Abstract
Memantine, an uncompetitive NMDA receptor antagonist, is a FDA approved drug used for the treatment of moderate to severe Alzheimer’s disease (AD). Several studies have documented protective roles of memantine against amyloid beta peptide (Aβ)– mediated damage to neurons in both in vitro and in vivo models. Memantine is also effective in reducing amyloid burden in the brain of APP transgenic mice. Currently the role of memantine in the Aβ– mediated neurodegenerative cascade, including APP metabolism is being understood [1]. Herein, we investigated the effect of memantine on levels of the secreted form of Aβ precursor protein (APP), secreted Aβ and cell viability markers under short/acute conditions. We treated neuronal SK-N-SH cells with 10μM memantine and measured levels of secreted total APP (sAPP), APPα isoform and Aβ (1-40) in a time dependent manner for up to 24 hours. Memantine significantly decreased the levels of the secreted form of sAPP, sAPPα and Aβ (1-40) compared to vehicle- treated cells. This change started as early as 8 hour and continued for up to 24 hour of drug treatment. Unlike sAPP, a slight non-significant increase in total intracellular APP level was observed in 24-hour treated memantine cells. Taken together, these results suggest a role for memantine in the transport or trafficking of APP molecules away from the site of their proteolytic cleavage by the secretase enzymes. Such a novel property of memantine warrants further study to define its therapeutic utility.
Keywords: Amyloid, Alzheimer’s drug, beta-peptide, dementia, neuronal cells, protein processing, sAPP, secretase, tissue culture
Alzheimer’s disease (AD) is characterized typically by deposition of amyloid β (Aβ) peptide -containing plaques in the brain parenchyma and associated neuro-inflammation, and by severe cholinergic neuronal and synapse loss [27]. Aβ peptides, which primarily consist of a short peptide of 40 amino acids in length (Aβ1–40) and a longer and more ‘amyloidogenic’ peptide of 42 residues Aβ(1–42), are proteolytic cleavage products of a transmembrane protein, amyloid-beta precursor protein (APP) by the enzymatic action of β and γ secretases [4, 18]. APP can also be processed by another enzyme, α secretase, to produce a truncated ectoplasmic APP, termed sAPPα, which is considered to be a product of a ‘non-amyloidogenic’ pathway [27]. Currently, cholinesterase inhibitors (ChEIs) and the uncompetitive N-methyl D-aspartate (NMDA) receptor antagonist, memantine, are FDA-approved treatment for AD [26]. Although the main purpose of ChEIs is to prevent hydrolysis of the neurotransmitter acetylcholine to augment its levels in brain, potent anti-Aβ properties have also been discovered for several ChEIs [16]. We have recently shown that posiphen, a newly developed analogue of a ChEI, dose dependently decreased levels of APP and Aβ in both cell culture and the brain of animal models [14]. Memantine is an uncompetitive NMDA receptor antagonist that is used for the treatment of moderate to severe AD. The NMDA receptor is a group of ionotropic channels that play important roles in synaptic plasticity and neurodegeneration through the Ca++ signaling pathway [5]. Impairment of the Mg++ blockade of the NMDA receptor not only leads to excessive influx of Ca++ in neurons, but may induce downstream activation of Ca++ dependent enzymes and protein kinase signaling pathways leading to excitatory damage of neuronal cells [12]. Several studies have revealed remarkably improved spatial learning in transgenic animals, and enhanced cognitive functions in AD patients treated with memantine [21, 24]. Although a number of studies have documented the effect of memantine in protecting neuronal cells from Aβ mediated damage both in cell culture and animals [7, 29], the action of memantine on levels of APP and Aβ remains to be fully elucidated. In this regard, we have recently shown a longer time (day) dependent decrease in sAPP and Aβ (1-40) levels in human SK-N-SH cells and also a decrease in Aβ (1-42) levels in AD transgenic mice following memantine [1]. In the present study, we treated human SK-N-SH cells with 10μM of memantine and measured levels of sAPP, sAPPα, intracellular APP and secreted Aβ (1-40) over a shorter time course to provide insight into memantine’s role in the processing of the APP molecule.
In several cell culture based studies, memantine exerted pharmacological activity between 1μM to 20μM doses depending upon the cell types [1, 2, 23]; we hence selected a single dose of 10μM memantine for the current study. Human SK-N-SH cells were grown in 100mm plates as described previously [20]. Once the cells became 80% confluent, 250,000 cells were transferred to each well of a six-well tissue culture plate (Corning Inc., NY; USA). Memantine (Sigma, MO; USA) was dissolved in low serum (1% FBS)-containing MEM medium at a final concentration of 10μM, and 2ml of the memantine containing medium was added into each of two wells (n=2). Similarly, 2ml of low serum medium without the drug was also added into each of two wells, which served as vehicle. The plate was incubated at 37°C, and 200 μl of conditioned media (CM) sample was collected from each well at eight hour intervals up to one day (8, 16 and 24 hours, respectively). Cells were harvested at the end of 24 hours and lysed by ‘IP’ lysis buffer, as described [17]. Cell viability in each well was determined by the luminescence based Cell Titer Glo (CTG, Promega Corporation, WI; USA) assay. CTG measures ATP concentrations, which reflects the cell number. Cell toxicity within the treatment groups was determined by LDH assay with 30μl of CM sample, as described [8]. For protein analysis, an equal volume of CM was mixed with Laemmli protein sample loading buffer and denatured at 95°C. Fifteen microliter (15μl) of the denatured CM was loaded into a polyacrylamide-SDS criterion gel (10%) (Bio-Rad, CA; USA) and electrophoresis was run for 1.2 hour. Proteins from the gel were electrophoretically transferred onto a PVDF membrane, which was blocked with 5% non-fat milk. After blocking, the membrane was probed with primary antibody (clone 22C11, Chemicon, MA; USA) against the N-terminus 66–81 residues of APP, and chemoluminescence signals were obtained. Protein band density was quantified by ‘Image J’ software (NIH, MA; USA). Afterwards, the same blot was stripped off by ‘Re-probe buffer’ (Pierce, IL; USA). The stripped blot was re-blocked in 5% non-fat milk, probed with secondary antibody (goat anti-mouse) and exposed in an X-ray film to ensure that previous primary antibody (22C11) was completely removed. The blot was probed with monoclonal antibody clone 6E10 (Covance, NJ, USA), which detects 1–17 residue of the Aβ sequence within the APP molecule. The specific protein band signals were obtained and analyzed in the same way. Bradford protein assay was performed to measure total protein content of cell lysate (CL) samples, and an equal amount of CL protein was loaded in another ‘Criterion’ western blotting gel to detect total intracellular APP by using 22C11 antibody. To normalize the APP signals, a specific strip of the blot was also probed with β-actin antibody (Sigma). Separately, Aβ (1-40) (N) ELISA assay was carried out with 50μl of each CM sample, as per the manufacturer’s protocol (IBL, Takasaki; Japan). Aβ (1-42) levels were also measured independently using an IBL ELISA kit with 50μl of the CM samples. However, Aβ (1-42) levels were below the detection level in these samples. Similar independent experiments in duplicate (n=2) was performed at a different time. Analyses of Western blot and ELISA were carried out by combining two independent experiments performed in duplicates i.e. (n=2) into one quadruplet (n=4) data set. For this, data of each individual experiment were divided by the mean of the entire data set of the same experiment. Statistical significance was determined by repeated measure ANOVA and independent t-test using SPSS 16.0 edition.
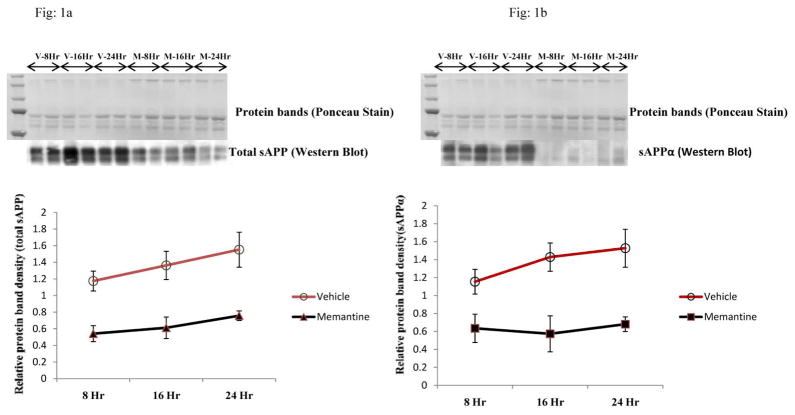
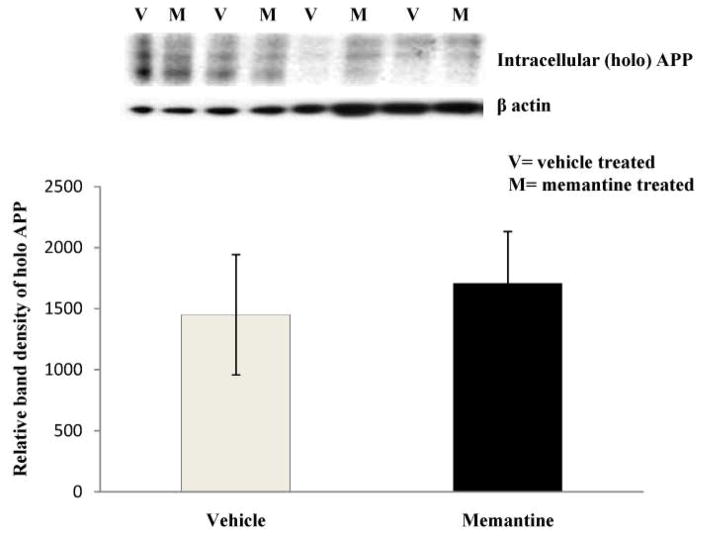
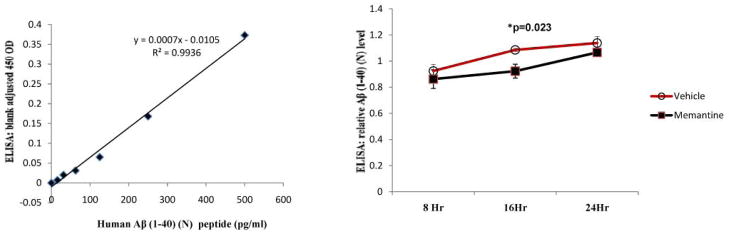
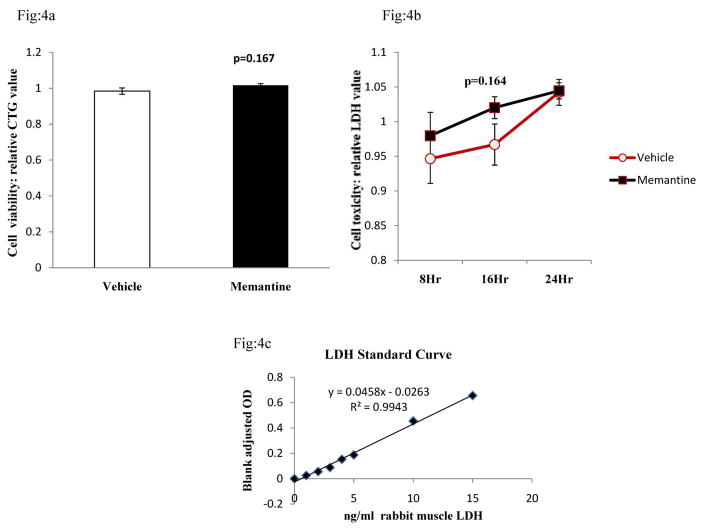
Following 10μM dose of memantine, we observed a significant decrease in the levels of both sAPP and sAPPα in all the time points studied (Fig. 1a and 1b). Significant decreases (approximately 50%) in levels of total sAPP and sAPPα in the CM of memantine treated cells were evident even at the 8 hour time point. These decreases were not due to an uneven transfer of proteins onto the membrane as assessed by Ponceau stain (Fig. 1a and 1b). Because of an increase in cell number and metabolic activity of cells, levels of sAPP and sAPPα in both vehicle and memantine treated cells increased gradually with the increase in duration of treatment. However, we quantified a non-significant slight increase in levels of total intracellular APP in the CL samples of memantine treated cells vs. vehicle treated cells after of 24-hour treatment period (Fig. 2). Interestingly, secreted Aβ (1-40) ELISA data showed a decrease in levels in the CM samples of memantine treated cells (Fig. 3). Results from the CTG assay revealed no change in cell viability between memantine and vehicle treated groups (Fig. 4a). Previously, we have observed a significant increase in cell viability of memantine treated embryonic primary cortical neurons [1]. However, such primary cortical neuronal cells were treated with memantine in nutritionally enriched medium. In our present experiment, memantine treatment was carried out in low serum medium, which restricts multiplication of cells. Under these conditions, there proved to be no significant change in toxicity in memantine treated cells, assessed by detecting LDH in the CM samples (Fig. 4b) suggesting that memantine was well tolerated in our experimental condition.
Fig 1. Levels of sAPP and sAPPα by Western immunoblotting of conditioned media (CM) samples from the human neuroblastoma cells (SK-N-SH) treated with vehicle or memantine at different time point.
a: The Western blot strip showed the protein bands belonging to total sAPP in memantine and vehicle treated CM samples. Levels of secreted total APP showed a decrease in memantine treated cells vs. vehicle-treated cells. A significant decrease in sAPP level was noted after 8 hour of treatment, which continued throughout the time course of the study. A protein band picture (Ponceau stain) of the blot after transfer is also shown, which indicates uniformity of protein loading in the gel or transfer onto the membrane. Results are plotted from four independent (n=4) sets of experiments and a representative Western blot with two sets of experiments is shown.
b: Secreted APPα was measured from the same blot after stripping off signals of 22C11 antibody , as mentioned in Fig. 1a. The Western blot picture shows a decrease in sAPPα levels in memantine treated samples vs. vehicle. This finding is similar to sAPP, which is shown in Fig. 1a. (‘V’ represents ‘vehicle’ and ‘M’ represents memantine).
Fig 2. Levels of total APP by Western immunoblotting of cell extracts from the human neuroblastoma cells treated with vehicle or memantine.
Western blot data showed a slight non-significant (p=0.701) increase in intracellular total APP (holo APP) levels in the lysate of memantine treated cells vs. vehicle. The relative density of each band was normalized with β− actin band, as indicated. Cell lysates from 4 experiments were analyzed.
Fig 3. ELISA determination of secreted Aβ (1-40) (N) peptide in conditioned media (CM) samples at different time points.
Levels of N terminus fragment of secreted Aβ (1-40) were measured from the CM samples of memantine and vehicle treated SK-N-SH cells by ELISA as per the manufacturer’s protocol. A standard curve performed with a known amount of N- terminus human Aβ (1-40) is also shown. The amount of detected Aβ (1-40) peptides in the CM range from 57 to 97 pg/ml. Results showed a decreasing trend of Aβ (1-40) in the CM of memantine treated cells. A statistically significant decrease was noticed at the 16 hour time point (P=0.023).
Fig 4. Assay of viability and toxicity in the cells treated with vehicle or memantine for 24 hours.
a. After memantine treatment, cells were lysed by adding 500 μl of ‘IP’ buffer to obtain cell lysate. Equal volumes (30μl) of cell lysates were mixed with an equal volume of CTG assay buffer, and luminescence signals were obtained in a luminometer. Data is plotted as relative luminescence units combining both experiments. CTG or cell viability data did not show any significant change between the memantine and vehicle-treated groups.
b. CM samples of memantine and vehicle treated cells were tested for membrane damage or toxicity using the LDH method. In our assay, LDH activity ranged from 0.8–1.08 ng/ml. Data plotted in the figure are relative LDH values combining two sets of identical experiments (relative value). We did not observe any significant toxic effect of memantine treatment vs. the vehicle.
c. LDH standard curve with known amount of LDH from rabbit muscle shows the linearity of the assay.
APP is a transmembrane glycoprotein that contains 695, 751 or 770 amino acid residues depending upon the splice variance [22]. Synthesis of APP together with its N-terminal glycosylation occurs within the endoplasmic reticulum (ER), immature APP then moves to the Golgi apparatus for maturation and, thereafter, it eventually is transported towards the cell membrane [10]. APP can be cleaved by α or β secretase enzyme activities to generate sAPPα and a C99 fragment, respectively, and the latter can be cleaved by presenilin dependent γ secretase to produce Aβ peptide of varying amino acid sequences. It is thought that cleavage of APP and subsequent generation of Aβ peptide occurs in subcellular acidic pH of Golgi and endosomes [31] or at the cell surface [3]. Any alteration in the trafficking of the APP molecule may result in a deficient production of total sAPP, sAPPα and Aβ peptide. In our study, we observed a greater than 50% decrease in the levels of both sAPP and sAPPα in each of the three time points studied within a 24-hour time period. Western blot data clearly revealed that the decrease in levels of sAPP and sAPPα in memantine-treated cells started at or before the 8 hour time period, and remained significantly lower than that of vehicle-treated cells. Additionally we observed a non-significant increase in intracellular total APP in the treatment group. Some amount of APP might have degraded by the lysosomal enzymes, which could not be detectable by the antibody used in this study. However, in a separate experiment, we observed a significant increase in intracellular APP levels with 20μM dose of memantine (data not shown). These circumstances may indicate that more APP is available in the cells, which may act as a substrate for β followed by γ secretase cleavage. Beta (β) and γ secretase cleavage of the APP molecule would be reflected as an increase in Aβ formation. Interestingly, we observed a significant decline in levels of the secreted N terminal fragment of Aβ (1-40) in the CM of memantine treated cells. Soluble oligomers of Aβ peptide have been shown to trigger Ca++ influx through NMDA receptors, which ultimately would lead to excitotoxic damage of cultured hippocampal neuronal cells and, interestingly, this can be prevented by memantine [6]. Furthermore, 4mM of Mg++, which is a low affinity NMDA receptor antagonist, has been shown to decrease Aβ burden in organotypic hippocampal cultures from APP/PS1 transgenic mice [11]. In this regard, a ‘pleotropic’ property of memantine has recently emerged as it has been documented that memantine treatment reduced Aβ plaques in APP/PS1 transgenic mice [28] and lowered the levels of Aβ (1-40) and secreted APP in human SK-N-SH cells [1]. In addition to neuronal cells, we have recently observed a decrease in the levels of total sAPP and Aβ in rat glioma (C6) cell cultures (Ray and Lahiri, unpublished data). The exact mechanism mediating memantine’s role in the reduction of APP and Aβ remains to be delineated as our described studies have not measured the levels of sAPPα or total intracellular APP, and thus it would be interesting to know whether memantine directs APP processing towards a non-amyloidogenic pathway or alters the level of total APP message. In our present study, we utilized undifferentiated human SK-N-SH cells, which lack a NR2B subunit and show no significant calcium influx when treated with NMDA [25]. Hence, the effect of memantine on APP processing may involve a component that is non-NMDA receptor mediated and we consider the following additional possibilities:
Following N- and O-glycosylation, nascent APP travels towards the cell surface followed by internalization into the endosomes where it can become the substrate for BACE-1 cleavage [30]. In contrast, APP is processed by α-secretase at the cell surface. Since we have found an increase in levels of total intracellular APP and a decrease in levels of total sAPP, sAPPα and Aβ, we speculate that memantine might play a role in the trafficking of APP molecules by either keeping immature APP in endoplasmic reticulum (ER) for a longer time or increasing its trafficking towards lysosomes. Lysosomal accumulation of memantine has already been proposed, which could provide an explanation to account for the reduced APP and Aβ secretion in memantine-treated cells [1]. In this context, cholesterol depleting agents have been shown to reduce Aβ production by interfering with intracellular APP trafficking [9, 10]. Similarly, the ChEI tacrine has been shown to effect the trafficking of APP to a lysosomal compartment, [19] and has therefore been suggested to have a lysosomotropic property [15]. Memantine has also been shown to reduce BACE activity in cultured human neuroblastoma cells [13]. Taken together, our results suggest that memantine decreases levels of secreted APP, sAPPα and Aβ (1-40) and may retain intracellular APP in human SK-N-SH cells. These results warrant further studies on the mechanism of action of memantine on the APP processing pathway.
Acknowledgments
We thank B.Maloney, J.Bailey and J.Long, and grants support from the Alzheimer’s Associations (Zenith Award) and the National Institutes of Health (AG18379 and AG18884) to DKL.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alley GM, Bailey JA, Chen D, Ray B, Puli LK, Tanila H, Banerjee PK, Lahiri DK. Memantine lowers amyloid-beta peptide levels in neuronal cultures and in APP/PS1 transgenic mice. J Neurosci Res. 2009 doi: 10.1002/jnr.22172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caumont AS, Octave JN, Hermans E. Amantadine and memantine induce the expression of the glial cell line-derived neurotrophic factor in C6 glioma cells. Neurosci Lett. 2006;394:196–201. doi: 10.1016/j.neulet.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 3.Chyung JH, Raper DM, Selkoe DJ. Gamma-secretase exists on the plasma membrane as an intact complex that accepts substrates and effects intramembrane cleavage. J Biol Chem. 2005;280:4383–4392. doi: 10.1074/jbc.M409272200. [DOI] [PubMed] [Google Scholar]
- 4.Citron M, Diehl TS, Gordon G, Biere AL, Seubert P, Selkoe DJ. Evidence that the 42- and 40-amino acid forms of amyloid beta protein are generated from the beta-amyloid precursor protein by different protease activities. Proc Natl Acad Sci U S A. 1996;93:13170–13175. doi: 10.1073/pnas.93.23.13170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cull-Candy S, Brickley S, Farrant M. NMDA receptor subunits: diversity, development and disease. Curr Opin Neurobiol. 2001;11:327–335. doi: 10.1016/s0959-4388(00)00215-4. [DOI] [PubMed] [Google Scholar]
- 6.De Felice FG, Velasco PT, Lambert MP, Viola K, Fernandez SJ, Ferreira ST, Klein WL. Abeta oligomers induce neuronal oxidative stress through an N-methyl-D-aspartate receptor-dependent mechanism that is blocked by the Alzheimer drug memantine. J Biol Chem. 2007;282:11590–11601. doi: 10.1074/jbc.M607483200. [DOI] [PubMed] [Google Scholar]
- 7.Dong H, Yuede CM, Coughlan C, Lewis B, Csernansky JG. Effects of memantine on neuronal structure and conditioned fear in the Tg2576 mouse model of Alzheimer's disease. Neuropsychopharmacology. 2008;33:3226–3236. doi: 10.1038/npp.2008.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghosh C, Lahiri DK. Increased vulnerability of neuronal cell lines to sodium nitroprusside-mediated toxicity is caused by the decreased level of nitric oxide metabolites. J Mol Neurosci. 1999;13:77–92. doi: 10.1385/JMN:13:1-2:77. [DOI] [PubMed] [Google Scholar]
- 9.Guardia-Laguarta C, Coma M, Pera M, Clarimon J, Sereno L, Agullo JM, Molina-Porcel L, Gallardo E, Deng A, Berezovska O, Hyman BT, Blesa R, Gomez-Isla T, Lleo A. Mild cholesterol depletion reduces amyloid-beta production by impairing APP trafficking to the cell surface. J Neurochem. 2009;110:220–230. doi: 10.1111/j.1471-4159.2009.06126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huttunen HJ, Peach C, Bhattacharyya R, Barren C, Pettingell W, Hutter-Paier B, Windisch M, Berezovska O, Kovacs DM. Inhibition of acyl-coenzyme A: cholesterol acyl transferase modulates amyloid precursor protein trafficking in the early secretory pathway. FASEB J. 2009 doi: 10.1096/fj.09-134999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kamenetz F, Tomita T, Hsieh H, Seabrook G, Borchelt D, Iwatsubo T, Sisodia S, Malinow R. APP processing and synaptic function. Neuron. 2003;37:925–937. doi: 10.1016/s0896-6273(03)00124-7. [DOI] [PubMed] [Google Scholar]
- 12.Kloda A, Martinac B, Adams DJ. Polymodal regulation of NMDA receptor channels. Channels (Austin) 2007;1:334–343. doi: 10.4161/chan.5044. [DOI] [PubMed] [Google Scholar]
- 13.Lahiri CDDK, Aelley GM, Banerjee PK. Memantine decreases beta-secretase activity in human neuroblastoma cells. The 10th International Conference on Alzheimer's Disease and Related Disorders; Madrid, Spain. 2006. [Google Scholar]
- 14.Lahiri DK, Chen D, Maloney B, Holloway HW, Yu QS, Utsuki T, Giordano T, Sambamurti K, Greig NH. The experimental Alzheimer's disease drug posiphen [(+)-phenserine] lowers amyloid-beta peptide levels in cell culture and mice. J Pharmacol Exp Ther. 2007;320:386–396. doi: 10.1124/jpet.106.112102. [DOI] [PubMed] [Google Scholar]
- 15.Lahiri DK, Farlow MR. Differential effect of tacrine and physostigmine on the secretion of the beta-amyloid precursor protein in cell lines. J Mol Neurosci. 1996;7:41–49. doi: 10.1007/BF02736847. [DOI] [PubMed] [Google Scholar]
- 16.Lahiri DK, Farlow MR, Hintz N, Utsuki T, Greig NH. Cholinesterase inhibitors, beta-amyloid precursor protein and amyloid beta-peptides in Alzheimer's disease. Acta Neurol Scand Suppl. 2000;176:60–67. doi: 10.1034/j.1600-0404.2000.00309.x. [DOI] [PubMed] [Google Scholar]
- 17.Lahiri DK, Farlow MR, Sambamurti K. The secretion of amyloid beta-peptides is inhibited in the tacrine-treated human neuroblastoma cells. Brain Res Mol Brain Res. 1998;62:131–140. doi: 10.1016/s0169-328x(98)00236-8. [DOI] [PubMed] [Google Scholar]
- 18.Lahiri DK, Farlow MR, Sambamurti K, Greig NH, Giacobini E, Schneider LS. A critical analysis of new molecular targets and strategies for drug developments in Alzheimer's disease. Curr Drug Targets. 2003;4:97–112. doi: 10.2174/1389450033346957. [DOI] [PubMed] [Google Scholar]
- 19.Lahiri DK, Lewis S, Farlow MR. Tacrine alters the secretion of the beta-amyloid precursor protein in cell lines. J Neurosci Res. 1994;37:777–787. doi: 10.1002/jnr.490370612. [DOI] [PubMed] [Google Scholar]
- 20.Lahiri DK, Nall C, Ge YW. Promoter activity of the beta-amyloid precursor protein gene is negatively modulated by an upstream regulatory element. Brain Res Mol Brain Res. 1999;71:32–41. doi: 10.1016/s0169-328x(99)00150-3. [DOI] [PubMed] [Google Scholar]
- 21.Minkeviciene R, Banerjee P, Tanila H. Memantine improves spatial learning in a transgenic mouse model of Alzheimer's disease. J Pharmacol Exp Ther. 2004;311:677–682. doi: 10.1124/jpet.104.071027. [DOI] [PubMed] [Google Scholar]
- 22.Newton JR, Parkinson D, Clench MR. Strategies for examination of Alzheimer's disease amyloid precursor protein isoforms. Anal Bioanal Chem. 2006;385:692–699. doi: 10.1007/s00216-006-0462-x. [DOI] [PubMed] [Google Scholar]
- 23.Pellegrini JW, Lipton SA. Delayed administration of memantine prevents N-methyl-D-aspartate receptor-mediated neurotoxicity. Ann Neurol. 1993;33:403–407. doi: 10.1002/ana.410330414. [DOI] [PubMed] [Google Scholar]
- 24.Peskind ER, Potkin SG, Pomara N, Ott BR, Graham SM, Olin JT, McDonald S. Memantine treatment in mild to moderate Alzheimer disease: a 24-week randomized, controlled trial. Am J Geriatr Psychiatry. 2006;14:704–715. doi: 10.1097/01.JGP.0000224350.82719.83. [DOI] [PubMed] [Google Scholar]
- 25.Pizzi M, Boroni F, Bianchetti A, Moraitis C, Sarnico I, Benarese M, Goffi F, Valerio A, Spano P. Expression of functional NR1/NR2B-type NMDA receptors in neuronally differentiated SK-N-SH human cell line. Eur J Neurosci. 2002;16:2342–2350. doi: 10.1046/j.1460-9568.2002.02403.x. [DOI] [PubMed] [Google Scholar]
- 26.Ray B, Lahiri DK. Neuroinflammation in Alzheimer's disease: different molecular targets and potential therapeutic agents including curcumin. Curr Opin Pharmacol. 2009;9:434–444. doi: 10.1016/j.coph.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 27.Sambamurti K, Greig NH, Lahiri DK. Advances in the cellular and molecular biology of the beta-amyloid protein in Alzheimer's disease. Neuromolecular Med. 2002;1:1–31. doi: 10.1385/NMM:1:1:1. [DOI] [PubMed] [Google Scholar]
- 28.Scholtzova H, Wadghiri YZ, Douadi M, Sigurdsson EM, Li YS, Quartermain D, Banerjee P, Wisniewski T. Memantine leads to behavioral improvement and amyloid reduction in Alzheimer's-disease-model transgenic mice shown as by micromagnetic resonance imaging. J Neurosci Res. 2008;86:2784–2791. doi: 10.1002/jnr.21713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song MS, Rauw G, Baker GB, Kar S. Memantine protects rat cortical cultured neurons against beta-amyloid-induced toxicity by attenuating tau phosphorylation. Eur J Neurosci. 2008;28:1989–2002. doi: 10.1111/j.1460-9568.2008.06498.x. [DOI] [PubMed] [Google Scholar]
- 30.Thinakaran G, Koo EH. Amyloid precursor protein trafficking, processing, and function. J Biol Chem. 2008;283:29615–29619. doi: 10.1074/jbc.R800019200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vassar R, Bennett BD, Babu-Khan S, Kahn S, Mendiaz EA, Denis P, Teplow DB, Ross S, Amarante P, Loeloff R, Luo Y, Fisher S, Fuller J, Edenson S, Lile J, Jarosinski MA, Biere AL, Curran E, Burgess T, Louis JC, Collins F, Treanor J, Rogers G, Citron M. Beta-secretase cleavage of Alzheimer's amyloid precursor protein by the transmembrane aspartic protease BACE. Science. 1999;286:735–741. doi: 10.1126/science.286.5440.735. [DOI] [PubMed] [Google Scholar]