Abstract
Inositol 1,4,5-trisphosphate receptors (InsP3R) and ryanodine receptors (RyR) are tetrameric intracellular Ca2+ channels1. For each, the pore is formed by C-terminal transmembrane domains and regulated by signals detected by the large cytosolic structures. InsP3R gating is initiated by InsP3 binding to the InsP3-binding core (IBC, residues 224-604 of InsP3R1)2 and it requires the suppressor domain (SD, residues 1-223)2-8. We present structures of the N-terminal region (NT) of InsP3R1 with (3.6 Å) and without (3.0 Å) InsP3 bound. The arrangement of the three NT domains, the SD, IBC-β and IBC-α, identifies two discrete interfaces (α and β) between the IBC and SD. Similar interfaces occur between equivalent domains (A, B and C) in RyR19. The orientations of the three domains docked into a tetrameric structure of InsP3R10 and of the ABC domains in RyR9 are remarkably similar. The importance of the α-interface for activation of InsP3R and RyR is confirmed by mutagenesis and, for RyR, by disease-causing mutations9,11,12. InsP3 causes partial closure of the clam-like IBC, disrupting the β-interface and pulling the SD towards the IBC. This reorients an exposed SD loop (HS-loop) that is essential for InsP3R activation7. The loop is conserved in RyR and includes mutations associated with malignant hyperthermia and central core disease9,11,12. The HS-loop interacts with an adjacent NT, suggesting that activation re-arranges inter-subunit interactions. The A-domain of RyR functionally replaced the SD in a full-length InsP3R, and an InsP3R in which its C-terminal transmembrane region was replaced by that from RyR1 was gated by InsP3 and blocked by ryanodine. Activation mechanisms are conserved between InsP3R and RyR. Allosteric modulation of two similar domain interfaces within an N-terminal subunit re-orients the first domain (SD or A-domain), allowing it, via interactions of the second domain of an adjacent subunit (IBC-β or B-domain), to gate the pore.
The essential role of the SD in linking InsP3 binding to InsP3R gating highlights the need to define the structural consequences of InsP3 binding to the NT (residues 1-604 of InsP3R1) (Supplementary Fig. 1). Because our attempts to crystallize the NT yielded poorly diffracting crystals, we expressed a Cys-less form of the NT (NTCysless). Native and Cys-less forms of the NT and IBC behaved indistinguishably (Supplementary Fig. 2 and Supplementary Tables 1-2), but NTCysless provided crystals with much improved diffraction (Supplementary Table 3). We determined crystal structures of NTCysless with (3.6 Å) and without (3.0 Å) InsP3 bound, showing three subdomains: the SD, IBC-β (residues 224-436) and IBC-α (residues 437-604) (Fig. 1a). The structures of these subdomains were nearly identical to those of isolated native SD and IBC2,3 (Supplementary Fig. 3).
Figure 1. Structure of the N-terminal region of InsP3R1 without InsP3 bound.
a, Structure of NTCysless at 3 Å resolution showing SD (blue), IBC-β (green) and IBC-α (yellow). Dashed lines show invisible regions in electron density. Positions of the three domains within a single InsP3R subunit are shown. b, c, Interfaces between SD/IBC-β (β-interface) (b) and SD/IBC-α domains (α-interface, with the hydrophobic core boxed and the 2Fo-Fc electron density map of key residues (contoured at 1.0 σ) shown as mesh) (c). d, Superposition of apo-NTCysless and RyR1-ABC (grey)9 structures by overlaying IBC-β and RyR1 B-domain. ‘Hot spot’ (HS) loop in RyR1 and corresponding region in InsP3R1 are highlighted (red). e, Close-up views of HS regions of InsP3R1 (blue) and RyR1 (grey, black lettering) with conserved residues depicted as sticks. Structure-based DALILite alignment of rat InsP3R and rabbit RyR show conserved residues in yellow, RyR1 disease-associated mutations in red, and hydrophobic residues implicated in activation of InsP3R in blue8.
The SD, IBC-β and IBC-α form a triangular structure, with the SD behind the InsP3-binding site (Fig. 1a). The SD interacts via two interfaces with the IBC, one with IBC-β (β-interface) and another with IBC-α (α-interface). A 310-like turn between the last strand of the SD and the first strand of IBC-β positions the IBC relative to the SD (Supplementary Fig. 4e). Within this connecting turn, a salt bridge (K225/D228) stabilizes the backbone conformation and so positions residues that form the β-interface. These interactions in the connecting turn and β-interface are augmented by a network of hydrophobic interactions within IBC-β (Fig. 1b). The α-interface forms a long ‘Velcro’-like structure that also involves a network of hydrophobic and electrostatic interactions (Fig. 1c). Intimate hydrophobic interactions between V33, and to a lesser extent L32, from the SD; and V452, F445, A449 and L476 from IBC-α are supported by bidentate salt bridges between R54/K127 in the SD and D444 in IBC-α (Fig. 1c). The V33K mutation at the α-interface almost abolished inhibition of InsP3 binding by the SD3,4 and reduced channel open probability4, confirming its importance. Mutation of neighbouring residues that contribute less to the α-interface (L32K, D34K, R36E, K127E) had lesser effects on InsP3 binding, while mutation of residues that do not contribute to the interface (D35K, K52E) had no effect (Supplementary Table 4)3,4. Hydrophobic and electrostatic interaction networks at the α- and β-interfaces contribute to a buried surface between the SD and IBC (~2040 Å2) that forms a hub connecting InsP3 binding to channel activation.
The structure of the NT is remarkably similar to that of the N-terminal of RyR19. The three NT domains of InsP3R1 (SD, IBC-β and IBC-α) can be individually superposed to corresponding domains of RyR1 (A, B and C) (Supplementary Fig. 3), and the relative orientation of the domains is nearly identical (Fig. 1d). Mutation of Y167A, located on an exposed loop of the SD opposite the IBC interfaces (‘HS-loop’11, residues 165-180, boxed in Fig. 1d), attenuates InsP3-evoked Ca2+ release8; and Ca2+, a co-regulator of InsP3R14, causes the loop to become accessible.15 The disease-associated ‘hot spot loop’ of RyR111 sits at the same location within the ABC structure9 (Fig. 1d) and a mimetic peptide causes RyR2 to become leaky16. Furthermore, the backbone and side chain conformation of this loop region superposes well in the two receptors (Fig. 1e). The HS-loop provides a critical link between InsP3 binding and gating.
The domain interfaces of InsP3R1-NT and RyR1-ABC are also similar. The bidentate salt bridges between R54/K127 and D444 at the InsP3R1 α-interface are preserved in RyR1 ABC, albeit in a reversed-charge manner between D40/D61 and R402 (Supplementary Fig. 4a). In RyR1, mutation of these residues (R402C, D61N) is associated with malignant hyperthermia and central core disease9, suggesting that disruption of the interaction perturbs RyR gating, as it does for InsP3R. The structural similarities extend also to the β-interface of InsP3R1 and corresponding A/B interface in RyR1 (Supplementary Fig. 4b-d).
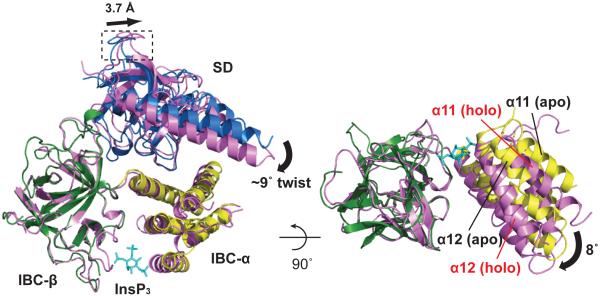
Our structures of NTCysless with and without InsP3 bound, together with that of the InsP3-bound IBC2 (Supplementary Fig. 5) reveal the structural changes evoked by InsP3 (Fig. 2). Side chains of nine residues become organized around InsP3 (Supplementary Fig. 5a), and the domain orientation angle between IBC-β and IBC-α is reduced (by ~8°) after InsP3 binding (Fig. 2 and Supplementary Fig. 5a). This InsP3-evoked ‘clam closure’, which is consistent with earlier predictions17 and small-angle X-ray scattering18, causes the distance across the entrance to the InsP3-binding pocket to decrease (Supplementary Fig. 5b, c). A similar agonist-evoked domain closure occurs in some glutamate receptor channels19. The SD and IBC remain associated after closure of the IBC (Fig. 2). InsP3 binding hardly changes the interactions across the extensive α-interface, but at the β-interface the SD residues move away from IBC-β (Supplementary Fig. 5d-f). With the SD glued to IBC-α by the α-interface, and the β-interface serving as a lubricant, InsP3 binding causes the SD to twist (by ~9°) and move closer to the top of the IBC (Fig. 2). This causes an amplified translational movement of the conserved HS-loop in the SD (Supplementary Fig. 5g). While our work was under review, 3.8 Å structures of apo- and InsP3-bound NT derived from a single crystal grown in excess InsP3 were published, showing similar InsP3-induced allostery in the interfaces between domains13. This confirms our observations, but our higher resolution structures reveal more detail of the α- and β-interfaces associated with this conformational change (Supplementary Discussion).
Figure 2. InsP3-evoked conformational changes.
Superposition of apo-NTCysless (SD, blue; IBC-α, yellow; IBC-β, green) and InsP3-bound NTCysless (3.6 Å resolution, magenta) by overlaying IBC-β domain. InsP3 binding causes the SD to rotate towards the IBC accompanied by a swing approximately perpendicular to the IBC ‘clam closure’. This twist (curved arrow) is measured as the angular difference between the SD arm helices in the apo- and bound states (~9°). Movement of the HS-loop (boxed) shows the distance between α-carbons of Y167 (~3.7 Å). A view rotated 90° about the x-axis is shown at right with only IBCs represented. The interdomain (IBC-β and IBC-α) angular difference between the free and bound states is ~8° (black arrow). Further details of InsP3 binding and its effects on the IBC and α- and β-interfaces in Supplementary Figure 5.
Docking the ABC structure into cryo-EM maps of RyR1 showed that the N-terminal domains form a central ring at the top of the mushroom-like RyR19. Rigid-body docking of our apo-NTCysless structure into a cryo-EM 10 Å structure of a closed InsP3R110 reveals an arrangement remarkably similar to that of RyR1 with a high docking contrast (Fig. 3 and Supplementary Fig. 6). The three domains of the four NTs, which form the upper cytoplasmic surface of the mushroom-like InsP3R, are arranged as four hillocks around a central bowl. This arrangement allows InsP3 unrestricted access to the IBC from the side of the cap (Fig. 3), and it is consistent with accessibility studies and binding sites for regulatory proteins (Supplementary Fig. 6c and Supplementary Table 5). Within the tetrameric InsP3R, the only contacts between NT subunits are via the critical HS-loop of the SD and a flexible loop (β20-β21, Supplementary Fig. 7) in IBC-β (Fig. 3c, d). The latter is longer in RyR and it lies ~10 Å further from the neighbouring hot spot loop9 (Fig. 3c, d). In InsP3R, the arm domains (residues 67-109) of each SD are the only NT structures that extend beyond the cap towards the pore (Fig. 3a), but these domains are neither essential for InsP3R activation8 nor conserved in RyR9,11.
Figure 3. Docking of the apo-NTCysless structure into the cryo-EM map of InsP3R1.
a, b, Side (a) and top (b) views of apo-NTCysless structure docked into the cryo-EM map (grey mesh) of InsP3R1 in a closed state10. Contour level corresponds to mass of InsP3R1 tetramer of 1.3 MDa (protein density 0.8 Da/Å3). Four molecules of the NT (SD, blue; IBC-β, green; IBC-α, yellow) are located at top of the cytoplasmic portion of the InsP3R1 tetramer. c, d, Dockings of the apo-NTCysless (coloured as in a) and ABC9 (grey) structures into cryo-EM structures of InsP3R110 and RyR19, respectively, are overlaid and presented to show only N-terminal structures. HS-loops of InsP3R (magenta) and RyR (orange) are highlighted. Enlargement of boxed area (d). Locations of other binding sites within NT of InsP3R1 are shown in Supplementary Figure 6.
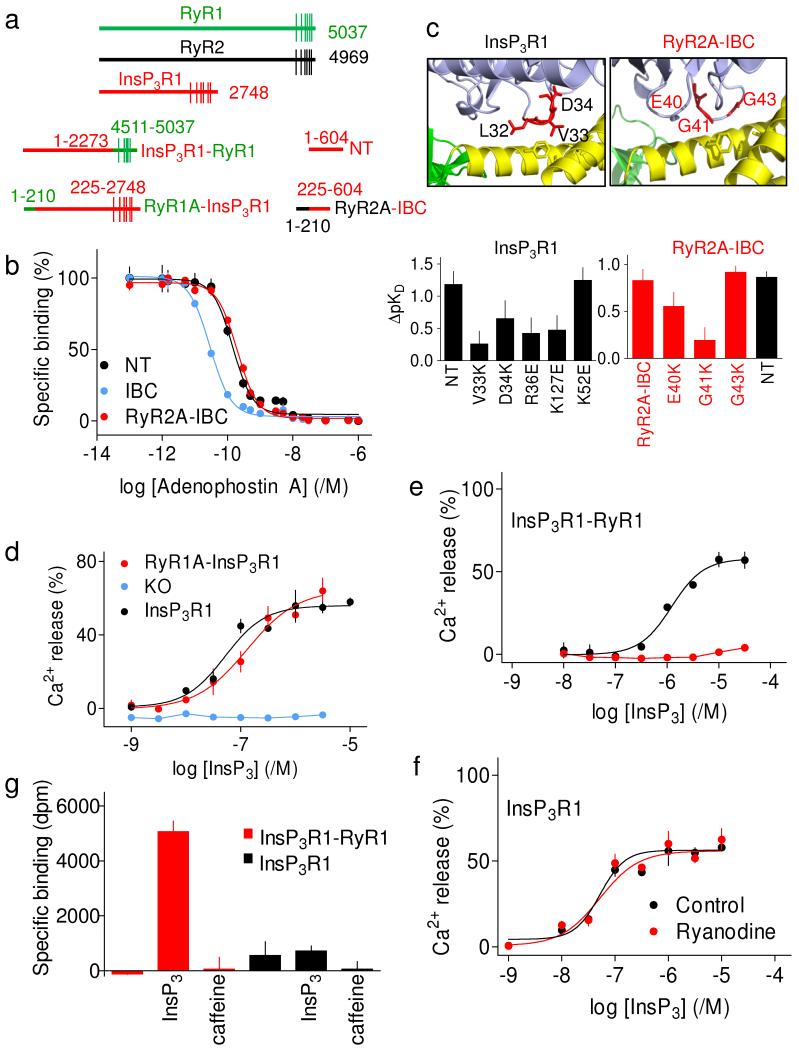
The structural similarities between the N-termini of RyR and InsP3R prompted us to examine whether the domains are functionally interchangeable. In a chimeric N-terminal fragment comprising the A-domain of RyR2 and IBC from InsP3R1 (RyR2A-IBC), the A-domain mimicked the SD by inhibiting InsP3 binding (Fig. 4a, b). Mutations within the A-domain loop that forms the A-B interface in RyR9 or the equivalent InsP3R loop in the SD attenuated this inhibition of binding (Fig. 4c, Supplementary Table 6 and Supplementary Fig. 8). InsP3 stimulated Ca2+ release via InsP3R1 or a chimeric InsP3R1 in which the SD was replaced by the A-domain of RyR1 (RyR1A-InsP3R1 (Fig. 4a, d and Supplementary Fig. 9). Both InsP3Rs were similarly expressed and they released similar fractions of the Ca2+ stores and with similar sensitivity to InsP3 (Supplementary Table 7). Opening of native InsP3R or RyR is restrained by interactions between cytosolic domains20,21. It is therefore significant that expression of InsP3R1 or RyR1A-InsP3R1 affected neither the Ca2+ content of the ER nor the Ca2+ leak from it (Supplementary Fig. 10), confirming that InsP3R and RyR1A-InsP3R1 have no detectable spontaneous activity. This demonstrates that the SD of InsP3R can be functionally substituted by the A-domain of RyR.
Figure 4. Functional chimeras of InsP3R and RyR.
a, Proteins used. b, Specific binding of 3H-InsP3 in presence of adenophostin A. c, Inhibition of 3H-InsP3 binding to IBC by SD or A-domain, and effects of mutations within equivalent loops. Affinities shown relative to IBC (ΔpKD). Structures show key residues within SD or A-domain at α-interface. d, Ca2+ release from DT40 cells expressing InsP3R1, RyR1A-InsP3R1 or lacking InsP3R (KO). e, f, Effect of ryanodine (10 μM) on Ca2+ release from DT40 cells expressing InsP3R1-RyR1 (e) or InsP3R1 (f). Ryanodine (≤10 μM) did not stimulate Ca2+ release via InsP3R1-RyR1 suggesting that TMDs may not alone mediate stimulation of RyR25. Results (d-f) are percentages of ATP-dependent Ca2+ uptake. g, Specific 3H-ryanodine binding (dpm, disintegrations/min) to membranes of DT40 cells expressing InsP3R1 or InsP3R1-RyR1 with caffeine (10 mM) or InsP3 (1 μM). Non-specific binding was 2245 ± 211 dpm. Results (b-g) are means ± s.e.m., n ≥ 3.
An InsP3R1 in which residues downstream of TMD1 were replaced by the equivalent region of RyR1 (InsP3R1-RyR1) also responded to InsP3 (Fig. 4a,e). Expression of InsP3R1-RyR1 increased Ca2+ leak from the ER, and this was reversed by ryanodine, which blocks the RyR pore22. However, the increased leak was insufficient to affect the steady-state Ca2+ content (Supplementary Fig. 10), suggesting that InsP3R1-RyR1 has minimal spontaneous activity. Expression of InsP3R1-RyR1 matched that of other InsP3Rs, but cells expressing InsP3R1-RyR1 were ~20-fold less sensitive to InsP3 (Supplementary Table 7). Because the TMDs minimally affect InsP3 binding23, this diminished response probably reflects a decrease in InsP3 efficacy. The increased Ca2+ leak and reduced efficacy of InsP3 suggest that within InsP3R1-RyR, communication between the SD and channel are slightly less effective than in native InsP3R. Nevertheless, it is remarkable that cytosolic domains of an InsP3R should so effectively regulate the pore of a RyR when the two receptors share only modest sequence identity and differ in the number of residues separating the NT from TMDs (Fig. 4a), and in the lengths and sequences of their C-terminal tails and the loops linking TMDs (Supplementary Fig. 11).
Ryanodine (10 μM) had no effect on InsP3R1 or RyR1A-InsP3R, but it abolished InsP3-evoked Ca2+ release via InsP3R1-RyR1 (Fig. 4e, f). Because ryanodine binds selectively to active RyR24, 3H-ryanodine binding is stimulated by agonists of RyR, like caffeine. Whereas caffeine had no effect on specific 3H-ryanodine binding to InsP3R1-RyR1, InsP3 stimulated it (Fig. 4g). InsP3 therefore causes conformational changes to the channel of InsP3R1-RyR1 that mimic those of native RyR in allowing binding of 3H-ryanodine.
Conservation of structure-function relationships between InsP3R and RyR (Fig. 1-4) allows comparisons between them to suggest possible mechanisms of InsP3R activation. For both receptors, gating requires that conformational changes in the large cytoplasmic structures pass to the TMDs10,22, but the N-terminal domains of InsP3R and RyR are at least 60 Å from these TMDs1,9,22 (Fig. 3a and Supplementary Fig. 6). Despite some evidence implicating direct interactions between the SD and TMD4-5 loop in gating InsP3R (Supplementary Discussion), we suggest, and in keeping with results from RyR20,26,27, that additional cytosolic domains couple the NT to opening of the pore. The exposed HS-loop in the SD (Fig. 1d and 3c,d) (hot spot-loop of RyR)9,11 is arranged similarly within the isolated N-terminal structures of InsP3R and RyR (Fig. 1d) and it reorients after InsP3 binding (Fig. 2). When the NT is docked into the InsP3R structure10, the HS-loop forms (with an exposed loop of IBC-β) the only interface between adjacent NT, however, the equivalent loop is displaced in the RyR9 (Fig. 3c, d). InsP3 binding closes the clam-like IBC, disrupting the β-interface and re-orienting the HS-loop (Fig. 2 and Supplementary Fig. 5). This, we suggest, disrupts interaction of the HS-loop with a neighbouring NT to cause a coordinated re-arrangement of the apical InsP3R structure (Fig. 3). The open state of RyR1 is associated with outward movement of protein density in regions that match the locations of docked ABC structures9,22 and with larger movements of peripheral ‘clamp domains’9,22 that are absent from InsP3R10. Movement of these apical domains in RyR is accompanied by re-arrangements within regions that taper towards the pore22 and which, in InsP3R, include the most flexible parts of its structure10. We suggest that similar rearrangements of the apical surface of InsP3R and RyR couple by shared mechanisms to additional cytosolic domains to gate the pore of each channel.
METHODS SUMMARY
The N-terminal (NT, residues 1-604) of rat InsP3R1 in which all Cys were replaced by Ala (NTCysless) was expressed in E. coli and purified. Crystals of NTCysless were grown by the hanging-drop vapour diffusion method in 0.1 M Hepes pH 7.0, 0.8-1.0 M (NH4)2SO4, and 3% (v/v) trimethylamine N-oxide for apo-state crystals, or 0.1 M Na citrate (pH 6.0), 8% (w/v) PEG-6000, 70 mM Li2SO4, 3% dimethyl sulfoxide for InsP3-bound crystals. Diffraction data were collected at 100 K on the 19-ID (apo-crystals) or 19-BM (InsP3-bound crystals) beam lines at the Advanced Photon Source Synchrotron facility (Argonne, IL) and processed with HKL200028. Structures of apo-NTCysless at 3.0 Å resolution and InsP3-bound NTCysless at 3.6 Å resolution were determined by molecular replacement using structures of the SD (PDB code: 1XZZ)3 and the IBC (1N4K)2 as search models with the program Phaser29. Iterative refinement and model building were performed with Refmac5 and Coot, respectively. Numbering of secondary structure motifs is in accord with Supplementary Figure 7. Binding of 3H-InsP3 or 3H-ryanodine to full-length InsP3R1, chimeras of InsP3R1 and RyR, and to related N-terminal fragments was defined using equilibrium-competition binding assays4. Functional properties of InsP3R1 and chimeras were characterized after stable expression in DT40 cells lacking endogenous InsP3R4. A luminal Ca2+ indicator was used to record InsP3-evoked Ca2+ release from the intracellular stores of permeabilized DT40 cells4.
Supplementary Material
Acknowledgements
We thank Paul Allen (Harvard University) and David MacLennan (University of Toronto) for kind gifts of plasmids encoding RyR2 and RyR1, respectively. C.W.T. thanks Taufiq Rahman and Vera Konieczny (University of Cambridge) for discussions. M.I. acknowledges Katsuhiko Mikoshiba and Takayuki Michikawa (RIKEN) for long-standing support and discussions. This work was supported by grants from the Heart and Stroke Foundation of Ontario (T-7181) to M.I., and the Wellcome Trust (085295), Biotechnology and Biological Sciences Research Council (BB/H009736) and Medical Research Council (G0900049) to C.W.T. M.S. is supported by postdoctoral fellowships from the Canadian Institutes of Health Research and the National Research Foundation of Korea (2009-352-E00006). A.M.R. is a fellow of Queens’ College, Cambridge. M.I. holds a Canadian Research Chair in Cancer Structural Biology.
Appendix
METHODS
Materials
InsP3 was from Enzo Life Sciences (Exeter, UK). Adenophostin A was from A. G. Scientific (San Diego, CA, USA). Ryanodine was from Ascent Scientific (Bristol, UK). Cyclopiazonic acid was from Sigma (St. Louis, MO). Sources of other materials are specified in earlier publications2-4,18 or in the descriptions that follow.
Cloning, expression and purification of N-terminal fragments of InsP3R1 and RyR2
The open reading frame (ORF) encoding the N-terminal fragment (NT, residues 1-604) of rat InsP3R1 (GenBank: GQ233032.1) was amplified by PCR from the full-length clone lacking the S1 splice site using forward 5′-CGGGATCCATGTCTGACAAAATGTCTAGT-3′ and reverse 5′-CGCGCTCGAGTCACTTTCGGTTGTTGTGGA-3′ primers. The PCR product was ligated into a pGEX-6P-2 vector (GE Healthcare, Little Chalfont, Bucks, UK) as a BamHI/XhoI fragment to give pGEX(NT), which includes an N-terminal GST tag followed by a PreScission-cleavage site. To generate NTCysless, a QuikChange multisite directed mutagenesis kit (Agilent, Stockport, UK) was used to mutate all Cys residues to Ala using pGEX-6P-2-(NT) as the template and the primers listed in Supplementary Table 8. Residues are numbered by reference to rat InsP3R1 containing the S1 splice site.
Plasmids encoding GST-tagged IBC (residues 224-604) and IBCCysless were generated using PCR to amplify the appropriate sequence from the ORF of full-length rat InsP3R1 or NTCysless using the following primers: forward 5′-CGGGATCCATGAAATGGAGTAACAAAG-3′ and reverse 5′-CGCGCTCGAGTCACTTTCGGTTGTTGTGGA-3′. Each PCR product was ligated into a pGEX-6P-2 vector as a BamHI/Xho I fragment to produce pGEX-6P-2-(IBC) and pGEX-6P-2-(IBCCysless), respectively. For analysis of the effects of mutations within the SD on InsP3 binding, the plasmids described previously were used to express His6-tagged NT and IBC4 and the His6-tag was cleaved prior to experiments4. Mutations were introduced using a QuikChange mutagenesis kit and the primers listed previously4 or in Supplementary Table 9.
The sequence encoding the A-domain of RyR2 was amplified by PCR from rabbit RyR2 (GenBank: GI164831)30 in pcDNA3 using the following primers: forward 5′-ACTAGTCTCGAGGTGCTCTTCCAGGGGCCCATGGCTGATGGGGGCGAA-3′ and reverse 5′-GATATCCTTCACTTCCTGAGCTGATGGG-3′. The ORF for the IBC of InsP3R1 was excised from pGEX-6P-2-(NT) as a BamHI/XhoI fragment and ligated into a pET41a vector to produce pET41a-(IBC). To generate a plasmid encoding a chimeric NT in which the A-domain of RyR2 (residues 1-210) was fused to the IBC of InsP3R1 (residues 225-604) (RyR2A-IBC), the PCR product from above was ligated into pET41a-(IBC) as a SpeI/EcoRV fragment to produce pet41a-(RyR2A-IBC). Mutations within the ORF of the A-domain of RyR2A-IBC were generated by site-directed mutagenesis using the QuikChange Lightning mutagenesis kit (Stratagene) using the primers listed in Supplementary Table 9. The complete coding sequences of all constructs were confirmed by sequencing. The sequences of the proteins used are summarized in Supplementary Table 1 and Figure 4a.
For structural studies, NTCysless was expressed as a GST-fusion protein in BL21-CodonPlus(DE3) E. coli strain. Transformed cells were first grown at 37°C until the OD600 reached ~1.0 and then induced with 0.5 mM IPTG at 15°C for ~18 h. Proteins were purified using glutathione sepharose 4B resin (GE Healthcare), and the GST tag was cleaved from the eluted proteins with PreScission protease (GE Healthcare) during overnight dialysis at 4°C in cutting buffer (20 mM Tris-HCl, pH 8.4, 300 mM NaCl, 5% glycerol, 2 mM DTT). The cleaved proteins were further purified with cation-exchange chromatography (Fractogel EMD SO3-resin, EM Industries Inc.) followed by size-exclusion chromatography (Superdex 200, GE Healthcare). Purified proteins were concentrated to 14 mg/ml in a buffer comprising 20 mM Tris-HCl, pH 8.4, 360 mM NaCl, 2.5% glycerol, 0.2 mM TCEP, 1 mM PMSF. Similar methods were used to express InsP3R fragments for binding studies, but with the following modifications: bacteria were initially grown at 22°C, the GST-tag was cleaved by incubation of bacterial lysates immobilized on glutathione sepharose 4B resin with PreScission for 5 h in PreScission-cleavage buffer (GE Healthcare). The eluant was then used for 3H-InsP3 binding analyses without further purification. Western blotting and silver-stained gels were used to verify expression and purification of NT fragments.
Western blotting
Western blotting of DT40 cells solubilized in TEM containing Triton-X100 (1% v/v) was performed as previously described31 using anti-peptide antisera corresponding to residues 240-253 within the IBC (AbNT, 1:1000) or 2733-2749 (AbCT, 1:500) of rat InsP3R1. The secondary antibody was HRP-conjugated donkey anti-rabbit antibody (1: 5000, Santa Cruz Biotechnology).
3H-InsP3 Binding
Equilibrium-competition binding assays were performed at 4°C in Tris-EDTA medium (TEM: 50 mM Tris, 1 mM EDTA, pH 8.3) containing 3H-InsP3 (0.75 nM, Perkin-Elmer Life Sciences), purified protein (1-4 μg) and unlabelled InsP3 in a final volume of 500 μl. After a 5-min incubation, during which equilibrium was attained, reactions were terminated by addition of 500 μl of TEM containing 30% poly(ethylene glycol) 8000 and γ-globulin (750 μg) followed by centrifugation (20,000 g, 5 min). For 3H-InsP3 binding to IBC, the amount of 3H-InsP3 was reduced to 0.25 nM, and incubation volumes were doubled. Pellets were solubilized in 200 μl of TEM containing 2% Triton-X 100 (v/v), mixed with EcoScintA scintillation liquid (National Diagnostics) and radioactivity was determined by liquid scintillation counting. Non-specific binding was determined in the presence of 10 μM InsP3. Binding results were fitted to a Hill equation (GraphPad Prism, version 5) from which pIC50 (-logIC50, where IC50 is the half-maximal inhibitory concentration) and thereby pKD (-logKD) values were calculated32.
3H-ryanodine binding
Microsomal membranes were prepared from DT40 cells by lysis with a glass homogenizer and sonication in cytosol-like medium (CLM) supplemented with protease inhibitors (Roche complete protease inhibitor cocktail), followed by centrifugation (50,000 g, 30 min). CLM had the following composition: 140 mM KCl, 20 mM NaCl, 1 mM EGTA, 20 mM Pipes, 2 mM MgCl2, 375 μM CaCl2 (free [Ca2+] ~ 220 nM), pH 7. Equilibrium-competition binding was performed with microsomal membranes (100 μg protein/ml) at 4°C in 200 μl of CLM supplemented with protease inhibitors and 3H-ryanodine (100 nM, Perkin-Elmer Life Sciences). Reactions were terminated after 90 min, and radioactivity was determined as described for 3H-InsP3 binding. Non-specific binding was defined by addition of 10 μM unlabelled ryanodine.
3H-ryanodine binding to RyR typically requires many hours to reach equilibrium24 because it binds only to the open state of the channel and spontaneous openings are rare. In our analyses of 3H-ryanodine binding to InsP3R1-RyR1 (Fig. 4g) equilibrium was attained within 90 min, perhaps because the modestly increased spontaneous activity of the chimeric channel (Supplementary Fig. 10b) contributed to an increased rate of 3H-ryanodine binding to the open state. In parallel comparisons, specific binding of 3H-ryanodine to InsP3R1-RyR1 expressed in DT40 cells and stimulated with InsP3 (1 μM) was (d.p.m.; mean [range] for 2 independent experiments): 4241 [4073-4409] after 90 min, 4941 [4825-5058] after 3 h, and 4410 [4108-4712] after 14 h. It is, however, important to note that our conclusion that InsP3 selectively stimulates 3H-ryanodine binding to InsP3R1-RyR1 is not dependent on having measured binding under equilibrium conditions.
Crystallization and data collection
Crystals of apo-NTCysless were grown by the hanging-drop vapour diffusion method at 293 K by mixing 1 μl of protein with an equal volume of reservoir solution (0.1 M Hepes, pH 7.0, 0.8-1.0 M (NH4)2SO4). Using an additives screen, 3% (v/v) trimethylamine N-oxide was identified as an important additive to obtain single rod-shaped crystals. After a series of microseeding trials, rod-shaped single crystals were obtained within 5 days. For crystallization of InsP3-bound NTCysless, five molar excess of InsP3 (~1 mM) was added before crystallization. Crystals of InsP3-bound NTCysless were grown using the same method except for the reservoir solution containing 0.1 M Na citrate (pH 6.0), 8% (w/v) PEG-6000, 70 mM Li2SO4 and 3% dimethyl sulfoxide.
For data collection, crystals were equilibrated in 25% glycerol cryo-protective solutions containing reservoir buffer, and flash frozen in liquid nitrogen. Diffraction data were collected at 100K on 19-ID beam line for apo-state crystals or 19-BM beam line for InsP3-bound crystals at the Advanced Photon Source Synchrotron facility (Argonne, IL), and were processed with HKL2000. Crystals of apo-NTCysless belong to the space group P1 with cell dimension a=63.1 Å, b=77.2 Å, c=101.5 Å, α=105.4°, β=100.0°, γ=101.0°. Crystals of InsP3-bound NTCysless belong to the space group C2 with cell dimension a=189.2 Å, b=78.7 Å, c=134.1 Å, α=90.0°, β=124.5°, γ=90.0°. Crystals of both apo- and InsP3-bound NTCysless contained two molecules in the asymmetric unit (Supplementary Table 3).
Structure determination and refinement
Structures of apo-NTCysless at 3.0 Å resolution and of InsP3-bound NTCysless at 3.6 Å resolution were determined by molecular replacement using structures of the SD (PDB code: 1XZZ)3 and the IBC (PDB code: 1N4K)2 as search models with the program Phaser29. Iterative refinement and model building were performed with Refmac533 and Coot34, respectively (Supplementary Table 3). Structures of the two molecules in the asymmetric unit of apo-NTCysless are virtually identical (rmsd value = 0.543 Å) except for a minor variation in the loop between β20 and β21 which does not affect the interpretation of our results. The low rmsd between chain A and chain B is maintained through the regions of the molecule which make up interface-α and interface-β, thus increasing the validity of our description of the ‘open-clam’ structure. The two molecules in the asymmetric unit of InsP3-bound NTCysless are more converged than those of the apo-structure (rmsd value = 0.134 Å), which also validates our description of the ‘closed-clam’ structure. The molecule of chain A for each state was used to generate figures, but the chain B molecule of apo-NTCysless was used for the side chain of D444 in Fig. 1c. All water molecules were modeled in Coot34. Initially, water molecules were detected using the automatic “find waters” function in the program. A 2F0-Fc map was used with a sigma cut-off value of 1.0, and minimum and maximum distances to protein atoms of 2.4 and 3.2 Å, respectively. We subsequently picked additional water molecules and deleted inappropriate water molecules by manually surveying the density in Coot. After refinement, all water molecules exhibiting negative electron density due to inconsistent modeling were deleted.
Circular dichroism (CD) analysis
CD spectra were collected on a Jasco J-720 spectrometer using a 1-mm path length cuvette at 20 °C. The NT and NTCysless (0.2 mg/ml) were prepared in a buffer (20 mM Tris-HCl, pH 8.4, 360 mM NaCl, 2.5% glycerol, 0.2 mM TCEP, 1 mM PMSF). CD spectra were obtained from 260 to 200 nm, with a 2-nm bandwidth, an 8-s response time, and scan speed of 50 nm/min. Data are averages of three consecutive scans.
Cloning and functional expression of chimeric InsP3R
To generate the plasmid encoding a chimeric InsP3R1 in which residues 2274-2748 of InsP3R1 (all residues downstream of those immediately before TMD1) were replaced by the equivalent region from RyR1 (residues 4511-5037) (InsP3R1-RyR1), the appropriate region of the ORF of rabbit RyR1 (GenBank: X15209)35 was amplified by PCR from the expression vector pcDNA3.2 using the following primers: forward, 5′-CGCGGGTTCGAAGTCCCCGAGGCCCCACCAGAACCCCCC-3′, and reverse 5′-CGGGGCGTCCTCGAGTCATTAGCTCAGCTGGTCCTCGTACTGCTTGCGGAAGC-3′. The PCR product was cloned in-frame as a BstBI/XhoI fragment into a pENTR1a vector containing nucleotides 1-6822 of rat InsP3R1. This construct was transferred into the Gateway-compatible expression vector, pcDNA3.2, to generate pcDNA3.2-(InsP3R1-RyR1). A plasmid encoding InsP3R lacking the SD was generated from ORFs for the full-length InsP3R1 lacking the S1 splice site (pENTR1A(InsP3R1)) and the IBC (pENTR1A(IBC)). Both plasmids were digested with NheI and KpnI, and the fragment from pENTR1A(IBC) was cloned into pENTR1A(InsP3R1). Site-directed mutagenesis was then used to silence 3 internal BamHI sites within this construct without affecting the coding sequence to generate the plasmid pENTR1A(InsP3R1ΔSD). A plasmid encoding a chimera in which the SD of InsP3R1 (residues 1-224) was replaced by the A-domain of RyR1 (residues 1-210) (RyR1A-InsP3R) was prepared by isolating the coding sequence for the A-domain of RyR1 by PCR from the the rabbit RyR1 ORF using the following primers: forward, 5′-GCTAGCATCATGGGTGACGGAGGA-3′ and reverse 5′-GGATCCTTCACAGCAGGAGCAGATG-3′. The PCR product was cloned as a NheI/BamHI fragment into pENTR1A(InsP3R1ΔSD). The complete coding sequences of all plasmids were verified by sequencing. Domain boundaries of the chimeric proteins are summarized in Supplementary Table 1.
DT40-KO cells were transfected by electroporation with linearized plasmids (10 μg DNA/106 cells) using the Neon (Invitrogen, Paisley, UK) or Amaxa (Lonza, Slough, UK) nucleofection systems. G418 (2 mg/ml) was used to select and amplify clones of G418-resistant cells. Stable cell lines were selected and InsP3R expression was measured by Western blotting. DT40 cells were cultured in RPMI 1640 medium with l-glutamine (Invitrogen) supplemented with 10% foetal bovine serum, 1% heat-inactivated chicken serum (both from Sigma) and 10 μM 2-mercaptoethanol at 37°C in humidified air containing 5% CO2. Cells (~2 ×106 cells/ml) were passaged every 2-3 days. Similar methods were used for transient transfections with RyR1 and InsP3R1CyslessNT, but with 50 μg DNA/3 × 106 cells.
Functional analyses of InsP3R in DT40 cells
Uptake of Ca2+ into the intracellular stores of saponin-permeabilized DT40 cells and its release by InsP3 were measured using a low-affinity Ca2+ indicator (Mag-fluo-4) trapped within the endoplasmic reticulum36. All experiments were performed at 20°C in CLM supplemented with 1.5 mM MgATP to allow active Ca2+ uptake. After the intracellular stores had loaded to steady-state with Ca2+ (~150 s), InsP3 was added with thapsigargin (1 μM) to prevent further Ca2+ uptake (Supplementary Fig. 9). The effects of InsP3 were assessed after a further 10-40 s. InsP3-evoked Ca2+ release is expressed as a fraction of the ATP-dependent Ca2+ uptake. Typical experiments are shown in Supplementary Figure 9.
Structural model of RyR2A-IBC
A structural homology model of RyR2A-IBC (Fig. 4c) was produced using UCSF Chimera37 to first superpose the backbone structures of apo-NTCysless and the ABC of RyR1 (PDB, 2XOA), the only RyR subtype for which there is a complete N-terminal structure9. This A-domain structure of RyR1 was then used to allow superposition of the A-domain from RyR2 (PDB, 3IM5)12, effectively achieving superposition of NTCysless onto a ‘virtual’ chimera of RyR2A with RyR1BC. The predicted structure of the RyR2A-IBC was then revealed by masking the SD of InsP3R1 and the BC domains of RyR1 (Fig. 4c).
Computational docking
Rigid-body docking of the apo-NTCysless structure into a ~10 Å cryo-EM density map of InsP3R110 was implemented using the six-dimensional search procedure in the Situs Program package38. The Laplacian filter was applied to the density maps to enhance the fitting contrast. Docking of the crystal structure of RyR1-ABC (PDB code: 2XOA) into a 9.6-Å cryo-EM density map (EMDB code: EMDB-1275)10, as previously described9, was repeated using the above procedure. The UCSF Chimera package37 was used to visualize the docking results with the density maps (Fig. 3 and Supplementary Fig. 6).
Footnotes
Full Methods and associated references are available in the online version of the paper.
Supplementary Information is linked to the online version of the paper.
Author Contributions M.S., N.I., P.B.S., M.I. and C.L. determined and analysed the structure of NT. S.V. prepared and characterized the full-length InsP3R and chimeras. A.M.R., S.A.K. and P.D. completed analyses of InsP3 binding and related molecular biology. J.B.A., M.I. and C.W.T. supervised work in their respective laboratories, coordinated the project and, with input from other authors, wrote the paper.
References
- 1.Serysheva I, editor. Structure and function of calcium release channels. Academic Press; San Diego: [Google Scholar]
- 2.Bosanac I, Alattia J-R, Mal TK, Chan J, Talarico S, Tong FK, Tong KI, Yoshikawa F, Furuichi T, Iwai M, Michikawa T, Mikoshiba K, Ikura M. Structure of the inositol 1,4,5-trisphosphate receptor binding core in complex with its ligand. Nature. 2002;420:696–700. doi: 10.1038/nature01268. [DOI] [PubMed] [Google Scholar]
- 3.Bosanac I, Yamazaki H, Matsu-ura T, Michikawa M, Mikoshiba K, Ikura M. Crystal structure of the ligand binding suppressor domain of type 1 inositol 1,4,5-trisphosphate receptor. Mol. Cell. 2005;17:193–203. doi: 10.1016/j.molcel.2004.11.047. [DOI] [PubMed] [Google Scholar]
- 4.Rossi AM, Riley AM, Tovey SC, Rahman T, Dellis O, Taylor EJA, Veresov VG, Potter BVL, Taylor CW. Synthetic partial agonists reveal key steps in IP3 receptor activation. Nat. Chem. Biol. 2009;5:631–639. doi: 10.1038/nchembio.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Uchida K, Miyauchi H, Furuichi T, Michikawa T, Mikoshiba K. Critical regions for activation gating of the inositol 1,4,5-trisphosphate receptor. J. Biol. Chem. 2003;278:16551–16560. doi: 10.1074/jbc.M300646200. [DOI] [PubMed] [Google Scholar]
- 6.Schug ZT, Joseph SK. The role of the S4-S5 linker and C-terminal tail in inositol 1,4,5-trisphosphate receptor function. J. Biol. Chem. 2006;281:24431–24440. doi: 10.1074/jbc.M604190200. [DOI] [PubMed] [Google Scholar]
- 7.Chan J, Yamazaki H, Ishiyama N, Seo MD, Mal TK, Michikawa T, Mikoshiba K, Ikura M. Structural studies of inositol 1,4,5-trisphosphate receptor: coupling ligand binding to channel gating. J. Biol. Chem. 2010;285:36092–36099. doi: 10.1074/jbc.M110.140160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamazaki H, Chan J, Ikura M, Michikawa T, Mikoshiba K. Tyr-167/Trp-168 in type1/3 inositol 1,4,5-trisphosphate receptor mediates functional coupling between ligand binding and channel opening. J. Biol. Chem. 2010;285:36081–36091. doi: 10.1074/jbc.M110.140129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tung CC, Lobo PA, Kimlicka L, Van Petegem F. The amino-terminal disease hotspot of ryanodine receptors forms a cytoplasmic vestibule. Nature. 2010;585:585–588. doi: 10.1038/nature09471. [DOI] [PubMed] [Google Scholar]
- 10.Ludtke SJ, Tran TP, Ngo QT, Moiseenkova-Bell VY, Chiu W, Serysheva II. Flexible architecture of IP3R1 by cryo-EM. Structure. 2011;19:1192–1199. doi: 10.1016/j.str.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amador FJ, Liu S, Ishiyama N, Plevin MJ, Wilson A, Maclennan DH, Ikura M. Crystal structure of type I ryanodine receptor amino-terminal β-trefoil domain reveals a disease-associated mutation “hot spot” loop. Proc. Natl. Acad. Sci. USA. 2009;106:11040–11044. doi: 10.1073/pnas.0905186106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lobo PA, Van Petegem F. Crystal structures of the N-terminal domains of cardiac and skeletal muscle ryanodine receptors: insights into disease mutations. Structure. 2009;17:1505–1514. doi: 10.1016/j.str.2009.08.016. [DOI] [PubMed] [Google Scholar]
- 13.Lin CC, Baek K, Lu Z. Apo and InsP3-bound crystal structures of the ligand-binding domain of an InsP3 receptor. Nat. Struct. Mol. Biol. 2011;18:1172–1174. doi: 10.1038/nsmb.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taylor CW, Tovey SC. IP3 receptors: toward understanding their activation. Cold Spring Harb. Perspect. Biol. 2010;2:a004010. doi: 10.1101/cshperspect.a004010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anyatonwu G, Joseph SK. Surface accessibility and conformational changes in the N-terminal domain of type I inositol trisphosphate receptors: studies using cysteine substitution mutagenesis. J. Biol. Chem. 2009;284:8093–8102. doi: 10.1074/jbc.M806932200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tateishi H, Yano M, Mochizuki M, Suetomi T, Ono M, Xu X, Uchinoumi H, Okuda S, Oda T, Kobayashi S, Yamamoto T, Ikeda Y, Ohkusa T, Ikemoto N, Matsuzaki M. Defective domain-domain interactions within the ryanodine receptor as a critical cause of diastolic Ca2+ leak in failing hearts. Cardiovasc. Res. 2009;81:536–545. doi: 10.1093/cvr/cvn303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sureshan KM, Riley AM, Rossi AM, Tovey SC, Dedos SG, Taylor CW, Potter BVL. Activation of IP3 receptors by synthetic bisphosphate ligands. Chem. Commun. 2009;14:1204–1206. doi: 10.1039/b819328b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chan J, Whitten AE, Jeffries CM, Bosanac I, Mal TK, Ito J, Porumb H, Michikawa T, Mikoshiba K, Trewhella J, Ikura M. Ligand-induced conformational changes via flexible linkers in the amino-terminal region of the inositol 1,4,5-trisphosphate receptor. J. Mol. Biol. 2007;373:1269–1280. doi: 10.1016/j.jmb.2007.08.057. [DOI] [PubMed] [Google Scholar]
- 19.Mayer ML. Glutamate receptors at atomic resolution. Nature. 2006;440:456–462. doi: 10.1038/nature04709. [DOI] [PubMed] [Google Scholar]
- 20.Hamada T, Bannister ML, Ikemoto N. Peptide probe study of the role of interaction between the cytoplasmic and transmembrane domains of the ryanodine receptor in the channel regulation mechanism. Biochemistry. 2007;46:4272–4279. doi: 10.1021/bi061557f. [DOI] [PubMed] [Google Scholar]
- 21.Ramos-Franco J, Galvan D, Mignery GA, Fill M. Location of the permeation pathway in the recombinant type-1 inositol 1,4,5-trisphosphate receptor. J. Gen. Physiol. 1999;114:243–250. doi: 10.1085/jgp.114.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Samso M, Feng W, Pessah IN, Allen PD. Coordinated movement of cytoplasmic and transmembrane domains of RyR1 upon gating. PLoS Biol. 2009;7:e85. doi: 10.1371/journal.pbio.1000085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iwai M, Michikawa T, Bosanac I, Ikura M, Mikoshiba K. Molecular basis of the isoform-specific ligand-binding affinity of inositol 1,4,5-trisphosphate receptors. J. Biol. Chem. 2007;282:12755–12764. doi: 10.1074/jbc.M609833200. [DOI] [PubMed] [Google Scholar]
- 24.Chu A, Diaz-Munoz M, Hawkes MJ, Brush K, Hamilton SL. Ryanodine as a probe for the functional state of the skeletal muscle sarcoplasmic reticulum calcium release channel. Mol. Pharmacol. 1990;37:735–741. [PubMed] [Google Scholar]
- 25.Lai FA, Meissner G. The muscle ryanodine receptor and its intrinsic Ca2+ channel activity. J. Bioenerg. Biomembr. 1989;21:227–246. doi: 10.1007/BF00812070. [DOI] [PubMed] [Google Scholar]
- 26.Liu Z, Wang R, Tian X, Zhong X, Gangopadhyay J, Cole R, Ikemoto N, Chen SR, Wagenknecht T. Dynamic, inter-subunit interactions between the N-terminal and central mutation regions of cardiac ryanodine receptor. J. Cell Sci. 2010;123:1775–1784. doi: 10.1242/jcs.064071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.George CH, Jundi H, Thomas NL, Scoote M, Walters N, Williams AJ, Lai FA. Ryanodine receptor regulation by intramolecular interactions between cytoplasmic and transmembrane domains. Mol. Biol. Cell. 2004;15:2627–2638. doi: 10.1091/mbc.E03-09-0688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Otwinowski Z, Minor W. Processing X-ray diffraction data collected in oscillation mode. Methods Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 29.McCoy AJ, Grosse-Kunstleve RW, Adams PD, Winn MD, Storoni LC, Read RJ. Phaser crystallographic software. J. Appl. Crystallogr. 2007;40:658–674. doi: 10.1107/S0021889807021206. [DOI] [PMC free article] [PubMed] [Google Scholar]
References
- 30.Otsu K, Willard HF, Khanna VK, Zorzato F, Green NM, MacLennan DH. Molecular cloning of cDNA encoding the Ca2+ release channel (ryanodine receptor) of rabbit cardiac muscle sarcoplasmic reticulum. J. Biol. Chem. 1990;265:13472–13483. [PubMed] [Google Scholar]
- 31.Tovey SC, Dedos SG, Rahman T, Taylor EJA, Pantazaka E, Taylor CW. Regulation of inositol 1,4,5-trisphosphate receptors by cAMP independent of cAMP-dependent protein kinase. J. Biol. Chem. 2010;285:12979–12989. doi: 10.1074/jbc.M109.096016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kenakin TP. Pharmacologic analysis of drug-receptor interactions. 3rd ed Lippincott, Williams and Wilkins; New York: 1997. [Google Scholar]
- 33.Murshudov GN, Vagin AA, Dodson EJ. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr D Biol Crystallogr. 1997;53:240–255. doi: 10.1107/S0907444996012255. [DOI] [PubMed] [Google Scholar]
- 34.Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 35.Zorzato F, Fujii J, Otsu K, Phillips M, Green NM, Lai FA, Meissner G, MacLennan DH. Molecular cloning of cDNA encoding human and rabbit forms of the Ca2+ release channel (ryanodine receptor) of skeletal muscle sarcoplasmic reticulum. J. Biol. Chem. 1990;265:2244–2256. [PubMed] [Google Scholar]
- 36.Tovey SC, Sun Y, Taylor CW. Rapid functional assays of intracellular Ca2+ channels. Nat. Protocol. 2006;1:259–263. doi: 10.1038/nprot.2006.40. [DOI] [PubMed] [Google Scholar]
- 37.Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE. UCSF Chimera - a visualization system for exploratory research and analysis. J. Comput. Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 38.Wriggers W, Milligan RA, McCammon JA. Situs: a package for docking crystal structures into low-resolution maps from electron microscopy. J. Struct. Biol. 1999;125:185–195. doi: 10.1006/jsbi.1998.4080. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.