speed of myogenic regulation of RBF was slowed ∼50% in the βENaC m/m model, which did not match the 75% loss in afferent arteriolar myogenic tone shown in the present investigation. We considered the possibility that our previous results did not reflect the full importance of βENaC-mediated myogenic control of RBF because of the potential confounding influence of TGF. The temporal separation in the onset of myogenic vs. TGF responses is not perfect. In addition, TGF may alter myogenic constriction (16, 17). Therefore, to minimize the confounding influence of TGF and gain additional insight into the importance of the myogenic mechanism on RBF control, we examined renal hemodynamic responses to a step increase in pressure under conditions where the contribution of TGF to vascular resistance was suppressed following acute volume expansion (9, 18, 28, 35–37). We avoided utilizing a more severe pharmacological approach to inhibit TGF, such as furosemide, since βENaC m/m mice have compensatory responses for a loss of tubular Na+ and fluid reabsorption due to the importance of βENaC in Na+/H20 transport in the distal nephron (27). Furthermore, furosemide can lower blood pressure independent of diuretic actions (31). Therefore, from our perspective, volume expansion to suppress the influence of TGF on vascular resistance was the best option. Consistent with our expectations, we found a greater deficit in the apparent myogenic speed in βENaC m/m mice with volume expansion. These findings suggest that our in vivo findings (loss of myogenic regulation of RBF) parallel our in vitro findings (loss of myogenic constriction in afferent arterioles) in βENaC m/m mice.
A limitation of the current study is that we are unable to compare the kinetics of the myogenic response between our in vivo and in vitro preparations. With the in vivo approach, we are able to assess the kinetics because we can elicit a rapid, single-step increase in renal perfusion pressure with ligation of the lower abdominal aorta. However, due to our experimental set-up of our in vitro preparation, we are not able to elicit a single-step increase in luminal pressure of consistent magnitude among samples. Multiple adjustments to the pressure generation system are required to achieve the 120-mmHg pressure at the arteriole perfusion pipette. This prevents an accurate dynamic assessment of the myogenic response in the in vitro preparation.
Potential involvement of compensatory responses to maintain volume homeostasis on renal myogenic responsiveness in βENaC m/m?
The loss in tubular Na+ and water retention in the βENaC m/m model likely evokes compensatory hormonal responses needed to maintain blood volume/pressure status. The mostly likely compensatory changes would be in the renin-angiotensin system. Angiotensin II (ANG II) is indeed an important potentiator of renal myogenic constriction (20). However, ANG II is likely to be elevated and thus not likely to account for loss of myogenic responsiveness. Aldosterone has been shown to suppress myogenic constriction in cerebral vessels, and thus may contribute to the loss of myogenic responsiveness (29). However, previous findings from our laboratory showing transient silencing of βENaC inhibits myogenic constriction in small renal arteries supports a role for βENaC independent of compensatory responses to maintain volume homeostasis.
Potential pathogenic impact of loss of myogenic constriction.
The fast nature of the myogenic response has led to the hypothesis that the myogenic response is a key protective mechanism against renal injury (23). In a previous investigation, we found that βENaC m/m mice have signs of renal inflammation, mild injury, and a small increase in blood pressure (6). We speculate that loss of myogenic constriction could increase the susceptibility of the βENaC m/m to injury or accelerate injury if a secondary stimulus, such as diabetes, end-stage renal disease, or greater increases in arterial pressure were superimposed. Understanding the importance of secondary factors on the protective ability of the myogenic response is an exciting area of future investigation.
In summary, the findings of the current investigation demonstrate that βENaC is expressed in small renal arteries and arterioles. The myogenic response in afferent arterioles and myogenic control of whole kidney blood flow are weakened in mice with reduced levels of βENaC. Our findings suggest βENaC is an important mediator of renal myogenic constriction.
GRANTS
The National Institutes of Health Grants HL-086996 (H. A. Drummond) and HL-51971 (Program Project Grant) supported this work.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: Y.G., K.P.G., M.G., B.M., and H.A.D. performed experiments; Y.G., K.P.G., and H.A.D. analyzed data; Y.G., K.P.G., R.L., and H.A.D. edited and revised manuscript; K.P.G. and H.A.D. prepared figures; R.L. and H.A.D. interpreted results of experiments; H.A.D. conception and design of research; H.A.D. drafted manuscript; H.A.D. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Bernard Rossier and Dr. Edith Hummler at the University of Lausanne for generously providing our laboratory with the βENaC m/m mouse model. We also thank Dr. Radu Iliescu at the University of Medicine and Pharmacy in Isai Romania for helpful comments.
Present address for K. Gannon: Department of Neurology, Hospital of the University of Pennsylvania, Philadelphia, PA 19104.
REFERENCES
- 1. Benos DJ, Awayda MS, Ismailov II, Johnson JP. Structure and function of amiloride-sensitive Na+ channels. J Membr Biol 143: 1–18, 1995 [DOI] [PubMed] [Google Scholar]
- 2. Bianchi L, Driscoll M. Protons at the gate: DEG/ENaC ion channels help us feel and remember. Neuron 34: 337–340, 2002 [DOI] [PubMed] [Google Scholar]
- 3. Cupples WA, Braam B. Assessment of renal autoregulation. Am J Physiol Renal Physiol 292: F1105–F1123, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Cupples WA, Novak P, Novak V, Salevsky FC. Spontaneous blood pressure fluctuations and renal blood flow dynamics. Am J Physiol Renal Fluid Electrolyte Physiol 270: F82–F89, 1996 [DOI] [PubMed] [Google Scholar]
- 5. Davis MJ, Hill MA. Signaling mechanisms underlying the vascular myogenic response. Physiol Rev 79: 387–423, 1999 [DOI] [PubMed] [Google Scholar]
- 6. Drummond HA, Grifoni SC, Abu-Zaid A, Gousset M, Chiposi R, Barnard JM, Murphey B, Stec DE. Renal inflammation and elevated blood pressure in a mouse model of reduced β-ENaC. Am J Physiol Renal Physiol 301: F443–F449, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Drummond HA, Grifoni SC, Jernigan NL. A new trick for an old dogma: ENaC proteins as mechanotransducers in vascular smooth muscle. Physiology (Bethesda) 23: 23–31, 2008 [DOI] [PubMed] [Google Scholar]
- 8. Drummond HA, Jernigan NL, Grifoni SC. Sensing tension: epithelial sodium channel/acid-sensing ion channel proteins in cardiovascular homeostasis. Hypertension 51: 1265–1271, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Elmarakby AA, Quigley JE, Imig JD, Pollock JS, Pollock DM. TNF-α inhibition reduces renal injury in DOCA-salt hypertensive rats. Am J Physiol Regul Integr Comp Physiol 294: R76–R83, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Goodman MB, Schwarz EM. Transducing touch in Caenorhabditis elegans. Annu Rev Physiol 65: 429–452, 2003 [DOI] [PubMed] [Google Scholar]
- 11. Grifoni SC, Chiposi R, McKey SE, Ryan MJ, Drummond HA. Altered whole kidney blood flow autoregulation in a mouse model of reduced β-ENaC. Am J Physiol Renal Physiol 298: F285–F292, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Guan Z, Pollock JS, Cook AK, Hobbs JL, Inscho EW. Effect of epithelial sodium channel blockade on the myogenic response of rat juxtamedullary afferent arterioles. Hypertension 54: 1062–1069, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hill MA, Zou H, Potocnik SJ, Meininger GA, Davis MJ. Invited review: arteriolar smooth muscle mechanotransduction: Ca2+ signaling pathways underlying myogenic reactivity. J Appl Physiol 91: 973–983, 2001 [DOI] [PubMed] [Google Scholar]
- 14. Jernigan NL, Drummond HA. Myogenic vasoconstriction in mouse renal interlobar arteries: role of endogenous β- and γENaC. Am J Physiol Renal Physiol 291: F1184–F1191, 2006 [DOI] [PubMed] [Google Scholar]
- 15. Jernigan NL, Drummond HA. Vascular ENaC proteins are required for renal myogenic constriction. Am J Physiol Renal Physiol 289: F891–F901, 2005 [DOI] [PubMed] [Google Scholar]
- 16. Just A, Arendshorst WJ. Dynamics and contribution of mechanisms mediating renal blood flow autoregulation. Am J Physiol Regul Integr Comp Physiol 285: R619–R631, 2003 [DOI] [PubMed] [Google Scholar]
- 17. Just A, Arendshorst WJ. A novel mechanism of renal blood flow autoregulation and the autoregulatory role of A1 adenosine receptors in mice. Am J Physiol Renal Physiol 293: F1489–F1500, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Kawata T, Ando A, Hatano M, Onuki T, Sugino N. Tubuloglomerular feedback resetting in different models of acute volume expansion. Kidney Int Suppl 32: S148–S152, 1991 [PubMed] [Google Scholar]
- 19. Kellenberger S, Schild L. Epithelial sodium channel/degenerin family of ion channels: a variety of functions for a shared structure. Physiol Rev 82: 735–767, 2002 [DOI] [PubMed] [Google Scholar]
- 20. Kirton CA, Loutzenhiser R. Alterations in basal protein kinase C activity modulate renal afferent arteriolar myogenic reactivity. Am J Physiol Heart Circ Physiol 275: H467–H475, 1998 [DOI] [PubMed] [Google Scholar]
- 21. Kizer N, Guo XL, Hruska K. Reconstitution of stretch-activated cation channels by expression of the alpha-subunit of the epithelial sodium channel cloned from osteoblasts. Proc Natl Acad Sci USA 94: 1013–1018, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu R, Ren Y, Garvin JL, Carretero OA. Superoxide enhances tubuloglomerular feedback by constricting the afferent arteriole. Kidney Int 66: 268–274, 2004 [DOI] [PubMed] [Google Scholar]
- 23. Loutzenhiser R, Bidani A, Chilton L. Renal myogenic response: kinetic attributes and physiological role. Circ Res 90: 1316–1324, 2002 [DOI] [PubMed] [Google Scholar]
- 24. Marsh DJ, Sosnovtseva OV, Pavlov AN, Yip KP, Holstein-Rathlou NH. Frequency encoding in renal blood flow regulation. Am J Physiol Regul Integr Comp Physiol 288: R1160–R1167, 2005 [DOI] [PubMed] [Google Scholar]
- 25. McDonald FJ, Yang B, Hrstka RF, Drummond HA, Tarr DE, McCray PB, Jr, Stokes JB, Welsh MJ, Williamson RA. Disruption of the beta subunit of the epithelial Na+ channel in mice: hyperkalemia and neonatal death associated with a pseudohypoaldosteronism phenotype. Proc Natl Acad Sci USA 96: 1727–1731, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Persson AE, Schnermann J, Wright FS. Modification of feedback influence on glomerular filtration rate by acute isotonic extracellular volume expansion. Pflugers Arch 381: 99–105, 1979 [DOI] [PubMed] [Google Scholar]
- 27. Pradervand S, Barker PM, Wang Q, Ernst SA, Beermann F, Grubb BR, Burnier M, Schmidt A, Bindels RJ, Gatzy JT, Rossier BC, Hummler E. Salt restriction induces pseudohypoaldosteronism type 1 in mice expressing low levels of the beta-subunit of the amiloride-sensitive epithelial sodium channel. Proc Natl Acad Sci USA 96: 1732–1737, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ren Y, D'Ambrosio MA, Liu R, Pagano PJ, Garvin JL, Carretero OA. Enhanced myogenic response in the afferent arteriole of spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol 298: H1769–H1775, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rigsby CS, Pollock DM, Dorrance AM. Spironolactone improves structure and increases tone in the cerebral vasculature of male spontaneously hypertensive stroke-prone rats. Microvasc Res 73: 198–205, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schnermann J, Briggs JP. Restoration of tubuloglomerular feedback in volume-expanded rats by angiotensin II. Am J Physiol Renal Fluid Electrolyte Physiol 259: F565–F572, 1990 [DOI] [PubMed] [Google Scholar]
- 31. Sechi LA, Palomba D, Bartoli E. Acute effects of furosemide on blood pressure in functionally anephric, volume-expanded rats. Am J Nephrol 13: 94–99, 1993 [DOI] [PubMed] [Google Scholar]
- 32. Syntichaki P, Tavernarakis N. Genetic models of mechanotransduction: the nematode Caenorhabditis elegans. Physiol Rev 84: 1097–1153, 2004 [DOI] [PubMed] [Google Scholar]
- 33. Tavernarakis N, Driscoll Degenerins M. At the core of the metazoan mechanotransducer? Ann NY Acad Sci 940: 28–41, 2001 [PubMed] [Google Scholar]
- 34. Tavernarakis N, Driscoll M. Molecular modeling of mechanotransduction in the nematode Caenorhabditis elegans. Annu Rev Physiol 59: 659–689, 1997 [DOI] [PubMed] [Google Scholar]
- 35. Thomson SC, Blantz RC, Vallon V. Increased tubular flow induces resetting of tubuloglomerular feedback in euvolemic rats. Am J Physiol Renal Fluid Electrolyte Physiol 270: F461–F468, 1996 [DOI] [PubMed] [Google Scholar]
- 36. Trujillo E, Alvarez de la Rosa D, Mobasheri A, Gonzalez T, Canessa CM, Martin-Vasallo P. Sodium transport systems in human chondrocytes II Expression of ENaC, Na+/K+/2Cl- cotransporter and Na+/H+ exchangers in healthy and arthritic chondrocytes. Histol Histopathol 14: 1023–1031, 1999 [DOI] [PubMed] [Google Scholar]
- 37. van Dokkum RP, Sun CW, Provoost AP, Jacob HJ, Roman RJ. Altered renal hemodynamics and impaired myogenic responses in the fawn-hooded rat. Am J Physiol Regul Integr Comp Physiol 276: R855–R863, 1999 [DOI] [PubMed] [Google Scholar]
- 38. VanLandingham LG, Gannon KP, Drummond HA. Pressure-induced constriction is inhibited in a mouse model of reduced betaENaC. Am J Physiol Regul Integr Comp Physiol 297: R723–R728, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang X, Takeya K, Aaronson PI, Loutzenhiser K, Loutzenhiser R. Effects of amiloride, benzamil, and alterations in extracellular Na+ on the rat afferent arteriole and its myogenic response. Am J Physiol Renal Physiol 295: F272–F282, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]