Abstract
The accumulation of insoluble proteins is a pathological hallmark of several neurodegenerative disorders. Tauopathies are caused by the dysfunction and aggregation of tau protein and an impairment of cellular protein degradation pathways may contribute to their pathogenesis. Thus, a deficiency in autophagy can cause neurodegeneration, while activation of autophagy is protective against some proteinopathies. Little is known about the role of autophagy in animal models of human tauopathy. In the present report, we assessed the effects of autophagy stimulation by trehalose in a transgenic mouse model of tauopathy, the human mutant P301S tau mouse, using biochemical and immunohistochemical analyses. Neuronal survival was evaluated by stereology. Autophagy was activated in the brain, where the number of neurons containing tau inclusions was significantly reduced, as was the amount of insoluble tau protein. This reduction in tau aggregates was associated with improved neuronal survival in the cerebral cortex and the brainstem. We also observed a decrease of p62 protein, suggesting that it may contribute to the removal of tau inclusions. Trehalose failed to activate autophagy in the spinal cord, where it had no impact on the level of sarkosyl-insoluble tau. Accordingly, trehalose had no effect on the motor impairment of human mutant P301S tau transgenic mice. Our findings provide direct evidence in favour of the degradation of tau aggregates by autophagy. Activation of autophagy may be worth investigating in the context of therapies for human tauopathies.
Keywords: autophagy, neurodegenerative disorders, neuroprotection, protein aggregation, tau
Introduction
Most neurodegenerative diseases are characterized by the accumulation of misfolded proteins. Thus, α-synuclein aggregates in Parkinson’s disease and other synucleinopathies, TDP-43 (TAR DNA-binding protein 43) in TDP-43 proteinopathies, huntingtin in Huntington’s disease and both β-amyloid and tau in Alzheimer’s disease (Goedert et al., 2010). Filaments made of hyperphosphorylated tau protein are a good predictor of the cognitive state in Alzheimer’s disease, because a negative correlation has been described between the number of neurofibrillary tangles, and the Mini-Mental score (Giannakopoulos et al., 2003). Tau deposits also constitute the defining pathological hallmark of several other diseases, including progressive supranuclear palsy, corticobasal degeneration, Pick’s disease and frontotemporal dementia and parkinsonism linked to chromosome 17 (Goedert and Jakes, 2005; Goedert and Spillantini, 2011). Since filament assembly is a concentration-dependent process, a reduction in the production and/or increased clearance of tau are potential targets.
In eukaryotic cells, there are two major systems responsible for the degradation of cytoplasmic proteins: the ubiquitin–proteasome system and the autophagy–lysosome pathway (Goldberg, 2003). The accumulation of ubiquitinated tau in intracellular filamentous inclusions suggests a possible impairment of tau degradation by the ubiquitin–proteasome system and/or the autophagy–lysosome pathway. Indeed, inactivation of the ubiquitin–proteasome system or the autophagy–lysosome pathway has been reported to lead to neurodegeneration associated with the presence of ubiquitinated aggregates (Hara et al., 2006; Komatsu et al., 2006; Bedford et al., 2008; Riley et al., 2010). Particularly, ubiquitin–proteasome system or autophagy–lysosome pathway inhibition induces the accumulation of tau (Blard et al., 2006; Ramesh Babu et al., 2008; Liu et al., 2009; Lee et al., 2010). Moreover, accumulation of autophagic vacuoles has been observed in Alzheimer’s disease brain and in a mouse model of tauopathy (Lin et al., 2003; Nixon et al., 2005), suggesting a possible disturbance of lysosomal proteolysis in tauopathy. Macroautophagy (hereafter referred to as autophagy) is the major form of autophagy. Although it is known that the suppression of autophagy causes neurodegeneration (Hara et al., 2006), relatively little is known about the role of autophagy in animal models of human tauopathy. In the triple transgenic mouse model of Alzheimer’s disease and in a mouse model expressing human mutant tau on a null background for the E3 ubiquitin ligase parkin, stimulation of autophagy promoted the degradation of insoluble tau (Caccamo et al., 2011; Majumder et al., 2011; Rodriguez-Navarro et al., 2011). However, these studies did not allow one to determine whether the effects of autophagy stimulation were due to a direct interaction between the autophagic machinery and tau or whether they were indirect. To date, there has been no report of the effect of increased autophagy in a mouse model of human tauopathy. We therefore administered trehalose, a mammalian target of rapamycin (mTOR)-independent activator of autophagy (Noda and Ohsumi, 1998; Sarkar et al., 2007a), to mice transgenic for human mutant P301S tau.
Materials and methods
Antibodies
We used phosphorylation-independent anti-tau antibodies BR133 and BR134 which recognize both murine and human tau isoforms. Phosphorylation-dependent anti-tau antibodies AT8 and AT100 (Innogenetics) were also used. AT8 recognizes tau protein phosphorylated at S202 and T205 (Goedert et al., 1995), whereas AT100 detects tau phosphorylated at T212, S214 and T217 (Yoshida and Goedert, 2006). Autophagy was assessed using an antibody against microtubule-associated protein light chain 3 (LC3) (Novus Biologicals). We also used an antibody against p62/SQSTM1 (Santa Cruz Biotechnology). To normalize protein levels, an anti-GAPDH (Millipore, Ltd.) antibody was used.
Animals and treatment
Homozygous human P301S tau transgenic mice (Allen et al., 2002) and age-matched wild-type C57BL/6 mice were used (n = 6 per group for western blot analysis; n = 3 per group for immunohistochemistry and stereology experiments). From weaning onwards, the animals were treated with 2% trehalose or 2% sucrose, which were used as the control. A separate group received normal drinking water (no treatment). The water solutions were changed twice weekly. Animals were checked daily and the quantity of water drunk and the animals’ weights were assessed twice a week. P301S tau transgenic mice develop motor deficits at ∼3–4 months of age, as shown using Rotarod (Scattoni et al., 2010). We routinely evaluated these deficits by visual inspection, as they form in a stereotypical manner consisting of an unstable gait followed by general stiffness and hindlimb paralysis. Furthermore, at 3–4 months of age, the human P301S tau mice are unable to extend their hindlimbs when lifted by the tail (Allen et al., 2002). In this study, we assessed the motor deficits by visual inspection and used the time when all the animals had developed hindleg stiffness and were unable to extend their hindlimbs when suspended by the tail as the endpoint (20 weeks of age).
Tissue extraction
To extract sarkosyl-insoluble proteins from brain and spinal cord, frozen tissues were weighed and homogenized in cold extraction buffer [10 mM Tris–HCl, pH 7.4, 800 mM NaCl, 1 mM EGTA, 0.1 M phenylmethylsulphonyl fluoride, 10% sucrose and one tablet of complete protease inhibitor cocktail (Roche)]. The homogenates were spun at 6000g for 20 min and the supernatants incubated with 1% sarkosyl for 1 h at room temperature. After a 1 h centrifugation at 80 000 g, the supernatants were discarded and the pellets resuspended in 50 mM Tris–HCl, pH 7.4 (300 µl/g tissue). For investigation of soluble tau, brains and spinal cords were homogenized in 25 mM Tris–HCl, pH 7.4, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM phenylmethylsulphonyl fluoride and one tablet of complete protease inhibitor cocktail. Samples were centrifuged at 80 000 g for 15 min. The resulting supernatant constituted the soluble fraction. The samples were analysed by SDS-PAGE.
Immunoblot analysis
After SDS-PAGE, the gels were blotted for 1 h at room temperature onto polyvinylidene difluoride membranes (Millipore). The membranes were blocked for 1 h at room temperature in 0.1 M phosphate buffer, pH 7.4, 0.2% Tween 20 and 5% milk, followed by an overnight incubation with the primary antibody in blocking buffer. Membranes were then washed in 0.1 M phosphate buffer, pH 7.4, 0.2% Tween 20 and incubated for 1 h at room temperature with peroxidase-conjugated secondary antibody (Pierce) in blocking solution. After washing, the blots were developed using enhanced chemiluminescence (Amersham Biosciences).
Quantification of proteins
Band intensities were quantified (Chemidoc™ XRS and Quantity One® software, Bio-Rad) and the amounts of soluble proteins normalized with respect to GAPDH. Insoluble tau was normalized relative to the tissue weight. Data were expressed as means ± SEM. Statistical analysis was performed using a one-way ANOVA test followed by Tukey’s post hoc comparisons (Prism, GraphPad Software, Inc.).
Immunohistochemistry
Mice were perfused transcardially with 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4. Brains and spinal cords were dissected and post-fixed overnight at 4°C, followed by cryoprotection in PBS containing 30% sucrose for at least 24 h. Serial sagittal brain sections (30 µm) were cut on a Leica SM2400 microtome (Leica Microsystems) and stored at 4°C in PBS containing 0.1% sodium azide. For fluorescence labelling, sections were permeabilized for 5 min with cold PBS containing 0.5% Triton X-100 and washed three times with PBS, 0.1% Triton X-100 (PBST). After 2 h blocking at room temperature with PBST containing 3% bovine serum albumin, sections were incubated with the primary antibodies in blocking solution for 24 h at 4°C. This was followed by three washes and a 2 h incubation at room temperature with Alexa Fluor® 488 (Molecular Probes) or Cy5 (Abcam) secondary antibodies in blocking solution. After washing and DAPI (4,6-diamidino-2-phenylindole) staining, the sections were mounted using Vectashield mounting medium (Vector Laboratories). They were analysed using a Leica DMRB fluorescence microscope (Leica) or a Radiance 2100 confocal microscope (Bio-Rad). Images were taken using a Sharp 2000 laser (Zeiss Bio-Rad) and were typically 512 × 512 pixels. Staining for neuronal nuclei (NeuN; Chemicon) (Mullen et al., 1992; Wolf et al., 1996; Lind et al., 2005) and tau phosphorylated at the AT100 epitope were carried out in brains of wild-type and transgenic mice using immunoperoxidase (Vectastain ABC kit, Vector Laboratories). Endogenous peroxidase was quenched for 15 min with 3% H2O2 in distilled water. After washing, sections were blocked for 1 h with normal horse serum in PBST and incubated overnight with the anti-NeuN antibody (1:500) in blocking solution at 4°C. After three rinses with PBST, sections were incubated with a biotin-conjugated anti-mouse antibody for 2 h at room temperature. Next, the avidin–biotin-conjugated complex was applied for a further 2 h. Finally, the antigen was visualized using the Vector® VIP substrate kit (Vector Laboratories). Sections were mounted on SuperFrost® glass slides (VWR international) and dehydrated using a series of ascending ethanol solutions (70, 95 and 100%) and xylene.
Stereology
The Stereo Investigator 9 (MBF Bioscience) was used to quantify the number of NeuN-positive cells. Sections through the brain were sampled in a systematic random manner using 1:12 series for quantification of NeuN-positive and AT100-positive neurons. The thickness of each section was determined using the Stereo Investigator 9 software. For a given section, the outline of the region of interest was traced under a ×10 objective and the enclosed area calculated by the software. Sections within the highlighted area were then sampled at random and cells counted under the ×40 objective to determine the total number of immunoreactive cells. Statistical analyses were performed using a one-way ANOVA test followed by Tukey’s post hoc comparisons (Prism, GraphPad Software, Inc.).
Results
Animal health
Water consumption of the three groups (no treatment, sucrose-treated and trehalose-treated) was similar. Sucrose and trehalose had no impact on the animals’ weights or coat aspects, suggesting that the health of the mice was similar among the three groups. The human P301S tau mice were sacrificed at 20 weeks of age, when they developed an unstable gait and motor abnormalities, which is typical of homozygous transgenic mice (Allen et al., 2002). The development of motor deficits was not significantly different between treated and non-treated human P301S tau transgenic mice. Thus, untreated mice developed the first motor deficits at 19 ± 0.19 weeks of age, which was not significantly different from the ages at which they appeared in sucrose-treated (18.8 ± 0.31 weeks) and trehalose-treated (19.25 ± 0.23 weeks) mice (Supplementary Fig. 1).
Effects of trehalose on autophagy activation
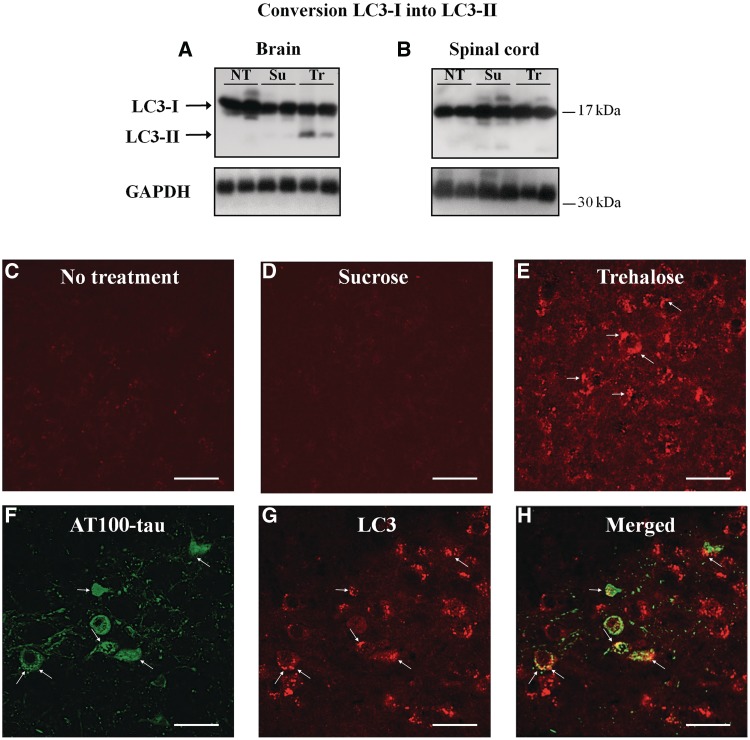
To investigate autophagy activation, we measured the conversion of LC3-I into LC3-II, a marker of autophagic vacuole formation (Kabeya et al., 2000; Mizushima et al., 2004; Tanida et al., 2004). We observed the conversion of 17.5 ± 2.3% LC3-I into LC3-II in the brains of trehalose-treated mice, while no detectable LC3-I conversion was seen in untreated or sucrose-treated mice (Fig. 1A). Unexpectedly, trehalose had no effect on LC3-I conversion in the spinal cord (Fig. 1B). We therefore focused our study on the brain. Immunohistochemistry of brain sections from mice transgenic for human mutant P301S tau showed the presence of LC3 puncta in trehalose-treated mice (Fig. 1E). The punctate distribution of LC3 was absent from control groups (Fig. 1C and D). In trehalose-treated transgenic mice, AT100-positive tau aggregates were also immunoreactive for LC3, indicating the co-localization of autophagic vacuoles and tau aggregates (arrows, Fig. 1F–H).
Figure 1.
Conversion of LC3-I into LC3-II was assessed by western blotting in the spinal cord (A) and the brain (B) of mice transgenic for human P301S tau. LC3-II staining was only present in brain where autophagic vacuoles were observed in trehalose-treated mice (arrow in E). Autophagic vacuoles were not present in the brains of non-treated (C) or sucrose-treated (D) mice. (F–H) Confocal analysis indicated that AT100-positive tau inclusions (F) were also immunoreactive for LC3-II (G), suggesting the presence of tau inclusions in autophagic vacuoles in trehalose-treated transgenic mice (H). NT = no treatment; Su = sucrose-treated; Tr = trehalose-treated. Scale bar = 20 µm.
Effects of trehalose on tau pathology and neuronal survival
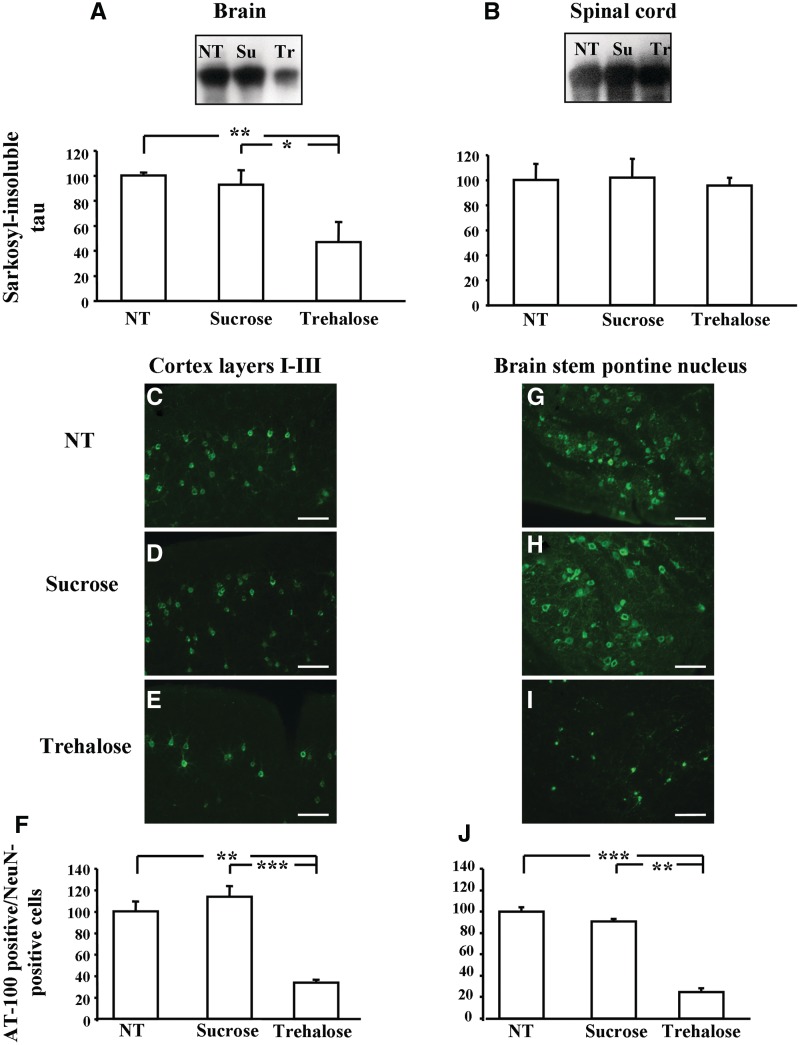
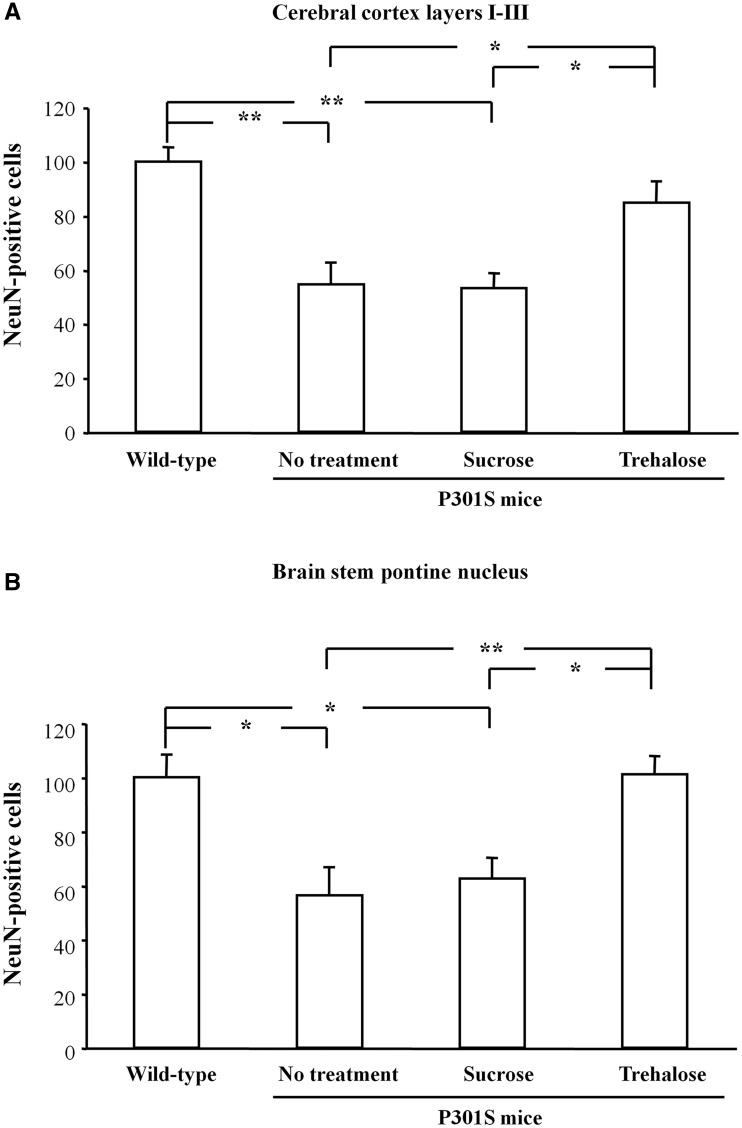
Total soluble tau and soluble tau phosphorylated at the AT8 epitope were not affected by trehalose treatment (Supplementary Fig. 2). In contrast, quantification of sarkosyl-insoluble tau in the brain indicated that trehalose decreased the amount of insoluble tau compared with untreated controls (−53%, P < 0.01) or to controls treated with sucrose (−46%, P < 0.05) (Fig. 2A). Trehalose treatment had no effect on the amount of sarkosyl-insoluble tau in the spinal cord (Fig. 2B). Immunofluorescence staining for anti-tau antibody AT100 showed a drastic reduction in the number of positive cells in layers I–III of the cerebral cortex (−66.5% compared with untreated animals, P < 0.01; −80.1% compared with sucrose-treated mice, P < 0.001) (Fig. 2C–F) and in the brainstem, particularly the pontine nucleus (−75% compared with untreated animals, P < 0.001; −58.6% compared with sucrose-treated mice, P < 0.01) (Fig. 2G–J). Nerve cell numbers were counted in layers I–III of the cerebral cortex and in the pontine nucleus. The number of neurons in layers I–III was higher in trehalose-treated P301S tau mice than in animals that received either no treatment (+55.5%, P < 0.05) or were treated with sucrose (+59.6%, P < 0.05) (Fig. 3A). The number of neurons in trehalose-treated mice and in untreated wild-type animals was not significantly different (Fig. 3A). In the pontine nucleus, the number of neurons increased in trehalose-treated animals compared with untreated (+79.2%, P < 0.01) and sucrose-treated controls (+61.6%, P < 0.05) (Fig. 3B). The number of NeuN-positive cells in the pontine nucleus was similar in trehalose-treated P301S tau mice and in wild-type controls (Fig. 3B).
Figure 2.
Sarkosyl-insoluble tau was decreased in the brain of trehalose-treated mice compared with non-treated or sucrose-treated animals (A), while trehalose treatment had no effect on sarkosyl-insoluble tau in the spinal cord (B). In trehalose-treated mice, the number of nerve cells with AT100-positive inclusions was drastically reduced in layers I–III of the cerebral cortex (C–F) and in the pontine nucleus of the brainstem (G–J). NT = no treatment; Su = sucrose-treated; Tr = trehalose-treated. *P < 0.05, **P < 0.01 and ***P < 0.001.
Figure 3.
Nerve cell numbers were assessed using stereology. Trehalose treatment increased the number of neurons in layers I–III of the cerebral cortex (A) and in the pontine nucleus of the brainstem (B). *P < 0.05 and **P < 0.01.
Effects of trehalose on p62/SQSTM1
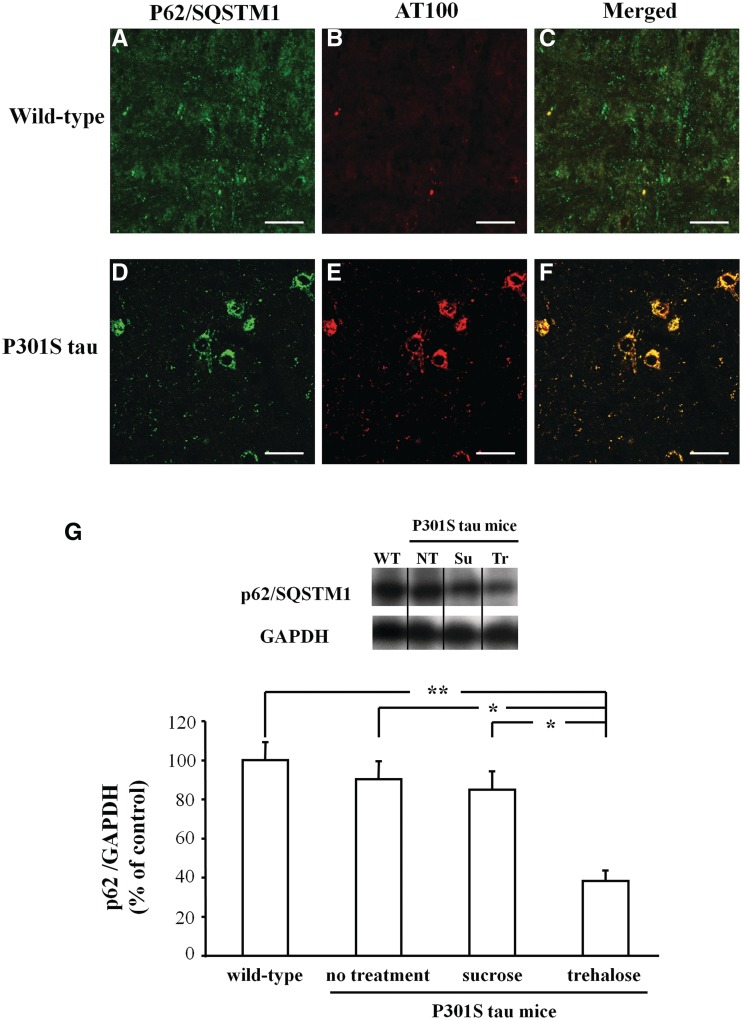
Immunohistochemistry revealed the co-localization of p62/SQSTM1 and AT100-positive tau aggregates in P301S tau transgenic mice (Fig. 4D–F), while no co-staining was observed in wild-type controls (Fig. 4A–C). We assessed the levels of p62/SQSTM1 in the brains of wild-type and transgenic P301S tau mice subjected to the different treatments. We observed a reduction in p62/SQSTM1 levels in mice treated with trehalose compared with mice that received either no treatment (−52.1%, P < 0.05) or sucrose (−46.7%, P < 0.05) (Fig. 4G). No significant difference was detected between wild-type controls and P301S tau transgenic mice (Fig. 4G).
Figure 4.
Immunohistochemistry for p62/SQSTM1 (A and D) and assembled tau (AT100; B and E) showed co-localization in P301S tau transgenic mice (F) but not in wild-type animals (C). Scale bar = 20 µm. (G) Western blot of p62/SQSTM1 levels in the brains of wild-type controls and mice transgenic for human mutant P301S tau that received either no treatment or were given sucrose or trehalose. The amount of p62/SQSTM1 signal is shown normalized with respect to GAPDH. *P < 0.05 and **P < 0.01.
Discussion
The assembly of normally soluble proteins into amyloid-like fibrils is a pathological hallmark of several neurodegenerative disorders, with tauopathies being the most common (Goedert et al., 2010). Herein, we report on the effects of stimulation of autophagy on tau inclusions and nerve cell survival in a mouse line transgenic for human mutant P301S tau. Trehalose activated autophagy in the brain, as shown by the increased formation of LC3-II, and reduced the level of sarkosyl-insoluble tau. However, it did not stimulate autophagy in the spinal cord and consequently had no effect on the level of insoluble tau. The mechanism of action of trehalose is not completely understood, but it is believed to activate autophagy in an mTOR-independent manner (Sarkar et al., 2007a). These findings suggest that autophagic pathways may differ between brain and spinal cord. The absence of a detectable effect of trehalose in the spinal cord probably accounted for the persistence of a pathological motor phenotype in treated P301S tau transgenic mice. We cannot exclude that a detailed behavioural assessment could have revealed subtle differences between treated and non-treated mice. However, the motor deficit characteristic of the human P301S tau transgenic mice probably results from the degeneration of spinal cord motor neurons, consequent to the development of a toxic filamentous tau pathology (Allen et al., 2002; Delobel et al., 2008), and possibly the sequestration of proteins important for motor behaviour. As tau pathology in the spinal cord was unchanged after trehalose administration, an improvement in motor function was unlikely. The reasons underlying our inability to activate autophagy in the spinal cord remain to be determined. They could be related to a less efficient delivery of trehalose to the spinal cord, although it has been shown that the blood–spinal cord barrier is more permeable to tracers and cytokines than the blood–brain barrier (Prockop et al., 1995; Pan et al., 1997). We hope that future experiments will allow us to overcome this limitation.
In the brains of trehalose-treated mice, we observed a decrease in sarkosyl-insoluble tau and the number of nerve cells with tau inclusions. Besides its ability to activate autophagy, trehalose has also been reported to act as a protective chaperone in a murine model of Huntington’s disease (Tanaka et al., 2004). However, the reduction in tau inclusions in our mouse line was probably due to the degradation of tau aggregates by trehalose-induced autophagy. Indeed, we observed a decrease in tau inclusions only in regions where autophagy was activated. Moreover, we showed co-localization between autophagic vacuoles and tau inclusions, suggesting that tau aggregates were engulfed by autophagosomes before degradation. Sucrose, a chemical chaperone, which has been shown to reduce the conversion of the cellular prion protein to its protease-resistant form (DebBurman et al., 1997), failed to reduce tau aggregates in the human mutant P301S tau transgenic mice, confirming that the effect of trehalose on tau aggregates was mediated through an increase in autophagy.
Altogether, our results indicate that the in vivo stimulation of autophagy reduced the amount of tau aggregates and improved nerve cell survival. A role for autophagy in the clearance of protein aggregates has been suggested for a number of proteins, including β-amyloid, α-synuclein, ataxin-3, prion protein and huntingtin (Webb et al., 2003; Sarkar et al., 2007b; Pickford et al., 2008; Heiseke et al., 2009; Casarejos et al., 2011; Majumder et al., 2011; Riedel et al., 2011; Spilman et al., 2011). Activation of autophagy has also shown protective effects in mouse and fly models of tauopathy (Berger et al., 2006; Caccamo et al., 2011; Majumder et al., 2011; Rodriguez-Navarro et al., 2011). However, in mice, tau was not the only engineered protein. In one study, the mice overexpressed human mutant tau and carried a deletion of the gene encoding parkin (Rodriguez-Navarro et al., 2011), a potential mediator of autophagy (Olzmann et al., 2007; Chin et al., 2011). Therefore, not surprisingly, restoration of autophagic activity decreased the number of tau aggregates and improved cell survival. In separate studies, autophagy was activated in triple transgenic mice exhibiting tau and β-amyloid pathologies (Caccamo et al., 2011; Majumder et al., 2011). It is well established that β-amyloid can promote tau pathology in some brain regions (Götz et al., 2001; Lewis et al., 2001; Oddo et al., 2003, 2004). However, direct evidence for the relevance of autophagy in modulating tau pathology was missing. We now show that the activation of autophagy markedly reduced the number of tau aggregates and nerve cell loss in a mouse model of human tauopathy.
We also investigated p62/SQSTM1 expression in wild-type and P301S tau transgenic mice. p62/SQSTM1 is a protein that acts as a receptor for selective autophagy, mainly because of its ability to bind both ubiquitin and LC3 (Pankiv et al., 2007; Lamark et al., 2009). It has previously been implicated in the degradation of protein inclusions (Bjorkoy et al., 2006; Watanabe and Tanaka, 2011) and has been found in tau inclusions in human neurodegenerative diseases (Kuusisto et al., 2001, 2002; Zatloukal et al., 2002). We now show co-localization of p62/SQSTM1 with tau inclusions in P301S tau transgenic mice, consistent with the human pathology. In addition, we observed a decrease in p62/SQSTM1 levels in trehalose-treated mice, suggesting that p62/SQSTM1 may be an adaptor molecule for tau inclusions before their degradation by autophagy. Although the majority of tau filaments in P301S tau transgenic are not ubiquitinated (Allen et al., 2002), p62/SQSTM1 may nonetheless be involved, as it has been shown to play a role in the autophagic degradation of non-ubiquitinated protein aggregates (Watanabe and Tanaka, 2011). The levels of p62/SQSTM1 were similar between P301S tau transgenic mice and wild-type controls, indicating that p62/SQSTM1 synthesis and basal autophagy were not affected in the transgenic mice. In conclusion, we show that activation of autophagy in mice transgenic for human mutant P301S tau decreased the number of tau inclusions and increased nerve cell survival in cerebral cortex and brainstem.
Funding
This work was supported by the UK Medical Research Council (MRC file reference number U105184291) and, in part, by Alzheimer's Research UK. M.T. and D.T.W. are supported by the Swiss National Science Foundation (310030_135214 to M.T. and 32323B_123812 to D.W.T.).
Supplementary material
Supplementary material is available at Brain online.
Acknowledgements
We thank Angela Middleton and Claire Knox for their help with mice and the Biomedical Services (MRC Laboratory of Molecular Biology) for assistance.
Glossary
Abbreviation
- LC3
microtubule-associated protein light chain 3
References
- Allen B, Ingram E, Takao M, Smith MJ, Jakes R, Virdee K, et al. Abundant tau filaments and nonapoptotic neurodegeneration in transgenic mice expressing human P301S tau protein. J Neurosci. 2002;22:9340–51. doi: 10.1523/JNEUROSCI.22-21-09340.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedford L, Hay D, Devoy A, Paine S, Powe DG, Seth R, et al. Depletion of 26S proteasomes in mouse brain neurons causes neurodegeneration and Lewy-like inclusions resembling human pale bodies. J Neurosci. 2008;28:8189–98. doi: 10.1523/JNEUROSCI.2218-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger Z, Ravikumar B, Menzies FM, Oroz LG, Underwood BR, Pangalos MN, et al. Rapamycin alleviates toxicity of different aggregate-prone proteins. Hum Mol Genet. 2006;15:433––42. doi: 10.1093/hmg/ddi458. [DOI] [PubMed] [Google Scholar]
- Bjorkoy G, Lamark T, Johansen T. p62/SQSTM1: a missing link between protein aggregates and the autophagy machinery. Autophagy. 2006;2:138–9. doi: 10.4161/auto.2.2.2405. [DOI] [PubMed] [Google Scholar]
- Blard O, Frebourg T, Campion D, Lecourtois M. Inhibition of proteasome and Shaggy/Glycogen synthase kinase-3β kinase prevents clearance of phosphorylated tau in Drosophila. J Neurosci Res. 2006;84:1107–15. doi: 10.1002/jnr.21006. [DOI] [PubMed] [Google Scholar]
- Caccamo A, Majumder S, Richardson A, Strong R, Oddo S. Molecular interplay between mammalian target of rapamycin (mTOR), amyloid-β, and Tau: effects on cognitive impairments. J Biol Chem. 2011;285:13107–20. doi: 10.1074/jbc.M110.100420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casarejos MJ, Solano RM, Gomez A, Perucho J, de Yebenes JG, Mena MA. The accumulation of neurotoxic proteins, induced by proteasome inhibition, is reverted by trehalose, an enhancer of autophagy, in human neuroblastoma cells. Neurochem Int. 2011;58:512–20. doi: 10.1016/j.neuint.2011.01.008. [DOI] [PubMed] [Google Scholar]
- Chin LS, Olzmann JA, Li L. Parkin-mediated ubiquitin signalling in aggresome formation and autophagy. Biochem Soc Trans. 2011;38:144–9. doi: 10.1042/BST0380144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DebBurman SK, Raymond GJ, Caughey B, Lindquist S. Chaperone-supervised conversion of prion protein to its protease-resistant form. Proc Natl Acad Sci USA. 1997;94:13938–43. doi: 10.1073/pnas.94.25.13938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delobel P, Lavenir I, Fraser G, Ingram E, Holzer M, Ghetti B, et al. Analysis of tau phosphorylation and truncation in a mouse model of human tauopathy. Am J Pathol. 2008;172:123–31. doi: 10.2353/ajpath.2008.070627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannakopoulos P, Herrmann FR, Bussiere T, Bouras C, Kovari E, Perl DP, et al. Tangle and neuron numbers, but not amyloid load, predict cognitive status in Alzheimer’s disease. Neurology. 2003;60:1495–500. doi: 10.1212/01.wnl.0000063311.58879.01. [DOI] [PubMed] [Google Scholar]
- Goedert M, Clavaguera F, Tolnay M. The propagation of prion-like protein inclusions in neurodegenerative diseases. Trends Neurosci. 2010;33:317–25. doi: 10.1016/j.tins.2010.04.003. [DOI] [PubMed] [Google Scholar]
- Goedert M, Jakes R. Mutations causing neurodegenerative tauopathies. Biochim Biophys Acta. 2005;1739:240–50. doi: 10.1016/j.bbadis.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Goedert M, Jakes R, Vanmechelen E. Monoclonal antibody AT8 recognises tau protein phosphorylated at both serine 202 and threonine 205. Neurosci Lett. 1995;189:167–9. doi: 10.1016/0304-3940(95)11484-e. [DOI] [PubMed] [Google Scholar]
- Goedert M, Spillantini MG. Pathogenesis of the Tauopathies. J Mol Neurosci. 2011;45:7. doi: 10.1007/s12031-011-9593-4. [DOI] [PubMed] [Google Scholar]
- Goldberg AL. Protein degradation and protection against misfolded or damaged proteins. Nature. 2003;426:895–9. doi: 10.1038/nature02263. [DOI] [PubMed] [Google Scholar]
- Götz J, Chen F, van Dorpe J, Nitsch RM. Formation of neurofibrillary tangles in P301L tau transgenic mice induced by Abeta 42 fibrils. Science. 2001;293:1491–5. doi: 10.1126/science.1062097. [DOI] [PubMed] [Google Scholar]
- Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R, et al. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006;441:885–9. doi: 10.1038/nature04724. [DOI] [PubMed] [Google Scholar]
- Heiseke A, Aguib Y, Riemer C, Baier M, Schatzl HM. Lithium induces clearance of protease resistant prion protein in prion-infected cells by induction of autophagy. J Neurochem. 2009;109:25–34. doi: 10.1111/j.1471-4159.2009.05906.x. [DOI] [PubMed] [Google Scholar]
- Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, et al. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000;19:5720–8. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu M, Waguri S, Chiba T, Murata S, Iwata J, Tanida I, et al. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature. 2006;441:880–4. doi: 10.1038/nature04723. [DOI] [PubMed] [Google Scholar]
- Kuusisto E, Salminen A, Alafuzoff I. Ubiquitin-binding protein p62 is present in neuronal and glial inclusions in human tauopathies and synucleinopathies. Neuroreport. 2001;12:2085–90. doi: 10.1097/00001756-200107200-00009. [DOI] [PubMed] [Google Scholar]
- Kuusisto E, Salminen A, Alafuzoff I. Early accumulation of p62 in neurofibrillary tangles in Alzheimer’s disease: possible role in tangle formation. Neuropathol Appl Neurobiol. 2002;28:228–37. doi: 10.1046/j.1365-2990.2002.00394.x. [DOI] [PubMed] [Google Scholar]
- Lamark T, Kirkin V, Dikic I, Johansen T. NBR1 and p62 as cargo receptors for selective autophagy of ubiquitinated targets. Cell Cycle. 2009;8:1986–90. doi: 10.4161/cc.8.13.8892. [DOI] [PubMed] [Google Scholar]
- Lee BH, Lee MJ, Park S, Oh DC, Elsasser S, Chen PC, et al. Enhancement of proteasome activity by a small-molecule inhibitor of USP14. Nature. 2010;467:179–84. doi: 10.1038/nature09299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis J, Dickson DW, Lin WL, Chisholm L, Corral A, Jones G, et al. Enhanced neurofibrillary degeneration in transgenic mice expressing mutant tau and APP. Science. 2001;293:1487–91. doi: 10.1126/science.1058189. [DOI] [PubMed] [Google Scholar]
- Lin WL, Lewis J, Yen SH, Hutton M, Dickson DW. Ultrastructural neuronal pathology in transgenic mice expressing mutant (P301L) human tau. J Neurocytol. 2003;32:1091–105. doi: 10.1023/B:NEUR.0000021904.61387.95. [DOI] [PubMed] [Google Scholar]
- Lind D, Franken S, Kappler J, Jankowski J, Schilling K. Characterization of the neuronal marker NeuN as a multiply phosphorylated antigen with discrete subcellular localization. J Neurosci Res. 2005;79:295–302. doi: 10.1002/jnr.20354. [DOI] [PubMed] [Google Scholar]
- Liu YH, Wei W, Yin J, Liu GP, Wang Q, Cao FY, et al. Proteasome inhibition increases tau accumulation independent of phosphorylation. Neurobiol Aging. 2009;30:1949–61. doi: 10.1016/j.neurobiolaging.2008.02.012. [DOI] [PubMed] [Google Scholar]
- Majumder S, Richardson A, Strong R, Oddo S. Inducing autophagy by rapamycin before, but not after, the formation of plaques and tangles ameliorates cognitive deficits. PLoS One. 2011;6:e25416. doi: 10.1371/journal.pone.0025416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima N, Yamamoto A, Matsui M, Yoshimori T, Ohsumi Y. In vivo analysis of autophagy in response to nutrient starvation using transgenic mice expressing a fluorescent autophagosome marker. Mol Biol Cell. 2004;15:1101–11. doi: 10.1091/mbc.E03-09-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen RJ, Buck CR, Smith AM. NeuN, a neuronal specific nuclear protein in vertebrates. Development. 1992;116:201–11. doi: 10.1242/dev.116.1.201. [DOI] [PubMed] [Google Scholar]
- Nixon RA, Wegiel J, Kumar A, Yu WH, Peterhoff C, Cataldo A, et al. Extensive involvement of autophagy in Alzheimer disease: an immuno-electron microscopy study. J Neuropathol Exp Neurol. 2005;64:113–22. doi: 10.1093/jnen/64.2.113. [DOI] [PubMed] [Google Scholar]
- Noda T, Ohsumi Y. Tor, a phosphatidylinositol kinase homologue, controls autophagy in yeast. J Biol Chem. 1998;273:3963–6. doi: 10.1074/jbc.273.7.3963. [DOI] [PubMed] [Google Scholar]
- Oddo S, Billings L, Kesslak JP, Cribbs DH, LaFerla FM. Aβ immunotherapy leads to clearance of early, but not late, hyperphosphorylated tau aggregates via the proteasome. Neuron. 2004;43:321–32. doi: 10.1016/j.neuron.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Kitazawa M, Tseng BP, LaFerla FM. Amyloid deposition precedes tangle formation in a triple transgenic model of Alzheimer’s disease. Neurobiol Aging. 2003;24:1063–70. doi: 10.1016/j.neurobiolaging.2003.08.012. [DOI] [PubMed] [Google Scholar]
- Olzmann JA, Li L, Chudaev MV, Chen J, Perez FA, Palmiter RD, et al. Parkin-mediated K63-linked polyubiquitination targets misfolded DJ-1 to aggresomes via binding to HDAC6. J Cell Biol. 2007;178:1025–38. doi: 10.1083/jcb.200611128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan W, Banks WA, Kastin AJ. Permeability of the blood-brain and blood-spinal cord barriers to interferons. J Neuroimmunol. 1997;76:105–11. doi: 10.1016/s0165-5728(97)00034-9. [DOI] [PubMed] [Google Scholar]
- Pankiv S, Clausen TH, Lamark T, Brech A, Bruun JA, Outzen H, et al. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J Biol Chem. 2007;282:24131–45. doi: 10.1074/jbc.M702824200. [DOI] [PubMed] [Google Scholar]
- Pickford F, Masliah E, Britschgi M, Lucin K, Narasimhan R, Jaeger PA, et al. The autophagy-related protein beclin 1 shows reduced expression in early Alzheimer disease and regulates amyloid beta accumulation in mice. J Clin Invest. 2008;118:2190–9. doi: 10.1172/JCI33585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prockop LD, Naidu KA, Binard JE, Ransohoff J. Selective permeability of [3H]-D-mannitol and [14C]-carboxyl-inulin across the blood-brain barrier and blood-spinal cord barrier in the rabbit. J Spinal Cord Med. 1995;18:221–6. doi: 10.1080/10790268.1995.11719399. [DOI] [PubMed] [Google Scholar]
- Ramesh Babu J, Lamar Seibenhener M, Peng J, Strom AL, Kemppainen R, Cox N, et al. Genetic inactivation of p62 leads to accumulation of hyperphosphorylated tau and neurodegeneration. J Neurochem. 2008;106:107–20. doi: 10.1111/j.1471-4159.2008.05340.x. [DOI] [PubMed] [Google Scholar]
- Riedel M, Goldbaum O, Schwarz L, Schmitt S, Richter-Landsberg C. 17-AAG induces cytoplasmic α-synuclein aggregate clearance by induction of autophagy. PLoS One. 2011;5:e8753. doi: 10.1371/journal.pone.0008753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley BE, Kaiser SE, Shaler TA, Ng AC, Hara T, Hipp MS, et al. Ubiquitin accumulation in autophagy-deficient mice is dependent on the Nrf2-mediated stress response pathway: a potential role for protein aggregation in autophagic substrate selection. J Cell Biol. 2010;191:537–52. doi: 10.1083/jcb.201005012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Navarro JA, Rodriguez L, Casarejos MJ, Solano RM, Gomez A, Perucho J, et al. Trehalose ameliorates dopaminergic and tau pathology in parkin deleted/tau overexpressing mice through autophagy activation. Neurobiol Dis. 2011;39:423–38. doi: 10.1016/j.nbd.2010.05.014. [DOI] [PubMed] [Google Scholar]
- Sarkar S, Davies JE, Huang Z, Tunnacliffe A, Rubinsztein DC. Trehalose, a novel mTOR-independent autophagy enhancer, accelerates the clearance of mutant huntingtin and α-synuclein. J Biol Chem. 2007a;282:5641–52. doi: 10.1074/jbc.M609532200. [DOI] [PubMed] [Google Scholar]
- Sarkar S, Perlstein EO, Imarisio S, Pineau S, Cordenier A, Maglathlin RL, et al. Small molecules enhance autophagy and reduce toxicity in Huntington’s disease models. Nat Chem Biol. 2007b;3:331–8. doi: 10.1038/nchembio883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scattoni ML, Gasparini L, Alleva E, Goedert M, Calamandrei G, Spillantini MG. Early behavioural markers of disease in P301S tau transgenic mice. Behav Brain Res. 2010;208:250–7. doi: 10.1016/j.bbr.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Spilman P, Podlutskaya N, Hart MJ, Debnath J, Gorostiza O, Bredesen D, et al. Inhibition of mTOR by rapamycin abolishes cognitive deficits and reduces amyloid-beta levels in a mouse model of Alzheimer’s disease. PLoS One. 2011;5:e9979. doi: 10.1371/journal.pone.0009979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Machida Y, Niu S, Ikeda T, Jana NR, Doi H, et al. Trehalose alleviates polyglutamine-mediated pathology in a mouse model of Huntington disease. Nat Med. 2004;10:148–54. doi: 10.1038/nm985. [DOI] [PubMed] [Google Scholar]
- Tanida I, Ueno T, Kominami E. LC3 conjugation system in mammalian autophagy. Int J Biochem Cell Biol. 2004;36:2503–18. doi: 10.1016/j.biocel.2004.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y, Tanaka M. p62/SQSTM1 in autophagic clearance of a non-ubiquitylated substrate. J Cell Sci. 2011;124:2692–701. doi: 10.1242/jcs.081232. [DOI] [PubMed] [Google Scholar]
- Webb JL, Ravikumar B, Atkins J, Skepper JN, Rubinsztein DC. α-Synuclein is degraded by both autophagy and the proteasome. J Biol Chem. 2003;278:25009–13. doi: 10.1074/jbc.M300227200. [DOI] [PubMed] [Google Scholar]
- Wolf HK, Buslei R, Schmidt-Kastner R, Schmidt-Kastner PK, Pietsch T, Wiestler OD, et al. NeuN: a useful neuronal marker for diagnostic histopathology. J Histochem Cytochem. 1996;44:1167–71. doi: 10.1177/44.10.8813082. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Goedert M. Sequential phosphorylation of tau protein by cAMP-dependent protein kinase and SAPK4/p38δ or JNK2 in the presence of heparin generates the AT100 epitope. J Neurochem. 2006;99:154–64. doi: 10.1111/j.1471-4159.2006.04052.x. [DOI] [PubMed] [Google Scholar]
- Zatloukal K, Stumptner C, Fuchsbichler A, Heid H, Schnoelzer M, Kenner L, et al. p62 is a common component of cytoplasmic inclusions in protein aggregation diseases. Am J Pathol. 2002;160:255–63. doi: 10.1016/S0002-9440(10)64369-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.