Abstract
Klotho is a recently discovered antiaging gene. Klotho is expressed in mouse pancreatic islets and in insulinoma β-cells (MIN6 β-cells). The purpose of this study was to investigate whether Klotho plays a role in the regulation of insulin secretion in MIN6 β-cells by overexpression and silencing of Klotho. It is interesting that overexpression of Klotho increased glucose-induced insulin secretion in MIN6 β-cells. Overexpression of mouse Klotho protein also significantly increased plasma membrane levels of transient receptor potential V2 (TRPV2), calcium entry, and the glucose-induced increase in intracellular calcium. On the other hand, knockdown of Klotho by siRNA significantly decreased plasma membrane levels of TRPV2 and attenuated glucose-induced calcium entry and insulin secretion. Tranilast, a selective inhibitor of TRPV2, abolished the promoting effects of overexpression of Klotho on glucose-induced calcium entry and insulin secretion in MIN6 cells. Thus, TRPV2 lies in the downstream of Klotho in the regulation of glucose-induced insulin secretion. This study demonstrated, for the first time, that Klotho may enhance glucose-induced insulin secretion by up-regulating plasma membrane levels of TRPV2 and thus glucose-induced calcium responses. These findings reveal a previously unidentified role of Klotho in the regulation of glucose-induced insulin secretion in MIN6 β-cells.
Insulin is essential in the regulation of blood glucose levels. It is produced exclusively by β-cells in the islet of Langerhans in the pancreas. The loss of pancreatic β-cell function is the primary etiology of type 1 diabetes. The β-cell dysfunction including decreased insulin secretion has also been considered as an important factor contributing to the pathogenesis of type 2 diabetes (1–3). The development of glucose intolerance and reduced β-cell function has been well recognized as an important process of the human aging process (4).
Insulin is stored in large dense-core granules in pancreatic β-cells and is secreted by Ca2+-dependent exocytosis in response to an elevation of the blood glucose level. In pancreatic β-cells, glucose stimulation evokes an increase in the ATP to ADP ratio, which triggers the closure of ATP-sensitive K+ channels and cell membrane depolarization. Activation of voltage-gated Ca2+ channels by membrane depolarization increases Ca2+ influx, leading to a rise in cytosolic free Ca2+ concentration, which stimulates insulin secretion (5, 6). Most recently the Ca2+-permeable cation channel transient receptor potential V2 (TRPV2) was reported to be involved in the regulation of calcium entry and glucose-induced insulin secretion in MIN6 cells (7, 8). TRVP2 is a member of the transient receptor potential channel family, which is well recognized as unique cellular sensors for mechano- and thermostimulations (8–10). Noxious heat, osmotic and mechanical stimulations, and some molecules have been reported to activate TRPV2 (11–14). It was reported that the cell surface retention of TRPV5 and the activity of TRPV5 in human embryonic kidney 293 cells and renal epithelial cells can be regulated by an antiaging gene, Klotho (15–17). It is not clear, however, whether Klotho regulates the plasma membrane levels of TRPV2 in MIN6 β-cells.
The Klotho gene was identified as a putative antiaging gene named after a Greek goddess Klotho who spins the thread of life (17–19). Overexpression of Klotho extended the life span in mice (19), whereas the mutation of the Klotho gene caused multiple premature-aging phenotypes and a shortened life span (17–19). Klotho is predominantly expressed in the kidney and the brain choroid plexus (17, 18, 20). Klotho-mutant mice displayed hypoinsulimia and pancreatic islet atrophy with decreased insulin storage (21). There are two forms of Klotho, the full-length Klotho (130 kDa) and the short-form Klotho (65 kDa). The full-length mouse Klotho gene contains five exons and encodes a single-pass transmembrane domain of 1014 amino acids (130 kDa). The majority of amino acids in the Klotho peptide resides in the amino-terminal extracellular domain, which is followed by 21 amino acids membrane-spanning domain, and an 11-amino acid short intracellular carboxyl terminus (18). The extracellular domain consists of two internal repeat sequences of 440 amino acids, named KL1 and KL2, respectively (18). The short-form Klotho can be generated by alternative RNA splicing or proteolytic cleavage (17, 18, 20). A spliced Klotho gene composed of exons 1, 2, and 3 encodes a putative secreted protein of 550 amino acids (corresponding to mKL1; calculated molecular mass at 65 kDa), lacking mKL2 and the transmembrane domain (20). Klotho is also found in blood, urine, and cerebrospinal fluid (17, 19, 22). In humans, the Klotho level decreases gradually with advancing age after 40 yr of age (23).
Our preliminary data showed that Klotho was expressed in mouse pancreatic β-cells and MIN6 β-cells. It remains unknown, however, whether Klotho has any function in β-cells. Whether Klotho is involved in the regulation of insulin secretion has never been investigated. The study of the regulation of insulin secretion by Klotho may reveal new insights into effective therapeutic strategies for patients with β-cell dysfunction.
The purpose of this study was to investigate whether Klotho plays a role in the regulation of insulin secretion in MIN6 β-cells by overexpression and silencing of Klotho.
Materials and Methods
Reagents and antibodies
For the sources of the reagents and antibodies, please refer to the Online Data Supplement, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org.
Immunohistochemical analysis of Klotho
The animal study was carried out according to the guidelines of the National Institutes of Health on the care and use of laboratory animals. The project was approved by the Institutional Animal Care and Use Committee. C57BL/6J mice (10 wk old) were euthanized by an overdose of sodium pentobarbital (100 mg/kg·liter, ip) and were perfused transcardiaclly using heparinized saline. After the perfusion, the kidney and pancreas were isolated. Kidneys were stored at −80 C. The pancreas was placed in 4% buffered paraformaldehyde for 24 h and then embedded in paraffin. The paraffin-embedded pancreas of the animal was cut at a thickness of 5 μm. The cross-sections of the mouse pancreas were incubated with anti-Klotho or anti-TRPV2 antibodies and then with horseradish peroxidase-conjugated secondary antibodies. Stable diaminobenzidine was used as a substrate for peroxidase. Hematoxylin was used as a counterstaining. The islet of Langerhans in the cross-section of the pancreas was identified under the microscopy (Eclipse Ti; Nikon, Tokyo, Japan). The images of the islets were collected at equal exposure conditions and at the same magnification.
Mouse pancreatic islet isolation
Mouse pancreatic islets were isolated with a modified protocol as described previously (24). Briefly, collagenase-P was injected into the common bile duct of a mouse. The pancreas was then excised and digested at 37 C. The islets were first purified with premixed Histopaque gradient and then purified by handpicking the separated islets with low-retention pipette tips under a dissecting microscope. When viewed under the microscope, spherical and golden-brown particles (darker color) with a diameter of 50–250 μm were considered as islets.
MIN6 cell culture
Pancreatic insulinoma MIN6 β-cells were kindly provided by Dr. Miyazaki (Kumamoto University Medical School, Kumamoto, Japan) and Dr. Steiner (University of Chicago, Chicago, IL) (25). MIN6 cells were cultured and maintained in DMEM containing 25 mm glucose, 10% fetal bovine serum, 1% penicillin/streptomycin, 2 mm glutamine, and 100 μm β-mercaptoethanol.
Transfection with plasmid DNA
pAAV-mKL with the full length of mouse Klotho cDNA driven by a cytomegalovirus promoter was constructed as described previously (26). Plasmid DNA including pAAV-mKL, pAAV vector, and pAAV-GFP was purified with QIAGEN maxikit (Valencia, CA). MIN6 cells cultured in a six-well plate, 12-well plate, or 10-cm dish were transfected with various plasmid DNA at the concentration of 0.064 μg/ml using Optifect reagent (Invitrogen, Grand Island, NY) according to the manufacturer's protocol, followed by 72 h incubation in DMEM with 10% fetal bovine serum at 37 C in a CO2 incubator.
Small interfering RNA (siRNA) transfection
The duplex of siRNA sequence against mouse Klotho gene (5′-GCGACTACCCAGAGAGTAT-3′) was used as described previously (27). siRNA sequences were synthesized by Ambion (Austin, TX). SiPORT Neo (Ambion) was used as transfection reagent (Invitrogen) according to the instruction. MIN6 cells were incubated with transfection reagent alone, 90 nm control siRNA, or 90 nm mouse Klotho (mKL) siRNA for 72 h.
RNA isolation and RT-PCR
Total RNA was purified from mouse kidney, isolated mouse pancreatic islets, and MIN6 cells using TRIzol reagent, followed by a QIAGEN RNeasy minikit. RNA (500 ng) was reverse transcribed using SuperScript III reverse transcriptase with OligodT20 (Invitrogen) in the presence of 10 μl deoxynucleotide triphosphate for 1 h at 50 C. The resulting cDNA were used as templates for PCR with oligonucleotide primers to amplify the Klotho mRNA and β-actin mRNA.
Two specific primer pairs for mouse Klotho mRNA were used. One pair target exons 1 and 2 of the mouse Klotho cDNA (forward, 5′-CCTGGTCGACCATTTCAG-3′ and reverse, 5′-AGCACAAAGTCGACAGACTTCTGGC-3′), which generated a PCR product of mouse Klotho with 710 bp (18). The other pair targets exons 4 and 5 of mouse Klotho cDNA (forward, 5′-GGGTGACTGGGTCAATCT-3′ and reverse, 5′-GCAAAGTAGCCACAAAGGC-3′), which generated a PCR product of mouse Klotho with 339 bp. The primers for the β-actin gene were used as the internal control. The PCR product for β-actin was 708 bp.
PCR reactions (50 μl volume) contained 3 μl of the above cDNA, 0.2 μm of the appropriate oligonucleotide primer pair, and 1× Taq 2× master mix (New England Biolabs, Beverly, MA). PCR amplification conditions were as follows: 5 min at 95 C followed by 30 cycles of 95 C for 1 min, optimized annealing temperature for each primer pair for 1 min, and 68 C for 1 min. The PCR products were separated on 1.5% agarose gels and stained with ethidium bromide. The bands were visualized using a ChemiDoc System Imager (Bio-Rad Laboratories, Hercules, CA) and quantified using ImageJ software (National Institutes of Health, Bethesda, MD).
Measurement of cytosolic free calcium concentration
Cytoplasmic-free calcium concentration ([Ca2+]c) was monitored using calcium orange as described previously (28, 29). Briefly, after transfection for 72 h, MIN6 cells were subcultured into 96-well plate (coated with 1% gelatin) for overnight incubation and then were washed twice with calcium-free KRB (125 mm NaCl; 4.74 mm KCl; 1.2 mm KH2PO4; 1.2 mm MgSO4; 5 mm NaHCO3; and 25 mm HEPES, pH 7.4). Cells were then incubated in Ca2+-free KRB, containing 10 μm calcium orange for 30 min at room temperature. After washing, calcium-free KRB or ca2+-containing KRB was added. Signals were recorded for 3–5 min to establish the basal line using a Synergy 2 fluorescence 96-well plate reader (BioTek, Winsookie, VT) with excitation wavelength at 530 nm and emission wavelength at 590 nm. The fluorescence signal was recorded for another 10 min after calcium or glucose was added to the well with final concentration of 2 or 25 mm. The relative [Ca2+]c change was calculated by the following formula (30):
where F is the dye fluorescence at any given time and Frest is the average fluorescence signal before an experimental manipulation (e.g. addition of calcium or glucose in the experiments).
Insulin secretion
After MIN6 cells were transfected for 72 h, MIN6 cells were grown in 12-well plate and were starved with KRB supplemented with 0.1% BSA and 2.8 mm glucose for 15 h. Cells were then washed with PBS and incubated with KRB supplemented with 2.8 mm glucose for 1 h. The medium was then changed to KRB buffer with glucose at various concentrations for 1 h. After incubation with glucose, the medium was collected and centrifuged. Cells were washed and lysed with radioimmunoprecipitation assay buffer for measuring the protein concentration using the Pierce bicinchoninic assay (BCA) assay (Rockford, IL). Insulin was measured in the medium with an ELISA kit ALPCO (Salem, NH), and the results were normalized to the amount of protein in the cell lysates.
Fractionation of plasma membrane proteins
A differential centrifugation procedure was used to isolate crude plasma membrane proteins from 70 to 80% confluent MIN6 cells as described previously (31). Four fractions identified by differential centrifugation include the plasma membrane, low-density microsomes, high-density microsomes, and cytosol. We collected the plasma membrane fraction in our study. Briefly, after transfection for 72 h, MIN6 cells were washed with PBS and incubated in serum-free DMEM for 2 h. Cells were then homogenized in HES buffer (20 mm HEPES; 1 mm EDTA; 250 mm sucrose, pH 7.4) containing protease inhibitors. The homogenate was centrifuged at 19,000 × g for 20 min at 4 C. The pellet was resuspended in HES buffer and centrifuged again at 19,000 × g for 20 min. The pellet was then resuspended in HES buffer and layered onto a sucrose cushion (38.5%) and centrifuged for 60 min at 100,000 × g. The plasma membrane fraction was collected from the top of the sucrose cushion (white fluffy band at the sucrose cushion interface), resuspended in HES, and repelleted by centrifugation at 40,000 × g for 20 min. The pellet of plasma membrane fractions were resuspended in HES buffer containing protease inhibitor and stored at −20 C. The protein concentration of these membrane fractions was determined using a Pierce BCA assay.
Western blotting analysis
Western blot analysis was performed as we described previously (2, 32). Briefly, at 70–80% confluence, MIN6 cells were washed twice with PBS. MIN6 cells, mouse kidneys, or isolated pancreatic islets were lysed with radioimmunoprecipitation assay buffer containing the protease inhibitor cocktail. Protein concentration was measured with the Pierce BCA assay. Lysates (40 μg protein/well) under the reducing condition were directly subjected to SDS-PAGE (4–20% Tris-HCL precast gel) followed by Western blotting with the Klotho antibody. The same blot was reprobed with antibody against β-actin after stripping the blot.
For the fractionated plasma membrane proteins, 3 μg of protein under the reducing condition was loaded on each well of SDS-PAGE, followed by Western blotting with antibody against TRPV2 or glucose transporter (GLUT)-2, respectively.
Statistical analysis
Data for Klotho, proinsulin, β-actin, [Ca2+]c, TRPV2, GLUT2, and insulin were analyzed by a two- or one-way ANOVA. The Newman-Keuls procedure was used to assess the significance of differences between means. A probability value with P < 0.05 was considered as statistically significant.
Results
Klotho is expressed in mouse pancreatic islets and MIN6 cells
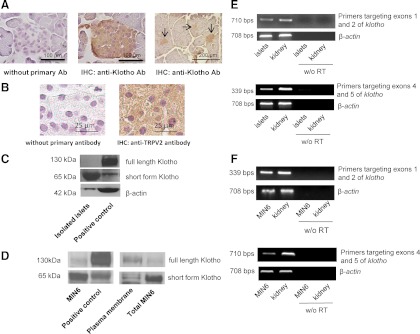
We first examined Klotho protein and mRNA expression in mouse pancreatic islets of Langerhans and MIN6 cells. As shown in the cross-sections of mouse pancreas stained with antimouse Klotho antibody, Klotho protein was detected specifically in pancreatic islets (Fig. 1A). TRPV2 protein was also expressed in pancreatic islets (Fig. 1B). The Western blot analysis showed that Klotho was mainly detected as a band with apparent molecular mass at approximately 65 kDa in the isolated pancreatic islets of Langerhans (Fig. 1C). In MIN6 cells, Klotho was detected as a major band with molecular mass of 65 kDa and a faint band of 130 kDa, respectively (Fig. 1D, left panel). Both full-length and short-form Klotho are expressed in plasma membrane in MIN6 cells (Fig. 1D, right panel). The RT-PCR analysis showed that the full-length Klotho mRNA was expressed in pancreatic islets and MIN6 cells as Klotho mRNA spanned over exons 1, 2, 4, and 5 (Fig. 1, E and F). Therefore, klotho is expressed in both mouse pancreatic islets and MIN6 cells.
Fig. 1.
Klotho is expressed in mouse pancreatic islets and in insulinoma β-cells (MIN6 cells). A, Representatives of Klotho staining of cross-sections of mouse pancreatic islets of Langerhans. Cross-sections of mouse pancreas were stained with antibody against mouse Klotho. Images were collected (×40 objective lens). B, Representatives of TRPV2 staining of cross-sections of mouse pancreatic islets of Langerhans. Cross-sections of mouse pancreas were stained with antibody against mouse TRPV2. Images were collected (×100 objective lens). C, Western blot analysis of Klotho protein expression in pancreatic islets. Lysates of the mouse kidney were used as a positive control because Klotho is mainly expressed in the kidneys. D, Western blot analysis of Klotho protein expression in MIN6 cells. E, RT-PCR analysis of Klotho mRNA in isolated mouse pancreatic islets. Two pairs of primers were used to detect Klotho mRNA. One pair of primers targeted exons 1 and 2 of the mouse Klotho gene. The other pair targeted exons 4 and 5 of mouse the Klotho gene. Klotho mRNA in mouse kidney was used as a positive control. F, RT-PCR analysis of Klotho mRNA in MIN6 cells (n = 3 independent experiments).
Overexpression of Klotho protein increased glucose-induced insulin secretion in MIN6 cells
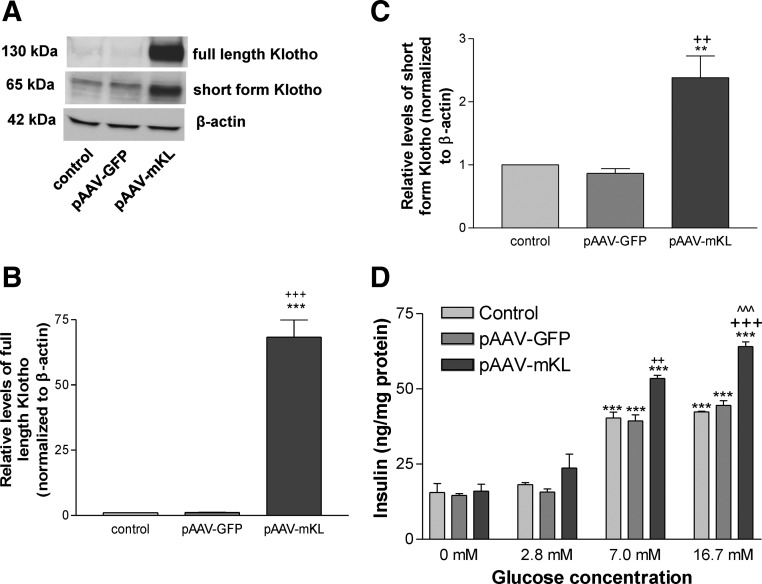
To explore the function of Klotho, we overexpressed Klotho protein in MIN6 cells by transfection with plasmid DNA pAAV-mKL (driven by the cytomegalovirus promoter). As shown in Fig. 2A, transfection with pAAV-mKL for 72 h significantly increased Klotho protein levels compared with the control group (transfection reagent) and the pAAV-GFP group. Klotho gene transfer resulted in both full-length (130 kDa) and short-form (65 kDa) Klotho protein expression (Fig. 2, A–C).
Fig. 2.
Overexpression of Klotho protein increased glucose-induced insulin secretion in MIN6 cells. MIN6 cells were transfected with pAAV-mKL or vehicles (transfection agent alone) for 72 h. A, Western blot analysis of Klotho protein expression. B, Quantification of full-length Klotho protein expression. C, Quantification of short-form Klotho protein expression. Results were standardized to β-actin and then expressed as fold changes vs. the control group (transfection reagent alone). Data are means ± sem (n = 4 independent experiments). **, P < 0.01, ***, P < 0.001 vs. the control group; ++, P < 0.01, +++, P < 0.001 vs. the pAAV-GFP group. D, Insulin secretion from MIN6 cells was determined by measuring insulin levels in the medium. Transfected cells were incubated in serum-free DMEM for 2 h before challenged with glucose at 2.8, 7.0, and 16.7 mm in KRB solution for 1 h (see Materials and Methods). Data are means ± sem (n = 4 independent experiments). **, P < 0.01, ***, P < 0.001 vs. control group (transfection reagent alone) at the glucose concentration of 2.8 mm; ++, P < 0.01, +++, P < 0.001 vs. the pAAV-GFP group at the glucose concentration of 7.0 or 16.7 mm; ^^^, P < 0.001 vs. the pAAV-mKL group at the glucose concentration of 7.0 mm.
Glucose at the concentrations of 7 and 16.7 mm simulated insulin secretion from MIN6 cells as evidenced by significant increases in insulin levels measured in the medium, whereas glucose at the concentration of 2.8 mm did not increase insulin secretion significantly (Fig. 2D). Overexpression of Klotho did not affect the baseline release of insulin (Fig. 2D). However, overexpression of Klotho significantly increased glucose-induced insulin secretion vs. the control groups (transfection reagent alone and pAAV-GFP) when the concentrations of glucose were 7 and 16.7 mm. Therefore, overexpression of Klotho protein enhanced glucose-induced insulin secretion (Fig. 2D). Glucose at a higher concentration (16.7 mm) did not further increase insulin secretion in the control groups vs. the glucose at a concentration of 7.0 mm (Fig. 2D). Notably, glucose at 16.7 mm resulted in a greater insulin release in the pAAV-mKL group vs. glucose at 7.0 mm, indicating that Klotho further enhanced insulin secretion induced by a higher concentration of glucose.
Overexpression of Klotho did not affect insulin mRNA expression or insulin protein levels in MIN6 cells at different concentrations of glucose (Supplemental Figs. 1 and 2, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org).
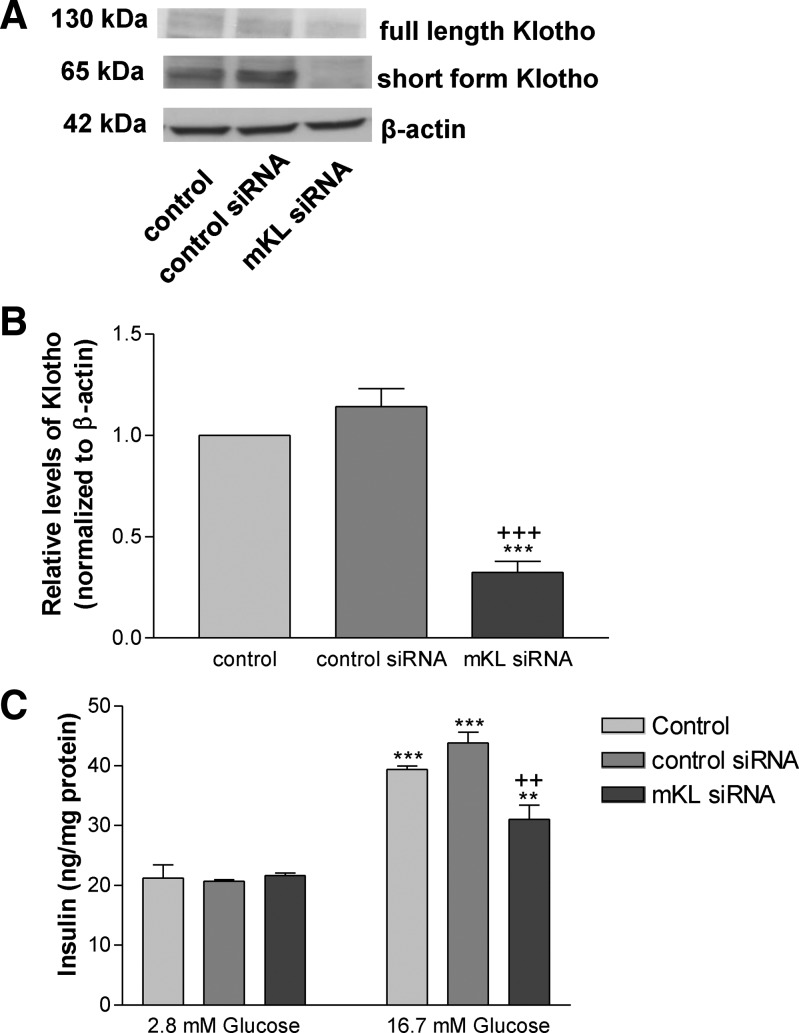
Suppression of Klotho expression attenuated glucose-induced insulin secretion in MIN6 cells
To explore the effect of suppression of Klotho expression on insulin secretion, we silenced Klotho gene expression by transfection of MIN6 cells with siRNA against mouse Klotho. As shown in Fig. 3, A and B, transfection with mKL siRNA for 72 h significantly decreased Klotho protein levels vs. both control groups (transfection reagent group and the pAAV-GFP group) in MIN6 cells, confirming effective silencing of the Klotho gene. Knockdown of Klotho did not affect the insulin release when glucose was at the concentration of 2.8 mm (Fig. 3C). Notably, suppression of Klotho expression significantly attenuated but did not abolish glucose-induced insulin secretion from MIN6 cells as evidenced by a significant decrease in the insulin levels in the medium (Fig. 3C). Therefore, Klotho is essential in the maintenance of normal glucose-induced insulin secretion in MIN6 cells. Silencing of Klotho did not alter insulin protein expression significantly (Supplemental Fig. 3).
Fig. 3.
Suppression of Klotho expression attenuated glucose-induced insulin secretion in MIN6 cells. MIN6 cells were transfected with mKL siRNA or vehicles (transfection agent) for 72 h. A, Western blot analysis of Klotho protein expression. B, Quantification of short-form Klotho protein expression. Results were standardized to β-actin and then expressed as fold changes vs. the control group (transfection agent alone). C, Insulin secretion from MIN6 cells was determined by measuring insulin levels in the medium. Transfected cells were incubated in serum-free DMEM for 2 h before challenged with glucose. Data are means ± sem (n = 4). **, P < 0.01; ***, P < 0.001 vs. the control group; ++, P < 0.01, +++, P < 0.001 vs. the control siRNA group.
Klotho modulated the distribution of TRPV2 on plasma membrane in MIN6 cells
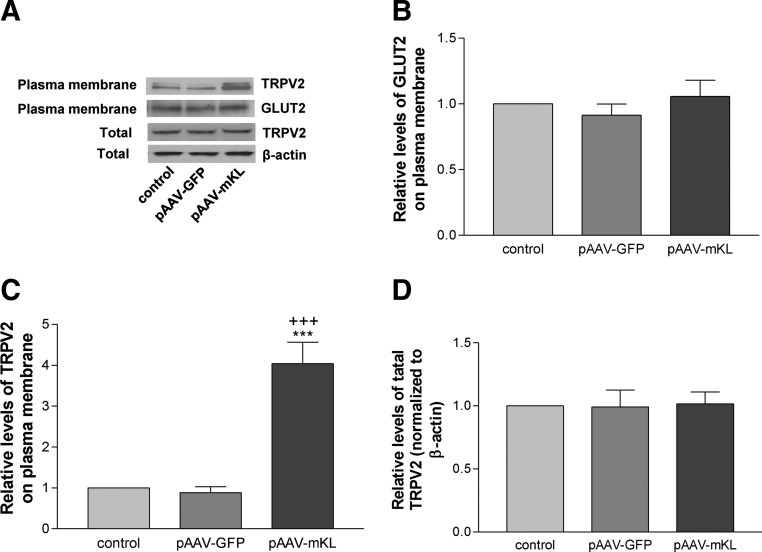
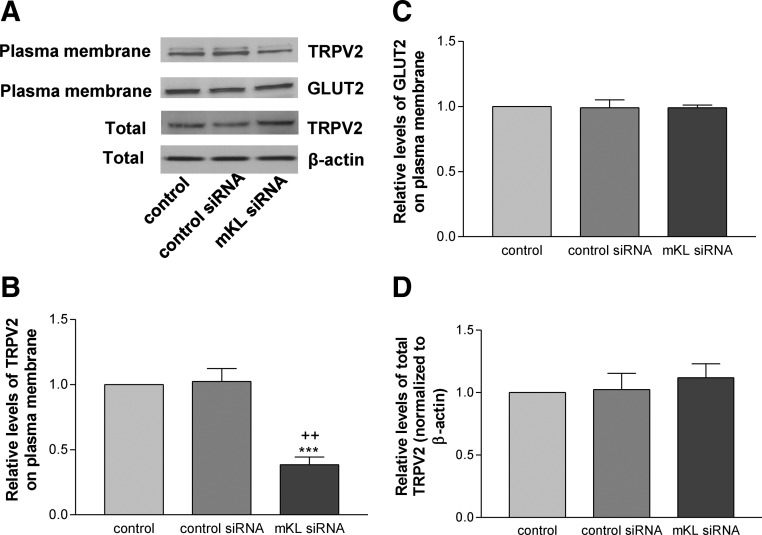
To further assess the molecular mechanism of the effects of klotho on glucose-induced insulin secretion, we examined the distribution TRPV2 on plasma membrane as TRPV2 translocation is involved in insulin secretion (7, 8). As shown in Fig. 4, A–C, overexpression of mouse Klotho significantly increased membrane retention of TRPV2 but did not alter membrane retention of GLUT2 compared with the control groups (transfection reagent alone and pAAV-GFP) in MIN6 cells. Overexpression of mouse Klotho did not affect total protein levels of TRPV2 in MIN6 cells (Fig. 4, A and D).
Fig. 4.
Overexpression of mouse Klotho increased plasma membrane retention of TRPV2 in MIN6 cells. Plasma membrane proteins were fractionated after MIN6 cells were transfected with pAAV-mKL or vehicles for 72 h. A, Western blot analysis of TRPV2 and GLUT2 protein expression in plasma membrane crude fractionation. Total TRPV2 protein level was measured from whole-cell lysates. B, Quantification of TRPV2 in plasma membrane fractionation. C, Quantification of GLUT2 in plasma membrane fractionation. D, Quantification of total TRPV2 in whole-cell lysates. Results were standardized to β-actin and then expressed as fold changes vs. the control group (transfection reagent alone). Data are means ± sem (n = 3). ***, P < 0.001 vs. the control group; +++, P < 0.001 vs. the pAAV-GFP group.
As shown in Fig. 5, A–C, knockdown of the Klotho gene significantly attenuated plasma membrane level of TRPV2 but did not affect membrane level of GLUT2 compared with the both control groups (transfection reagent alone and control siRNA) in MIN6 cells. Knockdown of Klotho did not change total protein levels of TRPV2 in cells (Fig. 5, A and D). These data indicated that Klotho may regulate plasma membrane distribution of TRPV2 in MIN6 cells.
Fig. 5.
Knockdown of Klotho decreased plasma membrane retention of TRPV2 in MIN6 cells. Plasma membrane proteins were fractionated after MIN6 cells were transfected with mKL siRNA or vehicles for 72 h. A, Western blot analysis of TRPV2 and GLUT2 protein expression in plasma membrane crude fractionation. Total TRPV2 protein level was measured from whole-cell lysates. B, Quantification of TRPV2 in plasma membrane fractionation. C, Quantification of GLUT2 in plasma membrane fractionation. D, Quantification of total TRPV2 in whole-cell lysates. Results were standardized to β-actin and then expressed as fold changes vs. the control group (transfection reagent alone). Data are means ± sem (n = 4). ***, P < 0.001 vs. the control group; ++, P < 0.01 vs. the control siRNA group.
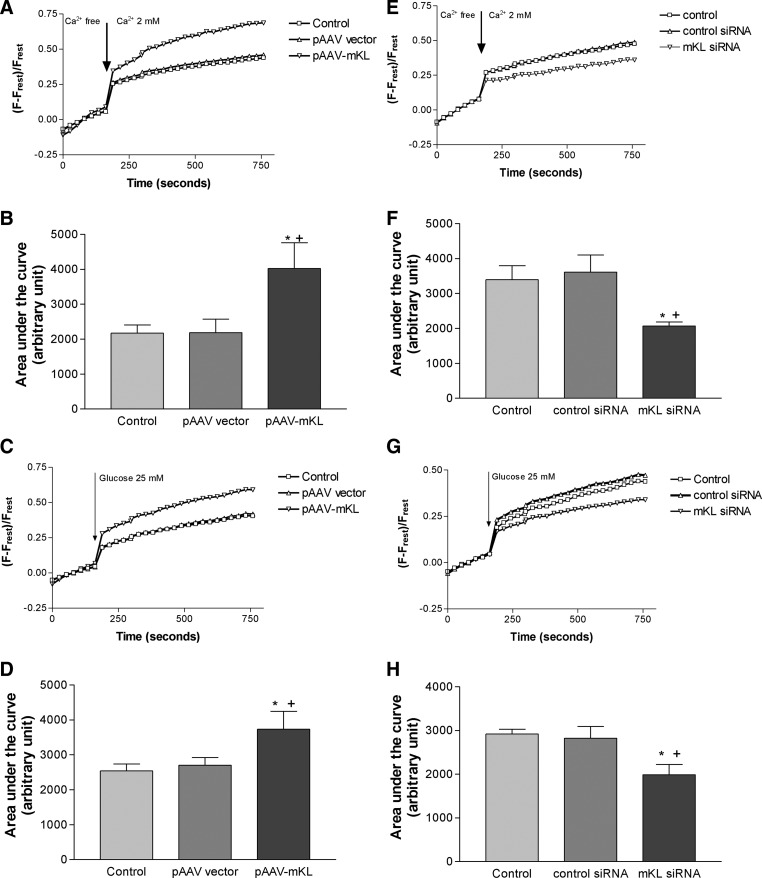
Klotho regulated calcium entry and glucose-induced intracellular calcium response
Calcium is a critical intracellular stimulator in the release of insulin (5, 6). We assessed calcium entry and glucose-induced intracellular calcium response after transfection of pAAV-mKL for 72 h. Overexpression of mouse Klotho significantly increased calcium entry and glucose-induced intracellular calcium response compared with the control groups (transfection reagent alone and pAAV-GFP) (Fig. 6, A–D), whereas knockdown of Klotho significantly attenuated calcium entry and glucose-induced intracellular calcium response compared with the control groups (transfection reagent alone and control siRNA) in MIN6 cells (Fig. 6, E–H). These data suggest that Klotho regulates cytosol free calcium levels in MIN6 cells.
Fig. 6.
Klotho regulated calcium entry and glucose-induced intracellular calcium responses. MIN6 cells were transfected with pAAV-mKL, mKL siRNA, or vehicles for 72 h and then seeded on a 96-well plate overnight. A, Average of calcium entry in cells with overexpression of mouse Klotho. [Ca2+]c change in calcium orange-loaded MIN6 cells was monitored for 3 min in calcium-free KRB and then measured for an additional 10 min after cells were challenged with 2 mm Ca2+. B, Quantification of calcium entry by measuring area under the curve of calcium entry in cells with overexpression of mouse Klotho (n = 4–5). C, Average of glucose-induced intracellular calcium changes in cells with overespression of mouse Klotho. [Ca2+]c change in calcium orange-loaded MIN6 cells was monitored for 3 min in KRB and then measured for an additional 10 min after cells were challenged with 25 mm glucose. D, Quantification of calcium entry by measuring area under the curve of glucose-induced calcium changes in cells with overexpression of mouse Klotho (n = 4–5). pAAV vector rather than pAAV-GFP was used to avoid interference of green fluorescence with calcium orange fluorescence. Data are means ± sem. *, P < 0.05 vs. the control group (transfection reagent alone); +, P < 0.05 vs. the pAAV vector group. E, Average of calcium entry in cells with knockdown of mouse Klotho. [Ca2+]c change was monitored as described in A. F, Quantification of calcium entry by measuring area under the curve of calcium entry in cells with knockdown of Klotho (n = 4–6). G, Average of glucose-induced intracellular calcium changes in cells with the knockdown of mouse Klotho. [Ca2+]c change was monitored as described in C. H, Quantification of calcium entry by measuring the area under the curve of glucose-induced calcium changes in cells with knockdown of Klotho (n = 4–5). Data are means ± sem. *, P < 0.05 vs. the control group (transfection reagent alone); +, P < 0.05 vs. the control siRNA group.
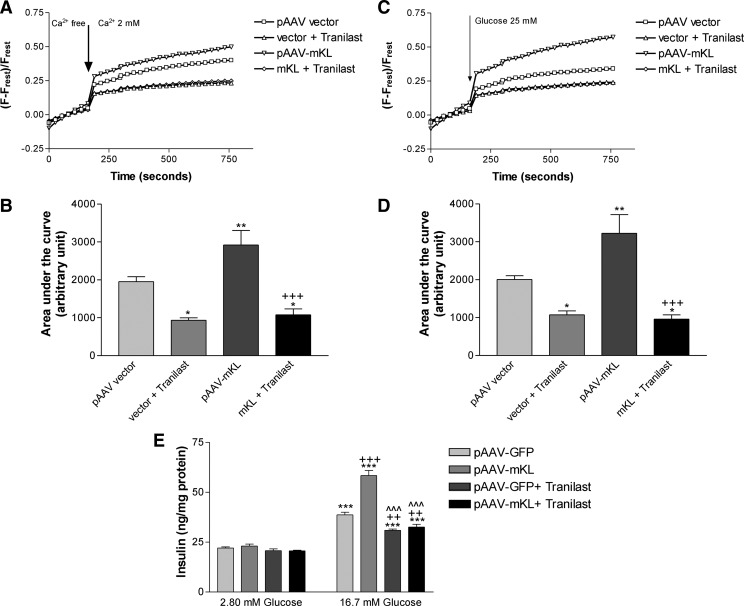
Inhibition of TRPV2 by Tranilast abolished the promoting effects of overexpression of Klotho on calcium entry and glucose-induced calcium responses and insulin secretion
We then examined cytosol-free calcium levels after inhibition of TRPV2 with Tranilast, a selective inhibitor of TRPV2 (7, 8, 33, 34). Treatments with Tranilast decreased basal calcium entry and glucose-induced intracellular calcium responses (vector + Tranilast) (Fig. 7, A–D). Notably, Tranilast abolished the promoting effects of overexpression of Klotho on calcium entry and glucose-induced intracellular calcium responses (Fig. 7, A–D). These results suggest that TRPV2 is a critical mediator for Klotho to regulate calcium entry and glucose-induced calcium responses in MIN6 cells.
Fig. 7.
Inhibition of TRPV2 by Tranilast abolished the promoting effects of overexpression of mouse Klotho on calcium entry and glucose-induced calcium responses and insulin secretion. MIN6 cells were transfected with pAAV-mKL or vehicles for 72 h and then seeded on a 96-well plate overnight. A, Average of calcium entry in cells. [Ca2+]c change in calcium orange-loaded MIN6 cells was monitored for 3 min in calcium-free KRB and then measured for an additional 10 min after 2 mm Ca2+ and/or 75 μm Tranilast was added. B, Quantification of calcium entry by measuring area under the curve of calcium entry (n = 4–5). Data are means ± sem. *, P < 0.05, **, P < 0.01 vs. the control group (pAAV vector); +++, P < 0.001 vs. the pAAV-mKL group. C, Average of glucose-induced intracellular calcium change. [Ca2+]c change in calcium orange-loaded MIN6 cells was monitored for 3 min in KRB and then measured for an additional 10 min after 25 mm glucose and/or 75 μm Tranilast was added. D, Quantification of calcium entry by measuring area under the curve of glucose-induced intracellular calcium change (n = 4–5). Data are means ± sem. *, P < 0.05, **, P < 0.01 vs. the control group (pAAV vector); +++, P < 0.001 vs. the pAAV-mKL group. E, Effects of Tranilast on the promoting effects of Klotho on glucose-induced insulin secretion. Insulin secretion was measured in the medium as described in Fig. 2D after treatments with glucose and/or Tranilast. Data are means ± sem (n = 3). ***, P < 0.001 vs. the control group (pAAV-GFP) with the concentration of glucose at 2.8 mm; ++, P < 0.01, +++, P < 0.001 vs. the control group (pAAV-GFP) with the concentration of glucose at 16.7 mm; ^^^, P < 0.001 vs. the pAAV-mKL group with the concentration of glucose at 16.7 mm.
Inhibition of TRPV2 with Tranilast did not affect insulin secretion from MIN6 cells when the glucose concentration was 2.8 mm (Fig. 7E). Tranilast decreased glucose-induced insulin secretion when the glucose concentration was 16.7 mm vs. the control group (pAAV-GFP) (Fig. 7E). Notably, Tranilast abolished the promoting effects of Klotho on glucose-induced insulin secretion (Fig. 7E), indicating that the enhancing effects of Klotho is TRPV2-dependent.
Discussion
Klotho is a recently discovered aging suppressing gene (17–19). We found that Klotho was expressed in mouse pancreatic islets and MIN6 cells as evidenced by expression of Klotho mRNA and protein in mouse pancreatic islets and insulinoma MIN6 β-cells (Fig. 1). The major Klotho protein in mouse pancreatic islets and MIN6 β-cells is the short-form Klotho with an apparent molecular mass of 65 kDa in Western blot. We therefore investigated whether Klotho plays a role in the regulation of β-cell function by overexpression and silencing of Klotho.
The present study revealed for the first time that overexpression of Klotho increased glucose-induced insulin secretion from MIN6 β-cells (Fig. 2D). However, overexpression of Klotho alone did not alter baseline insulin secretion. These results suggest that Klotho potentiated or enhanced glucose-induced insulin secretion rather than directly stimulated insulin secretion. On the other hand, knockdown of Klotho attenuated glucose-induced insulin secretion (Fig, 3C). Therefore, Klotho is a critical factor that determines the functional responses of MIN6 β-cells to glucose in term of insulin secretion. It is interesting that Klotho may regulate the MIN6 β-cell function. The enhancing effects of Klotho on glucose-induced insulin secretion may be due to an increase in insulin release rather than an increase in insulin synthesis because overexpression of Klotho did not alter Klotho mRNA and protein levels in MIN6 cells treated with glucose (Supplemental Figs. 1 and 2).
TRPV2 is exclusively expressed in β-cells (8). Most recently, TRPV2 has been shown to be involved in the regulation glucose-inducted insulin secretion (7, 8). To gain insights into the mechanisms of enhancing effects of Klotho on glucose-stimulated insulin release, we assessed plasma membrane level of TRPV2 in MIN6 cells. Overexpression of the Klotho gene increased the cell surface level of TRPV2, whereas knockdown of the Klotho gene attenuated cell surface retention of TRPV2 in MIN6 cells (Figs. 4 and 5). The effects of Klotho on TRPV2 distribution did not seem to be global because overexpression or knockdown of Klotho did not cause any changes in plasma membrane level of GLUT2, a glucose transporter in pancreatic β-cells (35). TRPV2 is a membrane calcium channel that determines calcium entry in β-cells (5, 6). It is noted that calcium entry and glucose-induced intracellular calcium responses were increased by overexpression of the Klotho gene but were down-regulated by knockdown of the Klotho gene (Fig. 6). Interestingly, the enhancing effects of overexpression of the Klotho on calcium entry and glucose-induced intracellular calcium responses and insulin secretion were abolished by the inhibition of TRPV2 with Tranilast (Fig. 7). These findings suggest, for the first time, that TRPV2 is essential for the potentiating effects of Klotho on glucose-induced insulin secretion in MIN6 cells. Activation of membrane TRPV2 increases Ca2+ influx leads to a rise in cytosolic-free Ca2+ concentration, which stimulates insulin secretion (5, 6). TRPV2 is a calcium-permeable channel that also permeates sodium (10). Thus, translocation of TRPV2 may increase sodium entry into cells as well, leading to depolarization of the plasma membrane and subsequent activation of the voltage-dependent calcium channels. Currently few specific antagonists for TRPV2 have been identified (36). Tranilast was used as a selective inhibitor of TRPV2 in our experiments because it had been shown to achieve the same inhibition of TRPV2 function as knockdown of TRPV2 with siRNA techniques in MIN6 cells (7, 8).
Despite the sequence homology to glycosidases, the endogenous substrates or interaction partners for Klotho remain controversial. It has been reported that Klotho exhibited weak β-glucuronidase activity in vitro (37). Treatments with Klotho have been shown to increase cell surface abundance of TRPV5 in human embryonic kidney 293 cells by hydrolysis of its extracellular N-linked glycans through glucuronidase activity of Klotho (16). Klotho also participated in removal of α2,6-linked sialic acids of TRPV5 through its sialidase activity in Chinese hamster ovary cells (15). Removal of terminal sialic acids from N-glycans exposes the underlying LacNAc for binding with galectin-1, leading to enhanced retention of TRPV5 at the cell surface (15). Given that TRPV2 does have a potential N-glycosylation site (10, 17), it would be interesting to investigate a hypothesis that Klotho targets this N-linked glycan or sialic acid on the N-glycan of TRPV2 to regulate the retention of TRPV2 on plasma membrane in MIN6 cells.
Most recently the importance of pancreatic β-cell dysfunction in the development of type 2 diabetes has been increasingly appreciated (1). It is now well accepted that when insulin resistance develops in response to metabolic dysfunction such as obesity, a subset of genetically predisposed individuals fails to adequately compensate for the increased insulin demand, and β-cell failure ensues (38). In addition, longitudinal studies in humans have clearly demonstrated that β-cell function deteriorates during the years after the diagnosis of type 2 diabetes, regardless of the therapeutic regimen (39). Chronic hyperglycemia and dislipidemia have been thought to contribute to the worsening of β-cell functions over time (40). The Klotho level was decreased in a mouse model of type 2 diabetes (41). Klotho-deficient mice exhibit hypoinsulinemia and pancreatic islet atrophy with diminished insulin protein and mRNA levels (decreased β-cell function) (21). Therefore, it is interesting to investigate whether the Klotho gene delivery can enhance insulin secretion in diabetic animal models. Such a study holds great promise for developing an effective therapeutic strategy for type 2 diabetes with β-cell dysfunction.
The Klotho gene was identified as a putative antiaging gene (17–19). Overexpression of Klotho extended the life span in mice (19), whereas the mutation of the Klotho gene caused multiple premature-aging phenotypes and shortened the life span (17–19). Genetic Klotho deficiency leads to hypoinsulimia and pancreatic β-cell dysfunction in mice (17, 21). In humans, the Klotho level declines with advancing age after 40 yr of age (17, 23). The human aging is characterized by reduced insulin secretion from β-cell and increased insulin resistance in the peripheral tissues (4). A further study is warranted to determine whether Klotho deficiency may contribute to the aging-related impairment in β-cell function.
The major endogenous Klotho protein expressed in MIN6 β-cells is the short-form Klotho protein (65 kDa) (Fig. 1). Interestingly, the transfer of the full-length Klotho gene led to expression of both full-length and short-form Klotho proteins with apparent molecular mass of 130 and 65 kDa, respectively, in MIN6 cells (Fig. 2). The limitation of this study is that it cannot differentiate whether the promoting effect of the Klotho gene transfer is due to expression of short-form klotho or full-length klotho. It should be mentioned that MIN6 may respond to Klotho differently vs. the primary β-cells in term of glucose-induced insulin secretion. Additional studies are needed to determine the role of Klotho in the other β-cell lines and in the in vivo conditions.
In summary, this study revealed a previously unidentified role of Klotho in the regulation of glucose-induced insulin secretion in MIN6 β-cells. Overexpression of mouse Klotho enhanced plasma retention of TRPV2, calcium entry, and glucose-induced intracellular calcium responses and insulin secretion, whereas knockdown of Klotho attenuated these events. Inhibition of TRPV2 with Tranilast abolished the enhancing effects of overexpression of Klotho on calcium entry and glucose-induced intracellular calcium responses and insulin secretion. These findings strongly suggest that Klotho enhances glucose-induced insulin secretion via up-regulating membrane retention of TRPV2.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grants R01 HL102074 and HL105302.
Disclosure Summary: Both authors declare that there are no conflicts of interest to disclose.
Footnotes
- BCA
- Bicinchoninic assay
- [Ca2+]c
- calcium concentration
- GLUT
- glucose transporter
- mKL
- mouse Klotho
- siRNA
- small interfering RNA
- TRPV2
- transient receptor potential V2.
References
- 1. Defronzo RA. 2009. Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 58:773–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lin Y, Sun Z. 2011. Thyroid hormone potentiates insulin signaling and attenuates hyperglycemia and insulin resistance in a mouse model of type 2 diabetes. Br J Pharmacol 162:597–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Marchetti P, Dotta F, Lauro D, Purrello F. 2008. An overview of pancreatic β-cell defects in human type 2 diabetes: implications for treatment. Regul Pept 146:4–11 [DOI] [PubMed] [Google Scholar]
- 4. Scheen AJ. 2005. Diabetes mellitus in the elderly: insulin resistance and/or impaired insulin secretion? Diabetes Metab 31(Spec No. 2):5S27–5S34 [DOI] [PubMed] [Google Scholar]
- 5. Henquin JC. 2009. Regulation of insulin secretion: a matter of phase control and amplitude modulation. Diabetologia 52:739–751 [DOI] [PubMed] [Google Scholar]
- 6. Wang Z, Thurmond DC. 2009. Mechanisms of biphasic insulin-granule exocytosis—roles of the cytoskeleton, small GTPases and SNARE proteins. J Cell Sci 122:893–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Aoyagi K, Ohara-Imaizumi M, Nishiwaki C, Nakamichi Y, Nagamatsu S. 2010. Insulin/phosphoinositide 3-kinase pathway accelerates the glucose-induced first-phase insulin secretion through TrpV2 recruitment in pancreatic β-cells. Biochem J 432:375–386 [DOI] [PubMed] [Google Scholar]
- 8. Hisanaga E, Nagasawa M, Ueki K, Kulkarni RN, Mori M, Kojima I. 2009. Regulation of calcium-permeable TRPV2 channel by insulin in pancreatic β-cells. Diabetes 58:174–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Caterina MJ, Rosen TA, Tominaga M, Brake AJ, Julius D. 1999. A capsaicin-receptor homologue with a high threshold for noxious heat. Nature 398:436–441 [DOI] [PubMed] [Google Scholar]
- 10. Kanzaki M, Zhang YQ, Mashima H, Li L, Shibata H, Kojima I. 1999. Translocation of a calcium-permeable cation channel induced by insulin-like growth factor-I. Nat Cell Biol 1:165–170 [DOI] [PubMed] [Google Scholar]
- 11. Iwata Y, Katanosaka Y, Arai Y, Komamura K, Miyatake K, Shigekawa M. 2003. A novel mechanism of myocyte degeneration involving the Ca2+-permeable growth factor-regulated channel. J Cell Biol 161:957–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Monet M, Gkika D, Lehen'kyi V, Pourtier A, Vanden Abeele F, Bidaux G, Juvin V, Rassendren F, Humez S, Prevarsakaya N. 2009. Lysophospholipids stimulate prostate cancer cell migration via TRPV2 channel activation. Biochim Biophys Acta 1793:528–539 [DOI] [PubMed] [Google Scholar]
- 13. Muraki K, Iwata Y, Katanosaka Y, Ito T, Ohya S, Shigekawa M, Imaizumi Y. 2003. TRPV2 is a component of osmotically sensitive cation channels in murine aortic myocytes. Circ Res 93:829–838 [DOI] [PubMed] [Google Scholar]
- 14. Qin N, Neeper MP, Liu Y, Hutchinson TL, Lubin ML, Flores CM. 2008. TRPV2 is activated by cannabidiol and mediates CGRP release in cultured rat dorsal root ganglion neurons. J Neurosci 28:6231–6238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cha SK, Ortega B, Kurosu H, Rosenblatt KP, Kuro-O M, Huang CL. 2008. Removal of sialic acid involving Klotho causes cell-surface retention of TRPV5 channel via binding to galectin-1. Proc Natl Acad Sci USA 105:9805–9810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chang Q, Hoefs S, van der Kemp AW, Topala CN, Bindels RJ, Hoenderop JG. 2005. The β-glucuronidase klotho hydrolyzes and activates the TRPV5 channel. Science (New York, NY) 310:490–493 [DOI] [PubMed] [Google Scholar]
- 17. Wang Y, Sun Z. 2009. Current understanding of klotho. Ageing Res Rev 8:43–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima YI. 1997. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 390:45–51 [DOI] [PubMed] [Google Scholar]
- 19. Kurosu H, Yamamoto M, Clark JD, Pastor JV, Nandi A, Gurnani P, McGuinness OP, Chikuda H, Yamaguchi M, Kawaguchi H, Shimomura I, Takayama Y, Herz J, Kahn CR, Rosenblatt KP, Kuro-o M. 2005. Suppression of aging in mice by the hormone Klotho. Science (New York, NY) 309:1829–1833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shiraki-Iida T, Aizawa H, Matsumura Y, Sekine S, Iida A, Anazawa H, Nagai R, Kuro-o M, Nabeshima Y. 1998. Structure of the mouse klotho gene and its two transcripts encoding membrane and secreted protein. FEBS Lett 424:6–10 [DOI] [PubMed] [Google Scholar]
- 21. Utsugi T, Ohno T, Ohyama Y, Uchiyama T, Saito Y, Matsumura Y, Aizawa H, Itoh H, Kurabayashi M, Kawazu S, Tomono S, Oka Y, Suga T, Kuro-o M, Nabeshima Y, Nagai R. 2000. Decreased insulin production and increased insulin sensitivity in the klotho mutant mouse, a novel animal model for human aging. Metabolism 49:1118–1123 [DOI] [PubMed] [Google Scholar]
- 22. Imura A, Iwano A, Tohyama O, Tsuji Y, Nozaki K, Hashimoto N, Fujimori T, Nabeshima Y. 2004. Secreted Klotho protein in sera and CSF: implication for post-translational cleavage in release of Klotho protein from cell membrane. FEBS Lett 565:143–147 [DOI] [PubMed] [Google Scholar]
- 23. Xiao NM, Zhang YM, Zheng Q, Gu J. 2004. Klotho is a serum factor related to human aging. Chin Med J (Engl) 117:742–747 [PubMed] [Google Scholar]
- 24. Carter JD, Dula SB, Corbin KL, Wu R, Nunemaker CS. 2009. A practical guide to rodent islet isolation and assessment. Biol Proced Online 11:3–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Miyazaki J, Araki K, Yamato E, Ikegami H, Asano T, Shibasaki Y, Oka Y, Yamamura K. 1990. Establishment of a pancreatic beta cell line that retains glucose-inducible insulin secretion: special reference to expression of glucose transporter isoforms. Endocrinology 127:126–132 [DOI] [PubMed] [Google Scholar]
- 26. Wang Y, Sun Z. 2009. Klotho gene delivery prevents the progression of spontaneous hypertension and renal damage. Hypertension 54:810–817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chihara Y, Rakugi H, Ishikawa K, Ikushima M, Maekawa Y, Ohta J, Kida I, Ogihara T. 2006. Klotho protein promotes adipocyte differentiation. Endocrinology 147:3835–3842 [DOI] [PubMed] [Google Scholar]
- 28. Prendergast MA, Harris BR, Mullholland PJ, Blanchard JA, 2nd, Gibson DA, Holley RC, Littleton JM. 2004. Hippocampal CA1 region neurodegeneration produced by ethanol withdrawal requires activation of intrinsic polysynaptic hippocampal pathways and function of N-methyl-d-aspartate receptors. Neuroscience 124:869–877 [DOI] [PubMed] [Google Scholar]
- 29. Xu Y, Krukoff TL. 2007. Adrenomedullin stimulates nitric oxide production from primary rat hypothalamic neurons: roles of calcium and phosphatases. Mol Pharmacol 72:112–120 [DOI] [PubMed] [Google Scholar]
- 30. Paredes RM, Etzler JC, Watts LT, Zheng W, Lechleiter JD. 2008. Chemical calcium indicators. Methods (San Diego, Calif) 46:143–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Elmendorf JS. 2003. Fractionation analysis of the subcellular distribution of GLUT-4 in 3T3-L1 adipocytes. Methods Mol Med 83:105–111 [DOI] [PubMed] [Google Scholar]
- 32. Dammanahalli JK, Wang X, Sun Z. 2011. Genetic interleukin-10 deficiency causes vascular remodeling via the upregulation of Nox1. J Hypertens 29:2116–2125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nie L, Kanzaki M, Shibata H, Kojima I. 1998. Activation of calcium-permeable cation channel by insulin in Chinese hamster ovary cells expressing human insulin receptors. Endocrinology 139:179–188 [DOI] [PubMed] [Google Scholar]
- 34. Nie L, Oishi Y, Doi I, Shibata H, Kojima I. 1997. Inhibition of proliferation of MCF-7 breast cancer cells by a blocker of Ca(2+)-permeable channel. Cell Calcium 22:75–82 [DOI] [PubMed] [Google Scholar]
- 35. Guillam MT, Dupraz P, Thorens B. 2000. Glucose uptake, utilization, and signaling in GLUT2-null islets. Diabetes 49:1485–1491 [DOI] [PubMed] [Google Scholar]
- 36. Vriens J, Appendino G, Nilius B. 2009. Pharmacology of vanilloid transient receptor potential cation channels. Mol Pharmacol 75:1262–1279 [DOI] [PubMed] [Google Scholar]
- 37. Tohyama O, Imura A, Iwano A, Freund JN, Henrissat B, Fujimori T, Nabeshima Y. 2004. Klotho is a novel β-glucuronidase capable of hydrolyzing steroid β-glucuronides. J Biol Chem 279:9777–9784 [DOI] [PubMed] [Google Scholar]
- 38. Prentki M, Nolan CJ. 2006. Islet β cell failure in type 2 diabetes. J Clin Invest 116:1802–1812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kahn SE, Haffner SM, Heise MA, Herman WH, Holman RR, Jones NP, Kravitz BG, Lachin JM, O'Neill MC, Zinman B, Viberti G. 2006. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med 355:2427–2443 [DOI] [PubMed] [Google Scholar]
- 40. Poitout V, Amyot J, Semache M, Zarrouki B, Hagman D, Fontés G. 2010. Glucolipotoxicity of the pancreatic β cell. Biochim Biophys Acta 1801:289–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhao Y, Banerjee S, Dey N, LeJeune WS, Sarkar PS, Brobey R, Rosenblatt KP, Tilton RG, Choudhary S. 2011. Klotho depletion contributes to increased inflammation in kidney of the db/db mouse model of diabetes via RelA (serine)536 phosphorylation. Diabetes 60:1907–1916 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.