Abstract
In steroidogenic tissues, cholesterol must be transported to the inner mitochondrial membrane to be converted to pregnenolone as the first step of steroidogenesis. Whereas steroidogenic acute regulatory protein has been shown to be responsible for the transport of cholesterol from the outer to the inner mitochondrial membrane, the process of how cholesterol moves to mitochondria from the cytoplasm is not clearly defined. The involvement of the cytoskeleton has been suggested; however, no specific mechanism has been confirmed. In this paper, using genetic ablation of an intermediate filament protein in mice, we present data demonstrating a marked defect in adrenal and ovarian steroidogenesis in the absence of vimentin. Cosyntropin-stimulated corticosterone production is decreased 35 and 50% in male and female Vimentin null (Vim−/−) mice, respectively, whereas progesterone production is decreased 70% in female Vim−/− mice after pregnant mare's serum gonadotropin and human chorionic gonadotropin stimulation, but no abnormalities in human chorionic gonadotropin-stimulated testosterone production is observed in male Vim−/− mice. These defects in steroid production are also seen in isolated adrenal and granulosa cells in vitro. Further studies show a defect in the movement of cholesterol from the cytosol to mitochondria in Vim−/− cells. Because the mobilization of cholesterol from lipid droplets and its transport to mitochondria is a preferred pathway for the initiation of steroid production in the adrenal and ovary but not the testis and vimentin is a droplet-associated protein, our results suggest that vimentin is involved in the movement of cholesterol from its storage in lipid droplets to mitochondria for steroidogenesis.
During steroidogenesis, cholesterol is converted to various steroid hormones in the adrenal and gonads (1). There are three sources from which cholesterol can be derived for use as a steroid precursor: de novo synthesis from acetate in the cell, hydrolysis from cholesterol ester (CE) stores within intracellular lipid droplets (LD), and uptake of cholesterol from plasma lipoproteins. Cholesterol within the cell, however, needs to be transported from its site of synthesis or release within the cytoplasm into mitochondria before it can be converted to pregnenolone as the initial enzymatic step in the synthesis of steroid hormones. The transfer of cholesterol from the cytoplasm into mitochondria is considered to be the rate-limiting step in steroidogenesis. The involvement of the cytoskeleton in this process has long been under investigation (2–5); however, no specific mechanisms involving the cytoskeleton have been confirmed. In fact, conflicting results have been obtained in studies using various cytoskeletal inhibitors, due partly to the lack of specificity of the inhibitors. Recent studies have shown that intermediate filament (IF) proteins, which constitute part of the cytoskeleton yet are distinct from microtubules and microfilaments, could be involved in dynamic scaffolding of the cytoskeleton in response to various stress conditions and appear to display cytoprotective and tissue specific functions (6).
Vimentin is an IF protein that is expressed in mesenchymal cells, including adrenal cells (7, 8). Several different reports of proteomic analyses of LD isolated from cells have consistently identified vimentin as an LD associated protein, and vimentin has been noted to be attached to and to form a capsule around LD (8–10). Vimentin has been shown to interact with several different proteins, including some with motor-like properties and sterol binding properties (11–13). In addition, vimentin has been reported to function as a reservoir for one of the soluble N-ethylmaleimide sensitive fusion factor attachment protein receptor components, synaptosomal-associated protein of 23 kDa, thus potentially linking vimentin indirectly to membrane fusion (14). Using a proteomics approach, vimentin was identified as an interacting partner of agonist stimulated β3-adrenergic receptors and this interaction was shown to be important for activation of ERK and stimulation of lipolysis (15), providing additional evidence for involvement of vimentin in LD metabolism. Additionally, we have provided evidence that vimentin interacts with hormone-sensitive lipase (HSL) and that this interaction influences lipolysis and movement of HSL to the LD (16). Vimentin null (Vim−/−) mice develop normally and can reproduce with no obvious phenotype (17).
In the current paper, using genetic ablation of vimentin in mice, we present data demonstrating a marked defect in steroidogenesis in the absence of vimentin that can be demonstrated under both in vivo and in vitro conditions. This blunted steroidogenic response is especially apparent under stimulated conditions, and appears to be due to a defect in cholesterol movement to the mitochondria.
Materials and Methods
Chemicals and reagents
Reagents were obtained from the following sources: cholesterol assay kit from Stanbio (Boerne, TX); bicinchoninic acid assay protein kit from Pierce Biotechnology, Inc. (Rockford, IL); organic solvents were from J. T. Baker (Phillipsburg, NJ); TRIzol reagent and SuperScript II from Invitrogen (Carlsbad, CA); RNeasy kit from QIAGEN (Valencia, CA); SyBr green Taqman PCR kit from Applied Biosystems (Foster City, CA); Odyssey blocking buffer, goat antimouse IgG-IRDye 800, donkey antigoat IgG-IRDye 680, and goat antirabbit IgG-IRDye 680 from Li-Cor Biosciences (Lincoln, NE). All other reagents were from Sigma (St. Louis, MO), unless otherwise noted.
Animals and treatments
Mice with homologous ablation of vimentin on a sv129 background were obtained from Dr. John Eriksson (Åbo Akademi University, Turku, Finland) (18). Mice were maintained in the animal facility at the Veterans Affairs Palo Alto Health Care System on a 12-h light, 12-h dark cycle. All procedures were in accordance with institution guidelines and approved by the institutional animal care and use committee of the Veterans Affairs Palo Alto Health Care System. For breeding experiments, mice heterozygous for the deleted vimentin allele were bred to generate Vim−/− and vimentin+/+ wild-type (WT) littermates. Genotyping was performed as described previously (19). For cosyntropin treatment, age-matched adult animals were treated with 0.8 μg/kg cosyntropin for 1 h before blood was drawn. To measure the cholesterol content in mitochondria, age-matched animals were treated with aminoglutethimide (0.5 g/kg) for 4 h before cosyntropin treatment for 1 h and collection of adrenals. After homogenization of intact adrenals, mitochondria were isolated by differential centrifugation and total cholesterol content measured using a kit from Stanbio (20).
Isolation of primary adrenocortical, ovarian granulosa, and testicular Leydig cells for in vitro steroid production
For isolation of adrenocortical cells, age matched WT and Vim−/− mice were killed and adrenals removed and cleaned free of fat. Adrenals from 10 mice were pooled and finely minced with scissors and digested with collagenase in a shaking incubator for 50 min. The resulting cell suspensions were washed three times before plating in medium 199 and incubated for 48 h. Cultured adrenocortical cells were treated with dibutyryl cAMP (Bt2cAMP) for an additional 24 h and media collected for analysis of corticosterone using RIA.
Granulosa cells from WT and Vim−/− mice were prepared as described previously (21). Briefly, immature female mice (22–25 d old) were injected once with 5 IU of pregnant mare's serum gonadotropin (PMSG) for 48 h. After hormone treatment, the ovaries were excised and placed in basal medium [DMEM:F12 with 20 mm HEPES (pH 7.4)] supplemented with 100 U/ml penicillin and 100 mg/ml streptomycin. Clumps of mural granulosa cells and oocyte-cumulus complexes were released into the medium by puncturing follicles with a 25-gauge needle. The mural granulosa cells were collected in DMEM/F12/HEPES/BSA medium and dispersed by being gently drawn in and out of a Pasteur pipette. The granulosa cells were washed and resuspended in fresh medium and cultured as described previously (22–24). The cells were maintained at 37 C up to 72 h in basal medium (DMEM:F12 supplemented with 2 μg/ml insulin, 5 μg/ml transferrin, 100 ng/ml hydrocortisone, and 2 μg/cm2 human fibronectin). They were then incubated with or without Bt2cAMP for 24 h and/or human apoE free high-density lipoprotein (hHDL3; 500 μg/ml) for an additional 24 h. Granulosa cells from PMSG-treated mice are partially luteinized. As such, these cells may be programmed to take in lipoprotein-derived CE. However, expression of a maximal secretory response in these cells requires further stimulation with 2.5 mm Bt2cAMP for 24–48 h. After the incubation, media were collected and progesterone production measured using RIA.
For isolation of Leydig cells, testicular interstitial cells containing Leydig cells were isolated by mechanical dispersion of decapsulated testis obtained from WT and Vim−/− mice. Highly purified (>85%) Leydig cell preparations were obtained by subjecting interstitial cell suspensions to Percoll density gradient centrifugation as previously described (25). To assay steroidogenesis, triplicate samples of freshly purified Leydig cells were incubated without (basal) or with human chorionic gonadotropin (hCG;10 ng/ml), Bt2cAMP (2.5 mm) or 20α-hydroxycholesterol (10 μm) for 3 h, and subsequently samples of incubation medium were frozen and stored until assayed for secreted testosterone by RIA (26).
Tissue section and staining of adrenal and ovary
Adrenals and ovaries were fixed in Tissue Tek optimal cutting temperature (Sakura Finetek USA, Torrance, CA) and processed into 10-μm sections before staining with oil-red-O and hematoxylin and eosin. The diameters of 100 adrenal cells and LD per animal were measured by microscopy and determined using ImageQuant TL software (Amersham Biosciences Corp., Piscataway, NJ).
Selective uptake using double-labeled lipoproteins
Human low-density lipoproteins, hHDL3, and rat high-density lipoprotein were isolated and characterized as previously described (22–24). For uptake and internalization studies, the lipoproteins were equipped with two nonreleasable labels, i.e. [125I]-labeled dilactitol tyramine to mark lipoprotein proteins and [3H]cholesteryl oleolyl ether to mark lipoprotein CE (22, 27). To measure cholesterol uptake into different organs, equal amounts of double particles per body weight were injected into age-matched WT and Vim−/− mice through tail veins, and animals were killed after 6 h and adrenal, ovary, and liver removed. Tissues were homogenized and processed to extract internalized and surface bound particles. Relative specific activities of double-labeled particles and estimates of protein to cholesterol ratios permit correction of data for surface binding of particles, internalization of intact particles (endocytic pathway), and internalization of CE without apolipoproteins (selective pathway) (21, 27) were calculated.
Immunoblotting
Immunoblotting was performed as described previously (28). Briefly, adrenals were homogenized in 50 mm Tris-HCl (pH 7.4), 8% sucrose, 1 mm EDTA, 0.1 mm Na3VO4, and 50 mm NaF with 10 μg/ml leupeptin, and protein concentrations were determined by bicinchoninic acid protein assays. Approximately 20-μg proteins were resolved by 4–15% gradient SDS-PAGE gel and blotted onto nitrocellulose membranes. Membranes were blocked with Odyssey blocking buffer (Li-Cor Biosciences) for 2 h at room temperature and were incubated with primary antibodies at the following dilutions: anti-scavenger receptor type B-I (SRB-I) (1:1000), anti- low density lipoprotein receptor (LDLR) (1:1000), anti-β-actin (1:1000). Membranes were incubated with the appropriate secondary antibody conjugated to infrared dye (goat antimouse IgG-IR dye 800cw, and donkey antigoat IgG-IR dye 680cw, goat antirabbit IgG-IR dye 680cw) at room temperature for 1 h, washed three times with PBS (0.1% Tween 20), rinsed with PBS, and then detected by an Odyssey infrared fluorescent imaging system (Li-Cor Biosciences).
RNA isolation and quantitative real time PCR analysis
For RNA isolation, adrenal samples were homogenized in 1 ml of TRIzol reagent using a power homogenizer (Ultra-Turrax T25; Labortechnik, Gottingen, Germany), and total RNA was isolated using TRIzol reagent. After the ethanol precipitation step, total RNA was dissolved in 30 μl ribonuclease-free water and reamplified to amplified RNA and then converted to cDNA for real-time PCR analysis. Real-time PCR was performed with the cDNA prepared as above using an ABI Prism 8500 System (Applied Biosystems) using SYBR green master mix reagent and the primer pairs used listed in Table 1. The relative mass of specific RNA was calculated by the comparative cycle of threshold detection method according to the manufacturer's instruction. Three independent sets of real-time PCR were performed using different RNA preparations. Genes examined included: SRB-I, LDLR, and LDL receptor related protein 1 (LRP1) for cholesterol uptake as well as genes involved in the initial steps of steroid hormone production, including CYP11A1, CYP17A, CYP11B1, CYP21A2, and 3βHSDII.
Table 1.
Primers for Taqman PCR
| Gene | Assession no. | Sequence (5-′–3′) |
|---|---|---|
| CYP17A | NM_007809 | GCCTGGGTACCACAACTGCAGTG |
| TCCTTGGTCCGACAAGAGGCCT | ||
| CYP11B1 | NM_001033229 | CGTGTTTGTGACGTTGCCGGA |
| CACACCACGTCCCAGGCCAC | ||
| CYP21A1 | NM_009995 | TTCCTACAGCCTAACCTTCCCATCT |
| GAGCAGAGGCAGCTGCATTCG | ||
| CYP21A2 | NM_000500 | TGGACGTGATTCCCTTTCTC |
| CACCCCTTGGAGCATGTAGT | ||
| 3ßHSDII | NM_153193 | TCCAGCTCAGTTGATGTTGC |
| TGCCTTCTCAGCCATCTTTT | ||
| SR-BI | NM_016741 | AAGTGGTCAACCCAAACGAG |
| ACGGTGTCGTTGTCATTGAA | ||
| LRP1 | NM_008512 | ACCACCAGCTACCTCATTGG |
| CCTGGCCACACTAATGGTCT | ||
| LDLR | NM_010700 | TCCTGGAGATGTGATGGACA |
| GAGCCATCTAGGCAATCTCG |
Statistics
Data are expressed as means ± sem. Statistical analyses were performed by two-way ANOVA using Prism 5 for Mac OS X (GraphPad Software, Inc., La Jolla, CA). Differences between groups were considered statistically significant when P < 0.05.
Results
Effects of ablation of vimentin on circulating steroid hormone levels
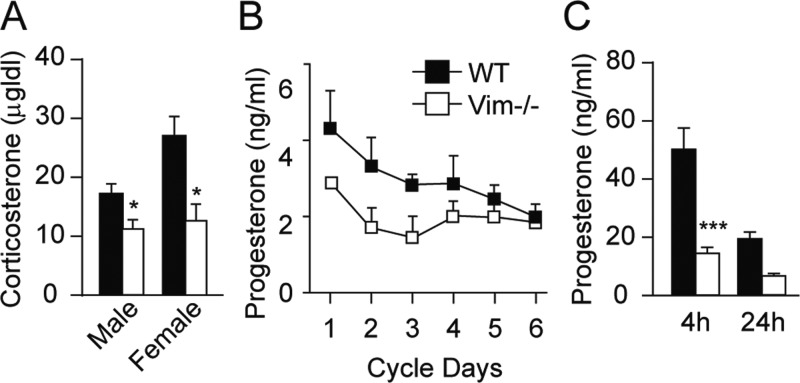
To analyze the involvement of the IF, vimentin, in the process of steroidogenesis, both male and female WT and Vim−/− mice were treated with cosyntropin and serum collected 1 h later for analysis of corticosterone levels. As shown in Fig. 1A, compared with WT mice, corticosterone levels are reduced 35% in males (P < 0.05) and more than 50% in female Vim−/− mice. Because the production of progesterone in female animals is affected by the estrus cycle, the progesterone levels in female animals were measured over a period of 6 d. As shown in Fig. 1B, the ablation of vimentin did not change the estrus cycle of the animals; however, the peak levels of progesterone in Vim−/− female mice were significantly lower than that of WT (P < 0.05). To evaluate the effect of vimentin deficiency on progesterone production further, female animals were stimulated with PMSG (5 U/mouse) for 56 h followed by administration of hCG (2.5 U/mouse), serum was collected at 4 and 24 h and progesterone levels analyzed. As shown in Fig. 1C, serum progesterone is 70% lower in Vim−/− animals compared with their WT littermates at 4 h (P < 0.001) and there is a trend of reduction (60%) at 24 h (P = 0.05). Examination of testosterone levels in the serum of male mice 4 h after hCG treatment revealed no difference between WT and Vim−/− animals (WT 54 ± 19 ng/ml vs. Vim−/− 45 ± 23.3 ng/ml, P = 0.4). Taken together, the reduction in circulating serum steroid levels in Vim−/− animals, particularly after acute stimulation, suggests a defect in steroidogenesis in the adrenal and ovary but not in the testis.
Fig. 1.
Serum corticosterone and progesterone levels. A, Age-matched male and female WT and Vim−/− mice (n = 3–5) were injected with 0.8 μg/kg cosyntropin and bloods were drawn 1 h later. Serum corticosterone levels were analyzed using RIA. B, Serum was collected daily from female WT and Vim−/− littermates (n = 5) at age 4 months and serum progesterone levels analyzed using RIA. C, Age-matched female WT and Vim−/− mice (n = 5) were injected with 5 IU of PMSG for 48 h before treatment with hCG. Serum was collected at 4 and 24 h and progesterone levels measured using RIA. *, P < 0.05; ***, P < 0.001.
Decreased steroidogenesis in primary adrenocortical and granulosa cells from Vim−/− mice
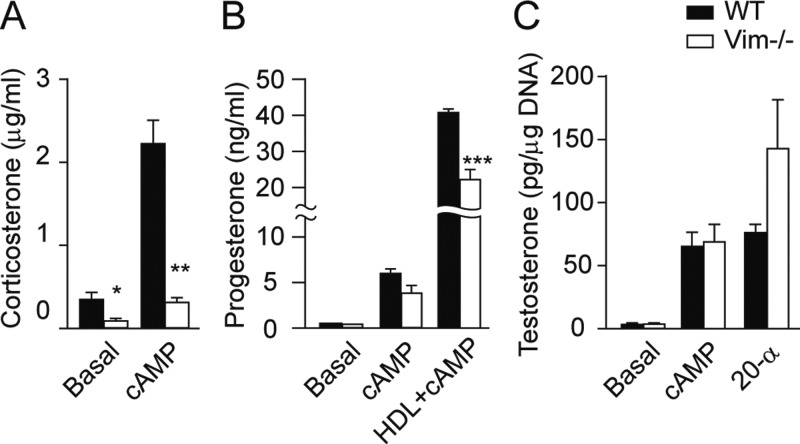
To further confirm the defects in steroidogenesis observed in circulating serum steroids of Vim−/− mice, primary adrenocortical and primary granulosa cells were isolated from Vim−/− and WT mice and steroidogenesis examined in the absence and presence of cAMP treatment. As shown in Fig. 2A, corticosterone production in adrenocortical cells from male Vim−/− mice was approximately 70% lower than that in WT cells under basal conditions and approximately 80% lower after cAMP stimulation (P < 0.05). As shown in Fig. 2B, progesterone production in luteinized granulosa cells is extremely low and similar between Vim−/− and WT under basal conditions and tends to be reduced in Vim−/− cells after cAMP stimulation, but this did not reach statistical significance. Nonetheless, when incubated in the presence of high-density lipoprotein together with cAMP, there is a 45% reduction in progesterone produced by granulosa cells from Vim−/− mice (P < 0.001). These data confirm in isolated primary cells in vitro the defects in steroidogenesis when vimentin is deficient that were observed in vivo.
Fig. 2.
Steroidogenesis in primary adrenocortical, granulosa, and Leydig cells from Vim−/− mice. Primary adrenocortical, granulosa, and Leydig cells were prepared as described in Materials and Methods. A, Adrenocortical cells were plated at a density of 2 × 105/well and treated with or without Bt2cAMP for 24 h and media collected for analysis of corticosterone using RIA. B, Granulosa cells were plated at a density of 2 × 105/well and incubated with or without Bt2cAMP and hHDL3 (500 μg/ml) for 48 h. Media were collected for analysis of progesterone using RIA. C, Leydig cells were plated at a density of 2 × 105/well and incubated with or without Bt2cAMP or 20-α-hydroxycholesterol. Media were collected for analysis of testosterone. All treatments were carried out in triplicate and the experiments were repeated three times. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Leydig cells were prepared from age matched WT and Vim−/− mice, and cells were treated with cAMP or 20-α-hydroxycholesterol. As shown in Fig. 2C, basal testosterone production from the Leydig cells is very low; however, cAMP treatment results in more than a 20-fold increase in testosterone production in cells isolated from both WT and Vim−/− mice. Similarly, there is no difference in testosterone production in Leydig cells from WT and Vim−/− mice treated with 20-α-hydroxycholesterol. Thus, vimentin deficiency was not associated with any abnormalities in testicular steroid production in vivo or in vitro, whereas defects in both adrenal and ovarian steroid production were observed.
Reduction in LD size and impaired cholesterol movement to mitochondria in Vim−/− animals
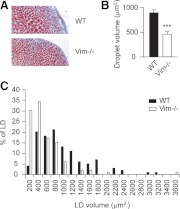
Because steroidogenesis in the adrenal and ovary depends heavily on lipoprotein- and LD-derived cholesterol, whereas steroidogenesis in the testis primarily uses endogenously synthesized cholesterol as a precursor (29, 30), the observed defects in steroidogenesis in the adrenal and ovaries of Vim−/− mice with normal testosterone production raised the possibility of the involvement of vimentin in processing lipoprotein- and/or LD-derived cholesterol. To explore the mechanisms underlying the defect in steroidogenesis in Vim−/− mice, adrenals and ovaries from age-matched WT and Vim−/− animals were collected and weighed. Although the weights of adrenals and ovaries vary, there are no significant differences between WT and Vim−/− mice. Cellular cholesterol expressed per milligram tissue weight is also not statistically different between WT and Vim−/− mice (Table 2). Frozen sections of adrenals from WT and Vim−/− mice were prepared and stained with oil-red-O to evaluate lipid accumulation (Fig. 3A). Interestingly, the volume of individual adrenal LD was significantly smaller in the Vim−/− mice than WT (P < 0.001) (Fig. 3B). The histogram of LD volume shows that there was also a considerably greater percentage of smaller LD in adrenals of Vim−/− mice than WT (Fig. 3C).
Table 2.
Weight and total cholesterol content in adrenal and ovary of WT and Vim−/− mice
| Weight (mg) |
TC (μg/mg) |
|||
|---|---|---|---|---|
| WT | Vim−/− | WT | Vim−/− | |
| Male adrenal | 6.0 ± 1.1 | 5.3 ± 0.1 | 30.9 ± 9.9 | 45.2 ± 16.0 |
| Female adrenal | 6.2 ± 0.8 | 6.4 ± 1.1 | 47.9 ± 10.5 | 48.0 ± 2.9 |
| Female ovary | 11.1 ± 2.1 | 9.42 ± 2.4 | 17.9 ± 4.9 | 18.4 ± 6.9 |
Adrenals and ovaries from 6-month-old animals (n = 5) were collected, weighed, and total cholesterol analyzed. TC, Total cholesterol.
Fig. 3.
Lipid droplet size in adrenals. Adrenals from WT and Vim−/− littermates were collected, sectioned, and stained with Oil-red-O. A, Oil-red-O staining of adrenal sections of WT and Vim−/− animals. B, Histogram of LD size of adrenal sections of WT and Vim−/− mice. ***, P < 0.001.
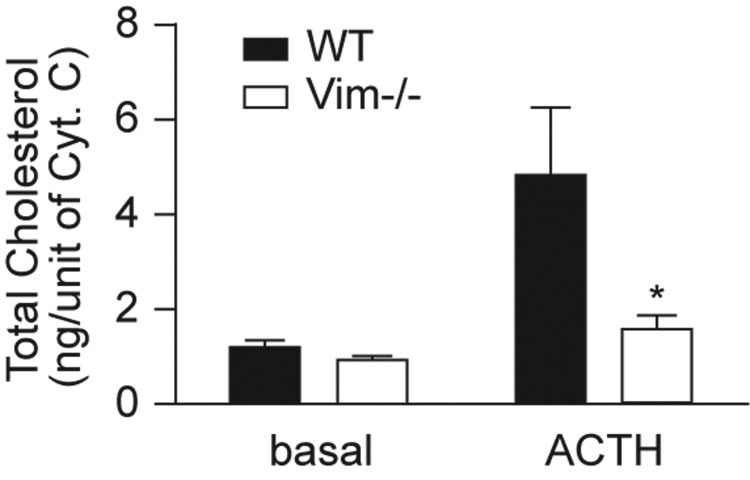
To examine the movement of cholesterol from the cytosol to mitochondria in cells, WT and Vim−/− mice were pretreated with aminoglutethimide before stimulation with ACTH or saline. One hour after treatment, the animals were killed, their adrenals removed, and mitochondria isolated and cholesterol content measured. As shown in Fig. 4, adrenal mitochondrial cholesterol content expressed per unit of cytochrome C was not different in WT and Vim−/− mice under basal conditions, whereas mitochondrial cholesterol content was approximately 65% lower in the Vim−/− mice compared with WT (P < 0.05) 1 h after treatment with ACTH. This result suggests that there could be a defect in cholesterol transfer to mitochondria in Vim−/− cells.
Fig. 4.
Cholesterol movement to mitochondria in Vim−/− cells. Six-month-old WT and Vim−/− animals (n = 7) were treated with aminoglutethemide for 4 h and cosyntropin for 1 h before the animals were killed. Adrenals were collected, mitochondria prepared by differential centrifugation, and cholesterol content measured. *, P < 0.05.
To examine the possibility that cholesterol delivery to the cells might be reduced in Vim−/− compared with WT animals, cholesterol uptake from lipoproteins into adrenal, ovary, and liver was studied using double-labeled lipoprotein particles with [125I]-labeled dilactitol tyramine to mark lipoprotein proteins and [3H]cholesteryl oleolyl ether to mark lipoprotein CE. Equal amounts of double-labeled particles were injected into mice through tail veins, and animals were killed after 6 h and adrenal, ovary, and liver removed. Tissues were homogenized and processed to extract internalized and surface bound particles, and selective uptake (uptake of CE without lipoprotein particle) was calculated. As shown in Table 3, both the selective and endocytic uptake of CE in adrenal, ovary, and liver were not significantly different between Vim−/− and WT animals. Thus, vimentin does not appear to be involved in the selective or endocytic uptake of lipoprotein-derived cholesterol.
Table 3.
Lipoprotein cholesteryl ester uptake in adrenal, ovary, and liver of WT and Vim−/− mice
| Adrenal |
Ovary |
Liver |
||||
|---|---|---|---|---|---|---|
| WT | Vim−/− | WT | Vim−/− | WT | Vim−/− | |
| Selective (ng/mg) | 54.64 ± 11.11 | 58.16 ± 9.88 | 11.21 ± 3.13 | 8.54 ± 5.31 | 45.72 ± 6.73 | 51.93 ± 8.95 |
| Endocytic (ng/mg) | 3.49 ± 0.48 | 4.27 ± 1.27 | 3.32 ± 0.38 | 4.99 ± 1.10 | 7.81 ± 4.58 | 3.21 ± 0.32 |
| Total (ng/mg) | 58.14 ± 11.46 | 62.43 ± 9.51 | 14.53 ± 2.93 | 13.52 ± 4.52 | 53.53 ± 4.32 | 55.14 ± 9.16 |
WT and Vim−/− mice (n = 6) were injected with equal amounts of double-labeled high-density lipoprotein particles/body weight and killed 6 h later. Tissues were homogenized and processed to extract internalized and surface bound particles. Selective, endocytic, and total cholesterol uptake were calculated.
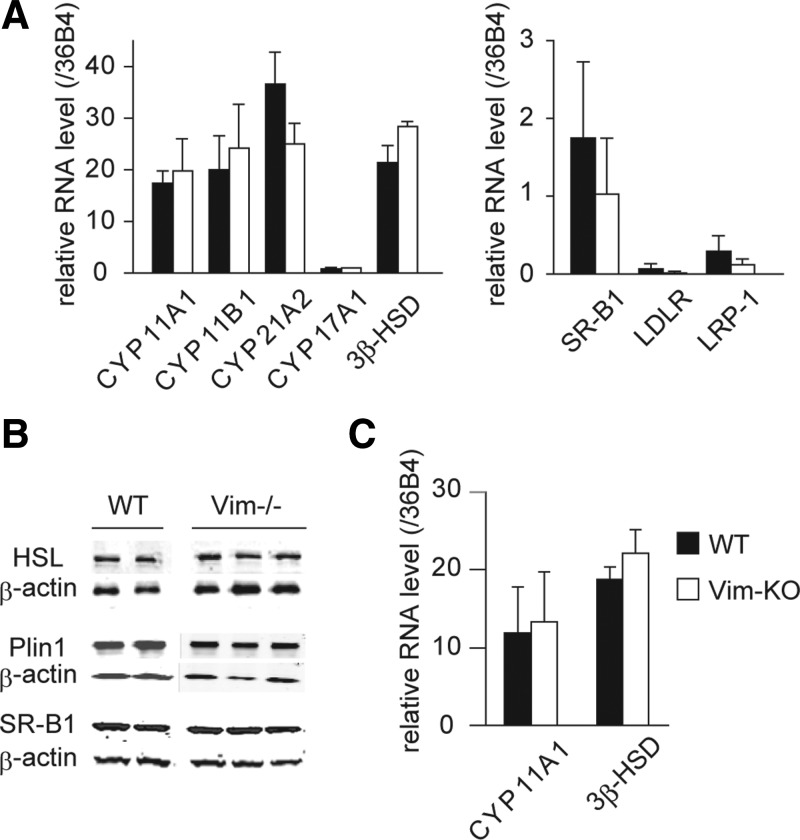
To evaluate the possibility that changes in pathways that are involved in cholesterol uptake or the initial steps of steroid hormone production might have occurred with the ablation of vimentin, we examined the mRNA levels of SRB-I, LDLR, and LRP1 as well as genes involved in the initial steps of steroid hormone production, including CYP11A1, CYP17A, CYP11B1, CYP21A2, and 3βHSDII, using RT-PCR. The relative level of expression of each of these genes is not significantly different in WT and Vim−/− adrenals (Fig. 5A). Levels of some of the proteins involved in LD and cholesterol uptake were also assessed using specific antibodies. As shown in Fig. 5B, the protein levels of HSL, perilipin, and SRB-I in the total cell extracts of adrenals from WT and Vim−/− mice were not different. We also examined the mRNA expression levels of CYP11A1 and 3βHSDII by RT-PCR in the ovaries of WT and Vim−/− mice 24 h after PMSG treatment. The relative levels of expression of both genes were not significantly different in the ovaries of WT and Vim−/− mice (Fig. 5C).
Fig. 5.
Gene expression and protein levels in adrenal and ovary of WT and Vim−/− mice. Adrenals from 6-month-old WT and Vim−/− animals (n = 3) were collected and used for analysis of gene expression levels (A) and protein levels (B). Ovaries from 6-month-old WT and Vim−/− animals (n = 3) were collected 24 h after treatment with 5 IU of PMSG, RNA extracted, and used for analysis of gene expression levels (C).
Discussion
Intermediate filaments are proteins that have a central coil-coil α-helical rod domain with an N-terminal head and a C-terminal tail domain of various lengths (18, 31). Based on their domain and sequence homology, IF proteins can be classified into five groups. Most IF proteins are regulated by posttranslational modifications and binding proteins and, in turn, change their polymerization and mechanical properties in cell- and tissue-specific expression patterns or associated with specific differentiation stages of cells. Together with microfilaments and microtubules, IF proteins are involved in maintaining the structure of the cellular cytoskeleton, as well as tissue integrity (32, 33). It is also known that IF proteins are involved in dynamic scaffolding of the cytoskeleton in response to various stress conditions and have cytoprotective and tissue-specific functions.
The initial indication of the involvement of IF in steroidogenesis came from studies showing that both functional mitochondria and cholesterol-enriched LD are specifically attached to IF in Y1 adrenal tumor cells (34, 35) and testicular Leydig cells (36), raising the possibility that such association may facilitate the transport of cholesterol to mitochondria for steroid synthesis (37). Later studies showed that overexpression of oxysterol binding protein related protein 4, which interacts with vimentin and causes its aggregation, results in a defect in cholesterol esterification (38). Likewise, adrenal cells lacking vimentin display a defect in the reesterification of low-density lipoprotein (LDL) cholesterol without any alterations in LDL receptor-mediated endocytosis (39, 40), suggesting that vimentin might possibly be involved in pathways of cholesterol movement from endosomes to the endoplasmic reticulum. In support of this, Rab9 has been reported to facilitate lipid movement from late endosomes in which Niemann Pick disease, type C 1 resides and to interact with vimentin (41). Although possible contributions of the cytoskeleton, including vimentin, to steroidogenesis have been suggested in studies using inhibitors, conflicting results have been generated (4, 42). Also, it is important to realize that the agents that were previously used in many of these studies to disrupt microfilaments, e.g. nocodazole, cytochalasin, and cycloheximide, affect tubulin and actin but have no effects on vimentin (43).
In our studies using Vim−/− animals and cells, we first demonstrate a marked defect in steroid hormone production in adrenals and ovaries both in vivo in intact mice and in vitro in isolated cells but normal hormone production in the testis. Studies into the possible underlying mechanisms show that there are no defects in the uptake of lipoprotein cholesterol into adrenals or ovaries. Although the LD in both the adrenal and ovary of Vim−/− mice are smaller, the weight of adrenals and ovaries and the amount of total cholesterol per total protein are similar in Vim−/− and WT mice. Because we did not observe an increase in the number of LD per cell but the size of the adrenals and cholesterol content were not different, it would seem that there might be an increase in the number of cells per adrenal (hyperplasia) in vimentin null mice. Moreover, our current studies show that when stimulated, there is a marked difference in the production of corticosterone and progesterone both in vivo and in vitro in isolated cells of Vim−/− mice. When cholesterol delivery to the cells was assayed using double-labeled lipoprotein particles, both the selective and endocytic uptake of CE into the adrenal, ovary, and liver were similar in WT and Vim−/−animals. Taken together, the observations that the uptake of cholesterol into tissues of WT and Vim−/− mice is not different and that the total cholesterol content is not different suggest that the defect in steroidogenesis is at the level of the mitochondria, with either a defect in cholesterol transport to mitochondria or a defect in mitochondrial processing of cholesterol. A survey of expression of genes involved in cholesterol uptake and cholesterol processing in mitochondria showed no significant differences between WT and Vim−/− mice. The demonstration that mitochondrial cholesterol content was markedly lower in adrenals of Vim−/− mice after stimulation by ACTH in the setting of inhibition of CYP11A by aminoglutethemide documents the defect to reside in the movement of cholesterol from the cytosol to mitochondria. It should be noted that we did not measure all hormones, for instance, aldosterone. Thus, even though both aldosterone and corticosterone production depend on cholesterol movement to mitochondria (44), our studies do not provide insights into the effects of vimentin deficiency on aldosterone production.
Different from the testis in rodents, in which free cholesterol is the main source of the precursor for steroid hormone production, an important reservoir of cholesterol for steroidogenesis in the rodent adrenal and ovary is in the form of cholesterol esters stored in LD (1, 29, 30), in which vimentin has been shown to form a capsule around the droplet (33). In fact, the mobilization of cholesterol from LD and transport to mitochondria is a preferred pathway for the initiation of steroid hormone production in the rodent adrenal and ovary. Our data showing a reduction in steroidogenesis with ablation of vimentin in the adrenal and ovary, but not in the testis, uncover a possible role of vimentin in the mobilization of cholesterol from LD to mitochondria. Previous data have shown that LD can have direct contact with mitochondria (11–13), and proteomic analyses of LD consistently have identified vimentin as a droplet-associated protein (9, 10, 45). More recently we have shown that vimentin can interact with HSL and modulate the rate of lipolysis on hormone stimulation in adipose tissue (16). Earlier investigations provided evidence that HSL can interact with steroidogenic acute regulatory protein to facilitate cholesterol transfer to mitochondria (20). Our observation of smaller LD in the adrenals of Vim−/− animals, as similarly observed for adipocytes (16), would imply altered lipid homeostasis in the cells where vimentin is ablated, suggesting that vimentin is important in determining the size or integrity of LD. Taking all of these facts into consideration leads us to suggest that vimentin is an important protein involved in mobilization of cholesterol from its storage in LD in cytosol to mitochondria for steroidogenesis. Through its binding to LD and its interaction with proteins that are involved in LD metabolism (vimentin-HSL) as well as through its interactions with proteins that target mitochondria (HSL-steroidogenic acute regulatory protein), vimentin is an important component of a network for facilitating the docking of LD to mitochondria and increasing the transport of cholesterol to mitochondria. With vimentin being the most abundant intermediate filament and its role in cellular organization, our studies, using vimentin ablation in mice, provide the first direct evidence for an important role of vimentin in maintaining lipid droplet homeostasis and participating in the movement of cholesterol for steroid hormone production.
Acknowledgments
This work was supported by the Department of Veterans Affairs (Office of Research and Development, Medical Research Service) and the National Institute of Health Grant 2RO1HL033881.
Disclosure Summary: The authors have nothing to declare.
Footnotes
- Bt2cAMP
- Dibutyryl cAMP
- CE
- cholesterol ester
- hCG
- human chorionic gonadotropin
- hHDL3
- human apoE free high-density lipoprotein
- HSL
- hormone-sensitive lipase
- IF
- intermediate filament
- LD
- lipid droplets
- LDL
- low-density lipoprotein
- LDLR
- low density lipoprotein receptor
- LRP 1
- LDL receptor related protein 1
- PMSG
- pregnant mare's serum gonadotropin
- SRB-I
- scavenger receptor type B-I
- Vim−/−
- Vimentin null
- WT
- wild type.
References
- 1. Miller WL, Bose HS. 2011. Early steps in steroidogenesis: intracellular cholesterol trafficking. J Lipid Res 52:2111–2135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Underwood KW, Jacobs NL, Howley A, Liscum L. 1998. Evidence for a cholesterol transport pathway from lysosomes to endoplasmic reticulum that is independent of the plasma membrane. J Biol Chem 273:4266–4274 [DOI] [PubMed] [Google Scholar]
- 3. Chou YH, Skalli O, Goldman RD. 1997. Intermediate filaments and cytoplasmic networking: new connections and more functions. Curr Opin Cell Biol 9:49–53 [DOI] [PubMed] [Google Scholar]
- 4. Sewer MB, Li DH. 2008. Regulation of steroid hormone biosynthesis by the cytoskeleton. Lipids 43:1109–1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhou Y, Shimabukuro M, Wang MY, Lee Y, Higa M, Milburn JL, Newgard CB, Unger RH. 1998. Role of peroxisome proliferator-activated receptor α in disease of pancreatic β cells. Proc Natl Acad Sci USA 95:8898–8903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Toivola DM, Strnad P, Habtezion A, Omary MB. 2010. Intermediate filaments take the heat as stress proteins. Trends Cell Biol 20:79–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Evans RM. 1998. Vimentin: the conundrum of the intermediate filament gene family. Bioessays 20:79–86 [DOI] [PubMed] [Google Scholar]
- 8. Schweitzer SC, Evans RM. 1998. Vimentin and lipid metabolism. Subcell Biochem 31:437–462 [PubMed] [Google Scholar]
- 9. Brasaemle DL, Dolios G, Shapiro L, Wang R. 2004. Proteomic analysis of proteins associated with lipid droplets of basal and lipolytically stimulated 3T3-L1 adipocytes. J Biol Chem 279:46835–46842 [DOI] [PubMed] [Google Scholar]
- 10. Wu CC, Howell KE, Neville MC, Yates JR, 3rd, McManaman JL. 2000. Proteomics reveal a link between the endoplasmic reticulum and lipid secretory mechanisms in mammary epithelial cells. Electrophoresis 21:3470–3482 [DOI] [PubMed] [Google Scholar]
- 11. Merry BJ. 1975. Mitochondrial structure in the rat adrenal cortex. J Anat 119:611–618 [PMC free article] [PubMed] [Google Scholar]
- 12. Murphy S, Martin S, Parton RG. 2009. Lipid droplet-organelle interactions; sharing the fats. Biochim Biophys Acta 1791:441–447 [DOI] [PubMed] [Google Scholar]
- 13. Stemberger BH, Walsh RM, Patton S. 1984. Morphometric evaluation of lipid droplet associations with secretory vesicles, mitochondria and other components in the lactating cell. Cell Tissue Res 236:471–475 [DOI] [PubMed] [Google Scholar]
- 14. Faigle W, Colucci-Guyon E, Louvard D, Amigorena S, Galli T. 2000. Vimentin filaments in fibroblasts are a reservoir for SNAP23, a component of the membrane fusion machinery. Mol Biol Cell 11:3485–3494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kumar N, Robidoux J, Daniel KW, Guzman G, Floering LM, Collins S. 2007. Requirement of vimentin filament assembly for β3-adrenergic receptor activation of ERK MAP kinase and lipolysis. J Biol Chem 282:9244–9250 [DOI] [PubMed] [Google Scholar]
- 16. Shen WJ, Patel S, Eriksson JE, Kraemer FB. 2010. Vimentin is a functional partner of hormone sensitive lipase and facilitates lipolysis. J Proteome Res 9:1786–1794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Colucci-Guyon E, Portier MM, Dunia I, Paulin D, Pournin S, Babinet C. 1994. Mice lacking vimentin develop and reproduce without an obvious phenotype. Cell 79:679–694 [DOI] [PubMed] [Google Scholar]
- 18. Eriksson JE, Dechat T, Grin B, Helfand B, Mendez M, Pallari HM, Goldman RD. 2009. Introducing intermediate filaments: from discovery to disease. J Clin Invest 119:1763–1771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Eckes B, Dogic D, Colucci-Guyon E, Wang N, Maniotis A, Ingber D, Merckling A, Langa F, Aumailley M, Delouvée A, Koteliansky V, Babinet C, Krieg T. 1998. Impaired mechanical stability, migration and contractile capacity in vimentin-deficient fibroblasts. J Cell Sci 111:1897–1907 [DOI] [PubMed] [Google Scholar]
- 20. Shen WJ, Patel S, Natu V, Hong R, Wang J, Azhar S, Kraemer FB. 2003. Interaction of hormone-sensitive lipase with steroidogenic acute regulatory protein: facilitation of cholesterol transfer in adrenal. J Biol Chem 278:43870–43876 [DOI] [PubMed] [Google Scholar]
- 21. Azhar S, Luo Y, Medicherla S, Reaven E. 1999. Upregulation of selective cholesteryl ester uptake pathway in mice with deletion of low-density lipoprotein receptor function. J Cell Physiol 180:190–202 [DOI] [PubMed] [Google Scholar]
- 22. Azhar S, Tsai L, Reaven E. 1990. Uptake and utilization of lipoprotein cholesteryl esters by rat granulosa cells. Biochim Biophys Acta 1047:148–160 [DOI] [PubMed] [Google Scholar]
- 23. Reaven E, Tsai L, Azhar S. 1995. Cholesterol uptake by the ‘selective’ pathway of ovarian granulosa cells: early intracellular events. J Lipid Res 36:1602–1617 [PubMed] [Google Scholar]
- 24. Reaven E, Tsai L, Azhar S. 1996. Intracellular events in the “selective” transport of lipoprotein-derived cholesteryl esters. J Biol Chem 271:16208–16217 [DOI] [PubMed] [Google Scholar]
- 25. Schumacher M, Schäfer G, Holstein AF, Hilz H. 1978. Rapid isolation of mouse Leydig cells by centrifugation in Percoll density gradients with complete retention of morphological and biochemical integrity. FEBS Lett 91:333–338 [DOI] [PubMed] [Google Scholar]
- 26. Cao L, Leers-Sucheta S, Azhar S. 2004. Aging alters functional expression of enzymatic and non-enzymatic antioxidant defense systems in testicular rat Leydig cells. J Steroid Biochem Mol Biol 88:61–67 [DOI] [PubMed] [Google Scholar]
- 27. Azhar S, Khan I, Gibori G. 1989. The influence of estradiol on cholesterol processing by the corpus luteum. Biol Reprod 40:961–971 [DOI] [PubMed] [Google Scholar]
- 28. Shen WJ, Patel S, Yu Z, Jue D, Kraemer FB. 2007. Effects of rosiglitazone and high fat diet on lipase/esterase expression in adipose tissue. Biochim Biophys Acta 1771:177–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Andersen JM, Dietschy JM. 1978. Relative importance of high and low density lipoproteins in the regulation of cholesterol synthesis in the adrenal gland, ovary and testis of rat. J Biol Chem 253:9024–9032 [PubMed] [Google Scholar]
- 30. Morris MD, Chaikoff IL. 1959. The origin of cholesterol in liver, small intestine, adrenal gland, and testis of the rat: dietary versus endogenous contributions. J Biol Chem 234:1095–1097 [PubMed] [Google Scholar]
- 31. Fuchs E, Weber K. 1994. Intermediate filaments: structure, dynamics, function, and disease. Annu Rev Biochem 63:345–382 [DOI] [PubMed] [Google Scholar]
- 32. Chou YH, Flitney FW, Chang L, Mendez M, Grin B, Goldman RD. 2007. The motility and dynamic properties of intermediate filaments and their constituent proteins. Exp Cell Res 313:2236–2243 [DOI] [PubMed] [Google Scholar]
- 33. Franke WW, Hergt M, Grund C. 1987. Rearrangement of the vimentin cytoskeleton during adipose conversion: formation of an intermediate filament cage around lipid globules. Cell 49:131–141 [DOI] [PubMed] [Google Scholar]
- 34. Almahbobi G, Hall PF. 1990. The role of intermediate filaments in adrenal steroidogenesis. J Cell Sci 97:679–687 [DOI] [PubMed] [Google Scholar]
- 35. Almahbobi G, Williams LJ, Hall PF. 1992. Attachment of steroidogenic lipid droplets to intermediate filaments in adrenal cells. J Cell Sci 101 383–393 [DOI] [PubMed] [Google Scholar]
- 36. Almahbobi G, Williams LJ, Han XG, Hall PF. 1993. Binding of lipid droplets and mitochondria to intermediate filaments in rat Leydig cells. J Reprod Fertil 98:209–217 [DOI] [PubMed] [Google Scholar]
- 37. Hall PF. 1997. The roles of calmodulin, actin, and vimentin in steroid synthesis by adrenal cells. Steroids 62:185–189 [DOI] [PubMed] [Google Scholar]
- 38. Wang C, JeBailey L, Ridgway ND. 2002. Oxysterol-binding-protein (OSBP)-related protein 4 binds 25-hydroxycholesterol and interacts with vimentin intermediate filaments. Biochem J 361:461–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Holwell TA, Schweitzer SC, Reyland ME, Evans RM. 1999. Vimentin-dependent utilization of LDL-cholesterol in human adrenal tumor cells is not associated with the level of expression of apoE, sterol carrier protein-2, or caveolin. J Lipid Res 40:1440–1452 [PubMed] [Google Scholar]
- 40. Sarria AJ, Panini SR, Evans RM. 1992. A functional role for vimentin intermediate filaments in the metabolism of lipoprotein-derived cholesterol in human SW-13 cells. J Biol Chem 267:19455–19463 [PubMed] [Google Scholar]
- 41. Walter M, Chen FW, Tamari F, Wang R, Ioannou YA. 2009. Endosomal lipid accumulation in NPC1 leads to inhibition of PKC, hypophosphorylation of vimentin and Rab9 entrapment. Biol Cell 101:141–152 [DOI] [PubMed] [Google Scholar]
- 42. Hall PF, Almahbobi G. 1997. Roles of microfilaments and intermediate filaments in adrenal steroidogenesis. Microsc Res Tech 36:463–479 [DOI] [PubMed] [Google Scholar]
- 43. Collot M, Louvard D, Singer SJ. 1984. Lysosomes are associated with microtubules and not with intermediate filaments in cultured fibroblasts. Proc Natl Acad Sci USA 81:788–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Capponi AM. 2004. The control by angiotensin II of cholesterol supply for aldosterone biosynthesis. Mol Cell Endocrinol 217:113–118 [DOI] [PubMed] [Google Scholar]
- 45. Bartz R, Zehmer JK, Zhu M, Chen Y, Serrero G, Zhao Y, Liu P. 2007. Dynamic activity of lipid droplets: protein phosphorylation and GTP-mediated protein translocation. J Proteome Res 6:3256–3265 [DOI] [PubMed] [Google Scholar]