Abstract
Maintenance of weight loss remains a challenge for most individuals, thus practical and effective weight loss maintenance (WTLM) strategies are needed. A two-group (WEV versus WEV+) 12-month WTLM intervention trial was conducted (June 2007–February 2010) to determine the feasibility and effectiveness of weight loss maintenance intervention for older adults using daily self-monitoring of body weight, step count, fruit/vegetable intake and water consumption. Forty weight-reduced (mean weight lost = 6.7 ± 0.6 kg; BMI 29.2 ± 1.1 kg/m2) individuals aged 63 ± 1 yrs, who had previously participated in a 12-week randomized controlled weight loss intervention trial, were instructed to record daily body weight (Weight), step count (Exercise), and fruit/vegetable intake (Vegetable). Experimental group (WEV+) participants were also instructed to consume 16 floz of water before each main meal (i.e., three times daily), and to record daily water intake. Outcome measures included weight change, diet/physical activity behaviors, theoretical constructs related to health behaviors, and other clinical measures. Statistical analyses included growth curve analyses and repeated measures ANOVA. Over 12 months, there was a linear decline in weight (β = −0.32, P < 0.001) and a quadratic trend (β = 0.02, P < 0.01) over time, but no group difference (β = −0.23, P = 0.08). Analysis of the 365 days of self-reported body weight for each participant determined that weight loss was greater over the study period in WEV+ than WEV, corresponding to weight changes of −0.67 kg and 1.00 kg respectively, and an 87% greater weight loss (β = −0.01, P < 0.01). Overall compliance to daily tracking was 76 ± 5%. Daily self-monitoring of weight, physical activity, and fruit/vegetable consumption is a feasible and effective approach for maintaining weight loss for 12 months, and daily self-monitoring of increased water consumption may provide additional WTLM benefits.
Keywords: Weight Loss Maintenance, Water, Self-Regulation, Physical Activity, Fruits and Vegetables
INTRODUCTION
Maintenance of weight loss remains a significant challenge (1). Few people recover from even minor weight gains of 1–2 kg (2) and most regain all weight lost within three years (1, 3). These poor long-term outcomes highlight the need for practical and effective intervention strategies to maintain weight loss (4, 5). Self-regulatory strategies, such as daily self-monitoring of body weight, physical activity, and fruit/vegetable consumption, facilitate weight loss maintenance (WTLM) (3, 6–10). Daily self-weighing is associated with less weight regain and healthier lifestyle behaviors (2, 3, 7–11) and the importance of daily physical activity in weight gain prevention is well known (2, 9, 12–18). With regard to dietary strategies, increasing fruit/vegetable consumption helps to maintain weight loss, in combination with other healthy lifestyle strategies (19–23).
Water consumers ingest ~200 fewer calories per day and consume more fruits, vegetables and dietary fiber; fewer sugar-sweetened beverages and added sugars; and a less energy-dense diet (kcal/gram weight of food) than non-consumers of water (24–26). Among middle-aged and older adults (55+), consuming 16 fl oz of water 30 minutes before an ad libitum meal reduced meal energy intake compared to a no-water condition (27, 28). When combined with a hypocaloric diet, consuming 16 fl oz of water prior to each meal led to ~ 2 kg greater weight loss over 12 weeks in older adults compared to a hypocaloric diet alone (29). However, to date no studies have examined daily self-monitoring of increased water consumption as a long-term WTLM strategy.
The above mentioned weight loss approach for middle-aged and older adults (aged 55–75) may be attractive for several reasons. First, the number of obese older adults has increased in the past decade (30), making this a population which warrants additional attention (31). Second, water is widely available and inexpensive, and it has been shown to decrease energy intake and increase weight loss in this population (27–29). Increasing water consumption may also reduce risk of dehydration in a population with reduced thirst sensations (32–34). Thus, daily self-monitoring of water consumption, in combination with other behaviors that may be self-monitored (body weight, step count, fruit/vegetable consumption), could be a practical and effective WTLM self-regulation approach.
The aim of this study was to determine the feasibility and effectiveness of a WTLM intervention for older adults using daily self-monitoring of body weight, step count, fruit/vegetable intake, and water consumption. It was hypothesized that self-monitoring of increased water intake, body weight, step count, and fruit/vegetable consumption (WEV+) would be more effective at maintaining body weight than self-monitoring of weight, step count, and fruit/vegetable intake (WEV) alone.
METHODS
Participants and Recruitment
Participants were invited to participate in a 12-month single-blinded WTLM intervention (June 2007–February 2010) following completion of a 12-week randomized controlled weight loss (WL) intervention trial (July 2006–July 2008). The WL participants were overweight and obese (BMI 25–40 kg/m2) adults aged 55–75 years who were recruited through local newspaper advertisements (29). To be included in the WL study, individuals were required to be weight stable (± 2kg, > one year), and non-smokers. Exclusion criteria were as follows: history of depression, eating disorders, diabetes, uncontrolled hypertension, heart/lung/kidney disease, cancer, or use of medications known to alter food intake or body weight (i.e. antidepressants, thyroid medications).
Procedures
Participants in the WL intervention trial were randomly assigned to one of two groups: 1) intervention group (1200–1500 kcal hypocaloric diet + 16 floz water prior to each daily main meal) or 2) control group (1200–1500 kcal hypocaloric diet alone), as previously described (29). The WL intervention did not include self-regulation, or self-monitoring strategies (29). The pre-meal water level was based upon prior research (27, 28). For the WTLM intervention, participants continued in their assigned treatment group (increased water consumption, “WEV+”; versus no increased water consumption, “WEV”); program characteristics are described below. This study protocol was approved by Virginia Tech Institutional Review Board. All participants provided written informed consent prior to study enrollment.
12-month WTLM intervention period
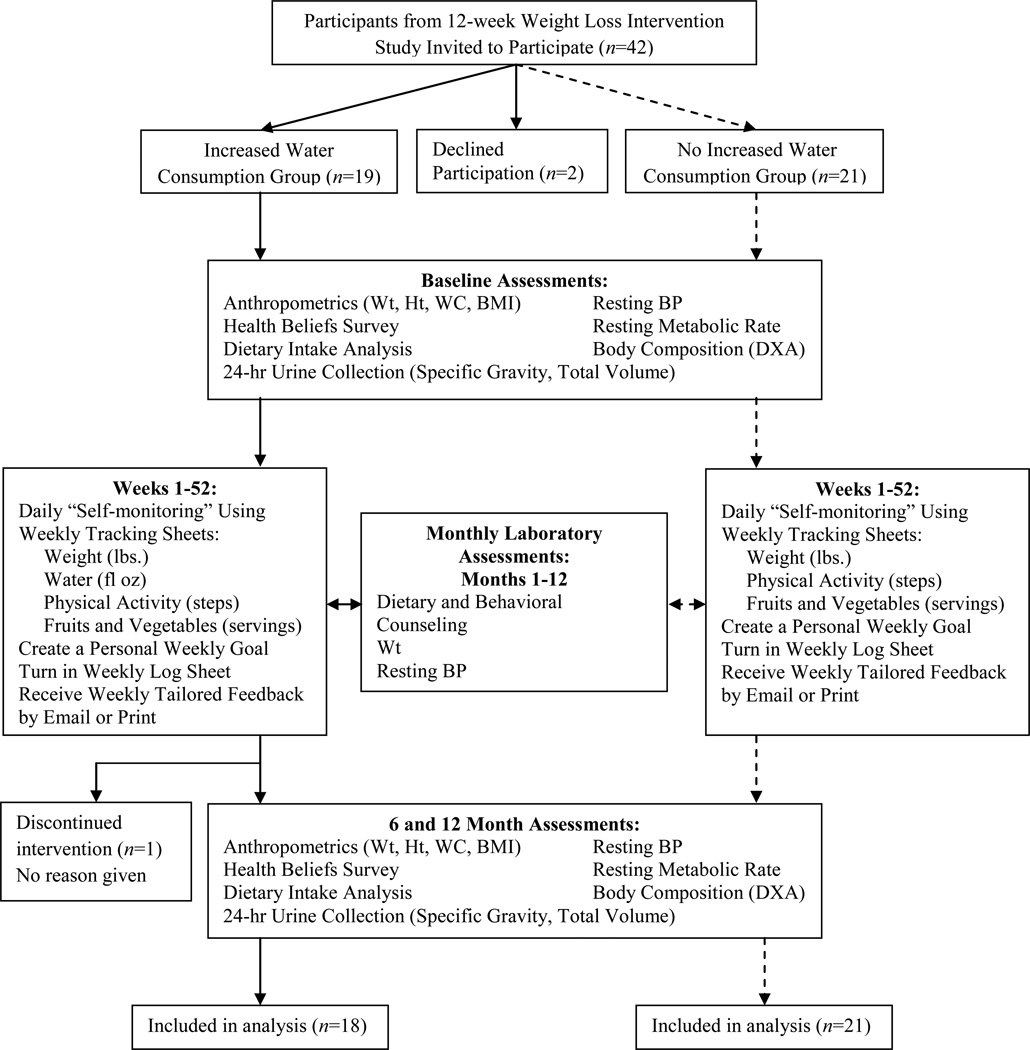
An overview of the study protocol is depicted in Figure 1. During the “WEV Changed” program, participants were instructed to record their body weight (W), daily physical activity (E) assessed by pedometer step count, and fruit/vegetable intake (V) using the self-monitoring tracking sheets. In addition, WEV+ was instructed to record daily water consumption. Participants were provided with a home-use digital scale (Tanita HD350, Tanita Inc., Arlington Heights, IL) and a pedometer (ACCUSPLIT Eagle AX120, San Jose, CA). WEV+ participants were provided with a 16 fl oz Nalgene water bottle with fl oz markings, and were instructed to continue consuming water as they did in the WL phase (16 fl oz, three times per day, 30 minutes prior to breakfast, lunch, and dinner). Targeted health behaviors included program and individual goals; individual goals were determined by the participant and discussed with the study coordinator. Program goals were as follows: ≥ 10,000 steps per day, ≥ five fruit and vegetable servings per day, remain at or below baseline “reduced” body weight (within 3 lbs.; 1.36kg), and consume at least 16 fl oz water three times per day (≥ 48 fl oz), prior to each main meal (WEV+ only). Although there is no standard definition of WTLM, changes in fluid balance can yield a daily weight change of ± 3 lbs (35). The intervention’s criterion of successful WTLM was consistent with this figure, and that of the Study to Prevent Regain (STOP Regain) Program (3).
Figure 1.
Study Procedures: 12-month weight loss maintenance (WTLM) intervention. Abbreviations used: DXA, dual-energy X-ray absorptiometry; BMI, body mass index; BP, blood pressure; Ht, height; WC, waist circumference; Wt, weight.
Participants were instructed to return tracking sheets weekly to the study coordinator for the duration of the 12-month study. Tracking sheets were returned by e-mail, or postal service if requested by the participant. For individuals who sent tracking sheets by postal service, pre-stamped and addressed envelopes were provided. Individualized feedback on goal achievement was provided weekly via e-mail or postal service for ongoing support. A feedback algorithm was utilized to standardize the feedback system.
Monthly laboratory-based assessments included body weight, four-day food intake records, resting blood pressure (BP), and an individualized counseling session with an RD. Counseling sessions varied with each participant and were based on the participant’s personal need each month (e.g., holiday eating, eating while traveling, physical activity routines). Baseline assessments were repeated at six and 12 months (Figure 1).
Laboratory Measures
Body weight and composition
Height (m) was measured without shoes using a wall mounted stadiometer, and weight (kg) was measured without shoes wearing light street clothes, to the nearest 0.1 kg on a digital scale (Scale-tronix 5002, White Plains, NY). BMI was calculated as weight (kg)/height (m)2. Waist circumference was measured to the nearest 0.5 cm at the umbilicus, using a tape measure (Gulick, Country Technology, Gays Mill, WI). Body composition was measured using dual-energy X-ray absorptiometry (DXA) (GE Lunar Prodigy; GE Healthcare, Madison, WI).
Clinical measures
Seated resting BP was measured using an automated blood pressure monitor (Colin Pressmate, Omron Co., San Antonio, TX). After a five-minute rest, measurements were taken every three minutes until two measurements were within six mmHg for systolic and diastolic BP. The average of two measurements is reported. To assess possible changes in metabolic rate over the 12-month WTLM intervention (36, 37), resting metabolic rate (RMR) was measured by indirect calorimetry using a ventilated hood and a metabolic system (Parvo Medics TrueOne 2400, Sandy, UT) (38, 39). Participants were tested 30 minutes upon awakening and after a 12-hour fast. After a 10-minute rest period on a hospital bed, inspired (O2) and expired (CO2) gases were collected and analyzed while participants rested quietly for a 45-minute testing period. Participants collected urine for one 24-h period for assessment of total urine volume, and specific gravity was determined using a refractometer (Fisher UriSystem; Fisher Scientific, Hampton, NH).
Health Beliefs Survey
Participants completed a questionnaire to measure the Social Cognitive Theory constructs of self-efficacy, social support, outcome expectations and self-regulation for diet and physical activity variables (40). The scales have adequate to high internal consistency (Cronbach’s α = 0.68–0.90).
Dietary intake
To assess habitual dietary intake, participants were instructed in proper methods to record four-day food intake records by an RD. Records were kept for three consecutive weekdays and one weekend day. Two-dimensional food diagrams were provided to assist participants in portion size determination. Records were reviewed for completeness and analyzed using the Nutrition Data System for Research nutrition analysis software (NDS-R 4.05, 2007, University of Minnesota, Minneapolis, MN). A second trained technician reviewed all diet analyses for data entry errors. To assess habitual beverage consumption, baseline, months six and 12 food intake records were manually reviewed to determine mean daily amounts (kcal, g) of water and other beverages consumed. Dietary energy density (kcal/g) was calculated using the baseline, six and 12-month food intake record analyses.
Statistical analyses
The sample size for the WL intervention (20 subjects/group) was determined based upon expected group differences in daily energy intake (180 kcals/day) and body weight reduction (2.0 kg) and associated standard deviations (energy intake: 200 kcal; body weight: 2.5 kg) with an alpha of 0.05 and 80% power (beta) (29). Independent samples t-tests were used to compare baseline demographic characteristics between groups using the Statistical Package for the Social Sciences software (SPSS version 12.0 for Windows, 2003, SPSS Inc, Chicago, IL). When baseline group differences were present (i.e. pre-study average weight loss), those variables were used as covariates in subsequent analyses. A random coefficients (multi-level) model (i.e. growth curve analysis) was used to determine the effects of the intervention on both self-reported (daily for 365 days) and laboratory-based measures of body weight (monthly for 12 months) among the WEV+ and the WEV groups. The growth curve model was fitted using STATA 9.1 xtmixed function. Growth curve analysis, as opposed to repeated-measures analysis of variance, is able to correct for measurement unreliability, uses all of an individual’s data, and utilizes individual trajectories as opposed to average value (41, 42). Two levels were used, with the first being an estimation of the individual regression for weight change over time. The second level included the predictors of the regression parameters of individual trajectories (assignment to the WEV+ or WEV condition). This model could also determine if there was a quadratic effect of time on weight change and whether there was an interaction between treatment condition and the quadratic effect of time. Because of the small sample size with the monthly measured data, bootstrapping using 1,000 samples was used to estimate bias-corrected confidence intervals by repeated re-estimation of the parameter estimates. To do this, a random samples with replacement from the original data was used (43). When data were analyzed by random coefficients model, full information maximum likelihood estimation was computed for the observed portion of each participant’s data, accumulated, and then maximized to address missing data (44). Analysis of the daily self-reported body weight data over the 365-day study period provided more statistical power to test for possible group differences in weight change; daily weight change was calculated as the difference from Day 1, and a quadratic growth curve model was fitted to the weight data for participants in two groups. To assess consistency between the two sets of body weight data (i.e., monthly laboratory weights and daily self-reported weights over 365 days), the 12 laboratory weights for each participant were compared to the same days’ self-reported weight using correlational analyses (Pearson’s r).
Differences between groups and over time (i.e., baseline, months six and 12) in other variables were assessed using repeated measures ANOVA; when significant differences were detected, paired t-tests were used for time measures and independent samples t-tests were used as post-hoc analyses for group differences. Program compliance was determined by number of tracking days completed divided by 365. Self-reported adherence to program goals (body weight, step count, water, and fruit and vegetable consumption) was determined by number of days reported adherent to each goal divided by the number of days recorded. Group differences in criteria for successful WTLM were assessed using a Pearson’s X2. Significance level was set at P ≤ 0.05.
RESULTS AND DISCUSSION
Baseline characteristics
Forty two individuals completed the WL intervention (29) and were invited to participate in the WTLM intervention. Of these, 40 individuals aged 62.7 ± 0.9 years enrolled, and 39 individuals completed the 12-month intervention (i.e., 98% retention; Figure 1). The sample was 95% Caucasian and 55% female. There was a group baseline difference in previous weight loss (−7.7 ± 1.0kg WEV+ versus −5.7 ± 0.6kg WEV), but no significant group differences in height, body weight, BMI, and waist circumference. Clinical measures and self reported diet and physical activity expenditures are reported in the Table.
Table.
Body composition, other clinical characteristics, and self-reported dietary intake and physical activity in the increased daily water consumption† and no increase in daily water consumption†† at baseline, six, and 12 months of the weight loss maintenance intervention*
| WEV+† | WEV†† | |||||
|---|---|---|---|---|---|---|
| Baseline | 6-month | 12-month | Baseline | 6-month | 12-month | |
| Body weight, kga | 83.7 ± 2.7 | 81.4 ± 2.5 | 81.8 ± 2.6 | 82.7 ± 3.7 | 81.2 ± 3.9 | 81.6 ± 4.3 |
| Body Mass Index, kg/m2 a, b | 29.1 ± 0.8 | 27.9 ± 0.7 | 28.6 ± 0.7 | 29.4 ± 1.3 | 29.0 ± 1.3 | 29.6 ± 1.5 |
| Waist circumference, cma, b | 99.0 ± 2.1 | 95.5 ± 1.7 | 98.4 ± 2.2 | 99.2 ± 2.8 | 95.4 ± 2.9 | 98.7 ± 3.5 |
| Body fat, %c, d | 36.2 ± 2.2 | 34.6 ± 2.0 | 36.0 ± 2.1 | 38.5 ± 1.9 | 36.8 ± 2.2 | 38.5 ± 2.3 |
| Total fat mass, kg | 28.2 ± 2.3 | 26.8 ± 1.8 | 28.4 ± 2.1 | 30.0 ± 2.0 | 29.9 ± 2.3 | 30.2 ± 2.5 |
| Total fat-free mass, kg | 51.1 ± 2.3 | 51.2 ± 2.4 | 50.6 ± 2.3 | 48.1 ± 3.0 | 49.0 ± 3.0 | 48.3 ± 3.4 |
| Systolic BP, mmHgc, d | 124 ± 2 | 125 ± 2 | 120 ± 1 | 117 ± 2 | 115 ± 3 | 118 ± 3 |
| Diastolic BP, mmHgc | 72 ± 2 | 74 ± 2 | 74 ± 1 | 66 ± 2 | 65 ± 2 | 65 ± 2 |
| RMR, kcal/day | 1604 ± 50 | 1563 ± 58 | 1588 ± 49 | 1582 ± 78 | 1523 ± 66 | 1482 ± 83 |
| RMR, kcal/FFM kgc | 31.1 ± 0.7 | 31.2 ± 0.8 | 31.5 ± 0.8 | 33.7 ± 0.8 | 32.5 ± 0.8 | 33.3 ± 1.2 |
| Urine volume, mle | 2452 ± 183 | 2356 ± 232 | 2621 ± 305 | 2173 ± 158 | 2196 ± 139 | 1938 ± 161 |
| Specific gravity, UG | 1.009 ± 0.000 | 1.011 ± 0.001 | 1.010 ± 0.001 | 1.011 ± 0.001 | 1.030 ± 0.016 | 1.013 ± 0.001 |
| Energy, kcal/da,d,f | 1466 ± 101 | 1481 ± 101 | 1726 ± 121 | 1490 ± 90 | 1678 ± 98 | 1654 ± 170 |
| Food Weight, g/dc,d,e | 3242 ± 189 | 3152 ± 189 | 3562 ± 267 | 2458 ± 175 | 2587 ± 170 | 2620 ± 198 |
| Carbohydrate, % energy | 50.7 ± 2.9 | 54.4 ± 2.3 | 53.4 ± 2.4 | 50.2 ± 2.5 | 53.5 ± 2.4 | 50.2 ± 2.1 |
| Protein, % energy | 18.2 ± 0.5 | 18.7 ± 1.0 | 18.6 ± 1.1 | 17.0 ± 0.6 | 15.6 ± 0.5 | 17.3 ± 1.0 |
| Fat, % energy | 31.5 ± 2.2 | 27.8 ±1.9 | 28.8 ± 1.9 | 31.5 ± 1.7 | 31.0 ± 1.8 | 30.8 ± 1.8 |
| Fiber, g/d | 20.5 ± 2.0 | 21.6 ± 2.1 | 23.9 ± 1.6 | 20.3 ± 1.6 | 22.9 ± 1.7 | 21.8 ± 2.2 |
| Energy density, kcal/g** | 0.48 ± 0.04 | 0.49 ± 0.04 | 0.51 ± 0.04 | 0.63 ± 0.05 | 0.69 ± 0.06 | 0.68 ± 0.06 |
| Beverages only: | ||||||
| Total beverages, kcal/d | 144 ± 16 | 174 ± 28 | 134 ± 17 | 195 ± 32 | 197 ± 34 | 211 ± 33 |
| Total beverages, g/dc,d,e | 2276 ± 166 | 2067 ± 204 | 2279 ± 206 | 1454 ± 138 | 1485 ±170 | 1493 ± 213 |
| Water, g/dc,d,e | 1548 ± 295 | 1073 ± 147 | 1241 ± 144 | 349 ± 96 | 495 ± 134 | 451 ± 132 |
| Non-water beverages, g/d | 1078 ± 161 | 1042 ± 99 | 1014 ± 120 | 1103 ± 112 | 1292 ± 298 | 1041 ± 149 |
| Weekly tracking sheet variables***: | ||||||
| Physical activity, steps/de | 9582 ± 605 | 8225 ± 752 | 9137 ± 762 | 8104 ± 735 | 7295 ± 729 | 7859 ± 711 |
| Fruit and vegetable, servings/d | 5.6 ± 0.4 | 5.4 ± 0.3 | 5.6 ± 0.3 | 5.6 ± 0.3 | 5.1 ± 0.4 | 5.2 ± 0.3 |
| Water, fl oz/d | 47.7 ± 4.4 | 49.9 ± 3.1 | 50.1 ± 4.3 | __________ | __________ | __________ |
Data are presented as mean ± standard error of the mean.
WEV+
WEV
Significant main effect of time, baseline to six months (P<0.01).
Significant main effect of time, six to 12 months (P<0.01).
Significant group difference at baseline (P<0.05).
Significant group difference at six months (P<0.05).
Significant group difference at 12 months (P<0.05).
Significant main effect of time, baseline to 12 months (P<0.05).
Abbreviations: BP, Blood pressure; FFM, Fat free mass; RMR, Resting metabolic rate; UG, Urine Specific Gravity
Calculated with all foods and beverages, including water.
Baseline data is the average of weeks one through four (month one), six month data is the average of weeks 25 through 28, and 12 month data is the average of weeks 49–52 of the weight loss maintenance intervention.
Intervention
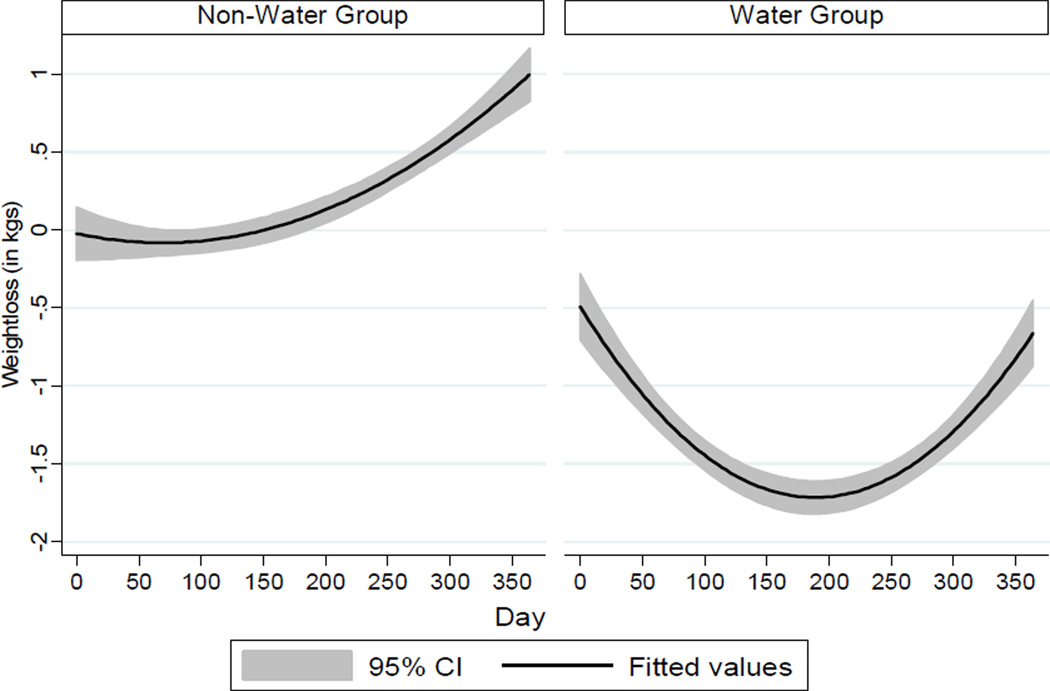
Using the definition of successful WTLM of 3% weight regain from baseline (35), 80% of all participants were categorized as successful. There was a significant linear decline in weight (β = −0.32, P < 0.001), indicating that participants in both groups lost weight over time. There was also a significant quadratic trend in weight change (β = 0.02, P < 0.01), indicating that weight change leveled off in the final months. There was no group difference in overall weight change using the laboratory-based weights (β = −0.23, P = 0.08). Monthly laboratory weights were closely associated with self-reported weights (r = 0.99, P < 0.001). Results utilizing the daily self-reported body weight data over the 365-day study period indicated that WEV+ exhibited greater weight loss over the 365 days than WEV, representing an 87% greater decline in weight (β = −0.01, P < 0.01) and mean weight changes of −0.67 kg and 1.00 kg, respectively (online supplemental Figure 2). Thus daily self-monitoring of pre-meal water consumption may be an effective WTLM self-regulation approach, beyond that achieved by daily monitoring of body weight, step count, and fruit/vegetable consumption. Previous investigations have shown that as part of a WL intervention, increasing water consumption can lead to greater weight loss (29, 45) and a reduction in meal and total daily energy intake (27, 28, 46). Yet, a recent cross-sectional survey of self-reported weight loss and WTLM maintenance practices did not identify water consumption (i.e., “drink plenty of water”) as a dietary strategy used more often among successful weight loss maintainers as compared to those unsuccessful at weight loss maintenance (47). The findings of this investigation are consistent with intervention trials reporting that self-monitoring of body weight and physical activity (48), and increasing fruit and vegetable consumption (49), are effective long-term weight loss and maintenance strategies. Taken together, these findings suggest that practitioners could recommend daily self-monitoring of increased water consumption, along with body weight, step count, and fruit/vegetable consumption, as a feasible and effective WTLM approach.
Clinical Measures and Behavioral Measures
As shown in the Table, WEV+ had significantly lower percent body fat at six months; however there were no other significant differences in body composition, BMI, or waist circumference. Water intake and 24-h urine volume was significantly higher in the WEV+ group compared to the WEV group at 12 months. WEV+ reported a higher mean yearly step count and fruit/vegetable intake (data not shown).
There were no group differences in scores for Social Cognitive Theory determinants of health behaviors, thus mean changes are presented. Strategies to improve self-regulation (0.2 ± 0.1), self monitoring of physical activity (0.6 ± 0.2), goal setting and planning of physical activity (0.5 ± 0.1), and friend support for dietary and physical activity behaviors increased (0.3 ± 0.1, 0.4 ± 0.2, respectively) (all P < 0.05). Negative dietary outcome expectations declined, while perceived positive benefits of physical activity increased (−0.3 ± 0.1, 1.4 ± 0.8, respectively). No changes were noted in self-efficacy for diet and physical activity behaviors. Collectively, improvements in these theory-based determinants of health behaviors are consistent with the intervention approach, which included self-regulation, self-monitoring, and goal setting. After four years, intervention participants in the Look AHEAD (Action for Health in Diabetes) trial achieved a mean WTLM of 4.7% of initial body weight, which suggests that behavioral interventions aimed at WTLM can achieve clinically significant long-term success (48). As with this intervention, the Look AHEAD intensive lifestyle intervention included self-weighing and diet/physical activity goal setting as interventions strategies (48).
Weekly Tracking Sheets
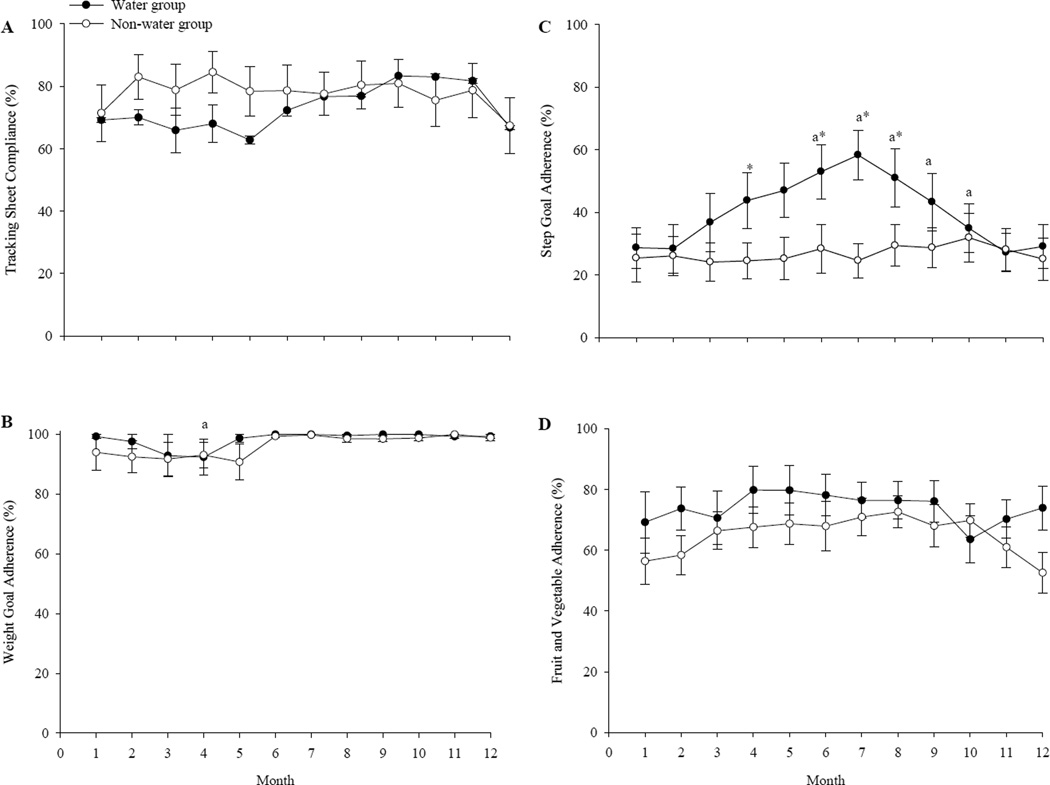
Overall compliance with returning tracking sheets was 76±5%, with no group difference (supplemental online Figure 3). Among WEV+ participants, self-reported adherence to the daily water intake regimen was 66±7%. The lowest level of self-reported adherence was to the physical activity goal; there were no group differences in yearly mean adherence to the fruit and vegetable intake goal and adherence to the daily weight goal (Figure 3). Compliance with returning tracking sheets was related to successful WTLM using the < 3% of baseline weight criterion (X2 = 5.516, P < 0.05). This finding is consistent with that reported by a nationally representative sample of successful weight-loss maintainers, who used similar behavioral strategies (i.e., tracking body, setting weight goals) (47).
Figure 3.
Monthly program compliance and self-reported goal adherence among the “WEV+” water group and “WEV” non-water group during a 12-month weight loss maintenance intervention: (A) compliance with turning in weekly tracking sheets, (B) adherence to the program body weight goal, (C) adherence to the program step count goal, and (D) adherence to the program fruit/vegetable intake goal. a Significant difference versus month one (P<0.05). *Group difference P<0.05. (Supplemental Online Figure)
Limitations
The sample size was limited and included primarily Caucasian middle-aged and older adults, who were recruited primarily through newspaper advertisements; all of which may limit generalizability. Further studies are warranted to determine program participation rate (i.e. intervention reach), in order to determine potential broader impacts of this intervention approach. Self-reported water intake compliance was less than ideal (i.e. 66%). The longer-term effectiveness (i.e. > 12 months) of this approach is not known; some have suggested that a WTLM intervention be assessed for at least five years (50), yet few trials have continued past 12 months (3, 4, 51, 52).
CONCLUSIONS
These findings indicate that daily monitoring of weight, physical activity, and fruit/vegetable consumption is an effective WTLM approach, and suggest that daily self-monitoring of increased water consumption may provide additional WTLM benefits. Future studies are warranted to determine the translation potential of effective WTLM interventions, such as within community-based settings, worksites, and medical systems, which also include formal cost analyses. Due to the December 2011 ruling by the Centers for Medicare and Medicaid which excluded registered dietitians (RD) from direct billing for intensive behavioral counseling for obesity, clinical evidence which demonstrates the effectiveness of RD-delivered WTLM interventions is clearly needed (53). These findings may serve as the methodological basis for designing large-scale, longer terms WTLM trials investigating daily self-monitoring of weight, step count, fruit/vegetable intake and water consumption.
Figure 2.
Body weight change according to daily self-reported body weight among “WEV+” water and “WEV” non-water group participants over the 365-day weight loss maintenance intervention period. (Supplemental Online Figure)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jeremy D. Akers, Email: akersjd@jmu.edu, James Madison University, 701 Carrier Drive, MSC 4301, Harrisonburg, VA 22807.
Rachel A. Cornett, Email: racornett@vt.edu, Virginia Tech, 228 War Memorial Hall (0351), Blacksburg, VA 25061, Phone: (540) 231-6469.
Jyoti S. Savla, Email: jsavla@vt.edu, Virginia Tech, 237 Wallace Hall (0426), Blacksburg, VA 24061, Phone: (540) 231-2348.
Kevin P. Davy, Email: kdavy@vt.edu, Virginia Tech, 215 War Memorial Hall (0351), Blacksburg, VA 24061, Phone: (540) 231-3487.
Brenda M. Davy, Email: bdavy@vt.edu, Virginia Tech, 221 Wallace Hall (0430), Blacksburg, VA 24061, Phone: (540) 231-6784, Fax: (540) 231-3916.
References
- 1.Wadden TA, Phelan S. Behavioral assessment of the obese patient. In: Wadden TA, Stunkard AJ, editors. Handbook of Obesity Treatment. New York: Guilford Press; 2002. pp. 186–226. [Google Scholar]
- 2.Wing RR, Phelan S. Long-term weight loss maintenance. Am J Clin Nutr. 2005;82(1 Suppl):222S–225S. doi: 10.1093/ajcn/82.1.222S. [DOI] [PubMed] [Google Scholar]
- 3.Wing RR, Tate DF, Gorin AA, Raynor HA, Fava JL. A self-regulation program for maintenance of weight loss. N Engl J Med. 2006;355(15):1563–1571. doi: 10.1056/NEJMoa061883. [DOI] [PubMed] [Google Scholar]
- 4.Svetkey LP, Stevens VJ, Brantley PJ, et al. Comparison of strategies for sustaining weight loss: the weight loss maintenance randomized controlled trial. JAMA. 2008;299(10):1139–1148. doi: 10.1001/jama.299.10.1139. [DOI] [PubMed] [Google Scholar]
- 5.Akers JD, Estabrooks P, Davy BM. Translational research: bridging the gap between long-term weight loss maintenance research and practice. J Am Diet Assoc. 2010;110(10):1511–1522. doi: 10.1016/j.jada.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burke LE, Wang J, Sevick MA. Self-monitoring in weight loss: a systematic review of the literature. J Am Diet Assoc. 2011;111(1):92–102. doi: 10.1016/j.jada.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Butryn ML, Phelan S, Hill JO, Wing RR. Consistent self-monitoring of weight: a key component of successful weight loss maintenance. Obesity. 2007;15(12):3091–3096. doi: 10.1038/oby.2007.368. [DOI] [PubMed] [Google Scholar]
- 8.Linde JA, Jeffery RW, French SA, Pronk NP, Boyle RG. Self-weighing in weight gain prevention and weight loss trials. Ann Behav Med. 2005;30(3):210–216. doi: 10.1207/s15324796abm3003_5. [DOI] [PubMed] [Google Scholar]
- 9.McGuire MT, Wing RR, Klem ML, Seagle HM, Hill JO. Long-term maintenance of weight loss: do people who lose weight through various weight loss methods use different behaviors to maintain their weight? Int J Obes Relat Metab Disord. 1998;22(6):572–577. doi: 10.1038/sj.ijo.0800627. [DOI] [PubMed] [Google Scholar]
- 10.Wing RR, Tate DF, Gorin AA, Raynor HA, Fava JL, Machan J. STOP regain: are there negative effects of daily weighing? J Consult Clin Psychol. 2007;75(4):652–656. doi: 10.1037/0022-006X.75.4.652. [DOI] [PubMed] [Google Scholar]
- 11.Linde JA, Jeffery RW, Finch EA, et al. Relation of body mass index to depression and weighing frequency in overweight women. Prev Med. 2007;45(1):75–79. doi: 10.1016/j.ypmed.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anderson JW, Konz EC, Frederich RC, Wood CL. Long-term weight-loss maintenance: a meta-analysis of US studies. Am J Clin Nutr. 2001;74(5):579–584. doi: 10.1093/ajcn/74.5.579. [DOI] [PubMed] [Google Scholar]
- 13.Franz MJ, VanWormer JJ, Crain AL, et al. Weight-loss outcomes: a systematic review and meta-analysis of weight-loss clinical trials with a minimum 1-year follow-up. J Am Diet Assoc. 2007;107(10):1755–1767. doi: 10.1016/j.jada.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 14.Jakicic JM, Marcus BH, Lang W, Janney C. Effect of exercise on 24-month weight loss maintenance in overweight women. Arch Intern Med. 2008;168(14):1550. doi: 10.1001/archinte.168.14.1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klem ML, Wing RR, Chang CC, et al. A case-control study of successful maintenance of a substantial weight loss: individuals who lost weight through surgery versus those who lost weight through non-surgical means. Int J Obes Relat Metab Disord. 2000;24(5):573–579. doi: 10.1038/sj.ijo.0801199. [DOI] [PubMed] [Google Scholar]
- 16.Klem ML, Wing RR, Lang W, McGuire MT, Hill JO. Does weight loss maintenance become easier over time? Obes Res. 2000;8(6):438–444. doi: 10.1038/oby.2000.54. [DOI] [PubMed] [Google Scholar]
- 17.Phelan S, Wyatt HR, Hill JO, Wing RR. Are the eating and exercise habits of successful weight losers changing? Obesity. 2006;14(4):710–716. doi: 10.1038/oby.2006.81. [DOI] [PubMed] [Google Scholar]
- 18.Tate DF, Jeffery RW, Sherwood NE, Wing RR. Long-term weight losses associated with prescription of higher physical activity goals. Are higher levels of physical activity protective against weight regain? Am J Clin Nutr. 2007;85(4):954–959. doi: 10.1093/ajcn/85.4.954. [DOI] [PubMed] [Google Scholar]
- 19.Kruger J, Blanck HM, Gillespie C. Dietary practices, dining out behavior, and physical activity, correlates of weight loss maintenance. Prev Chronic Dis. 2008;5(1):A11. [PMC free article] [PubMed] [Google Scholar]
- 20.Lanza E, Schatzkin A, Daston C, et al. Implementation of a 4-y, high-fiber, high-fruit- and- vegetable, low-fat dietary intervention: results of dietary changes in the Polyp Prevention Trial. Am J Clin Nutr. 2001;74(3):387–401. doi: 10.1093/ajcn/74.3.387. [DOI] [PubMed] [Google Scholar]
- 21.Rolls BJ, Ello-Martin JA, Carlton Tohill B. What can intervention studies tell us about the relationship between fruit and vegetable consumption and weight management? Nutr Rev. 2004;62:1–17. doi: 10.1111/j.1753-4887.2004.tb00001.x. [DOI] [PubMed] [Google Scholar]
- 22.Singh RB, Dubnov G, Niaz MA, et al. Effect of an Indo-Mediterranean diet on progression of coronary artery disease in high risk patients (Indo-Mediterranean Diet Heart Study): a randomized single-blind trial. The Lancet. 2002;360(9344):1455–1461. doi: 10.1016/S0140-6736(02)11472-3. [DOI] [PubMed] [Google Scholar]
- 23.Tohill BC, Seymour J, Serdula M, Kettel-Khan L, Rolls BJ. What epidemiologic studies tell us about the relationship between fruit and vegetable consumption and body weight. Nutr Rev. 2004;62:365–374. doi: 10.1111/j.1753-4887.2004.tb00007.x. [DOI] [PubMed] [Google Scholar]
- 24.Kant AK, Graubard BI, Atchison EA. Intakes of plain water, moisture in foods and beverages, and total water in the adult US population--nutritional, meal pattern, and body weight correlates: National Health and Nutrition Examination Surveys 1999–2006. Am J Clin Nutr. 2009;90(3):655–663. doi: 10.3945/ajcn.2009.27749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nielsen SJ, Popkin BM. Changes in beverage intake between 1977 and 2001. Am J Prev Med. 2004;27(3):205–210. doi: 10.1016/j.amepre.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 26.Raynor HA, Van Walleghen EL, Bachman JL, Looney SM, Phelan S, Wing RR. Dietary energy density and successful weight loss maintenance. Eating Behav. 2011;12(2):119–125. doi: 10.1016/j.eatbeh.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davy BM, Dennis EA, Dengo AL, Wilson KL, Davy KP. Water consumption reduces energy intake at a breakfast meal in obese older adults. J Am Diet Assoc. 2008;108(7):1236–1239. doi: 10.1016/j.jada.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van Walleghen EL, Orr JS, Gentile CL, Davy BM. Pre-meal water consumption reduces meal energy intake in older but not younger subjects. Obesity. 2007;15(1):93–99. doi: 10.1038/oby.2007.506. [DOI] [PubMed] [Google Scholar]
- 29.Dennis EA, Dengo AL, Comber DL, Flack KD, Savla J, Davy KP, Davy BM. Water consumption increases weight loss during a hypocaloric diet intervention in middle-aged and older adults. Obesity. 2010;18(2):300–307. doi: 10.1038/oby.2009.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.US Government Printing Office; 2008. Federal Interagency Forum on Aging-Related Statistics. Older Americans 2008: Key Indicators of Well-Being. [Google Scholar]
- 31.Goulding M, Rodgers M, Smith S. Public health and gaining: trends in aging- United States and worldwide. MMWR Morb Mortal Wkly Rep. 2003;52:101–106. [PubMed] [Google Scholar]
- 32.Ferry M. Strategies for ensuring good hydration in the elderly. Nutr Rev. 2005;63(6 Pt 2):S22–S29. doi: 10.1111/j.1753-4887.2005.tb00151.x. [DOI] [PubMed] [Google Scholar]
- 33.Phillips PA, Rolls BJ, Ledingham JG, et al. Reduced thirst after water deprivation in healthy elderly men. N Engl J Med. 1984;311(12):753–759. doi: 10.1056/NEJM198409203111202. [DOI] [PubMed] [Google Scholar]
- 34.Rolls BJ, Phillips PA. Aging and disturbances of thirst and fluid balance. Nutr Rev. 1990;48(3):137–144. doi: 10.1111/j.1753-4887.1990.tb02915.x. [DOI] [PubMed] [Google Scholar]
- 35.Stevens J, Truesdale KP, McClain JE, Cai J. The definition of weight maintenance. Int J Obes. 2006;30(3):391–399. doi: 10.1038/sj.ijo.0803175. [DOI] [PubMed] [Google Scholar]
- 36.Boschmann M, Steiniger J, Hille U, et al. Water-induced thermogenesis. J Clin Endocrinol Metab. 2003;88(12):6015–6019. doi: 10.1210/jc.2003-030780. [DOI] [PubMed] [Google Scholar]
- 37.Brown CM, Dulloo AG, Montani J-P. Water-induced thermogenesis reconsidered: The effects of osmolality and water temperature on energy expenditure after drinking. J Clin Endocrinol Metab. 2006;91(9):3598–3602. doi: 10.1210/jc.2006-0407. [DOI] [PubMed] [Google Scholar]
- 38.Bassett DR, Jr, Howley ET, Thompson DL, et al. Validity of inspiratory and expiratory methods of measuring gas exchange with a computerized system. J Appl Physiol. 2001;91(1):218–224. doi: 10.1152/jappl.2001.91.1.218. [DOI] [PubMed] [Google Scholar]
- 39.Jensen K, Jorgensen S, Johansen L. A metabolic cart for measurement of oxygen uptake during human exercise using inspiratory flow rate. Europ J Applied Phys. 2002;87(3):202–206. doi: 10.1007/s00421-002-0616-2. [DOI] [PubMed] [Google Scholar]
- 40.Winett RA, Anderson ES, Wojcik JR. Guide to Health: Nutrition and Physical Activity Outcomes of a Group-Randomized Trial of an Internet-Based Intervention in Churches. Ann Behav Med. 2007;33(3):251–261. doi: 10.1007/BF02879907. [DOI] [PubMed] [Google Scholar]
- 41.Raudenbush S, Bryk A. Hierarchical linear model: Applications and data analysis methods. 2nd edn. Thousand Oaks, CA: Sage; 2002. [Google Scholar]
- 42.Singer J, Willett J. Applied longitudinal model: Modeling change and event occurrence. New York: Oxford Press; 2003. [Google Scholar]
- 43.Mooney CZ, Duval RR. Bootstrapping: a nonparametric approach to statistical inference. Newbury Park, CA: Sage; 1993. [Google Scholar]
- 44.Arbuckle JL. Full information estimation in the presence of incomplete data. In: Marcoulides GA, Shumacker RE, editors. Advanced Structural Modeling: Issues and Techniques. Mahwah, NJ, USA: Lawrence Erlbaum Associates, Inc.; 1996. pp. 243–278. [Google Scholar]
- 45.Stookey JD, Constant F, Popkin BM, Gardner CD. Drinking water is associated with weight loss in overweight dieting women independent of diet and activity. Obesity. 2008;16(11):2481–2488. doi: 10.1038/oby.2008.409. [DOI] [PubMed] [Google Scholar]
- 46.Stookey JD, Constant F, Gardner CD, Popkin BM. Replacing sweetened caloric beverages with drinking water is associated with lower energy intake. Obesity. 2007;15(2):3013–3022. doi: 10.1038/oby.2007.359. [DOI] [PubMed] [Google Scholar]
- 47.Sciamanna CN, Kiernan M, Rolls BJ, et al. Practices associated with weight loss versus weight-loss maintenance results of a national survey. AM J Prev Med. 2011;41(2):159–166. doi: 10.1016/j.amepre.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 48.Wadden TA, Neiberg RH, Wing RR, et al. Four-year weight losses in the look AHEAD study: factors associated with long-term success. Obesity. 2011;19(10):1987–1998. doi: 10.1038/oby.2011.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Champagne CM, Broyles ST, Moran LD, et al. Dietary intakes associated with successful weight loss and maintenance during the weight loss maintenance trail. J AM Diet Assoc. 2011;111(12):1826–1835. doi: 10.1016/j.jada.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.National Heart, Lung, and Blood Institute. Washington, DC: National Institutes of Health; 2004. Think Tank on Enhancing Obesity Research at the National Heart, Lung, and Blood Institute. Department of Health and Human Services. (ed). [Google Scholar]
- 51.Leibbrand R, Fichter MM. Maintenance of weight loss after obesity treatment: is continuous support necessary? Behav Res Ther. 2002;40(11):1275–1289. doi: 10.1016/s0005-7967(01)00099-7. [DOI] [PubMed] [Google Scholar]
- 52.Riebe D, Blissmer B, Greene G, et al. Long-term maintenance of exercise and healthy eating behaviors in overweight adults. Prev Med. 2005;40(6):769–778. doi: 10.1016/j.ypmed.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 53.Anding R. Medicare coverage for obesity counseling decision. [Accessed December 2, 2011];Sports Cardiovascular and Wellness Nutrition Website Blog. http://scandpg.blogspot.com/2011/12/medicare-coverage-for-obesity.html. Published December 1, 2011.