Abstract
Background and Aims
Chronic infection with the bacterial pathogen Helicobacter pylori causes gastric disorders ranging from chronic gastritis to gastric adenocarcinoma. Only a subset of infected individuals will develop overt disease; the large majority remains asymptomatic despite lifelong colonization. This study aims to elucidate the differential susceptibility to H. pylori that is found both across and within populations.
Methods
We have established a C57BL/6 mouse model of H. pylori infection with a strain that is capable of delivering the virulence factor CagA into host cells through the activity of a Cag-pathogenicity island-encoded type IV secretion system.
Results
Mice infected at 5–6 weeks of age with CagA+ H. pylori rapidly develop gastritis, gastric atrophy, epithelial hyperplasia and metaplasia in a type IV secretion system-dependent manner. In contrast, mice infected during the neonatal period with the same strain are protected from preneoplastic lesions. Their protection results from the development of H. pylori-specific peripheral immunological tolerance, which requires TGF-β signalling and is mediated by long-lived, inducible regulatory T-cells, and which controls the local CD4+ T-cell responses that trigger premalignant transformation. Tolerance to H. pylori develops in the neonatal period due to a biased Treg to T-effector cell ratio, and is favoured by prolonged low-dose exposure to antigen.
Conclusions
Using a novel CagA+ H. pylori infection model, we report here that the development of tolerance to H. pylori protects from gastric cancer precursor lesions. The age at initial infection may thus account for the differential susceptibility of infected individuals to H. pylori-associated disease manifestations.
Keywords: Helicobacter pylori, gastric cancer, neonatal tolerance, regulatory T-cells
Introduction
The gastric gram-negative human pathogen Helicobacter pylori is typically acquired during childhood and persists for life.1 Whereas ~20% of infected individuals develop gastric disorders ranging in severity from chronic atrophic gastritis2 to gastric ulcers and gastric cancer,3 the majority remain asymptomatic. This differential risk of developing overt disease is only partly explained by bacterial strain variations, host genetic predisposition, nutrition and other lifestyle factors.4
Chronic infection with H. pylori strains harboring the virulence factor cytotoxin-associated gene A (CagA) increases the risk of developing gastric cancer over the risk associated with H. pylori infection alone.5 CagA is the only known protein substrate of a type IV secretion system encoded by the Cag pathogenicity island (Cag PAI), which allows the bacteria to deliver CagA directly into their host cells cytosol.6 Once inside the host cell, CagA is phosphorylated on C-terminal tyrosine residues by Src-family- and Abl kinases, leading to increased cell motility, elongation and scattering.7 CagA also affects components of tight and adherence junctions, loosening the contacts between neighboring cells, perturbing cell polarity and initiating a process referred to as epithelial-to-mesenchymal transition.8, 9 We have recently reported that the CagA-mediated disruption of host cell polarity and the local breakdown of intercellular contacts allows the bacteria to colonize the apical cell surface.10 The disruption of the epithelial barrier has been postulated to promote inflammation;10 this hypothesis is supported by more severe gastritis detected in individuals infected with CagA+ H. pylori 11 and in Mongolian gerbils infected with CagA delivery-proficient strains.12,13 Similarly, transgenic expression of CagA in the gastric epithelium predisposes mice to epithelial hyperplasia, gastric polyps and adenocarcinomas in a CagA phosphorylation-dependent manner,14 confirming a long-suspected oncogenic role for CagA. Despite these recent advances, a CagA-positive, genetically tractable infection model has remained elusive to date.
Here we provide evidence that a CagA+ clinical patient isolate can stably colonize the murine stomach, triggers a strong local and systemic immune response and rapidly induces gastric cancer precursor lesions. We have used this novel CagA+ infection model to investigate the mechanisms underlying the differential susceptibility of infected individuals to the development of H. pylori-associated preneoplastic gastric disease. We find that the development of immunological tolerance to H. pylori determines the extent of the host s immune response to the infection and influences gastric cancer risk.
Material and Methods
Animal experimentation and cell culture
C57BL6, TCR-β−/−BL6 and IL-10−/−BL6 mice were purchased from Charles River Laboratories (Sulzfeld, Germany). FoxP3-eGFP:DTR, CD4-dnTβRII and IL-10fl/flCD4-Cre mice were described previously.15–17 All animal experiments were approved by the cantonal veterinary office. Mice were maintained in individually ventilated cages and mixed gender groups were infected at either 7 days or 6 weeks of age with 1 orogastric dose of ~2×107 CFU H. pylori PMSS1 or PMSS1ΔcagE (see Supplemental Methods for strain details). For vaccination, mice received 4 weekly doses of 1mg H. pylori sonicate with 10μg of cholera toxin (List Biologicals, Campbell, CA, USA) prior to autologous challenge infection. Antibiotic eradication therapy was achieved by 2 weeks of daily orogastric treatment with 4.5mg/ml metronidazole, 10mg/ml tetracycline hydrochloride (both Sigma-Aldrich, Germany) and 1.2mg/ml bismuth subcitrate (Park-Davis, Australia). In vivo depletion of regulatory T-cells was achieved by weekly i.p. injections of 1×100 and 3×50μg of anti-CD25 antibody or by i.p. injections of 50ng diphtheria toxin per g of body weight at three day intervals (in the FoxP3-eGFP:DTR strain).15 For assessment of CagA translocation, AGS cells (ATCC CRL 1739) were infected for 16h prior to processing for 3D microscopy and immunoblotting as described in the Supplemental Methods.
Preparation of tissues and assessment of H. pylori colonization, gastric cytokine responses, serum antibodies and gastric histopathology
Upon sacrifice, the glandular stomach was retrieved, opened along the lesser curvature and dissected longitudinally into 6 equal pieces comprising identical proportions of antral and corpus tissue. Of every stomach, the same section was assigned to the same downstream processing (embedding, RNA, gDNA etc.) to minimize sampling error. For quantitative assessment of H. pylori colonization, one stomach section was homogenized in Brucella broth and serial dilutions were plated on horse blood plates for colony counting. Gastric IFN-γ, IP-10 and MIP-2 levels were determined by RT-PCR or real time RT-PCR and serum antibodies against H. pylori were measured by ELISA as described in the Supplemental Methods. IFN-γ production was measured either by ELISA according to the manufacturer s instructions (BD Biosciences, San Diego, CA, USA) or by flow cytometry as described in the Supplemental Methods. For the quantitative assessment of gastric histopathology, Giemsa-, Alcian blue- and Periodic Acid Schiff-stained paraffin-embedded stomach sections were scored on a scale of 0–6 for the parameters chronic inflammation, atrophy, epithelial hyperplasia and metaplasia as described previously;18 the entire corpus area from the forestomach/corpus junction to the corpus/antrum junction was taken into account. All pictures were taken with a Leica Leitz DM RB microscope equipped with a Leica DFC 420C camera. Images were acquired using the Leica Application Suite 3.3.0 software. Statistical data analysis is described in the Supplemental Methods.
Results
CagA+ H. pylori infection rapidly induces gastric cancer precursor lesions in a mouse model in a type IV secretion system-dependent manner
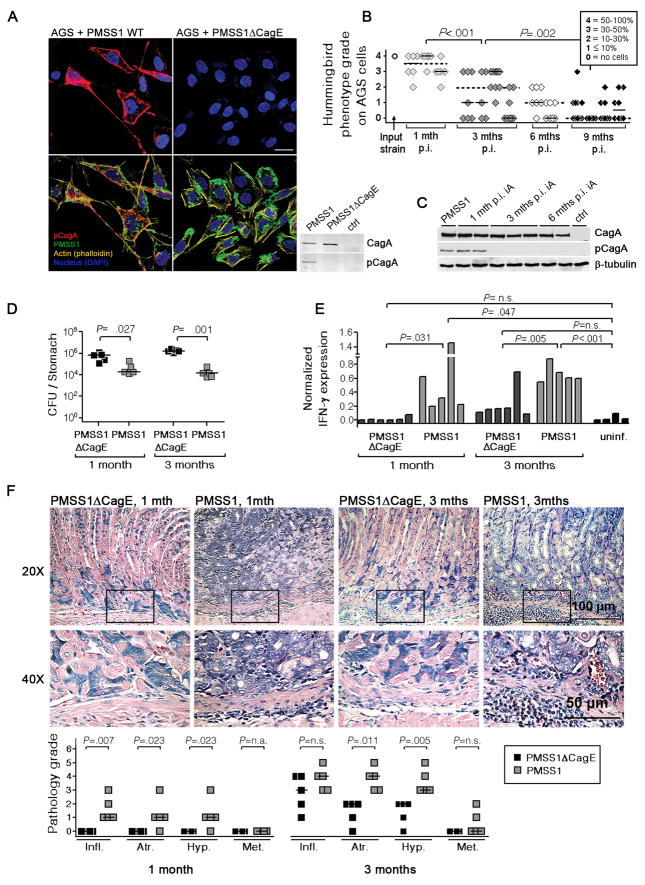
To establish a genetically tractable, CagA+ H. pylori infection model, we revisited a strain that was originally isolated from a duodenal ulcer patient and used to derive a highly mouse colonizing variant, Sydney strain 1 (SS1).19 The parental strain (here termed PMSS1 for “pre-mouse” SS1) persistently colonizes mice, albeit at significantly lower levels than SS1 (supplemental Figure 1A). In contrast to most other mouse-colonizing H. pylori strains,20 PMSS1 harbors a cagA gene, expresses the corresponding protein and is capable of injecting CagA into cultured human gastric epithelial cells (AGS; Figure 1A). CagA translocation by PMSS1 and its tyrosine phosphorylation inside the host cell causes cellular elongation and cell scattering (Figure 1A) as described previously for other CagA+ patient isolates.6,7 An isogenic mutant of PMSS1 lacking an essential component of the type IV secretion system (ΔCagE) fails to trigger this phenotype (Figure 1A), as does the mouse-passaged variant SS1 (supplemental Figure 1B). PMSS1 CagA has three predicted C-terminal EPIYA tyrosine phosphorylation motifs characteristic of the “Western” type (Supplemental Figure 2). The ability of PMSS1 to inject CagA into host cells is maintained in vivo for at least 1 month and decreases gradually thereafter as determined by quantitative assessment of AGS cell elongation and scattering induced by infection with re-isolates (Figure 1B) and by immunoblotting for phospho-CagA performed on re-isolate-infected AGS cell extracts (Figure 1C).
Figure 1.
H. pylori PMSS1 injects CagA into gastric epithelial cells, and rapidly induces gastric cancer precursor lesions in a type IV secretion system-dependent manner. (A) AGS cells were co-cultured with PMSS1 or PMSS1ΔCagE for 16 hours. 3D-confocal immunofluorescence images show elongation and scattering of cells co-cultured with the wild-type strain, but not the ΔCagE mutant. Extracts of the same cells were subjected to immunoblotting with anti-CagA and anti-phospho-CagA antibodies. (B) The hummingbird phenotype induced by clones re-isolated from 1, 3, 6 and 9 month infected mice was quantified on a scale of 0–4 and compared to the input strain. Between 6 and 11 independent re-isolated clones per animal were analyzed for 2 to 4 mice per time point. Individual mice are plotted on the x-axis; each square represents one re-isolated clone. Medians for all clones from one individual and for all mice of a time point are indicated by black and dotted bars. (C) CagA delivery and phosphorylation was confirmed by immunoblotting for selected re-isolates. (D–F) C57/BL6 mice were infected at 6 weeks of age with PMSS1 or PMSS1ΔCagE and sacrificed at 1 month and 3 months post infection (p.i.). (D) Colony-forming units (CFU) per stomach; bars indicate the medians. (E) Gastric IFN-γ expression as determined by real time RT-PCR compared to an age-matched uninfected control group. (F) Low and high magnification micrographs of one representative mouse per group; pathology scores were assigned for the four parameters chronic inflammation, atrophy, hyperplasia and metaplasia; every mouse is represented by four data points. Results representative of 2–3 independent experiments are shown for all panels except B and C.
Cag-PAI+ H. pylori strains are associated with more severe gastritis and a higher risk of gastric cancer development than strains not harbouring this important virulence trait.5, 21, 22 To assess the contribution of the type IV secretion system to the immunogenicity and virulence of PMSS1, we infected mice with PMSS1 or PMSS1ΔCagE for 1 and 3 months (Figure 1D–F). PMSS1ΔCagE colonizes mice at significantly higher levels than the parental strain (Figure 1D) and fails to trigger the local gastric immune responses characterized by high levels of IFN-γ transcript as seen with PMSS1 (Figure 1E). As a consequence of its lower immunogenicity, PMSS1ΔCagE does not induce the pathophysiological changes typically observed in PMSS1-infected stomachs, i.e. chronic inflammation, atrophy, compensatory epithelial hyperplasia and acidic mucus-positive metaplasia (Figure 1F). The PMSS1 derivative SS1 is similarly avirulent in this model (Supplemental Figure 1C, D). In conclusion, PMSS1 is a useful tool to study the consequences of Cag-PAI+ H. pylori infection in a genetically tractable rodent model.
Neonatally infected mice do not initiate local or systemic immune responses to H. pylori and are consequently protected from immunopathological gastric lesions
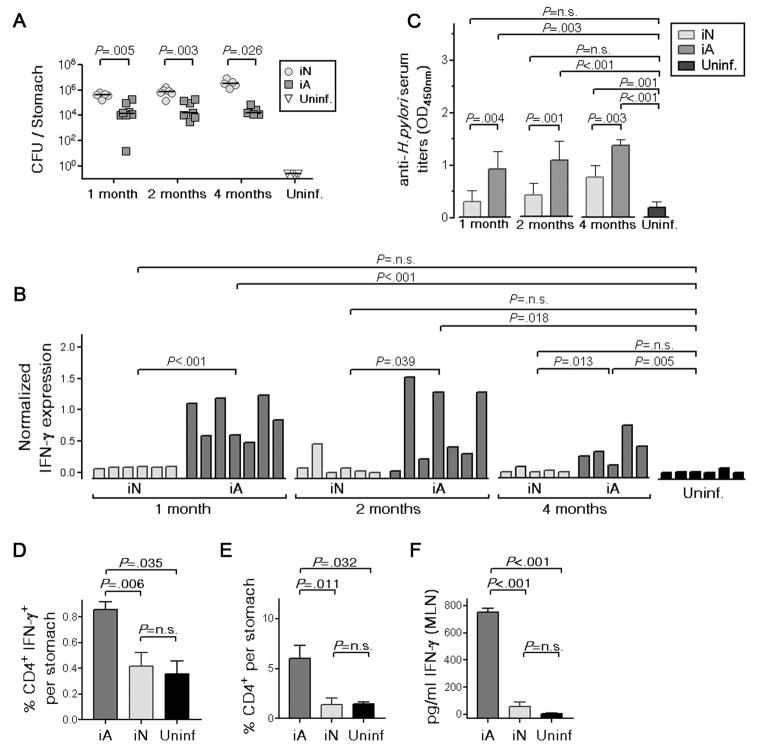
Based on reports that children are less prone to develop gastritis upon H. pylori infection than adults colonized with similar strains,23, 24 we hypothesized that PMSS1 infection outcome may differ depending on age at the time of infection. We infected groups of mice at 1 and 5 weeks of age with PMSS1 (i.e. as neonates and young adults, respectively), and assessed various parameters of infection outcome at 1, 2 and 4 months post infection (p.i.). Mice infected as neonates were colonized by ~two orders of magnitude more heavily than mice infected as adults at all 3 time points analyzed (Figure 2A). Neonatally infected mice further failed to initiate the gastric production of IFN-γ (Figure 2B), and of its downstream mediator IP-10 and the murine IL-8 analogue Mip-2 (Supplemental Figure 3). Serum antibodies against H. pylori were also produced at significantly lower levels in neonatally infected mice (Figure 2C). In line with the higher IFN-γ transcript levels found in adult-infected stomachs, significantly more CD4+IFN-γ+ cells and CD4+ T-cells in general were detected in single cell gastric mucosal preparations of adult-infected compared to neonatally infected mice (Figure 2D, E). Furthermore, IFN-γ secretion as determined by ELISA of single cell suspension culture supernatants of the corresponding mesenteric lymph nodes was significantly higher in the adult-infected compared to the neonatally infected group (Figure 2F). Indeed, neonatally infected mice were indistinguishable from uninfected controls with respect to these 3 parameters (Figure 2D–F).
Figure 2. Neonatally infected mice fail to mount local and systemic immune responses to H. pylori infection.
(A–F) C57BL/6 mice were infected with H. pylori PMSS1 at either 7 days (infected as neonates, iN) or 5 weeks of age (infected as adults, iA) and sacrificed at 1, 2 and 4 months p.i. A shared, uninfected control group consisted of mice that were 1, 2, 4 and 6 months old at the time of sacrifice. (A) CFU per stomach; bars indicate medians. (B) Gastric IFN-γ expression as determined by real time RT-PCR. (C) Serum titers to H. pylori as determined by ELISA. (D, E) Gastric mucosal infiltration of CD4+IFN-γ+ T-cells and all CD4+ T-cells as determined by intracellular cytokine and surface staining for IFN-γ and CD4 at 1 month p.i. (F) Production of IFN-γ by MLN cells after 4 days in culture as determined by ELISA. Data are representative of 5 experiments.
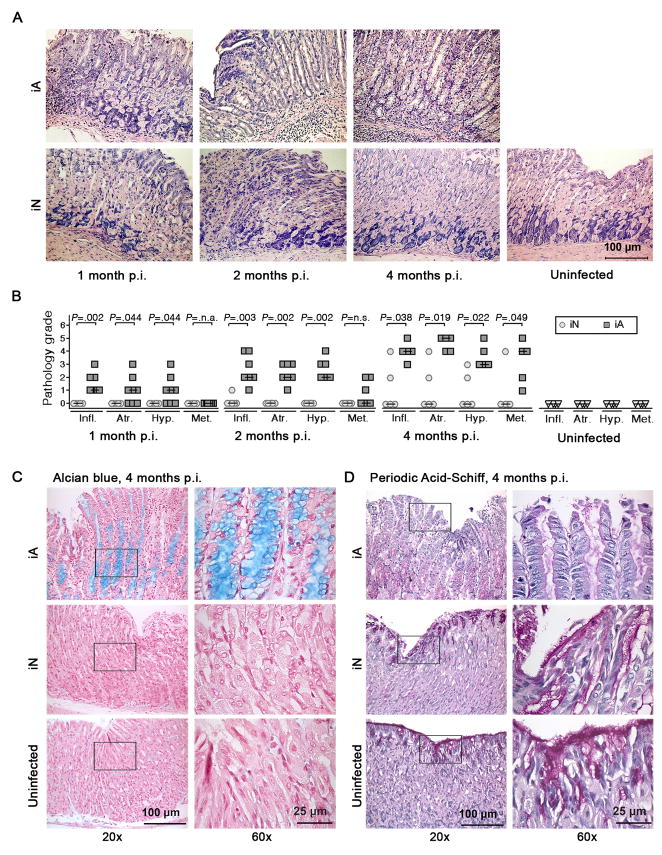
In agreement with our observation that H. pylori colonization levels and gastric pathology are inversely correlated (Figure 1), mice infected as neonates were protected from the chronic gastritis and preneoplastic lesions that characterize adult-infected mice at 1, 2 and 4 months p.i. (Figure 3). Neonatally infected mice developed only very moderate, if any, symptoms of corpus gastritis (Figure 3A, B), and never showed signs of metaplasia or atrophy of the corpus surface epithelium as determined by positive Alcian blue staining and loss of Periodic Acid-Schiff reactivity, respectively (Figure 3C, D). Consequently, neonatally infected mice were assigned significantly lower histopathology scores than mice infected as adults at all time points analyzed (Figure 3B) and were indistinguishable from uninfected mice. In contrast to the major age-dependent differences found in the corpus, the histopathology of the antrum was comparable between all groups (Supplemental Figure 4).
Figure 3. Neonatally infected mice are protected from gastric preneoplastic pathology.
(A–D) Gastric corpus histopathology of the neonatally and adult-infected mice described in Figure 2 was assessed on Giemsa-, Alcian blue- and Periodic Acid-Schiff-stained paraffin sections. Representative micrographs are shown in A, C and D and histopathology scores are shown in B. Low and high magnification micrographs are shown in C and D. Data are representative of 5 experiments.
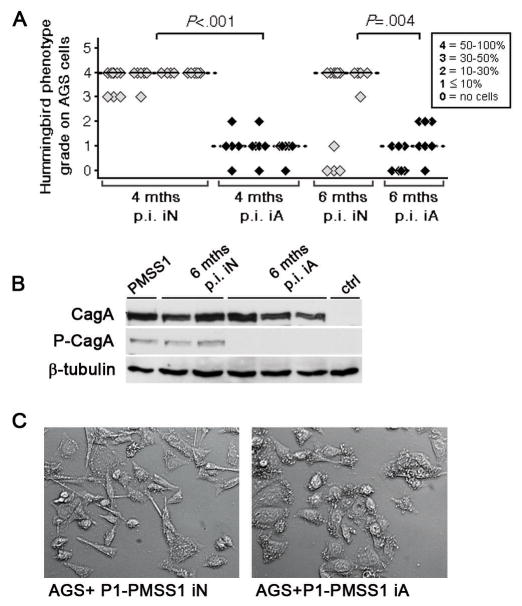
Interestingly, the loss of CagA delivery characteristic of re-isolates from adult-infected mice was observed much less frequently in re-isolates from neonatally infected mice (Figure 4A-C), implying that the negative selective pressure leading to CagA loss in adults is due to their strong local and/or systemic immune response to CagA+ infection. Interestingly, infection with PMSS1ΔCagE and H. felis mirrored our observations with PMSS1 (Supplemental Figure 5), suggesting that the age-related differences in pathology are independent of the type IV secretion system and can also be observed with other gastric Helicobacter species. In summary, the early acquisition of Helicobacter is beneficial for the host in this murine model, as the immunopathological consequences of infection are entirely prevented.
Figure 4. The ability of mouse-colonizing H. pylori to inject CagA in vitro is lost in mice infected as adults, but not in neonatally infected mice.
(A) H. pylori PMSS1 clones were isolated from neonatally or adult-infected mice 4 and 6 months p.i. (iN, 39 clones; iA, 31 clones). Re-isolates derived from 3 independent experiments were assessed quantitatively for hummingbird phenotype induction in AGS cells. Individual mice are plotted on the x-axis; each square represents one clone. Medians for individual mice and time points are indicated by black and dotted bars. (B) The delivery and phosphorylation of CagA by selected 6 month isolates was confirmed by phospho-Cag-specific immunoblotting of infected AGS cell extracts. (C) Representative micrographs of the analysis shown in A.
Helicobacter-specific effector T-cell populations develop normally in neonatally infected mice
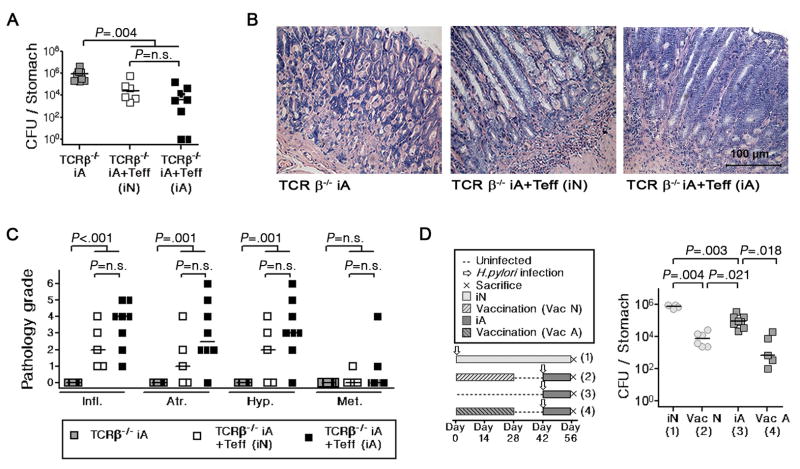
The phenotype of neonatally infected mice can either be explained by the development of H. pylori-specific immunological tolerance –either central or peripheral- or to a lack of “responsiveness” to the bacteria. To discriminate between these possibilities, we assessed whether H. pylori-specific CD4+ effector T-cells are generated normally in neonates or whether they are eliminated during an early stage of development. We chose to test this notion by adoptively transferring CD4+CD25− effector T-cells, which rapidly clear a Helicobacter infection and induce severe preneoplastic gastric pathology in immunodeficient recipients.18 Interestingly, effector cells isolated from the spleens of mice infected either as neonates or as adults reduce H. pylori colonization with similar efficiency in TCR-β−/− hosts (Figure 5A), and also induce indistinguishable levels of preneoplastic pathology (Figure 5B, C).
Figure 5. The priming of T-effector cell responses is not impaired in neonatally infected compared to adult-infected mice.
(A–C) CD4+CD25− effector T-cells from the spleens of neonatally infected and adult-infected animals (Teff (iN) and Teff (iA)) were adoptively transferred into 6 week old TCR-β−/− hosts. All recipients and controls were infected 1 day post-transfer. Colonization and gastric histopathology was determined 4 weeks p.i. Colony counts (A), representative micrographs (B) and histopathology scores (C) are shown. (D) Neonatal and adult mice were immunized 4 times in weekly intervals with an H. pylori vaccine (VacN and VacA) prior to challenge infection. All animals were sacrificed 2 weeks p.i. (see schematic, left panel) and colonization levels were compared to non-immunized control mice (iN, iA). Data are representative of 2 independent experiments.
Having determined that functional H. pylori-specific CD4+ effector T-cells are generated independent of age at infection, we next sought to clarify whether neonates would respond to vaccination against H. pylori. We administered 4 doses of a whole cell PMSS1 sonicate vaccine adjuvanted by cholera toxin to neonatal and adult groups of mice, and measured protective immunity upon challenge infection (Figure 5D). Immunization resulted in a strong reduction of bacterial colonization independent of age (Figure 5D), implying that neonatal vaccination induces protection rather than tolerance. Both results taken together indicate that neonates can develop immunity to H. pylori and suggest an active mechanism of peripheral tolerance induction.
Regulatory T-cells control Helicobacter-specific effector T-cell responses and gastric immunopathology in neonatally infected mice
Pursuing the possibility that peripheral regulatory T-cells (Treg) induce and maintain H. pylori-specific tolerance, we depleted Treg quantitatively during a 4 week time course of infection using an αCD25 mAb. Strikingly, this treatment was sufficient to prevent the development of tolerance and to restore the ability of neonatally infected mice to reduce the bacterial burden to the same or lower levels as found in adults (Supplemental Figure 6).
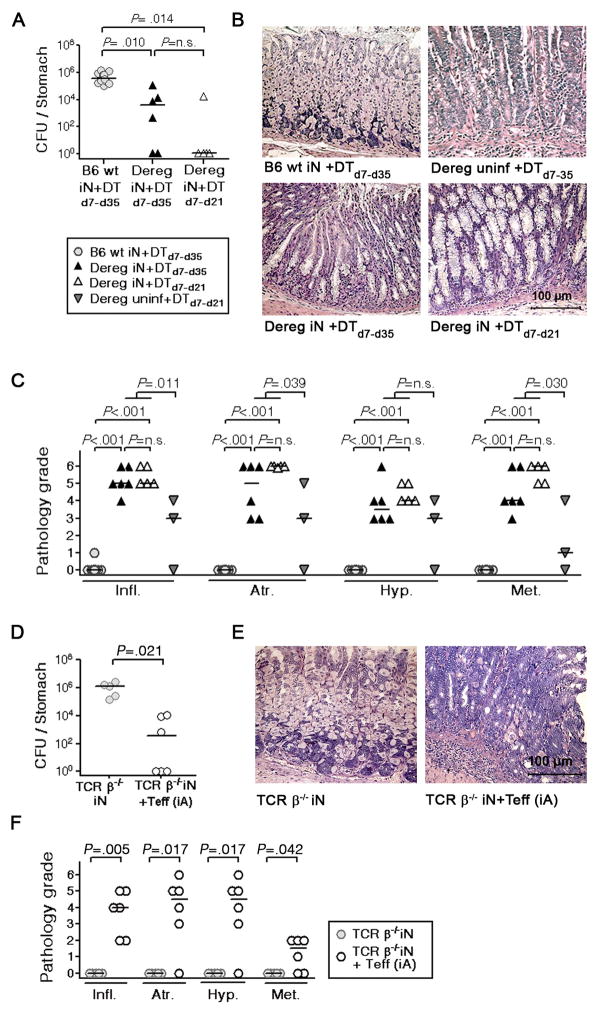
Not all Treg populations express CD25 at similar levels; conversely, CD25 is also up-regulated on activated effector T-cells.15 To verify our result in an alternative model, we took advantage of a transgenic strain expressing a diphtheria toxin (DT) receptor-eGFP fusion protein under the control of the foxp3 promoter and thus allowing for Foxp3+ Treg depletion by injection of DT.15 H. pylori-specific tolerance was prevented in mice depleted of Treg in this manner, resulting in complete clearance or a strong reduction of H. pylori (Figure 6A). It did not make a difference whether Treg were depleted during the entire course of the experiment, or only during the “tolerance window” of neonatal mice, which is generally believed to close at the time of weaning (Figure 6A).25 Clearance of H. pylori in Treg-depleted mice was accompanied by induction of preneoplastic gastric pathology, with widespread metaplasia and complete atrophy observed in all mice (Figure 6B, C).
Figure 6. Tolerance induction and maintenance requires peripheral regulatory mechanisms.
(A–C) Neonatal FoxP3-eGFP:DTR mice ( Dereg, triangles) and their non-transgenic littermates (B6 WT, circles) were infected with PMSS1. Treg were depleted by i.p. administration of diphtheria toxin (DT) starting on the day of infection, either for the entire 4 week time course of the experiment (DTd7–d35, black triangles) or only during the first 2 weeks of infection (DTd7–d21, white triangles). Treg depletion was on average 98% effective in the mesenteric lymph nodes draining the GI tract. All animals were assessed with respect to H. pylori colonization (A) and gastric histopathology (B, C) 4 weeks p.i. and compared to uninfected transgenic mice depleted of Treg for 4 weeks (inverted triangles). (D–F) TCR-β−/− mice were infected as neonates and, at 5 weeks of age, received immunomagnetically purified effector T-cells from the spleen of an adult infected animal (TCR-β−/−(iN) +Teff (iA), white circles). H. pylori colonization (D) and gastric histopathology (E, F) was assessed four weeks post adoptive transfer in comparison to a group that had not received cells (TCR-β−/−(iN), grey circles; compare also to Fig. 5A-C). All results are representative of 2–3 experiments.
To test independently whether H. pylori-specific tolerance can develop in the absence of T-cells, we infected TCR–β−/− mice as neonates and challenged them 4 weeks later with immunomagnetically isolated CD4+CD25− effector T-cells from age-matched, infected WT donors following the procedure shown in Figure 5. All neonatally infected recipients significantly reduced the bacterial burden (Figure 6D) and developed similar gastric pathology as observed in TCR–β−/− recipients infected as adults (compare Figure 6E, F and Figure 5B, C) suggesting that TCRα/β+ cells are absolutely required for tolerance development to H. pylori.
H. pylori-specific tolerance requires TGF-β signalling
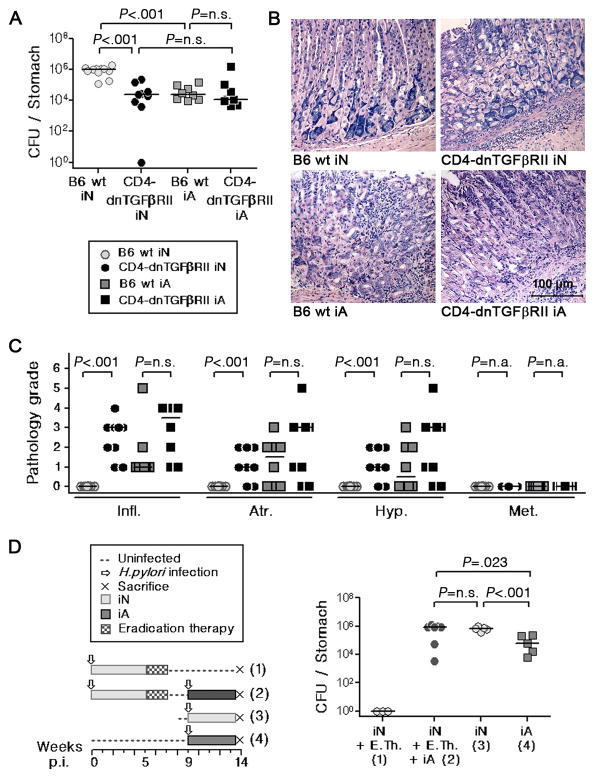
The anti-inflammatory cytokine TGF-β is an important mediator of Treg suppressor functions.26 To examine the role of TGF-β in Helicobacter-specific tolerance, we analyzed the phenotype of a mouse strain with T-cell–restricted, transgenic expression of a dominant-negative form of the TGF-β receptor II (CD4-dnTβRII).16 Natural (n)Treg develop in normal (or higher) numbers in these mice (supplemental Figure 7A), but the induction of iTreg is impaired in the periphery in vivo27 and also in vitro (supplemental Figure 7B). The T-cell–restricted loss of TGF-β signaling prevented tolerance induction in neonatally infected mice (Figure 7A–C), but had only moderate effects in adult-infected mice (Figure 7A–C). IL-10−/− mice, in which Treg also develop normally but lack crucial suppressor functions28 show a very similar, albeit slightly delayed phenotype with respect to H. pylori-specific tolerance (supplemental Figure 8). The phenotype of the IL-10−/− mouse is mirrored almost exactly by a strain in which the il-10 gene is conditionally deleted in the CD4+ and CD8+ T-cell compartments (supplemental Figure 8).17
Figure 7. Tolerance induction requires the activity of TGF-β-responsive, long-lived CD4+ T-cells.
(A–C) Neonatal CD4-dnTβRII mice (black circles) and their WT littermates (grey circles) were infected for 1 month. All mice were analyzed with respect to H. pylori colonization and gastric histopathology and compared to respective adults (grey and black squares). Colony counts (A), representative micrographs (B) and histopathology scores (C) are shown. (D) Neonatally infected mice were subjected to antibiotic eradication therapy followed by re-infection five weeks later (schematic, left panel). Eradication was verified in a control group (1). The remaining mice were killed 4 weeks after re-infection (2) along with groups that had been infected as neonates or adults in week 9 (3, 4). Colony counts are shown. Pooled data from two studies are shown in AC; data in D are representative of 2 independent experiments.
Based on the phenotype of CD4-dnTβRII mice, we hypothesized that tolerance may be mediated in this scenario by TGF-β-induced iTreg, which survive for long periods of time without antigenic stimulation.29 To test whether tolerance to H. pylori requires the continuous, uninterrupted exposure to antigen, we subjected neonatally infected mice to H. pylori eradication therapy starting at five weeks p.i., re-infected them 4 weeks after the onset of antibiotic therapy and compared them to neonatally and adult-infected control groups (Figure 7D). There was no difference in colonization between the mice that had been eradicated and subsequently re-infected and the group that had been continuously colonized for 14 weeks (Figure 7D), arguing that tolerance is maintained despite a >two week gap in exposure to H. pylori. The combined findings imply that long-lived Treg are essential mediators of H. pylori-specific tolerance.
As chronic low-level exposure to oral antigens is known to favor tolerance over immunity,30 we speculated that adults and neonates would differ with respect to their colonization levels during the first weeks p.i. Indeed, H. pylori colonization remains close to the detection limit during the first 2 weeks of neonatal infection, and only recovers around the time of weaning (supplemental Figure 9A), suggesting that the maternal milk efficiently limits bacterial replication. In contrast, adults are exposed to comparatively constant, and significantly higher, levels of bacteria in this time frame (supplemental Figure 9A).
One of the proposed differences between the neonatal and the adult immune systems is the ratio of effector to regulatory T-cell populations.25 Indeed, only 2–4% of the neonatal splenocyte population are CD4+ compared to 10–14% in adults (supplemental Figure 9B) and a significantly larger fraction of these CD4+ T-cells expresses FoxP3 (supplemental Figure 9C). In combination, our data suggest that the low level exposure to antigen that is a hallmark of neonatal infection with H. pylori may favor tolerance over immunity, especially in a setting that is inherently biased towards tolerance due to its abundant regulatory and limited effector T-cell populations.
Discussion
In this study, we provide evidence for an efficient mechanism of tolerance induction to H. pylori in the neonatal period, which strongly reduces the risk of developing gastric cancer precursor lesions later in life. H. pylori-specific tolerance in neonatally infected mice is evident in our PMSS1 infection model due to the strong immunogenicity of this strain in adult animals, which distinguishes it from the weakly immunogenic strains commonly used for experimental murine infection. The PMSS1 model mimics the human host s response to CagA+ H. pylori infection, which results in more severe gastritis11 and a higher risk of gastric adenocarcinoma than infection with CagA− strains.5, 21
PMSS1-specific pro-inflammatory and adaptive immune responses depend on the strain s type IV secretion system. A CagE-deficient, translocation-incompetent mutant of PMSS1 shows strongly reduced immunogenicity and pathogenicity compared to the parental strain. Our observations in the murine model are in line with previous results obtained in the Mongolian gerbil, which have revealed a similar link between gastric inflammation and preneoplastic lesions and a functional type IV secretion system.12, 13 PMSS1 constitutes a notable exception to the rule that CagA+ strains do not colonize mice, and mouse-colonizing strains are CagA-negative.20 Our model has allowed us to follow the fate of CagA-translocation proficient bacteria in vivo over time. Indeed, the ability of re-isolates to deliver CagA in vitro is gradually lost in mice infected as adults; this is not the case in tolerized mice.
Using the CagA+ infection model, we have made the unexpected observation that active tolerance development towards H. pylori is a major determinant of disease outcome. Neonatally infected mice reproducibly fail to launch the local and systemic responses to the bacteria that are readily observed in adult-infected mice as early as one month p.i. As a consequence, the mice are permanently protected from the development of gastric preneoplastic immunopathology. We attributed the tolerance of neonatally infected mice to the induction of peripheral tolerogenic Treg, which efficiently control effector T-cell responses against H. pylori. H. pylori-specific Treg are long-lived and independent of continuous antigenic stimulation, and require TGF-β signalling. Vaccination of neonatal mice with an adjuvanted vaccine overrides the suppressive capacity of Treg; this observation is clinically important as all vaccine development efforts targeting H. pylori envisage early childhood vaccination; it also confirms earlier data validating childhood vaccination with other, less immunogenic strains.31, 32 Indeed, neonatal tolerance to H. pylori is particularly evident with strains that are strongly immunogenic in adults, such as PMSS1 and H. felis. An earlier study using a mildly immunogenic isolate also found differences in colonization dependent on age at the time of infection, but did not link these differences to the differential susceptibility towards gastric cancer precursor lesions.32
The first weeks of life are characterized by an inherent bias towards tolerogenic over immunogenic responses, resulting in an increased susceptibility to viral and bacterial infections.25 Few experimental models exist that mirror neonatal infections in humans; in these models, neonatal tolerance is usually detrimental for the newborn host. For instance, Fernandez et al. showed that the neonatal Treg population actively suppresses CD8+ T-cell responses to herpes simplex virus 2.33 Several differences between the neonatal and adult immune systems have been used to explain the bias towards tolerogenic responses in neonates. A Treg-biased ratio of CD4+ T-cell subsets has been proposed,25 which we could substantiate in our model. Another striking difference between the infected neonates and adults was their bacterial load in the first two weeks of infection. The low dose exposure to antigen of neonatally infected mice is consistent with the preferential induction of tolerogenic over immunogenic responses.30 We conclude that the inherent tolerogenic bias of neonates together with low-dose antigen exposure may account for the development of tolerance rather than immunity in this age group. The risk of developing overt disease differs radically between H. pylori-infected individuals; whereas the majority remain asymptomatic, a subset of infected individuals develops clinical disease manifestations. Our data suggest that immunological tolerance to the infection protects from severe disease outcomes and that the acquisition of H. pylori early in life may be beneficial to the host. In summary, the unexpected finding that tolerant -rather than immune responsive- individuals are protected from H. pylori-induced gastric preneoplasia may have new implications for gastric cancer prevention strategies.
Supplementary Material
Acknowledgments
Grant support: This study was funded by grants from the Swiss National Science foundation, the UBS foundation and the Swiss Cancer League to A.M. Additional funding was supplied by the University Research Priority Program in Systems Biology.
We thank Adrian Lee for strain PMSS1. Stanley Falkow, Burkhard Becher, Joerg Huelsken, Wolf-Dietrich Hardt and Josef Jiricny are thanked for critical discussions or comments on the manuscript. We are grateful to Reto Schwendener, Isabella Toller, Esther Kohler, Iris Hitzler, Vanessa Craig and Ayca Sayi for experimental assistance and discussions.
Abbreviations
- MOI
multiplicity of infection
- CT
cholera toxin
- MLN
mesenteric lymph node
- p.i
post infection
- i.p
intraperitoneal
- i.v
intravenous
- CagA
cytotoxin-associated gene A
Footnotes
Disclosures: The authors declare no competing financial interests.
J.L., M.R.A., A.R., T.S. and R.A.F. contributed vital tools; I.A. and A.M. designed experiments, conducted research, analyzed data and wrote the manuscript. All authors discussed the results and commented on the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Weyermann M, Rothenbacher D, Brenner H. Acquisition of Helicobacter pylori infection in early childhood: independent contributions of infected mothers, fathers, and siblings. Am J Gastroenterol. 2009;104:182–9. doi: 10.1038/ajg.2008.61. [DOI] [PubMed] [Google Scholar]
- 2.Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;1:1311–5. doi: 10.1016/s0140-6736(84)91816-6. [DOI] [PubMed] [Google Scholar]
- 3.Parsonnet J, Friedman GD, Vandersteen DP, et al. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;325:1127–31. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- 4.Pritchard DM, Crabtree JE. Helicobacter pylori and gastric cancer. Curr Opin Gastroenterol. 2006;22:620–5. doi: 10.1097/01.mog.0000245539.50765.f6. [DOI] [PubMed] [Google Scholar]
- 5.Huang JQ, Zheng GF, Sumanac K, et al. Meta-analysis of the relationship between cagA seropositivity and gastric cancer. Gastroenterology. 2003;125:1636–44. doi: 10.1053/j.gastro.2003.08.033. [DOI] [PubMed] [Google Scholar]
- 6.Odenbreit S, Puls J, Sedlmaier B, et al. Translocation of Helicobacter pylori CagA into gastric epithelial cells by type IV secretion. Science. 2000;287:1497–500. doi: 10.1126/science.287.5457.1497. [DOI] [PubMed] [Google Scholar]
- 7.Segal ED, Cha J, Lo J, et al. Altered states: involvement of phosphorylated CagA in the induction of host cellular growth changes by Helicobacter pylori. Proc Natl Acad Sci U S A. 1999;96:14559–64. doi: 10.1073/pnas.96.25.14559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amieva MR, Vogelmann R, Covacci A, et al. Disruption of the epithelial apical-junctional complex by Helicobacter pylori CagA. Science. 2003;300:1430–4. doi: 10.1126/science.1081919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bagnoli F, Buti L, Tompkins L, et al. Helicobacter pylori CagA induces a transition from polarized to invasive phenotypes in MDCK cells. Proc Natl Acad Sci U S A. 2005;102:16339–44. doi: 10.1073/pnas.0502598102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tan S, Tompkins LS, Amieva MR. Helicobacter pylori usurps cell polarity to turn the cell surface into a replicative niche. PLoS Pathog. 2009;5:e1000407. doi: 10.1371/journal.ppat.1000407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peek RM, Jr, Miller GG, Tham KT, et al. Heightened inflammatory response and cytokine expression in vivo to cagA+ Helicobacter pylori strains. Lab Invest. 1995;73:760–70. [PubMed] [Google Scholar]
- 12.Rieder G, Merchant JL, Haas R. Helicobacter pylori cag-type IV secretion system facilitates corpus colonization to induce precancerous conditions in Mongolian gerbils. Gastroenterology. 2005;128:1229–42. doi: 10.1053/j.gastro.2005.02.064. [DOI] [PubMed] [Google Scholar]
- 13.Wiedemann T, Loell E, Mueller S, et al. Helicobacter pylori cag-Pathogenicity island-dependent early immunological response triggers later precancerous gastric changes in Mongolian gerbils. PLoS One. 2009;4:e4754. doi: 10.1371/journal.pone.0004754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ohnishi N, Yuasa H, Tanaka S, et al. Transgenic expression of Helicobacter pylori CagA induces gastrointestinal and hematopoietic neoplasms in mouse. Proc Natl Acad Sci U S A. 2008;105:1003–8. doi: 10.1073/pnas.0711183105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lahl K, Loddenkemper C, Drouin C, et al. Selective depletion of Foxp3+ regulatory T cells induces a scurfy-like disease. J Exp Med. 2007;204:57–63. doi: 10.1084/jem.20061852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gorelik L, Flavell RA. Abrogation of TGFbeta signaling in T cells leads to spontaneous T cell differentiation and autoimmune disease. Immunity. 2000;12:171–81. doi: 10.1016/s1074-7613(00)80170-3. [DOI] [PubMed] [Google Scholar]
- 17.Roers A, Siewe L, Strittmatter E, et al. T cell-specific inactivation of the interleukin 10 gene in mice results in enhanced T cell responses but normal innate responses to lipopolysaccharide or skin irritation. J Exp Med. 2004;200:1289–97. doi: 10.1084/jem.20041789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sayi A, Kohler E, Hitzler I, et al. The CD4+ T cell-mediated IFN-gamma response to Helicobacter infection is essential for clearance and determines gastric cancer risk. J Immunol. 2009;182:7085–101. doi: 10.4049/jimmunol.0803293. [DOI] [PubMed] [Google Scholar]
- 19.Lee A, O’Rourke J, De Ungria MC, et al. A standardized mouse model of Helicobacter pylori infection: introducing the Sydney strain. Gastroenterology. 1997;112:1386–97. doi: 10.1016/s0016-5085(97)70155-0. [DOI] [PubMed] [Google Scholar]
- 20.Philpott DJ, Belaid D, Troubadour P, et al. Reduced activation of inflammatory responses in host cells by mouse-adapted Helicobacter pylory isolates. Cell Microbiol. 2002;4:285–96. doi: 10.1046/j.1462-5822.2002.00189.x. [DOI] [PubMed] [Google Scholar]
- 21.Blaser MJ, Perez-Perez GI, Kleanthous H, et al. Infection with Helicobacter pylori strains possessing cagA is associated with an increased risk of developing adenocarcinoma of the stomach. Cancer Res. 1995;55:2111–5. [PubMed] [Google Scholar]
- 22.Peek RM, Jr, Blaser MJ. Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat Rev Cancer. 2002;2:28–37. doi: 10.1038/nrc703. [DOI] [PubMed] [Google Scholar]
- 23.Campbell DI, Warren BF, Thomas JE, et al. The African enigma: low prevalence of gastric atrophy, high prevalence of chronic inflammation in West African adults and children. Helicobacter. 2001;6:263–7. doi: 10.1046/j.1083-4389.2001.00047.x. [DOI] [PubMed] [Google Scholar]
- 24.Harris PR, Wright SW, Serrano C, et al. Helicobacter pylori gastritis in children is associated with a regulatory T-cell response. Gastroenterology. 2008;134:491–9. doi: 10.1053/j.gastro.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 25.Arnold B, Schuler T, Hammerling GJ. Control of peripheral T-lymphocyte tolerance in neonates and adults. Trends Immunol. 2005;26:406–11. doi: 10.1016/j.it.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 26.Li MO, Wan YY, Flavell RA. T cell-produced transforming growth factor-beta1 controls T cell tolerance and regulates Th1- and Th17-cell differentiation. Immunity. 2007;26:579–91. doi: 10.1016/j.immuni.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 27.Wan YY, Flavell RA. ‘Yin-Yang’ functions of transforming growth factor-beta and T regulatory cells in immune regulation. Immunol Rev. 2007;220:199–213. doi: 10.1111/j.1600-065X.2007.00565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee CW, Rao VP, Rogers AB, et al. Wild-type and interleukin-10-deficient regulatory T cells reduce effector T-cell-mediated gastroduodenitis in Rag−/− mice, but only wild-type regulatory T cells suppress Helicobacter pylori gastritis. Infect Immun. 2007;75:2699–707. doi: 10.1128/IAI.01788-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Apostolou I, Verginis P, Kretschmer K, et al. Peripherally induced Treg: mode, stability, and role in specific tolerance. J Clin Immunol. 2008;28:619–24. doi: 10.1007/s10875-008-9254-8. [DOI] [PubMed] [Google Scholar]
- 30.Friedman A, Weiner HL. Induction of anergy or active suppression following oral tolerance is determined by antigen dosage. Proc Natl Acad Sci U S A. 1994;91:6688–92. doi: 10.1073/pnas.91.14.6688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eisenberg JC, Czinn SJ, Garhart CA, et al. Protective efficacy of anti-Helicobacter pylori immunity following systemic immunization of neonatal mice. Infect Immun. 2003;71:1820–7. doi: 10.1128/IAI.71.4.1820-1827.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Minoura T, Kato S, Otsu S, et al. Influence of age and duration of infection on bacterial load and immune responses to Helicobacter pylori infection in a murine model. Clin Exp Immunol. 2005;139:43–7. doi: 10.1111/j.1365-2249.2005.02658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fernandez MA, Puttur FK, Wang YM, et al. T regulatory cells contribute to the attenuated primary CD8+ and CD4+ T cell responses to herpes simplex virus type 2 in neonatal mice. J Immunol. 2008;180:1556–64. doi: 10.4049/jimmunol.180.3.1556. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.