Abstract
Background
Pups of normally nourished dams that are cross-fostered after birth to dams fed a low protein (8% by weight) diet (postnatal low protein; PLP) grow slower during the suckling period and remain small and lean throughout adulthood. At weaning, they have increased expression in the arcuate nucleus (ARC) of the hypothalamus of the orexigenic neuropeptide Y (NPY) and decreased expression of pro-opiomelanocortin (POMC), the precursor of anorexigenic melanocortins.
Objectives and methods
We investigated, using third ventricle administration, whether 3-month-old male PLP rats display altered sensitivity to leptin with respect to food intake, NPY and the melanocortin 3/4-receptor agonist MTII, and using in situ hybridization or laser capture microdissection of the ARC followed by RT-PCR, whether the differences observed were associated with changes in the hypothalamic expression of NPY, or the leptin receptor, NPY receptors, and melanocortin receptors.
Results
PLP rats were smaller and had reduced percentage body fat content and plasma leptin concentration compared to control rats. Leptin (5 μg) reduced food intake over 0-48 h more in PLP than control rats (P<0.05). Submaximal doses of NPY increased food intake less in PLP rats than controls, whilst submaximal doses of MTII reduced food intake more in the PLP rats. Maximal responses did not differ between postnatal low protein and control rats. Leptin (ObRb) and melanocortin-3 (MC3R) receptor expression were increased in both ARC and ventromedial (VMH) hypothalamic nuclei in PLP animals compared to the controls. MC4R, NPY Y1R and Y5R and NPY expression were unchanged.
Conclusion
Postnatal undernourishment results in food intake in adult rats being more sensitive to reduction by leptin and melanocortins, and less sensitive to stimulation by NPY. We propose that this contributes to increased leptin sensitivity and resistance to obesity. Increased expression of ObRb and MC3R may partly explain these findings but other downstream mechanisms must also be involved.
Keywords: postnatal nutrition, obesity, hypothalamus, leptin sensitivity, neuropeptide Y (NPY), alpha melanocortin agonist (MTII)
Introduction
Growth patterns during early life influence the risk of developing metabolic diseases such as obesity in later life1. Rapid postnatal growth during early life is associated with long-term susceptibility to obesity2. In contrast, slow growth during early postnatal life has been associated with decreased obesity in later life. The Dutch Hunger Winter provides a natural experiment to directly examine the long-term health effects of profound nutritional changes at different stages of early life. Men exposed in utero during early gestation show an increased propensity to obesity; conversely those exposed in the last trimester of pregnancy or in early postnatal life to the Dutch Hunger Winter had a reduced risk of obesity as adults3, 4. Similarly, breastfed infants have a lower growth rate over the first year of life than formula-fed infants5, probably because their energy intake is lower and are less susceptible to obesity as adults6.
Developmental programming studies in rodents support these findings. Using a variety of rodent models, we and others have shown that reducing growth and weight gain during lactation results in a permanent reduction in body weight and food intake, leanness when fed on a chow diet, resistance to diet-induced obesity and improved insulin sensitivity7-11. The precise molecular mechanisms linking altered nutrition in early life and long-term energy balance are unclear. It has been suggested that programmed changes in the hypothalamus mediate some of these effects12. The hypothalamus differentiates in utero but continued maturation occurs into early postnatal life in both rodents and humans13, 14. Studies in animal models have shown that during this period the expression of neuropeptides and their receptors can be permanently altered by the maternal diet15-17.
We have demonstrated that maternal protein restriction during suckling leads to a permanent reduction in body weight and food intake even when animals (postnatal low protein [PLP] offspring) are weaned onto standard laboratory chow 8. At weaning, these animals had a low plasma concentration of the adipose tissue-derived, leptin, which should promote feeding, but increased ObRb expression (signalling form of the leptin receptor). Downstream targets of leptin action were also altered in postnatal low protein animals with increased neuropeptide Y (NPY) and decreased pro-opiomelanocortin (POMC) expression in the arcuate nucleus (ARC) of the hypothalamus 8. Since NPY promotes feeding, whilst α-melanocyte-stimulating hormone (α-MSH), which is derived from POMC, reduces feeding, these changes might be expected to promote weight gain. However, these rats remain lean with a reduced food intake throughout adulthood. This suggests that postnatal low protein rats do not respond appropriately to elevated levels of NPY or the reduced levels of POMC (and thus α-MSH).
We therefore hypothesised that leptin would be more effective at reducing food intake in postnatal low protein animals as a consequence of hypersensitivity to α-MSH and insensitivity to NPY and have investigated the effects of centrally administered leptin, NPY and the α-MSH mimetic MTII18. We also investigated whether differences in the expression of receptors for leptin, NPY and α-MSH in the hypothalamic nuclei could explain any differences in sensitivity to these neuropeptides.
Materials and Methods
Animals
Animal procedures were conducted in accordance with University of Cambridge and the University of Buckingham project licences, under the UK Animals (Scientific Procedures) Act (1986). Breeding was performed in the University of Cambridge. Control animals were the offspring of Wistar rat dams fed a 20% (by dry weight) protein diet during pregnancy and lactation. Postnatal low protein animals were the offspring of dams fed a 20% protein diet during pregnancy that were cross-fostered after birth to dams fed an isocaloric 8% protein diet, as described previously8. Offspring were weaned onto standard laboratory chow diet at 21 days of age and housed at 22 °C on a 12:12 h light: dark cycle. Half of the males from each litter were transferred to the University of Buckingham at six weeks of age for the central neuropeptide sensitivity studies. The other half remained in Cambridge for gene expression studies and magnetic resonance imaging (MRI) analysis (Echo MRI TM, Whole Body Composition Analyzer, Echo Medical Systems, Houston, Texas). All studies were conducted in 3-month-old male animals.
Measurements of leptin, NPY and MT11 sensitivity
A cannula was inserted into the third ventricle under gaseous anaesthetic (Isofluorane: Isoba, Schering-Plough, Animal Health, Summit, New Jersey, USA) using coordinates from the stereotactic rat brain atlas (coordinates based from the bregma: anterior-posterior (AP) −0.8 mm; Lateral (L) 0; dorsal-ventral (DV) −7.5 mm)19. Its position was verified by a positive drinking response over 15 min to angiotensin II (20 μg/ml in 2.5 μl). Four animals out of 84 did not show a positive drinking response and were excluded. For measurements of acute effects of leptin and MTII on food intake, rats were individually housed in wire-bottom cages, fasted for 4 h, dosed at the beginning of the dark period and re-fed. NPY was given at the beginning of the light cycle. Each peptide was given in 2.5 μl saline. Animals were dosed using a Latin square design. There were at least 4 days between doses and it was verified that normal feeding behaviour and body weight had been restored prior to administration of the next dose.
Recombinant rat leptin (Preprotech, London, UK) was administered at a dose of 5 μg because this dose was the lowest that reduced food intake over 4 h significantly in a preliminary study (data not shown). The doses of NPY and MTII (Bachem, Bubendorf, Switzerland) were based on published data and doses routinely used by ourselves20-22. Plasma was stored at −80 °C prior to assay of leptin by ELISA (Crystal Chem Inc. Immunoassay, Chicago, IL, USA).
In situ hybridisation
Brains were removed following an overnight fast, frozen immediately on dry ice and stored at −80°C. Riboprobes for MC4R, ObRb, SOCS3 and MC3R were generated using partial rat and human cDNA sequences respectively 23, 24. The in situ hybridization methodology has been described elsewhere 25, 26. Five sections per slide through the ARC and three sections per slide through the ventromedial hypothalamus (VMH) and paraventricular nucleus (PVN) were used for analysis. Briefly, slides were air dried and exposed to Kodak BioMax MR autoradiographic film (GE Healthcare, Little Chalfont, Bucks, UK). Film images were corrected for background and quantified. A standard curve was generated from the 14C autoradiographic microscales (Amersham Biosciences, UK.) using Image Proplus software (Media Cyernetics, Silver Spring, MD, USA). Integrated optical densities of areas of interest for each section were calculated using the Image Pro Plus system. Data are averaged for each slide for each animal.
Laser-captured microdissection (LCM) and Quantitative Real Time PCR analysis
LCM was performed using a P.A.L.M. MicrolaserSystem (P.A.L.M. Microlaser Technologies AG, Bernried, Germany) as described previously 27. Total RNA was isolated using the RNAqueous Micro RNA extraction kit (Ambion, Applied Biosystems, Life Technologies, Paisley, UK) and analysed using an Agilent BioAnalyzer (Agilent Technologies, Edinburgh, UK). RNA amplification of LCM ARC nuclei samples was performed using MegaScript T7 Amplification Kit (Ambion, Applied Biosystems, UK) in combination with the GeneChip sample CleanUp Module kit (Affymetrix, Santa Clara, California, USA). This method of amplification involves two rounds of T7-based in vitro transcription (IVT). NPY, and the NPY Y1 and Y5 receptors expression was measured using Micro Fluidic Cards (Applied Biosystems, UK). Quantitative PCR reactions were performed in duplicate using an ABI 7900HT (Applied Biosystems, UK). All procedures were carried out in accordance to the manufacturer’s recommendation.
Statistical analysis
Data were analysed using GraphPad Prism software, version 4.0, La Jolla, California, USA. Baseline food intake, body weight, body composition, the percentage reduction in food intake in response to leptin, in situ hybridization and real time PCR of gene expression data were analysed using an unpaired Student’s t test. The Mann-Whitney U-test was used to compare the food intakes after administration of leptin (Table 2), because variances were significantly different between control and PLP treatments. Plasma leptin levels were analysed by the Kruskal-Wallis test followed by Dunn’s multiple comparison test because variances differed between saline and leptin-treated rats. Absolute food intake in the NPY and MTII experiments was analysed by two-way ANOVA with maternal diet and administration of neuropeptide as the independent variables, followed by Bonferroni post hoc tests. ED50 values for the effects of NPY and MTII were calculated from dose-response curves for all NPY or MTII data (sigmoidal dose-response; variable slope) and analysed by unpaired Student’s t-tests. The Welch correction was applied where variances were significantly different between control and PLP groups, the Mann-Whitney U-test not being possible with this analysis. ED50 is the dose that increases (NPY) or decreases (MTII) food intake by half of the maximal effect as determined by sigmoidal dose-response curve fit (curve equation: Y = Bottom + (Top-Bottom)/(1 + 10(logED50-X)). Results are presented as means ± SEM. Statistical significance for effects in the tables and figures is given as *P<0.05; **P<0.01; ***P<0.001. For each outcome measured, no more than one animal per litter was included. Therefore n represents number of litters as well as number of animals.
TABLE 1.
Characteristics of developmentally programmed animals
| Measurement | Control | Postnatal low protein (PLP) |
|---|---|---|
| Body weight (g) | 411 ± 16 | 311 ± 10** |
| Body fat (g) | 54.4 ± 5.1 | 32.1 ± 1.7** |
| Body lean (g) | 278 ± 10 | 224 ± 8** |
| % Body fat | 13.1 ± 1.1 | 10.4 ± 0.5* |
| % Body lean | 67.6 ± 1.0 | 72.2 ± 1.0* |
| Food intake (g/24 h) | 30.0 ± 1.0 | 24.8 ± 0.7*** |
| Plasma leptin (pM) | 431 ± 36 | 271 ± 59* |
Body weight and composition (n = 8), 24-hour food intake and 4-hour fasted plasma leptin (n = 12) were measured in 3-month-old offspring. Students unpaired t-test
P < 0.05
P < 0.01.
P < 0.001.
TABLE 2.
Food intake following central administration of leptin
| Time (h) from i3v leptin | % Reduction in food intake |
|
|---|---|---|
| Control | Postnatal low protein | |
| 0–2 | 27.5 ± 3.9 | 24.1 ± 7.9 |
| 0–4 | 16.6 ± 6.0 | 25.7 ± 11.5 |
| 0–24 | 12.7 ± 2.0 | 24.2 ± 10.9 |
| 0–48 | 16.3 ± 1.9 | 36.11 ± 11.0* |
Three-month-old rats received saline or 5 μg recombinant leptin i3v. Data are expressed as the percentage reduction in food intake for each animal given leptin compared to when it was given saline (n= 9–13).
P = 0.035 for effect of leptin in PLP compared to control rats by Mann-Whitney U-test (n = 9–13). The reductions in food intake elicited by leptin were all significant (P < 0.05).
Results
Body weight, body composition, food intake and plasma leptin
At three months of age postnatal low protein rats were smaller and had a lower food intake than control rats. They also had lower body fat in both absolute terms and as a percentage of body weight, and lower plasma leptin (Table 1). Absolute lean body mass was lower than in control rats but percentage lean body mass was higher (Table 1).
Effect of leptin on food intake and plasma leptin concentration
Leptin reduced food intake in both the control and postnatal low protein groups over the first 2, 4, 24 and 48 hours (P<0.05 at all time points) (Table 2). It did not have a significant effect in the first hour or in the 72 to 96 h period post administration (results not shown). The effect of leptin was significantly greater over 0 to 48 h in the postnatal low protein than in the control rats (Table 2). Leptin reduced body weight in the control and postnatal low protein groups both at 24 hours and 48 hours post dosing (change in bodyweight at 24 hours post dosing: control saline −0.1 ± 1.9 g ; PLP saline −1.3 ± 2.1 g; control leptin −5.2 ± 1.9 g; PLP leptin −7 ± 2.6 g and 48 hours post dosing: control saline 2.3 ± 1.4 g; PLP saline 1.6 ± 4.3 g control leptin −6.9 ± 2 g; PLP leptin −7.5 ± 3.1 g) although the effect of leptin on body weight change in PLP versus controls was not significant at either time point.
The plasma leptin concentration rose following administration of i3v leptin, but there was no difference in the increase between control and postnatal low protein rats (increases at 2 h post-dosing: control 481 ± 118 pM; PLP 434 ± 88 pM) (Supplemental Figure 1). Studies using human leptin showed that the increase in plasma leptin was due to the injected leptin reaching the plasma, rather than an increase in endogenous leptin secretion (Supplemental Figure 2).
Effect of NPY on food intake
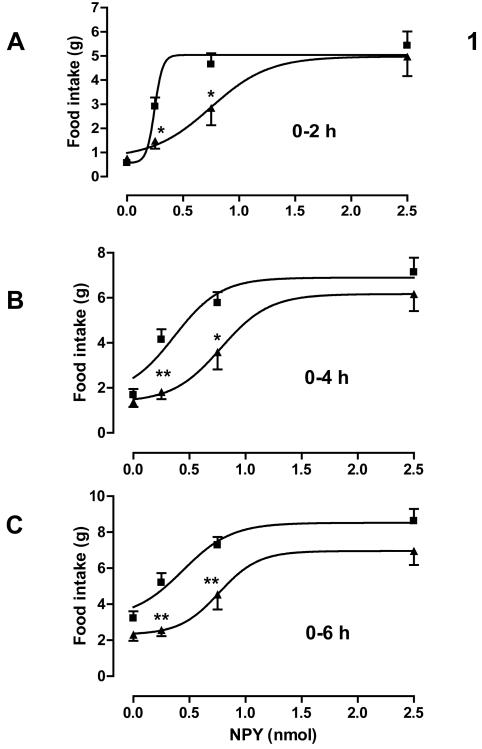
NPY dose-dependently stimulated food intake 2, 4 and 6 hours after dosing in both control and postnatal low protein rats (P < 0.001). Maternal diet significantly affected the dose-response curve over each time period (2 hours: P < 0.05; 4 and 6 hours: P < 0.0001). Compared to the control animals, the postnatal low protein rats showed a significantly blunted orexigenic response to the low (0.25 nmol) and mid (0.75 nmol) doses but not to the high (2.5 nmol) dose during the first 6 hours post dosing (Figure. 1). These blunted responses remained significant (P < 0.05) over 2 and 4 hours, but not over 6 hours, after correction for the difference in food intake between the groups after administration of vehicle. This was reflected in significantly increased ED50 values over each time period (Table 3). NPY had no significant effect on body weight in either control or postnatal low protein rats over the 6 hour period (data not shown).
Figure. 1.
Food intake following central administration of NPY. Food intake was measured 2, 4 and 6 hours after administering NPY to three-month-old control (■) and PLP (▲) rats (n = 13 to 18). ED50 values are given in Table 3 n=16-20 per group. *P<0.05; **P<0.01 for absolute food intake differences between control and PLP rats.
TABLE 3.
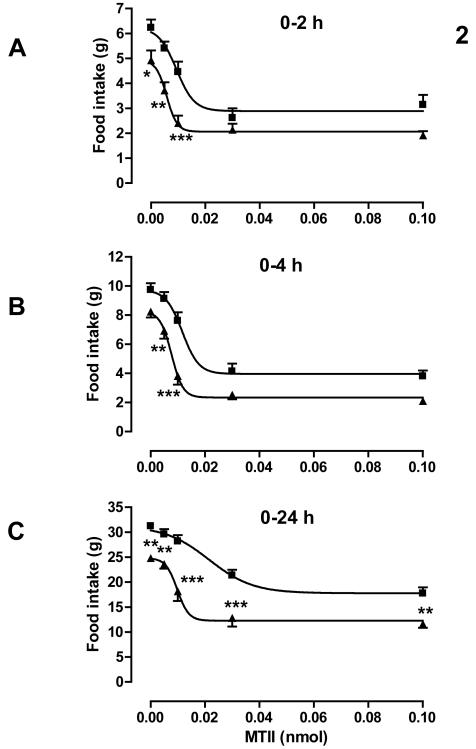
ED50a values for effects of NPY and MTII on food intake
| 0-2h |
0-4h |
0-6/24hb |
||||
|---|---|---|---|---|---|---|
| Control | PLP | Control | PLP | Control | PLP | |
| NPY | 250±30 | 750±160** | 370±90 | 780±120* | 440±100 | 760±110* |
| MTII | 9±1 | 6±1* | 12±2 | 8±1* | 21±3 | 10±1* |
Values expressed as mean (pM) ± SEM
0-6h for NPY; 0-24h for MTII
P < 0.05
P < 0.01 for PLP v control
Effect of MTII on food intake
MTII dose-dependently reduced food intake 2, 4 and 24 hours after dosing in both control and postnatal low protein rats (P < 0.001). Maternal diet significantly affected the dose-response curve over each time period (P < 0.0001). Food intake following the mid dose of 0.01 nmol MTII was most different between control and postnatal low protein rats. This difference was significant (P = 0.015) over 4 h after correction for the difference following administration of vehicle. Food intake over 0-24 h following the high dose (0.10 nmol) was lower in the postnatal low protein than in the control rats, but this difference was similar to that following administration of vehicle (Figure. 2). ED50 values for MTII were significantly lower in postnatal low protein than control rats over each time period (Table 3). MTII reduced body weight in the control and postnatal low protein groups by 24 hours post dosing (change in total bodyweight: control saline −1.2 ± 1.6 g ; PLP saline 0.4 ± 1.2 g; control MTII 0.01 nmol 1.2 ± 1.2 g; PLP MTII 0.01 nmol −5.2 ± 4 g and control MTII 0.03 nmol −6.6 ± 1.3 g; PLP MTII 0.03 nmol −12 ± 2.2 g) although the effect of MTII on body weight change in PLP versus controls was not significant.
Figure. 2.
Food intake following central administration of MTII. Food intake was measured 2, 4 and 24 hours after administering MTII to three-month-old control (■) and PLP (▲) rats. ED50 values are given in Table 3 n=13-18 per group. *P<0.05; **P<0.01; ***P<0.001 for absolute food intake differences between control and PLP rats.
Hypothalamic gene expression
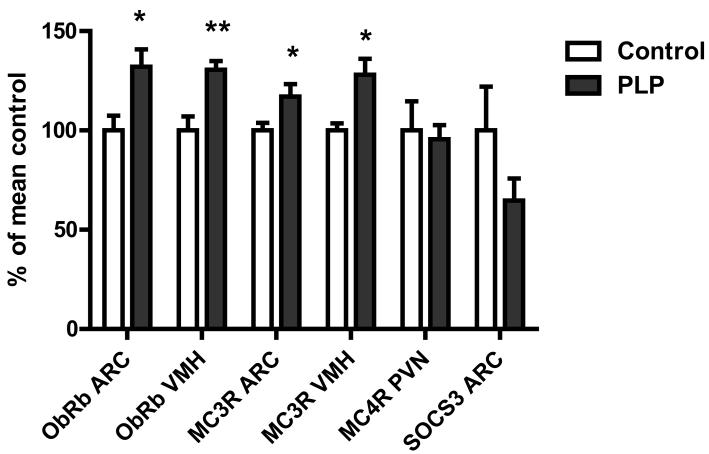
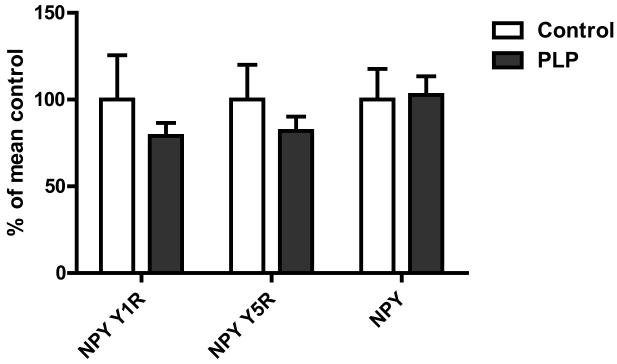
Expression of both ObRb and MC3R was higher in postnatal low protein than control rats in both the ARC (P < 0.05 for both) and the VMH (P < 0.01 for both) (Figure 3). Expression of MC4R in the PVN and NPY, NPY Y1R and NPY Y5R and suppressor of cytokine signalling 3 (SOCS3) in the ARC was not significantly different between groups (Figure 4).
Figure. 3.
Gene expression of ObRb, MC3R and MC4R and SOCS3 in hypothalamic nuclei. Gene expression was measured in serial brain sections from overnight fasted animals by in situ hybridisation. Data expressed as means ± SEM. n = 6-8 per group, *P<0.05; **P<0.01 for absolute differences between control and PLP rats.
Figure. 4.
Gene expression of NPY, NPY Y1R and NPY Y5R in hypothalamic nuclei. Gene expression was measured in laser capture microdisssected arcuate nuclei from overnight fasted animals by quantitative PCR. Data expressed as means ± SEM. n = 6-8 per group
Discussion
We have confirmed our finding8 that growth restriction during lactation, achieved by cross-fostering male rat pups to dams fed a low protein diet, results in permanently reduced body and fat pad weights, even when offspring are maintained on standard laboratory chow. We have now shown that, at three months of age, these offspring are hypersensitive to the anorectic effect of centrally administered leptin. This is consistent with their having a low percentage body fat despite having a low circulating plasma leptin concentration.
Hypersensitivity to leptin may be partly due to increased ObRb expression. However, it also involves neurons downstream of those that respond to leptin. Thus, the male postnatal low protein rats were hypersensitive to submaximal doses of the MC3/4R agonist MTII, and insensitive to NPY, as reflected in altered ED50 values for these peptides. These results are consistent with our previous finding that the male postnatal low protein rats are lean despite having increased NPY expression and decreased POMC expression in the ARC at weaning28 and a report that POMC expression is reduced in ‘middle-aged’ rats that are lean as a consequence of early postnatal food restriction28.
Hypersensitivity to MTII may be due to increased expression of MC3R. However, an involvement in the regulation of feeding is far better established for MC4R, the expression of which was unaltered, than for MC3R. Some studies suggest that MC3R plays no role at all in the regulation of feeding29. In contrast, MC3R is found in areas of the hypothalamus that regulate feeding (the arcuate nucleus and the ventromedial area), mice that lack MC3R are obese, a stable agonist of the human MC3R stimulates feeding in rats, and some studies have linked variants in MC3R with adiposity in humans. The stimulatory effect of the stable MC3R agonist seems inconsistent with MC3R knockout mice being obese. This response may be due to an autoinhibitory role of MC3R on POMC neurones, or it could be because the stable agonist is only a partial agonist at the native rat receptor and inhibits its constitutive activity30.
Just as leptin resistance is partly due to an elevated plasma leptin concentration31, 32, hypersensitivity to MTII may be a consequences of a decreased synaptic melanocortin expression, but if so it is surprising that MC3R but not MC4R expression increased. Insensitivity to NPY was not associated with altered expression of NPY Y1 or Y5 receptors. Mechanisms downstream of Y1, Y5 and possibly MC4R receptors therefore appear to play a role in altered sensitivity to MTII and NPY.
The phenomenon of leptin hypersensitivity that we have identified is not a simple mirror image of that of leptin resistance in obesity. Leptin resistance is associated with enhanced sensitivity (or at least no reduction in the response) to MTII33-38. Thus, resistance to leptin is not due to resistance to melanocortins, whereas in our model, enhanced sensitivity to leptin was associated with enhanced sensitivity to melanocortins. It is less clear whether altered sensitivity to NPY contributes to or opposes leptin resistance, and therefore mirrors or fails to mirror the contribution of NPY resistance to leptin hypersensitivity in postnatal low protein rats. A report that describes an enhanced feeding response to NPY in diet-induced obesity is in a minority39. These authors were also in a minority which found that elevated leptin was associated with decreased NPY expression.
Taking into account any differences in food intake between control and postnatal low protein rats following administration of saline, feeding responses to maximal doses of NPY and MTII did not differ between control and postnatal low protein rats: only responses to submaximal doses differed. This implies that there are “spare receptors” 40 that couple NPY and MTII to feeding. In other words, not all these receptors have to be activated in normal rats to elicit a maximal response. Therefore some reduction in the number of NPY receptors or their coupling to feeding can occur without there being a reduction in the maximum possible response; equally an increase in the number of melanocortin receptors will not increase the maximal response. Sensitivity to NPY and MTII will alter, however. This is important to bear in mind when studying sensitivity to NPY and MTII in situations of altered leptin sensitivity.
The experiments that we have described on the effect of leptin on feeding were conducted using central (third ventricle) administration, which is in close proximity to the ARC41, 42. This has allowed us to relate sensitivity to leptin to sensitivity to NPY and MTII, which have to be administered centrally to study their effects upon food intake. It might be argued that it is more physiologically relevant to administer leptin peripherally. It has been suggested that transport of leptin across the blood-brain barrier concentrates leptin in the ARC to a degree not seen after right intracerebroventricular injection43. Reduced transport of leptin across the blood-brain barrier is a possible cause of leptin resistance, and so increased transport might contribute to hypersensitivity to leptin. However, the effects of intraperitoneally administered leptin on food intake, especially rapid responses and those elicited by low doses, are partly mediated via the vagus nerve, possibly reflecting the role of gastric rather than adipocyte leptin44-47. Furthermore, blood levels of leptin achieved with doses of leptin that are usually given intraperitoneally to assess leptin sensitivity are far above physiological concentrations 48. Thus the intraperitoneal route may not model physiology better than the third ventricle route, and it may even be inferior.
We can discount the possibility that i3v-administered leptin elicited any of its effect on food intake by raising peripheral leptin levels. There was an increase in the plasma leptin concentration following administration of 5 μg leptin, but this was 2000-fold lower than the increase in plasma leptin in response to intraperitoneal administration of 1 mg/kg leptin, which is a dose below that (2 mg/kg, ip) which inhibits food intake significantly over 0-1 h and longer time periods (data not shown). Moreover, the increase in leptin concentration was not different following its administration to control and postnatal low protein rats. This provides no insight into the activity of the blood-brain barrier for leptin because transport of intracerebroventricularly administered leptin to the periphery appears to be due to reabsorption of cerebrospinal fluid43.
A recent study in rats that were selectively bred to be sensitive to diet-induced obesity has demonstrated that large litter rearing confers enhanced sensitivity to the anorectic effect of leptin and protects the offspring from becoming obese49. Large litter-reared diet-induced obese neonates had increased ARC binding of leptin to its extracellular receptors consistent with our finding of increased expression of ObRb. Voluntary exercise beginning from soon after weaning also provides protection from later obesity for at least 10 weeks after termination of exercise and increases sensitivity to leptin50-52. Thus the long term effects of exercise may involve similar mechanisms to those described here. In these same studies, restriction of energy intake pre-weaning provided long term protection from obesity, consistent with our results. Restriction of energy intake at later times was ineffective.
Administration of leptin to rat dams during pregnancy and lactation reduces postnatal (as well as in utero) growth and protects their offspring from diet-induced obesity 53, 54. However, administration of leptin to dams during pregnancy alone resulted in thinner offspring, even though postnatal growth was not reduced55. Thus the protective effect of leptin is not simply due to reduced postnatal growth.
In conclusion, leanness in rats achieved by cross-fostering to dams fed on a low protein diet is associated with increased sensitivity of feeding to centrally administered leptin and MTII, a melanocortin 3/4 receptor agonist, and with decreased sensitivity to neuropeptide Y. These effects may be partly due to increased expression of ObRb and MC3R, but other downstream mechanisms must also play a role. Early post-natal nutrition has a lifelong influence on hypothalamic function, the underlying mechanisms of which require further investigation, but which may involve changes in epigenetic regulation during early hypothalamic development.
Supplementary Material
Supplemental Figure 1. Plasma leptin kinetics following central administration of rat leptin. Plasma levels of rat leptin levels were measured (using a rat leptin ELISA, Crystal Chem Inc. Immunoassay, Chicago, IL, USA) following central administration of 5 μg recombinant rat leptin to three-month-old control (■) and PLP (▲) rats. Data expressed as means ± SEM. n = 7-8 rats. *P<0.05 per group; **P<0.01 for absolute differences between control and † P<0.05 †† P<0.01 PLP rats.
Supplemental Figure 2. Plasma leptin kinetics following central administration of human leptin. Plasma levels of human leptin levels were measured (using a human specific human leptin ELISA, RnD, UK) following central administration of 5 μg recombinant human leptin to three-month-old control rats. Data expressed as means ± SEM. n = 5 rats.
Acknowledgements
The authors would like to thank David Hislop, Anita Roberts, Adrian Wayman and Delia Hawkes for their excellent technical support. This work was supported by an Industrial partnered BBSRC research grant E007821/1 and E00797X/1. SEO is a British Heart Foundation Senior Fellow.
Grants: This work was funded by grants E007821/1 and E00797X/1 from the Biotechnology and Biological Sciences Research Council, UK.
Footnotes
Conflicts of interest The authors have no conflicts of interest.
Supplementary information is available at International Journal of Obesity website, Wargent and Stocker et al.
References
- 1.McMillen IC, Robinson JS. Developmental origins of the metabolic syndrome: prediction, plasticity, and programming. Physiol Rev. 2005;85:571–633. doi: 10.1152/physrev.00053.2003. [DOI] [PubMed] [Google Scholar]
- 2.Druet C, Ong KK. Early childhood predictors of adult body composition. Best Pract Res Clin Endocrinol Metab. 2008;22:489–502. doi: 10.1016/j.beem.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Ravelli GP, Stein ZA, Susser MW. Obesity in young men after famine exposure in utero and early infancy. N Engl J Med. 1976;295:349–353. doi: 10.1056/NEJM197608122950701. [DOI] [PubMed] [Google Scholar]
- 4.de Rooij SR, Painter RC, Phillips DI, Osmond C, Michels RP, Godsland IF, Bossuyt PM, Bleker OP, Roseboom TJ. Impaired insulin secretion after prenatal exposure to the Dutch famine. Diabetes Care. 2006;29:1897–1901. doi: 10.2337/dc06-0460. [DOI] [PubMed] [Google Scholar]
- 5.Ong KK, Preece MA, Emmett PM, Ahmed ML, Dunger DB. Size at birth and early childhood growth in relation to maternal smoking, parity and infant breast-feeding: longitudinal birth cohort study and analysis. Pediatr Res. 2002;52:863–867. doi: 10.1203/00006450-200212000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Harder T, Bergmann R, Kallischnigg G, Plagemann A. Duration of breastfeeding and risk of overweight: a meta-analysis. Am J Epidemiol. 2005;162:397–403. doi: 10.1093/aje/kwi222. [DOI] [PubMed] [Google Scholar]
- 7.Ozanne SE, Hales CN. Lifespan: catch-up growth and obesity in male mice. Nature. 2004;427:411–412. doi: 10.1038/427411b. [DOI] [PubMed] [Google Scholar]
- 8.Cripps RL, Martin-Gronert MS, Archer ZA, Hales CN, Mercer JG, Ozanne SE. Programming of hypothalamic neuropeptide gene expression in rats by maternal dietary protein content during pregnancy and lactation. Clin Sci (Lond) 2009;117:85–93. doi: 10.1042/CS20080393. [DOI] [PubMed] [Google Scholar]
- 9.Velkoska E, Cole TJ, Dean RG, Burrell LM, Morris MJ. Early undernutrition leads to long-lasting reductions in body weight and adiposity whereas increased intake increases cardiac fibrosis in male rats. J Nutr. 2008;138:1622–1627. doi: 10.1093/jn/138.9.1622. [DOI] [PubMed] [Google Scholar]
- 10.Jimenez-Chillaron JC, Hernandez-Valencia M, Lightner A, Faucette RR, Reamer C, Przybyla R, Ruest S, Barry K, Otis JP, Patti ME. Reductions in caloric intake and early postnatal growth prevent glucose intolerance and obesity associated with low birthweight. Diabetologia. 2006;49:1974–1984. doi: 10.1007/s00125-006-0311-7. [DOI] [PubMed] [Google Scholar]
- 11.Remmers F, Fodor M, Delemarre-van de Waal HA. Neonatal food restriction permanently alters rat body dimensions and energy intake. Physiol Behav. 2008;95:208–215. doi: 10.1016/j.physbeh.2008.05.021. [DOI] [PubMed] [Google Scholar]
- 12.Bouret SG. Early life origins of obesity: role of hypothalamic programming. J Pediatr Gastroenterol Nutr. 2009;48(Suppl 1):S31–38. doi: 10.1097/MPG.0b013e3181977375. [DOI] [PubMed] [Google Scholar]
- 13.Grove KL, Grayson BE, Glavas MM, Xiao XQ, Smith MS. Development of metabolic systems. Physiol Behav. 2005;86:646–660. doi: 10.1016/j.physbeh.2005.08.063. [DOI] [PubMed] [Google Scholar]
- 14.Glavas MM, Joachim SE, Draper SJ, Smith MS, Grove KL. Melanocortinergic activation by melanotan II inhibits feeding and increases uncoupling protein 1 messenger ribonucleic acid in the developing rat. Endocrinology. 2007;148:3279–3287. doi: 10.1210/en.2007-0184. [DOI] [PubMed] [Google Scholar]
- 15.Muhlhausler BS, Adam CL, Findlay PA, Duffield JA, McMillen IC. Increased maternal nutrition alters development of the appetite-regulating network in the brain. Faseb J. 2006;20:1257–1259. doi: 10.1096/fj.05-5241fje. [DOI] [PubMed] [Google Scholar]
- 16.Delahaye F, Breton C, Risold PY, Enache M, Dutriez-Casteloot I, Laborie C, Lesage J, Vieau D. Maternal perinatal undernutrition drastically reduces postnatal leptin surge and affects the development of arcuate nucleus proopiomelanocortin neurons in neonatal male rat pups. Endocrinology. 2008;149:470–475. doi: 10.1210/en.2007-1263. [DOI] [PubMed] [Google Scholar]
- 17.Chen H, Simar D, Lambert K, Mercier J, Morris MJ. Maternal and postnatal overnutrition differentially impact appetite regulators and fuel metabolism. Endocrinology. 2008 doi: 10.1210/en.2008-0582. [DOI] [PubMed] [Google Scholar]
- 18.Schioth HB, Bouifrouri AA, Rudzish R, Muceniece R, Watanobe H, Wikberg JE, Larhammar D. Pharmacological comparison of rat and human melanocortin 3 and 4 receptors in vitro. Regul Pept. 2002;106:7–12. doi: 10.1016/s0167-0115(02)00025-3. [DOI] [PubMed] [Google Scholar]
- 19.Paxinos G, Watson C, Pennisi M, Topple A. Bregma, lambda and the interaural midpoint in stereotaxic surgery with rats of different sex, strain and weight. J Neurosci Methods. 1985;13:139–143. doi: 10.1016/0165-0270(85)90026-3. [DOI] [PubMed] [Google Scholar]
- 20.Turnbull AV, Ellershaw L, Masters DJ, Birtles S, Boyer S, Carroll D, Clarkson P, Loxham SJ, McAulay P, Teague JL, Foote KM, Pease JE, Block MH. Selective antagonism of the NPY Y5 receptor does not have a major effect on feeding in rats. Diabetes. 2002;51:2441–2449. doi: 10.2337/diabetes.51.8.2441. [DOI] [PubMed] [Google Scholar]
- 21.Hagan MM, Rushing PA, Pritchard LM, Schwartz MW, Strack AM, Van Der Ploeg LH, Woods SC, Seeley RJ. Long-term orexigenic effects of AgRP-(83---132) involve mechanisms other than melanocortin receptor blockade. Am J Physiol Regul Integr Comp Physiol. 2000;279:R47–52. doi: 10.1152/ajpregu.2000.279.1.R47. [DOI] [PubMed] [Google Scholar]
- 22.Hwa JJ, Ghibaudi L, Gao J, Parker EM. Central melanocortin system modulates energy intake and expenditure of obese and lean Zucker rats. Am J Physiol Regul Integr Comp Physiol. 2001;281:R444–451. doi: 10.1152/ajpregu.2001.281.2.R444. [DOI] [PubMed] [Google Scholar]
- 23.Murray JF, Mercer JG, Adan RA, Datta JJ, Aldairy C, Moar KM, Baker BI, Stock MJ, Wilson CA. The effect of leptin on luteinizing hormone release is exerted in the zona incerta and mediated by melanin-concentrating hormone. J Neuroendocrinol. 2000;12:1133–1139. doi: 10.1046/j.1365-2826.2000.00577.x. [DOI] [PubMed] [Google Scholar]
- 24.Nilaweera KN, Ellis C, Barrett P, Mercer JG, Morgan PJ. Hypothalamic bHLH transcription factors are novel candidates in the regulation of energy balance. Eur J Neurosci. 2002;15:644–650. doi: 10.1046/j.1460-9568.2002.01894.x. [DOI] [PubMed] [Google Scholar]
- 25.Simmons DM, Arriza JL, Swanson LW. A complete protocol for in situ hybridization of messenger RNAs in brain and other tissues with radio-labelled single-stranded RNA probes. J Histotechn. 1989;12:169–181. [Google Scholar]
- 26.Mercer JG, Lawrence CB, Beck B, Burlet A, Atkinson T, Barrett P. Hypothalamic NPY and prepro-NPY mRNA in Djungarian hamsters: effects of food deprivation and photoperiod. Am J Physiol. 1995;269:R1099–1106. doi: 10.1152/ajpregu.1995.269.5.R1099. [DOI] [PubMed] [Google Scholar]
- 27.Jovanovic Z, Tung YC, Lam BY, O’Rahilly S, Yeo GS. Identification of the global transcriptomic response of the hypothalamic arcuate nucleus to fasting and leptin. J Neuroendocrinol. 22:915–925. doi: 10.1111/j.1365-2826.2010.02026.x. [DOI] [PubMed] [Google Scholar]
- 28.Remmers F, Verhagen LA, Adan RA, Delemarre-van de Waal HA. Hypothalamic neuropeptide expression of juvenile and middle-aged rats after early postnatal food restriction. Endocrinology. 2008;149:3617–3625. doi: 10.1210/en.2007-1388. [DOI] [PubMed] [Google Scholar]
- 29.Abbott CR, Rossi M, Kim M, AlAhmed SH, Taylor GM, Ghatei MA, Smith DM, Bloom SR. Investigation of the melanocyte stimulating hormones on food intake. Lack Of evidence to support a role for the melanocortin-3-receptor. Brain Res. 2000;869:203–210. doi: 10.1016/s0006-8993(00)02386-6. [DOI] [PubMed] [Google Scholar]
- 30.Renquist BJ, Lippert RN, Sebag JA, Ellacott KL, Cone RD. Physiological roles of the melanocortin MC receptor. Eur J Pharmacol. 2011;660:13–20. doi: 10.1016/j.ejphar.2010.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scarpace PJ, Matheny M, Zhang Y, Tumer N, Frase CD, Shek EW, Hong B, Prima V, Zolotukhin S. Central leptin gene delivery evokes persistent leptin signal transduction in young and aged-obese rats but physiological responses become attenuated over time in aged-obese rats. Neuropharmacology. 2002;42:548–561. doi: 10.1016/s0028-3908(02)00003-5. [DOI] [PubMed] [Google Scholar]
- 32.Arch JR. Central regulation of energy balance: inputs, outputs and leptin resistance. Proc Nutr Soc. 2005;64:39–46. doi: 10.1079/pns2004407. [DOI] [PubMed] [Google Scholar]
- 33.Enriori PJ, Evans AE, Sinnayah P, Jobst EE, Tonelli-Lemos L, Billes SK, Glavas MM, Grayson BE, Perello M, Nillni EA, Grove KL, Cowley MA. Diet-induced obesity causes severe but reversible leptin resistance in arcuate melanocortin neurons. Cell Metab. 2007;5:181–194. doi: 10.1016/j.cmet.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 34.Hansen MJ, Ball MJ, Morris MJ. Enhanced inhibitory feeding response to alpha-melanocyte stimulating hormone in the diet-induced obese rat. Brain Res. 2001;892:130–137. doi: 10.1016/s0006-8993(00)03246-7. [DOI] [PubMed] [Google Scholar]
- 35.Scarpace PJ, Matheny M, Zolotukhin S, Tumer N, Zhang Y. Leptin-induced leptin resistant rats exhibit enhanced responses to the melanocortin agonist MT II. Neuropharmacology. 2003;45:211–219. doi: 10.1016/s0028-3908(03)00158-8. [DOI] [PubMed] [Google Scholar]
- 36.Li G, Zhang Y, Wilsey JT, Scarpace PJ. Unabated anorexic and enhanced thermogenic responses to melanotan II in diet-induced obese rats despite reduced melanocortin 3 and 4 receptor expression. J Endocrinol. 2004;182:123–132. doi: 10.1677/joe.0.1820123. [DOI] [PubMed] [Google Scholar]
- 37.Zhang Y, Matheny M, Tumer N, Scarpace PJ. Aged-obese rats exhibit robust responses to a melanocortin agonist and antagonist despite leptin resistance. Neurobiol Aging. 2004;25:1349–1360. doi: 10.1016/j.neurobiolaging.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 38.van Dijk G, de Vries K, Nyakas C, Buwalda B, Adage T, Kuipers F, Kas MJ, Adan RA, Wilkinson CW, Thiele TE, Scheurink AJ. Reduced anorexigenic efficacy of leptin, but not of the melanocortin receptor agonist melanotan-II, predicts diet-induced obesity in rats. Endocrinology. 2005;146:5247–5256. doi: 10.1210/en.2005-0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hansen MJ, Jovanovska V, Morris MJ. Adaptive responses in hypothalamic neuropeptide Y in the face of prolonged high-fat feeding in the rat. J Neurochem. 2004;88:909–916. doi: 10.1046/j.1471-4159.2003.02217.x. [DOI] [PubMed] [Google Scholar]
- 40.Brown L, Deighton NM, Bals S, Sohlmann W, Zerkowski HR, Michel MC, Brodde OE. Spare receptors for beta-adrenoceptor-mediated positive inotropic effects of catecholamines in the human heart. J Cardiovasc Pharmacol. 1992;19:222–232. doi: 10.1097/00005344-199202000-00011. [DOI] [PubMed] [Google Scholar]
- 41.Schwartz MW, Seeley RJ, Woods SC, Weigle DS, Campfield LA, Burn P, Baskin DG. Leptin increases hypothalamic pro-opiomelanocortin mRNA expression in the rostral arcuate nucleus. Diabetes. 1997;46:2119–2123. doi: 10.2337/diab.46.12.2119. [DOI] [PubMed] [Google Scholar]
- 42.Mizuno TM, Kleopoulos SP, Bergen HT, Roberts JL, Priest CA, Mobbs CV. Hypothalamic pro-opiomelanocortin mRNA is reduced by fasting and [corrected] in ob/ob and db/db mice, but is stimulated by leptin. Diabetes. 1998;47:294–297. doi: 10.2337/diab.47.2.294. [DOI] [PubMed] [Google Scholar]
- 43.Maness LM, Kastin AJ, Farrell CL, Banks WA. Fate of leptin after intracerebroventricular injection into the mouse brain. Endocrinology. 1998;139:4556–4562. doi: 10.1210/endo.139.11.6319. [DOI] [PubMed] [Google Scholar]
- 44.Patel JD, Ebenezer IS. The effect of intraperitoneal administration of leptin on short-term food intake in rats. Eur J Pharmacol. 2008;580:143–152. doi: 10.1016/j.ejphar.2007.10.046. [DOI] [PubMed] [Google Scholar]
- 45.Peters JH, McKay BM, Simasko SM, Ritter RC. Leptin-induced satiation mediated by abdominal vagal afferents. Am J Physiol Regul Integr Comp Physiol. 2005;288:R879–884. doi: 10.1152/ajpregu.00716.2004. [DOI] [PubMed] [Google Scholar]
- 46.Cakir B, Kasimay O, Devseren E, Yegen BC. Leptin inhibits gastric emptying in rats: role of CCK receptors and vagal afferent fibers. Physiol Res. 2007;56:315–322. doi: 10.33549/physiolres.930865. [DOI] [PubMed] [Google Scholar]
- 47.Ellacott KL, Halatchev IG, Cone RD. Characterization of leptin-responsive neurons in the caudal brainstem. Endocrinology. 2006;147:3190–3195. doi: 10.1210/en.2005-0877. [DOI] [PubMed] [Google Scholar]
- 48.Niimi M, Sato M, Yokote R, Tada S, Takahara J. Effects of central and peripheral injection of leptin on food intake and on brain Fos expression in the Otsuka Long-Evans Tokushima Fatty rat with hyperleptinaemia. J Neuroendocrinol. 1999;11:605–611. doi: 10.1046/j.1365-2826.1999.00368.x. [DOI] [PubMed] [Google Scholar]
- 49.Patterson CM, Bouret SG, Park S, Irani BG, Dunn-Meynell AA, Levin BE. Large litter rearing enhances leptin sensitivity and protects selectively bred diet-induced obese rats from becoming obese. Endocrinology. 151:4270–4279. doi: 10.1210/en.2010-0401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Patterson CM, Dunn-Meynell AA, Levin BE. Three weeks of early-onset exercise prolongs obesity resistance in DIO rats after exercise cessation. Am J Physiol Regul Integr Comp Physiol. 2008;294:R290–301. doi: 10.1152/ajpregu.00661.2007. [DOI] [PubMed] [Google Scholar]
- 51.Patterson CM, Bouret SG, Dunn-Meynell AA, Levin BE. Three weeks of postweaning exercise in DIO rats produces prolonged increases in central leptin sensitivity and signaling. Am J Physiol Regul Integr Comp Physiol. 2009;296:R537–548. doi: 10.1152/ajpregu.90859.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Applegate EA, Upton DE, Stern JS. Exercise and detraining: effect on food intake, adiposity and lipogenesis in Osborne-Mendel rats made obese by a high fat diet. J Nutr. 1984;114:447–459. doi: 10.1093/jn/114.2.447. [DOI] [PubMed] [Google Scholar]
- 53.Stocker C, O’Dowd J, Morton NM, Wargent E, Sennitt MV, Hislop D, Glund S, Seckl JR, Arch JR, Cawthorne MA. Modulation of susceptibility to weight gain and insulin resistance in low birthweight rats by treatment of their mothers with leptin during pregnancy and lactation. Int J Obes Relat Metab Disord. 2004;28:129–136. doi: 10.1038/sj.ijo.0802476. [DOI] [PubMed] [Google Scholar]
- 54.Stocker CJ, Wargent E, O’Dowd J, Cornick C, Speakman JR, Arch JR, Cawthorne MA. Prevention of diet-induced obesity and impaired glucose tolerance in rats following administration of leptin to their mothers. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;292:R1810–1818. doi: 10.1152/ajpregu.00676.2006. [DOI] [PubMed] [Google Scholar]
- 55.Nilsson C, Swolin-Eide D, Ohlsson C, Eriksson E, Ho HP, Bjorntorp P, Holmang A. Reductions in adipose tissue and skeletal growth in rat adult offspring after prenatal leptin exposure. J Endocrinol. 2003;176:13–21. doi: 10.1677/joe.0.1760013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Plasma leptin kinetics following central administration of rat leptin. Plasma levels of rat leptin levels were measured (using a rat leptin ELISA, Crystal Chem Inc. Immunoassay, Chicago, IL, USA) following central administration of 5 μg recombinant rat leptin to three-month-old control (■) and PLP (▲) rats. Data expressed as means ± SEM. n = 7-8 rats. *P<0.05 per group; **P<0.01 for absolute differences between control and † P<0.05 †† P<0.01 PLP rats.
Supplemental Figure 2. Plasma leptin kinetics following central administration of human leptin. Plasma levels of human leptin levels were measured (using a human specific human leptin ELISA, RnD, UK) following central administration of 5 μg recombinant human leptin to three-month-old control rats. Data expressed as means ± SEM. n = 5 rats.