Abstract
Apical membrane antigen 1 (AMA1) is one of the leading candidate antigens for inclusion in a subunit vaccine against blood-stage malaria. However, the efficacy of antibody-inducing recombinant AMA1 protein vaccines in Phase IIa/b clinical trials to date remains disappointing. Here we describe the development of recombinant human adenovirus serotype 5 (AdHu5) and modified vaccinia virus Ankara (MVA) vectors encoding AMA1 from the Plasmodium chabaudi chabaudi strain AS (PccAS). These vectors, when used in a heterologous prime-boost regime in BALB/c mice, are capable of inducing strong transgene-specific humoral as well as cellular immune responses. We show that this vaccination regime is protective against a non-lethal PccAS blood-stage challenge, resulting in reduced peak parasitemias. The role of vaccine-induced, AMA1-specific antibodies and T cells in mediating anti-parasite effect was investigated by in vivo depletion of CD4+ T cells and adoptive transfer studies into naive and immunodeficient mice. Depletion of CD4+ T cells led to a loss of vaccine-induced protection. Adoptive transfer studies confirmed that efficacy is mediated by both CD4+ T cells and antibodies functioning in the context of an intact immune system. Unlike previous studies, these results confirm that antigen-specific CD4+ T cells, induced by a clinically relevant vaccine delivery platform, can make a significant contribution to vaccine blood-stage efficacy in the P. chabaudi model. Given cell-mediated immunity may also contribute to parasite control in human malaria, these data support the clinical development of viral vectored vaccines that induce both T cell and antibodies against P. falciparum blood-stage malaria antigens like AMA1.
INTRODUCTION
Malaria remains a significant burden on global public health, despite some recent and encouraging successes with regard to malaria control in certain parts of Africa. The development of a highly effective vaccine, which could help in the control and eventual elimination of this disease, thus remains an important goal (1). Vaccines against the asexual blood-stage of malaria infection have often aimed to reproduce natural immunity, and have generally targeted antigens associated with the merozoite surface or located in the apical organelles (2). AMA1 appears on the surface of merozoites after its release from the micronemes where it is a target of growth inhibitory antibodies in in vitro assays and in vivo malaria models. It is also frequently associated with naturally-acquired immunity, and has thus classically been one of the leading candidate antigens for inclusion in a subunit vaccine against the asexual blood-stage of the parasite (3). In an attempt to induce such protective antibodies, malaria vaccine developers have classically focused on recombinant protein-in-adjuvant vaccines. Typically these require multiple immunizations in animal models in order to induce antibody responses of a protective magnitude (4), and clinical trials of the efficacy of such candidate vaccines remain disappointing (2).
An increasingly recognized alternative to protein/adjuvant formulations is the use of viral vectored vaccines (5, 6), and in particular replication-defective adenoviruses of both human and simian serotype, as well as poxviruses, such as modified vaccinia virus Ankara (MVA). These vectors can express relatively large antigen constructs and when deployed in an adenovirus-MVA heterologous prime-boost regime, have been shown in mice and non-human primates to induce high levels of antigen-specific CD8+ and CD4+ T cell responses as well as high titers of antibody (7-12). The chimpanzee adenovirus ChAd63 and MVA have both displayed an excellent safety profile for use in humans as prophylactic vaccines (13, 14), and the recombinant adenovirus-MVA vaccine delivery platform has shown high-level efficacy against all three malaria life-cycle stages in pre-clinical models (9, 15-17).
There have been numerous studies suggesting that the induction of cellular immunity in conjunction with antibody responses may increase the efficacy of vaccines targeting classical blood-stage antigens, leading to recent calls for such an approach to be tested clinically (18). Studies using both sporozoite and infected red blood cell inoculation to immunize human volunteers have associated cellular immune responses against blood-stage parasites with protective outcome (19, 20). Similarly, three studies in the P. yoelii mouse model have associated CD8+ T cell responses against blood-stage parasites or the merozoite surface protein 1 (MSP1) antigen with protective immunity against pre-erythrocytic liver-stage infection (7, 21, 22), and a more recent study has even associated such CD8+ T cells with protective blood-stage immunity (23). Other studies with P. yoelii have shown that the adoptive transfer of CD4+ T cell lines, against antigens such as MSP133, into naïve mice can lead to the control of blood-stage parasitemia (24), although not all vaccinated or T cell-transfused recipients survive despite a significant reduction in parasite densities. We have also previously reported that an AdHu5-MVA vaccination regime against the P. yoelii MSP142 antigen can induce strong antigen-specific cellular and humoral immune responses. In this case, the CD8+ T cells against MSP133 provided partial efficacy against developing liver-stage parasites, however, depletion of either the CD8+ or CD4+ T cell subsets prior to blood-stage challenge of immunized mice had no effect on protective outcome (7, 9).
The PccAS parasite has provided an alternative and highly informative model for the study of blood-stage malaria immunity, in which both antibodies and CD4+ T cells (acting as helper cells for B cells, as well as through antibody-independent mechanisms) have been reported to be important (25). In the case of infection with this parasite, immunologically competent mice generate a protective immune response that involves both T helper-type 1 (Th1) and Th2-type CD4+ T cells (26), with the importance for such cells demonstrated in numerous depletion and adoptive transfer studies in immunodeficient mice (27). During the acute stage of infection, a Th1-type antibody-independent response has been shown to control initial peak parasitemia, with this effect mediated by production of pro-inflammatory cytokines and effector molecules such as IFN-γ and TNFα (27). Once parasitemia has been brought under control, a Th2-type CD4+ T cell response appears to predominate. These cells have been shown to produce IL-4, IL-5, IL-6 and IL-10 and to also provide essential help for antibody production by B cells (28, 29). B cell depletion studies have shown that these cells per se are not required for early control of parasitemia, but are important for the resolution of the chronic phase of infection and the clearance of the parasite (30).
In agreement with these data, immunization studies with recombinant AMA1 in the P. chabaudi adami model have also shown an important contribution of CD4+ T cells acting independently of antibodies in vaccine-induced protection (31). In this study, a significant decrease in acute anti-parasite immunity was seen if CD4+ T cells were depleted from immunized normal or B cell knockout mice prior to blood-stage challenge. Similarly, athymic (nu/nu) mice transfused with a CD4+ T cell line raised against a conserved cryptic epitope in AMA1 showed 50% survival following P. chabaudi adami challenge, and accelerated rates of de novo antibody production against AMA1 in some mice (32). Comparable observations were made in a similar study following adoptive transfer of transgenic CD4+ T cells that recognized a H-2 I-Ed epitope within P. chabaudi MSP133 (33). A more recent study has shown that low doses of killed blood-stage PccAS parasites, administered in aluminium hydroxide adjuvant plus the TLR9 agonist CpG oligodeoxynucleotide, can induce high-level cross-strain and even some cross-species immunity that is dependent on CD4+ T cells, IFN-γ and nitric oxide (34). However none of these studies using recombinant AMA1 protein formulated in Montanide ISA720 adjuvant, transferred CD4+ T cell lines or transgenic T cells, or a whole parasite blood product administered with CpG, represent vaccine formulations that are easily translatable with demonstrated adequate safety profiles for human use (35, 36).
Here we characterize the immunogenicity and protective efficacy of a prime-boost vaccination regime with viral vectored vaccines targeting the PccAS AMA1 antigen, and investigate the mechanisms of vaccine-induced protection following challenge with P. chabaudi blood-stage parasites. These vectors induce AMA1-specific T cell and antibody responses, and provide a system in which to dissect the protective contribution of both the cellular and humoral arms of the immune system. Depletion and adoptive transfer studies demonstrate an important contribution of both vaccine-induced CD4+ T cells and antibodies to protective immunity. These data, generated with a vaccine platform that is highly suited for human use (13, 14), support the further clinical translation of this approach. Given evidence that cell-mediated immunity may also contribute to parasite control in human malaria, the development of viral vectored vaccines targeting antigens such as P. falciparum AMA1, which induce strong cellular immune responses in conjunction with antibodies, may stand a greater chance of success in protecting humans against blood-stage parasites in comparison to vaccination strategies aiming to induce antibodies alone.
MATERIALS AND METHODS
Generation of viral vectors expressing AMA1
The PccF forward primer (5′-ATG AAA GAA ATA TAT TAT ATT GTA ATT TTG TGC-3′) and PccR reverse primer (5′-TTA ATA GTA TGG TTT TTC CAT CAA AAC TG-3′) were used to amplify the antigen from genomic PccAS DNA by PCR, prior to ligation into a plasmid and sequencing. The genomic DNA was obtained from Dr Jean Langhorne, NIMR, London, UK. DNA sequencing of these amplified regions was then completed to obtain the amino acid sequence for PccAS AMA1. The sequencing was done at the Wellcome Trust Centre for Human Genetics, Oxford University, UK and MWG Biotech (Ebensburg, Germany). The sequence of PccAS AMA1 differed from that of P. chabaudi adami (strain DS) by 34 amino acids (Figure S1). The vaccine construct encoded the ectodomain of P. chabaudi chabaudi strain AS AMA1 (PcAMA1) (amino acid (αα) 25-546), and included 4 αα substitutions to remove potential sites of N-linked glycosylation [(originalαα-position>newαα) S-136>A, T-191>A, S-233>A, S-251>A). The native parasite N-terminal signal sequence, transmembrane domain and the C-terminal cytoplasmic domain were not included. The construct was codon optimized for expression in mice and synthesized by GeneArt (Regensburg, Germany). The tissue plasminogen activator (tPA) leader sequence replaced the native AMA1 signal sequence as previously described (12). This construct was used to make recombinant AdHu5 and MVA by methods that have been previously described (9). The vector control vaccines were AdHu5 with the transgene promoter and polyA tail, but no antigenic insert, and MVA expressing GFP (37). AdHu5 was chosen as a model adenovirus without intellectual property restrictions that is suitable for pre-clinical vaccine work. The immunogenicity and efficacy of AdHu5 vaccines can be readily matched by clinically-applicable simian adenoviruses: we have recently shown that the immunogenicity of AdHu5 and various simian adenoviruses is comparable in animal studies (11, 12, 15, 38), and that ChAd63 viruses are highly immunogenic and safe in both non-human primate studies (8) and in human clinical trials (13, 14).
Animals and immunizations
Female BALB/c (H-2d) mice 5-6 weeks old (John Radcliffe Hospital, Oxford, UK or Harlan, UK) were used as stated in all experiments. RAG 1/2 knockout mice were a kind gift from Dr Nicholas Jones (Nuffield Department of Surgery, University of Oxford, UK). The nude mice were obtained from Harlan, UK. All procedures were performed according to the terms of the UK Animals (Scientific Procedures) Act Project License (PPL 30/2414) and Personal License (PIL 30/7792) and were approved by the University of Oxford Animal Care and Ethical Review Committee. The vaccines were diluted in endotoxin-free Dulbecco’s PBS prior to immunization. Vaccines were administered i.d. bilaterally into the ears in a total volume of 50μl for both recombinant adenovirus and MVA. The doses of recombinant adenovirus vaccines used were 5 × 1010 viral particles (vp), 1 × 1010 vp or 1 × 109 vp and the dose of recombinant MVA vaccine used was always 1 × 107 plaque forming units (pfu). The interval between the prime immunization with AdHu5-PcAMA1 and boosting with MVA-PcAMA1 was 8 weeks in all experiments.
Total IgG ELISA
The antibodies induced by vaccination were measured by ELISA as previously described (9). Briefly, recombinant PcAMA1 protein was adsorbed to 96 well Nunc-Immuno Maxisorp plates at a concentration of 5μg/ml in PBS. PcAMA1 protein was a kind gift from Dr Jean Langhorne (NIMR, London, UK). The PccAS parasite lysate preparation is described below and was coated at a concentration of 2μg/ml in PBS. After blocking, the serum was incubated for 2h and the bound antibodies were detected using alkaline phosphatase-conjugated goat anti-mouse IgG (whole molecule) (Sigma Aldrich, UK) diluted 1:5000. Serum antibody endpoint titers were taken as the x-axis intercept of the dilution curve at an absorbance value 3x standard deviations greater than the OD405 for naïve mouse serum (typical cut-off OD405 for positive sera = 0.15). Serum from naïve mice were pooled and used as controls for all the ELISAs and were always below the level of detection.
Multiparameter flow cytometry
Cytokine secretion by peripheral blood mononuclear cells (PBMC) and splenocytes were assayed by intracellular cytokine staining (ICS). PBMC and splenocytes were prepared and the ICS assay performed as previously described (7). Briefly, 100μl aliquots of cells were added to a 96-well U-bottom plate. 50μl of 1μg/ml Brefeldin A (GolgiPlug, BD Biosciences) and 50μl of peptide diluted in complete medium were added to the test wells. Individual or pooled peptides (Peptide Protein Research Ltd, UK) are described in Tables 1 and 2 and were added at a final concentration of 1μg/ml. In the control unstimulated wells, GolgiPlug and medium alone were added. Cells were incubated at 37°C 5% CO2 for 5h. After incubation the cells were left at 4°C overnight. The next day cells were surface stained for 30min at 4°C with PerCP-Cy5.5-conjugated anti-mouse CD8α (clone 53-6.7) and Pacific blue (PB)-labelled anti-mouse CD4 (clone LT34) diluted in PBS with 0.1% BSA at a dilution individually assessed by titration for each antibody. Cells were then permeabilized in 100μl 1x Cytofix/Cytoperm solution (BD Biosciences) for 20min at 4°C and then intracellularly stained for 30min at 4°C with APC-conjugated anti-mouse IFN-γ (clone XMG1.2), FITC-conjugated anti-mouse TNFα (clone 145-2C11) and PE-conjugated anti-mouse IL-2 (clone JES6-5H4). After staining, cells were resuspended in 200μl PBS containing 1% formalin. Samples were analyzed using a LSRII Flow Cytometer (BD Biosciences) and FlowJo v8.8 (Tree Star Inc, USA). The Boolean gate platform was used with individual gates to create response combinations. Pestle v1.6 and SPICE v5.0 software (39) were used to analyze the T cell response profiles. Background responses in unstimulated control cells were subtracted from the peptide stimulated response.
Table 1. PccAS AMA1 peptides tested by ICS.
The table shows the six peptide pools tested by ICS, and the αα sequence of each peptide. The far right column indicates if the peptide pools contained published CD4+ T cell epitopes (32) or predicted CD8+ T cell epitopes (using the epitope prediction section of the SYFPEITH website http://www.syfpeithi.de).
| POOL | PEPTIDE # | αα SEQUENCE | αα POSITION | CD4 or CD8 |
|---|---|---|---|---|
| POOL 1 | P7 | LINPWEKFMEKYDIE | 51-65 | CD4 |
| P8 | EKFMEKYDIEKVHGS | 56-70 | ||
| P9 | KYDIEKVHGSGIRVD | 61-75 | ||
| P10 | KVHGSGIRVDLGEDA | 66-80 | ||
| P11 | GIRVDLGEDARVENQ | 71-85 | ||
| P12 | LGEDARVENQDYRIP | 76-90 | ||
| P13 | RVENQDYRIPSGKCP | 81-95 | ||
| P14 | DYRIPSGKCPVMGKG | 86-100 | ||
| P15 | SGKCPVMGKGITIQN | 91-105 | ||
| P16 | VMGKGITIQNSKVSF | 96-110 | ||
| POOL 2 | P25 | ANLKLMYKDHKEILA | 141-155 | CD8 |
| P26 | MYKDHKEILALNDMS | 146-160 | ||
| POOL 3 | P29 | LCAKHASFYVPGTNV | 161-175 | CD8 |
| P30 | ASFYVPGTNVNTAYR | 166-180 | ||
| POOL 4 | P74 | SFPCDIYKKKIAEEI | 386-400 | CD8 |
| P75 | IYKKKIAEEIKVMNV | 391-405 | ||
| POOL 5 | P79 | GNGTIQFPRIFISDD | 411-425 | CD4 |
| P80 | QFPRIFISDDKESLK | 416-430 | ||
| POOL 6 | P85 | SSCNFFVCNCVEKRQ | 441-455 | CD4 |
| P86 | FVCNCVEKRQFISEN | 446-460 |
Table 2. Comparison of the peptide sequences containing the CD4+ T cell epitopes identified in AMA1 across various strains of P. chabaudi.
The table shows the sequence comparison across various P. chabaudi strains for the two CD4+ T cell epitopes identified in PccAS AMA1. The P16 and P79 peptides represent the αα sequence in the PccAS AMA1 vaccine. P16B and P79B represent peptides encoding the variant sequences identified in the heterologous strains. The αα shown in bold lettering differ between the homolgous and heterologous strains.
| PEPTIDE | PARASITE STRAIN | SEQUENCE | VACCINE STRAIN HOMOLOGY |
|---|---|---|---|
| P16 | P. chabaudi chabaudi AS | VMGKGITIQNSKVSF | Yes |
| P16 | P. chabaudi adami 556/DK | VMGKGITIQNSKVSF | Yes |
| P16 | P. chabaudi adami CB | VMGKGITIQNSKVSF | Yes |
| P16B | P. chabaudi adami DS | VMGKGITIQKSTKSF | No |
| P79 | P. chabaudi chabaudi AS | GNGTIQFPRIFISDD | Yes |
| P79B | P. chabaudi adami 556/DK | GNDTIKFPRIFISDD | No |
| P79B | P. chabaudi adami CB | GNDTIKFPRIFISDD | No |
| P79B | P. chabaudi adami DS | GNDTIKFPRIFISDD | No |
Parasites and blood-stage challenge
PccAS parasites were kindly provided by Dr Jean Langhorne (NIMR, London, UK). Five days prior to challenge, a frozen aliquot of PccAS-infected blood was thawed and injected intraperitoneally (i.p.) into a donor BALB/c mouse. On the day of challenge, the number of RBCs per μl of blood was counted (40) and the % parasitemia was measured by counting a Giemsa-stained thin-blood smear. This was used to calculate the number of parasitized red blood cells (pRBCs) per μl of blood. Blood from the donor mouse was diluted accordingly in KGS (PBS with 0.2% glucose) supplemented with 10% naïve mouse serum (Sigma-Aldrich) to give 1×107 pRBCs/ml, unless otherwise stated. Mice were infected with 100μl of this solution (1×106 pRBCs) intravenously (i.v.) via the tail vein. From day 4 post-challenge, the parasitemia was monitored daily by Giemsa-stained thin-blood smear and the health status was monitored twice daily during acute parasitemia. PccAS non-lethal infection in naïve wild-type BALB/c mice reaches a maximum parasitemia of 50-60% and is then controlled. P. chabaudi admai strain DS and strain DK parasites were kindly provided by Dr Alison Creasey (Institute of Immunology and Infection Research, Edinburgh University, UK). Challenges were performed as described for PccAS.
Blood-Stage PccAS Parasite Lysate
Mice were infected with PccAS-infected pRBC and the parasitemia was monitored until it reached ~20-30%. The mice were then sacrificed and bled by cardiac puncture into 10x heparin (final heparin concentration 30U/ml blood) and the blood was pooled and stored on ice. The blood was centrifuged at 2500 rpm for 5 min and the supernatant was removed carefully using a pipette. The pellet was then resuspended in 5ml of PBS and mixed by inverting the tube 5-6 times. A Plasmodipur filter (Euro-Diagnostika) was prepared with PBS and the sample was passed through the filter using a 20ml syringe. The original tube was washed with PBS and filtered twice. The cells were washed after filtration with 10ml of PBS and resuspended in 8ml PBS + 0.06% saponin, mixed and incubated at room temperature for 10 min. After the incubation the sample was centrifuged at 2500 rpm for 5 min and the pellet was washed twice in 5ml of PBS and resuspended in 1ml PBS. This was then freeze-thawed three times to lyse the cells, and centrifuged at 2500 rpm for 5 min before the supernatant was transferred to another tube. This was centrifuged at 12000rpm for 30 min to remove any remaining insoluble material. The protein concentration of the lysate was measured using a Nanodrop.
In vivo CD4+ T cell depletion
CD4+ T cells were depleted using anti-CD4 GK1.5 (rat IgG2a) monoclonal antibody (mAb) as previously described (7). The mAb was purified using protein G affinity chromatography from hybridoma culture supernatants. Mice were injected i.p. with 200μg of mAb diluted in PBS on days -2, -1 and 0 (with respect to challenge on day 0) and the control mice were given normal rat polyclonal IgG (Sigma Aldrich, UK) purified in the same way. The degree of in vivo CD4+ T cell depletion was assessed by flow cytometry in the PBMC of representative depleted and undepleted control mice, and was shown to be >98%. A different anti-CD4 mAb clone (LT34) was used to stain CD4, in order to confirm that the CD4+ T cells were depleted (rather than the staining mAb binding being blocked by GK1.5) (Figure S2).
Adoptive transfer of CD4+ T cells
Splenocytes were prepared from immunized mice (as described in the text) and CD4+ T cells were isolated using the CD4+ T Cell Isolation Kit (MACS; Miltenyi Biotec) by depletion of non CD4+ T cells (negative selection). Briefly, splenocytes were resuspended in 40ml of MACS buffer and the cells were counted using a CASY counter (Schärfe Systems, Reutlingen, Germany). Cells were then centrifuged at 300 xg for 10min and 40μl of MACS buffer per 1×107 cells were added. 10μl per 1×107 cells of “Biotin conjugated antibody cocktail” containing biotin-conjugated mAb against CD8α (Ly-2), CD45R (B220), DX5, CD11b (Mac-1) and Ter-119 were added to this, mixed and incubated for 10min at 4°C. After the incubation 30μl of MACS buffer and 20μl of Anti-Biotin Microbeads per 1×107 cells were added and incubated for 15min at 4°C. Cells were then washed with buffer by adding 10x the labelling volume and centrifuged at 300 xg for 10 min. The supernatant was discarded and the cell pellet was resuspended in 500μl of buffer per 1×108 total cells.
Magnetic separation of the cells was performed using a MACS separator and LS column (MACS; Miltenyi Biotec). Columns were attached to the magnets in the MACS separator, and prepared by rinsing with 3ml of MACS buffer. The cell suspension was added to the column in units of 10ml and allowed to pass through. The effluent was collected which contained the unlabelled cells, i.e. the CD4+ T cell enriched fraction. The columns were then washed four times with 3ml of MACS buffer and the flow through collected into the same tube as the effluent. The cells were then spun down, resuspended in PBS and the cell count was determined. After counting the cells, they were centrifuged at 300 xg for 5min and the pellet was resuspended in a suitable volume of PBS for injection. 9 × 107 CD4+ T cells were injected i.v. in a 200μl volume per mouse two days before pRBC challenge.
Statistical analysis
Data were analyzed for statistical significance using GraphPad Prism v5 for Windows (GraphPad Software, San Diego, CA). The normality of the data set was determined using the Kolmogorov-Smirnov one-sample test. For non-parametric data a Mann-Whitney U test was used to compare two groups. Parametric data were compared either using t-test (for comparing two groups) or one-way ANOVA (for more than two groups). A post-hoc Dunnet’s correction was used to compare test groups to one control group, whilst a Bonferroni correction was used when all the groups were compared to each other. ELISA titers were logarithmically (log10) transformed in order to normalize the data and enable a more stringent parametric analysis. The area under the curve (AUC) was calculated to analyze the challenge data and the different groups were compared by ANOVA. P ≤ 0.05 was considered significant in all cases (*P ≤ 0.05, ** P ≤ 0.01 and ***P ≤ 0.001).
RESULTS
P. chabaudi chabaudi AS AMA1 sequence and vaccine design
The complete coding sequence of PccAS AMA1 was not available, so primers were designed against conserved regions by comparing sequences from related strains (41) and the sequence was determined (GenBank accession number: JF690775) (Figure S1). A vaccine construct (termed PcAMA1) was subsequently designed that contained the ectodomain of PccAS AMA1 with four potential sites of N-linked glycosylation removed (described in Methods). The construct was codon optimized for expression in mice, synthesized and then used to generate recombinant AdHu5 and MVA vectors as described in Methods.
Antibody responses induced by AdHu5-MVA PcAMA1 prime-boost vaccination
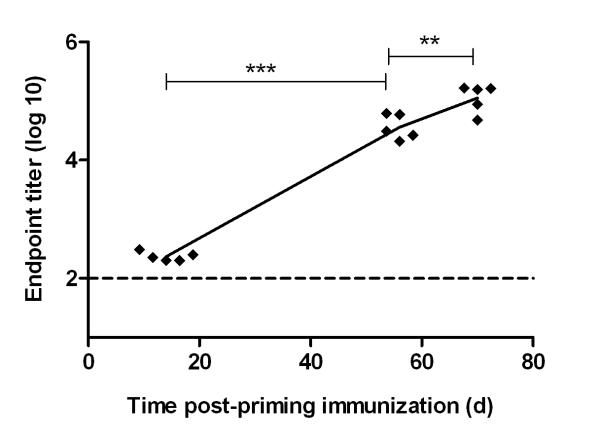
BALB/c mice were immunized intradermally (i.d.) with 5 × 1010 viral particles (vp) of AdHu5-PcAMA1 and boosted eight weeks later with 1 × 107 pfu of MVA-PcAMA1 (i.d.). This 8 week interval has been shown to be important for both antibody and T cell responses when using these two vectors in a prime-boost vaccination regime (9, 42). Serum was obtained two weeks post-prime (day 14), pre-boost (day 55) and two weeks post-boost (day 70). The total IgG response against the ectodomain of PccAS AMA1 was measured by ELISA. In agreement with other murine data for the AdHu5 vector, there were detectable AMA1-specific IgG responses two weeks after the AdHu5 priming immunization, and these increased over time to become significantly higher at day 55. MVA administration on day 56 boosted the antibody level significantly, as measured on day 70 (Figure 1).
Figure 1. Antibody responses induced by AdHu5-MVA PcAMA1 prime-boost vaccination.
BALB/c mice (n=5) were primed with 5 × 1010 vp of AdHu5-PcAMA1 and boosted 8 weeks later with 1 × 107 pfu of MVA-PcAMA1. The AMA1-specific total IgG response against recombinant PcAMA1 ectodomain was measured by ELISA in the serum at days 14 (post-prime), 55 (pre-boost) and 70 (post-boost). Each point indicates the response from an individual mouse and the line links the mean. Significant differences between the different time-points by paired t test are shown (** P ≤ 0.01 and *** P ≤ 0.001).
T cell responses induced by AdHu5-MVA PcAMA1 prime-boost vaccination
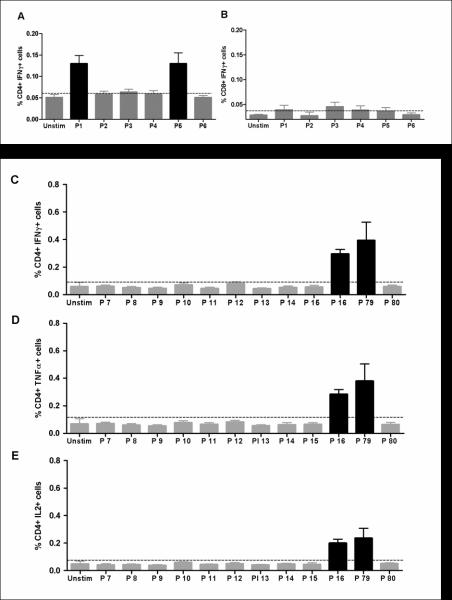
A previous study mapped both immunodominant and cryptic epitopes within P. chabaudi adami AMA1 following immunization of BALB/c mice with recombinant antigen (32). The T cell responses induced against PccAS AMA1 by AdHu5-MVA immunization were measured here by ICS following re-stimulation of splenocytes with peptides corresponding to the previously reported CD4+ T cell epitopes, as well as predicted CD8+ T cell epitopes. Six peptide pools containing 15 mer peptides overlapping by 10 αα were tested. The peptides were divided into pools according to whether they were reported to contain CD4+ T cell epitopes (32) or predicted CD8+ T cell epitopes, and also according to the position of the peptide within the antigen (Table 1). Pool 1 contained 10 peptides (P7 to P16) which included the known immunodominant CD4+ T cell epitope. Pool 2 (P25 and P26), Pool 3 (P29 and P30) and Pool 4 (P74 and P75) each contained two peptides and were predicted to be H-2d CD8+ T cell epitopes from known H-2 class I binding motifs. Pool 5 (P79 and P80) and Pool 6 (P84 and P85) also contained two peptides each, which were published to be CD4+ T cell immunodominant epitopes. Splenocytes from BALB/c mice, harvested two weeks after AdHu5-MVA PcAMA1 immunization (day 70), were re-stimulated with the peptide pools for five hours, surface stained for CD4 and CD8 and then intracellularly stained for IFN-γ, TNFα and IL-2. Pools 1 and 5 were identified as containing peptides that were recognized by CD4+ T cells leading to the production of IFN-γ (Figure 2A). Similarly, TNFα+ and IL-2+ CD4+ T cell responses were also measured in cells re-stimulated with Pool 1 and Pool 5 (data not shown). There were no CD8+ IFN-γ+ T cell responses detected to any of the peptide pools tested (Figure 2B). The individual epitopes within the responding peptide pools were then identified in a repeat experiment. This showed that the CD4+ IFN-γ+ T cell responses in BALB/c mice against the ectodomain of PccAS AMA1 were against epitopes present within two peptides – P16 (within Pool 1) and P79 (within Pool 5) (Figure 2C-E). These cells produced all three cytokines assayed but the levels of IFN-γ (Figure 2C) and TNFα secreting cells (Figure 2D) tended to be slightly higher than the levels of cells secreting IL-2 (Figure 2E).
Figure 2. Identification of splenic T cell responses to AMA1 following AdHu5-MVA PcAMA1 immunization.
Mice were immunized with the same regimen as Figure 1. Two weeks post-boost (day 70), splenocytes were isolated and re-stimulated with pools of over-lapping peptides (P1 to P6, see Table 1) or no peptide (Unstim). Cells were surface stained for CD4 and CD8, and intracellularly stained for IFN-γ and assessed by flow cytometry to determine the % of responding cells per total (A) CD4+ or (B) CD8+ T cell subset. The bars represent the mean response ± SEM (n=5). In a repeat experiment, splenocytes were re-stimulated with individual peptides from Pool 1 (P7 to P16), Pool 5 (P79 and P80) or no peptide (Unstim). Cells were surface stained for CD4 and intracellularly stained for (C) IFN-γ, (D) TNFα and (E) IL-2, and assessed by flow cytometry to determine % of responding cells per total CD4+ T cell subset. The bars represent the mean response ± SEM (n=5).
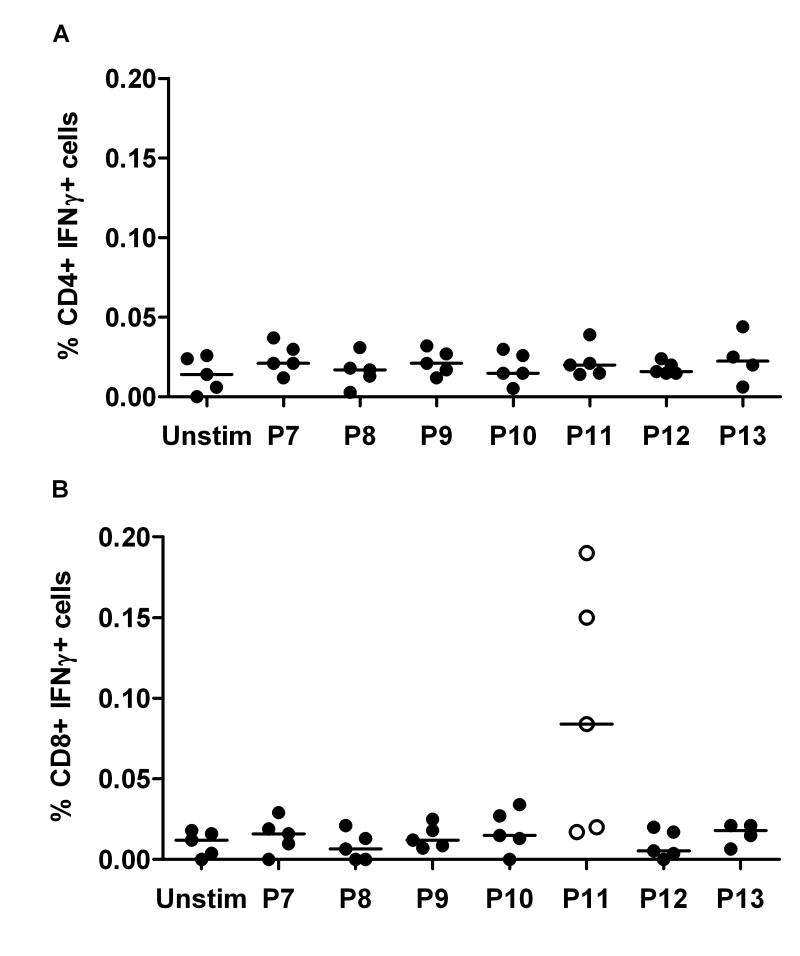
Two CD4+ T cell epitopes had now been identified, and these were in agreement with those reported to be induced following immunization with recombinant AMA1 protein in adjuvant (32). In this study using a viral vectored vaccination regime, it remained possible that T cell responses to other epitopes were induced in addition to those identified after protein immunization. In order to confirm whether any other T cell responses were induced against the AMA1 antigen, 15 mer peptides overlapping by 10 αα were synthesized that covered the entire ectodomain of PccAS AMA1 contained within the vaccine insert (Table S1). Splenocytes, isolated as before, were subsequently re-stimulated with seven overlapping peptide pools (10 peptides in each pool) or no peptide (Unstim) and assayed by ICS. The CD4+ T cell epitopes P16 and P79 identified in the previous set of experiments (Figure 2C) were included as positive controls. There were no further CD4+ IFN-γ+ T cell responses identified in any of the peptide pools (Figure 3A), but a very weak CD8+ IFN-γ+ T cell response was measured in three out of five mice in Pool 11 (Figure 3B).
Figure 3. T cell epitope mapping within the entire ectodomain of PccAS AMA1.
Mice were immunized with the same reigmen as Figure 1. Two weeks post-boost (day 70), splenocytes were re-stimulated for 5h with seven overlapping peptide pools containing 10 peptides each (Pool 7 to Pool 13, see Table S1) or no peptide (Unstim). The cells were then surface stained for CD4 or CD8 and intracellularly for IFN-γ. The figure shows the % of (A) CD4+ IFN-γ+ cells or (B) CD8+ IFN-γ+ cells. Responses from individual mice are shown and the lines represent the median.
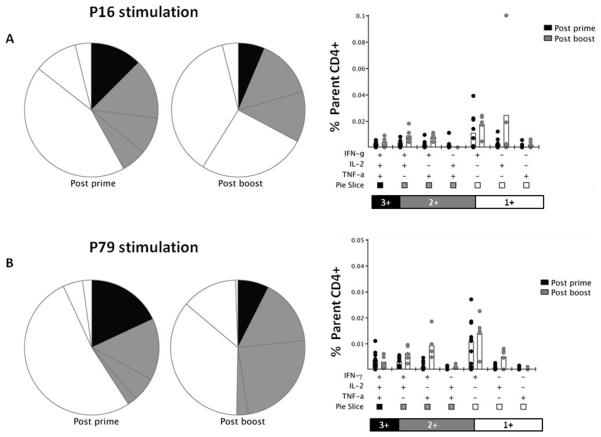
The functionality of the P16 and P79-specific splenic CD4+ T cells was also analyzed after the AdHu5-PcAMA1 prime (day 14) and the MVA-PcAMA1 boost (day 70) (Figure 4). “Triple positive cells” were defined as cells which simultaneously produce the three cytokines assayed (IFN-γ, TNFα, and IL-2) (43), “double positive cells” simultaneously produce any two of the three cytokines, and “single positive cells” are those which produce only one cytokine. For the P16 epitope, the number of triple positive cells producing IFN-γ, TNFα and IL-2 did not significantly change after the boost (Figure 4A). However, there was a significant increase (~2 to 5 fold) in the number of double positive cells producing IFN-γ and IL-2 or IFN-γ and TNFα (P = 0.02 and P = 0.004, respectively, Mann Whitney test). Cells producing only TNFα were not detectable, and there was no significant increase in the number of cells producing only IFN-γ or IL-2 after the MVA boost. For the P79 epitope, the functionality profile of the CD4+ T cells was highly similar to that of P16 (Figure 4B). Overall, these data showed an increased frequency of CD4+ T cells producing IFN-γ and IL-2 or IFN-γ and TNFα after the booster immunization, in conjunction with an increase in the overall magnitude of the antigen-specific response.
Figure 4. Multifunctionality of P16 and P79 specific CD4+ T cells.
Mice were immunized with the same regimen as described in Figure 1. Two weeks post-prime with AdHu5-PcAMA1 (day 14) and two weeks post-boost with MVA-PcAMA1 (day 70), splenocytes were re-stimulated with the P16 and P79 peptide or no peptide (Unstim) for 5h. Cells were surface stained for CD4 and intracellularly stained for IFN-γ, TNFα and IL-2, and assessed by flow cytometry to determine the % of responding cells per total CD4+ T cell subset. Toal responses of each cytokine to P16 (A) and P79 (B) are shown at each time-point. The multifunctionality or “quality” of CD4+ T cells was analyzed using the SPICE software.
Protective efficacy of AdHu5-MVA PcAMA1 vaccination against blood-stage challenge
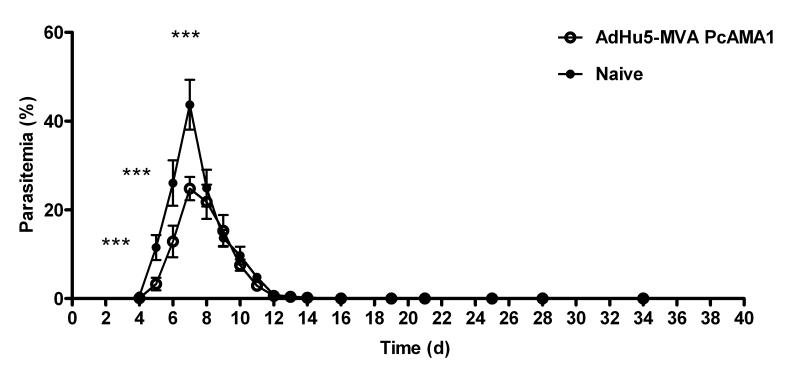
The protective efficacy of the AdHu5-MVA PcAMA1 prime boost vaccination regime was next assessed by blood-stage challenge with PccAS parasites. Immunized mice were challenged two weeks post-boost (d70) i.v. with 105 PccAS pRBC. Naïve unimmunized control BALB/c mice were challenged at the same time. Parasitemia was monitored from day 4 post-challenge by Giemsa-stained thin blood smears and results are expressed as the % infected RBCs (Figure 5). Given PccAS infection in naïve BALB/c mice is non-lethal, protective efficacy was assessed by parasitemia comparison between the groups. Significantly lower mean parasitemia was observed in the AdHu5-MVA PcAMA1 vaccinated mice in comparison to the naïve controls from the time of patency (day 4) until day 7. At the time of peak parasitemia (day 7), the vaccinated mice had significantly lower levels of infection (geomean ± SD = 24.6 ± 2.6% versus 43.4 ± 5.6 % in the naïve controls, P = 0.002). These data confirmed that the vaccine could lead to significant control of peak parasitemia in these mice, although the rate of parasite clearance following this was comparable between the vaccinees and controls. It was confirmed in a repeat experiment that this control of peak parasitemia occurs following antigen specific immunization. The same result was observed following challenge of mice immunized with non-recombinant / control AdHu5 and MVA vaccines (Supplemental Figure 3A).
Figure 5. Efficacy of AdHu5-MVA PcAMA1 immunization against blood-stage challenge.
BALB/c mice (n=6) were vaccinated with 5 × 1010 vp of AdHu5-PcAMA1 and boosted 8 weeks later with 1 × 107 pfu of MVA-PcAMA1. Two weeks after the MVA boost (day 70), these mice and six naïve unvaccinated controls were challenged i.v. with 105 non-lethal PccAS pRBCs. Parasitemia was monitored daily from day 4 by Giemsa-stained thin-blood smears and results are expressed as the % infected RBCs. The difference in parasitemia between the two groups was taken as the measure of vaccine efficacy. The mean percentage parasitemia ± SEM is shown. Significant differences by Mann-Whitney test (*** P ≤ 0.001) are indicated.
In vivo depletion of CD4+ T cells prior to challenge results in a loss of vaccine-induced protection
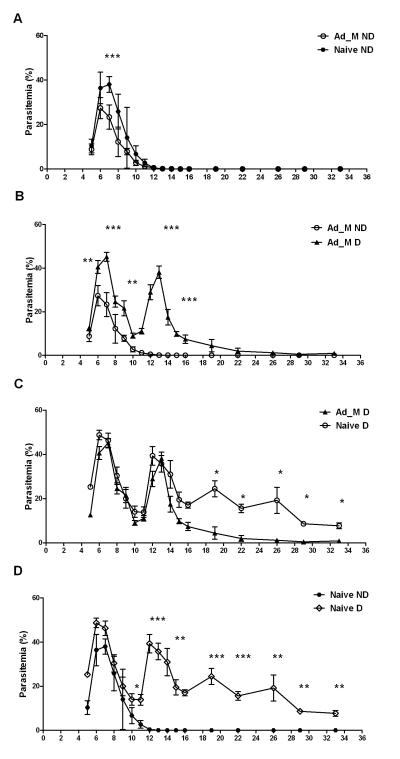
Previous work analyzing protective immune mechanisms in the P. chabaudi adami model has demonstrated a role for antibodies either induced by active immunization with recombinant AMA1 or passively transferred from the sera of immunized rabbits (41, 44), and for CD4+ T cells acting independently of or in concert with AMA1-specific antibodies (31, 32). The contribution of CD4+ T cells in vaccine-induced protection in this PccAS model was thus initially assessed by in vivo CD4+ T cell depletion prior to blood-stage challenge. The anti-mouse CD4 GK1.5 (rat IgG2a) mAb has been reported to deplete CD4+ T cells for up to 10 days, after which the CD4+ T cells are replenished to about 50% of the original level by 4 weeks (45). Two groups of mice were immunized as before with the AdHu5-MVA PcAMA1 vaccine regime. CD4+ T cells were depleted in one group of the vaccinated mice, as well as in one group of naïve controls, prior to challenge i.v. with 106 PccAS pRBCs. The undepleted vaccinated and naïve mice were administered the same amount of normal rat polyclonal IgG. In the case of this experiment, a similar degree of protective efficacy was observed for the AdHu5-MVA PcAMA1 vaccination regime in undepleted mice, even though the challenge dose was increased to 106 pRBC (Figure 6A) as compared to a challenge with 105 pRBC in the previous experiment (Figure 5). Mean peak parasitemia was again significantly reduced in the vaccinated mice in comparison to the naïve controls at days 6 and 7 post-challenge (Figure 6A). However, CD4+ T cell depletion resulted in the loss of vaccine efficacy in immunized mice, as seen by increased peak parasitemias on days 6-7 that were comparable to undepleted naïve controls (Figure 6B), thus confirming an important contributory role for vaccine-induced CD4+ T cells to protection in this model. Interestingly, the vaccinated depleted mice also failed to clear the parasites as early as the non-depleted mice, and a second wave of parasitemia was observed that peaked on day 13. An almost identical second wave was also observed in the naïve depleted animals, and overall they fared the worst, demonstrating a chronic patent parasitemia 30 days post-infection (Figure 6C). Also, in agreement with a previous report (31), the naïve CD4+ T cell depleted mice suffered the highest peak parasitemias on days 6-7 (Figure 6D).
Figure 6. Effect of in vivo CD4+ T cell depletion on vaccine efficacy.
Two groups of BALB/c mice (n=6/group) were vaccinated with 5 × 1010 vp of AdHu5-PcAMA1 and boosted 8 weeks later with 1 × 107 pfu of MVA-PcAMA1. These mice and two further groups of naïve controls were challenged i.v. 14 days post-boost with 106 pRBC. One group each of vaccinated and naïve mice were depleted of CD4+ T cells as described in Methods. Parasitemia was monitored daily by Giemsa-stained thin-blood smears and results are expressed as the % infected RBCs (mean ± SEM). The difference in parasitemia between the groups was taken as the measure of vaccine efficacy. (A) Vaccinated non-depleted (Ad_M ND) verus naïve non-depleted (Naive ND), (B) vaccinated depleted (Ad_M D) and non-depleted (Ad_M ND), (C) vaccinated depleted (Ad_M D) and naïve depleted (Naive D), and (D) naïve depleted (Naive D) and non-depleted (Naive ND). Significant differences in the mean % parasitemias between the different groups are indicated (Mann-Whitney test * P ≤ 0.05, ** P ≤ 0.01, and *** P ≤ 0.001).
Role of PcAMA1-specific and whole parasite antibodies in clearance
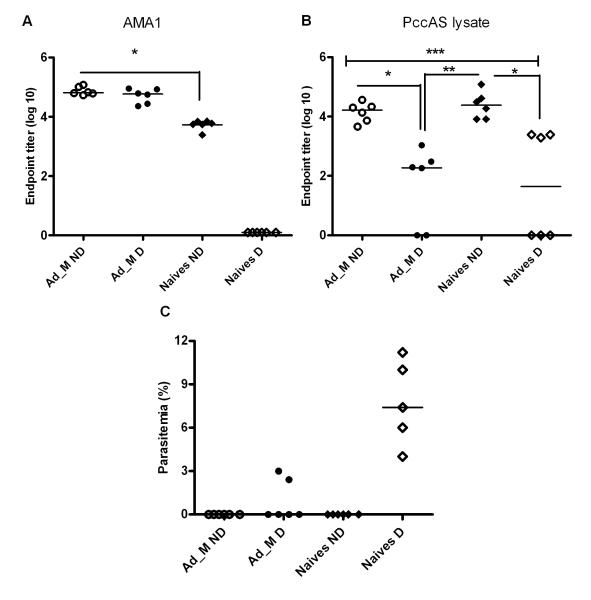
Unlike control of peak parasitemia, parasite clearance in the P. chabaudi model has been attributed to B cells and antibodies (27, 46). The faster rate of parasite clearance observed in the depleted vaccinated mice in comparison to naïve depleted controls (Figure 6C), could thus be due to the presence of vaccine-induced AMA1-specific antibodies in the vaccinated mice that are able to control and clear parasites in the later stage of infection. Total IgG serum antibody titers, against recombinant PcAMA1 protein and whole parasite lysate, were therefore measured by ELISA one-month post-challenge (Figure 7A). In agreement with the previously reported protein vaccine study (31), there was no difference in PcAMA1 specific antibody titers in the vaccinated depleted versus non-depleted mice. A de novo PcAMA1-specific antibody response was detected in the non-CD4+ T cell depleted naïve control mice, but the titers were significantly lower than those seen in the vaccinated mice. There was no detectable PcAMA1-specific antibody response in the naïve-depleted mice, suggesting that CD4+ T cell help is necessary for de novo PcAMA1 antibody production from B cells during infection.
Figure 7. PcAMA1- and parasite-specific antibody responses post-challenge.
Total IgG serum antibody levels in the mice shown in Figure 6 were analyzed by ELISA one month post-challenge (day 33). Individual responses plus the median are shown for (A) PccAS AMA1 ectodomain and (B) whole PccAS parasite lysate. Significant differences in antibody levels between the different groups are indicated (ANOVA * P ≤ 0.05, ** P ≤ 0.01**, and *** P ≤ 0.001). (C) The corresponding individual and median % parasitemias in the different groups on day 33 is shown.
Interestingly, the response to the whole PccAS parasite lysate was similar in the non-depleted vaccinated and naïve groups, whilst there was a significantly lower antibody response to the whole parasite post-challenge in the CD4+ T cell depleted groups as compared to the two non-depleted groups (Figure 7B). Given the naïve CD4+ T cell depleted mice were still parasitemic one month post-challenge (Figure 7C), it is interesting to note that these mice possessed no PcAMA1-specific antibodies but similar parasite lysate specific antibody levels when compared to the vaccinated depleted mice (who cleared the infection faster). However, both the vaccinated and the naïve non-depleted mice cleared their parasites at the same time (Figure 6A), even though the naïve mice possessed significantly lower levels of PcAMA1-specific antibodies (Figure 7A). Taken all together, these data suggest that both PcAMA1-specific antibodies and antibodies to other parasite antigens are able to contribute to parasite clearance.
Adoptive transfer of CD4+ T cells from vaccinated mice to naïve and immunocompromised mice
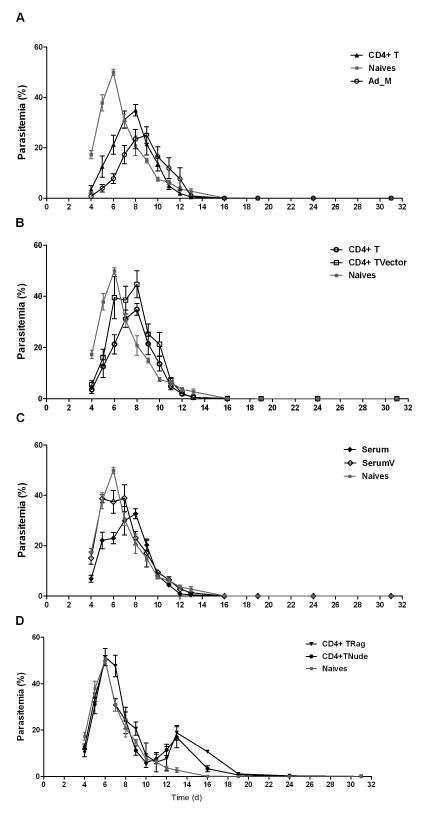
In order to further assess the contribution of vaccine-induced CD4+ T cells and antibodies to the initial control of blood-stage parasite densities, a number of adoptive transfer studies were undertaken. BALB/c mice were immunized exactly as before and, two weeks after the MVA boost, the spleen and serum were harvested from each mouse. Mice were either immunized with AdHu5-MVA vectors encoding PcAMA1, or with an identical regime using vector controls expressing no recombinant antigen (Ad_M C). These vector controls did not express PcAMA1, thus enabling an assessment of the protective role of PcAMA1-specific CD4+ T cells and to confirm that any protective effect observed was not due to viral vector immunization per se. Splenic CD4+ T cells from the vaccinated mice were isolated by negative selection (as described in Methods) and these or pooled sera were transferred into naïve BALB/c mice (Figure 8A-C). All mice were subsequently challenged with 106 PccAS pRBC. In agreement with the previous result (Figure 6A), there was a significantly lower parasitemia (area under the curve (AUC) analysis, P = 0.008) in the AdHu5-MVA PcAMA1 vaccinated control group in comparison to naïve controls (Figure 8A). This effect was also mirrored in the naïve wild-type mice that received the CD4+ T cell transfer from vaccinated mice (P = 0.008, AUC versus naïve controls). Moreover, although the mice which received the CD4+ T cells from the Ad_M C immunized controls showed transiently lower parasitemias than the naïve controls on days 4 and 5, there was subsequently no significant difference in the AUC analysis (P = 0.69), unlike that observed in the mice receiving CD4+ T cells from PcAMA1 immunized mice (Figure 8A,B). These data confirm that vaccine-induced PcAMA1-specific CD4+ T cells are an important contributing factor to the control of initial peak parasitemia in this model.
Figure 8. Adoptive transfer of CD4+ T cells and serum from vaccinated mice to normal and immunocompromised mice.
BALB/c mice were immunized as before with AdHu5-MVA PcAMA1. Two weeks after the MVA boost, spleens were harvested, pooled and the CD4+ T cells were isolated as described in Methods. 9 × 107 CD4+ T cells from vaccinated mice were injected i.v. into naïve wild-type mice (CD4+ T), RAG1/2−/− mice (CD4+ TRag), or BALB/c athymic nude (nu/nu) mice (CD4+ TNude). CD4+ T cells from vector control immunized mice were also isolated and transferred into naïve wild-type mice (CD4+ TVector) in the same manner. Serum was also harvested from each vaccinated and vector control mouse, pooled, and then transferred into two groups of naïve wild-type mice (labelled as Serum and SerumV respectively). 500μl of serum was injected i.p. on days -1 and 0 (total 1ml into each mouse), with respect to challenge on day 0. A group of naïve wild-type mice and vaccinated (Ad_M) control mice were also included. n=5 mice/group except for the serum transfer groups where n=4/group. All mice were then challenged with 106 PccAS pRBC and montiored as previously. In the CD4+ TRag group, 3 mice died (one on each of days 8, 9 and 11 denoted by +) and in the CD4+ TNude group one mouse died (day 10 denoted by ++). Panels (A-D) show comparisons between the various groups and the mean group % parasitemia ± sem over time.
Interestingly, serum transfers into normal naïve BALB/c mice from AMA1 or control vaccinated mice showed a similar result to the CD4+ T cell transfer (Figure 8C). The mice receiving serum from mice vaccinated with PcAMA1 had significantly lower initial parasite densities in comparison to naïve controls (AUC, P = 0.01). The serum transfer from vector control immunized mice led to no difference in parasitemia as compared to the naïve mice (AUC not calculated because one of the mice died on day 9 post challenge). Taken all together, these data suggest that both PcAMA1-specific CD4+ T cells and antibodies can contribute to the control of initial parasitemia in immunocompetent recipients.
CD4+ T cell transfer studies were also performed into athymic nude mice (BALB/c nu/nu) which lack T cells, and RAG1/2 knockout mice (BALB/c RAG1/2−/−) which lack T and B cells. Previous studies have shown that nude mice infected with PccAS develop a chronic parasitemia and show a high mortality rate (33, 47), with a similarly poor outcome in RAG2−/− knockout mice, many of which succumb within the first 15 days of infection (31, 33). In comparison to the previous reports for the lethal challenge of nude mice that were not reconstituted (31, 33), the transfer of the CD4+ T cells from PcAMA1 immunized mice was sufficient to control the initial peak parasitemias, albeit at a higher level than that seen when naïve normal mice were recipients (Figure 8D). A similar result was obtained in the RAG1/2− knockouts, and both immunocompromised strains showed comparable parasite densities up to day 11 followed by a second smaller wave of parasitemia peaking on day 13 – an observation previously attributed to sub-optimal transfer of CD4+ T cell numbers (47). However, only 2/5 RAG1/2 knockout mice ultimately survived in comparison to 4/5 nude mice and, in agreement with similar studies using both naïve wild-type or transgenic CD4+ T cells specific for MSP133 (33), the RAG knockout mice that survived did not clear the infection, whereas the surviving nude mice cleared the parasites by day 30. Given that nude mice possess B cells, unlike the RAG knockouts, the trend for better survival and parasite clearance is consistent with reports detailing the importance of B cells with regard to these two outcomes.
T cell response to heterologous peptides from other P. chabaudi strains
Sequence polymorphism has remained a significant obstacle to the development of antibody-inducing AMA1 protein subunit vaccines (3, 48). Antibody responses induced against AMA1 are allele specific and vaccination with one allele does not confer cross-strain protection – an effect clearly shown in assays of P. falciparum in vitro growth inhibition (49), as well as homologous versus heterologous strain challenge studies in mice following P. chabaudi AMA1 protein immunization (41). Having shown a protective contribution of vaccine-induced CD4+ T cells, it was therefore of interest to ascertain whether the induction of cellular immunity by the AdHu5-MVA vaccines could help to improve cross-strain protection.
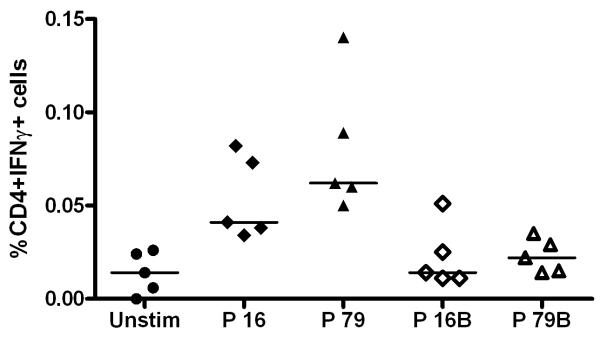
Initially, the αα sequences of the two CD4+ T cell epitopes, P16 and P79, were compared across various parasite strains: P. chabaudi chabaudi AS (vaccine strain), P. chabaudi adami 556/DK, P. chabaudi adami CB and P. chabaudi adami DS. As previously reported for the immunodominant epitopes mapped in P. chabaudi adami DS following AMA1 protein immunization, neither the P16 nor the P79 sequences were highly conserved between these four P. chabaudi strains. P16 was conserved in all but P. chabaudi adami DS, whereas the sequence of P79 was different to the vaccine PccAS strain in all of the other three strains compared (Table 2). The heterologous peptides P16B and P79B, encoding the alternative sequences, were used to re-stimulate splenocytes from immunized mice as described before, and as expected, there was no detectable response to these heterologous peptides (Figure 9). Given the P16 epitope is partially conserved, we sought to ascertain whether the response to this epitope alone (in the absence of strain-specific antibodies) could lead to initial control of parasite densities following heterologous parasite challenge. However, there was no protection observed in this experiment against the heterologous strain P. chabaudi adami DK (Supplemental Figure 3B).
Figure 9. T cell response to heterologous AMA1 peptide sequences from other P. chaubaudi strains.
BALB/c mice (n=5) were primed with 1 × 1010 vp AdHu5-PcAMA1 and boosted 8 weeks later with 1 × 107 pfu MVA-PcAMA1. Two weeks post-boost (day 70), splenocytes were isolated and re-stimulated with the peptides P16 and P79 (as described previously), or with P16B and P79B (heterologous peptides to P16 and P79, respectively), or no peptide (Unstim). Cells were surface stained for CD4 and intracellularly stained for IFN-γ, and then assessed by flow cytometry to determine % of responding cells per total CD4+ T cell subset. The figure shows the individual and median CD4+ IFN-γ+ responses.
DISCUSSION
The AMA1 antigen remains a leading candidate antigen for inclusion in a subunit vaccine against blood-stage P. falciparum malaria. However, despite extensive efforts, candidate protein-in-adjuvant vaccines tested for efficacy in Phase IIa/b trials to date have shown minimal or strain-specific efficacy (48, 50). Here we have characterized in detail the immunogenicity and protective efficacy of a prime-boost vaccination regime utilizing an alternative human-compatible vaccine delivery platform encoding the PccAS AMA1 antigen. The P. chabaudi rodent malaria model remains a key example of how cellular immunity, acting independently of antibody, can significantly contribute to immune control of ascending early-phase blood-stage parasitemia, and these data confirm that viral vectored vaccines are capable of inducing CD4+ T cell responses that can fulfil that role. In order to develop viral vectors encoding PccAS AMA1, the complete sequence of the antigen was obtained from sequencing a PCR fragment amplified from parasite genomic DNA. The entire ectodomain was subsequently included in the viral vaccine transgene insert, given this has been previously shown in this model to induce protective immune responses (31, 41). The AdHu5-PcAMA1 vaccine primed a detectable antibody response that increased over time and which was significantly boosted after the administration of MVA-PcAMA1. These data are in accordance with previous findings in the P. yoelii model using viral vectors expressing MSP142 or the circumsporozoite protein (CSP), where an 8-week interval between prime and boost was shown to induce of high-titer antibodies as well as strong T cell responses (9, 37). The kinetics of the antibody response are similar to those seen following vaccination with AdHu5 expressing other antigens such as Ebola virus glycoprotein (51) or ovalbumin (52), where the antibody response increased by a similar order of magnitude over time and then reached a plateau by six weeks post-vaccination.
Analysis of the T cell responses in the spleen of immunized BALB/c mice, using overlapping peptides spanning the entire length of the PccAS AMA1 antigen, revealed two CD4+ T cell epitopes. These peptides (P16 and P79) corresponded to those that were previously described by Amante et al. after immunization with recombinant AMA1 protein-in-adjuvant. The authors referred to these as immunodominant epitopes, in contrast to cryptic epitopes which only elicit detectable responses in vitro following immunization of mice with the relevant peptide(s) (32). The data generated here using viral vectored vaccines are thus in agreement with their previous observations, with the exception of a very weak CD8+ T cell response that was also detected in some mice to one of the peptide pools tested. Interestingly, unlike previously seen with the P. yoelii MSP133 antigen in BALB/c mice (7, 11, 53), no responses were measured to those peptides predicted to contain CD8+ T cell epitopes by the H-2d class I epitope motif analysis.
Similar to other studies with the AdHu5-MVA prime-boost regime in mice (10, 43), vaccination induced multifunctional T cell responses, whereby cells simultaneously produce various combinations of cytokines (such as IFN-γ, TNFα and IL-2) following re-stimulation in vitro. It has previously been shown that the frequency of CD4+ T cells producing all three of these cytokines associates with protection against Leishmania major in mice (43), but this has not been investigated in the context of a mouse model of blood-stage malaria in which CD4+ T cell responses are known to be critical for protection. In the analysis described here, it was shown that the multi-functional profile of the AMA1-speicifc CD4+ T cells in some cases differed between those cells measured 14 days after the AdHu5 prime, and those measured 14 days after the MVA boost. Because efficacy was only assessed here after AdHu5-MVA PcAMA1 immunization, and not following a single administration of AdHu5-PcAMA1, it is not possible to conclude whether these qualitative differences would translate into altered levels of vaccine efficacy. This assessment would also be confounded by the fact that the overall magnitudes of both the T cell and antibody responses are lower after a single AdHu5 immunization. Few model systems in fact provide the ability to assess the protective consequences of altered T cell “quality” in the context of constant overall quantity. This probably reflects the fact that T cell multi-functionality measured in vitro more likely represents a snapshot of the continuum of T cell phenotypes which occur following antigen exposure in vivo and which are also invariably accompanied by changes in the magnitude of the T cell response. It would nevertheless remain of interest in future studies to attempt to assess whether the so-called “quality” of such CD4+ T cell responses affected efficacy in this blood-stage malaria model.
The AdHu5-MVA PcAMA1 immunization regime described here was able to afford a level of protective efficacy against a PccAS blood-stage challenge that was only slightly less than that observed following immunization with recombinant AMA1 protein formulated in less clinically-relevant adjuvants such as Freund’s or Montanide ISA720 (41, 44). This efficacy was measured as a significant reduction in peak parasitemia between the AMA1 vaccinated and control groups, but a similar rate of clearance occurred thereafter. The early reduction in peak parasitemia in immunocompetent mice has been attributed in the literature to both conformation-dependent anti-AMA1 antibody responses (31, 44) as well as CD4+ T cell responses acting independently of antibodies (31). The evidence here that vaccine-induced AMA1-specific CD4+ T cells are capable of contributing to efficacy comes from the significant loss of this antiparasitic effect in immunized mice following the depletion of CD4+ T cells prior to PccAS pRBC challenge, whilst anti-AMA1-specific antibody titers remained unchanged post-challenge. These data concur with the observations of Xu et al. (31) but contrast our previous studies in the P. yoelii MSP142 model, where CD4+ T cells were shown to provide essential help for priming B cells at the time of vaccination (7), but depletion of CD4+ T cells prior to pRBC challenge failed to alter vaccine efficacy (9). The depletion experiments also suggested that PcAMA1-specific antibodies, as well as those targeting other parasite antigens, are capable of contributing to parasite clearance. Despite similar levels of anti-parasite antibodies, immunized and CD4+ T cell depleted mice were able to clear parasites faster than their naïve depleted counterparts. This enhanced rate of clearance was associated with higher levels of PcAMA1-specific antibodies (induced by prior vaccination). However, in apparent contradiction of this, both the vaccinated and the naïve non-depleted mice cleared their parasites at the same time, even though the naïve mice possessed significantly lower levels of PcAMA1-specific antibodies. These data suggest that both PcAMA1-specific antibodies and antibodies to other parasite antigens can contribute to parasite clearance, and agree with other studies suggesting that the rate of infection resolution correlates with the level of malaria-specific antibodies and the speed of their production (30). The data here suggest that the vaccine-induced anti-PcAMA1 antibodies are capable of contributing to this critical threshold of total antibody that is necessary for parasite clearance, but that antibodies of other antigen specificities are just as effective.
The fact that PcAMA1-specific antibodies were not detected in the naïve CD4+ T cell depleted mice also suggests that CD4+ T cell help is essential for production of PcAMA1-specific antibodies. CD4+ T cell help is also required for the production of antibodies to other parasite antigens, because both the vaccinated and naïve CD4+ T cell depleted mice showed significantly lower antibodies post-challenge to the whole parasite lysate in comparison to non-depleted mice. Previous data showing that CD4+ T cells for a cryptic epitope in PcAMA1 (32) as well as an epitope in PcMSP133 (33), can prime anamnestic antibody responses to their respective antigens following challenge support this important role for CD4+ T cell help for B cells.
Although transfer of immune sera alone into immunocompromised hosts is reported to be insufficient to control initial parasite densities (47), the studies performed here showed that both vaccine-induced CD4+ T cells and antibodies can be adoptively transferred into naïve immunocompetent mice leading to significant control of initial peak parasitemia upon pRBC challenge. These data emphasize the fact that both arms of the immune system can play an important contributory role when functioning within an intact immune system. Although control sera failed to show this effect, there was an apparent intermediate (but here not statistically significant) effect following transfer of CD4+ T cells from vector control immunized mice. These data are, however, in agreement with other studies showing that transfer of naïve CD4+ T cells into immunodeficient mice can have a protective effect, that this is both cell number dependent (47), and in some cases less effective than transfer of parasite specific CD4+ T cells (33). The ability of transferred cells to prolong and enhance the survival of nude mice again supports the importance of CD4+ T cells in mediating early control of parasite density in this model. Reduced potency in nude versus normal mice most likely reflects the overall lower absolute numbers of CD4+ T cells in the reconstituted nude mice. These experiments also did not dissect the contribution of naïve versus PcAMA1-specific CD4+ T cells in the immunocompromised hosts, although it has been already shown that prior activation of such transferred T cell subsets (e.g. by vaccination with antigen following cell transfer but preceding pRBC challenge) may be necessary to tease apart these differences in protective capacity between naïve and antigen-specific T cells (33).
A key rationale for developing such viral vectored vaccines was the possibility of inducing T cell responses to conserved epitopes, which could aid in better induction of cross-strain immunity by AMA1 subunit vaccines (in contrast to the well documented strain-specificity of anti-AMA1 antibody responses) (12, 41, 48). When mice are immunized with recombinant P. chabaudi chabaudi DS AMA1 protein it has been shown that there is no heterologous protection against P. chabaudi adami 556KA (possessing an AMA1 sequence that differs by 36 αα) (41). Unfortunately, of the two CD4+ T cell epitopes identified in this model, both were polymorphic with respect to other strains of P. chabaudi. The failure to detect any T cell responses to the heterologous peptides (P16B and P17B) in PccAS AMA1-immunized mice or protection against challenge with heterologous parasite strains (Supplemental Figure 3B) confirms that even slight variation (two αα) in the epitope sequence has a serious consequence with regard to immune recognition. The challenge data with P. chabaudi adami DK (where only the P16 epitope is conserved) indicate that responses against the P79 epitope alone or combined with those against P16 are important for mediating efficacy (possibly in conjunction with strain-specific antibodies). It is worth noting that following immunization, responses were slightly stronger against P79, and it may be that responses against P16 alone were too weak to confer efficacy against the heterologous parasite. Nevertheless, this work has been recently extended and similar viral vectored vaccines developed that target P. falciparum AMA1, and encode two alleles of AMA1 from the 3D7 and FVO strains. Importantly, following immunization of mice (12), rhesus macaques (9) and humans (14), T cell responses were measured against peptides that were conserved between the two alleles. Thus if cell-mediated immunity contributes to parasite control in P. falciparum malaria in a similar fashion to that observed for P. chabaudi, then the induction of such responses may benefit the induction of better cross-strain immunity by such subunit vaccination strategies.
The data presented here were generated with a vaccine platform that is highly suited for human use, particularly with the substitution of a simian adenovirus for the human adenoviral vector (13, 14). They have important implications for the future development of subunit blood-stage malaria vaccines. If the protective immune mechanisms observed in the P. chabaudi model are also reflected in human immunity to blood-stage malaria, then the development of viral vectored vaccines targeting antigens such as P. falciparum AMA1, which induce strong cellular immune responses in conjunction with antibodies, may stand a greater chance of success in protecting humans against blood-stage parasites in comparison to vaccination strategies aiming to induce antibodies alone.
Supplementary Material
Acknowledgements
We thank Simone de Cassan, Frances Pearson, Anna Goodman, Matthew Dicks, Julie Furze, Drew Worth and the Jenner Institute Vector Core Facility and Flow Cytometry Core Facility (Oxford, UK) for assistance; Asmahan Abdallah, Bill Jarra and Jean Langhorne (NIMR, London, UK) for providing PccAS AMA1 protein and parasites; Alison Creasey (Edinburgh University, UK) for providing the other strains of P. chabaudi parasites from the European Malaria Reference Reagent Repository (www.malariaresearch.eu); and Nicholas Jones (Nuffield Department of Surgery, University of Oxford) for providing the RAG 1/2 knockout mice.
SB was funded by MalParTraining, an FP6-funded Marie Curie Action under contract number MEST-CT-2005-020492. AAH is funded by the UK Medical Research Council (U117532067). This work was also funded in part by EVIMalaR, a European Community FP7-funded programme (Grant agreement No. 242095). SCG, AVSH and SJD are Jenner Investigators. AVSH was supported by a Wellcome Trust Principal Research Fellowship. SJD is a MRC Career Development Fellow (G1000527).
ABBREVIATIONS
- AMA1
Apical membrane antigen 1
- AdHu5
Adenovirus human serotype 5
- MVA
Modified vaccinia virus Ankara
- PccAS
Plasmodium chabaudi chabaudi strain AS
- MSP1
Merozoite surface protein 1
- mAb
Monoclonal antibody
- ICS
Intracellular cytokine staining
- pRBCs
Parasitized red blood cells
Footnotes
Accession Number GenBank accession number: JF690775 (http://www.ncbi.nlm.nih.gov/nuccore/JF690775 )
Competing Interest Statement SJD, EKF, SCG and AVSH are named inventors on patent applications covering malaria vectored vaccines and immunization regimes.
REFERENCES
- 1.Hill AV. Vaccines against malaria. Philosophical transactions of the Royal Society of London. 366:2806–2814. doi: 10.1098/rstb.2011.0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goodman AL, Draper SJ. Blood-stage malaria vaccines - recent progress and future challenges. Ann Trop Med Parasitol. 2010;104:189–211. doi: 10.1179/136485910X12647085215534. [DOI] [PubMed] [Google Scholar]
- 3.Remarque EJ, Faber BW, Kocken CH, Thomas AW. Apical membrane antigen 1: a malaria vaccine candidate in review. Trends Parasitol. 2008;24:74–84. doi: 10.1016/j.pt.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 4.Hirunpetcharat C, Tian JH, Kaslow DC, van Rooijen N, Kumar S, Berzofsky JA, Miller LH, Good MF. Complete protective immunity induced in mice by immunization with the 19-kilodalton carboxyl-terminal fragment of the merozoite surface protein-1 (MSP1[19]) of Plasmodium yoelii expressed in Saccharomyces cerevisiae: correlation of protection with antigen-specific antibody titer, but not with effector CD4+ T cells. J Immunol. 1997;159:3400–3411. [PubMed] [Google Scholar]
- 5.Draper SJ, Heeney JL. Viruses as vaccine vectors for infectious diseases and cancer. Nat Rev Microbiol. 2010;8:62–73. doi: 10.1038/nrmicro2240. [DOI] [PubMed] [Google Scholar]
- 6.Hill AV, Reyes-Sandoval A, O’Hara G, Ewer K, Lawrie A, Goodman A, Nicosia A, Folgori A, Colloca S, Cortese R, Gilbert SC, Draper SJ. Prime-boost vectored malaria vaccines: progress and prospects. Hum Vaccin. 2010;6:78–83. doi: 10.4161/hv.6.1.10116. [DOI] [PubMed] [Google Scholar]
- 7.Draper SJ, Goodman AL, Biswas S, Forbes EK, Moore AC, Gilbert SC, Hill AV. Recombinant viral vaccines expressing merozoite surface protein-1 induce antibody- and T cell-mediated multistage protection against malaria. Cell Host Microbe. 2009;5:95–105. doi: 10.1016/j.chom.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Draper SJ, Biswas S, Spencer AJ, Remarque EJ, Capone S, Naddeo M, Dicks MDJ, Faber BW, de Cassan SC, Folgori A, Nicosia A, Gilbert SC, Hill AVS. Enhancing Blood-Stage Malaria Subunit Vaccine Immunogenicity in Rhesus Macaques by Combining Adenovirus, Poxvirus, and Protein-in-Adjuvant Vaccines. J Immunol. 2010;185:7583–7595. doi: 10.4049/jimmunol.1001760. [DOI] [PubMed] [Google Scholar]
- 9.Draper SJ, Moore AC, Goodman AL, Long CA, Holder AA, Gilbert SC, Hill F, Hill AV. Effective induction of high-titer antibodies by viral vector vaccines. Nature medicine. 2008;14:819–821. doi: 10.1038/nm.1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Douglas AD, de Cassan SC, Dicks MD, Gilbert SC, Hill AV, Draper SJ. Tailoring subunit vaccine immunogenicity: maximizing antibody and T cell responses by using combinations of adenovirus, poxvirus and protein-adjuvant vaccines against Plasmodium falciparum MSP1. Vaccine. 2010;28:7167–7178. doi: 10.1016/j.vaccine.2010.08.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goodman AL, Epp C, Moss D, Holder AA, Wilson JM, Gao GP, Long CA, Remarque EJ, Thomas AW, Ammendola V, Colloca S, Dicks MD, Biswas S, Seibel D, van Duivenvoorde LM, Gilbert SC, Hill AV, Draper SJ. New candidate vaccines against blood-stage Plasmodium falciparum malaria: prime-boost immunization regimens incorporating human and simian adenoviral vectors and poxviral vectors expressing an optimized antigen based on merozoite surface protein 1. Infect Immun. 2010;78:4601–4612. doi: 10.1128/IAI.00315-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Biswas S, Dicks MD, Long CA, Remarque EJ, Siani L, Colloca S, Cottingham MG, Holder AA, Gilbert SC, Hill AV, Draper SJ. Transgene optimization, immunogenicity and in vitro efficacy of viral vectored vaccines expressing two alleles of Plasmodium falciparum AMA1. PLoS One. 2011;6:e20977. doi: 10.1371/journal.pone.0020977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sheehy SH, Duncan CJ, Elias SC, Collins KA, Ewer KJ, Spencer AJ, Williams AR, Halstead FD, Moretz SE, Miura K, Epp C, Dicks MD, Poulton ID, Lawrie AM, Berrie E, Moyle S, Long CA, Colloca S, Cortese R, Gilbert SC, Nicosia A, Hill AV, Draper SJ. Phase Ia clinical evaluation of the Plasmodium falciparum blood-stage antigen MSP1 in ChAd63 and MVA vaccine vectors. Mol Ther. 2011;19:2269–2276. doi: 10.1038/mt.2011.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sheehy SH, Duncan CJA, Elias SC, Biswas S, Collins KA, O’Hara GA, Halstead FD, Ewer KJ, Mahungu T, Spencer AJ, Miura K, Poulton ID, Dicks MJD, Edwards NJ, Berrie E, Moyle S, Colloca S, Cortese R, Gantlett K, Long CA, Lawrie AM, Gilbert SC, Doherty T, Nicosia A, Hill AVS, Draper SJ. Phase Ia Clinical Evaluation of the Safety and Immunogenicity of the Plasmodium falciparum Blood-Stage Antigen AMA1 in ChAd63 and MVA Vaccine Vectors. PLoS ONE. 2012 doi: 10.1371/journal.pone.0031208. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reyes-Sandoval A, Berthoud T, Alder N, Siani L, Gilbert SC, Nicosia A, Colloca S, Cortese R, Hill AV. Prime-boost immunization with adenoviral and modified vaccinia virus Ankara vectors enhances the durability and polyfunctionality of protective malaria CD8+ T-cell responses. Infect Immun. 78:145–153. doi: 10.1128/IAI.00740-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reyes-Sandoval A, Sridhar S, Berthoud T, Moore AC, Harty JT, Gilbert SC, Gao G, Ertl HC, Wilson JC, Hill AV. Single-dose immunogenicity and protective efficacy of simian adenoviral vectors against Plasmodium berghei. Eur J Immunol. 2008;38:732–741. doi: 10.1002/eji.200737672. [DOI] [PubMed] [Google Scholar]
- 17.Goodman AL, Blagborough AM, Biswas S, Wu Y, Hill AV, Sinden RE, Draper SJ. A viral vectored prime-boost immunization regime targeting the malaria pfs25 antigen induces transmission-blocking activity. PLoS One. 6:e29428. doi: 10.1371/journal.pone.0029428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Good MF, Engwerda C. Defying malaria: Arming T cells to halt malaria. Nature medicine. 2011;17:49–51. doi: 10.1038/nm0111-49. [DOI] [PubMed] [Google Scholar]
- 19.Pombo DJ, Lawrence G, Hirunpetcharat C, Rzepczyk C, Bryden M, Cloonan N, Anderson K, Mahakunkijcharoen Y, Martin LB, Wilson D, Elliott S, Eisen DP, Weinberg JB, Saul A, Good MF. Immunity to malaria after administration of ultra-low doses of red cells infected with Plasmodium falciparum. Lancet. 2002;360:610–617. doi: 10.1016/S0140-6736(02)09784-2. [DOI] [PubMed] [Google Scholar]
- 20.Roestenberg M, McCall M, Hopman J, Wiersma J, Luty AJ, van Gemert GJ, van de Vegte-Bolmer M, van Schaijk B, Teelen K, Arens T, Spaarman L, de Mast Q, Roeffen W, Snounou G, Renia L, van der Ven A, Hermsen CC, Sauerwein R. Protection against a malaria challenge by sporozoite inoculation. The New England journal of medicine. 2009;361:468–477. doi: 10.1056/NEJMoa0805832. [DOI] [PubMed] [Google Scholar]
- 21.Kawabata Y, Udono H, Honma K, Ueda M, Mukae H, Kadota J, Kohno S, Yui K. Merozoite surface protein 1-specific immune response is protective against exoerythrocytic forms of Plasmodium yoelii. Infect Immun. 2002;70:6075–6082. doi: 10.1128/IAI.70.11.6075-6082.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Belnoue E, Voza T, Costa FT, Gruner AC, Mauduit M, Rosa DS, Depinay N, Kayibanda M, Vigario AM, Mazier D, Snounou G, Sinnis P, Renia L. Vaccination with live Plasmodium yoelii blood stage parasites under chloroquine cover induces cross-stage immunity against malaria liver stage. J Immunol. 2008;181:8552–8558. doi: 10.4049/jimmunol.181.12.8552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Imai T, Shen J, Chou B, Duan X, Tu L, Tetsutani K, Moriya C, Ishida H, Hamano S, Shimokawa C, Hisaeda H, Himeno K. Involvement of CD8+ T cells in protective immunity against murine blood-stage infection with Plasmodium yoelii 17XL strain. Eur J Immunol. 2010;40:1053–1061. doi: 10.1002/eji.200939525. [DOI] [PubMed] [Google Scholar]
- 24.Wipasa J, Hirunpetcharat C, Mahakunkijcharoen Y, Xu H, Elliott S, Good MF. Identification of T cell epitopes on the 33-kDa fragment of Plasmodium yoelii merozoite surface protein 1 and their antibody-independent protective role in immunity to blood stage malaria. J Immunol. 2002;169:944–951. doi: 10.4049/jimmunol.169.2.944. [DOI] [PubMed] [Google Scholar]
- 25.Stevenson MM, Riley EM. Innate immunity to malaria. Nat Rev Immunol. 2004;4:169–180. doi: 10.1038/nri1311. [DOI] [PubMed] [Google Scholar]
- 26.Langhorne J. The role of CD4+ T-cells in the immune response to Plasmodium chabaudi. Parasitol Today. 1989;5:362–364. doi: 10.1016/0169-4758(89)90113-0. [DOI] [PubMed] [Google Scholar]
- 27.Taylor-Robinson AW. Regulation of immunity to Plasmodium: implications from mouse models for blood stage malaria vaccine design. Exp Parasitol. 2010;126:406–414. doi: 10.1016/j.exppara.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 28.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. 1986. J Immunol. 2005;175:5–14. [PubMed] [Google Scholar]
- 29.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 30.von der Weid T, Kitamura D, Rajewsky K, Langhorne J. A dual role for B cells in Plasmodium chabaudi chabaudi (AS) infection? Res Immunol. 1994;145:412–419. doi: 10.1016/s0923-2494(94)80170-3. [DOI] [PubMed] [Google Scholar]
- 31.Xu H, Hodder AN, Yan H, Crewther PE, Anders RF, Good MF. CD4+ T cells acting independently of antibody contribute to protective immunity to Plasmodium chabaudi infection after apical membrane antigen 1 immunization. J Immunol. 2000;165:389–396. doi: 10.4049/jimmunol.165.1.389. [DOI] [PubMed] [Google Scholar]
- 32.Amante FH, Crewther PE, Anders RF, Good MF. A cryptic T cell epitope on the apical membrane antigen 1 of Plasmodium chabaudi adami can prime for an anamnestic antibody response: implications for malaria vaccine design. J Immunol. 1997;159:5535–5544. [PubMed] [Google Scholar]
- 33.Stephens R, Albano FR, Quin S, Pascal BJ, Harrison V, Stockinger B, Kioussis D, Weltzien HU, Langhorne J. Malaria-specific transgenic CD4(+) T cells protect immunodeficient mice from lethal infection and demonstrate requirement for a protective threshold of antibody production for parasite clearance. Blood. 2005;106:1676–1684. doi: 10.1182/blood-2004-10-4047. [DOI] [PubMed] [Google Scholar]
- 34.Pinzon-Charry A, McPhun V, Kienzle V, Hirunpetcharat C, Engwerda C, McCarthy J, Good MF. Low doses of killed parasite in CpG elicit vigorous CD4+ T cell responses against blood-stage malaria in mice. The Journal of clinical investigation. 120:2967–2978. doi: 10.1172/JCI39222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCarthy JS, Good MF. Whole parasite blood stage malaria vaccines: a convergence of evidence. Hum Vaccin. 6:114–123. doi: 10.4161/hv.6.1.10394. [DOI] [PubMed] [Google Scholar]
- 36.Roestenberg M, Remarque E, de Jonge E, Hermsen R, Blythman H, Leroy O, Imoukhuede E, Jepsen S, Ofori-Anyinam O, Faber B, Kocken CH, Arnold M, Walraven V, Teelen K, Roeffen W, de Mast Q, Ballou WR, Cohen J, Dubois MC, Ascarateil S, van der Ven A, Thomas A, Sauerwein R. Safety and immunogenicity of a recombinant Plasmodium falciparum AMA1 malaria vaccine adjuvanted with Alhydrogel, Montanide ISA 720 or AS02. PLoS One. 2008;3:e3960. doi: 10.1371/journal.pone.0003960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Forbes EK, Biswas S, Collins KA, Gilbert SC, Hill AV, Draper SJ. Combining liver- and blood-stage malaria viral-vectored vaccines: investigating mechanisms of CD8+ T cell interference. J Immunol. 2011;187:3738–3750. doi: 10.4049/jimmunol.1003783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goodman AL, Blagborough AM, Biswas S, Wu Y, Hill AV, Sinden RE, Draper SJ. A viral vectored prime-boost immunization regime targeting the malaria pfs25 antigen induces transmission-blocking activity. PLoS One. 2011;6:e29428. doi: 10.1371/journal.pone.0029428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roederer M, Nozzi JL, Nason MC. SPICE: exploration and analysis of post-cytometric complex multivariate datasets. Cytometry A. 79:167–174. doi: 10.1002/cyto.a.21015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sinden RE, Butcher GA, Beetsma AL. Maintenance of the Plasmodium berghei life cycle. Methods in molecular medicine. 2002;72:25–40. doi: 10.1385/1-59259-271-6:25. [DOI] [PubMed] [Google Scholar]
- 41.Crewther PE, Matthew ML, Flegg RH, Anders RF. Protective immune responses to apical membrane antigen 1 of Plasmodium chabaudi involve recognition of strain-specific epitopes. Infect Immun. 1996;64:3310–3317. doi: 10.1128/iai.64.8.3310-3317.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bruna-Romero O, Gonzalez-Aseguinolaza G, Hafalla JC, Tsuji M, Nussenzweig RS. Complete, long-lasting protection against malaria of mice primed and boosted with two distinct viral vectors expressing the same plasmodial antigen. Proc Natl Acad Sci U S A. 2001;98:11491–11496. doi: 10.1073/pnas.191380898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Darrah PA, Patel DT, De Luca PM, Lindsay RW, Davey DF, Flynn BJ, Hoff ST, Andersen P, Reed SG, Morris SL, Roederer M, Seder RA. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nature medicine. 2007;13:843–850. doi: 10.1038/nm1592. [DOI] [PubMed] [Google Scholar]
- 44.Anders RF, Crewther PE, Edwards S, Margetts M, Matthew ML, Pollock B, Pye D. Immunisation with recombinant AMA-1 protects mice against infection with Plasmodium chabaudi. Vaccine. 1998;16:240–247. doi: 10.1016/s0264-410x(97)88331-4. [DOI] [PubMed] [Google Scholar]
- 45.Qin S, Cobbold S, Tighe H, Benjamin R, Waldmann H. CD4 monoclonal antibody pairs for immunosuppression and tolerance induction. Eur J Immunol. 1987;17:1159–1165. doi: 10.1002/eji.1830170813. [DOI] [PubMed] [Google Scholar]
- 46.Taylor-Robinson AW, Phillips RS. B cells are required for the switch from Th1- to Th2-regulated immune responses to Plasmodium chabaudi chabaudi infection. Infect Immun. 1994;62:2490–2498. doi: 10.1128/iai.62.6.2490-2498.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cavacini LA, Long CA, Weidanz WP. T-cell immunity in murine malaria: adoptive transfer of resistance to Plasmodium chabaudi adami in nude mice with splenic T cells. Infect Immun. 1986;52:637–643. doi: 10.1128/iai.52.3.637-643.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thera MA, Doumbo OK, Coulibaly D, Laurens MB, Ouattara A, Kone AK, Guindo AB, Traore K, Traore I, Kouriba B, Diallo DA, Diarra I, Daou M, Dolo A, Tolo Y, Sissoko MS, Niangaly A, Sissoko M, Takala-Harrison S, Lyke KE, Wu Y, Blackwelder WC, Godeaux O, Vekemans J, Dubois MC, Ballou WR, Cohen J, Thompson D, Dube T, Soisson L, Diggs CL, House B, Lanar DE, Dutta S, Heppner DG, Jr., Plowe CV. A field trial to assess a blood-stage malaria vaccine. The New England journal of medicine. 2011;365:1004–1013. doi: 10.1056/NEJMoa1008115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hodder AN, Crewther PE, Anders RF. Specificity of the protective antibody response to apical membrane antigen 1. Infect Immun. 2001;69:3286–3294. doi: 10.1128/IAI.69.5.3286-3294.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ouattara A, Mu J, Takala-Harrison S, Saye R, Sagara I, Dicko A, Niangaly A, Duan J, Ellis RD, Miller LH, Su XZ, Plowe CV, Doumbo OK. Lack of allele-specific efficacy of a bivalent AMA1 malaria vaccine. Malar J. 9:175. doi: 10.1186/1475-2875-9-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sullivan NJ, Geisbert TW, Geisbert JB, Xu L, Yang ZY, Roederer M, Koup RA, Jahrling PB, Nabel GJ. Accelerated vaccination for Ebola virus haemorrhagic fever in non-human primates. Nature. 2003;424:681–684. doi: 10.1038/nature01876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.de Cassan SC, Forbes EK, Douglas AD, Milicic A, Singh B, Gupta P, Chauhan VS, Chitnis CE, Gilbert SC, Hill AV, Draper SJ. The requirement for potent adjuvants to enhance the immunogenicity and protective efficacy of protein vaccines can be overcome by prior immunization with a recombinant adenovirus. J Immunol. 2011;187:2602–2616. doi: 10.4049/jimmunol.1101004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sridhar S, Reyes-Sandoval A, Draper SJ, Moore AC, Gilbert SC, Gao GP, Wilson JM, Hill AV. Single-dose protection against Plasmodium berghei by a simian adenovirus vector using a human cytomegalovirus promoter containing intron A. Journal of virology. 2008;82:3822–3833. doi: 10.1128/JVI.02568-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.