Abstract
β-1,4-endoglucanses, a.k.a. cellulases, are parasitism genes that facilitate root penetration and migration by plant-parasitic nematodes. Rotylenchulus reniformis is a sedentary semi-endoparasite for which little molecular data has been collected. In this report, we describe the isolation and characterization of a predicted glycosyl hydrolase family 5 cellulase from R. reniformis that we have named Rr-eng-1. The Rr-eng-1 cDNA was 1,341 bp long and was comprised of a 19 bp 5′-untranslated region (UTR), a 1,245 bp open reading frame (ORF), and an 80 bp 3′-UTR. The Rr-eng-1 genomic sequence was 2,325 bp. Alignment of the cDNA and genomic sequences revealed seven introns and eight exons for Rr-eng-1. BLASTN analysis showed the Rr-eng-1 cDNA was most homologous to the Hg-eng-6 mRNA from Heterodera glycines. Southern blot analysis indicated that at least three Rr-eng-1-like sequences were present in the R. reniformis genome. Translation of the Rr-eng-1 ORF yielded a 414 amino acid peptide (Rr-ENG-1) having an N-terminal signal sequence for secretion. No cellulose binding module (CBM) was detected in Rr-ENG-1; however, a putative CBM linker sequence N-terminal to the catalytic domain was present. Rr-ENG-1 was most homologous to Hg-ENG-6 but also shared a number of intron splice positions with Mi-ENG-2. Quantitative RT-PCR indicated that Rr-eng-1 was highly expressed in the J2 and adult vermiform life-stages with a sharp decline in expression detected in sedentary females.
Keywords: cellulase, endoglucanase, reniform nematode, host parasitic relationship, molecular biology, parasitism gene, phylogenetics, Rotylenchulus reniformis
The reniform nematode (Rotylenchulus reniformis Linford & Oliveira) is a sedentary semi-endoparasitic nematode that infects the roots of more than 300 plant species and is found throughout the world's tropical, subtropical, and warm temperate areas (Robinson et al., 1997). The beginning of the R. reniformis life-cycle is like that of other plant-parasitic nematodes (PPNs) with the J1 molt to the J2 occurring in the egg and the emergence of the J2 upon hatching (Robinson et al., 1997). However, unlike most other PPNs, the R. reniformis J2 is not the infective life-stage; instead, the J2 enters a series of three superimposed molts without feeding (Robinson et al., 1997). The final molt then produces either an adult male or immature vermiform female. R. reniformis adult male nematodes do not feed. Infective vermiform females penetrate the host root epidermis and establish a feeding site, called a syncytium, in the stelar region of the root (Robinson et al., 1997). The syncytium provides the nutrients required for the female to mature and produce eggs upon fertilization by the male. Syncytia begin as an initial syncytial cell (ISC), usually endodermal in origin, which quickly expands to encompass 100-200 cells through extensive cell wall dissolution and cytoplasmic fusion (Heald, 1975; Rebois et al., 1975). Syncytia show increased metabolic activity, hypertrophied nuclei, and granular, densely staining cytoplasm (Agudelo et al., 2005; Heald, 1975; Rebois et al., 1975; Vovlas and Lamberti, 1990). These characteristics of syncytia formed by R. reniformis are also observed in syncytia formed by the sedentary endoparasitic cyst nematode species Heterodera and Globodera (Williamson and Hussey, 1996). Syncytia formation in cyst nematodes occurs when effector proteins, encoded by parasitism genes that are expressed within the esophageal gland cells, are injected into or near the ISC through the nematode stylet (Baum et al., 2007).
The first line of defense for a potential host plant when confronted by a nematode pathogen is the root epidermal cell wall. The plant cell wall is primarily composed of cellulose, which can account for up to 40% of the total cell wall mass (Liepman et al., 2010). In addition to cellulose, the cell wall contains hemicelluloses, xyloglucan, pectin, and various proteoglycans (Liepman et al., 2010). In order to penetrate the root epidermis and move throughout the root, PPNs couple mechanical force with the activity of cell wall degrading enzymes that are secreted by the esophageal gland cells (Baum et al., 2007). Within this collection of cell wall degrading enzymes, glycosyl hydrolase family 5 (GHF5) β-1,4-endoglucanases, a.k.a. cellulases, are well-represented and have been isolated from many PPN species (Baum et al., 2007). The characterization of Gr-eng-1 and Gr-eng-2 from G. rostochiensis and Hg-eng-1 and Hg-eng-2 from H. glycines represented the first report of cellulase genes within the animal kingdom (Smant et al., 1998). Additional GHF5 cellulase genes have been isolated from cyst nematodes (De Meutter et al., 2001; Gao et al., 2002, 2004; Goellner et al., 2000; Rehman et al., 2009; Yan et al., 2001), root-knot nematode (Meloidogyne incognita) (Ledger et al., 2006; Rosso et al., 1999), and migratory PPNs including Radopholus similis (Haegeman et al., 2008), Pratylenchus coffeae (Kyndt et al., 2008), P. penetrans (Uehara et al., 2001), and Ditylenchus africanus (Kyndt et al., 2008). An important role for cellulases in facilitating plant parasitism has been confirmed by immunolocalization studies in planta (Goellner et al., 2001; Wang et al., 1999) and by observed decreased nematode infectivity when cellulase genes are silenced by RNA-interference (Bakhetia et al., 2007; Chen et al., 2005; Rehman et al., 2009). PPN cellulases possess an N-terminal signal peptide for secretion and in situ hybridization experiments have shown that their expression is restricted to the esophageal gland cells. The presence of a signal peptide and esophageal gland cell-specific expression are defining characteristics of parasitism genes (Baum et al., 2007).
Glycosyl hydrolases have been classified into families based on sequence similarity and hydrophobic cluster analyses (Henrissat et al., 1989; Henrissat, 1991). In general, bacterial and fungal GHF5 β-1,4-endoglucanases contain a catalytic domain having a GHF5-specific signature, a short stretch of proline and/or hydroxyamino acids termed a linker sequence, and a cellulose binding module (CBM) (Gilkes et al., 1991). All plant-parasitic nematode GHF5 cellulases possess the catalytic domain but the presence or absence of a linker sequence and CBM can vary significantly. For example, Hg-eng-1 contains the catalytic, linker, and CBM sequences (Smant et al., 1998), while Hg-eng-6 has only the catalytic domain with no linker or CBM present (Gao et al., 2004). Another structural variation is exhibited by Hg-eng-4 which possesses the catalytic and linker domains but lacks a CBM (Gao et al., 2004). Similar variation in cellulase structural features has been observed for M. incognita and G. rostochiensis (Ledger et al., 2006). Due to their similarity to bacterial cellulases and their absence from other nematodes and animals, it has been hypothesized that PPNs acquired cellulases and other cell wall degrading genes from prokaryotes through horizontal gene transfer (Keen and Roberts, 1998; Yan et al., 1998). Recently, this hypothesis has been strongly supported by the work of Danchin et al. (2010) who demonstrated that many independent horizontal gene transfer events likely occurred between PPNs and different bacterial species.
An EST project initiated by our laboratory revealed the presence of cellulase-like sequences expressed by sedentary parasitic R. reniformis females (Wubben et al., 2010). In this report, we describe the identification and characterization of a GHF5 β-1,4-endoglucanase from R. reniformis that we have named Rr-eng-1. BLAST and phylogenetic analyses showed that Rr-ENG-1 was most homologous to Hg-ENG-6 at the nucleotide and amino acid level but also showed homology to Mi-ENG-2 with respect to the relative positions of exon/intron junctions within the protein sequence. Quantitative RT-PCR indicated that Rr-eng-1 was highly expressed in the J2 and adult vermiform life-stages with a sharp decline in expression detected in sedentary females. In addition, we show that Rr-ENG-1, while lacking a CBM, contains a unique linker sequence N-terminal to the catalytic domain, a feature that has not been described in plant-parasitic nematode GHF5 cellulases.
Materials and Methods
Nematode culture and life-stage collection: A Rotylenchulus reniformis stock culture was maintained on cotton plants in the greenhouse. R. reniformis eggs were collected from infected cotton roots according to Hussey and Barker (1973). In brief, roots were gently washed free of soil and cut into 1-2 cm pieces. Root pieces were then soaked for 3-min in a 1% (v/v) sodium hypochlorite solution. Eggs were washed from the roots and collected on a 25 μM sieve. Contaminating root debris and fine soil material were removed from the egg solution via centrifugal-floatation (Jenkins, 1964). The purified eggs were pelleted by centrifugation, flash-frozen with liquid nitrogen, and stored at -80°C until RNA extraction.
J2 nematodes were collected by suspending R. reniformis eggs over a 38 μM sieve in tap-water in a plastic container and incubating them in the dark at 30°C. Hatched J2 were then collected from the plastic container at regular daily intervals, washed three times with sterile MilliQ water (Millipore, Billerica, MA, USA), pelleted, and flash-frozen with liquid nitrogen. J3 nematodes were collected by continuing to incubate hatched J2 at 30°C until they entered the second molting event. J3 were identified based on physical attributes described by Nakasono (1973), specifically, the assumption of a non-motile crescent shape and the presence of the hyaline J2 cuticle. J3 nematodes were washed three times with sterile MilliQ water (Millipore, MA), pelleted, and flash-frozen with liquid nitrogen. A mixture of J3 and J4 nematodes was collected by allowing the J3 to enter the third molting event while being incubated at 30°C. J4 were distinguished from J3 by the presence of both the J2 and J3 hyaline cuticles (Nakasono, 1973). In our samples, not all of the J3 molted to the J4 stage before a portion of the J4 nematodes entered the fourth molting event; therefore, total RNA was extracted from a mixture of J3 and J4. A sample of mixed vermiform stages was collected as described by Wubben et al. (2008). Sedentary females were collected as previously described by Wubben et al. (2010).
DNA and RNA isolation: R. reniformis genomic DNA was isolated from eggs according to Blin and Stafford (1976). Total RNA was isolated from R. reniformis life-stages with Trizol Reagent (Invitrogen, Carlsbad, CA, USA) per manufacturer's instructions using a glass/glass Kontes-20 homogenizer for tissue disruption. Contaminating genomic DNA was removed using the DNA-free™ Kit (Ambion, Austin, TX, USA).
Rr-eng-1 genomic and cDNA clone isolation: The full-length Rr-eng-1 genomic sequence was identified by DNA walking from the original EST sequence (GenBank Accession No. GT737677.1) using the DNA Walking SpeedUp™ Premix Kit I and Kit II (Seegene, Rockville, MD, USA) per the manufacturer's instructions. PCR products from each DNA walking experiment were excised from an agarose gel and purified using the MinElute Gel Extraction Kit (Qiagen, Valencia, CA, USA). The purified PCR product was then cloned into the TOPO pCR4.0 TA cloning vector and clones suitable for sequencing generated using the TOPO TA Cloning Kit for Sequencing (Invitrogen, CA) per the manufacturer's instructions. Plasmid DNAs were isolated and bi-directionally sequenced from universal M13 priming sites at the USDA-ARS MidSouth Area Genomics Facility (Stoneville, MS, USA). Clone sequence processing and assembly of DNA walking sequences into the full-length Rr-eng-1 genomic clone was accomplished using Sequencher v4.7 software (Gene Codes Corp., Ann Arbor, MI, USA). Forward (RrENG1-F, 5′-CGGCTCAATCCTCATCAAGATGCGTCT-3′) and reverse (RrENG1-R, 5′-GAGAAGGGGGAGGAGAGCTTTCAA-3′) primers were designed from the full-length genomic clone that were used to isolate the Rr-eng-1 cDNA by RT-PCR.
Southern blot: A Southern blot was prepared having 10 μg of R. reniformis genomic DNA digested with the following single enzymes and enzyme combinations per the manufacturer's instructions (New England Biolabs, Ipswich, MA, USA): EcoRI, HindIII, EcoRI+BamHI, EcoRI+HindIII, BamHI+HindIII, and EcoRI+BamHI+HindIII. Digests were resolved on a 0.8% agarose/TAE gel and transferred to a nylon membrane via TurboBlotter (Schleicher and Schuell Bioscience, Dassel, Germany). DIG (digoxigenin-11-dUTP) probe labeling, prehybridization, hybridization (48°C), blot washing, and immunological detection was performed in accordance with the DIG High Prime DNA Labeling and Detection Starter Kit I (Roche Applied Science, Mannheim, Germany).
Quantitative real-time RT-PCR (qRT-PCR): First-strand cDNA was reverse-transcribed from approximately 500 ng of DNase-treated total RNA from each R. reniformis life-stage using the SuperScript™ First-Strand Synthesis System for RT-PCR (Invitrogen, CA) per the manufacturer's instructions with the following modification: In addition to 0.5 μg of an oligo(dT)12-18 primer, each 20 μL reaction contained 0.1 μM of a R. reniformis 18S-specific primer (5′-AACCAGGGCGCTCATTGAGTCTTA-3′) to facilitate normalization of Rr-eng-1 expression levels. Reverse transcription reactions were diluted 1:10 to produce templates for qRT-PCR. qRT-PCR reactions were performed in triplicate in 96-well plates on a CFX96™ Real-Time System (Bio-Rad, Hercules, CA). Each qRT-PCR reaction was comprised as follows: 12.5 μL 2X iQ™ SYBR® Green Supermix (Bio-Rad, CA), 0.625 μL 10 μM forward+reverse primer stock, 10.875 μL MilliQ water (Millipore, MA), and 1 μL cDNA template. qRT-PCR cycling parameters for Rr-eng-1 and Rr18S primers were an initial denaturation step of 95°C for 3 min followed by 40 cycles of 95°C for 10 sec and 60°C for 30 sec. qRT-PCR primer sequences for Rr-eng-1 and Rr18S were as follows (5′→3′): qRrENG1-F (AAGCCCAGCAGGCCATTGATAA), qRrENG1-R (ATGCCGGAGTTGTGCCGTATT), qRr18S-F (TCGCCACACTAACAAACCGT), and qRr18S-R (GCAACAACTGCTCAACAACGCA). Standard curve slope and R2 values for qRrENG1 and qRr18S were (-3.77, 0.995) and (-3.54, 0.996), respectively. Normalized fold expression of Rr-eng-1 was calculated using the ΔΔCt method as part of the Bio-Rad CFX™ Manager Software package (Bio-Rad, CA).
Phylogenetic analysis of cellulase catalytic domains: A phylogenetic tree was constructed from nematode cellulase catalytic domain sequences by maximum parsimony using MEGA4 (Tamura et al., 2007). A bootstrap consensus tree was created from 1000 replicates. Catalytic domains were identified and extracted from the full-length protein sequences using the conserved domain search function of BLASTP. The GenBank Accession Nos. of the proteins used in our analysis are as follows: Mi-ENG-1 (AAK21882), Mi-ENG-2 (AAK21883), Mi-ENG-3 (AAR37374), Mi-ENG-4 (AAR37375), Gr-ENG-1 (AAC63988), Gr-ENG-2 (AAC63989), Gr-ENG-3 (AAN03645), Gr-ENG-4 (AAN03648), Gt-ENG-1 (AAD56392), Gt-ENG-2 (AAD56393), Hg-ENG-1 (AAC15707), Hg-ENG-2 (AAC15708), Hg-ENG-3 (AAC33860), Hg-ENG-4 (AAP88024), Hg-ENG-5 (AAP97436), Hg-ENG-6 (AAO25506), Hs-ENG-1 (CAC12958), Hs-ENG-2 (CAC12959), Pp-ENG-1 (BAB68522), Pp-ENG-2 (BAB68523), Rs-ENG-1 (ACB38289), Rs-ENG-2 (ABV54448), Rs-ENG-3 (ABV54449), Rs-ENG-1B (ABV54447), Rs-ENG-1A (ABV54446), Da-ENG-1 (ABY52965), Dd-ENG-1B (ACJ60676), and Dd-ENG-2 (ACP20205).
Results
Identification of Rr-eng-1 and BLAST analyses: We had previously isolated a 528 bp EST (GenBank Accession No. GT737677.1) from sedentary parasitic R. reniformis females that showed significant similarity to PPN GHF5 β-1,4-endoglucanases (Wubben et al., 2010). BLASTX analysis of GT737677.1 showed that bp 2-247 aligned with amino acids 251-335 of the H. glycines cellulase protein Hg-ENG-6 (GenBank Accession No. AAO25506). The full-length R. reniformis cellulase gene, hereafter referred to as Rr-eng-1, was isolated by three sequential rounds of DNA walking. BLASTX analyses of newly acquired genomic sequence and the presence of a signal peptide at the N-terminus of the predicted protein, as determined by SignalP 3.0 (Bendtsen et al., 2004), were used to determine when we had spanned the complete Rr-eng-1 genomic sequence. The full-length Rr-eng-1 genomic sequence was 2,325 bp. RT-PCR using primers designed from the genomic sequence amplified a Rr-eng-1 cDNA of 1,341 bp with an open reading frame (ORF) of 1,245 bp that was 53.98% G+C (Fig. 1). The Rr-eng-1 cDNA sequence was submitted to GenBank under Accession No. HQ132749. BLASTN analysis showed that the Rr-eng-1 cDNA was most homologous to the Hg-eng-6 mRNA (GenBank Accession No. AY163572; E = 5e-121). The only other BLASTN alignment at E ≤ 1e-15 was to the GHF5 cellulase gene Rs-eng-1A from Radopholus similis (GenBank Accession No. EF693940; E = 2e-18).
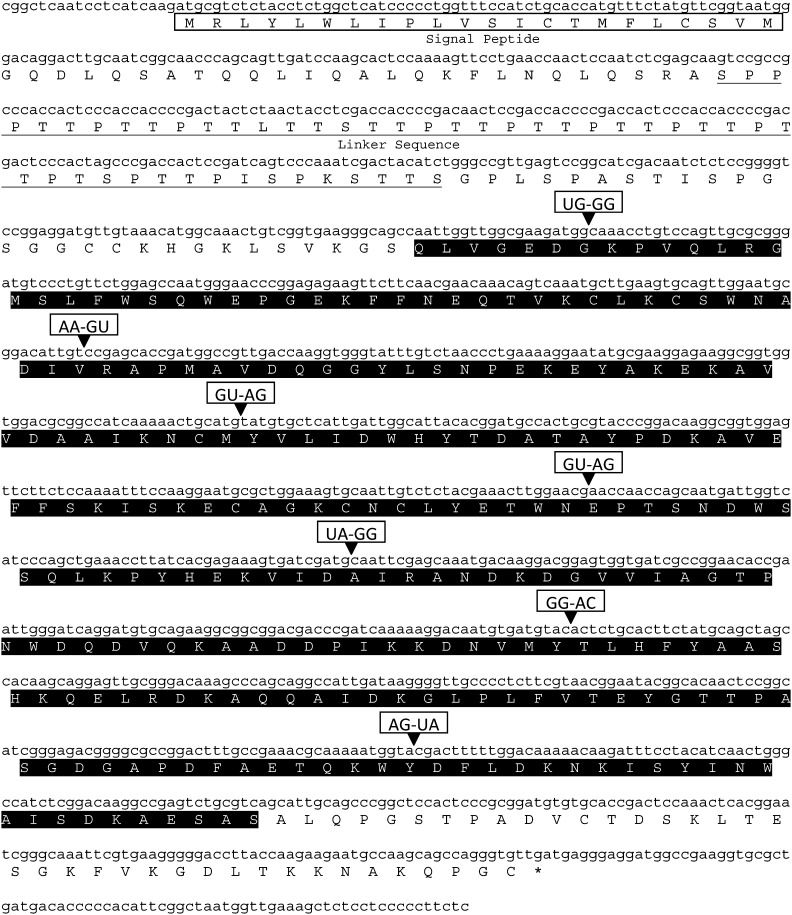
Fig. 1.
The full-length Rr-eng-1 cDNA nucleotide sequence (GenBank Accession No. HQ132749) and predicted polypeptide. Signal peptide and linker sequences are boxed and underlined, respectively. The glycosyl hydrolase family 5 (PF00150) predicted catalytic domain is indicated by the shaded amino acid sequence. Intron splice junctions along with the splice donor-acceptor dinucleotide sequences are indicated by (▾).
Alignment of the Rr-eng-1 genomic and cDNA sequences revealed the presence of eight exons and seven introns (Fig. 1). A similar gene structure was found for Hg-eng-2, -3, -6, and Gr-eng-2 (Gao et al., 2004; Smant et al., 1998; Yan et al., 2001). In addition, 19 bp of the 5′-untranslated region (UTR) and 80 bp of the 3′-UTR were identified (Fig. 1). No 5′-spliced leader sequence was detected for Rr-eng-1. Introns 1 (57 bp), 3 (47 bp), 4 (61 bp), 5 (67 bp), and 7 (95 bp) were similar in size to that observed on average in Caenorhabditis elegans genes (Blumenthal and Steward, 1997), while introns 2 (248 bp) and 6 (409 bp) were significantly larger. Introns 3 and 4 followed the GU-AG consensus sequence normally observed for cis-spliced introns in nematodes (Blumenthal and Steward, 1997; Kyndt et al., 2008); however, the remaining five introns showed variable 5′-donor and 3′-acceptor sites (Fig. 1). Collectively, Rr-eng-1 introns were 66.46% A+T. Among the Rr-eng-1 exons, exon 1 was the largest at 389 bp, while the remaining seven exons ranged from 60 to 184 bp in length (Fig. 1).
The predicted Rr-ENG-1 protein was 414 amino acids long with an estimated molecular weight of 45.3 kDa and theoretical pI of 6.25. A signal peptide probability of 0.996 was calculated by SignalP-NN and SignalP-HMM (Bendtsen et al., 2004) with the most likely cleavage site being between the 22nd and 23rd amino acids (Fig. 1). A signal peptide at the N-terminus of Rr-ENG-1 was also detected by iPSORT (Bannai et al., 2002). A CBM linker sequence was present at the N-terminus of Rr-ENG-1 which consisted of proline and serine/threonine amino acids (Fig. 1). Rr-ENG-1 contained the GHF5 cellulase conserved domain (pfam00150) from amino acids 124-376. BLASTP analysis of Rr-ENG-1 identified Hg-ENG-6 as most homologous (GenBank Accession No. AAO25506; E = 1e-127) with a shared identity of 66% and similarity of 78%. Following Hg-ENG-6, cellulases from migratory PPNs were most similar to Rr-ENG-1 and included Da-ENG-1 (GenBank Accession No. ABY52965; E = 4e-92) from D. africanus, Rs-ENG-1a (GenBank Accession No. ABV54446; E = 5e-92) from R. similis, Dd-ENG-1b (GenBank Accession No. ACJ60676; E = 4e-90) from D. destructor, Rs-ENG-2 (GenBank Accession No. ABV54448; E = 3e-87), Pp-ENG-1 (GenBank Accession No. BAB68522; E = 4e-85) from Pratylenchus penetrans, and Dd-ENG-2 (GenBank Accession No. ACP20205; E = 5e-85). The most homologous non-nematode cellulase was PhEG (GenBank Accession No. BAB86867; E = 1e-80) from the beetle Psacothea hilaris which showed a shared identity of 49% across the entire length of the protein. A large number of prokaryotic cellulase sequences were also homologous to Rr-ENG-1, the most significant of which being a protein from the bacterium Cytophaga hutchinsonii (GenBank Accession No. YP_678708; E = 4e-78).
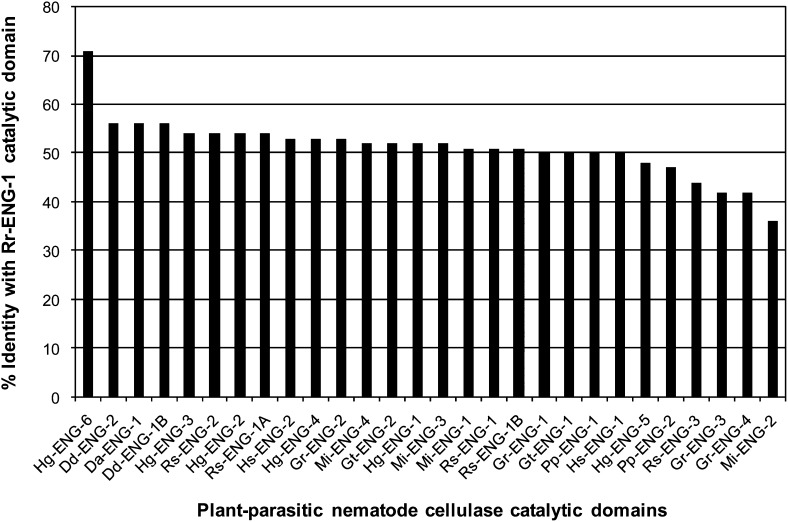
PPN cellulases vary in regards to the presence or absence of a linker and/or CBM sequence; therefore, we aligned the GHF5 catalytic domain of Rr-ENG-1 to the catalytic domains of other PPN cellulases by CLUSTALW. Hg-ENG-6 remained the most homologous sequence at 71% shared identity (Fig. 2). Not counting Hg-ENG-6, a group of twenty-one cellulase catalytic domains shared ≥50% identity with Rr-ENG-1 (Fig. 2). At the top of this group were three cellulases from Ditylenchus spp. that all showed 56% identity with Rr-ENG-1 (Fig. 2). The most divergent catalytic domain relative to Rr-ENG-1 corresponded to the root-knot nematode cellulase Mi-ENG-2 (GenBank Accession No. AAK21883), which shared only 36% identity with Rr-ENG-1 (Fig. 2).
Fig. 2.
Percent identity shared between the Rr-ENG-1 catalytic domain and the catalytic domains of other selected cellulases. Percent shared identity was determined using CLUSTALW. Gr (Globodera rostochiensis), Gt (G. tabacum), Hg (Heterodera glycines), Hs (H. schachtii), Rs (Radopholus similis), Dd (Ditylenchus destructor), Da (D. africanus), Mi (Meloidogyne incognita), Pp (Pratylenchus penetrans).
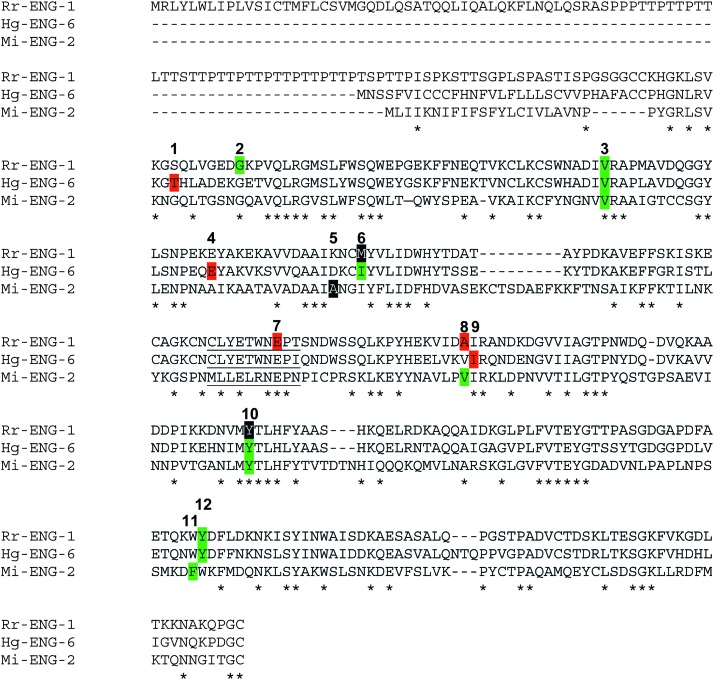
Exon-intron structure and phylogenetic analysis: BLASTP analysis demonstrated that Rr-ENG-1 was most homologous to the cyst nematode cellulase Hg-ENG-6. It has been shown that Hg-ENG-6 shares a number of intron splice positions with Mi-ENG-2 (GenBank Accession No. AAK21883) but not other PPN cellulases, and that these two cellulases form a separate cluster in PPN cellulase phylogenetic analyses (Kyndt et al., 2008; Ledger et al., 2006). Rr-ENG-1 shares only 35% identity with Mi-ENG-2 (Fig. 2); however, we were curious as to the level of intron splice site conservation between these two proteins and Hg-ENG-6. We found that intron splice positions 3 and 10 were conserved among all three proteins (Fig. 3). In addition, the splice position for intron 8 was conserved between Rr-ENG-1 and Mi-ENG-2, while the splice position of intron 9 for Hg-ENG-6 was off by only one amino acid (Fig. 3). A similar situation was found for intron 11 of Mi-ENG-2 and intron 12 for Rr-ENG-1 and Hg-ENG-6 (Fig. 3). In total, four intron splice positions were identified that were conserved among these three proteins. In addition to these four positions, Rr-ENG-1 and Hg-ENG-6 also shared the intron 6 splice position; however, this splice position was in phase 0 for Rr-ENG-1 compared to phase 2 for Hg-ENG-6 (Fig. 3). Interestingly, Rr-ENG-1 showed an intron splice junction within the GHF5 signature sequence, which was not present for either Hg-ENG-6 or Mi-ENG-2 (Fig. 3).
Fig. 3.
CLUSTALW alignment of Rr-ENG-1, Hg-ENG-6, and Mi-ENG-2 identifies shared intron splice positions. Intron splice positions are numbered 1-12 above the amino acid sequence. Amino acids highlighted in: black = phase 0 (splice site is after the 3rd bp of the codon), red = phase 1 (splice site is between the 1st and 2nd bp of the codon), and green = phase 2 (splice site is between the 2nd and 3rd bp of the codon). Positions of shared identity among the three proteins are indicated by asterisks. The glycosyl hydrolase family 5 signature sequence is underlined.
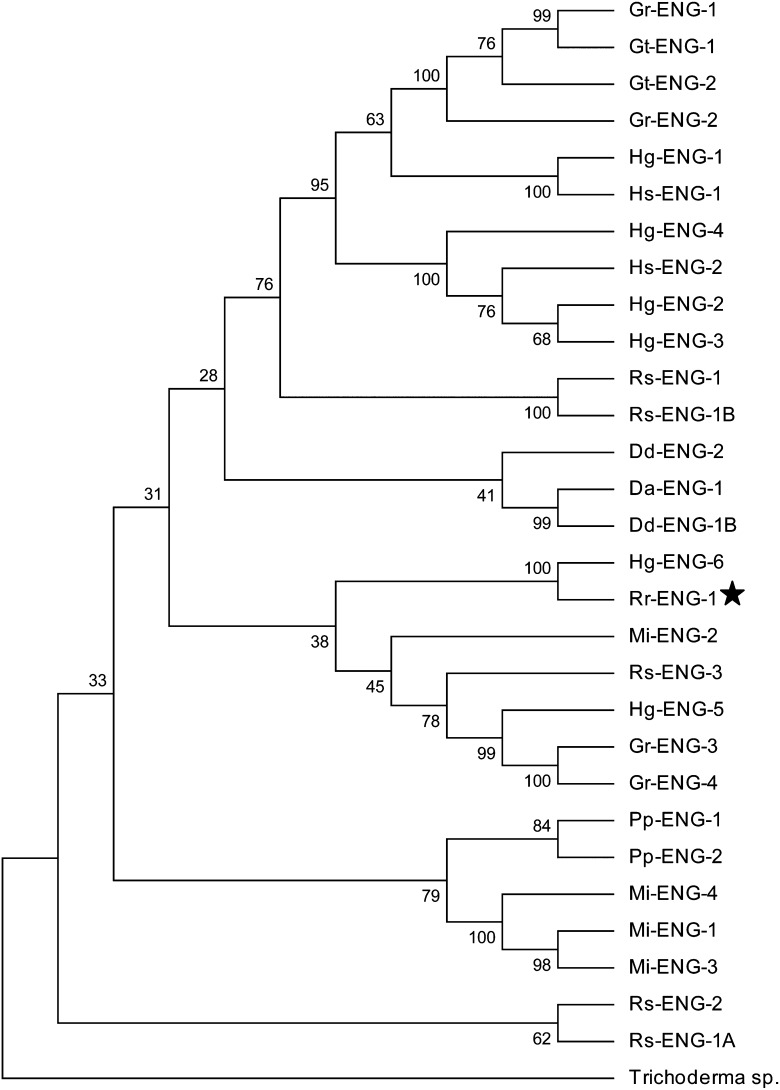
To gain further insight into the evolutionary history of Rr-eng-1 with respect to other PPN cellulase genes, a phylogenetic tree was constructed by maximum parsimony using GHF5 catalytic domain amino acid sequences from cyst nematodes (Heterodera and Globodera spp.), root-knot nematode (M. incognita), migratory PPNs (Ditylenchus, Pratylenchus, and Radopholus spp.), and Rr-ENG-1. A GHF5 β-1,4-endoglucanase from the fungus Trichoderma sp. C-4 was used as an outlier to root the tree because fungal sequences are the most divergent from PPN cellulases (Kyndt et al., 2008). As expected, Rr-ENG-1 and Hg-ENG-6 formed a tight cluster with a high bootstrap value, implying a paralogous relationship between these proteins (Fig. 4). The Rr-ENG-1/Hg-ENG-6 cluster was grouped closest to Mi-ENG-2; however, this arrangement was seen in less than 50% of all possible trees (Fig. 4). Overall, the topology of the consensus tree generally reflected that which had been observed in other evolutionary studies of PPN cellulases (Gao et al., 2004; Kyndt et al., 2008; Ledger et al., 2006; Uehara et al., 2001).
Fig. 4.
Phylogenetic analysis of plant-parasitic nematode GHF5 cellulase catalytic domains. Maximum parsimony was used to create a bootstrap consensus tree inferred from 1000 replicates. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test are shown next to the branches. Placement of Rr-ENG-1 within the tree is indicated by (★). Gr (Globodera rostochiensis), Gt (G. tabacum), Hg (Heterodera glycines), Hs (H. schachtii), Rs (Radopholus similis), Dd (Ditylenchus destructor), Da (D. africanus), Mi (Meloidogyne incognita), Pp (Pratylenchus penetrans).
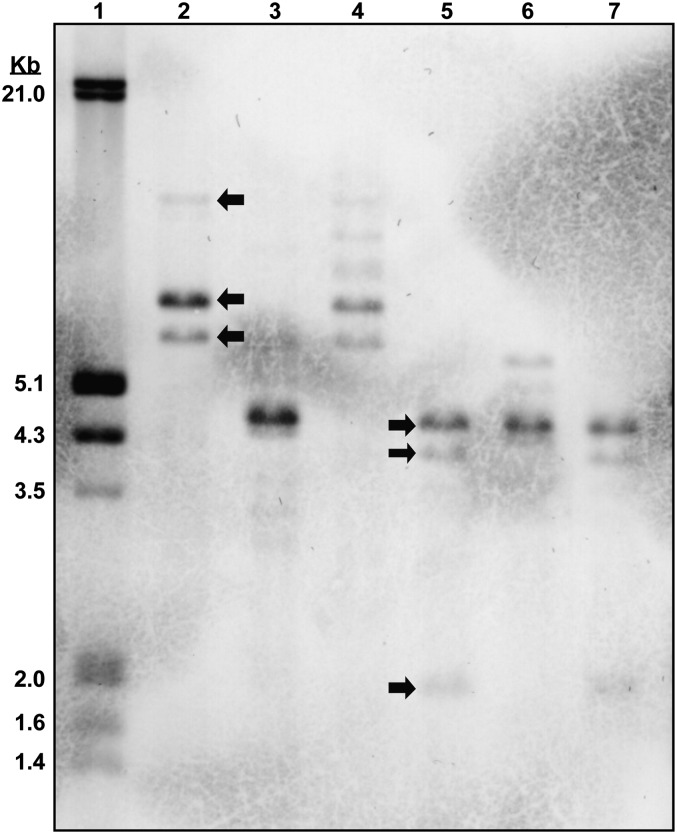
Rr-eng-1 copy number and developmental expression: To determine a minimum number of Rr-eng-1-like sequences in the R. reniformis genome, a DIG (digoxigenin-11-dUTP)-labeled Rr-eng-1 cDNA probe was hybridized to a Southern blot containing restriction enzyme-digested R. reniformis genomic DNA (Fig. 5). Double- and triple-digestions with three different enzymes suggested the presence of at least three homologous Rr-eng-1-like sequences in the R. reniformis genome (Fig. 5).
Fig. 5.
Southern blot of digested Rotylenchulus reniformis genomic DNA probed with a DIG-labeled Rr-eng-1 cDNA. Examples of hybridizing bands are indicated by arrows in lanes 2 and 5. 10 μg digested DNA were loaded in lanes 2-7. Lane 1 (DIG marker), lane 2 (EcoRI), lane 3 (HindIII), lane 4 (BamHI + EcoRI), lane 5 (EcoRI + HindIII), lane 6 (BamHI + HindIII), and lane 7 (BamHI + EcoRI + HindIII).
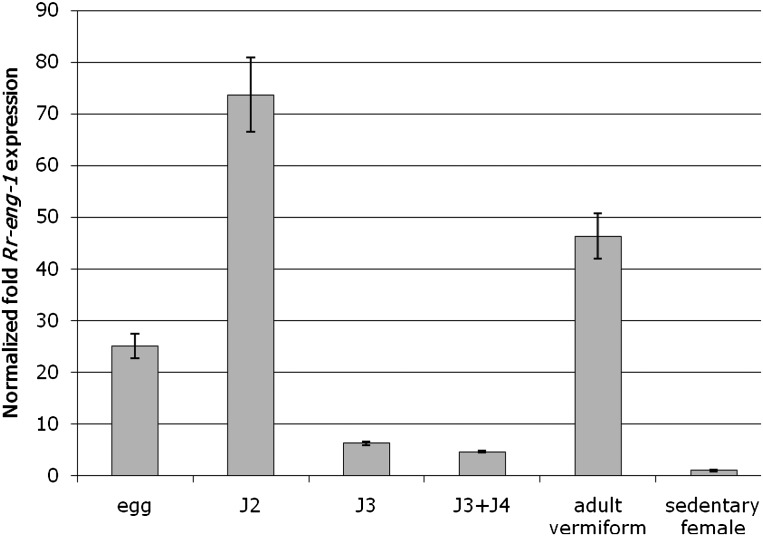
The relative fold-expression of Rr-eng-1 was determined across multiple R. reniformis life-stages by quantitative real-time RT-PCR (qRT-PCR). Total RNA was isolated from the following life-stages: (i) eggs, (ii) J2, (iii) J3, (iv) a mixture of J3 and J4, (v) a mixture of adult vermiform males and infective vermiform females, and (vi) sedentary parasitic females. The corresponding cDNAs were used as template in qRT-PCR reactions with Rr-eng-1-specific primers. We determined that Rr-eng-1 was expressed to some degree in all life-stages; however, relative to sedentary females, the highest levels of Rr-eng-1 expression were observed in eggs (25-fold), J2 (73-fold), and vermiform adults (46-fold) (Fig. 6). Following the J2 stage, Rr-eng-1 expression decreased sharply during the J3 and J3+J4 molting stages (Fig. 6). Rr-eng-1 expression then increased in the sample containing adult male and female vermiform nematodes. Sedentary parasitic females showed the least amount of Rr-eng-1 expression.
Fig. 6.
Expression of Rr-eng-1 in Rotylenchulus reniformis life-stages as determined by quantitative real-time PCR. Presented are the mean ± standard error fold expression values from three technical replicates of Rr-eng-1 relative to that in sedentary female nematodes (set = 1.0). cDNA was generated from R. reniformis eggs, J2, J3, J3+J4, adult vermiform (a mixture of males and pre-parasitic females), and sedentary females. Rr-eng-1 expression was normalized to the level of 18S rRNA in each sample. This experiment was repeated twice with similar results.
Discussion
The plant cell wall represents a formidable barrier that must be overcome by nematodes before parasitism can be initiated. The ability of nematodes to produce a variety of plant cell wall degrading enzymes is a unique adaptation specifically for plant parasitism; consequently, cellulases are referred to as the first parasitism genes identified in plant-parasitic nematodes (Davis et al., 2008). GHF5 cellulases have been identified from many different PPN species, regardless of parasitic life-style; therefore, it should not be surprising that reniform nematode, despite the unusual nature of its sedentary semi-endoparasitic life-cycle, would also possess cellulase genes.
Southern blot analysis of R. reniformis genomic DNA probed with the Rr-eng-1 cDNA identified three hybridizing bands, suggesting that Rr-eng-1 is part of a gene family. Cellulase gene families are common among plant-parasitic nematodes. For example, cellulase gene families have been identified in H. glycines (Gao et al., 2004), P. penetrans (Uehara et al., 2001), and R. similis (Haegeman et al., 2008). In fact, sequencing of the M. incognita genome revealed the presence of 21 GHF5 cellulase genes (Abad et al., 2008). Recently, Danchin et al. (2010) has hypothesized that the selective advantage conferred by cellulases, and other cell wall degrading enzymes, to nematodes following lateral gene transfer from bacteria promoted their duplication in the genome. Different members of a cellulase gene family can be differentially expressed during development. This observation suggests that members of a particular cellulase gene family may play specific roles during plant parasitism (Gao et al., 2004; Haegeman et al., 2008). Based on these observations in other PPN species, it would seem likely that a similar collection of cellulases would exist in the R. reniformis genome.
In situ hybridizations of PPN cellulases have consistently shown their expression to be specific to the subventral esophageal gland cells (Gao et al., 2002; Gao et al., 2004; Goellner et al., 2000; Ledger et al., 2006; Rosso et al., 1999; Smant et al., 1998). For the sedentary endoparasitic species, most cellulases exhibit their highest level of expression during the pre-parasitic or early sedentary parasitic J2 stage when the requirement for cellulase activity would likely be greatest and when subventral gland cell gene expression is at its peak (Elling et al., 2009; Gao et al., 2004; Goellner et al., 2000). Using expression in sedentary females as a baseline for comparison, we found Rr-eng-1 to be highly expressed in eggs, freshly hatched J2, and in a sample containing a mixture of adult males and infective vermiform females. Because R. reniformis males do not feed, elevated Rr-eng-1 expression in the adult vermiform sample can most likely be attributed to the presence of infective females that would use cellulolytic enzymes during root penetration. The observation that cellulase expression was elevated in R. reniformis J2 was unexpected since the J2 stage is not the infective life-stage for this nematode. Cellulase expression in eggs has also been found for Hg-eng-1 and Hg-eng-4 (Gao et al., 2004) and for some Meloidogyne cellulases (Ledger et al., 2006; Rosso et al., 1999). Morphological and developmental studies on R. reniformis have indicated that esophageal gland structure varies in its distinctness during the juvenile molting events (Bird, 1984; Nakasono, 1973). Our observations suggest that the R. reniformis esophageal gland cells remain intact and transcriptionally active during juvenile development, particularly in J2; however, in situ hybridizations of Rr-eng-1 would need to be performed to confirm this conclusion.
The evolutionary relationship among GHF5 cellulases from sedentary and migratory PPNs has previously been studied in detail. Ledger et al. (2006) hypothesized that because of their shared intron splice positions, Mi-eng-2 and Hg-eng-6 likely evolved from a common ancestral GHF5 gene. A similar conclusion was reached by Kyndt et al. (2008) who hypothesized that PPN GHF5 cellulases originated from two ancestral paralogs, type A and type B, that appeared following the duplication of a cellulase having both linker and CBM sequences in addition to the catalytic domain. Mi-eng-2 and Hg-eng-6 were thought to correspond to the type A ancient paralog which lost its linker sequence and CBM shortly after the duplication event (Kyndt et al., 2008). The conservation of several intron splice positions between Hg-eng-6, Mi-eng-2, and Rr-eng-1 suggests that Rr-eng-1 also corresponds to the type A ancestral cellulase; however, the presence of a linker sequence in Rr-eng-1 may indicate a closer resemblance to the original type A gene, if the evolutionary model proposed by Kyndt et al. (2008) is followed. A complicating factor is that the Rr-eng-1 linker sequence is N-terminal to the catalytic domain whilst all other PPN GHF5 cellulases that have a linker sequence show a C-terminal orientation with respect to the catalytic domain (Kyndt et al., 2008; Ledger et al., 2006). The unusual position of the Rr-eng-1 linker sequence relative to the catalytic domain may have occurred through a process known as domain shuffling. In D. africanus, domain shuffling has been proposed in order to explain the unusual domain structure exhibited by the expasin-like gene Da-exp-1 (Haegeman et al., 2010). The similarity between CBMs found within expansin and GHF5 cellulases in plant-parasitic nematodes also implies instances of domain shuffling (Danchin et al., 2010). Furthermore, the Rr-eng-1 linker, being comprised of proline-threonine-threonine repeats, represents a new sequence variant for PPNs. PPN linker sequences identified to date are rich in serine and glycine residues or they resemble linker histone1- and 5-like motifs (Rehman et al., 2009).
The high level of identity shared between Rr-ENG-1 and Hg-ENG-6 may also hint at the potential substrate specificity of Rr-ENG-1. Gao et al. (2004) determined that Hg-ENG-6 showed high levels of activity on crystalline cellulose and xylan despite lacking a CBM. These enzymatic activities stood in stark contrast to the activities exhibited by most other H. glycines cellulases, even among those cellulases having a CBM (Gao et al., 2004). The authors concluded that the significant differences in substrate activity between Hg-ENG-6 and other PPN cellulases was reflected in the high level of sequence divergence in the catalytic domain of Hg-ENG-6 (Gao et al., 2004). While the catalytic domain of Rr-ENG-1 is 66% identical to that of Hg-ENG-6, it is difficult to predict how Rr-ENG-1 activity would be affected by its N-terminal linker sequence.
Literature Cited
- Abad P, et al. Genome sequence of the metazoan plant-parasitic nematode Meloidogyne incognita. Nature Biotechnology. 2008;26:909–915. doi: 10.1038/nbt.1482. [DOI] [PubMed] [Google Scholar]
- Agudelo P, Robbins RT, Stewart JM, Bell A, Robinson AF. Histological observations of Rotylenchulus reniformis on Gossypium longicalyx and interspecific cotton hybrids. Journal of Nematology. 2005;37:444–447. [PMC free article] [PubMed] [Google Scholar]
- Bakhetia M, Urwin PE, Atkinson HJ. qPCR analysis and RNAi define pharyngeal gland cell-expressed genes of Heterodera glycines required for initial interactions with the host. Molecular Plant-Microbe Interactions. 2007;20:306–312. doi: 10.1094/MPMI-20-3-0306. [DOI] [PubMed] [Google Scholar]
- Bannai H, Tamada Y, Maruyama O, Nakai K, Miyano S. Extensive feature detection of N-terminal protein sorting signals. Bioinformatics. 2002;18:298–305. doi: 10.1093/bioinformatics/18.2.298. [DOI] [PubMed] [Google Scholar]
- Baum TJ, Hussey RS, Davis EL. Root-knot and cyst nematode parasitism genes: The molecular basis of plant parasitism. In: Setlow JK, editor. Genetic Engineering Volume 28. Springer Science; 2007. pp. 17–43. [DOI] [PubMed] [Google Scholar]
- Bendtsen JD, Nielsen H, von Heijne G, Brunak S. Improved prediction of signal peptides: SignalP 3.0. Journal of Molecular Biology. 2004;340:783–795. doi: 10.1016/j.jmb.2004.05.028. [DOI] [PubMed] [Google Scholar]
- Bird AF. Growth and moulting in nematodes: Moulting and development of the hatched larva of Rotylenchulus reniformis. Parasitology. 1984;89:107–119. [Google Scholar]
- Blin N, Stafford DW. A general method for isolation of high molecular weight DNA from eukaryotes. Nucleic Acids Research. 1976;3:2303–2308. doi: 10.1093/nar/3.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal T, Steward K. RNA processing and gene structure. In: Riddle DL, Blumenthal T, Meyer BJ, Priess JR, editors. C. Elegans II. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1997. pp. 117–149. [PubMed] [Google Scholar]
- Chen Q, Rehman S, Smant G, Jones JT. Functional analysis of pathogenicity proteins of the potato cyst nematode Globodera rostochiensis using RNAi. Molecular Plant-Microbe Interactions. 2005;18:621–625. doi: 10.1094/MPMI-18-0621. [DOI] [PubMed] [Google Scholar]
- Danchin EGJ, Rosso M-N, Vieira P, de Almeida-Engler J, Coutinho PM, Henrissat B, Abad P. Multiple lateral gene transfers and duplications have promoted plant parasitism ability in nematodes. Proceedings of the National Academy of Science of the United States of America. 2010;107:17651–17656. doi: 10.1073/pnas.1008486107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EL, Hussey RS, Mitchum MG, Baum TJ. Parasitism proteins in nematode-plant interactions. Current Opinion in Plant Biology. 2008;11:360–366. doi: 10.1016/j.pbi.2008.04.003. [DOI] [PubMed] [Google Scholar]
- De Meutter J, Vanholme B, Bauw G, Tytgat T, Gheysen G, Gheysen G. Preparation and sequencing of secreted proteins from the pharyngeal glands of the plant parasitic nematode Heterodera schachtii. Molecular Plant Pathology. 2001;2:297–301. doi: 10.1046/j.1464-6722.2001.00078.x. [DOI] [PubMed] [Google Scholar]
- Elling AA, Mitreva M, Gai X, Martin J, Recknor J, Davis EL, Hussey RS, Nettleton D, McCarter JP, Baum TJ. Sequence mining and transcript profiling to explore cyst nematode parasitism. BMC Genomics. 2009;10:58–74. doi: 10.1186/1471-2164-10-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao B, Allen R, Maier T, Davis EL, Baum TJ, Hussey RS. Identification of a new β-1,4-endoglucanase gene expressed in the esophageal gland cells of Heterodera glycines. Journal of Nematology. 2002;34:12–15. [PMC free article] [PubMed] [Google Scholar]
- Gao B, Allen R, Davis EL, Baum TJ, Hussey RS. Developmental expression and biochemical properties of a β-1,4-endoglucanase family in the soybean cyst nematode, Heterodera glycines. Molecular Plant Pathology. 2004;5:93–104. doi: 10.1111/j.1364-3703.2004.00209.x. [DOI] [PubMed] [Google Scholar]
- Gilkes NR, Henrissat B, Kilburn DG, Miller RC, Jr, Warren RAJ. Domains in microbial β-1,4-glycanases: Sequence conservation, function, and enzyme families. Microbiological Reviews. 1991;55:303–315. doi: 10.1128/mr.55.2.303-315.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goellner M, Smant G, De Boer JM, Baum TJ, Davis EL. Isolation of beta-1,4-endoglucanase genes from Globodera tabacum and their expression during parasitism. Journal of Nematology. 2000;32:154–165. [PMC free article] [PubMed] [Google Scholar]
- Goellner M, Wang X, Davis EL. Endo-β-1,4-glucanase expression in compatible plant-nematode interactions. The Plant Cell. 2001;13:2241–2255. doi: 10.1105/tpc.010219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haegeman A, Jacob J, VanHolme B, Kyndt T, Gheysen G. A family of GHF5 endo-1,4-beta-glucanases in the migratory plant-parasitic nematode Radopholus similis. Plant Pathology. 2008;57:581–590. [Google Scholar]
- Haegeman A, Kyndt T, Gheysen G. The role of pseudo-endoglucanases in the evolution of nematode cell wall-modifying proteins. Journal of Molecular Evolution. 2010;70:441–452. doi: 10.1007/s00239-010-9343-1. [DOI] [PubMed] [Google Scholar]
- Heald CM. Pathogenicity and histopathology of Rotylenchulus reniformis infecting cantaloup. Journal of Nematology. 1975;7:149–152. [PMC free article] [PubMed] [Google Scholar]
- Henrissat B. A classification of glycosyl hydrolases based on amino acid sequence similarities. Biochemical Journal. 1991;280:309–316. doi: 10.1042/bj2800309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrissat B, Claeyssens M, Tomme P, Lemesle L, Mornon J-P. Cellulase families revealed by hydrophobic cluster analysis. Gene. 1989;81:83–95. doi: 10.1016/0378-1119(89)90339-9. [DOI] [PubMed] [Google Scholar]
- Hussey RS, Barker KR. A comparison of methods of collecting inocula of Meloidogyne spp., including a new technique. Plant Disease Reporter. 1973;57:1025–1028. [Google Scholar]
- Jenkins WR. A rapid centifugal-floatation technique for separating nematodes from soil. Plant Disease Reporter. 1964;48:692. [Google Scholar]
- Keen NT, Roberts PA. Plant parasitic nematodes: Digesting a page from the microbe book. Proceedings of the National Academy of Science of the United States of America. 1998;95:4789–4790. doi: 10.1073/pnas.95.9.4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyndt T, Haegeman A, Gheysen G. Evolution of GHF5 endoglucanase gene structure in plant-parasitic nematodes: no evidence for an early domain shuffling event. BMC Evolutionary Biology. 2008;8:305–320. doi: 10.1186/1471-2148-8-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledger TN, Jaubert S, Bosselut N, Abad P, Rosso M-N. Characterization of a new β-1,4-endoglucanase gene from the root-knot nematode Meloidogyne incognita and evolutionary scheme for phytonematode family 5 glycosyl hydrolases. Gene. 2006;382:121–128. doi: 10.1016/j.gene.2006.06.023. [DOI] [PubMed] [Google Scholar]
- Liepman AH, Wightman R, Geshi N, Turner SR, Scheller HV. Arabidopsis – a powerful model system for plant cell wall research. The Plant Journal. 2010;61:1107–1121. doi: 10.1111/j.1365-313X.2010.04161.x. [DOI] [PubMed] [Google Scholar]
- Nakasono K. Studies on post-embryonic development of the reniform nematode, Rotylenchulus reniformis Linford and Oliveira (Nematoda: Rotylenchulidae) I. Morphological changes of the molting larva of a parthenogenetic population. Applied Entomology and Zoology. 1973;8:83–96. [Google Scholar]
- Rebois RV, Madden PA, Eldridge BJ. Some ultrastructural changes induced in resistant and susceptible soybean roots following infection by Rotylenchulus reniformis. Journal of Nematology. 1975;7:122–139. [PMC free article] [PubMed] [Google Scholar]
- Rehman S, Butterbach P, Popeijus H, Overmars H, Davis EL, Jones JT, Goverse A, Bakker J, Smant G. Identification and characterization of the most abundant cellulases in stylet secretions from Globodera rostochiensis. Phytopathology. 2009;99:194–202. doi: 10.1094/PHYTO-99-2-0194. [DOI] [PubMed] [Google Scholar]
- Robinson AF, Inserra RN, Caswell-Chen EP, Vovlas N, Troccoli A. Rotylenchulus species: Identification, distribution, host ranges, and crop plant resistance. Nematropica. 1997;27:127–180. [Google Scholar]
- Rosso M-N, Favery B, Piotte C, Arthaud L, De Boer JM, Hussey RS, Bakker J, Baum TJ, Abad P. Isolation of a cDNA encoding a β-1,4-endoglucanase in the root-knot nematode Meloidogyne incognita and expression analysis during plant parasitism. Molecular Plant-Microbe Interactions. 1999;12:585–591. doi: 10.1094/MPMI.1999.12.7.585. [DOI] [PubMed] [Google Scholar]
- Smant G, Stokkermans JPWG, Yan Y, De Boer JM, Baum TJ, Wang X, Hussey RS, Gommers FJ, Henrissat B, Davis EL, Helder J, Schots A, Bakker J. Endogenous cellulases in animals: Isolation of β-1,4-endoglucanase genes from two species of plant-parasitic cyst nematodes. Proceedings of the National Academy of Science of the United States of America. 1998;95:4906–4911. doi: 10.1073/pnas.95.9.4906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Molecular Biology and Evolution. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- Uehara T, Kushida A, Momota Y. PCR-based cloning of two beta-1,4-endoglucanases from the root-lesion nematode Pratylenchus penetrans. Nematology. 2001;3:335–341. [Google Scholar]
- Vovlas N, Lamberti F. Histological alterations induced by Rotylenchulus reniformis on Coffea arabica roots. Nematologia Mediterranea. 1990;18:77–81. [Google Scholar]
- Wang X, Meyers D, Yan Y, Baum T, Smant G, Hussey R, Davis E. In planta localization of a β-1,4-endoglucanase secreted by Heterodera glycines. Molecular Plant-Microbe Interactions. 1999;12:64–67. doi: 10.1094/MPMI.1999.12.1.64. [DOI] [PubMed] [Google Scholar]
- Williamson VM, Hussey RS. Nematode pathogenesis and resistance in plants. The Plant Cell. 1996;8:1735–1745. doi: 10.1105/tpc.8.10.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wubben MJ, Callahan FE, Hayes RW, Jenkins JN. Molecular characterization and temporal expression analyses indicate that the MIC (Meloidogyne Induced Cotton) gene family represents a novel group of root-specific defense-related genes in upland cotton (Gossypium hirsutum L.) Planta. 2008;228:111–123. doi: 10.1007/s00425-008-0723-3. [DOI] [PubMed] [Google Scholar]
- Wubben MJ, Callahan FE, Scheffler BE. Transcript analysis of sedentary parasitic females of the semi-endoparasitic nematode Rotylenchulus reniformis. Molecular and Biochemical Parasitology. 2010;172:31–40. doi: 10.1016/j.molbiopara.2010.03.011. [DOI] [PubMed] [Google Scholar]
- Yan Y, Smant G, Stokkermans J, Qin L, Helder J, Baum T, Schots A, Davis E. Genomic organization of four β-1,4-endoglucanase genes in plant-parasitic cyst nematodes and its evolutionary implications. Gene. 1998;220:61–70. doi: 10.1016/s0378-1119(98)00413-2. [DOI] [PubMed] [Google Scholar]
- Yan Y, Smant G, Davis E. Functional screening yields a new β-1,4-endoglucanase gene from Heterodera glycines that may be the product of recent gene duplication. Molecular Plant-Microbe Interactions. 2001;14:63–71. doi: 10.1094/MPMI.2001.14.1.63. [DOI] [PubMed] [Google Scholar]