Abstract
In CHO-K1 cells, heat shock strongly activated reporter-gene expression driven by the cytomegalovirus immediate-early (CMV-IE) promoter from adenoviral and plasmid vectors. Heat shock treatment (2 hrs at 42.5°C) significantly enhanced the promoter DNA-binding activity in nuclear extracts. In CHO cells expressing mGluR1a and mGluR5a receptors under the control of the CMV promoter, heat shock increased receptor protein expression, mRNA levels and receptor function estimated by measurement of PI hydrolysis, intracellular Ca2+ and cAMP. Hyperthermia increased average amplitudes of Ca2+ responses, the number of responding cells, and revealed the toxic properties of mGluR1a receptor. Heat shock also effectively increased the expression of EGFP. Hence, heat shock effects on mGluR expression and function in CHO cells may be attributed to the activation of the CMV promoter. Moreover, this effect was not limited to CHO cells as heat shock also increased EGFP expression in PC-12 and HEK293 cells. Heat shock treatment may be a useful tool to study the function of proteins expressed in heterologous systems under control of the CMV promoter. It may be especially valuable for increasing protein expression in transient transfections, for enhancing receptor expression in drug screening applications and to control the expression of proteins endowed with toxic properties.
Keywords: heat shock, CMV promoter, metabotropic glutamate receptor, PI hydrolysis, toxicity, transfection
1. Introduction
Metabotropic glutamate receptors (mGluRs) belong to the family of G protein-coupled receptors and have been classified into 3 groups (Conn and Pin, 1997). Group I includes mGluR1 and mGluR5. Agonist stimulation of both receptors leads to the activation of phospholipase C followed by inositol phosphates (IP) formation and intracellular Ca2+ release. In addition, mGluR1a is also able to stimulate cAMP formation and arachidonic acid release (Aramori and Nakanishi, 1992;Abe et al., 1992). In contrast, in heterologous expression systems group II (mGluR2 and 3) and III (mGluR4, 6, 7 and 8) receptors are negatively coupled to adenylyl cyclase, thus their stimulation leads to inhibition of cAMP formation (Conn and Pin, 1997). Group I mGluRs have been implicated in glutamate-induced excitotoxicity that leads to neuronal cell death associated with acute brain ischemia and neurotrauma, and may contribute to a variety of neurodegenerative diseases (Nicoletti et al., 1996)
To study the potential toxic properties of the mGluR1a we have expressed the receptor in Chinese hamster ovary cells (CHO). The receptor gene expression was controlled by the cytomegalovirus immediate-early (CMV-IE) gene enhancer/promoter. The CMV-IE promoter is one of the most commonly used promoters in eukaryotic expression vectors allowing high levels of protein expression in most mammalian cells. The potency of the CMV-IE promoter is attributed primarily to its enhancer element, which is located between nucleotides –118 and –524. This strong enhancer has little cell type preference and contains consensus binding sites for transcription factors such as nuclear factor 1 (NF1) (Niller and Hennighausen, 1991), and 90 (NF90) (Reichman et al., 2002), NF-kappaB/Rel (Sun et al., 2001), CREB/ATF (Stamminger et al., 1990), AP-1 (Rideg et al., 1994), SP-1 (Luu and Flores, 1997), ELK-1 (Chan et al., 1996), retinoic acid receptor and RAR-RXR family members (Angulo et al., 1996). Hence, the CMV-IE promoter can be stimulated by a wide range of different stimuli, such as phorbol myristate acetate, forskolin (Maass et al., 2003) and dibutyryl-cAMP (Zhang et al., 2003), prostaglandin E2, tumor necrosis factor alfa and other cytokines (Kline et al., 1998;Prosch et al., 1995) and lipopolysaccharide (Lee et al., 2004). In addition to the pharmacological stimulation, it has been shown that heat shock (HS) enhanced CMV-IE promoter driven gene expression transduced by adenovirus (Lee et al., 2002).
To enhance the yield of mGluR expression, we explored the inducible properties of the CMV-IE promoter in different transfection vectors. While the presence of a HS-element motif within the CMV-IE promoter/enhancer has not been reported, here we present evidence that HS treatment considerably enhanced the expression of several receptor proteins driven by the CMV-IE promoter in CHO cell lines and revealed the toxic properties of the mGluR1a receptor.
This report describes two aspects of the HS effect. One is the effect that HS plays in a direct stimulation of the CMV promoter. The other explores how the HS sensitivity of the CMV promoter can be used to enhance the expression of transfected proteins, including neurotransmitter receptors. This approach may be useful to reveal functional properties of receptors expressed at low densities and provides a HS-inducible system to study proteins endowed with toxic properties.
2. Materials and Methods
2.1. Materials
Fura-2/AM and Pluronic F-127 were obtained from Molecular Probes, Inc. (Eugene, OR). All restriction enzymes were from New England Biolabs (Beverly, MD). DMEM, fetal bovine serum, antibiotic/antimycotic and G-418 were purchased from Invitrogen (Carlsbad, CA). L-Quisqualic acid, (1S,3R)-1-aminocyclopentane-1,3-dicarboxylic acid (ACPD), (S)-3,5-dihydroxyphenylglycine (DHPG) were from Tocris Cookson, (Ellisville, MO). All other chemicals were from Sigma (St. Louis, MO).
2.2. Vector construction and cell cultures
Rat mGluR1a and mGluR5a cDNAs were subcloned into BamHI/NotI or EcoRI/NotI restriction sites of mammalian expression vector pcDNA3.1 (Invitrogen), respectively. This vector contains the CMV-IE promoter/enhancer and the neomycin resistance gene allowing stably transfected cells to be selected using G-418. Chinese hamster ovary (CHO)-K1 cells were transfected by using Effectene transfection reagent (Qiagen, Hilden, Germany). Individual cell lines were isolated by limited dilution and cultured in DMEM supplemented with 10% fetal bovine serum, 1% L-proline, 0.8 mg/ml G-418 and antibiotic/antimycotic in a humidified 6% CO2 atmosphere at 37°C. The mGluR2 sequence was cloned into pcDNAI/Amp (Invitrogen) plasmid under control of the CMV promoter (Wroblewska et al., 1997). The cells were plated into 96 well plates or 35 mm culture dishes 3-4 days before heating.
2.3. Construction of Adenoviral vectors
mGluR1a cDNA was cloned in frame with EGFP (enhanced green fluorescent protein) sequence into SalI/XbaI restriction sites of pEGFP-C2 vector (Clontech, Palo Alto, CA). Then, NheI/XbaI cDNA fragment, containing GFP-mGluR1a sequence, was cloned in the pShuttle vector (Clontech). The resulting vector was digested with I-CeuI and PI-SceI restriction enzymes and the fragment, containing sequences of both GFP and mGluR1a under control of the CMV-IE promoter/enhancer, was cloned into Adeno-X viral vector (Clontech). Adenoviruses were isolated from transfected human epithelial kidney (HEK)-293 cells using BD Adeno-X virus purification kit (BD Biosciences, Palo Alto, CA). Purified adenoviruses containing EGFP or EGFP-mGluR1a cDNAs were added at indicated multiplicity of infection (MOI) to the cells 24 hours before HS. Expression of EGFP and EGFP-mGluR1a fusion protein was monitored by fluorescence measurement at 485 nm (excitation)/520 nm (emission) wavelength using MFX Microtiter Plate Fluorometer (Dynex, Chantilly, VA).
2.4. Heat shock treatment
Typically, cell cultures were incubated for 2 hours in a CO2 (6%) incubator at 42.5°C, and after treatment were returned to 37°C for 24 hours. This temperature was shown previously to achieve the highest level of expression without inducing toxicity (Shchepotin et al., 1997). Cells were cultured in 96 well plates (in the volume 100 μl) or in 35 mm dishes (2 ml). In preliminary experiments cells were incubated for 2 hours at 42.5°C in a water bath without additional CO2. Both treatments gave similar results.
2.5. Immunoblots
Cells were harvested in 25 mM Tris-HCl buffer, pH 7.5, containing protease inhibitors cocktail (Sigma), 1mM EDTA and 1mM Na3VO4. Membrane proteins were solubilized in sample buffer, containing 2.5% SDS and 20 mM DTT, and resolved by SDS-PAGE on a 7.5% gel (Invitrogen). Proteins were transferred to Hybond ECL nitrocellulose membrane (Amersham Pharmacia Biotech, Buckinghamshire, UK) and were probed with specific antibodies (anti-mGluR1a and anti-mGluR5a from Upstate Biotechnology, Waltham, MA, anti-mGluR2 from Chemicon, Temecula, CA, anti-GFP from Clontech). Receptor proteins were visualized by incubation with horseradish peroxidase-coupled goat anti-rabbit secondary antibody (Amersham) followed by exposure to a chemiluminescent chromogen (ECL-Amersham).
2.6. Quantitative real-time RT-PCR
Total RNA was isolated from cells by using TRIzol reagent, (Invitrogen). Reverse transcription (RT) was carried out from 1 μg of total RNA in the volume of 20 μl. Real-time PCR was performed form 1 μl of RT-mixture on MX3000P Real-Time PCR System (Stratagene, La Jolla, CA) with TaqMan Universal PCR Master Mix, primers and fluorescent probe for mGluR1a from Assays-on-Demand TaqMan Gene Expression Assays (Applied Biosystems, Branchburg, NJ). For amplification, initial denaturation at 95°C for 10 min was performed, followed by 40 cycles: 15 sec at 95°C and 60 sec at 60°C. All samples were analyzed in triplicates, and for all samples the level of the housekeeping gene glyceraldehyde 3-phosphate dehydrogenase was analyzed.
2.7. Electromobility shift assay (EMSA)
DNA corresponding to CMV-IE promoter/enhancer was isolated from pEGFP-C2 vector (Clontech) by digestion with AseI/AgeI restrictases and purification on LMP-agarose. Both restriction enzymes generate 5’ overhang (TA and CCGG respectively), which can be labeled in fill-in reaction. Labeling of the DNA-probe was done by incorporation of 20μCi [32P]-dCTP with 0.5 units of Pfu DNA polymerase and 100 μM dNTPs at 72°C for 30 minutes. Nuclear extracts were prepared from CHO cells. Cell pellets were resuspended in 400 μl of cold buffer containing 10 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA, 1 mM DTT, 0.5 mM phenylmethanesulfonyl fluoride (PMSF) and 10 mM Hepes pH 7.9. To disrupt plasma membranes 25 μl of 10% solution of Nonidet NP-40 were added with vigorous vortexing. After centrifugation, the nuclear pellets were resuspended in 50 μl of ice-cold buffer containing 0.4 M NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM DTT, 1 mM PMSF and 20mM Hepes pH 7.9. The extracts (5 μg protein) were incubated for 1 hr with the 32P-labeled DNA probe (60 000 cpm) in binding buffer (10% glycerol, 50 mM KCl, 0.5 mM DTT and 10 mM Tris-HCl, pH 7.5) in presence of 0.5 unit/ml Poly dI/dC to prevent nonspecific binding. The formation of DNA-protein complexes leading to a “shift” in molecular weight of the labeled DNA-probes was analyzed on 6% polyacrylamide gels.
2.8. Assessment of signal transduction and cell viability
Phosphoinositide (PI) hydrolysis, cAMP assay and intracellular calcium ([Ca2+]i) measurements were performed as described previously (Surin et al., 2007). Cell viability was measured by using the colorimetric MTT (thiazolyl blue tetrazolium) assay (Sigma) (Mosmann, 1983). MTT is converted by active mitochondria into a colored formazan product proportional to the number of live cells. Following 1 h of incubation of cells with MTT (0.2 mg/ml of medium) the produced formazan was extracted in DMSO and the absorbance was measured at 570 nm.
3. Results
3.1. Effect of heat shock on the CMV-IE promoter activity
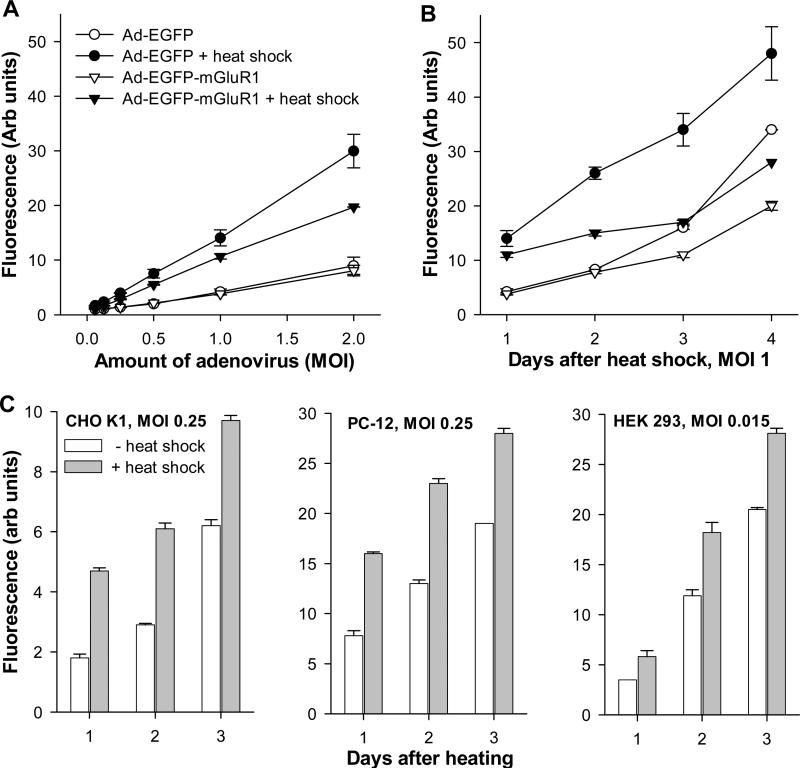
To assess the effect of heat shock on the CMV-IE promoter-driven gene expression we constructed adenoviral vectors containing the CMV-IE promoter/enhancer carrying cDNAs corresponding to EGFP and EGFP-mGluR1a fusion protein (EGFP was attached to the N-terminus of the mGluR1a). CHO cells were infected by adenoviruses at various titers 3 days after plating. HS treatment (42.5°C for 2 hrs) was performed on the next day after infection. As estimated 24 hrs after the treatment, HS significantly facilitated the expression of both EGFP and EGFP-mGluR1a proteins delivered by the adenovirus (Fig. 1A), as manifested by an increased number of fluorescent cells (not shown) and the measured overall intensity of fluorescence. The elevated expression of the fluorescent proteins persisted for at least 4 days after HS treatment (Fig. 1B). The stimulatory effect of HS on protein expression was not limited to CHO cells, as similar results were seen in other cell lines such as PC-12 and HEK 293 (Fig. 1C). However, in HEK 293 cells, lower adenoviral titer was used because of their ability to allow adenovirus replication leading to rapid cell death.
Fig. 1.
Effect of heat shock on expression of adenovirus-delivered EGFP and EGFP-mGluR1a. (A) CHO-K1 cells were infected one day before heating using different titers (MOI) of adenovirus and fluorescence of the expressed proteins was measured 24 hrs after HS treatment. (B) Time course of protein expression in CHO-K1 cells at different days after HS treatment, performed on day 0. (C) Effect of HS on adenovirus-mediated EGFP expression in different cell lines. CHO-K1 and PC-12 cells (infected at MOI 0.25), and HEK 293 cells (infected at MOI 0.015) were treated and monitored as in (B). Cells were heated for 2 hrs at 42.5°C.
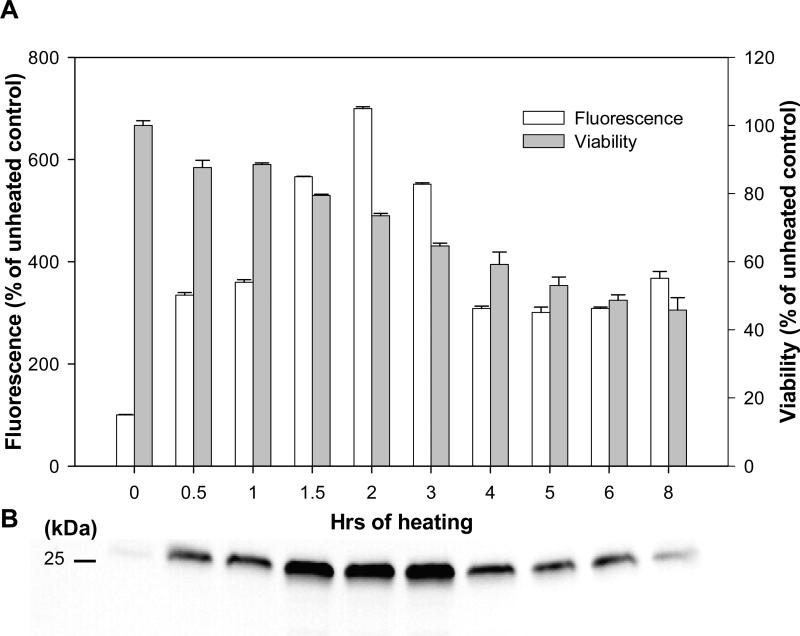
In subsequent experiments, we assessed the stimulatory role of HS on protein expression in stable cell lines transfected with CMV-driven plasmid vectors. CHO cells were transfected with the commercially available vector pEGFP, which expresses the EGFP gene under control of the CMV-IE promoter/enhancer. HS treatments were applied 1-2 days after the cells reached confluency. As shown in Fig. 2, heating at 42.5°C for 2 hrs considerably increased EGFP expression, as estimated on the next day after treatment by fluorescence measurements (Fig. 2A) and by Western blotting (Fig. 2B). Longer treatments did not additionally stimulate EGFP expression but rather led to significant toxicity (Fig. 2A). Hence, in further experiments, 2 hrs were used as the optimal duration of HS treatments.
Fig. 2.
Effect of heat shock on the reporter gene expression driven by the CMV-IE promoter/enhancer in transfected CHO-K1 cells. CHO-K1 cells were permanently transfected with pEGFP-C2 vector expressing EGFP as a reporter gene under control of the CMV-IE promoter/enhancer. The cells were heated at 42.5°C for the indicated time. (A) Activity of the promoter was evaluated by measurement of the reporter gene expression (i.e. EGFP fluorescence) 24 hrs later. Cell viability was measured by MTT assay in the same samples. (B) Whole cell extracts were collected next day after heating. Samples (5 μg of total protein) were resolved on 12 % PAGE. EGFP expression was measured by Western blotting with specific anti-GFP antibodies. Each protein band corresponds to the time-point indicated in (A).
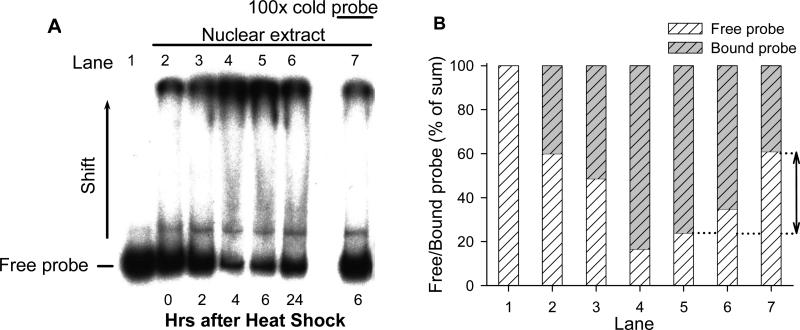
The observed stimulatory effects suggest that HS may enhance gene expression through the activation of the CMV-IE promoter. The possibility that HS may enhance DNA-binding of some transcription factor(s) to the CMV-IE promoter/enhancer was tested by using the electromobility shift assay. For this purpose, nuclear extracts from CHO cells heated for 2 h and a 32P-labeled DNA probe corresponding to the promoter sequence was used. As shown in Fig. 3, HS treatment stimulated the formation of CMV-IE promoter/enhancer DNA-nuclear protein complexes leading to a significant shift in molecular weight of the labeled DNA-probes. The nuclear protein-DNA binding increased after HS in a time-dependent manner and reached maximum at 4-6 hrs after treatment. Competition reaction with a 100-fold excess of the cold DNA-probe significantly reversed the mobility shift, confirming the specificity of interactions. These experiments allowed us to conclude that HS treatment caused enhanced binding of nuclear proteins to the CMV-IE promoter, followed by activation of EGFP reporter gene expression.
Fig. 3.
Heat shock induces DNA-binding activity to the CMV promoter in nuclear extracts from CHO-K1 cells. (A) Nuclear extracts were obtained from the cells before heat shock (Lane 2) and 2, 4, 6 and 24 hrs after heating (Lanes 3-7). Heat shock treatment was performed for 2 hrs. Binding activity of nuclear proteins to the radiolabeled CMV promoter probe was estimated by the electromobility shift assay. The extracts were incubated with [P32]-probe for 60 min and resolved on 6% PAGE. The lower bands correspond to the free probe, the upper – to the protein-DNA complexes. Specificity of binding to the CMV promoter was examined in a competition reaction with a 100-fold excess of cold probe added to the binding reaction (Lane 7). Lane 1 contains only free [P32]-probe without nuclear extracts. (B) Quantification of ratio (in % of sum) of free and bound probe. Lanes correspond to those in (A). The arrow indicates the effect of cold probe addition (Lane 7 should be compared with Lane 5 as indicated by dotted line).
3.2. Effect of heat shock on mGluR expression
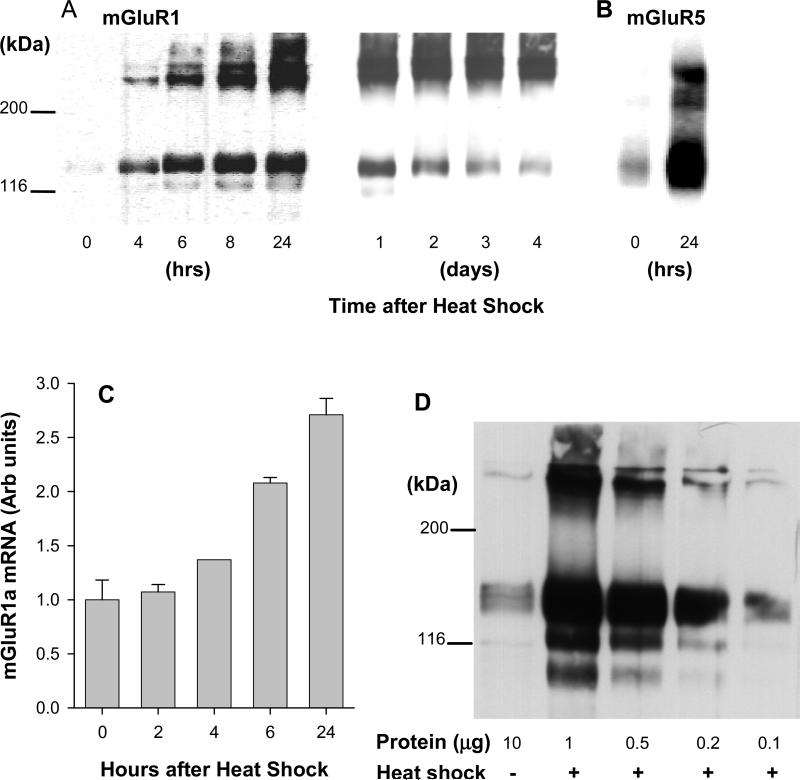
To establish stable cell lines expressing mGluRs, CHO-K1 cells were transfected with pcDNA3.1-based vectors containing the appropriate cDNAs under control of the CMV-IE promoter/enhancer. In case of group I receptors expression levels of both mGluR1a and mGluR5a proteins were relatively low, which correlated well with a weak receptor response (as will be shown in Fig.5). Application of HS to cells expressing mGluR1a caused a time-dependent increase of receptor protein, as seen on Western blots (Fig. 4A). Expression was the highest at 24 h after HS treatment, and then gradually declined, but remained elevated at 4 days after treatment. A similarly increased expression, 24 hrs after HS treatment, was observed in cells transfected with mGluR5a (Fig. 4B). Measurements of mGluR1a mRNA by real-time PCR showed also increased mRNA levels (Fig. 4C). To assess the magnitude of the HS effect on mGluR1a expression, we performed Western blots using a series of dilutions of the treated sample. As shown in Fig. 4D, the intensity of receptor band containing 0.1 μg of protein from HS-treated cells corresponds to the band representing 10 μg of protein from untreated cells, indicating that, 24 h after treatment, HS increased mGluR1a expression about 100-fold, as compared to unheated cells.
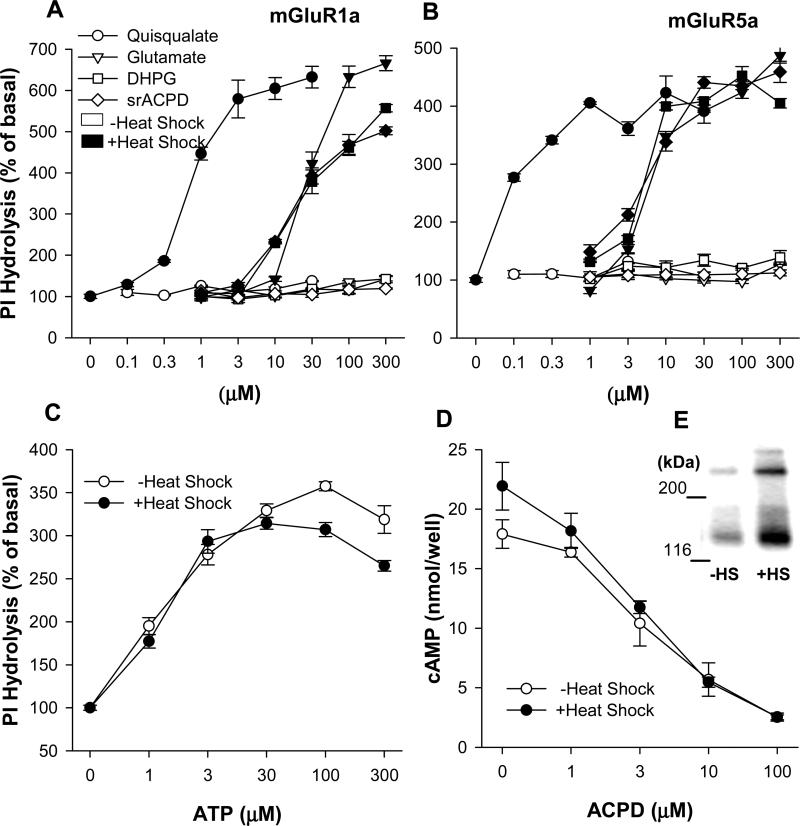
Fig. 5.
Heat shock increases PI hydrolysis in mGluR1a and mGlur5a transfected CHO-K1 cells. Permanently transfected cells cultured on 96 well plates were heated for 2 hrs at 42.5°C. Measurement of PI hydrolysis was performed on the next day. (A) mGluR1a-transfected cells were treated with mGluR agonists ACPD, DHPG, glutamate and quisqualate together with 20 mM LiCl in Locke's buffer. Open and black symbols represent IP accumulation in untreated cells and in heated cells respectively. (B) PI hydrolysis in mGluR5a-transfected cells. (C) Heat shock treatment did not induce enhancement of PI hydrolysis in untransfected CHO-K1 cells stimulated by ATP, an agonist of endogenous purinoceptors. (D and E) Heat shock increases mGluR2 expression but does not potentiate physiological response of the receptor in permanently transfected CHO-K1 cells. Adenylyl cyclase activity was measured next day after heat shock treatment. cAMP accumulation was induced by 10 μM forskolin in the presence of 300 μM IBMX. Activation of mGluR2 receptors by mGluR agonist ACPD inhibited cAMP accumulation and was measured by radioimmunoassay using a magnetic Amerlex RIA kit. The receptors expression was measured by Western blotting in control unheated (-HS) and heated (+HS) cells 24 hrs after heat shock treatment. The receptor appears on the blot as combination of two bands corresponding to monomeric and dimeric forms.
Fig. 4.
Heat shock induces mGluR1a and mGluR5a expression in stably transfected CHO-K1 cells. Cells were heated for 2 hrs at 42.5°C. (A) Cell membranes were collected at the indicated times after the treatment and mGluR1a expression was determined by Western blotting with specific anti-mGluR1a antibodies. The receptors appear on the blot as a combination of two bands corresponding to monomeric (145 kDa) and dimeric (290 kDa) forms of the mGluR1a. One μg of membrane protein was loaded into each well. (B) mGluR5a expression was measured similarly with anti-mGluR5a antibodies. (C) mGluR1a mRNA expression in transfected cells was measured by quantitative real-time PCR. The first bar (0 hrs) represents mRNA expression in control unheated cells. mGluR1a mRNA levels were measured in triplicates and normalized to the levels of mRNA for housekeeping gene (GAPDH). (D) Heat shock increases mGluR1a expression in CHO-K1 cells nearly 100-fold. Membrane proteins were isolated 24 hrs after heating and resolved by Western blotting. In order to estimate the magnitude of stimulation of the receptors expression by heat shock the dilution curve of the samples was done. Indicated amounts of membrane protein were loaded into the gel. Lane 1, membranes from control unheated CHO-K1 cells; lanes 2-5, membranes from heat shock-treated cells.
3.3. Effect of heat shock on the properties of group I mGluRs
In untreated CHO cells expressing mGluR1a and mGluR5a receptors, agonists (glutamate, quisqualate, ACPD and DHPG) stimulated PI hydrolysis only up to about 1.5-fold above the basal level, however, at 24 h after HS treatment, agonist-induced PI hydrolysis was elevated up to about 5-6 fold above the basal level (Fig. 5A,B). The absolute levels of maximal PI hydrolysis determined in cells expressing mGluR1a and mGluR5a were similar (1263±34 and 1206±32 cpm/well respectively), but the stimulation, as compared to the basal level, was lower in mGluR5a-expressing cells due to an elevated basal PI hydrolysis (248±9 cpm/well vs. 190±10 cpm/well in mGluR1a-expressing cells). A high basal PI hydrolysis may result from higher culture density seen in mGluR5a expressing cells, as compared to mGluR1a expressing cells. HS treatment failed to affect PI hydrolysis stimulated by ATP, the agonist of PLC-coupled purinoceptors present in untransfected CHO cells (Iredale and Hill, 1993) (Fig. 5C). The above results indicate that the effect of HS is specific to transfected receptors and does not involve a direct enhancement of PLC activity. We also observed that increases in the level of receptors expression may not, necessarily, lead to enhanced receptor response. In CHO cells expressing mGluR2 receptors, which are negatively coupled to adenylyl cyclase, HS treatment produced no significant enhancement in receptor activity, as measured by its ability to inhibit cAMP formation (Fig. 5D), even though the levels of receptor protein were increased (Fig. 5E).
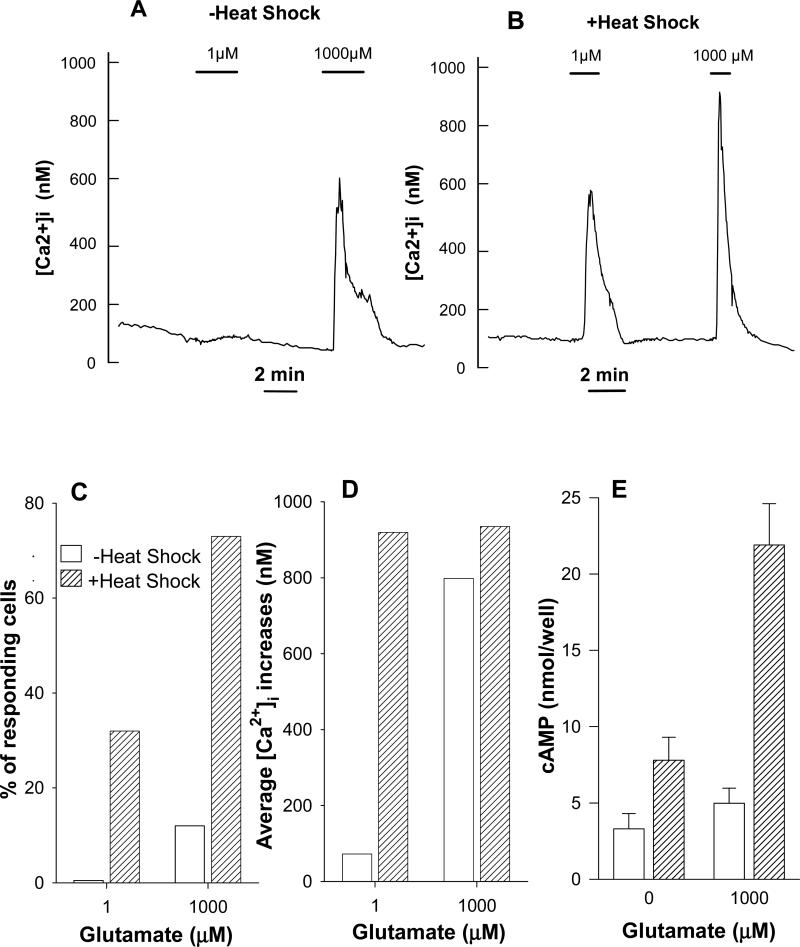
Measurements of intracellular Ca2+ concentrations in mGluR1a-transfected CHO cells showed that HS treatment increased the sensitivity to the agonist, as low concentrations of glutamate (1 μM) produced a robust response in heated cells (Fig. 6B) but not in control cells (Fig. 6A). HS treatment also increased the number of responding cells. While in control cells 1 M glutamate increased [Ca2+]i in very few, if any, cells and 1 mM glutamate induced responses in about 10% of cells, after HS treatment 30% of cells responded to 1 μM glutamate and 73% of cells were stimulated by 1 mM glutamate (Fig. 6C). The average amplitude of [Ca2+]i signals in responding cells was similar in treated and untreated cells, when saturating concentrations of glutamate (1 mM) were used. At low glutamate concentrations (1 μM) HS treatment caused an 8-fold increase as compared to untreated cells (Fig. 6D). The observed effects of HS treatment on [Ca2+]i release signals reflect the significant increase in the level of receptor expression revealed by PI hydrolysis and Western blot analysis.
Fig. 6.
In mGluR1a transfected CHO cells heat shock increases both agonist potency and proportion of cells responding to agonist. (A) Average Ca2+ signals of responding cells stimulated by glutamate under control conditions (10 responding cells of 82 in the field of observation). (B) Cells were heated at 42.5°C for 2 hrs 1 day before measurements were performed (27 responding cells of 51 in the field of observation). Data of two representative experiments are shown; similar results were obtained in 3-5 other experiments. (C) Summarized data indicating proportion of cells responding to different glutamate concentrations under control conditions (17 out of 222 cells in 3 independent experiments) and after heating (141 out of 243 cells in 5 independent experiments). (D) Average [Ca2+]i signals of the same (C) responding cells. (E) Heat shock enhances cAMP formation in mGluR1a expressing CHO-K1 cells. Cells were heated for 2 hours at 42.5°C. After additional 24 hours cells were used for determination of cAMP accumulation stimulated by agonist in presence of phosphodiesterase inhibitor IBMX.
The increase of mGluR1a protein levels by HS was followed also by elevated cAMP levels mediated through the activation of adenylyl cyclase (Fig. 6E). While in untreated CHO cells expressing mGluR1a saturating concentrations of glutamate induced a small increase of cAMP formation (20% above basal), HS treatment increased this response to 3-fold of the basal cAMP levels. Hence, HS treatment enhanced the functional responses of mGluR1a receptors mediated through different coupling mechanisms: PLC activation and stimulation of adenylyl cyclase.
3.4. Effect of heat shock on toxic properties of mGluR1a
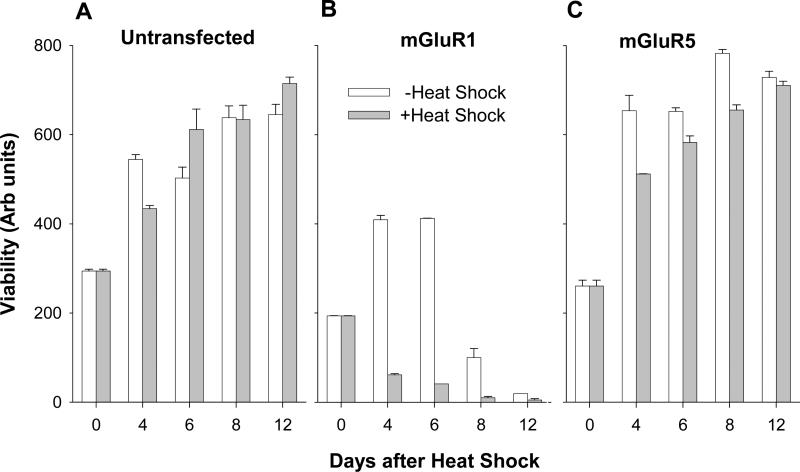
The mGluR1 receptor has been reported to induce toxicity when transiently expressed in heterologous systems (Dale et al., 2000). In order to study the functional consequences of the elevated receptor expression we assessed cell viability after HS treatment. Cells, plated at the same densities in 96-well tissue culture plates, were subjected to HS treatment after reaching near-confluency at 4 DIV. In untransfected CHO cells (Fig. 7A), as well as in cells expressing mGluR5a (Fig. 7C), HS treatment caused a transient small decrease in viability at 4 days after treatment. This decrease was most probably due to a slowing of the cell growth rate, rather than toxicity, as the cells completely recovered within 12 days of the treatment. In contrast, CHO cells expressing mGluR1a showed decreased viability even in control conditions, however, HS treatment greatly accelerated the rate of cell death (Fig. 7B), with most cells dying within 4 days after treatment. Hence, the activation of the CMV promoter by HS not only increases mGluR1a expression and mGluR1a-mediated signaling but also reveals the toxic properties this receptor.
Fig. 7.
Heat-shock enhances toxic properties of mGluR1a in transfected CHO cell lines. Untransfected and transfected with mGluR1a or mGluR5a cells were seeded at the same density in 96 well plates. Cell viability in control and heated cultures was measured by MTT assay. The first measurement (at DIV 4) was done immediately after heat shock treatment.
4. Discussion
4.1. Effect of heat shock on mGluR expression
We have determined that the expression of transfected proteins, under control of the CMV-IE promoter/enhancer can be greatly enhanced by HS. This was true for the expression of EGFP, as well as for three metabotropic glutamate receptors. The effect was not dependent on the type of cells used as HS was effective in CHO-K1, HEK-293 and in PC12 cells. The effectiveness of HS depended on the time of application and at 42.5°C the highest protein expression was between 1.5 and 3 hours of HS without significantly impacting the viability of the cells. The application of HS to CHO-K1 cells permanently transfected with mGluR1a, mGluR5 and mGluR2 caused a robust increase in the expression level of these receptors. The increase in the mGluR1a mRNA which followed the HS treatment indicates that the effect of HS occurred at the transcriptional level, increasing receptor synthesis. It should be noted that, as discussed below, heat shock sensitivity was the property of the CMV promoter and not of the receptor themselves, therefore HS is effective only with transfected receptors and does not affect native receptors present in neuronal cells. However, the degree to which enhanced receptor expression was reflected in the stimulation of their functional properties depended on the particular mechanisms of signal transduction. In the case of group I mGluRs, mGluR1 and mGluR5, HS caused a robust increase in receptor-mediated PI hydrolysis and downstream effects on intracellular calcium release. The smaller effect of HS treatment on PI hydrolysis as compared with a 100-fold increase of mGluR1a protein expression indicates that the increase of receptor activity may be limited by the availability of G proteins or other elements of the signal transduction cascade. This was especially visible with transfected mGluR2 receptors, where HS increased protein expression without enhancing its ability to inhibit cAMP formation. Hence, if the expression of certain receptors in transfected cells is high enough to fully saturate the critical components of the downstream signaling cascade, the effect of HS treatment may be visible only at the level of protein expression but not at the level of the functional response. Alternatively, the apparent agonist potency may be increased (lower EC50), due to high receptor reserve.
HS treatment also revealed the toxic properties of mGluR1, while the increase of mGluR5 expression failed to cause toxicity. The toxicity caused by the increased expression of mGluR1a has been studied in detail previously (Pshenichkin et al. 2008). Those studies showed that mGluR1a toxicity was not induced by receptor agonists nor was it inhibited by its antagonists, instead it was controlled by the levels of receptor expression and prevented by glutamate, therefore exhibiting the properties of a dependence receptor. Here, we used this receptor as an example of a potentially toxic protein which can be expressed at a low, nontoxic level to maintain the viability of transfected cells. When expressed under the control of the CMV promoter HS can be used to enhance receptor expression and fully reveal its toxic properties.
4.2. Effect of HS on the CMV-IE promoter
Our results from EMSA demonstrate that HS significantly increased the formation of nuclear protein-CMV promoter complexes. Although a direct effect of HS factors on the CMV-IE promoter cannot be excluded, as a considerable homology to the HS element core consensus (GA--TCC) within 18 bp elements in IE enhancer has been pointed out (Geelen et al., 1987), HS might regulate its activity indirectly, through induction of various transcription factors including those activating the CMV-IE promoter. For example, HS activates AP-1 in variety of cell types (Sikora et al., 1993; Harada et al., 2003), CREB in rat intestinal epithelial cells (Harada et al., 2003), C/EBP-beta in human intestinal epithelial cells (Hungness et al., 2002) in vitro, and AP-1 and CREB (Tacchini et al., 1999) in rat liver in vivo. The effect of HS on NF-kappaB is somewhat controversial and depends on cell type and conditions, since both activation (Sikora et al., 1993) and inhibition (Santoro, 2000) have been reported. One of the candidates, which may participate in HS-mediated activation of the CMV-IE promoter, may be BAG-1M, capable of binding Bcl-2. It has been shown that BAG-1M directly binds to the CMV promoter and its interaction with Hsp70/Hsc70 proteins is necessary for enhancing the CMV promoter activity (Takahashi et al., 2001).
Our results demonstrate the stimulatory effect of HS treatment on CMV-driven expression of foreign proteins in cells transfected with plasmids or viral vectors. In earlier studies, it has been suggested that HS may activate the regulatory sequence in the integrated latent human CMV-IE gene, leading to viral reactivation during cellular distress (Geelen et al., 1987). HS was also shown to induce the CMV-IE promoter-driven expression of viral IE gene and chloramphenicol acetyltransferase reporter-gene expression in stably transfected cell lines (Boom et al., 1988). These data were interpreted in the context of the reactivation of the human CMV virus, but not as inherent properties of the widely used CMV-IE promoter, and failed to promote the use of HS in various heterologous expression systems.
Our findings further expand this area of research in several ways. In agreement with previous data, we have found that adenovirus-mediated, CMV-driven, expression of delivered proteins was enhanced after HS treatment also in CHO, PC-12 and HEK 293 cells, as monitored by fluorescence measurements. These experiments allowed us to conclude that HS treatment caused enhanced binding of nuclear proteins to the CMV-IE promoter, followed by activation of EGFP reporter gene expression. We have also shown the possibility of using of the CMV-IE promoter, in combination with HS, for practical applications in a variety of existing heterologous expression systems. We observed the stimulatory effects of HS on CMV-driven expression of mGluR1a protein in 3 cell lines: CHO-K1, HEK-293 and PC-12 cells. In addition, the effect of HS was observed not only in stably transfected cell lines but also in transiently transfected cells (data not shown). This approach may find application in a variety of systems utilizing the CMV-IE promoter-driven gene expression. However, some limitations may exist. It has been reported that hyperthermia failed to stimulate the CMV promoter in THP-1 cells, a monocytic leukemia cell line (Iwamoto et al., 1999), suggesting that the HS effect may be, to some extent, cell-specific. In addition, the adenoviral CMV-IE promoter has been shown in several human tumor cell lines to be sensitive to chemical DNA damaging agents but not to HS treatment (Zacal et al., 2005). This apparent discrepancy with our data probably results from a much shorter duration of the treatment (15-30 min), which was applied before viral infection, whereas we treated cells for at least 2 hrs and, most importantly, after transfection or infection.
The use of HS treatment to enhance protein expression may be especially beneficial in case of proteins endowed with toxic activity. It has been reported that transient expression of mGluR1a in heterologous systems might be toxic (Dale et al., 2000) and in some studies chemically inducible systems were used (Nash et al., 2001). Generally, in order to maintain stable cell lines expressing mGluR1a, the receptor expression must remain low and the cells must be frequently passaged. Compared to other inducible systems HS seems to be a preferred approach because it does not require any chemical treatment, allows precise control by varying temperature and the time of exposure, and can be used with any vector containing the CMV promoter. Therefore, HS not only increases expression of proteins of interest, but also allows achieving inducible expression of potentially toxic proteins.
4.3. Potential implications
Further studies are necessary to define the location of the CMV promoter region responsible for sensitivity to HS treatment. Our findings allow us to suggest that hyperthermic stress may activate the CMV-IE promoter and, in turn, facilitate the expression of delivered genes, at least in certain cell types. While the molecular mechanism of the HS effect on the CMV-IE promoter remains unclear, the importance of this effect is stressed by the widespread use of CMV promoter-based vectors in various expression systems. HS treatment may be especially useful in transient transfection experiments where the number of transfected cells is often too low to obtain reliable data on the functional properties of the transfected proteins. High, inducible, expression of desired proteins in permanent cell lines provides valuable screening tools for discovery of novel receptor ligands. Our observations also suggest the potential for the use of HS treatment in gene therapy to enhance local expression of target genes controlled by the CMV promoter. Because hyperthermia can be applied locally to a restricted mass of tissue, even with systemic administration, the CMV-driven genes will be highly expressed within the treated area, while remaining at much lower level of expression in cells located outside of the heated area. This may be particularly important for expression of highly toxic proteins.
Acknowledgements
This work was supported by a grant from the National Institutes of Health (NS37436).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abe T, Sugihara H, Nawa H, Shigemoto R, Mizuno N, Nakanishi S. Molecular Characterization of a Novel Metabotropic Glutamate Receptor MGluR5 Coupled to Inositol Phosphate/Ca2+ Signal Transduction. J Biol Chem. 1992;267:13361–8. [PubMed] [Google Scholar]
- Angulo A, Suto C, Heyman RA, Ghazal P. Characterization of the Sequences of the Human Cytomegalovirus Enhancer That Mediate Differential Regulation by Natural and Synthetic Retinoids. Mol Endocrinol. 1996;10:781–93. doi: 10.1210/mend.10.7.8813719. [DOI] [PubMed] [Google Scholar]
- Aramori I, Nakanishi S. Signal Transduction and Pharmacological Characteristics of a Metabotropic Glutamate Receptor, MGluR1, in Transfected CHO Cells. Neuron. 1992;8:757–65. doi: 10.1016/0896-6273(92)90096-v. [DOI] [PubMed] [Google Scholar]
- Boom R, Sol CJ, Minnaar RP, Geelen JL, Raap AK, van der Noordaa J. Induction of Gene Expression Under Human Cytomegalovirus Immediate Early Enhancer-Promoter Control by Inhibition of Protein Synthesis Is Cell Cycle-Dependent. J Gen Virol. 1988;69(Pt 6):1179–93. doi: 10.1099/0022-1317-69-6-1179. [DOI] [PubMed] [Google Scholar]
- Chan YJ, Chiou CJ, Huang Q, Hayward GS. Synergistic Interactions Between Overlapping Binding Sites for the Serum Response Factor and ELK-1 Proteins Mediate Both Basal Enhancement and Phorbol Ester Responsiveness of Primate Cytomegalovirus Major Immediate-Early Promoters in Monocyte and T-Lymphocyte Cell Types. J Virol. 1996;70:8590–605. doi: 10.1128/jvi.70.12.8590-8605.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn PJ, Pin JP. Pharmacology and Functions of Metabotropic Glutamate Receptors. Annu Rev Pharmacol Toxicol. 1997;37:205–37. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- Dale LB, Bhattacharya M, Anborgh PH, Murdoch B, Bhatia M, Nakanishi S, Ferguson SS. G Protein-Coupled Receptor Kinase-Mediated Desensitization of Metabotropic Glutamate Receptor 1A Protects Against Cell Death. J Biol Chem. 2000;275:38213–20. doi: 10.1074/jbc.M006075200. [DOI] [PubMed] [Google Scholar]
- Geelen JL, Boom R, Klaver GP, Minnaar RP, Feltkamp MC, van Milligen FJ, Sol CJ, van der Noordaa J. Transcriptional Activation of the Major Immediate Early Transcription Unit of Human Cytomegalovirus by Heat-Shock, Arsenite and Protein Synthesis Inhibitors. J Gen Virol. 1987;68(Pt 11):2925–31. doi: 10.1099/0022-1317-68-11-2925. [DOI] [PubMed] [Google Scholar]
- Harada T, Koyama I, Kasahara T, Alpers DH, Komoda T. Heat Shock Induces Intestinal-Type Alkaline Phosphatase in Rat IEC-18 Cells. Am J Physiol Gastrointest Liver Physiol. 2003;284:G255–62. doi: 10.1152/ajpgi.00244.2002. [DOI] [PubMed] [Google Scholar]
- Hungness ES, Robb BW, Luo GJ, Hershko DD, Hasselgren PO. Hyperthermia-Induced Heat Shock Activates the Transcription Factor C/EBP-Beta and Augments IL-6 Production in Human Intestinal Epithelial Cells. J Am Coll Surg. 2002;195:619–26. doi: 10.1016/s1072-7515(02)01342-x. [DOI] [PubMed] [Google Scholar]
- Iredale PA, Hill SJ. Increases in Intracellular Calcium Via Activation of an Endogenous P2-Purinoceptor in Cultured CHO-K1 Cells. Br J Pharmacol. 1993;110:1305–10. doi: 10.1111/j.1476-5381.1993.tb13960.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto GK, Ainsworth AM, Moseley PL. Hyperthermia Enhances Cytomegalovirus Regulation of HIV-1 and TNF-Alpha Gene Expression. Am J Physiol. 1999;277:L1051–6. doi: 10.1152/ajplung.1999.277.5.L1051. [DOI] [PubMed] [Google Scholar]
- Kline JN, Hunninghake GM, He B, Monick MM, Hunninghake GW. Synergistic Activation of the Human Cytomegalovirus Major Immediate Early Promoter by Prostaglandin E2 and Cytokines. Exp Lung Res. 1998;24:3–14. doi: 10.3109/01902149809046050. [DOI] [PubMed] [Google Scholar]
- Lee Y, Sohn WJ, Kim DS, Kwon HJ. NF-KappaB- and C-Jun-Dependent Regulation of Human Cytomegalovirus Immediate-Early Gene Enhancer/Promoter in Response to Lipopolysaccharide and Bacterial CpG-Oligodeoxynucleotides in Macrophage Cell Line RAW 264.7. Eur J Biochem. 2004;271:1094–105. doi: 10.1111/j.1432-1033.2004.04011.x. [DOI] [PubMed] [Google Scholar]
- Lee YJ, Lee H, Borrelli MJ. Gene Transfer into Human Prostate Adenocarcinoma Cells With an Adenoviral Vector: Hyperthermia Enhances a Double Suicide Gene Expression, Cytotoxicity and Radiotoxicity. Cancer Gene Ther. 2002;9:267–74. doi: 10.1038/sj.cgt.7700433. [DOI] [PubMed] [Google Scholar]
- Luu P, Flores O. Binding of SP1 to the Immediate-Early Protein-Responsive Element of the Human Cytomegalovirus DNA Polymerase Promoter. J Virol. 1997;71:6683–91. doi: 10.1128/jvi.71.9.6683-6691.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maass A, Langer SJ, Oberdorf-Maass S, Bauer S, Neyses L, Leinwand LA. Rational Promoter Selection for Gene Transfer into Cardiac Cells. J Mol Cell Cardiol. 2003;35:823–31. doi: 10.1016/s0022-2828(03)00140-8. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Nash MS, Selkirk JV, Gaymer CE, Challiss RA, Nahorski SR. Enhanced Inducible MGlu1alpha Receptor Expression in Chinese Hamster Ovary Cells. J Neurochem. 2001;77:1664–7. doi: 10.1046/j.1471-4159.2001.00405.x. [DOI] [PubMed] [Google Scholar]
- Nicoletti F, Bruno V, Copani A, Casabona G, Knopfel T. Metabotropic Glutamate Receptors: a New Target for the Therapy of Neurodegenerative Disorders? Trends Neurosci. 1996;19:267–71. doi: 10.1016/S0166-2236(96)20019-0. [DOI] [PubMed] [Google Scholar]
- Niller HH, Hennighausen L. Formation of Several Specific Nucleoprotein Complexes on the Human Cytomegalovirus Immediate Early Enhancer. Nucleic Acids Res. 1991;19:3715–21. doi: 10.1093/nar/19.13.3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosch S, Staak K, Stein J, Liebenthal C, Stamminger T, Volk HD, Kruger DH. Stimulation of the Human Cytomegalovirus IE Enhancer/Promoter in HL-60 Cells by TNFalpha Is Mediated Via Induction of NF-KappaB. Virology. 1995;208:197–206. doi: 10.1006/viro.1995.1143. [DOI] [PubMed] [Google Scholar]
- Pshenichkin S, Dolinska M, Klauzinska M, Luchenko V, Grajkowska E, Wroblewski JT. Dual neurotoxic and neuroprotective role of metabotropic glutamate receptor 1 in conditions of trophic deprivation. Neuropharmacology. 2008;55:500–8. doi: 10.1016/j.neuropharm.2008.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichman TW, Muniz LC, Mathews MB. The RNA Binding Protein Nuclear Factor 90 Functions As Both a Positive and Negative Regulator of Gene Expression in Mammalian Cells. Mol Cell Biol. 2002;22:343–56. doi: 10.1128/MCB.22.1.343-356.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rideg K, Hirka G, Prakash K, Bushar LM, Nothias JY, Weinmann R, Andrews PW, Gonczol E. DNA-Binding Proteins That Interact With the 19-Base Pair (CRE-Like) Element From the HCMV Major Immediate Early Promoter in Differentiating Human Embryonal Carcinoma Cells. Differentiation. 1994;56:119–29. doi: 10.1046/j.1432-0436.1994.56120119.x. [DOI] [PubMed] [Google Scholar]
- Santoro MG. Heat Shock Factors and the Control of the Stress Response. Biochem Pharmacol. 2000;59:55–63. doi: 10.1016/s0006-2952(99)00299-3. [DOI] [PubMed] [Google Scholar]
- Shchepotin IB, Soldatenkov V, Wroblewski JT, Surin A, Shabahang M, Buras RR, Nauta RJ, Pulyaeva H, Evans SRT. Apoptosis induced by hyperthermia and verapamil in vitro in a human colon cancer line. Int. J. Hyperthermia. 1997;13:547–557. doi: 10.3109/02656739709023553. [DOI] [PubMed] [Google Scholar]
- Sikora E, Grassilli E, Radziszewska E, Bellesia E, Barbieri D, Franceschi C. Transcription Factors DNA-Binding Activity in Rat Thymocytes Undergoing Apoptosis After Heat-Shock or Dexamethasone Treatment. Biochem Biophys Res Commun. 1993;197:709–15. doi: 10.1006/bbrc.1993.2537. [DOI] [PubMed] [Google Scholar]
- Stamminger T, Fickenscher H, Fleckenstein B. Cell Type-Specific Induction of the Major Immediate Early Enhancer of Human Cytomegalovirus by Cyclic AMP. J Gen Virol. 1990;71(Pt 1):105–13. doi: 10.1099/0022-1317-71-1-105. [DOI] [PubMed] [Google Scholar]
- Sun B, Harrowe G, Reinhard C, Yoshihara C, Chu K, Zhuo S. Modulation of Human Cytomegalovirus Immediate-Early Gene Enhancer by Mitogen-Activated Protein Kinase Kinase Kinase-1. J Cell Biochem. 2001;83:563–73. doi: 10.1002/jcb.1251. [DOI] [PubMed] [Google Scholar]
- Surin A, Pshenichkin S, Grajkowska E, Surina E, Wroblewski JT. Cyclothiazide selectively inhibits mGluR1 receptors interacting with a common allosteric site for non-competitive antagonists. Neuropharmacology. 2007;52:744–54. doi: 10.1016/j.neuropharm.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tacchini L, Radice L, Bernelli-Zazzera A. Differential Activation of Some Transcription Factors During Rat Liver Ischemia, Reperfusion, and Heat Shock. J Cell Physiol. 1999;180:255–62. doi: 10.1002/(SICI)1097-4652(199908)180:2<255::AID-JCP13>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Takahashi N, Sasaki R, Takahashi J, Takayama S, Reed JC, Andoh T. BAG-1M, an Isoform of Bcl-2-Interacting Protein BAG-1, Enhances Gene Expression Driven by CMV Promoter. Biochem Biophys Res Commun. 2001;286:807–14. doi: 10.1006/bbrc.2001.5473. [DOI] [PubMed] [Google Scholar]
- Wroblewska B, Wroblewski JT, Pshenichkin S, Surin A, Sullivan SE, Neale JH. N-Acetylaspartylglutamate Selectively Activates MGluR3 Receptors in Transfected Cells. J Neurochem. 1997;69:174–81. doi: 10.1046/j.1471-4159.1997.69010174.x. [DOI] [PubMed] [Google Scholar]
- Zacal NJ, Francis MA, Rainbow AJ. Enhanced Expression From the Human Cytomegalovirus Immediate-Early Promoter in a Non-Replicating Adenovirus Encoded Reporter Gene Following Cellular Exposure to Chemical DNA Damaging Agents. Biochem Biophys Res Commun. 2005;332:441–9. doi: 10.1016/j.bbrc.2005.04.148. [DOI] [PubMed] [Google Scholar]
- Zhang B, Liu Y, Harbrecht BG. Cyclic AMP Regulate Adenovirus Mediated Inducible Nitric Oxide Synthase Expression and CMV Promoter Activity in Cultured Hepatocytes. Nitric Oxide. 2003;8:262–5. doi: 10.1016/s1089-8603(03)00041-7. [DOI] [PubMed] [Google Scholar]