Abstract
Objective
We examined whether HIV-1 testing using a rapid assay increases the proportion of pregnant women obtaining HIV-1 results and the uptake of perinatal HIV-1 interventions.
Methods
Pregnant women attending public health clinics in Nairobi were offered voluntary counselling and testing for HIV-1. Consenting women were randomly assigned to receive either rapid or conventional HIV-1 testing. Women randomly assigned to rapid testing were allowed to receive same-day results or to return later. The results for women randomly assigned to conventional enzyme-linked immunosorbent assay (ELISA) testing were available after 7 days. HIV-1-infected women were referred for antiretroviral prophylaxis to prevent mother-to-child transmission of HIV-1.
Results
Among 1282 women offered voluntary HIV-1 testing and counselling, 1249 accepted testing, of whom 627 were randomly assigned to rapid testing and 622 to conventional testing. The median duration between testing and obtaining results was 0 days for women who received rapid testing compared with 11 days for women who received conventional testing. The percentage receiving HIV-1 results was significantly higher among women who received rapid testing compared with conventional testing. Of 161 HIV-1-seropositive women, only 24 received antiretroviral prophylaxis. The uptake of perinatal HIV-1 interventions did not differ between HIV-1-seropositive women randomly assigned to rapid testing or conventional ELISA testing.
Conclusion
Rapid HIV-1 testing significantly increased the proportion of women receiving HIV-1 results, which is important for sexual and perinatal HIV-1 prevention. The challenge remains to improve the uptake of perinatal HIV-1 interventions among HIV-1-seropositive women.
Keywords: Africa, perinatal HIV interventions, prevention of mother-to-child transmission of HIV, randomized clinical trial, rapid HIV testing, voluntary counselling and testing
Introduction
Short-course antiretroviral regimens have been shown to reduce mother-to-child transmission of HIV-1, and are being implemented in developing countries [1–4]. Despite antenatal HIV-1 seroprevalence rates of 15–35% in parts of sub-Saharan Africa [5], routine voluntary HIV-1 counselling and testing (VCT) is not available in many public health settings. Although 75–95% of pregnant women accept HIV-1 testing in antenatal settings offering VCT, between 25 and 55% of women consenting to antenatal HIV-1 testing do not obtain their results [6,7].
Strategies to increase the proportion of pregnant women who obtain HIV-1 results are urgently needed. Similarly, it is important to know whether HIV-1-infected pregnant women who obtain their results will comply with perinatal HIV-1 interventions. Unlike conventional enzyme-linked immunosorbent assay (ELISA) testing, which is relatively more expensive and time-consuming and requires clients to return for results several days later, rapid HIV-1 testing allows individuals to receive same-day results. To date, there are no randomized studies on the effect of rapid HIV-1 testing on uptake rates of perinatal HIV-1 interventions.
In this study, we compared the frequency of obtaining test results and the uptake of perinatal HIV-1 interventions among women receiving rapid versus conventional ELISA testing. In addition, we identified correlates of obtaining HIV-1 results and the acceptance of referral for perinatal HIV-1 interventions.
Methods
Study protocol
This trial was conducted between October 1999 and September 2000 in two public health centers in Nairobi, Kenya. The study population consisted of pregnant women who attended antenatal care at these centers. The protocol was reviewed and approved by the Institutional Review Boards of the University of Nairobi and the University of Washington. All participating women gave written informed consent. Study staff received training on counselling for HIV-1 testing and mother-to-child transmission of HIV-1 during the month before the study initiation.
On their first antenatal visit, women were offered group education on the prevention of maternal–infant HIV-1 infection by study staff. Following group education, each woman was counselled individually and offered HIV-1 testing by the study nurse. Women were informed that if they were found to be HIV-1 infected, they would be referred to a nearby research clinic to receive free antiretroviral drugs to prevent mother-to-child transmission of HIV-1.
Women completed a pre-test questionnaire concerning demographic characteristics, sexual history, and knowledge of HIV-1 transmission and prevention. Women who accepted HIV-1 testing were individually randomly assigned to either rapid HIV-1 or conventional ELISA testing using a block randomization scheme and sealed envelopes. Rapid HIV-1 testing was conducted in the clinic by trained laboratory staff. Women who received rapid HIV-1 testing were given the option to receive same-day results or to return on another day. Blood for conventional ELISA testing was taken to a laboratory at the University of Nairobi. Women randomly assigned to conventional ELISA testing were asked to return for their results 7 days later. All women were referred to clinic staff for routine antenatal care, including syphilis testing and treatment.
HIV-1 testing algorithm
Blood for rapid HIV-1 testing was screened using Capillus HIV-1/HIV-2 (Cambridge Biotech Corp., Worcester, MA, USA). Negative results were reported as HIV-1/HIV-2 seronegative. Specimens that tested HIV-1 positive using the Capillus assay were confirmed with Abbott Determine HIV-1/HIV-2 (Abbott Laboratories, Abbott Park, IL, USA). If the specimen tested positive on the second rapid test, it was reported as HIV-1/HIV-2 seropositive. Specimens testing negative on Abbott Determine were reported as HIV-1/HIV-2 seronegative. Indeterminate results were retested using conventional ELISA testing. Blood for conventional ELISA testing was screened using Detect HIV I/II assay (BioChem Immunosystems Inc., Montreal, Quebec, Canada) and specimens testing negative were reported as HIV-1/HIV-2 seronegative. Specimens that tested positive on screening ELISA were confirmed with Recombigen HIV-1/2 enzyme immunoassay (Cambridge Biotech).
Post-test counselling and referral for antiretroviral drugs
Women who returned for HIV-1 results completed a post-test questionnaire before receiving results and received individual post-test counselling. The individual administering the post-test questionnaire was blinded to the HIV-1 status of the participant. HIV-1-seropositive women received counselling on the use of antiretroviral drugs to prevent mother-to-child HIV-1 transmission. During post-test counselling, HIV-1-seropositive women were offered referral to a nearby clinic for antiretroviral prophylaxis. Women accepting referral were given referral letters. Women who declined referral were requested to return to receive further counselling and interventions for the prevention of infant HIV-1 on-site. Women presenting at the secondary clinic before 36 weeks’ gestation received further counselling on antiretroviral drugs and infant feeding practices, and were randomly assigned either to single-dose nevirapine prophylaxis (HIVNET 012 regimen) [4] or zidovudine prophylaxis (Thai-CDC) [2]. Women presenting after 36 weeks’ gestation received nevirapine prophylaxis.
Statistical analysis
Baseline characteristics were compared between randomization groups using the Mann–Whitney U test for continuous variables and Chi-square or Fisher’s exact tests for categorical variables. Correlates of obtaining HIV-1 results and referral for perinatal HIV-1 interventions were analysed using similar tests. Odds ratios (OR) and 95% confidence intervals were obtained with Chi-square tests for women in the two randomization groups for the two endpoints: received HIV-1 results and accepted referral for antiretroviral prophylaxis.
Results
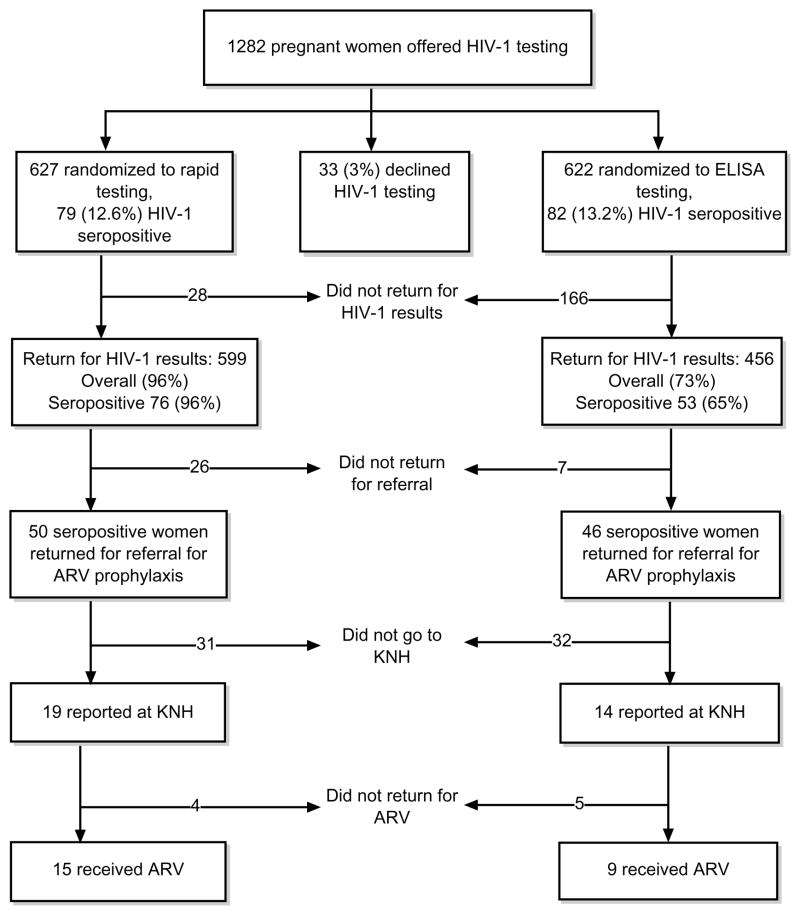
Of 1282 pregnant women offered HIV-1 testing, 1249 (97%) accepted testing. Among women accepting HIV-1 testing, 627 were randomly assigned to rapid HIV-1 testing, and 622 to conventional ELISA testing (Fig. 1). Among the randomly assigned women, the mean age was 23 years (range 18–44) and 88% were married. Ninety-six per cent had primary or higher education, and only 31% were employed. The median age of sexual debut was 18 years (range 9–27). A history of greater than three lifetime sex partners was reported by 14% of women, and a history of previous sexually transmitted disease was reported by 10% of women. Eighty-two per cent said they had never used condoms with their current partner.
Fig. 1. Study profile.
ARV, Antiretroviral regimen; ELISA, enzyme-linked immunosorbent assay; KNH, Kenyatta National Hospital.
The characteristics of the pregnant women randomly assigned to rapid HIV-1 testing were similar to those of the women randomly assigned to conventional ELISA testing with respect to age, marital status, education, occupation, history of condom use, and HIV-1 seropositivity (Table 1). The median waiting period between HIV-1 testing and obtaining results was 0 days (range 0–77) for women who received rapid testing compared with 11 days (range 3–148) for women who received conventional ELISA testing (P < 0.001). Women randomly assigned to rapid HIV-1 testing were more likely to obtain their results than women randomly assigned to conventional ELISA testing (96 versus 73%, OR 1.3; 95% CI 1.2–1.4, P < 0.001). Although rapid testing was associated with a significantly increased rate of obtaining results, there was no significant difference in the overall uptake of perinatal HIV-1 interventions between women randomly assigned to rapid testing and women randomly assigned to conventional ELISA testing [15/79 (19%) versus 9/82 (11%), OR 1.7; 95% CI 0.8–3.7, P = 0.2] (Fig. 1).
Table 1.
Characteristics of participating women by randomization group.
| Characteristics | Rapid HIV-1 testing (n = 627) | Conventional HIV-1 testing (n = 622) | P value |
|---|---|---|---|
| Median age in years (range) | 23 (18–43) | 23 (18–44) | 1.0 |
| Marital status: | |||
| Single | 67 (11%) | 62 (10%) | 0.7 |
| Married | 548 (88%) | 554 (89%) | 0.3 |
| Othera | 10 (2%) | 4 (1%) | 0.1 |
| Education level: | |||
| None | 23 (4%) | 23 (4%) | 1.0 |
| Primary | 383 (61%) | 373 (60%) | 0.7 |
| Secondary | 195 (31%) | 210 (34%) | 0.3 |
| College | 24 (4%) | 15 (2%) | 0.2 |
| Occupation: | |||
| Housewife | 368 (59%) | 382 (62%) | 0.3 |
| Unemployed | 64 (10%) | 46 (8%) | 0.1 |
| Parity (range) | 1 (0–9) | 1 (0–8) | 0.7 |
| Median age of sexual debut (range) | 18 (9–27) | 18 (9–27) | 0.3 |
| History of condom use by current sex partner | 112 (18%) | 118 (19%) | 0.6 |
| Lifetime sex partners (range) | 2 (1–31) | 2 (1–30) | 0.4 |
| HIV-1 seropositivity | 79 (13%) | 82 (13%) | 0.8 |
| Time to obtain HIV-1 results (days) (range) | 0 (0–77) | 11 (3–148) | <0.0001 |
| Number receiving results | 599 (96%) | 456 (73%) | <0.0001 |
Separated/divorced/widowed.
Women who obtained HIV-1 results were slightly older than those who did not receive their results (P = 0.08). Obtaining test results was not associated with marital status, education level, or a history of a previous HIV-1 test. Among women randomly assigned to conventional ELISA testing, obtaining results was higher among HIV-1-seronegative compared with HIV-1-seropositive women (75 versus 65%, P = 0.06). Of 161 women diagnosed with HIV-1, 96 (60%) accepted referral for perinatal HIV-1 interventions and only 24 (15%) eventually received antiretroviral drugs (Fig. 1). Among HIV-1-seropositive women who received their results (n = 129), those randomly assigned to rapid testing (n = 50) were significantly less likely to accept referral for antiretroviral prophylaxis compared with women randomly assigned to conventional ELISA testing (66 versus 87%, P = 0.007). Other factors (being married, uneducated, unemployed) were not significantly associated with accepting referral for perinatal HIV-1 interventions. Among women accepting referral for interventions, no factors were identified that were associated with the uptake of perinatal HIV-1 interventions (data not shown).
Discussion
Acceptance of HIV-1 VCT was high (97%) in this setting. This is similar to or higher than what has been observed previously in other antenatal settings in Africa [5,6,8]. Our findings suggest that if VCT services were offered routinely in antenatal settings, a significant proportion of pregnant women may utilize them. Furthermore, it reinforces the view that routine voluntary HIV-1 counselling and rapid on-site HIV-1 testing can be integrated in antenatal settings in developing countries [9,10].
This study demonstrates that rapid HIV-1 testing substantially increases the proportion of women who obtain HIV-1 results in the antenatal clinic compared with conventional ELISA testing. This observation is consistent with studies carried out in other settings and, to our knowledge, is the first randomized study to compare these test options [9,11,12]. Rapid on-site HIV-1 testing leads to the identification of HIV-1-infected pregnant women who can be offered perinatal HIV-1 interventions. Equally important, it helps to identify HIV-1-uninfected women who receive counselling to prevent primary HIV-1 infection among themselves and their partners.
Surprisingly, significantly fewer HIV-1-seropositive women who received rapid testing accepted referral for perinatal HIV-1 interventions than women who received conventional testing. Women may have been unable to cope with the HIV/AIDS information on the same day as receiving their HIV-1 test results. With rapid HIV-1 testing, there may be limited time for women to comprehend the implications of a positive diagnosis and to decide on what action to take. In contrast, conventional testing provides ample time for women to decide on their readiness to receive HIV-1 results, and those who choose to get their results are prepared to accept the diagnosis, the implications of a positive test, and referral for perinatal HIV-1 interventions.
A significant percentage of HIV-1-seropositive women referred for perinatal HIV-1 interventions did not attend the secondary clinic. Reasons for low attendance at the secondary clinic may include travel costs, the fear of the disclosure of HIV-1 results to other providers, a preference to deliver in a rural home, and fear of partner notification. Obstacles to attending a secondary clinic may be overcome by providing on-site interventions.
If all HIV-1-seropositive women identified in this study were given rapid testing and received nevirapine at the time of obtaining the results, we would have reached 96% of all women diagnosed with HIV-1. This would ensure that women who did not return to the clinic for continued counselling could take nevirapine if they elected to do so. However, for HIV-1-seropositive women who continue clinic attendance, it is important to provide multiple counselling sessions, because HIV-1 diagnosis in pregnancy and the issues involved in preventing infant HIV-1 infection are too complex for pregnant women to comprehend in one counselling session. Thorough post-test counselling for HIV-1-infected pregnant women who receive same-day results is important for such women to understand their role in the prevention of infant HIV-1. Finally, the overall uptake of perinatal HIV-1 interventions may be improved if women of reproductive age are educated about the prevention of mother-to-child transmission of HIV-1 before they become pregnant. Widespread education about the two-dose nevirapine regimen may be useful to ensure familiarity with the regimen for women who may not attend antenatal clinics.
Conclusion
We have shown that rapid on-site HIV-1 testing is associated with an increased proportion of women obtaining HIV-1 test results in the antenatal clinic. However, the challenge remains to improve the uptake of antiretroviral drugs to prevent mother-to-child transmission of HIV-1.
Acknowledgments
The authors would like to thank the mothers who participated in this study and the staff of Westlands and Kangemi Health Centers for their invaluable assistance. The authors also thank Regina Mbaji for assistance with patient counselling; Ruth Nduati and Dorothy Mbori-Ngacha for their encouragement and advice; and Nairobi City Council for permission to use the health centers for the study.
Sponsorship: This work was supported by the International AIDS Research and Training Program through a grant from the Fogarty International Center, National Institutes of Health (D43-TW00007).
Footnotes
Note: I.M.M. was working for the Department of Medical Microbiology, University of Nairobi, as a Fogarty IARTP scholar when the study was done.
References
- 1.Wiktor SZ, Ekpini E, Karon JM, Nkengasong J, Maurice C, Severin ST, et al. Short-course oral zidovudine for prevention of mother-to-child transmission of HIV-1 in Abidjan, Côte d’Ivoire: a randomized trial. Lancet. 1999;353:781–785. doi: 10.1016/S0140-6736(98)10412-9. [DOI] [PubMed] [Google Scholar]
- 2.Shaffer N, Chuachoowong R, Mock PA, Bhadrakom C, Siriwasin W, Young NK, et al. Short-course zidovudine for perinatal HIV-1 transmission in Bangkok, Thailand: a randomised controlled trial. Bangkok Collaborative Perinatal HIV Transmission Study Group. Lancet. 1999;353:773–780. doi: 10.1016/s0140-6736(98)10411-7. [DOI] [PubMed] [Google Scholar]
- 3.The PETRA Study Team. Efficacy of three short-course regimens of zidovudine and lamivudine in preventing early and late transmission of HIV-1 from mother to child in Tanzania, South Africa, and Uganda: a randomised, double-blind, placebo-controlled trial. Lancet. 2002;359:1178–1186. doi: 10.1016/S0140-6736(02)08214-4. [DOI] [PubMed] [Google Scholar]
- 4.Guay LA, Musoke P, Fleming T, Bagenda D, Allen M, Nakabiito C, et al. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: HIVNET 012 randomised trial. Lancet. 1999;354:795–802. doi: 10.1016/S0140-6736(99)80008-7. [DOI] [PubMed] [Google Scholar]
- 5.Dabis F, Newell ML, Fransen L, Saba J, Lepage P, Leroy V, et al. Prevention of mother-to-child transmission of HIV in developing countries: recommendations for practice. The Ghent International Working Group on Mother-To-Child Transmission of HIV. Health Policy Plan. 2000;15:34–42. doi: 10.1093/heapol/15.1.34. [DOI] [PubMed] [Google Scholar]
- 6.Cartoux M, Meda N, Van de Perre P, Newell ML, de Vincenzi I, Dabis F. Acceptability of voluntary HIV testing by pregnant women in developing countries: an international survey. The Ghent International Working Group on Mother-To-Child Transmission of HIV. AIDS. 1998;12:2489–2493. doi: 10.1097/00002030-199818000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cartoux M, Msellati P, Meda N, Welffens-Ekra C, Mandelbrot L, Leroy V, et al. Attitude of pregnant women towards HIV testing in Abidjan, Côte d’Ivoire and Bobo-Dioulasso, Burkina Faso. AIDS. 1998;12:2337–2344. doi: 10.1097/00002030-199817000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kiarie J, Nduati R, Musia J, John G. HIV-1 testing in pregnancy: acceptability and correlates of return for test results. AIDS. 2000;14:1468–1470. doi: 10.1097/00002030-200007070-00030. [DOI] [PubMed] [Google Scholar]
- 9.Kassler WJ, Dillon BA, Haley C, Jones WK, Goldman A. On-site, rapid HIV testing with same-day results and counseling. AIDS. 1997;11:1045–1051. doi: 10.1097/00002030-199708000-00014. [DOI] [PubMed] [Google Scholar]
- 10.Wilkinson D, Wilkinson N, Lombard C, Martin D, Smith A, Floyd K, et al. On-site HIV testing in resource-poor settings: is one rapid test enough? AIDS. 1997;11:377–381. doi: 10.1097/00002030-199703110-00016. [DOI] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention. Update: HIV counseling and testing using rapid tests – United States, 1995. MMWR. 1998;47:211–215. [PubMed] [Google Scholar]
- 12.Kassler WJ, Alwano-Edyegu MG, Marum E, Biryahwaho B, Kataaha P, Dillon B. Rapid HIV testing with same-day results: a field trial in Uganda. Int J STD AIDS. 1998;9:134–138. doi: 10.1258/0956462981921882. [DOI] [PubMed] [Google Scholar]