Background: Inhibitors of the enzyme that inactivates the peptide transmitter N-acetylaspartylglutamate reduce behaviors induced by PCP in animal models of schizophrenia.
Results: NAAG peptidase inhibition reduces PCP-induced glutamate release in two brain areas implicated in this disorder.
Conclusion: Peptidase-mediated inhibition of glutamate release is consistent with the glutamate model of this disorder.
Significance: NAAG peptidase inhibitors warrant further biochemical characterization as potential antipsychotic drugs.
Keywords: Dopamine, Glutamate, Glutamate Receptors Metabotropic, Neurotransmitter Release, Schizophrenia, N-Acetylaspartylglutamate (NAAG), PCP, ZJ43, Nucleus Accumbens, Prefrontal Cortex, mGluR3
Abstract
The “glutamate” theory of schizophrenia emerged from the observation that phencyclidine (PCP), an open channel antagonist of the NMDA subtype of glutamate receptor, induces schizophrenia-like behaviors in humans. PCP also induces a complex set of behaviors in animal models of this disorder. PCP also increases glutamate and dopamine release in the medial prefrontal cortex and nucleus accumbens, brain regions associated with expression of psychosis. Increased motor activation is among the PCP-induced behaviors that have been widely validated as models for the characterization of new antipsychotic drugs. The peptide transmitter N-acetylaspartylglutamate (NAAG) activates a group II metabotropic receptor, mGluR3. Polymorphisms in this receptor have been associated with schizophrenia. Inhibitors of glutamate carboxypeptidase II, an enzyme that inactivates NAAG following synaptic release, reduce several behaviors induced by PCP in animal models. This research tested the hypothesis that two structurally distinct NAAG peptidase inhibitors, ZJ43 and 2-(phosphonomethyl)pentane-1,5-dioic acid, would elevate levels of synaptically released NAAG and reduce PCP-induced increases in glutamate and dopamine levels in the medial prefrontal cortex and nucleus accumbens. NAAG-like immunoreactivity was found in neurons and presumptive synaptic endings in both regions. These peptidase inhibitors reduced the motor activation effects of PCP while elevating extracellular NAAG levels. They also blocked PCP-induced increases in glutamate but not dopamine or its metabolites. The mGluR2/3 antagonist LY341495 blocked these behavioral and neurochemical effects of the peptidase inhibitors. The data reported here provide a foundation for assessment of the neurochemical mechanism through which NAAG achieves its antipsychotic-like behavioral effects and support the conclusion NAAG peptidase inhibitors warrant further study as a novel antipsychotic therapy aimed at mGluR3.
Introduction
The glutamate theory of schizophrenia emerged from the discovery that open channel NMDA receptor antagonists and dissociative anesthetics, such as phencyclidine (PCP),3 induced schizophrenia-like behaviors in humans. Subsequent studies demonstrated that PCP increases extracellular levels of glutamate and dopamine in the medial prefrontal cortex (mPFC) and nucleus accumbens (NAc) (1), presumably as a result of decreased activation of NMDA receptors on GABAergic interneurons. Excess glutamate activation of AMPA receptors appears to be involved in the effects of PCP because an AMPA antagonist blocks the behavioral effects of PCP in animal models (2–5). As a result, the glutamate theory of schizophrenia supports the development of drugs that reduce PCP-induced glutamate release as novel antipsychotic therapies.
The activation of group II metabotropic receptors (mGluR2 and mGluR3) by heterotropic agonists has long been associated with presynaptic inhibition of transmitter release, including glutamate release (6). Among these receptors, mGluR3 is distributed on peri-, pre-, and postsynaptic neuronal membranes and astrocytes (7). The peptide neurotransmitter N-acetylaspartylglutamate (NAAG) is an mGluR3-selective agonist that negatively regulates levels of cAMP and cGMP (8–12). Acting on presynaptic mGluR3 receptors, NAAG inhibits the release of transmitters, including glutamate and GABA (13–17). Synaptically released NAAG is inactivated by an extracellular glial enzyme, glutamate carboxypeptidase II (18, 19). Inhibitors of this enzyme (20–22) increase the extracellular concentration of synaptically released NAAG in vivo (13, 16).4 We found that potent glutamate carboxypeptidase II inhibitors also reduced the behavioral effects of PCP, dizocilpine and d-amphetamine in validated animal models of positive, negative, and cognitive schizophrenia-like behaviors (24–26).5 In contrast to heterotropic group II agonists, NAAG is a selective mGluR3 agonist (12), a fact supported by our finding that the efficacy of NAAG peptidase inhibitors in blocking PCP-induced behaviors is observed in mGluR2 knock-out mice but not in mGluR3 knockouts (26).
Heterotropic mGluR2/3 receptor agonists also reduced the effects of PCP in animal models of schizophrenia and reduced the PCP-induced release of glutamate in the mPFC and NAc (1, 28–30). The efficacy of heterotropic agonists in this PCP model was observed in mGluR3 but not mGluR2 knock-out mice, consistent with their activation of mGluR2 in vitro (31–34). Although mGluR2/3 agonists and mGluR2-positive allosteric modulators represent a potentially efficacious mGluR2-targeted antipsychotic therapy (35, 36), data from animal models of schizophrenia suggest that NAAG peptidase inhibition represents a related but distinctly different pharmacotherapy based on activation of mGluR3 (12, 26). The present study was initiated to test the hypothesis that, consistent with the glutamate theory of this disorder, NAAG peptidase inhibitors also block the PCP-induced increase in glutamate release in the mPFC and NAc, brain regions associated with the behavioral and neurochemical effects of PCP and schizophrenia (1, 37–40).
EXPERIMENTAL PROCEDURES
Animals
The experimental protocols used in this research were approved by the Georgetown University Animal Care and Use Committee consistent with guidelines of the National Institutes of Health. Male Sprague-Dawley rats (Taconic, Germantown, MD) weighing 300–350 g were used in this study. They were housed in groups of two and maintained with a 12-h/12-h light/dark cycle (dark 6 p.m. to 6 a.m.). Food and water were available ad libitum. Experiments were performed between 9 a.m. and 4 p.m.
Drugs
ZJ43 (N-[[[(1S)-1-carboxy-3-methylbutyl]amino]carbonyl]-l-glutamic acid) was provided by Alan Kozikowski (41), and 2-(phosphonomethyl)pentane-1,5-dioic acid (2-PMPA) (22) and the mGluR2/3 antagonist LY341495 (42) were from Tocris Cookson Ltd. (Bristol, UK). PCP was from Sigma-Aldrich. All compounds were dissolved in sterile saline solution and administered by intraperitoneal injection.
Microdialysis
On the day of surgery, rats were anesthetized with ketamine (80 mg/kg)/xylazine (5 mg/kg) (intraperitoneally (i.p.)) and placed in a stereotaxic apparatus (KOPF Instruments, Tujunga, CA) for surgical implantation of a guide cannula. The guide cannula (SciPro Inc., Sanborn, NY) was positioned to both the mPFC according to the coordinates (AP, +3.2; ML, +0.8; DV, 2.6) and the NAc according to the coordinates (AP, +1.7; ML, −1.0; DV, 6.0) in the same rats (two simultaneous dialysis probes per rat). The guide cannula was secured to the skull with dental cement anchored by two stainless steel screws. After surgery, each rat was individually housed and allowed to recover for at least 24 h before the microdialysis experiment. Microdialysis experiments were carried out on conscious, freely moving rats. On the day of the experiment, rats were lightly anesthetized to facilitate manual insertion of the microdialysis probe into the guide cannula. The stylet in the guide cannula was replaced with the microdialysis probe (outer diameter, 0.6 mm; exposed tip, 3.0 mm for mPFC and 2.0 mm for NAc; cut-off of 6 kDa; SciPro Inc.). Rats were tethered to the awake animal system by means of a plastic collar (CMA Microdialysis AB). The probe was perfused at 2 μl/min with artificial cerebrospinal fluid (147 mm NaCl, 1.2 mm CaCl2, 2.7 mm KCl, 0.85 mm MgCl2; CMA Microdialysis AB). All fluid connections were made using FEP tubing (internal volume of 1.2 μl/10 cm; SciPro Inc.). After at least a 2-h equilibration period, dialysate samples were collected every 30 min. Three base-line fractions were collected before ZJ43, 2-PMPA, or saline injection. Pretreatment of ZJ43 (150 mg/kg, i.p.), 2-PMPA (50 mg/kg, i.p.), ZJ43 (150 mg/kg, i.p.) + LY341495 (1 mg/kg, i.p.), 2-PMPA (50 mg/kg, i.p.) + LY341495 (1 mg/kg, i.p.), or saline occurred 20 min before PCP (5 mg/kg, i.p.) or saline injection. Samples were subsequently analyzed for either DA or its metabolites (DOPAC and HVA) or glutamate by high performance liquid chromatography (HPLC) and NAAG by radioimmunoassay.
DA Analysis
Dialysate samples (20 μl) were directly applied to the HPLC column and monitored with electrochemical detection (HPLC-ECD, Waters). A reversed-phase column (Atlantis T3, ODS, 4.6 × 150 mm, 3 μm; Waters) was used, and the mobile phase for detection of DA and its metabolites consisted of 0.1 m citric acid-sodium acetate, 0.05 mm EDTA, and 65 mg/ml l-octanesulfonic acid with 15% methanol (v/v) in water adjusted to pH 3.8 with 1 m citric acid. The flow rate was 1.0 ml/min. The column temperature was kept at 30 °C, and the applied potential was +750 mV. Reagents used were analytical or HPLC grade.
Glutamate Analysis
Glutamate was derivatized with o-phthalaldehyde (Sigma), as described by Rowley et al. (43). o-Phthalaldehyde (2.2 mg) was dissolved in 0.05 ml of absolute ethanol, and then the solution was mixed with 0.9 ml of sodium tetraborate buffer (0.1 m). After that, the 0.05 ml of sodium sulfite (1 m) was added into the solution. Fifteen μl of microdialysate sample was mixed with 3 μl of o-phthalaldehyde solution for 10 min at room temperature before detection of glutamate. After derivatization, glutamate samples (15 μl) were detected by HPLC-ECD. A reversed-phase column (Atlantis T3, ODS, 4.6 × 150 mm, 3 μm; Waters) was used, and the mobile phase for detection of glutamate was composed of 0.1 m citric acid-sodium acetate and 0.05 mm EDTA with 19% methanol (v/v) in water adjusted to pH 3.8 with 1 m citric acid. The flow rate was 1.0 ml/min. The column temperature was kept at 30 °C, and the applied potential was +850 mV.
NAAG Analysis
Dialysate samples were analyzed for NAAG content using radioimmunoassay, as described previously (44), with small modifications. Briefly, 20-μl samples were diluted to 50 μl with PBS and incubated overnight at 4 °C with 25 μl of NAAG antisera (1:25) and 25 μl of [3H]NAAG (50,000 cpm, ∼5 pmol). After incubation, 900 μl of −20 °C methanol was added, and precipitated proteins were separated by sedimentation at 15,000 × g for 15 min. Levels of tritium in the pellets and supernatants were measured by liquid scintillation, and [3H]NAAG bound to antibody in unknown samples was compared with that of standards. A standard curve (0.033–3.3 μm NAAG) was performed with each assay. Highly specific NAAG antiserum was produced in rabbits by repeated immunizations with NAAG coupled to thyroglobulin and to colloidal gold particles. This antiserum was highly specific for NAAG versus N-acetylaspartate, glutamate, GABA, and aspartate (44).
Verification of Probe Placement
At the completion of each experiment, rats were killed by CO2 inhalation, and brains were removed and fixed in 10% formalin and dehydrated in 30% sucrose solution. After that, coronal sections of 30 μm were cut frozen, and the position of the probe was determined by Nissl's staining. The microdialysis data were analyzed only if the probe was found to be located inside the mPFC or NAc.
Behaviors
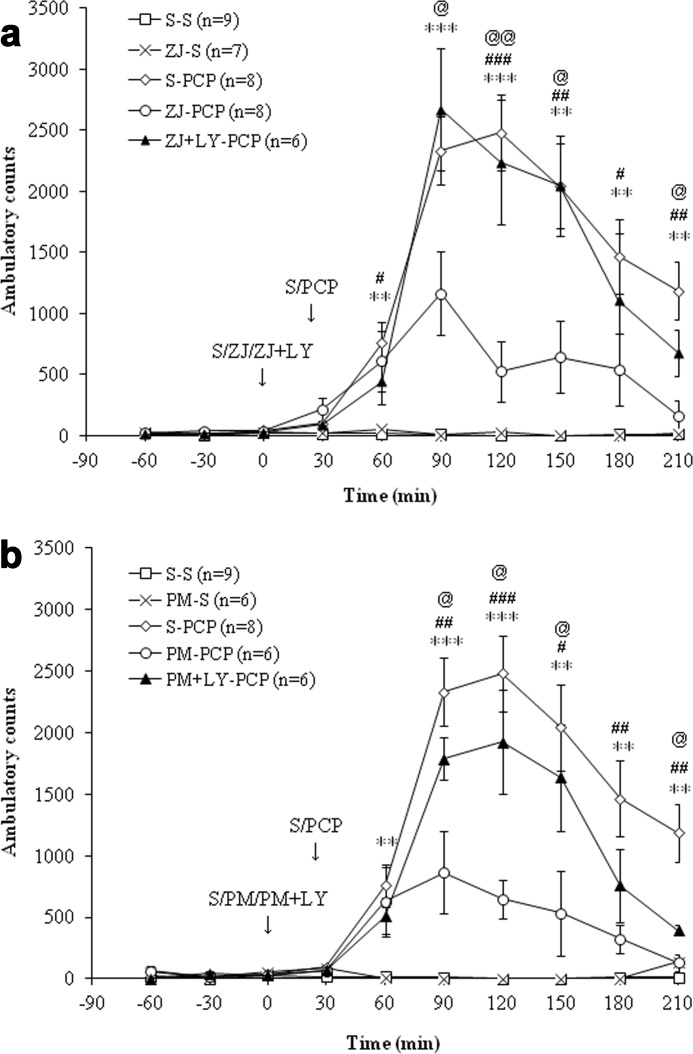
The rat was placed into a Med Associates (St. Albans, VT) ENV-515 open field chamber (43 × 43 cm, with three 16-beam infrared beams and detectors) to allow microdialysis sampling in the rat brain while simultaneously recording the locomotor activity of the rat. Motor activity was recorded as distance traveled and as ambulatory counts (the number of infrared beam breaks/test interval). Equivalent data were obtained from the two measures. Ambulatory counts are presented in Fig. 8, a and b.
FIGURE 8.
a, effects of saline (S) or ZJ43 (ZJ; 150 mg/kg, i.p.) with or without LY341495 (LY; 1 mg/kg, i.p.) on PCP (5 mg/kg, i.p.)-induced ambulatory counts in rats. The S-PCP treatment induced a significant increase in ambulatory counts (p < 0.001 versus S-S). ZJ-S had no effect on ambulatory counts compared with the S-S group (p = 0.97). However, ZJ43 pretreatment significantly inhibited PCP-induced hyperactivity (p < 0.001). LY341495 significantly antagonized the inhibitory effect of ZJ43 on PCP-induced hyperactivity (p < 0.01). **, p < 0.01; ***, p < 0.001, S-S versus S-PCP. #, p < 0.05; ##, p < 0.01; ###, p < 0.001, ZJ-PCP versus S-PCP. @, p < 0.05; @@, p < 0.01, ZJ-LY-PCP versus ZJ-PCP. b, effects of saline or 2-PMPA (PM; 50 mg/kg, i.p.) with or without LY341495 on PCP-induced ambulatory counts in rats. The S-PCP treatment induced a significant increase in ambulatory counts compared with the S-S treatment (p < 0.001), whereas PM-S had no effect on ambulatory counts (p = 0.88). 2-PMPA pretreatment significantly inhibited PCP-induced hyperactivity (p < 0.001). LY341495 pretreatment significantly reduced the inhibitory effect of PM on PCP-induced hyperactivity (p < 0.01). The S-S and S-PCP data are the same as found in a. **, p < 0.01; ***, p < 0.001, S-S versus S-PCP. #, p < 0.05; ##, p < 0.01; ###, p < 0.001, S-PCP versus PM-PCP. @, p < 0.05, PM-PCP versus PM-LY-PCP.
Data Analysis
Microdialysis data are expressed as a percentage of basal values (calculated as means of the three samples before injections). The basal concentrations in the dialysis were expressed in μm. All data are given as means ± S.E. and not corrected for “recovery” of the dialysis procedure. Rare samples whose values varied by more than 2 S.D. values from the mean were removed from consideration. The general linear model with repeated measurements (SPSS19 for Windows) was used to examine the time-dependent effect of drugs on each neurotransmitter in the two brain regions and behavior with treatment group as a fixed factor and time as the within-subject factor. In case of significant interactions, post hoc comparisons were performed by a Duncan test. The level of significant difference was set at p < 0.05. A one-way analysis of variance with post hoc comparisons was used for every time point analysis.
Antibodies and Immunohistochemistry
NAAG-specific antisera were prepared and immunohistochemistry performed as described previously (45, 46). Polyclonal NAAG antisera were purified in stages by both affinity chromatography and negative affinity adsorption against related protein-coupled molecules, including N-acetylaspartate, N-acetylglutamate, glutamate, aspartate, and GABA (46). Rats were transcardially perfused with 6% carbodiimide and 5% DMSO. Brains were post fixed with 4% paraformaldehyde, saturated with 30% sucrose, frozen and sectioned (20 μm). Sections were treated with 2% normal goat serum prior to overnight incubation with purified anti-NAAG rabbit serum (1:2,000). Antibodies were visualized with peroxidase-labeled avidin-biotin complex (Vectastain, Vector Laboratories) and developed with H2O2 and diaminobenzidine. Controls consisted of incubating the primary antibodies with 5 μg/ml protein-coupled NAAG before performing immunohistochemistry, which blocked all antibody binding to tissue sections. In contrast, incubating the antibodies with 5 μg/ml protein-coupled glutamate, N-acetylaspartate, N-acetylglutamate, aspartate, or GABA had no effect on NAAG immunoreactivity in tissue sections. Images were acquired using an Olympus DP71 camera and were adjusted for contrast and brightness using PC-based software (Adobe Systems). Additional software was used for extended depth of field by taking multiple images at different focal points in a tissue slice and combining them into a single focused image (Media Cybernetics).
RESULTS
NAAG Immunoreactivity in Rat mPCF and NAc
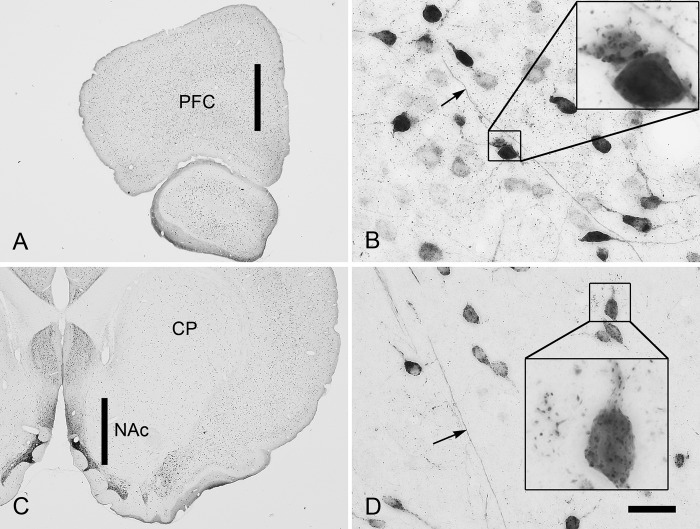
NAAG immunoreactivity was present exclusively in neurons, their processes, and in small punctate structures that appeared to be synapses (Fig. 1). That NAAG is present in synaptic terminals and vesicles has been demonstrated by ultrastructural analysis (47) and evoked release studies (48). NAAG-positive neurons were numerous in cortical structures, such as the mPFC (Fig. 1A). Neurons ranged from very lightly to intensely stained, and most immunoreactive neurons were generally small in size. Larger pyramidal neurons were only lightly stained or unstained. Some axons were stained, especially in the deeper layers of cortex. Synaptic-like structures stained for NAAG were present throughout the neuropil and were often concentrated on neuronal cell bodies and proximal dendrites (Fig. 1B). Fewer NAAG-immunoreactive neurons were observed in the caudate/putamen and NAc, but the pattern of staining was similar to that seen in the cortex (Fig. 1C). Scattered neurons ranged from lightly to strongly stained, and neuronal processes, including some axons (arrow in D) and dendrites, also were immunoreactive. NAAG-positive synaptic-like structures were observed in the neuropil and were particularly concentrated on some neuronal cell bodies and dendrites (Fig. 1D, inset).
FIGURE 1.
NAAG immunoreactivity in the medial prefrontal cortex and nucleus accumbens in the rat. The vertical black bar in A shows the approximate location of the dialysis probe in the medial prefrontal cortex. Numerous neurons were immunoreactive for NAAG in all layers of the prefrontal cortex (PFC) and ranged from lightly to intensely stained (B). Some axons were immunoreactive for NAAG (arrow in B), as were many putative synapses in the neuropil and on neuronal somata and dendrites (inset in B). The vertical black bar in C shows the approximate placement of the dialysis probe in the NAc. Scattered NAAG-immunoreactive neurons were observed throughout the accumbens (D). Some axons were immunoreactive for NAAG (arrow in D) as were some putative synapses in the neuropil and on the cell bodies and dendrites of neurons (inset in D). CP, caudate/putamen. Extended depth of field was used in B and D; scale bar, 850 μm (A and C), 32 μm (B and D), and 8 μm (insets).
Basal Extracellular DA, DOPAC, HVA, Glutamate, and NAAG Levels in mPFC and NAc
The base-line samples were collected after at least 2 h of perfusion. The basal extracellular levels of DA, DOPAC, HVA, Glutamate, and NAAG in the dialysates from mPFC and NAc are shown in Table 1. There were no significant differences in basal extracellular levels of these compounds among treatment groups. The basal level of extracellular NAAG in the NAc were ∼10% lower than the level detected in the mPFC, a finding that was consistent with the immunohistochemical results showing higher expression of NAAG in the mPFC than in the NAc.
TABLE 1.
Basal extracellular DA, DOPAC, HVA, glutamate, and NAAG levels in the mPFC and NAc (n = 55–57)
| mPFC | NAc | |
|---|---|---|
| DA (nm) | 0.434 ± 0.012 | 1.249 ± 0.142 |
| DOPAC (nm) | 41.020 ± 2.783 | 247.126 ± 37.352 |
| HVA (nm) | 35.992 ± 2.450 | 126.699 ± 15.216 |
| Glutamate (μm) | 2.190 ± 0.211 | 1.578 ± 0.141 |
| NAAG (μm) | 0.102 ± 0.051 | 0.088 ± 0.040 |
Effects of ZJ43 on NAAG and Glutamate Levels in mPFC and NAc
Analysis of variance results on the main group effects and group × time interactions for the following data are presented in Table 2.
TABLE 2.
Main effects and interactions, analysis of variance results
| Figure | Overall group effect | Group × time interaction |
|---|---|---|
| Fig. 2a | F(4,32) = 7.102, p < 0.001 | F(24,168) = 3.640, p < 0.001 |
| Fig. 2b | F(4,32) = 10.080, p < 0.001 | F(24,192) = 5.080, p < 0.001 |
| Fig. 3a | F (3,23) = 16.590, p < 0.001 | F(18,138) = 7.193, p < 0.001 |
| Fig. 3b | F(4,33) = 5.382, p < 0.01 | F(24,198) = 1.875, p < 0.05 |
| Fig. 4a | F(4,32) = 21.383, p < 0.001 | F(24,168) = 15.214, p < 0.001 |
| Fig. 4b | F(4,32) = 11.126, p < 0.001 | F(24,192) = 3.203, p < 0.001 |
| Fig. 5a | F(3,22) = 25.859, p < 0.001 | F(18,132) = 7.673, p < 0.001 |
| Fig. 5b | F(4,29) = 6.641, p < 0.01 | F(24,174) = 2.372, p < 0.001 |
| Fig. 6a | F(4,32) = 10.080, p < 0.001 | F(24,192) = 5.080, p < 0.001 |
| Fig. 6b | F(4,32) = 8.030, p < 0.001 | F(24,192) = 2.534, p < 0.01 |
| Fig. 7a | F(4,27) = 9.885, p < 0.001 | F(24,162) = 7.314, p < 0.001 |
| Fig. 7b | F(4,29) = 6.597, p < 0.01 | F(24,174) = 2.618, p < 0.001 |
| Fig. 8a | F(4,34) = 21.180, p < 0.001 | F(24,204) = 9.377, p < 0.001 |
| Fig. 8b | F(4,31) = 31.622, p < 0.001 | F(24,186) = 8.460, p < 0.001 |
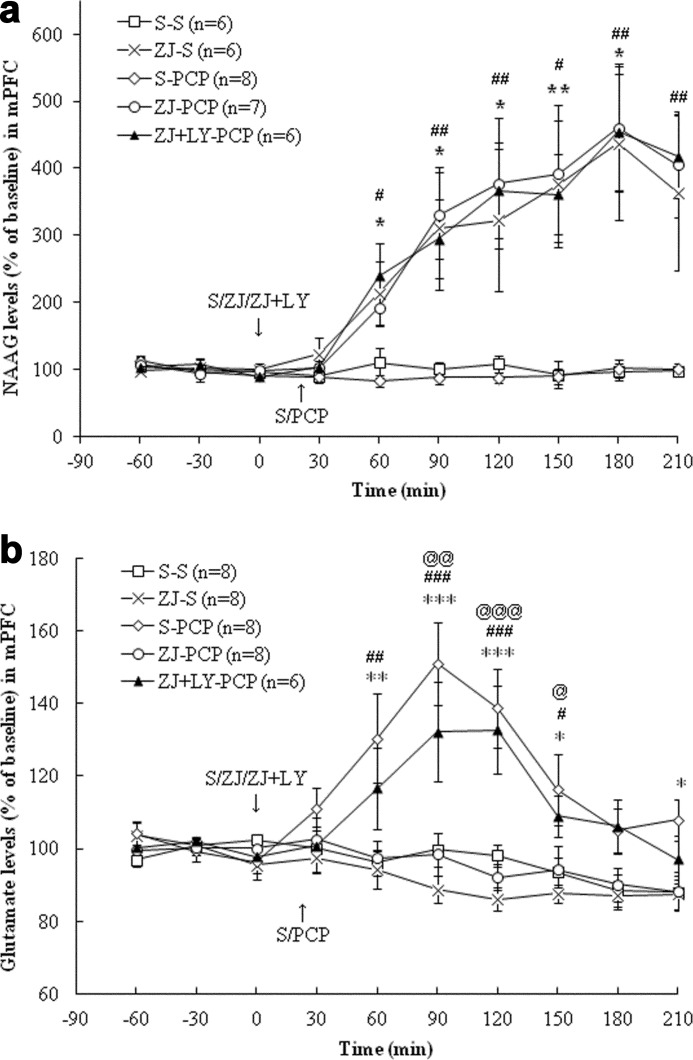
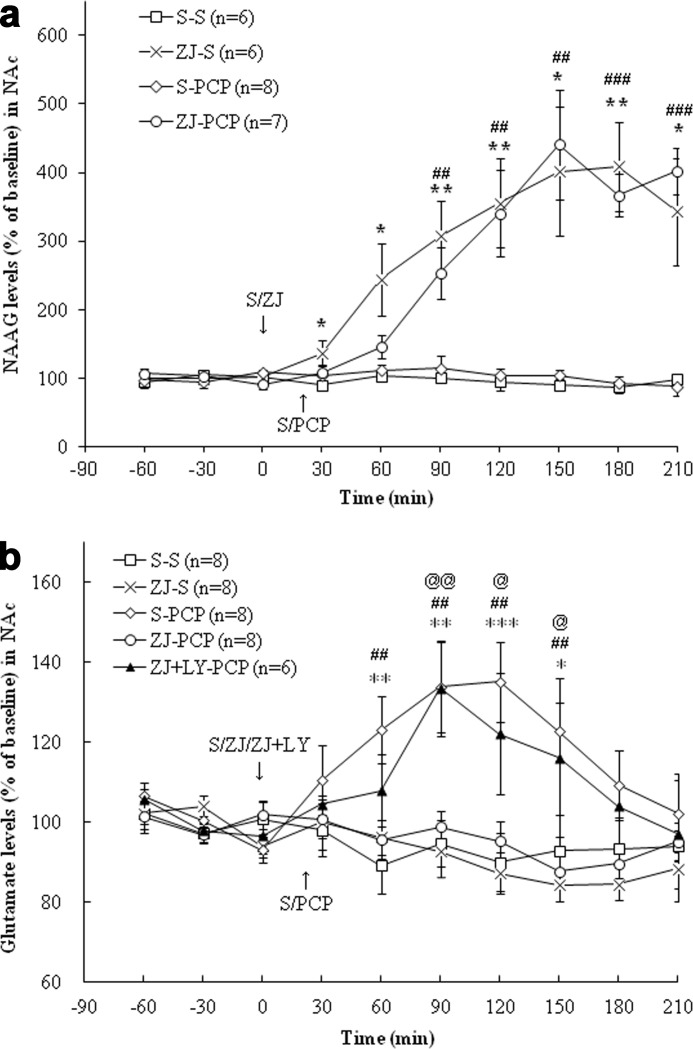
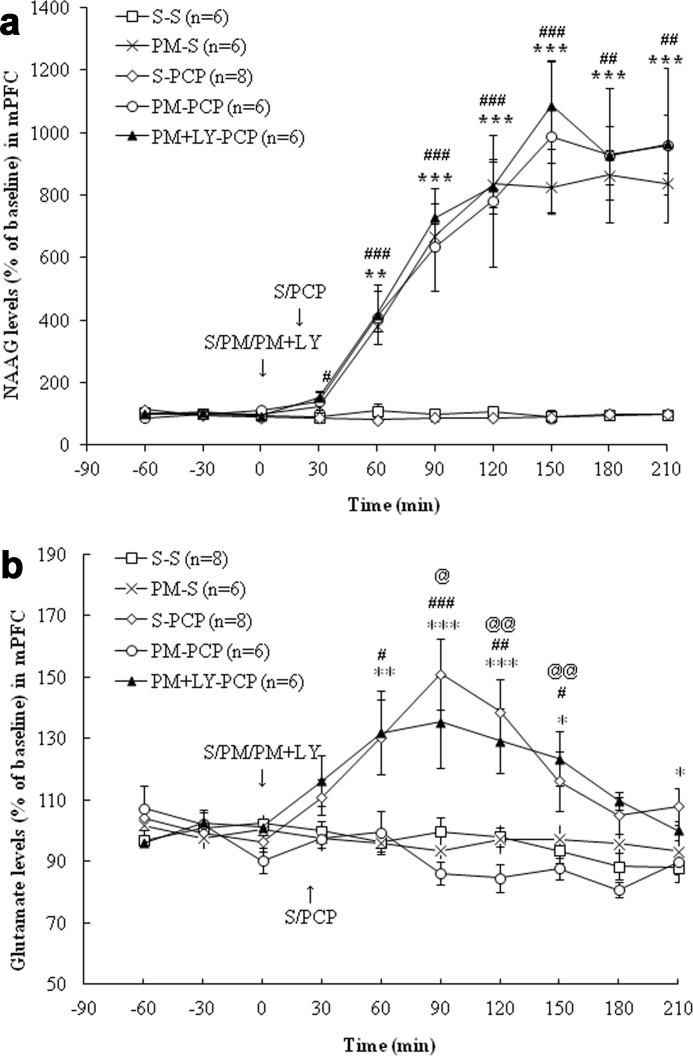
In the mPFC, treatment with PCP (5 mg/kg, i.p.; this dose was used in all studies described below) had no effect on extracellular NAAG levels (Fig. 2a). Treatment with ZJ43 (150 mg/kg, i.p.; this dose was used in all ZJ43 studies described below) increased extracellular NAAG levels (p < 0.01). PCP treatment increased glutamate levels (p < 0.001), and this increase was blocked by ZJ43 pretreatment (p < 0.001). As predicted, the group II (mGluR2/3) antagonist LY341495 (1 mg/kg, i.p., in all experiments) had no effect on ZJ43-induced increases in NAAG levels but blocked the effects of ZJ43 on PCP-induced glutamate release (Fig. 2, a and b). In the NAc (Fig. 3a), PCP had no significant effect on extracellular NAAG levels, whereas ZJ43 with saline or PCP increased extracellular NAAG levels (p < 0.001). PCP increased glutamate levels relative to saline (p < 0.01) (Fig. 3b), an effect that was blocked by ZJ43 pretreatment (p < 0.01). The mGluR2/3 antagonist again blocked the inhibitory effect of ZJ43 on PCP-induced glutamate release (p < 0.05).
FIGURE 2.
a, effects of (first treatment) saline (S), ZJ43 (ZJ; 150 mg/kg, i.p.), or ZJ43 with LY341495 (LY; 1 mg/kg, i.p.) and (second treatment) saline or PCP (5 mg/kg, i.p.) on NAAG release in the mPFC. S-PCP had no effect on extracellular NAAG levels compared with the S-S group (p = 0.90). ZJ-S and ZJ-PCP significantly increased extracellular NAAG levels compared with the S-S treatment group (p < 0.01). LY341495 pretreatment did not affect the ZJ43-induced increase in NAAG levels (p = 0.96). *, p < 0.05; **, p < 0.01, S-S versus ZJ-S. #, p < 0.05; ##, p < 0.01, ZJ-PCP versus S-PCP. b, effects of (first treatment) saline (S), ZJ43 (ZJ; 150 mg/kg, i.p.), or ZJ43 with LY341495 (LY; 1 mg/kg, i.p.) and (second treatment) saline or PCP (5 mg/kg, i.p.) on glutamate release in the mPFC. S-PCP treatment produced a significant glutamate increase compared with the S-S group (p < 0.001). ZJ-S had no effect on glutamate levels in the mPFC compared with the S-S group (p = 0.38), whereas ZJ43 pretreatment significantly inhibited PCP-induced glutamate release (p < 0.001). LY341495 pretreatment with ZJ43 significantly antagonized the inhibitory effect of ZJ43 on PCP-induced glutamate release (p < 0.01). *, p < 0.05; **, p < 0.01; ***, p < 0.001, S-S versus S-PCP. #, p < 0.05; ##, p < 0.01; ###, p < 0.001, ZJ-PCP versus S-PCP. @, p < 0.05; @@, p < 0.01; @@@, p < 0.001, ZJ-LY-PCP versus ZJ-PCP.
FIGURE 3.
a, effects of (first treatment) saline (S) or ZJ43 (ZJ; 150 mg/kg, i.p.) and (second treatment) saline or PCP (5 mg/kg, i.p.) on NAAG release in the NAc. S-PCP had no effect on extracellular NAAG levels relative to the S-S group (p = 0.84). ZJ-S significantly increased extracellular NAAG levels compared with the S-S treatment (p < 0.001). ZJ43 treatment with PCP also resulted in a significant NAAG level increase compared with S-PCP (p < 0.001). *, p < 0.05; **, p < 0.01, S-S versus ZJ-S. ##, p < 0.01; ###, p < 0.001, ZJ-PCP versus S-PCP. b, effects of (first treatment) saline (S), ZJ43 (ZJ; 150 mg/kg, i.p.), or ZJ43 with LY341495 (LY; 1 mg/kg, i.p.) and (second treatment) saline or PCP (5 mg/kg, i.p.) on glutamate release in the NAc. S-PCP produced a significant glutamate increase compared with the S-S group (p < 0.01). ZJ-S had no effect on glutamate levels in the NAc relative to the S-S group (p = 0.73). ZJ pretreatment significantly inhibited PCP-induced glutamate release (p < 0.01). LY431495 pretreatment with ZJ43 blocked the inhibition effect of ZJ43 on PCP-induced glutamate release (p < 0.05). *, p < 0.05; **, p < 0.01; ***, p < 0.001, S-S versus S-PCP. ##, p < 0.01, ZJ-PCP versus S-PCP. @, p < 0.05; @@, p < 0.01, ZJ-LY-PCP versus Z-PCP.
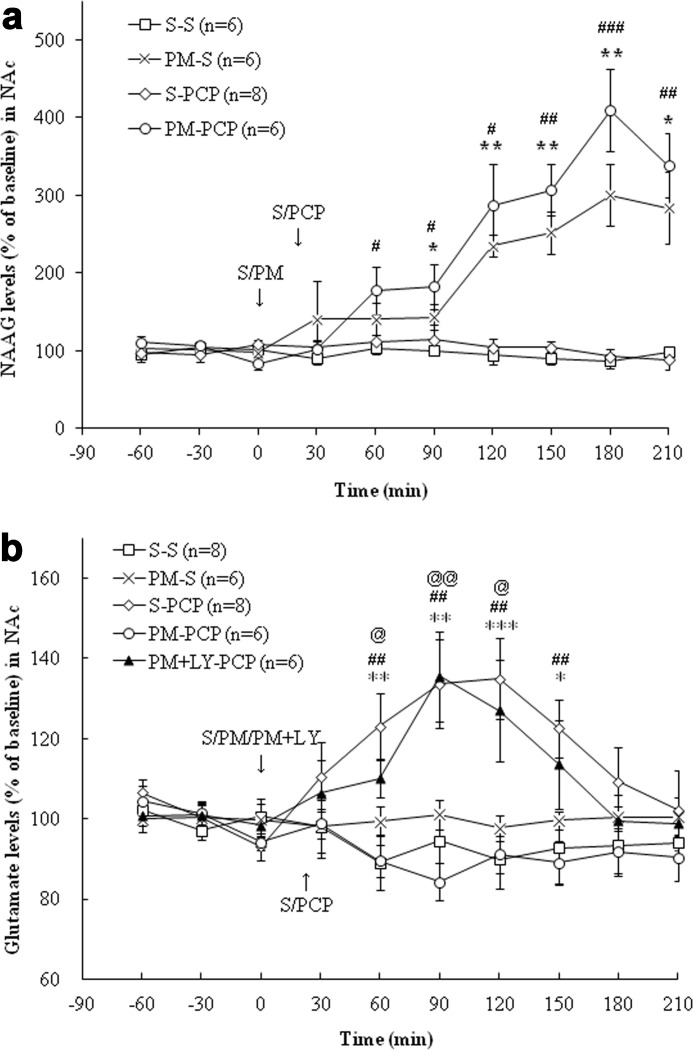
Effects of 2-PMPA on NAAG and Glutamate Levels in mPFC and NAc
In the mPFC, 2-PMPA (50 mg/kg, i.p.; this dose was used in all PMPA experiments) with saline, PCP, or PCP and LY341495 significantly increased extracellular NAAG levels compared with the saline + saline group (p < 0.001) (Fig. 4a). LY341495 had no effect on 2-PMPA-induced increases in NAAG levels. PCP increased glutamate levels compared with the saline treatment (p < 0.001), and this effect was blocked by 2-PMPA (p < 0.001) (Fig. 4b). In the NAc, 2-PMPA with or without PCP increased extracellular NAAG levels compared with the saline group (p < 0.001, p < 0.01) (Fig. 5a). Although 2-PMPA had no effect on basal glutamate levels in the NAc, it inhibited PCP-induced glutamate release (p < 0.001) (Fig. 5b). Again, the effect of the NAAG peptidase inhibitor on PCP-induced glutamate levels was blocked by the group II mGluR antagonist in both the mPFC and the NAc (p < 0.01, p < 0.001) (Figs. 4b and 5b).
FIGURE 4.
a, effects of (first treatment) saline (S), 2-PMPA (PM; 50 mg/kg, i.p.), or 2-PMPA with LY341495 (LY; 1 mg/kg, i.p.) and (second treatment) saline or PCP (5 mg/kg, i.p.) on NAAG levels in the mPFC. S-PCP had no effect on extracellular NAAG levels in the mPFC compared with S-S treatment group (p = 0.936). PM-S significantly increased extracellular NAAG levels compared with S-S group (p < 0.001). After 2-PMPA pretreatment, PCP administration also had a significant NAAG level increase compared with the S-PCP group (p < 0.001). LY341495 did not affect the increase of NAAG levels observed in the PM-PCP treatment group (p = 0.93). The S-S and S-PCP data are the same as presented in Fig. 2a. **, p < 0.01; ***, p < 0.001, S-S versus PM-S. #, p < 0.05; ##, p < 0.01; ###, p < 0.001, PM-PCP versus S-PCP. b, effects of (first treatment) saline (S), 2-PMPA (PM; 50 mg/kg, i.p.), or 2-PMPA with LY341495 (LY; 1 mg/kg, i.p.) and (second treatment) saline or PCP (5 mg/kg, i.p.) on glutamate levels in the mPFC. PM-S had no effect on glutamate levels compared with the S-S group (p = 0.88). PM pretreatment significantly inhibited PCP-induced glutamate release (p < 0.001), and LY341495 blocked the inhibitory effect of PM on PCP-induced glutamate release (p < 0.001). The S-S and S-PCP data are the same as presented in Fig. 2b. *, p < 0.05; **, p < 0.01; ***, p < 0.001, S-S versus S-PCP. #, p < 0.05; ##, p < 0.01; ###, p < 0.001, PM-PCP versus S-PCP. @, p < 0.05; @@, p < 0.01, PM-LY-PCP versus PM-PCP.
FIGURE 5.
a, effects of (first treatment) saline (S) or 2-PMPA (PM; 50 mg/kg, i.p.) and (second treatment) saline or PCP (5 mg/kg, i.p.) on NAAG levels in the NAc. S-PCP had no effect on extracellular NAAG levels in the NAc compared with the S-S treatment group (p = 0.71). The 2-PMPA significantly increased NAAG levels (versus S-S group, p < 0.001), as did the PM-PCP treatment. The S-S and S-PCP data are the same as presented in Fig. 3a. (p < 0.001). *, p < 0.05; **, p < 0.01, S-S versus PM-S. #, p < 0.05; ##, p < 0.01, PM-PCP versus S-PCP. b, effects of (first treatment) saline (S), 2-PMPA (PM; 50 mg/kg, i.p.), or 2-PMPA with LY341495 (LY; 1 mg/kg, i.p.) and (second treatment) saline or PCP (5 mg/kg, i.p.) on glutamate levels in the NAc. S-PCP induced a significant glutamate increase compared with the S-S treatment group (p < 0.001), whereas PM-S had no effect on glutamate levels (p = 0.37). 2-PMPA pretreatment significantly inhibited PCP-induced glutamate release (p < 0.001). LY431495 blocked the effect of 2-PMPA-induced glutamate release (PM-PCP versus PM + LY + PCP, p < 0.01). The S-S and S-PCP are the same as presented in Fig. 3b. *, p < 0.05; **, p < 0.01; ***, p < 0.001, S-S versus S-PCP. ##, p < 0.01, PM-PCP versus S-PCP. @, p < 0.05; @@, p < 0.01, PM-LY-PCP versus PM-PCP.
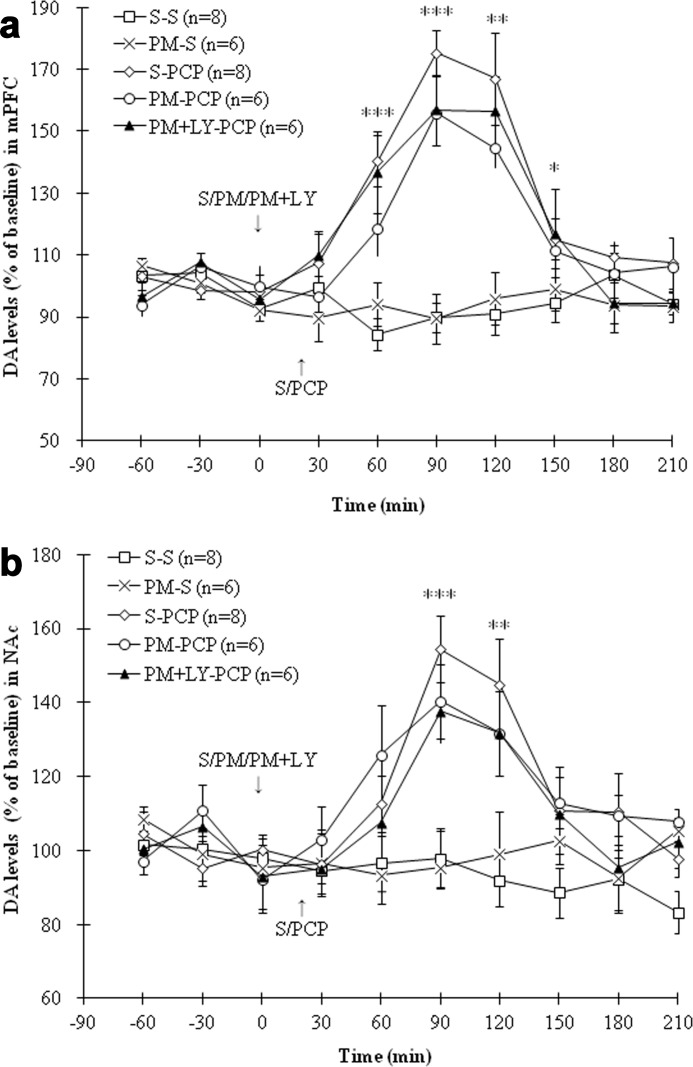
ZJ43 and 2-PMPA Do Not Block PCP-induced Increases in Extracellular DA
PCP produced a significant increase in DA levels in the mPFC and the NAc (p < 0.001), and ZJ43 failed to affect this increase (Figs. 6, a and b). 2-PMPA similarly failed to significantly affect PCP-induced increases in dopamine levels in the mPFC and NAc (Fig. 7, a and b).
FIGURE 6.
a, effects of (first treatment) saline (S), ZJ43 (ZJ; 150 mg/kg, i.p.), or ZJ43 with LY341495 (LY; 1 mg/kg, i.p.) and (second treatment) saline or PCP (5 mg/kg, i.p.) on dopamine levels in the mPFC. S-PCP produced a significant DA increase compared with the S-S treatment group (p < 0.001). The effect of ZJ-S on DA levels did not reach significance in the mPFC (p = 0.22 versus S-S group). Neither ZJ nor ZJ + LY pretreatment had an effect on PCP-induced DA release (p = 0.73 and p = 0.68, respectively). *, p < 0.05; **, p < 0.01; ***, p < 0.001, S-S versus S-PCP. b, effects of (first treatment) saline (S), ZJ43 (ZJ; 150 mg/kg, i.p.), or ZJ43 with LY341495 (LY; 1 mg/kg, i.p.) and (second treatment) saline or PCP (5 mg/kg, i.p.) on dopamine levels in the NAc. S-PCP treatment produced a significant DA increase (p < 0.001 versus S-S treatment). ZJ-S had no effect on DA levels compared with the S-S treatment group (p = 0.90). Neither ZJ nor ZJ + LY pretreatment had a significant effect on PCP-induced DA release (p = 0.99 and p = 0.96, respectively). **, p < 0.01; ***, p < 0.001, S-S versus S-PCP.
FIGURE 7.
a, effects of (first treatment) saline (S), 2-PMPA (PM; 50 mg/kg, i.p.), or 2-PMPA with LY341495 (LY; 1 mg/kg, i.p.) and (second treatment) saline or PCP (5 mg/kg, i.p.) on DA levels in the mPFC. The S-PCP treatment produced a significant DA increase compared with the S-S treatment group (p < 0.001). PM-S had no effect on DA levels (p = 0.98 versus S-S). PM-PCP pretreatment did not reach significance versus S-PCP (p = 0.14), whereas PM + LY had no significant effect on PCP-induced DA release (p = 0.31). The S-S and S-PCP data presented here are the same as in Fig. 6a. *, p < 0.05; **, p < 0.01; ***, p < 0.001, S-S versus S-PCP. b, effects of (first treatment) saline (S), 2-PMPA (PM; 50 mg/kg, i.p.), or 2-PMPA with LY341495 (LY; 1 mg/kg, i.p.) and (second treatment) saline or PCP (5 mg/kg, i.p.) on DA levels in the NAc. S-PCP produced a significant DA increase compared with S-S group (p < 0.001). PM-S treatment had no effect on DA levels compared with S-S group (p = 0.41). Pretreatment with PM or PM + LY also had no detectable effect on PCP-induced DA release (p = 0.94 and p = 0.33, respectively). The S-S and S-PCP data presented here are the same as in Fig. 6b. **, p < 0.01; ***, p < 0.001, S-S versus S-PCP.
DA Metabolites DOPAC and HVA in Microdialysis Samples
It is possible that the rapid conversion of DA to DOPAC and HVA in extracellular space of the mPFC and NAc obscured the effect of PCP or NAAG peptidase inhibition on DA flux in these two brain regions. To test this, the concentrations of these two metabolites were examined in the same samples that were tested for DA. We found that the changes in concentration of DOPAC and HVA over the time course of the sampling closely paralleled the changes in concentration of DA in samples taken from rats in each of the treatment groups (see supplemental Figs. S1–S8 with statistical assessments). These data demonstrate that there were no significant effects on NAAG peptidase inhibition on PCP-induced elevation of these DA metabolites in the extracellular space.
ZJ43 and 2-PMPA Block PCP-induced Motor Activation Model
In the same groups in which the effects of ZJ43 were assessed on NAAG and glutamate levels, the open field motor activation induced by PCP also was recorded (Fig. 8a). PCP-induced motor activation is a validated model for testing potential antipsychotic drugs. PCP induced a significant increase in ambulatory counts (number of infrared beam breaks detected in the open field chamber) compared with the saline group (p < 0.001). ZJ43 + saline had no significant effect on ambulatory counts compared with the saline + saline group. ZJ43 pretreatment significantly inhibited PCP-induced hyperactivity (p < 0.001) and LY341495 blocked the effects of ZJ43 on this PCP-induced hyperactivity (p < 0.01).
PCP-induced motor activation also was studied in the groups of animals that were assayed for the effects of 2-PMPA on NAAG and glutamate levels. The same saline + saline and saline + PCP data are presented in Fig. 8, a and b. As was observed with ZJ43, 2-PMPA had no effect on ambulatory counts, whereas pretreatment with 2-PMPA significantly inhibited PCP-induced hyperactivity (p < 0.001). LY341495 blocked the effect of both NAAG peptidase inhibitors on PCP-induced hyperactivity (p < 0.01) (Fig. 8, a and b).
DISCUSSION
NAAG, a prevalent and widely distributed peptide co-transmitter, is found in neurons and presumptive synaptic endings in the mPFC and NAc (Fig. 1), two brain regions that are widely documented to be involved in schizophrenia and in PCP models of this disorder (1, 37–40). NAAG immunoreactivity in synaptic endings is localized in synaptic vesicles (47). The NAAG receptor, mGluR3 (12), also is expressed in these brain regions (49), and polymorphisms in the gene for this receptor are associated with expression of schizophrenia in some patients with this disorder (50–53).
The most widely used and highly validated animal models for testing the development of antipsychotic drugs are based on the neurochemical and behavioral effects of PCP or d-amphetamine (54–56). NAAG peptidase inhibitors have been shown to reduce positive, negative, and cognitive behaviors elicited by PCP and d-amphetamine in these models (25, 26, 47, 57).5
The data presented here represent the first demonstration of the neurochemical effects of NAAG peptidase inhibition in brain regions associated with schizophrenia. Their efficacy in dramatically elevating extracellular levels of NAAG and in blocking PCP-induced motor activation and increases in glutamate, but not dopamine release, in the mPFC and NAc provides a framework for defining the molecular pathway that mediates their behavioral effects.
Two structurally distinct NAAG peptidase inhibitors were tested in order to confirm that the effects of the drugs were the result of this inhibitory effect and the consequent elevation of NAAG levels. The different structures of the urea-based ZJ43 and pentanedioic acid-based 2-PMPA militate against an unknown common second order function being responsible for this behavioral and neurochemical activity. In these experiments, additional confirmation of the mechanism of action of the inhibitors derives from the blockade of their effects by the group II mGluR antagonist LY431495 and by the failure of ZJ43 and 2-PMPA to directly activate mGluR2 or mGluR3 (58, 59).
Heterotropic group II mGluR2/3 agonists have behavioral effects in PCP and d-amphetamine models of schizophrenia that are similar to those of NAAG peptidase inhibitors (1, 28–30, 60, 61). However, studies in mGuR2 and mGluR3 knock-out mice demonstrate that the heterotropic agonists act via mGluR2 to block the effects of PCP (31, 34), whereas the effects of the 2-PMPA on PCP in these knock-out mice are mediated by NAAG activation of mGluR3 (26), consistent with a substantial body of data demonstrating that NAAG is an mGluR3-selective agonist (12). The data presented here on the NAAG peptidase inhibition-induced elevation of NAAG levels in the mPFC and NAc together with the resulting blockade of PCP-induced increases in glutamate release establish further the parallels between the effects of the heterotropic agonists and those of NAAG peptidase inhibitors in these schizophrenia models. As a result, it appears that similar behavioral and neurochemical effects are obtained by activation of either mGluR2 or mGluR3, and thus there is potential for these two pharmacological approaches to act additively. Particularly interesting in this respect would be combination therapies using mGluR2-positive allosteric modulators (62) with NAAG peptidase inhibitors.
Currently approved antipsychotics target DA, norepinephrine, and serotonin receptors. These drugs have limited efficacy in treating schizophrenia in many individuals, particularly for cognitive and negative symptoms. Given the differences in their targets from those of the typical and atypical antipsychotic drugs, the discovery of the efficacy of group II mGluR agonists and NAAG peptidase inhibitors in these animal models of schizophrenia and in clinical trials using the agonists represents a potentially important breakthrough in antipsychotic drug design. Interestingly, it may turn out that current antipsychotic treatments may already achieve some of their effects via NAAG in as much as clozapine and haloperidol increase NAAG synthesis in a neuroblastoma cell line (23).
In parallel with the glutamate theory of schizophrenia, the DA theory of this disorder emerged from the discovery of the antipsychotic efficacy of DA receptor antagonists and is modeled in animals by d-amphetamine- and PCP-induced increases in dopamine release in humans and animals (54). ZJ43 and 2-PMPA failed to affect the increase in DA release that was induced by PCP (Figs. 6 and 7). A similar lack of effect on PCP-induced DA release was reported for a heterotropic group II mGluR agonist (1). In contrast, a group II agonist blocked d-amphetamine-induced motor activation and increases in DA release but not electrically evoked DA release in the mPAG, bringing into question its mechanism of action (27, 29). Because ZJ43 and 2-PMPA also reduce d-amphetamine-induced motor activation,5 it will be interesting to determine if the peptidase inhibitors affect DA release in the d-amphetamine model.
This demonstration of the efficacy of glutamate carboxypeptidase inhibitors in elevating NAAG levels and blocking PCP-induced increases in glutamate release suggests that their mechanisms of action in the behavioral studies may reside in part or wholly within the mPFC and NAc because these areas have previously been implicated in the behavioral effects of PCP. Testing this will require microinjection of ZJ43 or 2-PMPA directly into these brain regions and observation of their effects on PCP- and d-amphetamine-induced behaviors.
The data presented here contribute to proof of the concept that NAAG peptidase inhibition represents a significant new approach to developing antipsychotic drugs with targets that differ from those of currently approved drugs and thus warrants further study.
This work was supported, in whole or in part, by National Institutes of Health Grant R01 MH 79983. This work was also supported by an endowment and generous gifts from Nancy and Daniel Paduano. The patent for ZJ43 is held by Georgetown University, but the authors have no proprietary interest in this compound.

This article contains supplemental Figs. S1–S8.
Yamada, T., Zuo, D., Yamamoto, T., Olszewski, R. T., Bzdega, T., Moffett, J. R., and Neale J. H. (2012) Mol. Pain, in press.
Olszewski, R. T., Janczura, K. J., Ball, S. R., Madore, J. C., Lavin, K. M., Lee, J. C., Lee, M. J., Der, E. K., Bzdega, T., and Neale J. H. (2012) Transl. Psychiatry, in press.
- PCP
- phencyclidine
- DA
- dopamine
- MPFC
- medial prefrontal cortex
- NAc
- nucleus accumbens
- NAAG
- N-acetylaspartylglutamate
- 2-PMPA
- 2-(phosphonomethyl)pentane-1,5-dioic acid
- DOPAC
- 3,4-dihydroxyphenylacetic acid
- HVA
- homovanillic acid
- ECD
- electrochemical detection
- ZJ43
- N-[[[(1S)-1-carboxy-3-methylbutyl]amino]carbonyl]-l-glutamic acid.
REFERENCES
- 1. Moghaddam B., Adams B. W. (1998) Reversal of phencyclidine effects by a group II metabotropic glutamate receptor agonist in rats. Science 281, 1349–1352 [DOI] [PubMed] [Google Scholar]
- 2. Sebban C., Tesolin-Decros B., Ciprian-Ollivier J., Perret L., Spedding M. (2002) Effects of phencyclidine (PCP) and MK 801 on the EEGq in the prefrontal cortex of conscious rats; antagonism by clozapine, and antagonists of AMPA, α1, and 5-HT2A receptors. Br. J. Pharmacol. 135, 65–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Deutsch S. I., Rosse R. B., Billingslea E. N., Bellack A. S., Mastropaolo J. (2002) Topiramate antagonizes MK-801 in an animal model of schizophrenia. Eur. J. Pharmacol. 449, 121–125 [DOI] [PubMed] [Google Scholar]
- 4. Ninan I., Jardemark K. E., Wang R. Y. (2003) Olanzapine and clozapine but not haloperidol reverse subchronic phencyclidine-induced functional hyperactivity of N-methyl-d-aspartate receptors in pyramidal cells of the rat medial prefrontal cortex. Neuropharmacology 44, 462–472 [DOI] [PubMed] [Google Scholar]
- 5. Katayama T., Jodo E., Suzuki Y., Hoshino K. Y., Takeuchi S., Kayama Y. (2007) Activation of medial prefrontal cortex neurons by phencyclidine is mediated via AMPA/kainate glutamate receptors in anesthetized rats. Neuroscience 150, 442–448 [DOI] [PubMed] [Google Scholar]
- 6. Di Iorio P., Battaglia G., Ciccarelli R., Ballerini P., Giuliani P., Poli A., Nicoletti F., Caciagli F. (1996) Interaction between A1 adenosine and class II metabotropic glutamate receptors in the regulation of purine and glutamate release from rat hippocampal slices. J. Neurochem. 67, 302–309 [DOI] [PubMed] [Google Scholar]
- 7. Tamaru Y., Nomura S., Mizuno N., Shigemoto R. (2001) Distribution of metabotropic glutamate receptor mGluR3 in the mouse CNS. Differential location relative to pre- and postsynaptic sites. Neuroscience 106, 481–503 [DOI] [PubMed] [Google Scholar]
- 8. Wroblewska B., Wroblewski J. T., Pshenichkin S., Surin A., Sullivan S. E., Neale J. H. (1997) N-Acetylaspartylglutamate selectively activates mGluR3 receptors in transfected cells. J. Neurochem. 69, 174–181 [DOI] [PubMed] [Google Scholar]
- 9. Wroblewska B., Santi M. R., Neale J. H. (1998) N-Acetylaspartylglutamate activates cyclic AMP-coupled metabotropic glutamate receptors in cerebellar astrocytes. Glia 24, 172–179 [DOI] [PubMed] [Google Scholar]
- 10. Wroblewska B., Wegorzewska I. N., Bzdega T., Olszewski R. T., Neale J. H. (2006) Differential negative coupling of type 3 metabotropic glutamate receptor to cyclic GMP levels in neurons and astrocytes. J. Neurochem. 96, 1071–1077 [DOI] [PubMed] [Google Scholar]
- 11. Wroblewska B., Wegorzewska I. N., Bzdega T., Neale J. H. (2011) Type 2 metabotropic glutamate receptor (mGluR2) fails to negatively couple to cGMP in stably transfected cells. Neurochem. Int. 58, 176–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Neale J. H. (2011) N-Acetylaspartylglutamate (NAAG) is an agonist at mGluR3 in vivo and in vitro. J. Neurochem. 119, 891–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Slusher B. S., Vornov J. J., Thomas A. G., Hurn P. D., Harukuni I., Bhardwaj A., Traystman R. J., Robinson M. B., Britton P., Lu X. C., Tortella F. C., Wozniak K. M., Yudkoff M., Potter B. M., Jackson P. F. (1999) Selective inhibition of NAALADase, which converts NAAG to glutamate, reduces ischemic brain injury. Nat. Med. 5, 1396–1402 [DOI] [PubMed] [Google Scholar]
- 14. Zhao J., Ramadan E., Cappiello M., Wroblewska B., Bzdega T., Neale J. H. (2001) NAAG inhibits KCl-induced [3H]GABA release via mGluR3, cAMP, PKA and L-type calcium conductance. Eur. J. Neurosci. 13, 340–346 [PubMed] [Google Scholar]
- 15. Sanabria E. R., Wozniak K. M., Slusher B. S., Keller A. (2004) GCP II (NAALADase) inhibition suppresses mossy fiber-CA3 synaptic neurotransmission by a presynaptic mechanism. J. Neurophysiol. 91, 182–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhong C., Zhao X., Van K. C., Bzdega T., Smyth A., Zhou J., Kozikowski A. P., Jiang J., O'Connor W. T., Berman R. F., Neale J. H., Lyeth B. G. (2006) NAAG peptidase inhibitor increases dialysate NAAG and reduces glutamate, aspartate, and GABA levels in the dorsal hippocampus following fluid percussion injury in the rat. J. Neurochem. 97, 1015–1025 [DOI] [PubMed] [Google Scholar]
- 17. Adedoyin M. O., Vicini S., Neale J. H. (2010) Endogenous N-acetylaspartylglutamate (NAAG) inhibits synaptic plasticity/transmission in the amygdala in a mouse inflammatory pain model. Mol. Pain. 6, 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cassidy M., Neale J. H. (1993) N-Acetylaspartylglutamate catabolism is achieved by an enzyme on the cell surface of neurons and glia. Neuropeptides 24, 271–278 [DOI] [PubMed] [Google Scholar]
- 19. Berger U. V., Luthi-Carter R., Passani L. A., Elkabes S., Black I., Konradi C., Coyle J. T. (1999) Glutamate carboxypeptidase II is expressed by astrocytes in the adult rat nervous system. J. Comp. Neurol. 415, 52–64 [DOI] [PubMed] [Google Scholar]
- 20. Neale J. H., Olszewski R. T., Gehl L. M., Wroblewska B., Bzdega T. (2005) The neurotransmitter N-acetylaspartylglutamate in models of pain, ALS, diabetic neuropathy, CNS injury, and schizophrenia. Trends Pharmacol. Sci. 26, 477–484 [DOI] [PubMed] [Google Scholar]
- 21. Zhou J., Neale J. H., Pomper M. G., Kozikowski A. P. (2005) NAAG peptidase inhibitors and their potential for diagnosis and therapy. Nat. Rev. Drug Discov. 4, 1015–1026 [DOI] [PubMed] [Google Scholar]
- 22. Tsukamoto T., Wozniak K. M., Slusher B. S. (2007) Progress in the discovery and development of glutamate carboxypeptidase II inhibitors. Drug Discov. Today 12, 767–776 [DOI] [PubMed] [Google Scholar]
- 23. Arun P., Madhavarao C. N., Moffett J. R., Namboodiri A. M. (2008) Antipsychotic drugs increase N-acetylaspartate and N-acetylaspartylglutamate in SH-SY5Y human neuroblastoma cells. J. Neurochem. 106, 1669–1680 [DOI] [PubMed] [Google Scholar]
- 24. Olszewski R. T., Bukhari N., Zhou J., Kozikowski A. P., Wroblewski J. T., Shamimi-Noori S., Wroblewska B., Bzdega T., Vicini S., Barton F. B., Neale J. H. (2004) NAAG peptidase inhibition reduces locomotor activity and some stereotypes in the PCP model of schizophrenia via group II mGluR. J. Neurochem. 89, 876–885 [DOI] [PubMed] [Google Scholar]
- 25. Olszewski R. T., Wegorzewska M. M., Monteiro A. C., Krolikowski K. A., Zhou J., Kozikowski A. P., Long K., Mastropaolo J., Deutsch S. I., Neale J. H. (2008) Phencyclidine and dizocilpine induced behaviors reduced by N-acetylaspartylglutamate peptidase inhibition via metabotropic glutamate receptors. Biol. Psychiatry 63, 86–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Olszewski R. T., Bzdega T., Neale J. H. (2012) mGluR3 and not mGluR2 receptors mediate the efficacy of NAAG peptidase inhibitor in PCP model of schizophrenia. Schizophr. Res. 136, 160–161 [DOI] [PubMed] [Google Scholar]
- 27. Pehrson A. L., Moghaddam B. (2010) Impact of metabotropic glutamate 2/3 receptor stimulation on activated dopamine release and locomotion. Psychopharmacology 211, 443–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cartmell J., Monn J. A., Schoepp D. D. (1999) The metabotropic glutamate 2/3 receptor agonists LY354740 and LY379268 selectively attenuate phencyclidine versus d-amphetamine motor behaviors in rats. J. Pharmacol. Exp. Ther. 291, 161–170 [PubMed] [Google Scholar]
- 29. Cartmell J., Monn J. A., Schoepp D. D. (2000) Attenuation of specific PCP-evoked behaviors by the potent mGlu2/3 receptor agonist, LY379268 and comparison with the atypical antipsychotic, clozapine. Psychopharmacology 148, 423–429 [DOI] [PubMed] [Google Scholar]
- 30. Cartmell J., Monn J. A., Schoepp D. D. (2000) The mGlu(2/3) receptor agonist LY379268 selectively blocks amphetamine ambulations and rearing. Eur. J. Pharmacol. 400, 221–224 [DOI] [PubMed] [Google Scholar]
- 31. Fell M. J., Svensson K. A., Johnson B. G., Schoepp D. D. (2008) Evidence for the role of metabotropic glutamate (mGlu)2 not mGlu3 receptors in the preclinical antipsychotic pharmacology of the mGlu2/3 receptor agonist (−)-(1R,4S,5S,6S)-4-amino-2-sulfonylbicyclo[3.1.0]hexane-4,6-dicarboxylic acid (LY404039). J. Pharmacol. Exp. Ther. 326, 209–217 [DOI] [PubMed] [Google Scholar]
- 32. Monn J. A., Valli M. J., Massey S. M., Hansen M. M., Kress T. J., Wepsiec J. P., Harkness A. R., Grutsch J. L., Jr., Wright R. A., Johnson B. G., Andis S. L., Kingston A., Tomlinson R., Lewis R., Griffey K. R., Tizzano J. P., Schoepp D. D. (1999) Synthesis, pharmacological characterization and molecular modeling of heterobicyclic amino acids related to (+)-2-aminobicyclo[3.1.0] hexane-2,6-dicarboxylic acid (LY354740). Identification of two new potent, selective, and systemically active agonists for group II metabotropic glutamate receptors. J. Med. Chem. 42, 1027–1040 [DOI] [PubMed] [Google Scholar]
- 33. Rorick-Kehn L. M., Johnson B. G., Burkey J. L., Wright R. A., Calligaro D. O., Marek G. J., Nisenbaum E. S., Catlow J. T., Kingston A. E., Giera D. D., Herin M. F., Monn J. A., McKinzie D. L., Schoepp D. D. (2007) Pharmacological and pharmacokinetic properties of a structurally novel, potent, and selective metabotropic glutamate 2/3 receptor agonist. In vitro characterization of agonist (−)-(1R,4S,5S,6S)-4-amino-2-sulfonylbicyclo[3.1.0]-hexane-4,6-dicarboxylic acid (LY404039). J. Pharmacol. Exp. Ther. 321, 308–317 [DOI] [PubMed] [Google Scholar]
- 34. Woolley M. L., Pemberton D. J., Bate S., Corti C., Jones D. N. (2008) The mGlu2 but not the mGlu3 receptor mediates the actions of the mGluR2/3 agonist, LY379268, in mouse models predictive of antipsychotic activity. Psychopharmacology 196, 431–440 [DOI] [PubMed] [Google Scholar]
- 35. Patil S. T., Zhang L., Martenyi F., Lowe S. L., Jackson K. A., Andreev B. V., Avedisova A. S., Bardenstein L. M., Gurovich I. Y., Morozova M. A., Mosolov S. N., Neznanov N. G., Reznik A. M., Smulevich A. B., Tochilov V. A., Johnson B. G., Monn J. A., Schoepp D. D. (2007) Activation of mGlu2/3 receptors as a new approach to treat schizophrenia. A randomized Phase 2 clinical trial. Nat. Med. 13, 1102–1107 [DOI] [PubMed] [Google Scholar]
- 36. Kinon B. J., Zhang L., Millen B. A., Osuntokun O. O., Williams J. E., Kollack-Walker S., Jackson K., Kryzhanovskaya L., Jarkova N., and the HBBI Study Group (2011) A multicenter, inpatient, phase 2, double-blind, placebo-controlled dose-ranging study of LY2140023 monohydrate in patients with DSM-IV schizophrenia. J. Clin. Psychopharmacol. 31, 349–355 [DOI] [PubMed] [Google Scholar]
- 37. Karch S., Pogarell O., Mulert C. (2012) Functional Magnetic Resonance Imaging and treatment strategies in schizophrenia. Curr. Pharm. Biotechnol., in press [DOI] [PubMed] [Google Scholar]
- 38. Arnsten A. F. (2011) Prefrontal cortical network connections. Key site of vulnerability in stress and schizophrenia. Int. J. Dev. Neurosci. 29, 215–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mikell C. B., McKhann G. M., Segal S., McGovern R. A., Wallenstein M. B., Moore H. (2009) The hippocampus and nucleus accumbens as potential therapeutic targets for neurosurgical intervention in schizophrenia. Stereotact. Funct. Neurosurg. 87, 256–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Adams B. W., Moghaddam B. (2001) Effect of clozapine, haloperidol, or M100907 on phencyclidine-activated glutamate efflux in the prefrontal cortex. Biol. Psychiatry 50, 750–757 [DOI] [PubMed] [Google Scholar]
- 41. Kozikowski A. P., Zhang J., Nan F., Petukhov P. A., Grajkowska E., Wroblewski J. T., Yamamoto T., Bzdega T., Wroblewska B., Neale J. H. (2004) Synthesis of urea-based inhibitors as active site probes of glutamate carboxypeptidase II. Efficacy as analgesic agents. J. Med. Chem. 47, 1729–1738 [DOI] [PubMed] [Google Scholar]
- 42. Kingston A. E., Ornstein P. L., Wright R. A., Johnson B. G., Mayne N. G., Burnett J. P., Belagaje R., Wu S., Schoepp D. D. (1998) LY341495 is a nanomolar potent and selective antagonist of group II metabotropic glutamate receptors. Neuropharmacology 37, 1–12 [DOI] [PubMed] [Google Scholar]
- 43. Rowley H. L., Martin K. F., Marsden C. A. (1995) Determination of in vivo amino acid neurotransmitters by high performance liquid chromatography with o-phthalaldehyde-sulfite derivatization. J. Neurosci. Methods 57, 93–99 [DOI] [PubMed] [Google Scholar]
- 44. Fuhrman S., Palkovits M., Cassidy M., Neale J. H. (1994) The regional distribution of N-acetylaspartylglutamate (NAAG) and peptidase activity against NAAG in the rat nervous system. J. Neurochem. 62, 275–281 [DOI] [PubMed] [Google Scholar]
- 45. Moffett J. R., Namboodiri M. A., Neale J. H. (1993) Enhanced carbodiimide fixation for immunohistochemistry. Application to the comparative distributions of N-acetylaspartylglutamate and N-acetylaspartate immunoreactivities in rat brain. J. Histochem. Cytochem. 41, 559–570 [DOI] [PubMed] [Google Scholar]
- 46. Moffett J. R., Namboodiri M. A. (1995) Differential distribution of N-acetylaspartylglutamate and N-acetylaspartate immunoreactivities in rat forebrain. J. Neurocytol. 24, 409–433 [DOI] [PubMed] [Google Scholar]
- 47. Williamson L. C., Neale J. H. (1988) Ultrastructural localization of N-acetylaspartylglutamate in synaptic vesicles of retinal neurons. Brain Res. 456, 375–381 [DOI] [PubMed] [Google Scholar]
- 48. Neale J. H., Bzdega T., Wroblewska B. (2000) N-Acetylaspartylglutamate. The most abundant peptide neurotransmitter in the mammalian central nervous system. J. Neurochem. 75, 443–452 [DOI] [PubMed] [Google Scholar]
- 49. Makoff A., Volpe F., Lelchuk R., Harrington K., Emson P. (1996) Molecular characterization and localization of human metabotropic glutamate receptor type 3. Brain Res. Mol. Brain Res. 40, 55–63 [DOI] [PubMed] [Google Scholar]
- 50. Egan M. F., Straub R. E., Goldberg T. E., Yakub I., Callicott J. H., Hariri A. R., Mattay V. S., Bertolino A., Hyde T. M., Shannon-Weickert C., Akil M., Crook J., Vakkalanka R. K., Balkissoon R., Gibbs R. A., Kleinman J. E., Weinberger D. R. (2004) Variation in GRM3 affects cognition, prefrontal glutamate, and risk for schizophrenia. Proc. Natl. Acad. Sci. U.S.A. 101, 12604–12609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Harrison P. J., Lyon L., Sartorius L. J., Burnet P. W., Lane T. A. (2008) The group II metabotropic glutamate receptor 3 (mGluR3, mGlu3, GRM3). Expression, function, and involvement in schizophrenia. J. Psychopharmacol. 22, 308–322 [DOI] [PubMed] [Google Scholar]
- 52. Sartorius L. J., Weinberger D. R., Hyde T. M., Harrison P. J., Kleinman J. E., Lipska B. K. (2008) Expression of a GRM3 splice variant is increased in the dorsolateral prefrontal cortex of individuals carrying a schizophrenia risk SNP. Neuropsychopharmacology 33, 2626–2634 [DOI] [PubMed] [Google Scholar]
- 53. Baune B. T., Suslow T., Beśte C., Birosova E., Domschke K., Sehlmeyer C., Konrad C. (2010) Association between genetic variants of the metabotropic glutamate receptor 3 (GRM3) and cognitive set shifting in healthy individuals. Genes Brain Behav. 9, 459–466 [DOI] [PubMed] [Google Scholar]
- 54. Carlsson A., Carlsson M. L. (2006) A dopaminergic deficit hypothesis of schizophrenia. The path to discovery. Dialogues Clin. Neurosci. 8, 137–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Javitt D. C. (2007) Glutamate and schizophrenia. Phencyclidine, N-methyl-d-aspartate receptors, and dopamine-glutamate interactions. Int. Rev. Neurobiol. 78, 69–108 [DOI] [PubMed] [Google Scholar]
- 56. Bondi C., Matthews M., Moghaddam B. (2012) Glutamatergic Animal Models of Schizophrenia. Curr. Pharm. Des. 18, 1593–1604 [DOI] [PubMed] [Google Scholar]
- 57. Takatsu Y., Fujita Y., Tsukamoto T., Slusher B. S., Hashimoto K. (2011) Orally active glutamate carboxypeptidase II inhibitor 2-MPPA attenuates dizocilpine-induced prepulse inhibition deficits in mice. Brain Res. 1371, 82–86 [DOI] [PubMed] [Google Scholar]
- 58. Yamamoto T., Hirasawa S., Wroblewska B., Grajkowska E., Zhou J., Kozikowski A., Wroblewski J., Neale J. H. (2004) Antinociceptive effects of N-acetylaspartylglutamate (NAAG) peptidase inhibitors ZJ-11, ZJ-17, and ZJ-43 in the rat formalin test and in the rat neuropathic pain model. Eur. J. Neurosci. 20, 483–494 [DOI] [PubMed] [Google Scholar]
- 59. Yamamoto T., Saito O., Aoe T., Bartolozzi A., Sarva J., Zhou J., Kozikowski A., Wroblewska B., Bzdega T., Neale J. H. (2007) Local administration of N-acetylaspartylglutamate (NAAG) peptidase inhibitors is analgesic in peripheral pain in rats. Eur. J. Neurosci. 25, 147–158 [DOI] [PubMed] [Google Scholar]
- 60. Schlumberger C., Schäfer D., Barberi C., Morè L., Nagel J., Pietraszek M., Schmidt W. J., Danysz W. (2009) Effects of a metabotropic glutamate receptor group II agonist LY354740 in animal models of positive schizophrenia symptoms and cognition. Behav. Pharmacol. 20, 56–66 [DOI] [PubMed] [Google Scholar]
- 61. Profaci C. P., Krolikowski K. A., Olszewski R. T., Neale J. H. (2011) Group II mGluR agonist LY354740 and NAAG peptidase inhibitor effects on prepulse inhibition in PCP and d-amphetamine models of schizophrenia. Psychopharmacology 216, 235–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Gregory K. J., Dong E. N., Meiler J., Conn P. J. (2011) Allosteric modulation of metabotropic glutamate receptors. Structural insights and therapeutic potential. Neuropharmacology 60, 66–81 [DOI] [PMC free article] [PubMed] [Google Scholar]